Abstract
Objective:
Increasing evidence suggests that radiologically determined sarcopenia prior to treatment can serve as a prognostic marker in various tumors. However, there are conflicting conclusions about the prognostic role of sarcopenia in urological tumors. We performed a meta-analysis to assess the association between radiologically determined sarcopenia before treatment and survival outcomes in urological tumors.
Methods:
A systematically literature search in PubMed, Cochrane databases, and EMBASE was performed. We estimated hazard ratios (HRs) for overall survival (OS) and cancer-specific survival (CSS). Hazard ratios (HR) with 95% confidence interval (CI) were calculated using STATA 12.0 software.
Results:
A total of 16 studies enrolling 2264 patients with urologic tumors were included in our meta-analysis. Among these studies, 13 studies with 1941 patients explored the association between sarcopenia and OS, and 10 studies with 1790 patients investigated the relationship between sarcopenia and OS. The synthesized result suggested that sarcopenia was significantly associated with poor OS (Fixed-effect model, HR 1.73, 95% CI: 1.48–2.01, P <.05; heterogeneity: P = .064; I2 = 40.5%), and poor CSS (Fixed-effect model, HR: 1.85, 95% CI: 1.51–2.28, P <.05, heterogeneity: P = .053; I2 = 46.2%).
Conclusion:
This meta-analysis showed that sarcopenia was associated with poor OS and CSS, suggesting that sarcopenia may serve as a promising prognostic marker in urologic cancer patients. Considering several limitations in our study, in the future more high-quality studies on this topic should be conducted to confirm our findings.
Keywords: kidney neoplasms, mortality, prognosis, sarcopenia, urinary bladder neoplasms
1. Introduction
Urologic cancers prevail worldwide. There were estimated 108,450 males and 49,770 females newly diagnosed with urologic cancers, and 33,420 patients died in United States in 2019.[1] Currently, TNM staging system is the most widely recognized method to predict the prognosis and guide therapy in cancer patients. However, in clinical practice, urologists are always confused by the situation that urologic cancer patients with the identical TNM stage, may have diverse oncological outcomes. Hence, it remains imperative to develop additional biomarkers to more accurately predict the prognosis and optimize the individualized treatment in patients with urologic cancers.
Sarcopenia, first introduced by Irwin Rosenberg,[2] is a common component of geriatric syndrome and is a major challenge to healthy aging.[3,4] Sarcopenia is featured with muscle mass loss alone or coupled with increased fat mass, and it is defined by loss of muscle, low muscle strength and/or low physical performance according to the European Working Group on Sarcopenia in Older People (EWGSOP).[5] Aging inversely affects protein synthesis, and skeletal muscle mass reduces progressively year by year in elderly people. This aging-associated sarcopenia is defined as primary sarcopenia, while sarcopenia that does not result from aging is defined as second sarcopenia. Several factors are reported to cause secondary sarcopenia, including inflammatory disease, endocrine dysfunction, malnutrition, chronic kidney disease, chronic liver disease, and malignancy.[6,7] In addition, cachexia is also considered to be a key cause of sarcopenia in oncological patients.[6] However, a recent review by Cederholm et al pointed out that sarcopenia and cachexia are overlapping syndromes from the perspective of malnutrition in phenotype, but they also have their own distinct etiologic contribution to cancer progression.[8] To date, there is no single tool or criteria that is appropriate for both sarcopenia and cachexia.[8] Therefore, before such a tool or criteria is established, it is necessary to explore the impact of sarcopenia or cachexia alone on survival in cancer patients, which may be used to predict prognosis and conduct individualized treatment in cancer patients. To date, it is widely accepted that pretreatment CT scan is an effective tool to determine whether patients are in the sarcopenic status or not.[9,10] In this method, 2 imaging parameters are often used to assess the sarcopenic status, one of which is psoas muscle index (PMI) defined as total psoas area (TPA) at the level 3 lumbar divided by height (m2), and the other is skeletal muscle index (SMI) defined as the muscle area at the third lumbar level, controlled by height (m2). Additionally, several parameters that reflect muscle strength and physical function, such as timed up and go test, short physical performance battery, gait speed, chair stand, and grip strength, are also recommended to define sarcopenia.[5] In recent years, numerous studies reported that cancer patients usually have a high risk of suffering from sarcopenia.[11–13] Furthermore, a large amount of evidence demonstrated that sarcopenia is an unfavorable prognostic factor in several cancers,[14–19] including esophageal cancer, lung cancer, gastric cancer, pancreatic cancer, colorectal cancer, and primary liver cancer.
Although the prognostic value of sarcopenia in patients with urologic cancer has also been studied, the conclusions remain conflicting. For instance, some studies[20–23] indicated that there was no significant correlation between pretreatment sarcopenia and overall survival (OS) or cancer-specific survival (CSS). Inversely, other studies[24–32] demonstrated that sarcopenia was an unfavorable prognostic parameter in patients with urologic cancers. Therefore, it is very imperative to conduct a systematic review and meta-analysis to comprehensively assess the prognostic value of pretreatment sarcopenia in patients with urologic tumors.
