Abstract
Thus far, all clinical trials evaluating the efficacy of embryo transfer strategies have selectively delayed the first frozen embryo transfer (FET) by at least 1 menstrual cycle. Nevertheless, this approach, which is based solely on clinical experience, may create unnecessary psychological stress on infertile patients who are anxious to conceive as soon as possible. This study aimed to investigate whether the time interval between oocyte retrieval and subsequent FET affects reproductive outcomes.
We implemented a large retrospective cohort study in a single assisted reproductive technology (ART) unit at a university-based hospital, including 1540 autologous FET cycles performed in freeze-all cycles. The beginning of the FET was classified as either ‘cycle 1’ (performing FET within the first menstrual cycle) or ‘cycle ≥2’ (performing FET after one or more menstrual cycles). Live birth rate (LBR) was the primary outcome of our study.
The mean interval for ‘cycle 1’ and ‘cycle ≥2’ FETs was 25.72 ± 5.10 days and 75.33 ± 24.85 days, respectively (P < .001). The type of controlled ovarian hyperstimulation (COH) and endometrial preparation protocols differed significantly between groups (P = .008 and P = .004, respectively). However, FET groups were similar in many ways. Univariate analysis showed that there was no significant difference in LBR between the different cycles (33.1% after ‘cycle 1’ FET vs 34.2% after ‘cycle ≥2’ FET, P = .68). To evaluate whether LBR remained unchanged after adjustment for potential confounders, we performed multivariate logistic regression. FET timing had no significant impact on LBR in the first FET (odds ratio [OR]: 1.06, 95% confidence interval [CI]: 0.80–1.39).
In accordance with the present study, it might not be necessary for clinicians to wait more than 1 menstrual cycle before performing FET. This allows us to reduce otiose deferment in FET, without adversely affecting reproductive outcomes.
Keywords: frozen embryo transfer (FET), live birth rate, timing of FET
1. Introduction
ART has evolved dramatically over the last 4 decades.[1] However, despite these advances, most treatment cycles fail to lead to a live birth.[2] The live birth of a healthy baby is the ultimate target ART treatment. However, the failure of ART to lead to a live birth may be due to a number of factors, including embryo factors[3] or a decline in endometrial receptivity.[4,5] Reduced levels of endometrial receptivity following a fresh embryo transfer may be theoretically supported by abnormal histological and genetic alterations in the endometrium (because of supra-physiological levels of estrogen resulting from COH and an elevation in serum progesterone levels resulting in the over-maturation of the endometrium and “embryo-endometrial asynchrony”.[6–8]
Performing frozen-thawed embryo transfer (FET) in a subsequent cycle can mitigate this issue, maintaining increased safety levels while demonstrating improved pregnancy rates and better obstetric and perinatal outcomes.[9,10] Nevertheless, at present, we have not ascertained the optimal time interval between egg retrieval and FET. Furthermore, we do not know if longer delays lead to better pregnancy outcomes. Over recent years, many studies have compared the outcomes of 2 different schemes, namely immediate FET (within the first menstrual cycle) and delayed FET (following 1 or more menstrual cycles), and discussed options for the specific FET timing in the ‘freeze-all’ strategy.[11–16] Unsurprisingly, whether delaying FET is beneficial following the freeze-all protocol remains controversial.
In the present study, we carried out a cohort study to compare pregnancy outcomes between patients who underwent FET during the first cycle and those who underwent FET after subsequent cycles. We did this for several reasons: to investigate whether endometrial receptivity is impaired within the first menstrual cycle after oocyte retrieval; to assess whether it is worth delaying the timing from oocyte retrieval to FET, and to investigate the potential influence of timing on reproductive outcomes following the freeze all’ strategy.
2. Material & methods
2.1. Patients, inclusion criteria, and exclusion criteria
We implemented an observational retrospective cohort study between January 2016 and September 2018 in a single ART unit at a university-based reproductive medicine center, only including the first autologous FET following oocyte retrieval after a freeze-all protocol. The study was approved by the Health Authorities and Ethics Committees of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine (Grant No. TCM20180901011). All subjects signed the informed consent prior to being included in the study.
