Supplemental Digital Content is available in the text.
Summary:
The preliminary experience and results of an innovative surgical technique that incorporated a single-port and 3-dimensional (3D) videoscope system for endoscopic nipple-sparing mastectomy (E-NSM) were reported. The medical records of patients who underwent single-port 3D E-NSM for breast cancer during the period of August 2018 to October 2018 in a single institution were collected prospectively and analyzed. Patients’ reported cosmetic outcome results were also obtained. During the study period, 11 patients received 15 procedures of single-port 3D E-NSM. The mean operation time for single-port 3D E-NSM and immediate prosthesis breast reconstruction was 181.8 ± 32.4 minutes. The mean blood loss was 38.3 ± 45.3 ml (15–60). In the postoperative morbidity evaluation, 1 patient (6.7%) had delayed axillary wound healing and 2 (13.4%) had transient nipple ischemia, but there were no cases of total nipple areolar complex necrosis and implant loss observed. In addition, there were no cases with margin involvement or locoregional recurrence during the follow-up period. In patient-reported cosmetic outcomes, high (93.3%) satisfaction rates were observed in terms of postoperative scar appearance, location, and length. All patients who had received 3D E-NSM and immediate prosthesis breast reconstruction reported that they would choose the same operation again if given the chance to do so. From our preliminary experience, single-port 3D E-NSM is a feasible and safe procedure with good cosmetic results. Hence, this technique could be a promising new technique for patients with breast cancer indicated for nipple-sparing mastectomy.
INTRODUCTION
Minimally invasive surgery has become the mainstream of breast cancer surgeries, and surgical innovations of nipple-sparing mastectomy (NSM) such as endoscopic-assisted NSM (E-NSM)1–4 or robotic NSM (R-NSM)5–7 are increasingly applied in the surgical treatment of breast cancer.
Conventional 2-dimensional (2D) E-NSM was performed with 2 separate incisions over the axilla and peri-areolar regions.1,3,8,9 New technique modifications of E-NSM focused on single axillary incision,2,4,10 which could be performed with retraction-type endoscopic instruments4 or with a single-port and insufflation system.2 The inconsistent and suboptimal optical window achieved by 2D endoscopic camera and limited internal mobility with rigid endoscopic instruments resulted in a potentially difficult operation and thereby limited its widespread use.8,11
Three-dimensional (3D) imaging had been shown to enhance depth perception and thereby facilitate endoscopic or laparoscopic operations.12,13 In this study, we reported the technique, preliminary experience, and clinical outcome of single-port 3D E-NSM in the management of breast cancer. Indications for single-port 3D E-NSM were early breast cancer, tumor size of less than 5 cm with no evidence of multiple lymph node metastases, and skin or chest wall invasion. Women with large (breast cup size larger than E or breast mastectomy weight >600 g) and ptotic breasts are not good candidates for single-port 3D E-NSM and immediate prosthesis breast reconstruction due to technical difficulty and suboptimal cosmetic outcomes.
SINGLE-PORT 3D E-NSM TECHNIQUE
Preoperative marking was performed with the patient in the standing position. After induction, the patient was placed in a supine position with ipsilateral arm abducted at 90°. The ipsilateral shoulder was then elevated to 30° to facilitate access. A tumescent solution containing lactated Ringer’s solution with lidocaine 0.05% and epinephrine 1:1,000,000 was injected subcutaneously into the whole breast to minimize bleeding. An approximately 4–5 cm oblique axillary incision was made over the extramammary region near the anterior axillary line. Axillary staging procedure (sentinel lymph node biopsy and/or axillary lymph node dissection) was carried out as indicated.
APPLICATION OF SINGLE-PORT AND 3D SYSTEM
To create the working space for the placement of the single port (Glove Port; Nelis, Gyeonggi-do, Korea), the subcutaneous flap was dissected under direct vision for 3–4 cm. After port placement, carbon dioxide insufflation with air pressure at 8 mm Hg was performed to create space for mastectomy.2,5 A 30° 10-mm diameter camera TIPCAM 1 S 3D VIDEO Endoscope (KARL STORZ, Germany) was used. The position of the endoscopic stack, surgeon, anesthetist, and patient are shown in Figure 1A (Intraoperative layout of 3D endoscopic nipple sparing mastectomy). Dissection was carried out with laparoscopic curved metzenbaum scissors (KARL STORZ, Germany), whereas countertraction was carried out with a laparoscopic grasping forceps.
Fig. 1.