2. Materials and methods
2.1. Literature search and selection
A comprehensive literature search was performed in PubMed, EMBASE, and Cochrane databases for eligible studies that evaluated the prognostic role of sarcopenia in patients with urologic cancers from inception to December, 2018. The detailed search strategy used in PubMed was as following: ((((((((((“Carcinoma, Transitional Cell”[Mesh]) OR “Ureteral Neoplasms”[Mesh]) OR “Prostatic Neoplasms”[Mesh]) OR “Kidney Neoplasms”[Mesh]) OR “Urinary Bladder Neoplasms”[Mesh]) OR “Urologic Neoplasms”[Mesh])) OR (((((((((((cancer[Title/Abstract]) OR tumor[Title/Abstract]) OR tumors[Title/Abstract]) OR carcinoma[Title/Abstract]) OR neoplasm[Title/Abstract]) OR neoplasms[Title/Abstract]) OR malignancy[Title/Abstract] OR cancers[Title/Abstract]))) AND (((((((((((((((urologic[Title/Abstract]) OR urinary[Title/Abstract]) OR urological[Title/Abstract]) OR renal[Title/Abstract]) OR kidney[Title/Abstract]) OR bladder[Title/Abstract]) OR vesical[Title/Abstract]) OR ureteral[Title/Abstract]) OR ureter[Title/Abstract]) OR upper tract[Title/Abstract]) OR upper urinary tract[Title/Abstract]) OR urothelial[Title/Abstract]) OR transitional cell[Title/Abstract]) OR vesical[Title/Abstract]) OR prostate[Title/Abstract]))))) AND ((((((((sarcopenia[Title/Abstract]) OR grip strength[Title/Abstract]) OR chair stand[Title/Abstract]) OR low muscle quantity[Title/Abstract]) OR gait speed[Title/Abstract]) OR appendicular skeletal muscle mass[Title/Abstract]) OR short physical performance battery[Title/Abstract]) OR (timed up and go test[Title/Abstract]))).
Studies were included if they conformed to the following criteria:
-
1)
Diagnosis of urologic cancers was histo-pathologically confirmed;
-
2)
The oncological prognosis, including overall survival (OS), or cancer-specific survival (CSS), were compared in urologic cancer patients with sarcopenia versus non-sarcopenia;
-
3)
Hazard ratios (HRs) and 95% confidence intervals (CIs) for OS or CSS were available;
-
4)
Pretreatment sarcopenia was assessed using image technology; and
-
5)
Studies were published in English.
Studies were excluded if they accorded with the following criteria:
-
1)
The studies were editorials, letters, reviews, conference abstracts or case reports;
-
2)
The studies enrolled overlapped patients;
-
3)
The studies focused on investigating the relationship between sarcopenia and non-urological tumors; and
-
4)
HRs and CIs could not be obtained.
2.2. Data extraction and quality evaluation
All potentially eligible studies were independently reviewed by 2 investigators (Jialin Li and Yinan Deng) and divergences in data extraction were resolved by the corresponding author. The following datum was extracted: the name of first author, year of publication, cancer type, cancer stage, number of patients, age, follow-up time, methods of assessing sarcopenia, cut-offs of defining sarcopenia, the percentage of patients with sarcopenia, and HRs and CIs for OS and CSS. If hazard ratios from both univariable and multivariable analysis were available in articles, hazard ratios from multivariable analysis were extracted preferentially. If HRs were not directly provided in article, we estimated HRs 95% and their CIs from Kaplan–Meier curves by the Tierney methods. The Newcastle–Ottawa Scale (NOS)[33] was used to assess the quality of the included studies. In this meta-analysis, we regarded studies obtaining 6 or more scores as high-quality ones. Any divergence was addressed by the corresponding author.
2.3. Statistical analysis
STATA version 12.0 (Stata Corporation, College Station, TX) was used to perform statistical analysis. Random effect model was applied to synthesize data if there was significant heterogeneity among the included studies (I2 >50%), otherwise, fixed-effect model was applied.[34] Subgroup analysis would be conducted to test the stability of our pooled results according to tumor type, country, tumor stage, assessment method, and analysis type. Meta-regression analysis was conducted based on tumor type, country, tumor stage, assessment method, and analysis type to explore the sources of heterogeneity in this meta-analysis. Additionally, sensitivity analysis was also conducted by sequentially omitting single included study to test the stability of our pooled results. Publication bias was assessed by Egger test and Begg test.[35,36] When there was significant publication bias, trim-and-fill method was used to evaluate whether publication bias significantly affected the reliability of our pooled results.[37] All statistical tests were 2-sided, and P value <.05 was considered statistically significant.
2.4. Ethics approval
Although this study did not utilize any human specimen, it has been approved by the Hospital Research Ethics Committee of Peking Union Medical College Hospital.
3. Results
3.1. Literature search and selection
The initial search identified a total of 630 records with 379 from EMBASE, 188 from PubMed, and 63 from Cochrane databases. After excluding 177 duplicated records, a total of 453 studies were further screened by titles and abstracts. In this process, 410 records were excluded due to irrelevant topics. Next, we screened the remained 43 studies by full-text and further excluded 27 studies owing to no available data on survival outcomes (n = 3), conference abstracts (15), and reviews (n = 9). Finally, a total of 16 studies were included for this meta-analysis. The Flow diagram of literature selection was displayed in Figure 1.