Generating a database from our electronic records, we included all cycles for women undergoing ovarian stimulation and IVF/intra-cytoplasmic sperm injection (ICSI) who met the following inclusion criteria:
-
(1)
age <45 years at the time of oocyte retrieval;
-
(2)
stimulation cycle completed with a freeze-all protocol rather than a fresh embryo transfer; and
-
(3)
day 3 (D3) cleavage stage embryo transferred instead of the blastocyst.
Patients with all diagnoses were included, as listed in Table 1.
Table 1.
Baseline demographic and cycle features of FETs that were carried out either within the immediate cycle following OPU (Cycle 1) or subsequently (Cycle ≥2) after a freeze-all protocol.
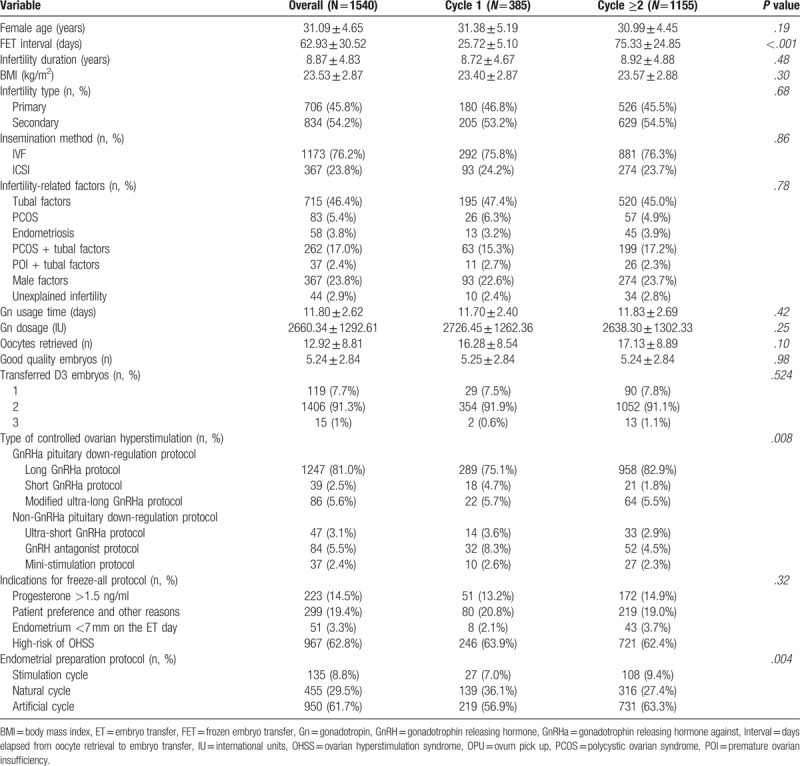
We excluded patients who did not undergo a stimulation cycle prior to FET, such as those receiving donor oocytes. Patients whose embryos were derived from a vitrified oocyte procedure, or preceding cycles with missing data, were also excluded.
Two groups were generated based on the number of cycles after COH:
-
(1)
a group for which the FET was performed within the first menstrual cycle after oocyte retrieval (‘cycle 1’) and
-
(2)
a group for which FET took place following 1 or more menstrual cycles (‘cycle ≥2’).
2.2. Ovarian stimulation
The following COH protocols were used in accordance with our institutional clinical protocols, with 150–450 IU/day of recombinant FSH (Puregon, MSD, Courbevoie, France; Gonal-F, Merck-Serono, Lyon, France) and urinary FSH (hMG, Menotrophin for Injection, Livzon Pharmaceutical Group Inc, Guangdong, China):
-
(1)
an ultra-short GnRH agonist protocol;
-
(2)
a short GnRH agonist protocol;
-
(3)
a long GnRH agonist protocol;
-
(4)
a modified ultra-long GnRH agonist protocol;
-
(5)
a GnRH antagonist protocol; and
-
(6)
a mini-stimulation protocol.
