Abstract
Although some studies found that an increased monocyte count is a predictive, short-term marker of unfavorable outcomes for patients with acute heart failure (HF), others have reported that monocytosis predicts prolonged survival.
The current follow-up study aimed to identify different monocyte count patterns and their prognostic association with HF outcomes.
Baseline blood samples for complete blood counts, differential counts, renal function tests, and lipid profiles of 303 chronic HF patients (average NYHA classification 2.8) were prospectively obtained to evaluate whether there is an association between monocyte count and clinical outcomes.
Mean follow-up was 11.3 years (range 1 month to 16 years) and 111 (36.6%) patients died during follow-up. Mean monocyte count was 10.6 ± 5.5 and mean left ventricular ejection fraction (LVEF) was 36%. Patients with low monocyte counts (≤6%) had significantly lower survival rates than did those with monocyte counts 6.1% to 14%, or >14% (14.3% vs 70.2% vs. 88%, P < .001). Poorest survival was predicted for patients with NYHA class 3 to 4 and monocyte counts ≤6. Regression analysis showed that monocyte levels, NYHA class, and LVEF values were predictors of mortality, in decreasing importance.
The total monocyte count was found to be an important prognostic factor that was inversely associated with predicted long-term mortality among patients with chronic HF. A low total monocyte count was strongly correlated with NYHA class and B-type natriuretic peptide levels, but no correlation was found with LVEF and oxidized low-density lipoproteins. It emerged as an independent risk factor for mortality in patients with chronic HF.
Keywords: biomarkers, heart failure, prognostic factors, total monocyte count
1. Introduction
Several biomarkers and tests have been established as useful for assessing the prognosis of patients with acute heart failure (HF)[1] and various types of inflammatory cells, including monocytes, lymphocytes, eosinophils, and neutrophils, have been involved in coronary heart disease.[2,3] Experimental data showed that an increased monocyte count may be useful as a predictive biomarker, and as an indicator of unfavorable outcomes among patients with acute coronary syndromes, post-infarction, HF, coronary artery disease (CAD), and atherosclerosis. It is also associated with increased in-hospital mortality.[4–14] Monocytes play an important role in inflammatory, vasculogenetic processes, as well as in regeneration of the vascular wall and in the development of HF.[4,5,9–15]
Characterization of 3 subgroups of monocytes and their related contribution to either aggravation or amelioration of HF needs further elucidation. In depth understanding of the mechanisms involved in these effects is needed as well.[4]
Shahid et al[2] recently provided a comprehensive analysis of the role of monocytes in heart failure. They highlighted the stimulating but commonly antagonizing effects of monocyte-mediated myocardial inflammation, that is regeneration and remodeling, as opposed to dilatation and fibrosis.
Monocyte platelet aggregates (MPA) are increased in patients with HF and believed to be associated with monocytes from Mon1 and Mon2 subgroups (also called “classical monocytes”).[6] The Mon1 subgroup monocytes (Mon1, antigen processing and presentation CD14++CD16–CCR2+) are recognized as phagocytes and play a role in first-line fortification and defense processes.
Mon3 stimulate “patrolling” function and tissue repair. The Mon3 (Ly-6C–) subgroup of monocytes promotes myocardial healing through myofibroblast accumulation, angiogenesis, and collagen deposition.[1] Ly-6C– monocytes, notwithstanding, have been found to have anti- inflammatory properties. This subset promotes myocardial healing after an infarction through the processes of myoblast activation, angiogenesis, and collagen formation. In the absence of inflammation, Ly6C+ transforms into Ly6C–, which predominates in the circulation, binding to vascular endothelium using CX3CR1 receptors. In response to bacterial infection, Ly-6C– cells release anti-inflammatory cytokines (namely, IL-10).[1,15] The response to inflammation triggers the differentiation of monocytes into M2 macrophages, which in turn, release anti-inflammatory cytokines central to tissue repair.[15]
This study investigated whether monocytes have an adverse or defensive effect on outcomes among patients with long-term, chronic HF. Most previous studies did not differentiate between chronic and acute HF. In acute HF, monocytes Mon1 and Mon2 predominate and increase during exacerbations.
2. Methods
2.1. Study design
This historical cohort, long-term, follow-up study included all patients treated in the special HF Unit at the Tel Aviv Sourasky Medical Center from January 1, 2000 to June 30, 2001 who met the study inclusion criteria. These patients were followed until January 1, 2017 or until death, if sooner.
2.2. Exclusion criteria
Excluded were patients with conditions known to affect white blood cell count, especially monocyte count (malignant disease with diffuse metastases, chemo- and/or radiotherapy), medications known to affect blood count (steroids), acute renal failure, active hepatic disease, severe chronic obstructive pulmonary disease, or acute infectious disease. Patients with conditions that could cause acute HF (those who in less the previous 3 months had acute MI, percutaneous coronary intervention, heart surgery, or those hospitalized for any reason) were also excluded.
Baseline blood samples for complete blood counts (CBC), including automatic differential counts, renal function tests, and lipid profile were obtained at the first visit. Clinical and laboratory measurements were obtained at subsequent follow-up visits.
Systolic HF (reduced ejection fraction) was defined as a LVEF ≤ 40%,[1,16] based on echocardiography or radionuclide ventriculography. LVEF >40% was defined as HF with preserved ejection fraction (diastolic dysfunction).
During their first visit to the HF Unit, all participants were examined and medical history was obtained, including current medications. Patients underwent physical examination that included resting blood pressure, heart rate, and weight measurements. NYHA classification was determined. Echocardiography and radionuclide ventriculography were performed to determine LVEF. Ischemic or valvular heart disease was defined according to previous history of confirmed MI, coronary artery bypass grafting, or coronary angiography with percutaneous coronary intervention and echocardiography. Patients were followed by a HF specialist at least once every 3 months.
The study endpoint was all-cause mortality.