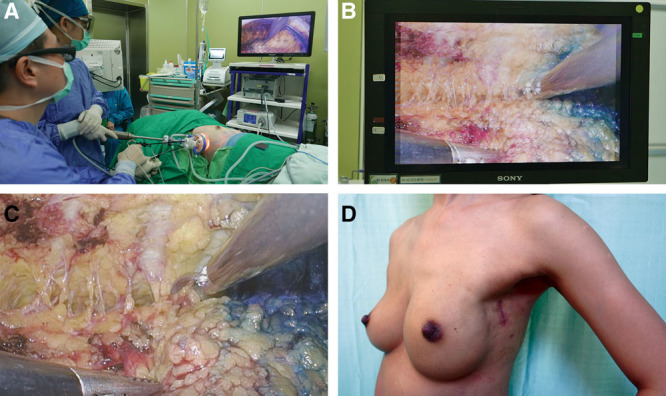
Intraoperative layout, endoscopic view of skin flap dissection and post operative view of 3D endoscopic nipple sparing mastectomy. A, Intraoperative layout demonstrating the position of surgeon, anesthetist, patient, and endoscopic stack system. B, Endoscopic view of intraoperative dissection of skin flap without 3D glasses. C, Endoscopic view of intraoperative dissection of skin flap with 3D glasses. D, Postoperative lateral view of patient A showing a well-healed scar hidden in the axilla.
The port placement of 3D scope, scissors, or grasping forceps was flexible and could be adjusted as necessary during the operation. To facilitate skin flap dissection, tunneling technique was applied,3,9 and the septum between the skin flap and parenchyma was subsequently dissected using laparoscopic metzenbaum scissors. During skin flap dissection, a 30° upward facing 3D endoscope with reverse 180° imaging was used to produce a clear 3D vision (Fig. 1). The angle and field of vision could be adjusted with either upward, downward, or reverse motion of the image by the 3D endoscope when necessary. For dissection near or beneath the nipple areolar complex (NAC) region, laparoscopic hook scissor (Snowden Pencer; BD, USA) was used to cut the dense glandular tissue. After which, a sub-nipple biopsy for frozen section was performed by taking 2 separate specimens (inner and outer part) under the NAC.14 If cancer cell invasion was found in the sub-nipple area, the entire NAC was removed and skin-sparing mastectomy was performed instead.3,9,14
After completion of superficial skin flap dissection, the peripheral and posterior dissection was carried out. Perforator vessels were clearly identified and adequately coagulated to achieve hemostasis. After the completion of dissection, the entire breast specimen was removed through the axillary incision.
BREAST RECONSTRUCTION
After removal of the breast, the single port was reinserted for submuscular pocket dissection. The laparoscopic grasping forceps were used to lift the pectoralis major muscle, and laparoscopic metzenbaum scissors or spatula tip suction coagulator was used to cut and dissect the plane. After completion of pocket dissection, implant was then inserted via the axillary incision. Two drains were placed (one beneath the skin flap and the other over the submuscular pocket) subsequently.
RESULTS
During the study period, 11 patients received 15 procedures of single-port 3D E-NSM. Ten patients (66.7%) had immediate prosthesis breast reconstruction with Gel implant (Mentor; Santa Barbara, California). The mean age of the patients was 50.9 ± 7.3 years old (37.9–66.9 years old). The mean tumor size was 3.41 ± 2.33 cm (0.4–6.9 cm). Majority of the cases were performed for DCIS (stage 0) (n = 4, 26.7%), followed by stage I (n = 3, 20%), stage II (n = 2, 13.3%), and stage III breast cancer (n = 2, 13.3%).
The mean operative time was 181.8 ± 32.4 minutes, and mean blood loss was 38.3 ± 45.3 ml. One patient (6.7%) had delayed axillary wound healing and 2 patients (13.4%) developed transient nipple ischemia. No total NAC necrosis or implant loss was observed. No patients (0%) had surgical margin involvement or locoregional recurrence.
The cosmetic outcomes of single-port 3D E-NSM were assessed via a self-administered questionnaire. All patients (100%) were satisfied with the location of the incision, and 93.3% were satisfied with the postoperative scar appearance and the wound length (Fig. 1D) (see figure, Supplemental Digital Content 1, which displays preoperative and postoperative photos of patient A: (A) preoperative front view of patient A; (B) preoperative lateral view of patient A; (C) postoperative front view of patient A; and (D) postoperative lateral view of patient A, http://links.lww.com/PRSGO/B175; see figure, Supplemental Digital Content 2, which displays preoperative and postoperative photos of patient B: (A) preoperative front view of patient B; (B) preoperative lateral view of patient B; (C) postoperative front view of patient B; and (D) postoperative lateral view of patient B, http://links.lww.com/PRSGO/B176; and video, which displays the technique of 3D videoscope-assisted endoscopic NSM in the management of breast cancer. All patients would agree that they would choose the same operation again if given a choice to do so.