Gonadotropin doses, and the type of COH protocol, were determined according to the individual patient's characteristics. Final oocyte maturation was triggered when ≥3 ovarian dominant follicles of ≥17 mm were visible by ultrasound and when E2 levels were ≥1000 pg/mL. Final oocyte maturation was achieved using either a single injection of 0.2 mg of GnRH agonist (Triptoreline, Decapeptyl, Ipsen, France) or 250 μg of recombinant hCG (rhCG, Ovitrelle, Serono, France), according to the COH protocol. Oocyte retrieval was performed 35–36 hours later by transvaginal aspiration under ultrasound guidance.
2.3. Oocyte retrieval and embryo culture
Oocyte collection and embryo culture was performed using BD Falcon IVF medium (Becton, Dickinson and Company, Franklin Lakes, NJ), with no change of media during culture. Incubation conditions were set at 6% CO2, 5% O2, and 37.0 (C200 CO2 Incubator, Labotect Labor-Technik-Göttingen GmbH, Göttingen, Germany). Oocytes were cultured for 4 hours post-harvest before being inseminated for IVF or decumulated for ICSI.
All good quality embryos were cryopreserved via vitrification using a closed vitrification system with high-security straws (CBS-ViT-HS, CryoBioSystem, L’Aigle, France). Dimethylsulfoxide and ethylene glycol were used as cryoprotectants (Irvine Scientific Freeze Kit, Irvine Scientific, Newtown mount Kennedy, Ireland and Vitrification Kit 101, Cryotech, Tokyo, Japan). Embryos were vitrified as cleavage stage embryos on D3.
2.4. Endometrial preparation and FET
The artificial endometrial preparation consisted of sequential administration of E2 valerate and injectable progesterone. In summary, 2 mg of E2 valerate was administered at least twice daily for 14 to 16 days, and the dose was later adjusted according to the endometrial thickness measured by vaginal ultrasonography. If the endometrial thickness was ≥7 mm, injectable progesterone supplementation was initiated. If the endometrial thickness was <7 mm, patients continued to take oral E2 until the endometrium reached the necessary threshold, at which point progesterone supplementation was commenced.
Progesterone injection was administered at 20 mg daily, and after 3 days, FET was performed. Embryos were transferred under ultrasound guidance using a soft embryo transfer catheter. The choice to transfer 1 or more embryos was made by the attending clinician depending upon female age and embryo quality.
2.5. Main outcome measure, sample size estimation, and statistics analysis
Basic demographic characteristics were compared between the women who underwent FET in the first menstrual cycle (‘cycle 1’) or after subsequent cycles (‘cycle ≥2’), using the t test (for continuous variables) or the χ2 test (for categorical variables). Live birth rate (LBR), defined as the delivery of a live infant after ≥20 gestation weeks, was the primary outcome of our study.
PASS software version 11.0 (NCSS, LLC. Kaysville, UT) was used to calculate sample sizes for both groups. The LBR in ‘cycle 1’ group is assumed to be 0.3 under the null hypothesis and 0.4 under the alternative hypothesis. The LBR in ‘cycle ≥2’ group is 0.3. The test statistic used is the two-sided Z test with pooled variance. The significance level of the test was targeted at .05. Group sample sizes of 315 in ‘cycle 1’ group and 945 in ‘cycle ≥2’ group achieve 90% power to detect a difference between the group proportions of 0.1. Assuming that the follow-up loss rate of study subjects is 10%, sample size N1 = 315÷0.9 = 350 cases, N2 = 945÷0.9 = 1050 cases. Finally, 385 subjects were included in the ‘cycle 1’ group and 1155 in the ‘cycle ≥2’ group.
To identify potential confounding variables that could be independently associated with LBR, we performed bivariate logistic regression analysis. In the bivariate regression analysis, we accounted for variables that were either unevenly distributed amongst the study groups or presumed to be potential confounders, namely female age (≥37 and <37 years), body mass index (BMI; ≥25 and <25 kg/m2), infertility type (primary and secondary), insemination methods (IVF and ICSI), infertility-related factors (tubal and non-tubal factors), type of COH (GnRH agonist and non-GnRH agonist pituitary down-regulation protocols), indications for freeze-all protocol (progesterone >1.5 ng/ml, preferred elective freeze-all, patient preference and other reasons, endometrium <7 mm on the embryo transfer day and a high-risk of ovarian hyperstimulation syndrome [OHSS]), number of oocytes retrieved (≤5, 6–15 and >15), the number of good quality embryos transferred (2–3 and 1) and endometrial preparation protocol (stimulation, natural and artificial cycle).
