Abstract
An international consensus for rheumatoid arthritis (RA) patients at risk of developing interstitial lung disease (ILD) is still lacking. The aims of study were to evaluate: the prevalence of ILD involvement in RA over high-resolution computed tomography (HRCT); the relationships between pulmonary function tests (PFTs), patient-centered measurements, and ILD; and the potential risk factors contributing to RA-ILD patients.
Data regarding the clinical characteristics (age, sex, age at onset of RA), laboratory findings (rheumatoid factor [RF] and anti-citrullinated protein antibodies [ACPA]), respiratory functional assessment (forced vital capacity [FVC] and carbon monoxide diffusion capacity [DLCO]), patient-centred measures of dyspnea (PCMD), Health Assessment Questionnaire—Disability Index (HAQ-DI), and HRCT have collected retrospectively. HRCT abnormalities were evaluated using a conventional visual reader-based score (CoVR) and a computer-aided method (CaM). The relationships between the 2 HRCT scores—PFTs and PCMD—were calculated using Pearson correlation. The area under the receiving-operating characteristic (AUC-ROC) curve was calculated to determine the discriminatory performance of measurements between patients with and without ILD. The multivariate regression model was used to evaluate the association force between ILD and RA characteristics.
In all, 151 patients (45 males and 106 females, mean age 53.4 ± 7.6 years) were included. ILD had been detected in 29 patients out of 151 (19.2%). Usual interstitial pneumonia was the most common HRCT. RA-ILD patients were older, and older at RA onset (both P < .01), with a higher HAQ-DI (P < .05) than patients without ILD. ACPA positivity and titer were higher in the RA-ILD group (P = .02). Extent and severity of ILD, and total CoVR and CaM score closely related to DLCO and PCMD (both P < .0001). A reduced DLCO was the most sensitive test for predicting the presence of ILD on HRCT (AUC-ROC 0.811 ± 0.037). Advanced age (P < .0001), age at RA onset (P = .025), ACPA titer (P = .004), and smoking (P = .008) were independent explanatory variables of HRCT damage in multivariate analysis.
The RA-ILD is associated with age and older age of RA onset, smoking, and ACPA titer. DLCO seems to be the most sensitive parameter to predict ILD on HRCT, followed by PCMD.
Keywords: computer-aided method, high-resolution computed tomography, interstitial lung disease, pulmonary function tests, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a progressive systemic autoimmune disorder, characterized by joint and extra-articular manifestations, affecting about 0.5% of the Italian adult population.[1] Lung complications related to RA are the most common extra-articular manifestations of the disease,[2] including pulmonary nodules, pleural effusion, bronchiectasis, and interstitial pulmonary disease (ILD).[3] RA patients are also at risk of secondary lung complications due to immunosuppressive therapy, such as drug toxicity or opportunistic infections.[4]
The prevalence of ILD in RA patients (RA-ILD) varies widely from 1% to 6% in conventional chest radiography studies, and from 5% to 67.3% in high-resolution computed tomography (HRCT) studies.[5–8] Few studies have identified a higher post mortem incidence of RA-ILD,[9,10] and a prospective study has shown that RA-ILD is the second leading cause of death in these patients, overcoming the risk of death from malignant tumors.[11]
Many studies have been published in recent years on the determination of risk factors for the development of RA-ILD,[12–15] but the results are discordant, and an international consensus has not yet been reached.
High-resolution computed tomography chest imaging provides valuable information on ILD, including the pattern and extent of the disease, the evaluation of disease progression over time, and the evaluation of extra-parenchymal abnormalities.[16]
Methods for quantifying ILD damage are complex, operator-dependent, and difficult to apply in clinical practice. Computer-aided methods (CaMs) applied to HRCT images allow greater objectivity, sensitivity, and repeatability in the evaluation of quantitative changes in lung characteristics.[17–19]
In the present study, the following has been investigated: the prevalence of ILD in a cohort of RA patients, measured by chest HRCT; the relationships between patient-centered measurements, pulmonary function tests (PFTs), and ILD; and the potential risk factors that contribute to ILD susceptibility in RA patients.
2. Methods
2.1. Study population
This is a retrospective analysis carried out in RA patients attending the Rheumatology Clinic, Università Politecnica delle Marche, Italy, from January 1, 2014 to June 30, 2018, stored in a dedicated database. All patients had signed a written consent to allow the use of their data for clinical investigation.