Figure 1.
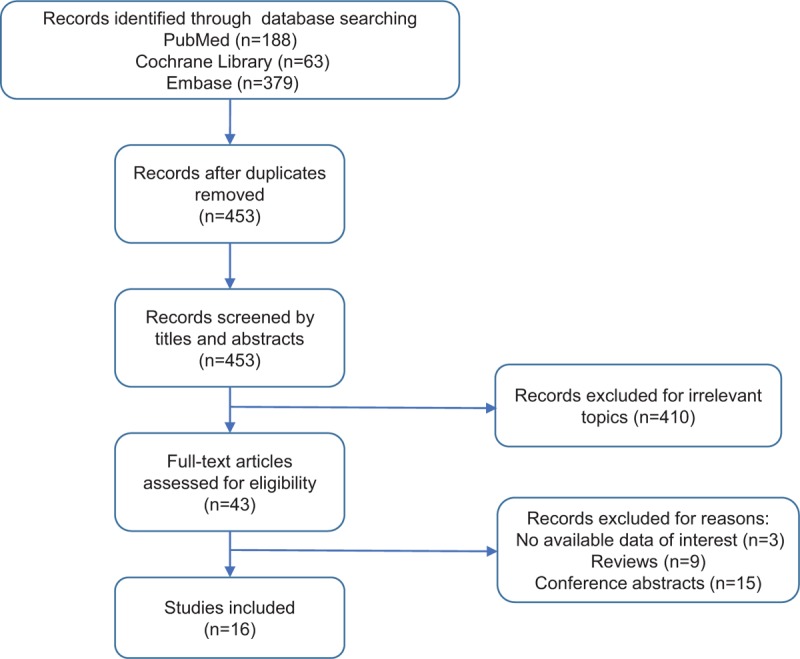
Flow diagram of literature selection.
3.2. Characteristics of included studies
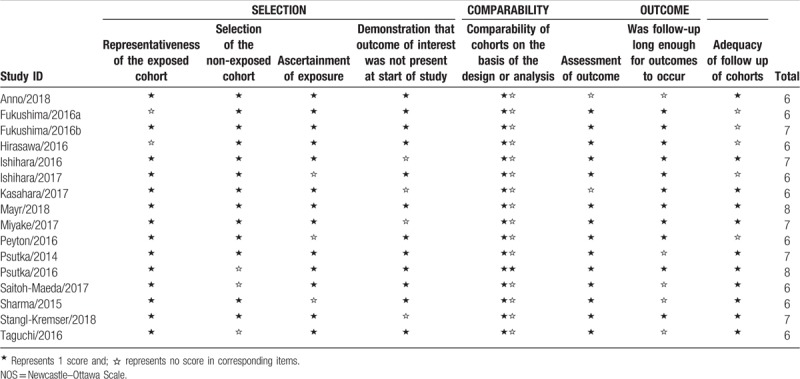
Characteristics of the included studies are presented in Table 1. All 16 studies were retrospective and published between 2014 and 2018. The number of participants ranged from 27 to 500, with a sum of 2264. Twelve studies used the SMI as the indicator of sarcopenia while 4 studies used the PMI. Most studies defined the patients as sarcopenic and non-sarcopenic using a threshold SMI of <41 cm2/m2 among women, <43 cm2/m2 among men with a body mass index (BMI) of <25 kg/ m2, and <53 cm2/m2 among men with a BMI of >25 kg/m2, which was first proposed by Martin et al[38] Besides, a few of included studies employed a threshold SMI of < 55 cm2/m2 for men and <39 cm2/m2 for women.[39] A total of 13 studies evaluated OS, and 10 studies evaluated CSS. The scores of Newcastle–Ottawa scale ranged from 6 to 7, indicating that the quality of the included studies was moderate to high and suitable for synthesized analysis (Table 2).
Table 1.
The main characteristics of the included studies.
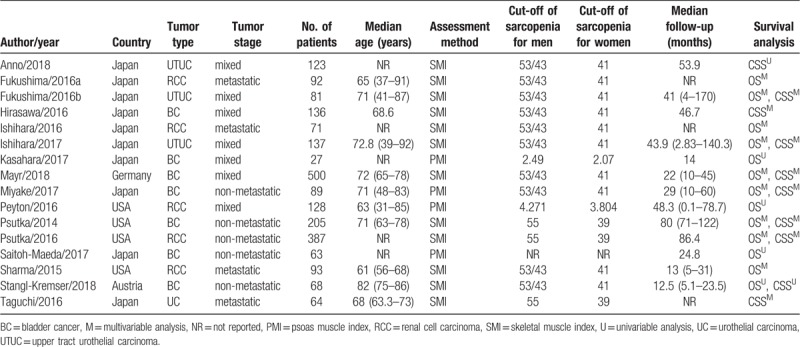
Table 2.
The NOS quality assessment of the enrolled studies.