A P value <.05 was considered to be statistically significant. For the statistical analysis, we used SPSS software version 22.0 (IBM Corp, Armonk, NY) and GraphPad prism 7.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Study population
Overall, 1540 autologous deferred FETs were analyzed in this study. There were 385 FET cycles performed within the first menstrual cycle following oocyte retrieval (‘cycle 1’) and 1155 FET cycles performed following 1 or more menstrual cycles (‘cycle ≥2’).
3.2. Demographic and FET cycle characteristics
Demographic and baseline information are presented in Table 1, showing comparable baseline characteristics between study groups; data are presented as mean ± standard deviation unless otherwise mentioned. The FET interval for cycle 1 and cycle ≥2 FETs was 25.72 ± 5.10 days and 75.33 ± 24.85 days, respectively (P < .001). The type of COH and endometrial preparation protocol differed significantly between groups (P = .008 and P = .004, respectively). However, beyond these factors, the 2 FET groups were similar.
3.3. Reproductive outcomes
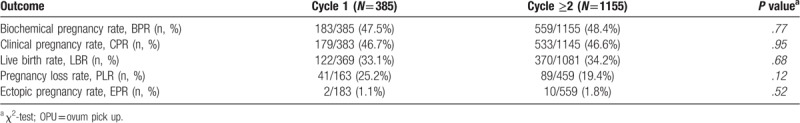
Table 2 shows overall reproductive outcomes according to each FET group. No significant differences were noted in our primary outcome (LBR) between ‘cycle 1’ and ‘cycle ≥2’ groups (33.1% vs 34.2%, P = .68). Similar results also occurred in terms of positive pregnancy rate (47.5% vs 48.4%, P = .77), clinical pregnancy rate (46.7% vs 46.6%, P = .95), pregnancy loss rate (25.2% vs 19.4%, P = .12) and ectopic pregnancy rate (1.1% vs 1.8%, P = .52).
Table 2.
Results of the univariate analysis comparing FETs that were carried out either within the immediate cycle following OPU (Cycle 1) or subsequently (Cycle ≥2) after a freeze-all protocol.
3.4. Variables independently associated with LBR
A multivariate analysis was performed to identify variables that were independently associated with LBR (Fig. 1). This multivariate model included female age, FET interval, BMI, infertility type, insemination methods, infertility-related factors, type of COH, number of oocytes retrieved, number of transferred D3 embryos, indications for freeze-all protocol and endometrial preparation protocol. A multivariate analysis was then performed to adjust for potential confounding factors, and results are presented in Figure 1. The only variables that showed a significant impact on LBR were female age (≥37 vs <37 years), number of transferred D3 embryos (2–3 vs 1), BMI (≥25 vs <25), and the type of COH (GnRH agonist and non-GnRH agonist pituitary down-regulation protocols). Performing FET within the first menstrual cycle (‘cycle 1’) compared to subsequent cycles (‘cycle ≥2’) after oocyte retrieval did not have a significant effect on LBR.
Figure 1.
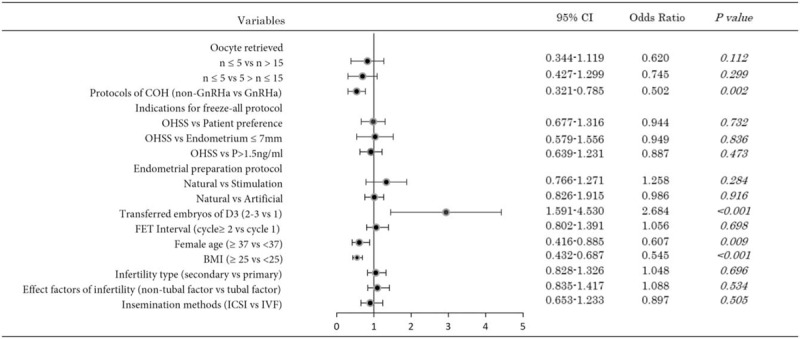
Multivariate logistic regression analysis results of factors affecting live birth rate after frozen-thawed embryo transfer.