2.3. Statistical analysis
Data are described using mean and standard deviation (SD) for continuous variables and frequencies for categorical variables. We considered 3 groups of patients based on quartiles of the distribution. The “lower” group included 25% of the patients with the lowest values of the variable, the “middle” group included 50% of the patients with the values of the variable between its first and third quartile, and the “upper” group included 25% of the patients with the highest values of the variable. Because of the small number of patients in NYHA class 1 and the unequal number of patients in NYHA classes 1 and 4, we defined 3 NYHA groups, namely NYHA 1–2, NYHA 2.5–3 and NYHA 3.5–4.
In all regressions, we took the lower part as the reference to show the trend for hazard of death. This approach is widely used to study the effect of a continuous variable for the following reasons:
It avoids assumption of linearity;
It provides a natural way to divide a population in the absence of officially accepted cut-off points; and
Sometimes it is preferable to study a small or moderate sample, even when there are accepted cut-offs, to increase the power if some categories include a small proportion of the sample.
Specifically, the parameters were grouped as: monocytes (Group 1: ≤6%, Group 2: 6.1%–14%, and Group 3: >14%); LVEF (group 1: ≤40% and group 2: >40%). The Kaplan–Meier method was used to compute the product-limit estimate survival function of mortality for each of the 3 groups. This made it possible to compare the effect of various variables on survival time. In order to show trends, the middle group was used as the reference in all regressions and included in the tables with hazard ratio (HR) = 1.
According to the goal of the study, we investigated prognostic values of the initial tests. This approach is standard in studies of specific subpopulations. Using initial values allowed us to build survival curves for the selected subgroups and to define the subpopulations at higher risk for poor outcomes.
We used expert controlled backward elimination in multiple Cox proportional hazard regressions where the least significant variable at each step was excluded if it agreed with expert opinion.
All calculations were done using STATA SE software. All tests were two-sided, and P values <0.05 were considered statistically significant.
There were clinical suspicions that the effect of monocytes might differ between patients with nonischemic cardiomyopathy (NICM) as compared to the other (non-NICM) patients. The cohort included 28 NICM patients. We calculated the effects of monocytes in both NICM and non-NICM groups. They were almost identical. In addition, we repeated the multiple regression with the non-NICM group and results were similar to those of the entire data set. Therefore, we present the results of the entire patient population.
3. Results
A total of 338 consecutive outpatients with congestive HF (ischemic or valvular origin) were included in this retrospective study. Thirty five were excluded due to technical reasons, noncompliance, or lack of follow-up information.
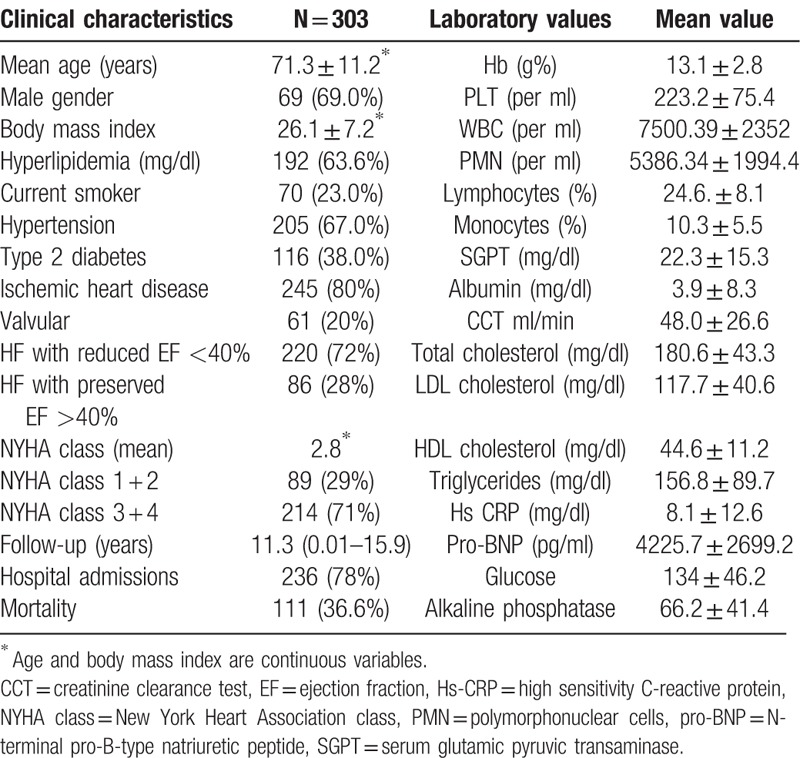
The remaining 303 patients had a mean ± SD age of 71.3 ± 11.2 years and they made up the study cohort. Mean follow-up was 11.3 years (range 1 month to 16 years) and 78% of the participants were admitted to the hospital during their course of follow-up. Clinical and laboratory findings at the first hospitalization of all the participants are presented in Table 1. The mean NYHA classification was 2.8. Due to the small number of patients in NYHA class 1 and the unequal number of patients in NYHA classes 1 and 4, we divided the study sample into 3 categories (statistically), that is, NYHA 1–2, NYHA 2.5–3, and NYHA 3.5–4. The mean monocyte count (percent of total white blood cells) was 10.3 ± 5.5% and the mean LVEF was 36%.
Table 1.
Patients’ clinical and laboratory characteristics at baseline.
