Abstract
The aim of this study was to evaluate the application of transthoracic echocardiography for the diagnosis of infective endocarditis (IE) to provide a basis for the better treatment of IE. From October 2016 to October 2018, 87 consecutive patients with IE at our hospital were selected for this study. All the patients were subjected to transthoracic echocardiography. The morphology, structure, activity, and closure of the patients’ heart valves were observed for vegetation identification, and the size, number, location, morphology, and echo intensity of vegetation, as well as degree of valve involvement, were determined.
The 87 patients investigated in this study included 38 cases of congenital heart disease, 27 cases of nonrheumatic valvular heart disease, 12 patients who underwent valve surgery, 5 cases of rheumatic valvular heart disease, and 5 patients with no obvious signs of heart disease. The most common clinical manifestations were heart murmur in 80 cases and fever in 60 cases. The most common complications were heart failure in 35 cases, followed by organ embolism in 12 cases. There were 36 cases of positive blood cultures, including 26 cases of Gram-positive cocci and 10 cases of Gram-negative bacilli. Echocardiography showed aortic valve involvement in 37 cases, mitral valve involvement in 34 cases, tricuspid valve involvement in 10 cases, pulmonary valve involvement in 2 cases, and the involvement of an artificial valve in 5 cases. Twenty-six of these cases showed multiple valve involvement, and 20 patients exhibited serious complications. No significant differences were found between echocardiography and actual surgical observations with respect to their accuracy in detecting the size, number, and location of vegetation in the 69 patients who underwent surgery (P > .05). Echocardiography could detect the occurrence of severe complications, namely, the rupture of chordae tendineae, valve prolapse, valve perforation, and paravalvular abscess, and no significant difference in diagnostic accuracy was found between echocardiography and surgical observations (P > .05).
Transthoracic echocardiography can rapidly and accurately detect IE vegetation and its complications and has important clinical value for guiding clinical treatment and determining prognosis.
Keywords: echocardiography, infective endocarditis, vegetations
1. Introduction
Infective endocarditis (IE) is a rare, life-threatening disease that has long-lasting effects even among patients who survive and are cured.[1] IE disproportionately affects those with underlying structural heart disease and is increasingly associated with health care contact, particularly in patients who have intracardiac prosthetic material. In the setting of bacteraemia with a pathogenic organism, an infected vegetation may form as the end result of complex interactions between invading microorganisms and the host immune system.[2] Once established, IE can involve almost any organ system in the body.
IE is an inflammatory disease of the valve, endocardium, and vascular intima that is caused by infection by pathogenic microorganisms such as bacteria, fungi, viruses, and chlamydia. The basic lesion involves the corresponding damage of the valve and the formation of vegetation. Vegetation shedding can cause arterial embolism as well as ischemia and necrosis in important tissues and organs, resulting in a high mortality rate.[3] In recent years, along with the aging of the population and the increase in the number of elderly patients with degenerative valve disease, the application of prosthetic heart valve replacement, cardiac pacemaker implantation, and various endovascular treatment technologies has led to an increasing incidence of IE. In addition, intravenous administration has increased the risk of right-heart IE.[4] Due to the poor prognosis and high mortality of IE, early diagnosis and early intervention have important clinical significance. In general, the clinical diagnostic methods for IE are blood cultures and transesophageal echocardiography, and transesophageal echocardiography can indicate and evaluate the morphology, spatial structure, and activity status of each structure of the heart as well as the state of blood flow through the heart. Transthoracic echocardiography is the most commonly used imaging method for the clinical diagnosis of IE, which is characterized by the detection of vegetation, and can provide an important basis for clinical diagnosis and treatment. The present study referenced Duke criteria for IE, retrospectively analyzed the sonographic characteristics and clinical data of 87 IE patients admitted to and treated at our hospital to improve the accuracy of transthoracic echocardiography for the diagnosis of IE.