Rheumatoid arthritis has been defined according to the classification criteria of the American College of Rheumatology, 2010.[20] Patients with RA with a history or a suspicion of ILD were included, who underwent rheumatological and pneumological evaluation (dyspnea scale, PFTs, chest HRCT), performed no more than 3 months before the visit. Exclusion criteria included concomitant respiratory infections, pulmonary hypertension, congestive heart failure, or clinically significant pulmonary abnormalities other than ILD identified by chest x-ray or HRCT.
For the purposes of this study, RA patients have been categorized into 2 clinical subsets: elderly-onset RA (EORA) and young-onset RA (YORA), using the age of onset of 60 as the cut-off value.[21] Current smokers were those who had smoked more than 5 cigarettes a day in the previous 6 months, and nonsmokers had smoked less than 20 packs of cigarettes during their lifetime.[22] Reference data included age, sex, duration of disease, concomitant therapy with conventional synthetic or biological disease-modifying anti-rheumatic drugs (csDMARDs or bDMARDs) and glucocorticoids. The data collected are those recorded closest to the HRCT evaluation.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee (Comitato Etico Unico Regionale) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
2.2. Clinical and laboratory data
The clinical and laboratory data included the following single items of the activity indices of RA: 28 joint counts for swollen (SJC) and tender (TJC) joints, evaluation of the patient general health status (PaGH), and erythrocyte sedimentation rate (ESR), and the C-reactive protein (CRP) level. These variables were used to calculate Disease Activity Score-28 joint (DAS28, for the purposes of this study we considered DAS28-ESR).[23] The DAS28 ranges from 0 (totally inactive disease) to 9.4 (very active disease). Functional ability was assessed by the Health Assessment Questionnaire—Disability Index (HAQ-DI), with a range from 0 (no disability) to 3 (severe disability).[24] The laboratory evaluation was completed considering the serum levels of rheumatoid factor (RF) and of anti-citrullinated protein antibodies (ACPA).
2.3. Patient-centred measures of dyspnea
The following patient-centred measures of dyspnea (PCMD) were included: the modified Borg Dyspnea Index (BDI) and the visual analog scale (VAS) for breathing. The BDI is a numerical scale for evaluating perceived dyspnea on a scale from 0 (no dyspnea) to 10 (maximum dyspnea),[25] whereas the VAS for breathing is a scale with a range from 0 to 100.
2.4. Pulmonary function tests
The PFTs were performed while the patient was at rest in a seated position. Pulmonary function was measured using a flow-sensing spirometer connected to a computerized lung analyzer (MasterScreen Diffusion, Jaeger GmbH, Höchber, Germany). The forced vital capacity (FVC, % expected) and the diffusion capacity of the single-breath carbon monoxide in the lung (DLCO, % expected, corrected for hemoglobin) were obtained. PFTs were carried out on the basis of published guidelines,[26] and were expressed as a percentage of the predicted value. At least 3 measurements were made for each variable to ensure repeatability.
2.5. HRCT assessment and visual reader-based disease quantification
The latest HRCT scan of each patient, carried out within 3 months of the clinical evaluation (available in the electronic imaging system of the Department of Radiology of the “Carlo Urbani” Hospital, Jesi [Ancona], Italy) was used for the interpretation. Thin-section volumetric CT examinations were performed using a CT 64 GE light Speed VCT power scanner. The parenchymal abnormalities were examined and evaluated by 2 experienced radiologists (M.C. and A.G.), unaware of the clinical data, using a conventional visual reader-based score (CoVR) and the CaM, reaching consensus. Each HRCT examination was evaluated as defined usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), and diffuse alveolar damage (DAD) based on previously published guidelines.[27] Representative HRCT images of subjects with RA-ILD are shown in Fig. 1.
Figure 1.