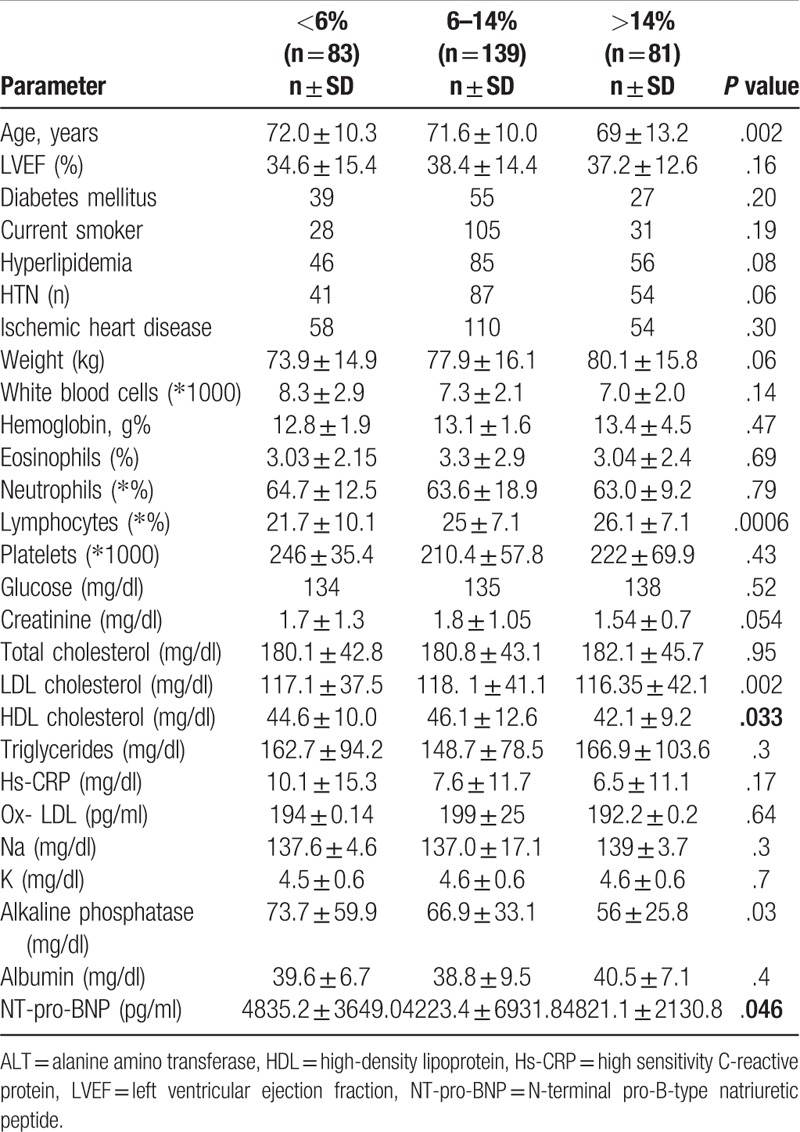
The general characteristics of the patients and the mean distribution of selected clinical and laboratory parameters according to the 3 monocyte groups are shown in Table 2. The monocyte groups differed in lymphocyte counts (P = .0006), high density lipoprotein (HDL) level (P = .033), and alkaline phosphatase level (P = .03). Patients in monocyte Group 3 (monocytes >14%) were somewhat younger than those in the other 2 monocyte groups, and slightly more were in a higher NYHA class (3.5–4). Group 2 patients had lower NT-pro-BNP levels (P = .046) and higher HDL levels (P = .033). There were no differences between monocyte groups in the prevalence of diabetes mellitus, hypertension, or ischemic heart disease. Hemoglobin, glucose albumin, creatinine, creatinine clearance test and C-reactive protein levels were similar for all 3 groups. Higher HDL level was associated with longer survival.
Table 2.
Mean values of the clinical and main laboratory parameters according to monocyte distribution groups (6%, 6%–14%, >14%).
There were no differences in the use of most medications (including those which prolong survival, such as beta blockers, ACE inhibitors) between the monocyte groups, except for warfarin (P = .04), which was much less frequent among group 3 patients (Table 3).
Table 3.
Frequency of medications according to monocyte groups (<6%, 6%–14%, >14%).
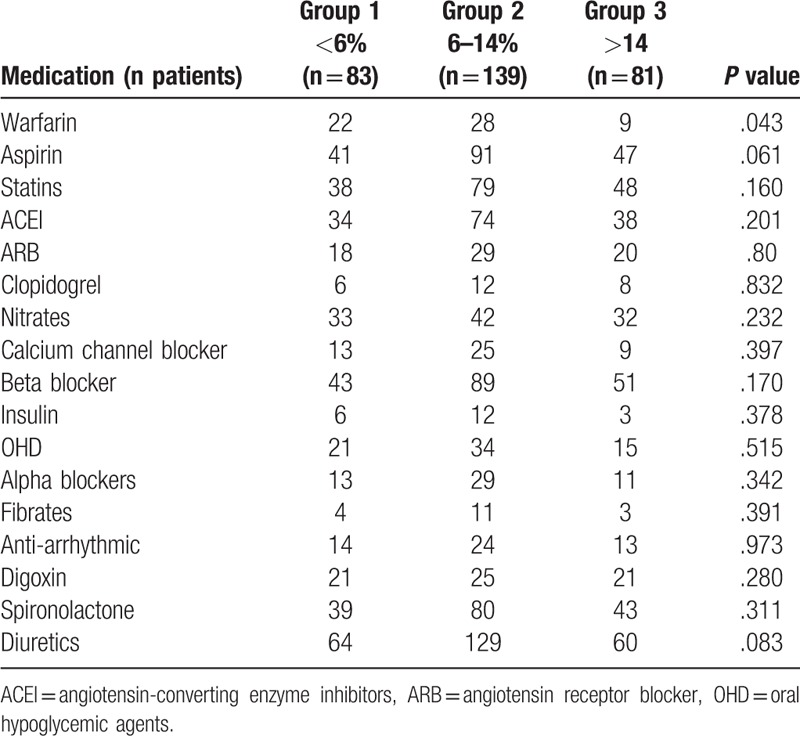
Figure 1 displays the Kaplan–Meier survival mortality curves according to the 3 monocyte groups. Group 3 (monocyte count >14%) had the best long-term survival (88% survived) and Group 1 (monocyte count ≤6%) had the poorest outcomes. This trend remained consistent for shorter periods, as well. Figure 2 illustrates the Kaplan–Meier mortality curves according to LVEF (≤40%) and monocyte count of Group 1 patients (28% and 18%, respectively) and was significantly less than that of the Group 3 patients (P < .001). Group 3 patients survived significantly longer (above 83%) independent of LVEF value (>40% or ≤40%). The highest survival was observed in the subgroup with monocytes >14% and EF >40%.