3.3. Synthesized analysis of the prognostic value of sarcopenia
A total of 13 studies with 1941 patients, which explored the association between sarcopenia and OS in urologic tumors, were included in our meta-analysis. As Figure 2 shown, the synthesized result suggested that sarcopenia was significantly associated with poor OS (Fixed-effect model, HR 1.73, 95% CI: 1.48–2.01, P <.05; heterogeneity: P = .064; I2 = 40.5%). Additionally, there were 10 eligible studies with 1790 patients [23–26,28,29,32,40–42] that evaluated the relationship between sarcopenia and CSS in urologic tumors. Our synthesized analysis of these studies showed that there was also a significant correlation between sarcopenia and poor CSS (Fixed-effect model, HR: 1.85, 95% CI: 1.51–2.28, P <.05, heterogeneity: P = .053; I2 = 46.2%) (Fig. 3).
Figure 2.
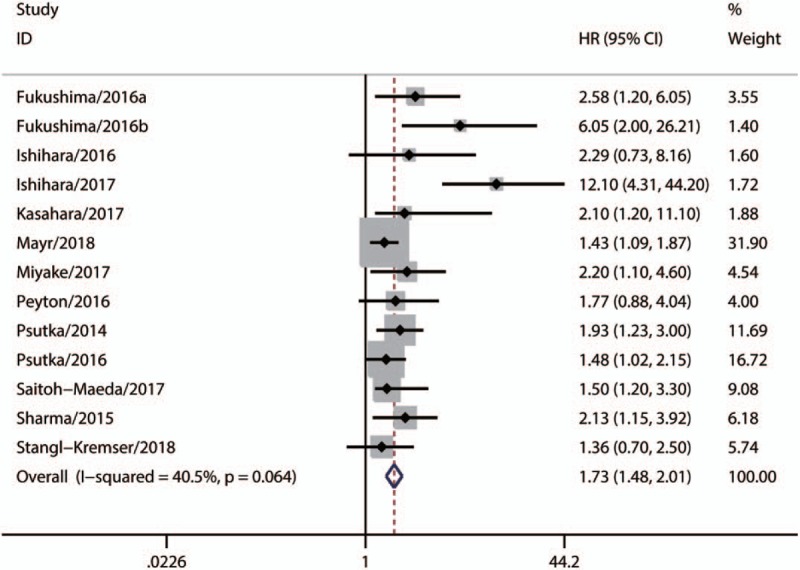
Synthesized analysis of the prognostic value of sarcopenia for OS in urologic tumors. OS = overall survival.
Figure 3.
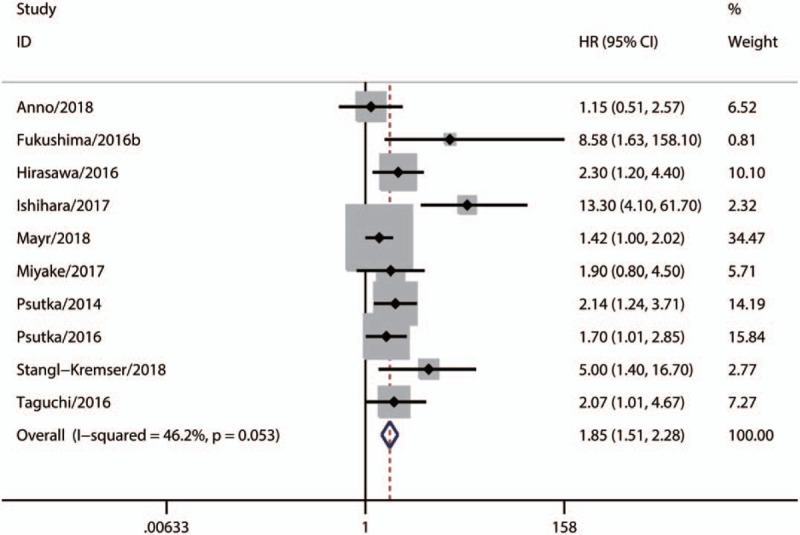
Synthesized analysis of the prognostic value of sarcopenia for CSS in urologic tumors. CSS = cancer-specific cancer.
3.4. Subgroup analysis
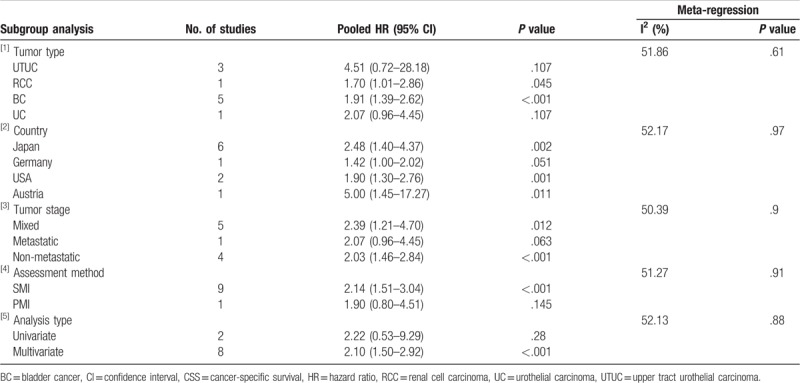
We performed subgroup analysis, according to tumor type, country, tumor stage, assessment method, and analysis type, to test the stability of our synthesized results. Overall, statistically significant synthesized HR values for OS (Table 3) and CSS (Table 4) were consistently calculated in each subgroup, indicating the stability of our synthesized results.