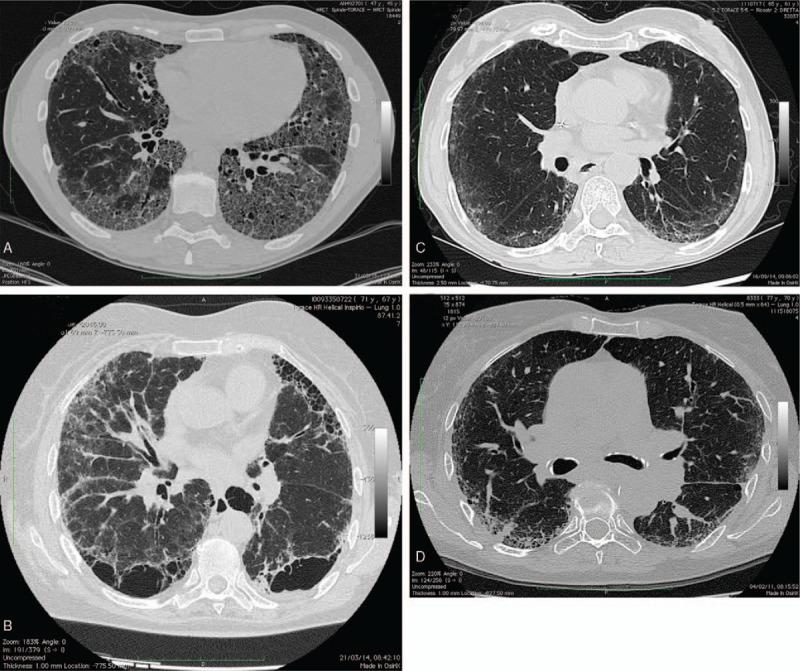
Representative high-resolution computed tomography images from the subjects with rheumatoid arthritis-interstitial lung disease. (A) A 64-year-old female with fibrotic changes of usual interstitial pneumonia pattern, reticular and ground glass opacity, septal thickening diffusely, and traction bronchiectasis. (B) A 69-year-old male revealing septal thickening diffusely and extensive macrocystic honeycombing. (C) A 65-year-old male with bilateral peripheral ground glass opacity and typical subpleural sparing representing the nonspecific interstitial pneumonia pattern. (D) A 70-year-old female showing evidence of bilateral peripheral reticular and ground glass opacity of the left lower lobe with peripheral consolidations typical of organizing pneumonia.
2.5.1. Conventional visual reader-based score
The CoVR refers to the classification system defined by Warrick et al.[28] This method categorizes the extent and severity of lung disease, obtaining a total score with a range from 0 to 30 points. The intraclass correlation coefficients (ICCs) for the level of agreement between radiologists on total HRCT scores proved to be good.[18] Although there is no official consensus, CoVR fibrotic scores, defined according to Warrick et al,[28] were classified into 2 groups as follows: <7 (normal or mild pulmonary fibrosis) and ≥7 (severe pulmonary fibrosis).[29] A minimum score of 7 on the semiquantitative system was required to consider HRCT abnormalities of ILD, identifying 2 groups: patients with ILD and patients without ILD.
2.5.2. Computer-aided method
The HRCT images were also analyzed by OsiriX MD 7—a DICOM display software (OsiriX MD version 7, 64-bit format) on a Mac Mini (2.8 GHz Intel Core 2 Duo Desktop Computer, 16 GB random access memory; Apple Computer, Cupertino, CA) with Mac OSX 10.12.2 operating system.[19] Through OsiriX a mask is applied that requires a minimum of intervention by the operator to exclude hilar large blood vessels and bronchi. According to Shin et al,[30] −700 Hounsfield unit (HU) was selected as the default threshold value for normal lung regions. There was a total agreement between the first and second measurements of CaM scores (95% of agreement limits 0–0, ICC 1).[18,19]
2.6. Statistical analysis
All data was entered into a Microsoft Excel database developed for the management of all data. The data were analyzed using the MedCalc version 18.6; 64-bit (MedCalc Software, Mariakerke, Belgium). Values in this study were expressed both as mean ± standard deviation (SD) and median (interquartile range). A parametric 2-sample t test and analysis of variance test were used to compare continuous variables, and the chi-square test was used to compare categorical variables between patients. The relationships among the lung segmentation analysis, the readers, and the PFT results were calculated using univariate regression analysis and Pearson product moment correlation (Pearson “r” values). To analyze the predictive potential of patient-centered measures and PTFs for RA-ILD, a receiver-operating characteristic (ROC) curve analysis was conducted. The area under the ROC (AUC-ROC) curve was calculated to quantify the discriminative accuracy. AUC from 0.50 to about 0.70 represent poor accuracy, those from 0.70 and 0.90 are “useful for some purposes,” and higher values represent high accuracy. The nonparametric Wilcoxon signed-rank test was used for calculation and comparison of the AUC-ROCs.
Finally, we performed a multivariate regression analysis adjusted for covariates to identify the potential factors that contributed to ILD in RA patients. HRCT-CaM quantification was taken as dependent variable. Age, sex, disease duration, disease onset (EORA or YORA), smoking habit, titre of RF and ACPA, DAS-28-ESR, and HAQ-DI were included in the model as covariates. The results were expressed as multivariate regression coefficient (R) and square regression coefficient corrected (R2) for the number of variables entered in the analysis. This enables to calculate the predictivity of each multivariate model according to the number of variables entered in the model itself. Significance was set at P < .05.