2. Materials and methods
2.1. Patients
From October 2016 to October 2018, 87 consecutive patients with IE at our hospital were selected for this study. The inclusion criteria were as follows. The patients met the improved diagnostic criteria of the European Society of Cardiology (ESC) 2015, which are based on the improved Duke diagnostic criteria, as well as the following: damage to the valve edge was found as the main diagnostic criterion by cardiac CT examination, positron emission tomography computed tomography (PET-CT) or single-photon emission computed tomography (SPECT) revealed abnormal nuclide concentrations at the edge of the prosthetic valve (prosthetic valve implantation >3 months) as the main diagnostic criteria, and recent asymptomatic embolization or infectious aneurysms found by medical imaging were secondary diagnostic criteria. IE detected by surgery or vegetation in the heart valve was confirmed by transthoracic echocardiography. In addition, the patients suffered from fever of an unexplained origin and with a duration of >7 days, and this symptom was accompanied by embolism, splenomegaly, heart murmur, skin petechiae, progressive anemia, and other symptoms. The exclusion criteria were as follows: patients who experienced heart failure; patients with immune system diseases or hematological diseases; and patients with severe liver and kidney dysfunction. Heart failure and acute renal dysfunction can be consequences of IE and immune problems can be part in its pathogenesis. The indications for surgery were based on the 2015 ESC guidelines for the diagnosis and treatment of IE. This study was approved by the ethics committee, which is equivalent to an institutional review board, at our institution, and informed consent from the patients was obtained after the procedure had been fully explained.
2.2. Equipment
A Philips IE33 color Doppler ultrasonic diagnostic system (Philips Medical Systems, Andover, MA) was used in this study. This ultrasound system is equipped with a 2.0-to-4.0-MHz transducer.
2.3. Procedure
Transthoracic echocardiography was performed with the patient in the supine or lateral position and was monitored by electrocardiography. Multi-section scans were performed on the parasternal line, on the apex of the heart, beneath the xiphoid process, and on the suprasternal fossa, which mainly included the following conventional sections: long-axis view of the left ventricle (observing the mitral valve, aortic valve, and left heart chamber), short axial section of the large artery (observing the aortic valve, pulmonary valve, tricuspid valve, left atrial, and right heart chambers), and 4-chamber or 5-chamber views (observing the mitral valve, tricuspid valve, aortic valve, and left and right heart chambers). ZOOM was used for magnification of the section to allow for local observation. The morphology, structure, activity, and closure of the valve were observed from multiple angles. The observations were focused on the vegetation size, number, location, shape, internal echo, and activity as well as on whether the vegetation was accompanied by complications such as rupture of the chordae tendineae, valve prolapse, valve perforation, and paravalvular abscess. In addition, we also observed whether the heart was associated with basic heart disease such as congenital heart disease, rheumatic heart disease, and vascular calcification.
2.4. Comparison with intraoperative findings
Of the 87 patients, 69 underwent surgical treatment, and the echocardiographic results were compared with observations during surgery.
2.5. Statistical analysis
All statistical analyses were performed with the SPSS 16.0 (SPSS Inc., Chicago, IL) software package. Continuous variables are expressed as the means ± standard deviations (SDs). The Chi-square test or Fisher exact test was used to compare the echocardiographic and surgical results. P < .05 was considered to indicate a significant difference, P < .01 was considered to indicate a very significant difference, and P > .05 was considered to indicate no significant difference.
3. Results
3.1. Clinical presentation
The 87 patients included 50 men and 37 women with a mean age ± SD of 45.2 ± 10.2 years (range, 6–73 years). Sixty patients experienced different degrees of fever, 80 patients had a heart murmur, 16 patients had anemia, 13 patients had hepatosplenomegaly, and 7 patients exhibited skin and mucous membrane petechia or ecchymosis. The most common complication was heart failure (35 cases), followed by organ embolism (12 cases), including cerebral embolism (7 cases), splenic embolism (3 cases), lower extremity arterial embolization (2 cases), and arrhythmia (4 cases).