Figure 1.
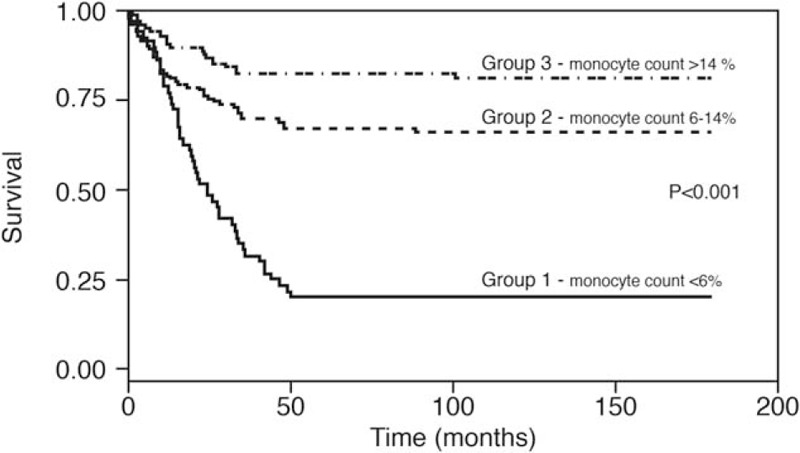
Kaplan–Meier survival-mortality curves according to the 3 groups of monocytes.
Figure 2.
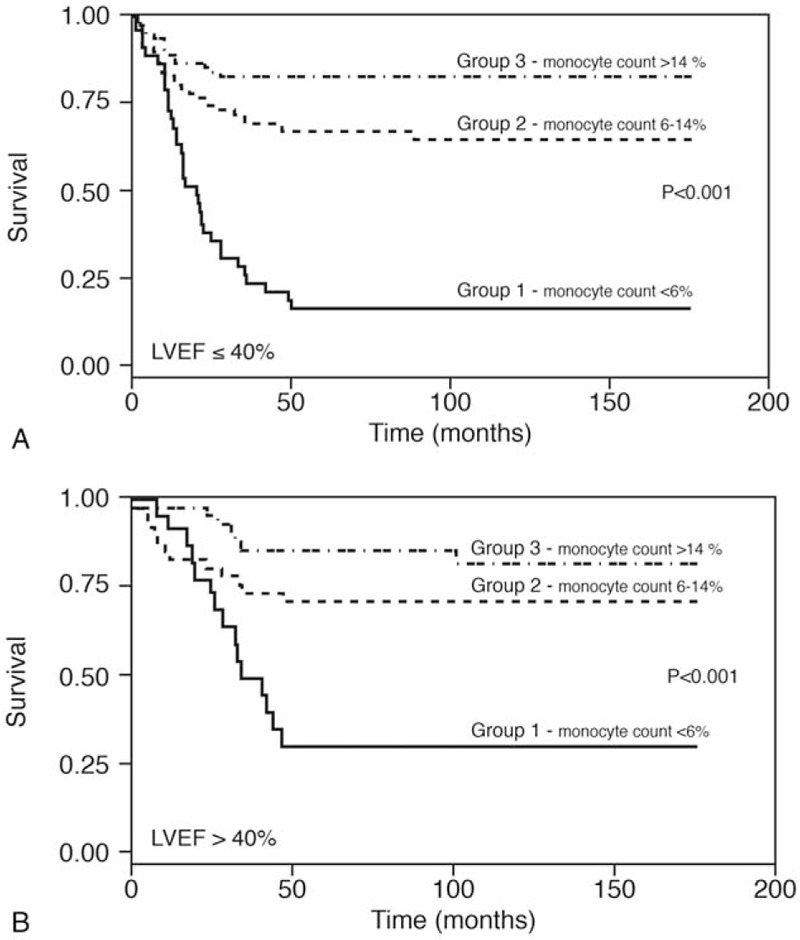
A. Kaplan–Meier survival curves of the 3 monocyte groups, among patients with ejection fraction ≤40%. B. Kaplan–Meier survival curves of the 3 monocyte groups among patients with ejection fraction >40%.
Figure 3A, B, C show the survival rates of the 3 monocyte groups according to NYHA Class. Patients in the NYHA 1 + 2 group with a monocyte count <6.0% had a poorer survival rate (29%) than did the patients in Group 2 (80% survival) and those in Group 3 (>85%; P < .001) (Fig. 3A). The same trend was observed in patients with NYHA Class 2.5–3 (Fig. 3B). Survival in Group 1 was 22%, in Group 2, 69% and in Group 3 it was more than 82% (P < .001). For NYHA Class above 3, the survival rate for Group 1 was 12%, Group 2, 27% and in Group 3, 50% (P < .001; Fig. 3C).
Figure 3.