Table 3.
Subgroup analysis of synthesized HRs for OS.
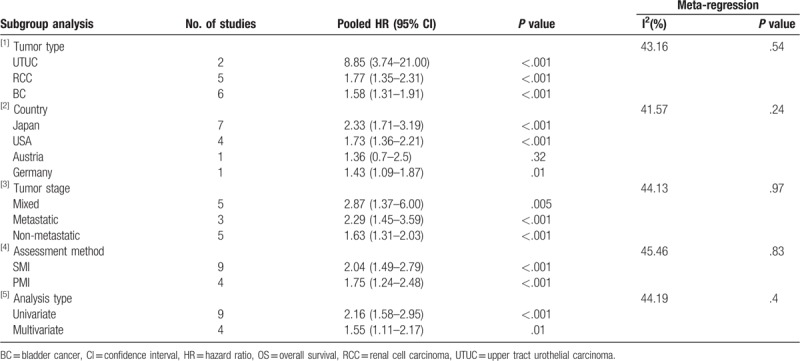
Table 4.
Subgroup analysis of synthesized HRs for CSS.
3.5. Meta-regression analysis
Our overall combined analyses showed moderate heterogeneity for OS (I2 = 40.5%) and CSS (I2 = 46.2%). Thus, we conducted meta-regression analysis based on tumor type, country, tumor stage, assessment method, and analysis type to explore the potential sources of the heterogeneity. As shown in Table 3 and Table 4, none of these factors could explain the heterogeneity for OS and CSS.
3.6. Sensitive analysis
We also performed sensitivity analysis by excluding 1 study in each step to further test the stability of our synthesized results. The results showed that the pooled HR values for OS (Fig. 4A) and CSS (Fig. 4B) did not change significantly when any individual study was excluded, which further indicated that our synthesized results in this meta-analysis were stable.
Figure 4.
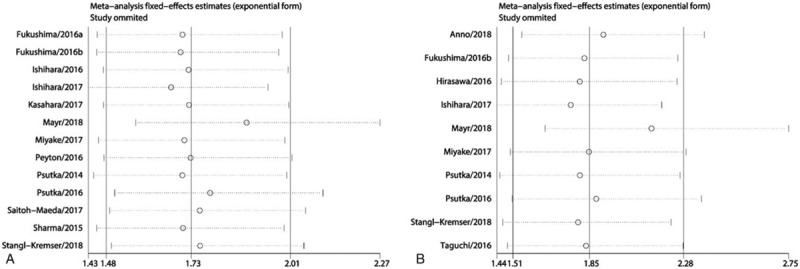
A. The sensitivity analysis for the synthesized HR values assessing the prognostic value of sarcopenia for OS in urologic tumors. B. The sensitivity analysis for the synthesized HR values assessing the prognostic value of sarcopenia for CSS in urologic tumors. CSS = cancer-specific cancer, HR = hazard ratio, OS = overall survival.
3.7. Publication bias
The publication bias was assessed using Begg and Egger tests. As the Begg tests shown, the funnel plots that assessed the publication bias in the included studies about OS (Fig. 5A) and CSS (Fig. 5B) were asymmetric. Meanwhile, the P values of Begg and Egger tests were also <.05. These results indicated that there might be significant publication bias in the included studies about OS and CSS. Thus, we performed trim-and-fill analysis to determine whether the publication bias significantly affected the reliability of the pooled results about OS and CSS. The results showed that the adjusted HR values for both OS and CSS were still more than 1 (OS: random-effects model, HR: 1.56, 95% CI: 1.22–2.00, P <.001; CSS: random-effects model, HR: 1.66, 95% CI: 1.17–2.37, P = .005), suggesting that the publication bias did not significantly affect the reliability of the pooled results about OS and CSS. Furthermore, the adjusted funnel plots that assessed the publication bias in the included studies about OS (Fig. 6A) and CSS (Fig. 6B) became symmetric. In view of the above results, the publication bias determined by Begg and Egger tests did not substantially affect the reliability and stability of our synthesized results.
Figure 5.
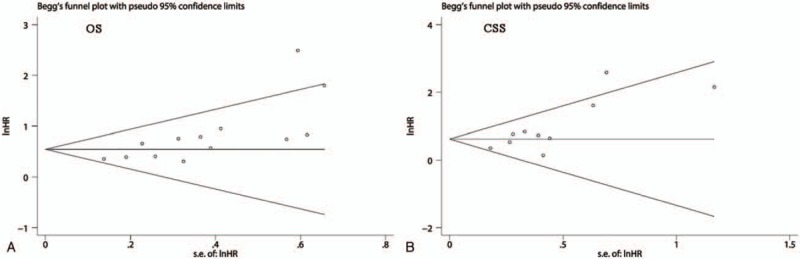
The funnel plots of Begg's test for assessing the publication bias in the included studies about OS (A) and CSS (B). CSS = cancer-specific cancer, OS = overall survival.