3.2. Underlying heart disease
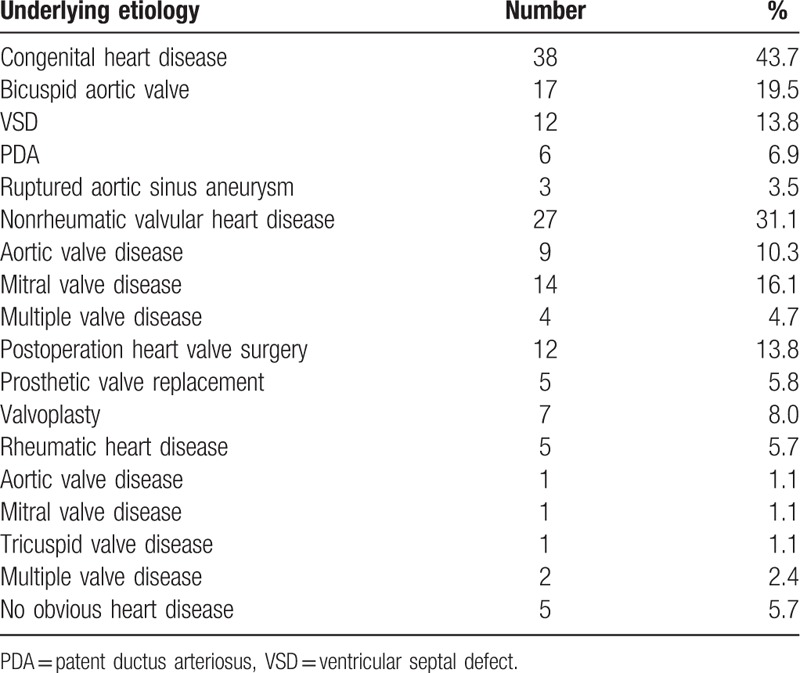
The 87 patients investigated in this study included 38 cases of congenital heart disease, 27 cases of nonrheumatic valvular heart disease, 12 cases of postoperative heart valve surgery, 5 cases of rheumatic valvular heart disease, and 5 patients showing no obvious heart disease (Table 1).
Table 1.
Underlying heart disease in 87 IE patients.
3.3. Pathogen distribution
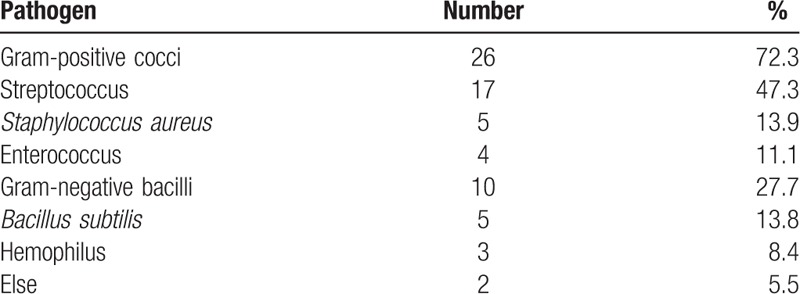
The 87 patients included 36 cases of positive blood cultures, which consisted of 26 cases of gram-positive cocci and 10 cases of gram-negative bacilli (Table 2).
Table 2.
Pathogen distribution.
3.4. Echocardiography
Echocardiography showed that the diameter of the vegetation was 2 to 25 mm and that it was in the form of flocculent, cord-like, lumps with an irregular morphology. The fresh vegetation exhibited low echoes and good activity. Increases in the disease duration were associated with a gradual increase in the extended echo and a reduced vegetation activity (Fig. 1). The vegetation was either single or multiple in number. The echocardiography results for the patients revealed 37 cases involving the aortic valve, 34 cases involving the mitral valve, 10 cases involving the tricuspid valve, 2 cases involving the pulmonary valve, and 5 cases involving an artificial valve. Twenty-six of these cases had multiple valve involvement. Twenty of the patients in this study experienced severe complications, including chordae tendineae rupture (7 cases), valve prolapse (6 cases), valve perforation (3 cases) (Fig. 2), and paravalvular abscess (4 cases) (Fig. 3).
Figure 1.