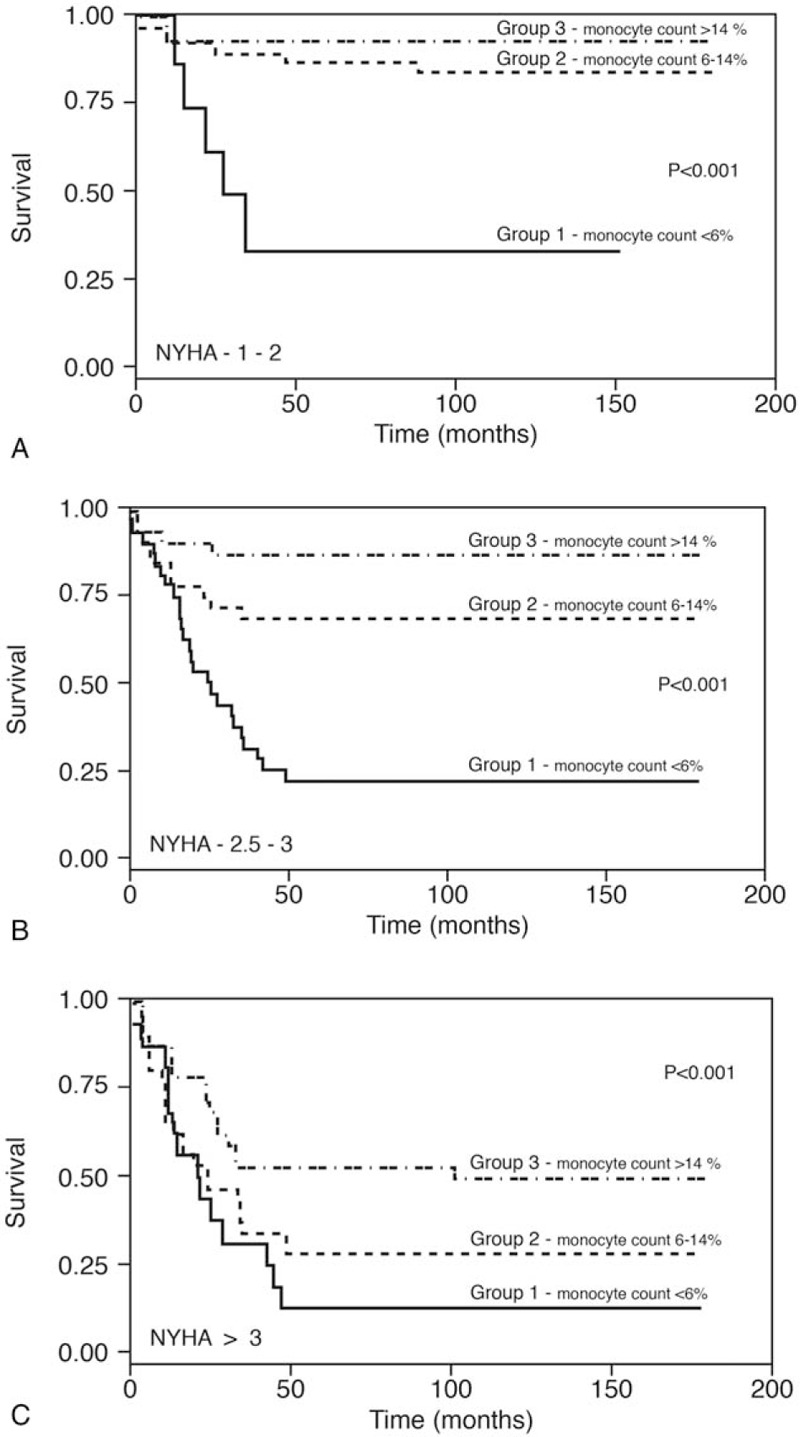
A. Kaplan–Meier survival curves of patients with various monocyte counts among patients with NYHA 1–2. B. Kaplan–Meier survival curves of patients with various monocyte counts among patients with NYHA 2.5–3. C. Kaplan–Meier survival curves of patients with various monocyte counts among patients with NYHA 3.5–4.
Table 4 demonstrates the mortality hazard ratio (HR) of main clinical and laboratory parameters, adjusted for age and gender. It can be seen that, as related to the middle group, the lower group of monocytes had the highest hazard ratio (5.053) and the upper group had the lowest hazard ratio (0.042).
Table 4.
Mortality Hazard Ratio (HR) of main clinical and laboratory parameters.
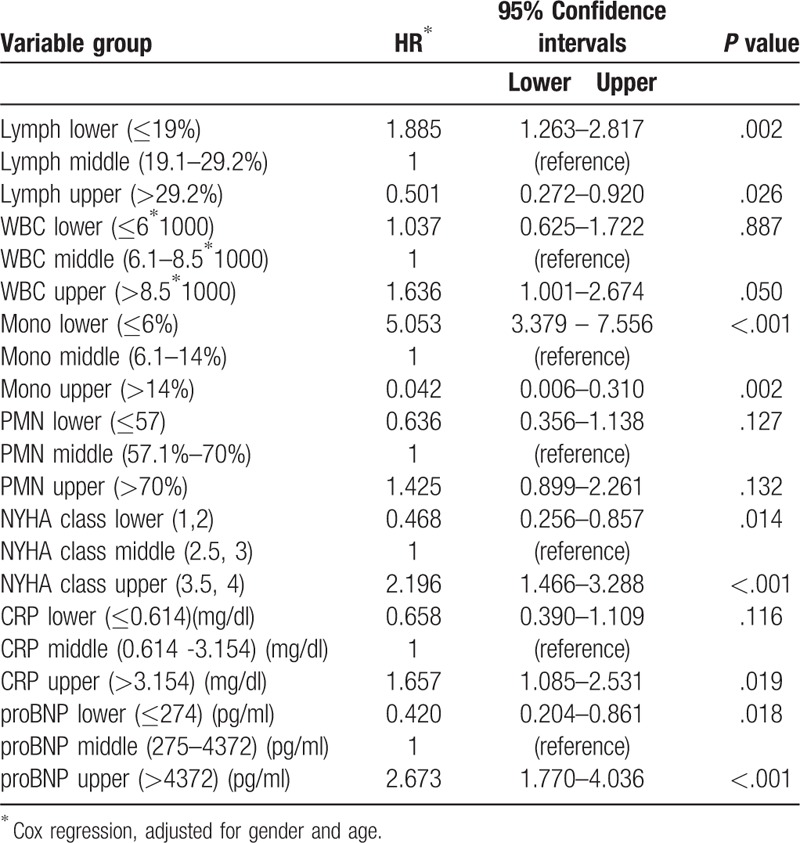
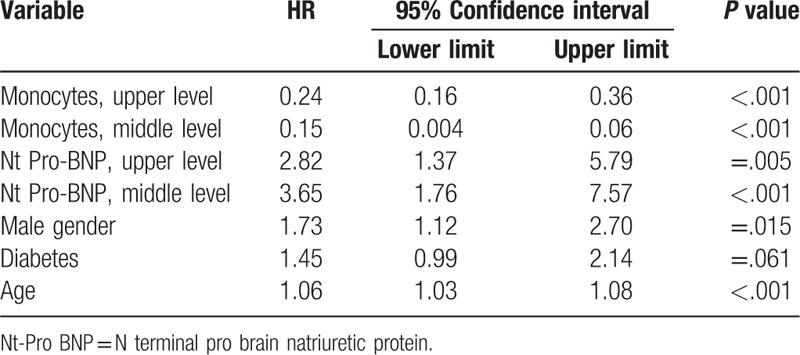
Table 5 shows a concise proportional regression model of mortality and significant hazard ratios of the main clinical and laboratory parameters. Monocytes had the highest HR as did Nt-Pro-BNP that reached a level of significance. After periods of 1 year, 3 years, 5 years, and 10 to 12 years, the mortality was higher in Group 1: 69 of the 83 patients (83%) died during follow-up, as compared to 40 of 139 patients (29%) in Group 2, and 2 of 81 (2.5%) in Group 3.