4. Discussions
The number of elective FET procedures carried out has increased significantly over recent years, largely because of accompanying improvements in cryo-techniques.[17] Furthermore, there has been a clear improvement in reproductive outcomes resulting from elective FET, which reduces the endometrial impairment that is often observed during a COH cycle. Moreover, delaying embryo transfer may result in better synchrony between embryo development and the endometrial window of implantation.[18,19] Although the adverse effects of COH on reproductive outcomes are obvious, there has been no specific study to certify how long it takes for the endometrial immune environment and gene expression patterns to recover their pre-COH functionality. Traditionally, elective FET has been performed 1 to 2 months after oocyte retrieval, or longer.[20] Furthermore, the majority of couples, especially females, receiving ART treatment, often show psychological negative emotions during their treatment, at least to a certain degree.[21,22] Following COH, delaying FET until the endometrium has been restored to an optimal pre-COH state may add to the psychological pressure on patients, particularly those who are desperate for an immediate FET following oocyte retrieval.[23]
In our study, we found no significant difference in terms of BPR, CPR, PLR, EPR, and LBR between the ‘cycle ≥2’ and ‘cycle 1’ groups in our study population after a freeze-all strategy. Furthermore, when controlling for potential confounders in multivariate analysis, LBR did not differ significantly, which meant that the time interval for FET had no eventual impact on LBR. Similarly, 4 previous studies showed that the reproductive outcomes from FET performed immediately following oocyte retrieval were not compromised after adjusting for specific confounding factors.[11,14–16] However, in contrast to our study, 3 of these previous studies only assessed FET involving an artificial cycle protocol,[14–16] while 2 of the studies only allowed the transfer of frozen-thawed blastocyst.[11,15] Hence, the generalizability of evidence arising from these studies is restricted. Our study, however, showed quite clearly that the endometrial preparation protocol did not cause any significant effect on LBR.
The effects of female age,[24–26] D3 embryo transfer,[27] and BMI[28,29] on pregnancy outcomes in patients undergoing in vitro fertilization have been demonstrated in many previous studies. Therefore, it is no longer necessary to explain the influence of the above factors on LBR.
After adjusting for confounding factors, compared with the non-GnRH agonist pituitary down-regulation protocol, the LBR of FET increased significantly after GnRH agonist pituitary down-regulation protocols were adopted. This result shows that GnRH agonists may be advantageous in improving endometrial receptivity.[30,31] However, Lattes et al[14] found no impact of the stimulation protocol on reproductive outcomes including LBR. Their result showed that the undesirable effects of COH on endometrial receptivity ceases after the following withdrawal bleeding, regardless of the COH protocol. However, Lattes et al studied only 2 COH protocols, and the sample size of their study (N = 512) is likely to have limited selection and statistical bias.
In contrast to our present study, 2 studies drew completely opposing conclusions.[12,13] First, Higgins et al,[12] in their multivariate analysis, found LBR to be significantly higher in an immediate FET group compared to a delayed FET group (OR, 1.31, 95% CI [1.02–1.67]). Second, while considering a range of predictive factors for ongoing pregnancy rate, regarding the benefits of delayed FET, Kaye et al demonstrated an adjusted OR of 1.74 [95% CI 1.00–3.03] that approached statistical significance, regardless of GnRH agonist or hCG triggers.[13] Interpreting these results is complex due to the high level of heterogeneity in the study populations, observational end point, and particularly in light of the various stages of embryo development that they analyzed.