Video.
DISCUSSION
In the current study, our preliminary experience of using single-port air insufflation system and 3D videoscope to perform E-NSM has shown favorable results in terms of clinical safety and patient satisfaction. The strength of this technique lies in the fact that it addresses the technical difficulty in 2D E-NSM and compares favorably to R-NSM in terms of cost. The limitations are the inferiority of conventional rigid endoscopic instruments compared with the more flexible instruments in R-NSM. Second, it requires an assistant to hold and control the camera compared with R-NSM whereby the need for assistance is obviated.
ACKNOWLEDGMENTS
The authors thank Ya-Ling Lin, Yun-Ting Chang, Shu-Hsin Pai, Yi-Ru Ke, and Shun-Ing Tsai for the assistance in this study.
Footnotes
Published online 19 August 2019.
Supported by the Ministry of Science and Technology of Taiwan, and the number of this funding was MOST 107-2314-B-371-006. This study was also sponsored by research funding provided by the Changhua Christian Hospital 104-CCH-ICO-006, 106-CCH-IRP-014, and 106-CCH-IRP-015.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
This study was approved by the Institutional Review Board of the Changhua Christian Hospital (CCH IRB No. 141224).
REFERENCES
- 1.Sakamoto N, Fukuma E, Higa K, et al. Early results of an endoscopic nipple-sparing mastectomy for breast cancer. Ann Surg Oncol. 2009;16:3406–3413. [DOI] [PubMed] [Google Scholar]
- 2.Tukenmez M, Ozden BC, Agcaoglu O, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction. J Laparoendosc Adv Surg Tech A. 2014;24:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai HW, Chen ST, Chen DR, et al. Current trends in and indications for endoscopy-assisted breast surgery for breast cancer: results from a six-year study conducted by the Taiwan Endoscopic Breast Surgery Cooperative Group. Plos One. 2016;11:e0150310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai HW, Lin SL, Chen ST, et al. Single-axillary-incision endoscopic-assisted hybrid technique for nipple-sparing mastectomy: technique, preliminary results, and patient-reported cosmetic outcome from preliminary 50 procedures. Ann Surg Oncol. 2018;25:1340–1349. [DOI] [PubMed] [Google Scholar]
- 5.Lai HW, Chen ST, Lin SL, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26:42–52. [DOI] [PubMed] [Google Scholar]
- 6.Sarfati B, Struk S, Leymarie N, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. [DOI] [PubMed] [Google Scholar]
- 7.Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leff DR, Vashisht R, Yongue G, et al. Endoscopic breast surgery: where are we now and what might the future hold for video-assisted breast surgery? Breast Cancer Res Treat. 2011;125:607–625. [DOI] [PubMed] [Google Scholar]
- 9.Lai HW, Wu HS, Chuang KL, et al. Endoscopy-assisted total mastectomy followed by immediate pedicled transverse rectus abdominis musculocutaneous (TRAM) flap reconstruction: preliminary results of 48 patients. Surg Innov. 2015;22:382–389. [DOI] [PubMed] [Google Scholar]
- 10.Lai HW. ASO author reflections: single axillary incision endoscopic-assisted hybrid technique for nipple-sparing mastectomy. Ann Surg Oncol. 2018;25(Suppl 3):626–627. [DOI] [PubMed] [Google Scholar]
- 11.Ingram D. Is it time for breast cancer surgeons to embrace endoscopic-assisted mastectomy? ANZ J Surg. 2008;78:837–838. [DOI] [PubMed] [Google Scholar]
- 12.Yoon J, Kang SI, Kim MH, et al. Comparison of short-term outcomes between 3D and 2D imaging laparoscopic colectomy with D3 lymphadenectomy for colon cancer. J Laparoendosc Adv Surg Tech A. 2019;29:340–345. [DOI] [PubMed] [Google Scholar]
- 13.Harada H, Kanaji S, Hasegawa H, et al. The effect on surgical skills of expert surgeons using 3D/HD and 2D/4K resolution monitors in laparoscopic phantom tasks. Surg Endosc. 2018;32:4228–4234. [DOI] [PubMed] [Google Scholar]
- 14.Chan SE, Liao CY, Wang TY, et al. The diagnostic utility of preoperative breast magnetic resonance imaging (MRI) and/or intraoperative sub-nipple biopsy in nipple-sparing mastectomy. Eur J Surg Oncol. 2017;43:76–84. [DOI] [PubMed] [Google Scholar]