Table 5.
Concise model of mortality and significant hazard ratios of main clinical and laboratory parameters.
Additional analyses were performed using absolute monocyte counts adjusted to hematocrit level. Since hematocrit does not change the proportion of monocytes, we adjusted only for absolute monocyte counts. We corrected for hematocrit in 2 ways. First, we defined a new variable equal to the absolute monocyte count divided by hematocrit and did the analysis using categories defined by this variable. Next, we included hematocrit in the Cox regression, in addition to absolute count and other covariates. As expected, there were strong correlations between the percent of monocytes and absolute monocyte count (0.819) and hematocrit-adjusted monocyte count (0.795). We found that the percent of monocytes had the strongest effect among all 3 definitions. In the Cox regression of survival on gender, age, and the 3 groups of monocytes, the effect of monocytes was similar.
4. Discussion
Increased levels of eosinophils,[7,8] monocytes,[9–11] neutrophils,[12,18–23] and decreased levels of lymphocytes[7–9,12,17–19,21,24–26] have been associated with increased risk of coronary heart disease and heart failure.[9,18,24] The results of the Japanese Adult Health Study showed a relationship between the total WBC count (including the eosinophil, neutrophil, and monocyte counts) and the incidence of coronary heart disease.[7,12–14] In the current study, an increased monocyte count/percent had the best correlation with survival, as compared to other selected laboratory parameters. The following prognostic parameters were similar between survivors and non-survivors in all 3 groups: smoking, LVEF, diabetes, HTN, hyperlipidemia, hemoglobin level, CRP, renal function (which was moderately impaired in all groups, with a mean creatinine clearance of 42 ml/minute), albumin level, and oxidized LDL. However, lymphocyte count, Pro-BNP, and HDL levels differed among groups (P < .05).
Several biomarkers are useful for prognosis prediction. These include NT Pro BNP, OX LDL, and CRP, which were measured in our study group. While CRP and OX LDL levels are useful when assessing the inflammatory process associated with atherosclerotic cardiovascular disease, NT Pro BNP is a biomarker that expresses poor hemodynamics associated with the failing heart. An emerging, new method that provides insight into the etiology of end-stage heart failure is based on the measurement of etiology-specific transcoronary concentration gradients of miRNA.
Our long-term results are in contrast to those reported in many studies that supported the theory that increasing monocyte count is associated with worse outcomes.[21,12–14,26–32] The results of these reports were explained by increased inflammatory activity (i.e., neurohumoral and oxidative stress with activation of inflammatory stress cells, such as neutrophils, eosinophils, and monocytes).[25,26] Monocytosis was shown to be an independent marker of risk for coronary artery disease. This was based on significantly higher Mon1 and Mon2 in acute exacerbation of HF, as compared with stable chronic HF. In accord with this, patients with stable coronary artery disease had decreased amounts of classical monocytes (Mon1 and Mon2). In contrast “nonclassical” monocytes (Mon 3) were higher (and may be responsible for monocytosis) in stable coronary artery disease[29] and prevalent (90%) in healthy subjects with normal coronary arteries, as determined by angiography.[29] The findings of the current study indicated trends of an association of low total monocyte counts with increased mortality in well-controlled HF. In agreement with our findings, Shimoni et al[27] reported that patients with severe aortic stenosis have lower monocyte counts than did patients with less prominent stenosis. The long-term prognostic value of low total monocyte count was examined in several studies that noted an association with increased risk for mortality in HF patients.[28–36] In other inflammatory and malignant diseases, such as follicular lymphoma, increased absolute monocyte count was associated with longer survival.[22] Monocytopenia was also reported in conditions with poor prognosis, such as ischemic stroke and Alzheimer's disease.[37–39]
There was no association between low monocyte counts and other prognostic parameters, such as NYHA class, LVEF, C-reactive protein level and others, and no other correlations were found, except for Pro-BNP level, lymphocyte count (%) and HDL. More Group 3 patients (monocyte count >14%) survived (above 83%), in both LVEF groups- (≤40 and >40). In contrast, the fewest survivors were observed among Group 1 patients (with monocyte count ≤6%, regardless of LVEF); 18% for ≤40 and 28% for >40. NYHA Class 1 + 2 group with a monocyte count <6.0 had poorer survival (29%) rate than the patients in Group 2 and Group 3 did. In NYHA class 2.5–3, the best survival was observed among Group 3 patients, at 82%. NYHA class >3, Group 3 patients with a monocyte count >14% showed 50% survival (P < .001). Our patients who survived longer than 15.9 years had a 50% higher monocyte count as compared to patients with shorter survival. The major finding of the current long-term, longitudinal study, in contrast to previous reports,[14,15] is that a low monocyte count appears to be an important predictor for mortality in chronic, well-controlled, heart failure but not in exacerbations or acute heart failure.