The main strengths of our research are derived from the fact that we included data from a large sample size (n = 1540) and accounted for numerous potential confounding factors. The retrospective design of our study represents a potential limitation; however, the data were recorded prospectively in a standardized manner, hence alleviating any risk of recall bias. In addition, the inclusion criteria, as in a randomized controlled clinical trial,[32] were strict so that various confounding factors may not have been taken into account, to the extent that the enrolled patient population is relatively inextensive. Therefore, retrospective cohort studies are indispensable at this stage. Finally, we would like to highlight that the present study only assessed the impact of the timing for FET following the transfer of D3 cleavage stage embryos and, thus, the results should not be extrapolated to other patients undergoing blastocyst transplantation.
5. Conclusions
This study demonstrated that FET performed within the first menstrual cycle is no worse than FETs following 1 or more menstrual cycles after a freeze-all protocol, regardless of the indication for the freeze-all protocol. Consequently, this study provides a simplified but potentially clinically relevant alternative to improve patient satisfaction in pursuit of a live birth as safety and quickly as possible.
Acknowledgments
The authors thank all the colleagues engaged in medical statistics and patients involved in this study.
Author contributions
Conceptualization: Zhengao Sun.
Data curation: Jingyan Song.
Formal analysis: Shan Xiang.
Software: Jingyan Song.
Validation: Zhengao Sun.
Visualization: Shan Xiang.
Writing – original draft: Jingyan Song.
Writing – review & editing: Shan Xiang, Zhengao Sun.
Footnotes
Abbreviations: ART = assisted reproductive technology, BMI = body mass index, BPR = biochemical pregnancy rate, COH = controlled ovarian hyperstimulation, CPR = clinical pregnancy rate, EPR = ectopic pregnancy rate, FET = frozen embryo transfer, FSH = follicle stimulating hormone, GnRH-a = gonadotrophin releasing hormone agonist, hCG = human chorionic gonadotrophin, ICSI = intracytoplasmic sperm injection, IVF = in vitro fertilization, LBR = live birth rate, OHSS = ovarian hyperstimulation syndrome, OPU = ovum pick up, PLR = pregnancy loss rate.
How to cite this article: Song J, Xiang S, Sun Z. Frozen embryo transfer at the cleavage stage can be performed within the first menstrual cycle following the freeze-all strategy without adversely affecting the live birth rate. Medicine. 2019;98:38(e17329).
This work was supported by the National Natural Science Foundation of China under Grant [No: 81373676; 81674018].
The authors have no conflicts of interests to disclose.
References
- [1].Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–26. [DOI] [PubMed] [Google Scholar]
- [2].European IVFmC, European Society of Human R, Embryology, Calhaz-Jorge C, et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod 2017; 32:1957–73. [DOI] [PubMed] [Google Scholar]
- [3].Martins WP, Nastri CO, Rienzi L, et al. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol 2017;49:583–91. [DOI] [PubMed] [Google Scholar]
- [4].Ribeiro VC, Santos-Ribeiro S, De Munck N, et al. Should we continue to measure endometrial thickness in modern-day medicine? The effect on live birth rates and birth weight. Reprod Biomed Online 2018;36:416–26. [DOI] [PubMed] [Google Scholar]
- [5].Zhao J, Zhang Q, Wang Y, et al. Endometrial pattern, thickness and growth in predicting pregnancy outcome following 3319 IVF cycle. Reprod Biomed Online 2014;29:291–8. [DOI] [PubMed] [Google Scholar]
- [6].Vladimirov IK, Tacheva D, Diez A. Theory about the embryo cryo-treatment. Reprod Med Biol 2017;16:118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Venetis CA, Kolibianakis EM, Bosdou JK, et al. Estimating the net effect of progesterone elevation on the day of hCG on live birth rates after IVF: a cohort analysis of 3296 IVF cycles. Hum Reprod 2015;30:684–91. [DOI] [PubMed] [Google Scholar]
- [8].Zapantis G, Szmyga MJ, Rybak EA, et al. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum Reprod 2013;28:3292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Legro RS. Introduction: evidence-based in vitro fertilization treatment of fresh versus frozen embryo transfer: peeling away the layers of the onion. Fertil Steril 2016;106:239–40. [DOI] [PubMed] [Google Scholar]