3. Results
In all, 151 patients were included, 45 (29.8%) males and 106 (70.3%) females, with a mean age of 53.4 ± 7.6 years. The mean ± SD disease duration was 7.5 ± 3.8 years. There were 110 (72.8%) RF-positive patients and 92 (60.9%) ACPA-positive patients. The mean BMI was 26.9 (range 18.5–44.4). Fifty-five (36.5%) patients were classified as having EORA, whereas 96 (63.5%) patients were classified as having YORA. Also, 145 (96%) patients were treated with at least a DMARD, of which 78 (51.6%) patients were treated with methotrexate and 101 (69.6%) with a combination of methotrexate and a biologic drug, respectively; 21 (13.9%) patients were taking adalimumab, 18 (11.9%) etanercept, 18 (11.9%) abatacept, 16 (10.6%) tocilizumab, 12 (7.9%) certolizumab pegol, 10 (6.6%) infliximab, and 6 (3.9%) golimumab. The most frequently reported concomitant drugs were: systemic corticosteroids in 102 (67.5%) patients, proton pump inhibitor in 72 (47.7%) patients, and nonsteroidal anti-inflammatory drugs in 67 (44.3%) patients.
3.1. RA-ILD prevalence
Twenty-nine (19.2%) patients showed HRCT features indicative of ILD (at the CoVR: mean disease extent = 9.75 ± 2.14, mean disease severity = 10.93 ± 3.23, mean total HRCT score = 20.69 ± 5.00). Analysis of the ILD subtypes showed that UIP was found in 18 (62.1%) patients, NSIP in 7 (24.1%) patients, OP in 1 (3.5%) patient, and a combination of NSIP and OP in 3 (10.3%) patients. No patient showed a DAD pattern.
Data from the RA-ILD group and the non-RA-ILD group are summarized in Table 1. Patients with ILD were older (P = .04) than those without ILD, and were predominantly in the EORA group (P < .03), had a higher ACPA titer (P < .001), and a higher percentage of smokers (34.5% compared with 21.3%; P = .03). In addition, patients with ILD had a higher HAQ-DI (1.05 ± 0.39 vs 0.78 ± 0.36; P < .005), a higher BDI (1.89 ± 0.82 vs 1.74 ± 0.89; P < .01), and a higher VAS for breathing (36.2 ± 15.44 vs 23.03 ± 13.02; P < .01) than patients without ILD. We found no statistically significant differences in the evaluation of DAS28-ESR, ESR, CRP, and RF levels.
Table 1.
Comparison of characteristics of the interstitial lung disease (ILD) group and the non-ILD group.
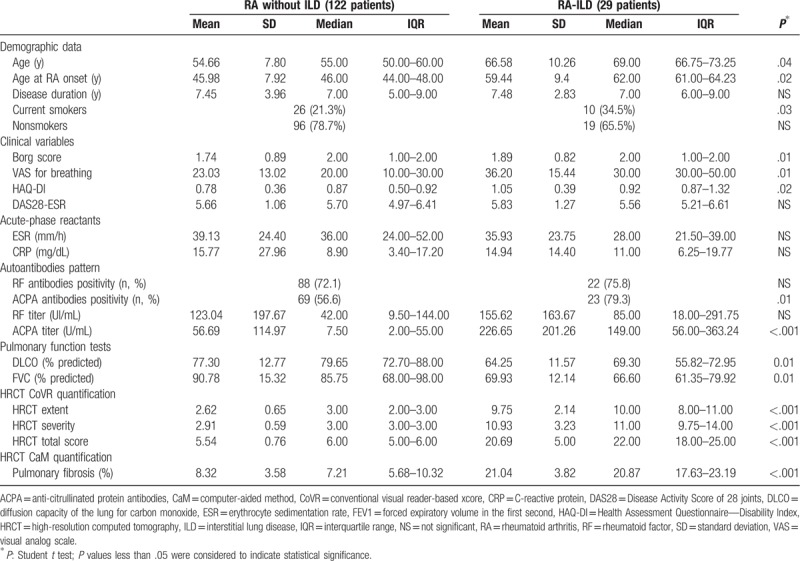
In the study cohort the mean DLCO was 74.80 ± 13.53% of the expected and mean FVC was 86.77 ± 8.35% of the expected. DLCO and FVC were statistically different (P = .01) in the 2 patient groups, as were HRCT scores (P < .001), both in extension and severity, using CoVR quantification and in extension of fibrosis using CaM quantification. (21.04 ± 3.82% vs 8.32 ± 3.58; P < .001). In terms of treatment history, we found no correlation between RA-ILD and drugs.