Figure 6.
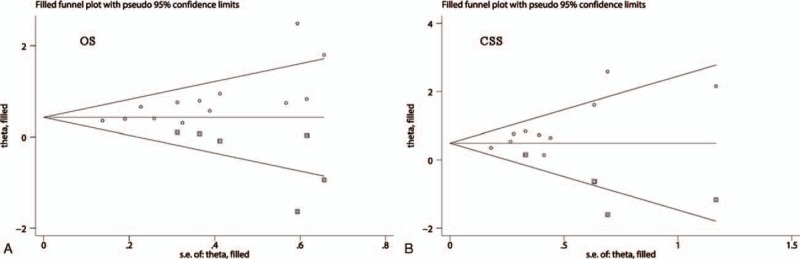
The adjusted funnel plots of Begg's test for assessing the publication bias in the included studies about OS (A) and CSS (B). CSS = cancer-specific cancer, OS = overall survival.
4. Discussion
Numerous studies have investigated the correlation between sarcopenia and the prognosis of urologic cancers. However, the results were inconsistent. Thus, we conducted this meta-analysis to assess the prognostic significance of sarcopenia in patients with urologic cancers. Our meta-analysis included 16 studies with 2264 patients, which focused on unveiling the predictive value of sarcopenia for the outcome of urologic tumors. From the results of the present study, we found that sarcopenia was significantly correlated with decreased OS and CSS in urologic cancer patients. Furthermore, the results of our subgroup and sensitivity analyses indicated that the pooled HR values in this meta-analysis were stable and reliable. Thus, sarcopenia may serve as a promising marker for assessing the prognosis of urologic cancers.
There are several mechanisms that may underlie the prognostic value of sarcopenia in cancer patients. Some researchers came up with the hypothesis that sarcopenia may be induced by systematic inflammation and malnutrition.[28,29,43] In the systematic inflammation process, the body tends to decrease the protein synthesis and increase protein degradation,[44] which may do harm to the discovery of patients. More importantly, a plenty of evidence suggested that the systemic inflammation could promote cancer progression and result in a poor prognosis.[45,46] Cytokines play a vital role in systemic inflammation associated with cancers and were found to participate in many metabolic pathways responsible for skeletal muscle wasting,[47] thus probably facilitating the development of sarcopenia. Additionally, it was considered that malnutrition-related to cancer progression and side effects of treatments could lead to anorexia in cancer patients, which subsequently promotes the development of sarcopenia.[31,48,49] Cachexia is a multisystem syndrome featured with weight loss, loss of muscle mass, systemic inflammation, anorexia, insulin resistance, and functional decline,[50] and there is a consensus definition of cachexia: weight loss of ≥5% of body weight in the past 6 months or ≥2% loss in patients with body mass index (BMI) of <20 kg/m239. Numerous studies demonstrated that cachexia could significantly worsen prognosis in cancer patients.[51–55] Furthermore, cachexia is considered to be important cause of sarcopenia in oncological patients,[6] which may also partly account for the positive association between sarcopenia and poor survival in patients with urologic tumors. Overall, close relationships of sarcopenia with systematic inflammation, malnutrition and cachexia strongly support our findings in this meta-analysis.
Our findings have some clinical significance. On 1 hand, our meta-analysis demonstrated that pretreatment sarcopenia was associated with inferior OS and CSS in urologic cancer patients, suggesting that sarcopenia may be used to stratify urologic cancer patients with low and high risk, and predict prognosis. On other hand, our study may provide evidence that pretreatment sarcopenia may be used to guide individualized treatments for patients with urologic cancers. Because malnutrition, anorexia, systemic inflammation, and cachexia were considered as important causes of sarcopenia, many therapeutic strategies have been applied to deal with these abnormalities associated with sarcopenia. For example, nutritional intervention and appetite stimulants are commonly used to alleviate skeleton muscle waste. Additionally, various anti-inflammatory drugs and anabolic agents have been being tested in clinical trials,[56,57] including COX-2 inhibitors, immunomodulator, Omega-3 supplements, Ghrelin analogs, and Janus kinase 1 and Janus kinase 2 inhibitor, to treat cancer cachexia.
To our best knowledge, this is the first meta-analysis to comprehensively evaluate the prognostic value of sarcopenia in urological cancers. Compared to individual included studies, our meta-analysis conquered the limitation of small sample size by synthesizing data of 16 eligible studies and thus may provide more strong evidence with larger statistical power. We found no significant heterogeneity in our analysis. Nevertheless, there were still several limitations that should be considered cautiously. First, all the included studies were designed retrospectively and thus could not draw robust conclusions about how plasma sarcopenia affects survival outcomes. Only the relationship between sarcopenia and poor survival outcome could be deduced. Second, we only estimated OS and CSS, but did not evaluate other survival outcomes, such as disease-free survival and progression-free survival, which was mainly due to a lack of data in the included studies. Third, we only considered studies published in English, which might lead to language bias. Fourth, some of the included studies lacked multivariate analyses, so we could only use HR values from univariate analyses to calculate the synthesized HR value. However, univariate analysis may overestimate effect sizes due to confounder bias. Fifth, the results of Begg and Egger tests suggested that our meta-analysis had significant publication bias. In fact, studies with positive results are usually more easily to be published that those with negative results due to many factors, such as the preference of authors and journal editors and the manipulation of fund provider, which may mainly account for the publication bias. Although there was significant bias in our meta-analysis, our trim-and-fill analysis suggested that the publication bias did not significantly affect the reliability of the pooled results about OS and CSS.