- [10].Roque M, Haahr T, Geber S, et al. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2–14. [DOI] [PubMed] [Google Scholar]
- [11].Bourdon M, Santulli P, Maignien C, et al. The interval between oocyte retrieval and frozen-thawed blastocyst transfer does not affect the live birth rate and obstetrical outcomes. PLoS One 2018;13:e0206067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Higgins C, Healey M, Jatkar S, et al. Interval between IVF stimulation cycle and frozen embryo transfer: Is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol 2018;58:217–21. [DOI] [PubMed] [Google Scholar]
- [13].Kaye L, Marsidi A, Rai P, et al. Frozen blastocyst transfer outcomes in immediate versus delayed subsequent cycles following GnRH agonist or hCG triggers. J Assist Reprod Genet 2018;35:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lattes K, Checa MA, Vassena R, et al. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod 2017;32:368–74. [DOI] [PubMed] [Google Scholar]
- [15].Ozgur K, Bulut H, Berkkanoglu M, et al. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet 2018;35:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Santos-Ribeiro S, Polyzos NP, Lan VT, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod 2016;31:2541–8. [DOI] [PubMed] [Google Scholar]
- [17].Shapiro BS, Daneshmand ST, Garner FC, et al. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril 2014;102:3–9. [DOI] [PubMed] [Google Scholar]
- [18].Horcajadas JA, Minguez P, Dopazo J, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab 2008;93:4500–10. [DOI] [PubMed] [Google Scholar]
- [19].Shapiro BS, Daneshmand ST, Garner FC, et al. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril 2011;96:344–8. [DOI] [PubMed] [Google Scholar]
- [20].Shapiro BS, Daneshmand ST, Garner FC, et al. Freeze-all at the blastocyst or bipronuclear stage: a randomized clinical trial. Fertil Steril 2015;104:1138–44. [DOI] [PubMed] [Google Scholar]
- [21].Milazzo A, Mnatzaganian G, Elshaug AG, et al. Depression and anxiety outcomes associated with failed assisted reproductive technologies: a systematic review and meta-analysis. PLoS One 2016;11:e0165805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Slade P, Emery J, Lieberman BA. A prospective, longitudinal study of emotions and relationships in in-vitro fertilization treatment. Hum Reprod 1997;12:183–90. [DOI] [PubMed] [Google Scholar]
- [23].An Y, Sun Z, Li L, et al. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet 2013;30:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101:633–4. [DOI] [PubMed] [Google Scholar]
- [25].Shufaro Y, Schenker JG. The risks and outcome of pregnancy in an advanced maternal age in oocyte donation cycles. J Matern Fetal Neonatal Med 2014;27:1703–9. [DOI] [PubMed] [Google Scholar]
- [26].Kenny LC, Lavender T, McNamee R, et al. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One 2013;8:e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Preutthipan S, Amso N, Curtis P, et al. The influence of number of embryos transferred on pregnancy outcome in women undergoing in vitro fertilization and embryo transfer (IVF-ET). J Med Assoc Thai 1996;79:613–7. [PubMed] [Google Scholar]
- [28].Van Der Linden EL, Browne JL, Vissers KM, et al. Maternal body mass index and adverse pregnancy outcomes: a ghanaian cohort study. Obesity (Silver Spring) 2016;24:215–22. [DOI] [PubMed] [Google Scholar]
- [29].Zhang J, Liu H, Mao X, et al. Effect of body mass index on pregnancy outcomes in a freeze-all policy: an analysis of 22,043 first autologous frozen-thawed embryo transfer cycles in China. BMC Med 2019;17:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Orvieto R, Meltzer S, Rabinson J, et al. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertil Steril 2008;90:1294–6. [DOI] [PubMed] [Google Scholar]
- [31].Ruan HC, Zhu XM, Luo Q, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod 2006;21:2521–9. [DOI] [PubMed] [Google Scholar]
- [32].Li H, Li L, Lu X, et al. Comparison of the effect of immediate versus delayed transfer following a stimulated IVF cycle on the ongoing pregnancy rate of frozen-thawed embryo transfer cycles: a study protocol for a randomised controlled trial. BMJ Open 2018;8:e020507. [DOI] [PMC free article] [PubMed] [Google Scholar]