The EORA patients had a higher percentage of pulmonary fibrosis in the HRCT-CaM assessment (HRCT-CaM 13.7 ± 4.9 vs 9.2 ± 3.7; P < .001) (Fig. 2A), HAQ-DI (HAQ-DI 0.98 ± 0.38 vs 0.75 ± 0.34,; P < .001) (Fig. 2B), and ESR (44.5 ± 22.9 vs 36.3 ± 20.7; P = .048) higher than YORA. There were no differences between the EORA and YORA groups in terms of acute-phase proteins and DAS28-ESR scores, although the YORA scores varied more widely. In addition, there were no differences between the groups in FR and ACPA titer.
Figure 2.
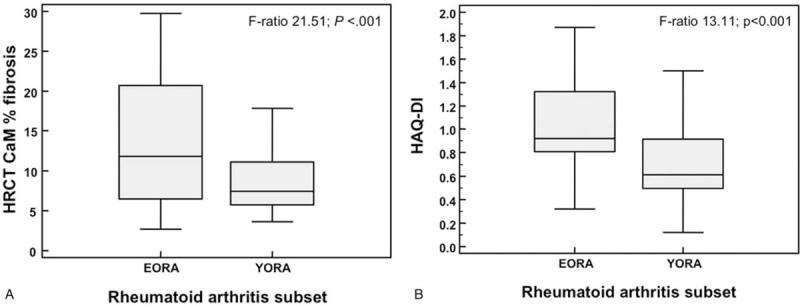
(A) Box-and-Whisker plot of percentage of lung fibrosis evaluated by high-resolution computed tomography (HRCT) computer-aided method (CaM), and (B) Health Assessment Questionnaire—Disability Index (HAQ-DI) values for each clinical subsets, respectively, elderly-onset rheumatoid arthritis (EORA) and young-onset rheumatoid arthritis (YORA). The boxes represent the values from 25th to 75th percentiles. The middle lines inside boxes are the medians (Kruskall-Wallis test).
3.2. Correlation between the HRCT score and clinical and functional data
The mean extension and severity of ILD, the mean total HRCT score, and the mean extension of fibrosis on CaM showed a highly significant negative correlation with DLCO (all at P < .001), and positive with BDI and VAS for breathing (all at P < .001). There was also a close correlation between HRCT and HAQ-DI results (all at P < .001). HRCT CoVR and CaM results showed no significant correlation with FVC (Table 2).
Table 2.
Correlations among high-resolution computed tomography scores, diffusion capacity of the lung for carbon monoxide, forced vital capacity, and patient-centered measures.
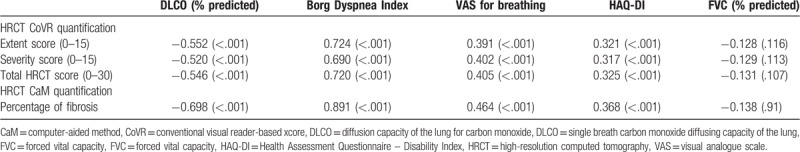
Figure 3 shows the ROC curves of discriminative ability power of PCMD, HAQ-DI, and PFTs to detect RA-ILD. By choosing parameters with maximum diagnostic accuracy, a reduced DLCO was the most sensitive test to predict the presence of ILD on the HRCT scan (AUC-ROC 0.811 ± 0.037), followed by FVC (Fig. 3).
Figure 3.