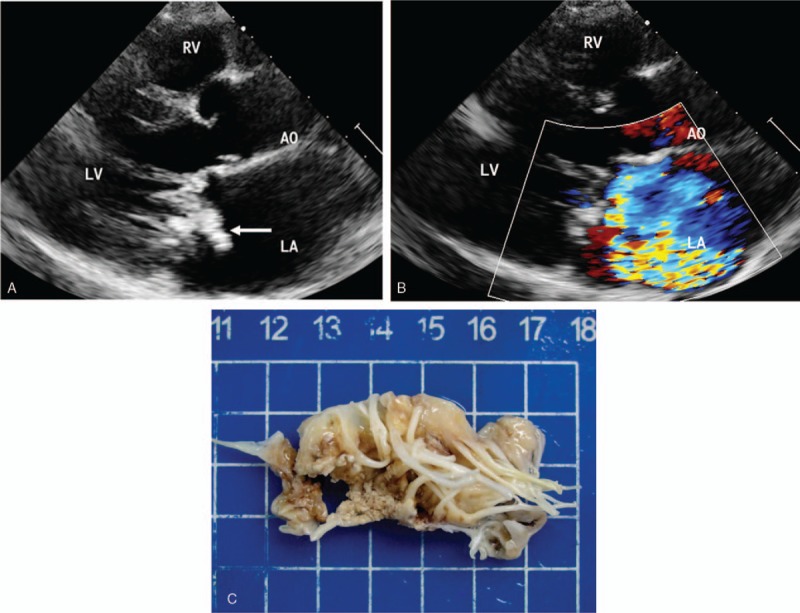
IE in a 50-year-old man. (A) Echocardiography in the left ventricle parasternal long-axis view shows a large complex vegetation on the mitral valve (arrows). (B) Color Doppler shows severe mitral regurgitation. (C) Specimen shows a large number of vegetations on the mitral valve. AO = aortic, LA = left atrium, LV = left ventricle, RV = right ventricle.
Figure 2.
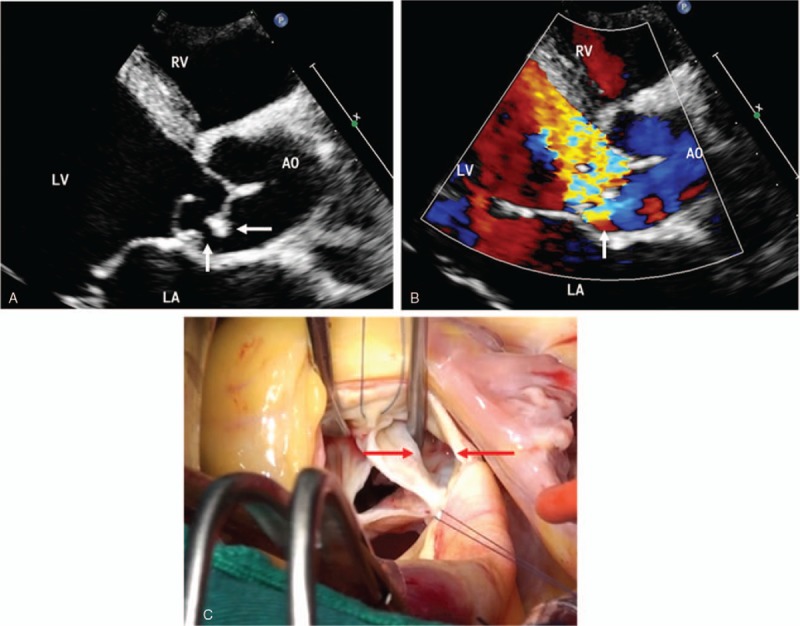
IE in a 61-year-old man. (A) Echocardiography in the parasternal long-axis view shows numerous vegetation clumps in the aortic valve (arrows), the continuous echo at the root of the valve was interrupted, and the valve leaflets were shed to the left ventricular outflow tract, which suggested valve perforation and valve prolapse. (B) Severe aortic regurgitation through the perforation is observed on Doppler color views. (C) Intraoperative images show an aortic valve leaflet with perforation. AO = aortic, LA = left atrium, LV = left ventricle, RV = right ventricle.
Figure 3.