In conclusion, our meta-analysis indicated that sarcopenia was associated with poor OS and CSS in urologic cancer patients, suggesting that sarcopenia may serve as a promising prognostic marker. Considering several limitations in our study, in the future more high-quality studies on this topic should be conducted to confirm our findings.
Author contributions
Conceptualization: Jialin Li, Yusheng Cheng.
Data curation: Jialin Li, Yinan Deng, Menghui Zhang.
Formal analysis: Yinan Deng.
Methodology: Jialin Li, Yinan Deng, Yusheng Cheng, Xin Zhao.
Resources: Zhigang Ji.
Software: Jialin Li, Yinan Deng, Menghui Zhang.
Supervision: Zhigang Ji.
Visualization: Jialin Li, Yusheng Cheng, Xin Zhao.
Writing – original draft: Jialin Li.
Writing – review & editing: Yinan Deng.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CSS = cancer-specific survival, HR = hazard ratios, OS = overall survival, PMI = psoas muscle index, SMI = skeletal muscle index.
How to cite this article: Li J, Deng Y, Zhang M, Cheng Y, Zhao X, Ji Z. Prognostic value of radiologically determined sarcopenia prior to treatment in urologic tumors. Medicine. 2019;98:38(e17213).
JL and YD contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Rosenberg IH. Epidemiologic and methodologic problems in determining nutritional-status of older persons - proceedings of a conference held in albuquerque, new mexico, october 19-21, 1988 - summary comments. Am J Clin Nutr 1989;50:1231–3. [PubMed] [Google Scholar]
- [3].Carnevale V, Castriotta V, Piscitelli PA, et al. Assessment of skeletal muscle mass in older people: comparison between 2 anthropometry-based methods and dual-energy X-ray absorptiometry. J Am Med Dir Assoc 2018;19:793–6. [DOI] [PubMed] [Google Scholar]
- [4].Chen LK, Lee WJ, Peng LN, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2016;17:767.e1–7. [DOI] [PubMed] [Google Scholar]
- [5].Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Keller K. Sarcopenia. Wien Med Wochenschr 2019;169:157–72. [DOI] [PubMed] [Google Scholar]
- [7].Ohara M, Ogawa K, Suda G, et al. L-carnitine suppresses loss of skeletal muscle mass in patients with liver cirrhosis. Hepatol Commun 2018;2:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jensen GL, Cederholm T. The malnutrition overlap syndromes of cachexia and sarcopenia: a malnutrition conundrum. Am J Clin Nutr 2018;108:1157–8. [DOI] [PubMed] [Google Scholar]
- [9].Shen W, Punyanitya M, Wang ZM, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004;97:2333–8. [DOI] [PubMed] [Google Scholar]
- [10].Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- [11].Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol 2015;112:403–7. [DOI] [PubMed] [Google Scholar]
- [12].Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One 2012;7:e37563.doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol Oncol 2015;33:17–23. [DOI] [PubMed] [Google Scholar]
- [14].Zhang G, Meng S, Li R, et al. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget 2017;8:102474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sun G, Li Y, Peng Y, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 2018;33:1419–27. [DOI] [PubMed] [Google Scholar]
- [16].Mintziras I, Miligkos M, Wachter S, et al. Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg 2018;59:19–26. [DOI] [PubMed] [Google Scholar]
- [17].Kamarajah SK, Bundred J, Tan BHL. Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2019;22:10–22. [DOI] [PubMed] [Google Scholar]
- [18].Deng HY, Zha P, Peng L, et al. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta-analysis. Dis Esophagus 2019;32: doi: 10.1093/dote/doy115. [DOI] [PubMed] [Google Scholar]
- [19].Deng HY, Hou L, Zha P, et al. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol 2019;45:728–35. [DOI] [PubMed] [Google Scholar]
- [20].Peyton CC, Heavner MG, Rague JT, et al. Does sarcopenia impact complications and overall survival in patients undergoing radical nephrectomy for stage iii and iv kidney cancer. J Endourol 2016;30:229–36. [DOI] [PubMed] [Google Scholar]
- [21].Sharma P, Zargar-Shoshtari K, Caracciolo JT, et al. Sarcopenia as a predictor of complications in penile cancer patients undergoing inguinal lymph node dissection. World J Urol 2015;33:1585–92. [DOI] [PubMed] [Google Scholar]
- [22].Ishihara H, Kondo T, Omae K, et al. Sarcopenia and the modified glasgow prognostic score are significant predictors of survival among patients with metastatic renal cell carcinoma who are receiving first-line sunitinib treatment. Target Oncol 2016;11:605–17. [DOI] [PubMed] [Google Scholar]
- [23].Miyake M, Morizawa Y, Hori S, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology 2017;93:259–69. [DOI] [PubMed] [Google Scholar]
- [24].Taguchi S, Akamatsu N, Nakagawa T, et al. Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer 2016;14:237–43. [DOI] [PubMed] [Google Scholar]
- [25].Psutka SP, Carrasco A, Schmit GD, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer 2014;120:2910–8. [DOI] [PubMed] [Google Scholar]