One possible answer to the question of why different studies present conflicting results is that different subsets of monocytes (e.g., Mon3) are predominant in well-controlled, chronic HF.[7,21,29,37–39] Monocytes play an important defensive role in several host functions, including initiating phagocytosis as a response to immune stimulation, removing cellular debris (“professional phagocytes”), secreting cytokines, and various substances that function as a self-defense mechanism and have antitumor effects, as well.[28,29] Thus, stable coronary artery disease is associated with an increase in the non-classical, Mon3 monocyte subset, and increased expression of inflammatory markers on the monocytes.[29] Hence, we consider that stable, chronic HF is associated with increases in the non-classical monocyte subset.[21]
The reported association between severe aortic stenosis with decreased total monocyte count[27] is in agreement with the current results. These findings may provide further clues to the mechanism underlying the pathogenesis of aortic stenosis.[27] To the best of our knowledge, this is the first report that describes a positive, predictive association of monocyte count and prognosis of stable, chronic HF.
A major limitation of this study is that monocyte subclasses were not analyzed prospectively. Using our set of inclusion criteria, we might have selected a subpopulation of participants who were at higher risk of mortality during the first 50 months from the beginning of the study. However, comparison of the subgroups that survived after this period showed significantly higher risk of mortality in the group with fewer monocytes despite the small number of events. This indicates that the effect of lower monocyte count was not limited to the initial period.[40]
Additional, well-controlled, studies of heart failure patients and measuring Mon3 in subgroups will provide important clues as to whether monocyte counts can serve as a reliable, simple, easily available, and inexpensive prognostic parameter.
5. Conclusion
Patients with low monocyte counts were found to have lower rates of survival, as compared to those with higher counts, based on long-term follow-up. A high monocyte count was independently associated with better prognosis for these patients.
Author contributions
Conceptualization: Gideon Charach, Ori Rogowski, Itamar Grosskopf, Ilya Novikov.
Data curation: Gideon Charach, Eli Karniel, Lior Charach, Itamar Grosskopf.
Formal analysis: Itamar Grosskopf.
Investigation: Gideon Charach, Eli Karniel, Lior Charach, Itamar Grosskopf.
Methodology: Ori Rogowski, Eli Karniel, Itamar Grosskopf, Ilya Novikov.
Resources: Lior Charach.
Supervision: Gideon Charach, Ori Rogowski, Ilya Novikov.
Validation: Eli Karniel.
Writing – original draft: Gideon Charach.
Gideon Charach orcid: 0000-0002-0696-9889.
Footnotes
Abbreviations: ACE = angiotensin converting enzyme, CAD = coronary artery disease, CBC = complete blood counts, HDL = high density lipoprotein, HR = hazard ratio, IL-10, interleukin-10, LVEF = left ventricular ejection fraction, MI = myocardial infarction, MPA = monocyte platelet aggregates, Nt pro-BNP = N-terminal pro-B-type natriuretic peptide, NYHA = New York Heart Association class, TNFα = tumor necrosis factor-alpha.
How to cite this article: Charach G, Rogowski O, Karniel E, Charach L, Grosskopf I, Novikov I. Monocytes may be favorable biomarker and predictor of long-term outcome in patients with chronic heart failure. Medicine. 2019;98:38(e17108).
The study was approved by the Ethics Committee of the Tel Aviv Medical Center (0338-10TLV). All participants provided written informed consent prior to data collection.
The authors give herein consent to publish the presented paper.
This study was funded by internal departmental resources.
The authors declare that they have no competing interests whatsoever.
Raw data are available in the Supporting Information files.