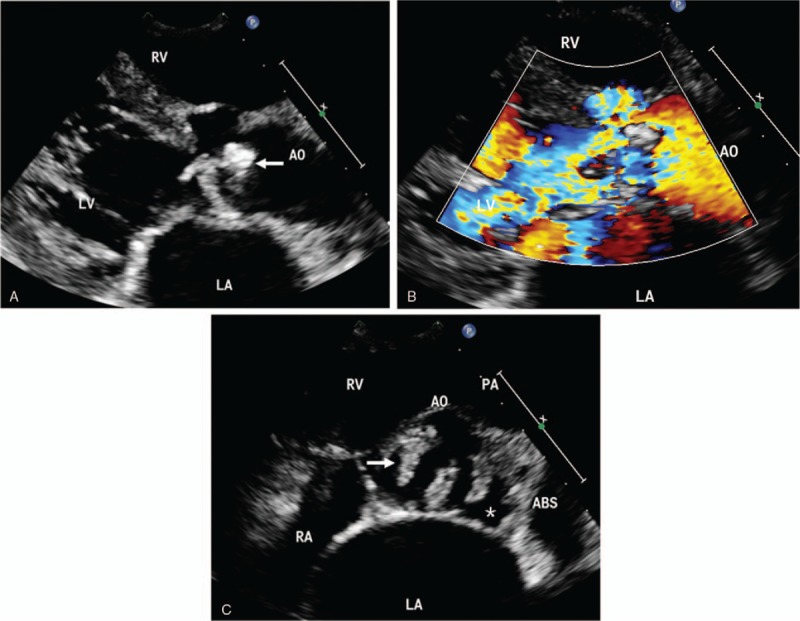
IE in a 71-year-old man. (A) Echocardiography in the left ventricle parasternal long-axis view shows numerous clumps of vegetation in the aortic valve (arrows). (B) Color Doppler shows severe aortic regurgitation. (C) The large artery parasternal short-axis view demonstrates native aortic valve vegetation (arrows) and paravalvular abscess (white asterisk). ABS = abscess, AO = aortic, LA = left atrium, LV = left ventricle, PA = pulmonary artery, RV = right ventricle.
3.5. Treatment and outcome
Among the 87 patients, 69 underwent surgical treatment, 12 had significantly decreased vegetation numbers after undergoing anti-infective therapy using intensive antibiotic therapy, and 5 patients died due to cardiogenic shock (3 patients), multiple organ failure (1 patient), and cardiac arrest (1 patient).
3.6. Comparison with intraoperative findings
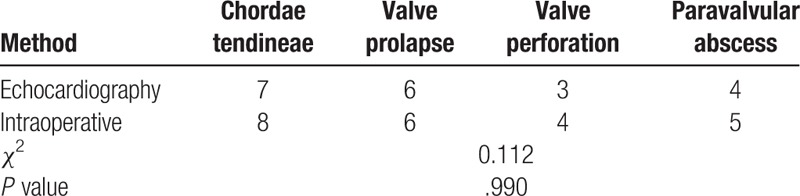
The comparison of echocardiography and actual surgical observations revealed no significant differences in the accuracy of the detections of the size, number, and location of vegetation in the 69 patients who underwent surgery (P > .05; Table 3). Echocardiography could detect the occurrence of various severe complications, namely, chordae tendineae rupture, valve perforation, and paravalvular abscess, and no statistically significant difference in diagnostic accuracy was found between echocardiography and surgical observation (P > .05; Table 4).
Table 3.
Comparison of the echocardiography detection of vegetation and surgical observation.
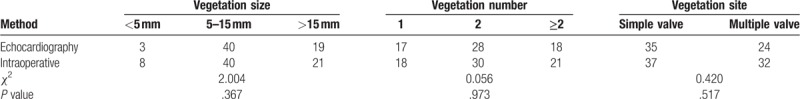
Table 4.
Comparison of the detected complications between echocardiography and surgical observation.