- [26].Psutka SP, Boorjian SA, Moynagh MR, et al. Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol 2016;195:270–6. [DOI] [PubMed] [Google Scholar]
- [27].Kasahara R, Kawahara T, Ohtake S, et al. A low psoas muscle index before treatment can predict a poorer prognosis in advanced bladder cancer patients who receive gemcitabine and nedaplatin therapy. BioMed Res Int 2017;2017:7981549.doi: 10.1155/2017/7981549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ishihara H, Kondo T, Omae K, et al. Sarcopenia predicts survival outcomes among patients with urothelial carcinoma of the upper urinary tract undergoing radical nephroureterectomy: a retrospective multi-institution study. Int J Clin Oncol 2017;22:136–44. [DOI] [PubMed] [Google Scholar]
- [29].Hirasawa Y, Nakashima J, Yunaiyama D, et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann Surg Oncol 2016;23suppl 5:1048–54. [DOI] [PubMed] [Google Scholar]
- [30].Fukushima H, Nakanishi Y, Kataoka M, et al. Prognostic significance of sarcopenia in patients with metastatic renal cell carcinoma. J Urol 2016;195:26–32. [DOI] [PubMed] [Google Scholar]
- [31].Fukushima H, Yokoyama M, Nakanishi Y, et al. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS One 2015;10:e0115895.doi: 10.1371/journal.pone.0115895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fukushima H, Nakanishi Y, Kataoka M, et al. Prognostic significance of sarcopenia in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Cancer Med 2016;5:2213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [34].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [36].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [38].Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol Off J Am Soc Clin Oncol 2013;31:1539–47. [DOI] [PubMed] [Google Scholar]
- [39].Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–95. [DOI] [PubMed] [Google Scholar]
- [40].Anno T, Kikuchi E, Fukumoto K, et al. Preoperative sarcopenia status is associated with lymphovascular invasion in upper tract urothelial carcinoma patients treated with radical nephroureterectomy. Can Urol Assoc J 2018;12:E132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mayr R, Gierth M, Zeman F, et al. Sarcopenia as a comorbidity-independent predictor of survival following radical cystectomy for bladder cancer. J Cachexia Sarcopenia Muscle 2018;9:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Stangl-Kremser J, D’Andrea D, Vartolomei M, et al. Prognostic value of nutritional indices and body composition parameters including sarcopenia in patients treated with radiotherapy for urothelial carcinoma of the bladder. Urol Oncol 2019;37:372–9. [DOI] [PubMed] [Google Scholar]
- [43].Richards CH, Roxburgh CS, MacMillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One 2012;7:e41883.doi: 10.1371/journal.pone.0041883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–66. [DOI] [PubMed] [Google Scholar]
- [45].Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22. [DOI] [PubMed] [Google Scholar]
- [46].Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer 2013;132:2721–9. [DOI] [PubMed] [Google Scholar]
- [47].White JP, Puppa MJ, Sato S, et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle 2012;2:14.doi: 10.1186/2044-5040-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Argiles JM, Busquets S, Stemmler B, et al. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol 2015;22:100–6. [DOI] [PubMed] [Google Scholar]
- [49].Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr 2010;91:1123S–7S. [DOI] [PubMed] [Google Scholar]
- [50].Advani SM, Advani PG, VonVille HM, et al. Pharmacological management of cachexia in adult cancer patients: a systematic review of clinical trials. BMC Cancer 2018;18:1174.doi: 10.1186/s12885-018-5080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vazeille C, Jouinot A, Durand JP, et al. Relation between hypermetabolism, cachexia, and survival in cancer patients: a prospective study in 390 cancer patients before initiation of anticancer therapy. Am J Clin Nutr 2017;105:1139–47. [DOI] [PubMed] [Google Scholar]
- [52].Stephens NA, Skipworth RJ, Gallagher IJ, et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2015;6:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Orell-Kotikangas H, Osterlund P, Makitie O, et al. Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Otolaryngol 2017;137:778–85. [DOI] [PubMed] [Google Scholar]
- [54].Hendifar AE, Chang JI, Huang BZ, et al. and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J Gastrointest Oncol 2018;9:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dominguez N, Canada T. Considerations in hypermetabolism, cachexia, and survival in cancer. Am J Clin Nutr 2017;106:957–8. [DOI] [PubMed] [Google Scholar]
- [56].Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011;62:265–79. [DOI] [PubMed] [Google Scholar]
- [57].Dingemans AM, de Vos-Geelen J, Langen R, et al. Phase II drugs that are currently in development for the treatment of cachexia. Expert Opin Investig Drugs 2014;23:1655–69. [DOI] [PubMed] [Google Scholar]