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Naylor S. Biomarkers: current perspectives and future prospects. Expert Rev Mol Diagn 2003;3:525–9. [DOI] [PubMed] [Google Scholar]
- [2].Shahid F, Lip GYH, Shantsila E. Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc 2018;7:e007849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wong KL, Yeap WH, Tai JJ, et al. The three human monocyte subsets: implications for health and disease. Immunol Res 2012;53:41–57. [DOI] [PubMed] [Google Scholar]
- [4].Wrigley BJ, Shantsila E, Tapp LD, et al. CD14++CD16+ monocytes in patients with acute ischaemic heart failure. Eur J Clin Invest 2013;43:121–30. [DOI] [PubMed] [Google Scholar]
- [5].Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res 2010;85:649–60. [DOI] [PubMed] [Google Scholar]
- [6].Wrigley BJ, Shantsila E, Tapp LD, et al. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail 2013;6:127–35. [DOI] [PubMed] [Google Scholar]
- [7].Prentice RL, Szatrowski TP, Fujikura T, et al. Leukocyte counts and coronary heart disease in a Japanese cohort. Am J Epidemiol 1982;116:496–509. [DOI] [PubMed] [Google Scholar]
- [8].Umemoto S, Suzuki N, Fujii K, et al. Eosinophil counts and plasma fibrinogen in patients with vasospastic angina pectoris. Am J Cardiol 2000;85:715–9. [DOI] [PubMed] [Google Scholar]
- [9].Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol 1993;137:49–53. [DOI] [PubMed] [Google Scholar]
- [10].Yun KH, Oh SK, Park EM, et al. An increased monocyte count predicts coronary artery spasm in patients with resting chest pain and insignificant coronary artery stenosis. Korean J Intern Med 2006;21:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Afiune Neto A, Mansur Ade P, Avakian SD, et al. Monocytosis is an independent risk marker for coronary artery disease [in Portuguese]. Arq Bras Cardiol 2006;86:240–4. [DOI] [PubMed] [Google Scholar]
- [12].Wheeler JG, Mussolino ME, Gillum RF, et al. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J 2004;25:1287–92. [DOI] [PubMed] [Google Scholar]
- [13].Avanzas P, Quiles J, Lopez de Sa E, et al. Neutrophil count and infarct size in patients with acute myocardial infarction. Int J Cardiol 2004;97:155–6. [DOI] [PubMed] [Google Scholar]
- [14].Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J 2013;40:17–29. [PMC free article] [PubMed] [Google Scholar]
- [15].Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14++CD16+monocytes as a third monocyte subset. Blood 2011;118:e50–61. [DOI] [PubMed] [Google Scholar]
- [16].Shantsila E, Wrigley B, Tapp L, et al. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology. J Thromb Haemost 2011;9:1056–66. [DOI] [PubMed] [Google Scholar]
- [17].Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 2011;13:1161–71. [DOI] [PubMed] [Google Scholar]
- [18].Grau AJ, Boddy AW, Dukovic DA, et al. Leukocyte count as an independent predictor of recurrent ischemic events. Stroke 2004;35:1147–52. [DOI] [PubMed] [Google Scholar]
- [19].Sweetnam PM, Thomas HF, Yarnell JW, et al. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol 1997;145:416–21. [DOI] [PubMed] [Google Scholar]
- [20].Avanzas P, Arroyo-Espliguero R, Cosin-Sales J, et al. Markers of inflammation and multiple complex stenoses (pancoronary plaque vulnerability) in patients with non-ST segment elevation acute coronary syndromes. Heart 2004;90:847–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Van Craenenbroeck AH, Van Ackeren K, Hoymans VY, et al. Acute exercise-induced response of monocyte subtypes in chronic heart and renal failure. Mediators Inflamm 2014;216534.doi: 10.1155/2014/216534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kawaguchi H, Mori T, Kawano T, et al. Band neutrophil count and the presence and severity of coronary atherosclerosis. Am Heart J 1996;132:9–12. [DOI] [PubMed] [Google Scholar]
- [23].Biasucci LM, D’Onofrio G, Liuzzo G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol 1996;27:611–6. [DOI] [PubMed] [Google Scholar]
- [24].Gillum RF, Mussolino ME, Madans JH. Counts of neutrophils, lymphocytes, and monocytes, cause-specific mortality and coronary heart disease: the NHANES-I epidemiologic follow-up study. Ann Epidemiol 2005;15:266–71. [DOI] [PubMed] [Google Scholar]
- [25].Thompson S, McMahon L, Nugent C. Endogenous cortisol a regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol 1980;17:506–14. [DOI] [PubMed] [Google Scholar]
- [26].Acanfora D, Gheorghiade M, Trojano L, et al. Relative lymphocyte count; a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J 2001;142:167–73. [DOI] [PubMed] [Google Scholar]
- [27].Shimoni S, Meledin V, Bar I, et al. Circulating CD14(+) monocytes in patients with aortic stenosis. J Geriatr Cardiol 2016;13:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Akiyama Y, Miller PJ, Thurman GB, et al. Stevenson HC characterization of a human blood monocyte subset with low peroxidase activity. J Clin Invest 1983;72:1093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tallone T, Turconi G, Soldati G, et al. Heterogeneity of human monocytes: an optimized four-color flow cytometry protocol for analysis of monocyte subsets. J Cardiovasc Transl Res 2011;4:211–9. [DOI] [PubMed] [Google Scholar]
- [30].Li Zhu, Yigang Yin, Ruifang Zhou, et al. Changes of monocyte subsets in patients with acute coronary syndrome and correlation with myocardial injury markers. Int J Clin Exp Pathol 2015;8:7266–71. [PMC free article] [PubMed] [Google Scholar]
- [31].Figdor CG, Bont WS, Touw I, et al. Isolation of functionally different human monocytes by counter flow centrifugation elutriation. Blood 1982;60:46–53. [PubMed] [Google Scholar]
- [32].Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16+ Monocytes Predict Cardiovascular Events. Circ Cardiovasc Genet 2012;5:122–31. [DOI] [PubMed] [Google Scholar]
- [33].Hristov M, Weber C. Differential role of monocyte subsets in atherosclerosis. Thromb Haemost 2011;106:757–62. [DOI] [PubMed] [Google Scholar]
- [34].Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 2010;10:427–39. [DOI] [PubMed] [Google Scholar]
- [35].Wrigley BJ, Shantsila E, Tapp LD. Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail 2013;6:127–35. [DOI] [PubMed] [Google Scholar]
- [36].van der Laan AM, Hirsch A, Robbers LF, et al. A proinflammatory monocyte response is associated with myocardial injury and impaired functional outcome in patients with ST-segment elevation myocardial infarction: monocytes and myocardial infarction. Am Heart J 2012;163:57–65.e2. [DOI] [PubMed] [Google Scholar]
- [37].Wilcox RA, Ristow K, Habermann TM, et al. The absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma 2012;53:575–80. [DOI] [PubMed] [Google Scholar]
- [38].Naert G, Rivest S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer's disease. J Molecular Cell Biol 2013;5:284–93. [DOI] [PubMed] [Google Scholar]
- [39].Bao Y, Eunhee Kim E, Sangram Bhosle S, et al. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflamm 2010;7:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].De Rosa S, Eposito F, Carella C, et al. Transcoronary concentration gradients of circulating microRNAs in heart failure. Eur J Heart Fail 2018;20:1000–10. [DOI] [PubMed] [Google Scholar]