4. Discussion
IE is an inflammatory disease of the valve, endocardium, and vascular intima caused by pathogenic microorganisms, and this disease symptoms accompanied by cardiac lesions.[5] In recent years, due to the widespread use of antibiotics and the application of invasive examination for the diagnosis of cardiovascular disease, the clinical signs of IE have become mostly atypical and are sometimes ignored, which can lead to missed diagnosis.[6] Currently, the diagnosis of IE is mainly based on clinical manifestations, echocardiography, and blood culture.[7,8] The early clinical manifestations of IE are diverse and nonspecific, and the results of this study showed that heart murmur, fever, and anemia remain the most common clinical manifestations of IE, consistent with the findings in China, whereas Osler nodes, Janeway lesions, and Roth spots were not common. The most common complication of IE is heart failure. As reported in the literature, acute heart failure is related to IE mortality. The second most common complication is organ embolism, which is commonly observed as cerebral embolism and splenic embolism. Studies have revealed that vegetation in the left section of the heart can fall off into the brain, kidney, spleen, lung, peripheral blood vessels, and coronary arteries to cause the embolization of various target organs. The incidence of embolism is approximately 15% to 35%, and embolism of the spleen is found in 40% of these cases. In addition, embolization might still occur 1 to 2 years after recovery. The vegetation in the right section of the heart can fall off into the pulmonary artery and cause pulmonary embolism. Therefore, IE should be suspected when unexplained embolism occurs in clinical practice.[9,10]
IE usually occurs in patients with underlying heart disease. In patients with underlying heart disease, shear stress generated by blood turbulence damages the endocardium, which makes it easier for pathogenic microorganisms to attach to the damaged endocardium and for the formation of vegetation from thrombosis.[11] The results of this study showed that 82 of 87 IE patients had underlying heart disease, including congenital heart disease (38 patients) and rheumatic heart disease (5 patients). Previous studies have shown that 50% to 80% of IE cases occur primarily due to rheumatic heart disease. In contrast, recent data show that the proportion of IE cases with rheumatic heart disease as the underlying etiology is significantly decreased, and as a result, congenital heart disease has become the most common etiology of IE. This result might be related to improvements in living standards, the widespread application of antibiotics, the significantly reduced incidence and recurrence of acute rheumatic fever, and the decreased incidence of rheumatic heart disease.[12]
The pathogens of IE are mainly streptococcus and staphylococcus, and infections with microbes such as bacilli, fungi, and viruses are occasionally observed. The increasing use of antibiotics, interventional procedures, endoscopic techniques, cardiac implant devices, and venous catheters in clinical settings has substantially changed the epidemiological characteristics of IE, that is, medically relevant infection of the intracardiac system. The proportion of membranous inflammation is increasing rapidly, and for the first time, the proportion of positive blood cultures is continuously decreasing. In this study, 36 cases, accounting for 41.37% of the cases (36/87), were positive for blood culture, and false negatives might have been obtained if the patient had used antibiotics before the specimen was collected, the specimen did not meet the requirements or the patient had a specific pathogen infection. Because some patients showed negative results after repeated blood cultures, these patients were diagnosed late, and the rate of missed diagnosis was high. Therefore, the improved Duke diagnostic criteria for IR are increasingly focusing on the role of imaging studies.
Echocardiography for the diagnosis of IE mainly relies on the detection of vegetation, evaluation of the degree of vascular damage, and the resulting hemodynamic abnormalities and observation of complications. Vegetation is a specific indicator for the diagnosis of IE. The ultrasound detection of definite vegetation often plays a very important role in the diagnosis of suspected IE.[13] According to statistical analyses, the sensitivity of vegetation detection by transthoracic echocardiography is 60% to 75%, and the sensitivity of vegetation detection by transesophageal echocardiography is >95%. Transthoracic echocardiography can rapidly and accurately detect vegetation and accurately determine the vegetation size, number, and location. Left ventricular vegetation was more common in the 87 patients diagnosed with IE by transthoracic echocardiography in this study (aortic and mitral valve involvement was detected in 37 and 34 patients, respectively). Published studies have shown that the frequency of valve infection is positively proportional to the risk of valve injury. Valves under high-pressure blood flow are subjected to the highest shear stress, and thus, left heart valve involvement has been observed in most IE patients.[14] The mitral and aortic valves receive the greatest blood pressure and are thus the most susceptible to infection, which is consistent with the findings obtained in this study.
In addition to the formation of valvular vegetation, the disease course of IE is accompanied by other complications, such as chordae tendineae rupture, valve prolapse, valve perforation, and paravalvular abscess.[15] Two patients in this study experienced vegetation shedding that caused peripheral arterial embolism. It has been reported that the vegetation echoes, size, and activity can be used as independent factors in the prediction of embolism, particularly for a vegetation size >10 mm, which is associated with the highest incidence of embolism.[16] The diagnosis of small valve perforation in 1 patient in this study was missed, which might have been caused by the difficulty associated with differential diagnosis among small perforation, valve prolapse, and valve regurgitation. Echocardiography has important value for the diagnosis of complications.
In this study, 69 of the 87 IE patients underwent surgery for vegetation removal and treatment for the underlying heart disease. The surgical rate in this study was 79.3% higher than the reported rate of 50%, and the overall mortality rate was 5.7%, which was lower than the rate of 17.7% reported in the literature.[17] The prognosis associated with active surgical treatment is significantly better than that associated with simple medical therapy, which shows the importance of timely surgical treatment at an early stage for the successful treatment of IE. Therefore, the pros and cons of the 2 aspects must be weighed when determining the timing of surgery; specifically, if the patient's condition is progressively aggravated and heart failure cannot be controlled after medical treatment, the efficacy of surgery will be better than that of conservative treatment. However, antibiotic treatment for IE should still be applied throughout the entire course of treatment. Once diagnosed, a sufficient dose of antibiotics should be administered, and a patient should continue to undergo antibiotic treatment for 4 to 6 weeks after surgery until sufficient treatment is achieved.
IE vegetation should be differentiated from calcified lesions in the valve, primary small tumors of cardiac valve leaflets, and thrombosis. Cardiac valve calcification is common in elderly patients with atherosclerosis or long-term rheumatic heart disease. Calcifications primarily appear as dense echoes visible as spots and lumps, whereas the vegetation mostly moves with valve opening and closing. The echo associated with vegetation is relatively weak, and the vegetation structure is relatively loose. Calcification develops in the vegetation over time, and the vegetation echo might thus be enhanced.[18] Primary small tumors of cardiac valves can be myxomas and fibroelastomas, for example, and primarily appear as single lesions with a normal morphology, that is, often or mostly round. However, most vegetation includes multiple lesions and has an irregular shape. The diagnosis of vegetation through echocardiography also needs to be combined with changes in clinical manifestations and disease conditions. The vegetation often shows changes during the course of treatment, whereas small tumors do not exhibit significant changes.[19] Thrombosis is more common in dilated cardiomyopathy, acute myocardial infarction with ventricular aneurysm formation, and mitral stenosis in rheumatic heart disease. Thrombosis is characterized by a relatively large volume, fewer morphological changes, lower activity, and low echo in cases of new thrombus formation. The echo is enhanced after thrombus formation.[20]
Transthoracic echocardiography still has some limitations. The detection rate of vegetation in patients with infective endocarditis is greatly reduced and affected by obesity, chronic obstructive pulmonary disease, chest wall deformity, and the acoustic shadow of an artificial mechanical valve. Therefore, a clinician may suspect that a patient has IE, but the transthoracic echocardiography might not show abnormalities, and transesophageal echocardiography should be used for a patient who meet these conditions.
5. Conclusions
In summary, the underlying etiology and clinical manifestations of IE have changed, but transthoracic echocardiography, as a noninvasive imaging method, exhibits high accuracy for the detection of vegetation and complications. Transthoracic echocardiography provides a basis for the accurate diagnosis of IE and is of great significance for guiding the development of clinical treatment programs and prognosis determination.
Acknowledgments
The authors greatly appreciate the assistance provided by the Department of Thoracic and Cardiovascular Surgery, The First Affiliated Hospital of Nanchang University, and thank the staff for their efforts.
Author contributions
Conceptualization: XinChun Yuan, Ming Liu, Jia Hu, Xi Zeng, AiYun Zhou, Li Chen.
Data curation: XinChun Yuan, Ming Liu, Jia Hu, Xi Zeng, AiYun Zhou, Li Chen.
Methodology: XinChun Yuan, Ming Liu, Jia Hu.
Writing – original draft: XinChun Yuan, Ming Liu, Jia Hu, Xi Zeng.
Writing – review & editing: XinChun Yuan.
Footnotes
Abbreviations: ABS = abscess, AO = aortic, ESC = European Society of Cardiology, IE = infective endocarditis, LA = left atrium, LV = left ventricle, PA = pulmonary artery, PDA = patent ductus arteriosus, PET-CT = positron emission tomography computed tomography, RV = right ventricle, SPECT = single-photon emission computed tomography, VSD = ventricular septal defect.
How to cite this article: Yuan Xc, Liu M, Hu J, Zeng X, Zhou Ay, Chen L. Diagnosis of infective endocarditis using echocardiography. Medicine. 2019;98:38(e17141).
X-cY and ML contributed equally to this work.
This study was approved by the ethical review committee of The First Affiliated Hospital of Nanchang University, and written informed consent was obtained from the patients.
The authors have no conflicts of interest to disclose.
References
- [1].Evangelista A, Gonzalez-Alujas M. Echocardiography in infective endocarditis. Heart 2004;90:614–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- [3].Rozich JD, Edwards WD, Hanna RD, et al. Mechanical prosthetic valve-associated strands: pathologic correlates to transesophageal echocardiography. J Am Soc Echocardiogr 2003;16:97–100. [DOI] [PubMed] [Google Scholar]
- [4].Vilacosta I, Gomez J. Complementary role of MRI in infectious endocarditis. Echocardiography 1995;12:673–6. [DOI] [PubMed] [Google Scholar]
- [5].Cabell CH, Jollis JG, Peterson GE, et al. Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 2002;162:90–4. [DOI] [PubMed] [Google Scholar]
- [6].Chen JJ, Manning MA, Frazier AA, et al. Ct angiography of the cardiac valves: normal, diseased, and postoperative appearances. Radiographics 2009;29:1393–412. [DOI] [PubMed] [Google Scholar]
- [7].Morris CD, Reller MD, Menashe VD. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA 1998;279:599–603. [DOI] [PubMed] [Google Scholar]
- [8].Hill EE, Herijgers P, Claus P, et al. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J 2007;28:196–203. [DOI] [PubMed] [Google Scholar]
- [9].Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010;11:202–19. [DOI] [PubMed] [Google Scholar]
- [10].Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009;53:436–44. [DOI] [PubMed] [Google Scholar]
- [11].Saghir S, Ivey T, Kereiakes D, et al. Anterior mitral valve leaflet aneurysm due to infective endocarditis detected by cardiac magnetic resonance imaging. Rev Cardiovasc Med 2006;7:157–9. [PubMed] [Google Scholar]
- [12].Wong D, Keynan Y, Rubinstein E. Comparison between transthoracic and transesophageal echocardiography in screening for infective endocarditis in patients with Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2014;33:2053–9. [DOI] [PubMed] [Google Scholar]
- [13].Dursun M, Yilmaz S, Yilmaz E, et al. The utility of cardiac MRI in diagnosis of infective endocarditis: preliminary results. Diagn Interv Radiol 2015;21:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bruun NE, Habib G, Thuny F, et al. Cardiac imaging in infectious endocarditis. Eur Heart J 2014;35:624–32. [DOI] [PubMed] [Google Scholar]
- [15].Hyafil F, Rouzet F, Lepage L, et al. Role of radiolabelled leucocyte scintigraphy in patients with a suspicion of prosthetic valve endocarditis and inconclusive echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:586–94. [DOI] [PubMed] [Google Scholar]
- [16].Thuny F, Grisoli D, Cautela J, et al. Infective endocarditis: prevention, diagnosis, and management. Can J Cardiol 2014;30:1046–57. [DOI] [PubMed] [Google Scholar]
- [17].Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- [18].Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075–123.26320109 [Google Scholar]
- [19].Champey J, Pavese P, Bouvaist H, et al. Value of brain MRI in infective endocarditis: a narrative literature review. Eur J Clin Microbiol Infect Dis 2015;35:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahmed FZ, James J, Memmott MJ, et al. Radionuclide imaging of cardiovascular infection. Cardiol Clin 2016;34:149–65. [DOI] [PubMed] [Google Scholar]