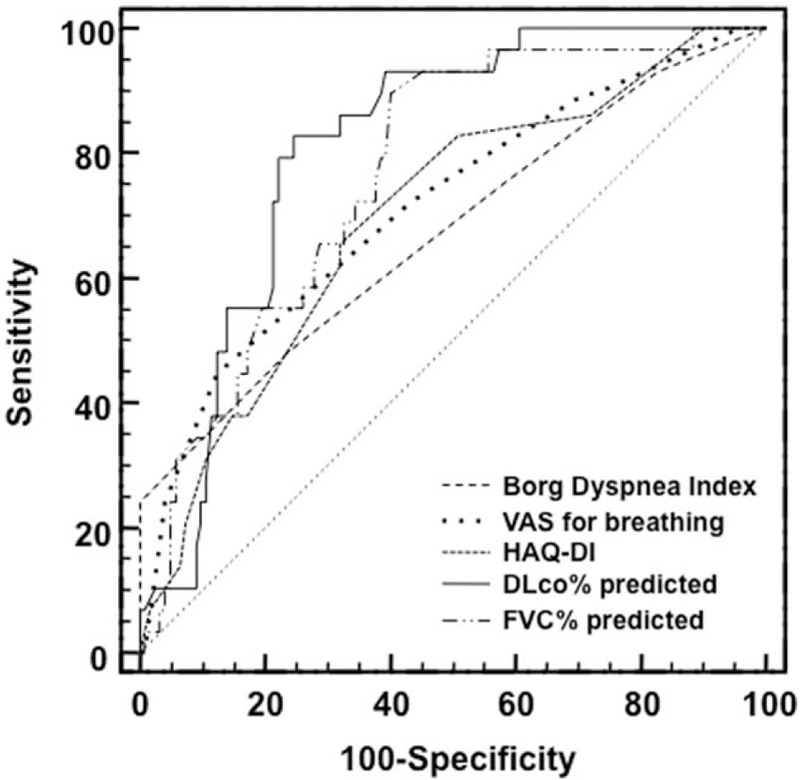
Receiver-operating characteristic curves of patient-centered measures of dyspnea, disability index, and pulmonary function tests to detect rheumatoid arthritis-interstitial lung disease.
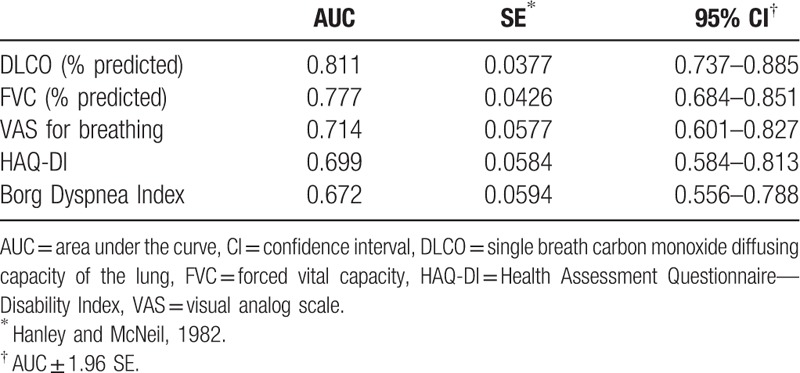
The discriminatory power of BDI and VAS for breathing was moderate, with an AUC of 0.672 (95% confidence interval [CI] 0.556–0.788) and 0.714 (95% CI 0.601–0.827), respectively (difference between areas 0.042, SE 0.072, P = .580) (Table 3).
Table 3.
Discriminatory power of patient-centered measures of dyspnea, Health Assessment Questionnaire—Disability Index, and pulmonary function tests in rheumatoid arthritis-interstitial lung disease.
3.3. Potential risk factors for RA-ILD
The results of the multivariate regression analysis indicated that the combination of age (t = 4.409, P < .0001), ACPA titer (t = 2.924, P = .004), age at RA onset (t = −2.260, P = .0253), and smoking status (t = 2.660, P = .008) explains 60.4% of the variance in the percentage of pulmonary fibrosis by the CaM method. Sex, BMI, disease duration, drugs, FR level, DAS28-ESR, and HAQ-DI were not significantly associated with HRCT damage in this patient population.
4. Discussion
Our study revealed first of all that the prevalence of ILD is very common in RA patients. A prevalence of 19.2% is similar to previously published data.[6,8,31,32] Different researches report a prevalence ranging from 4% to 68%.[8,15] This wide range depends mainly on the methods of detection and the design of the research. A post mortem study showed that ILD is the most common manifestation of lung involvement in RA patients, observed in up to 34% of biopsies.[17] In our cohort, patients underwent chest HRCT if there was a clinical or instrumental suspicion of ILD, so our results could underestimate the true prevalence of ILD-RA, because subclinical lung involvement was not considered.[33] Johnson et al[31] elaborated the results of 2 large cohort studies performed to estimate the prevalence of cardiovascular abnormalities in RA population (Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis study) and no RA-population (Multi-Ethnic Study of Atherosclerosis study): they found a significant correlation between smoking and lung injury with high attenuation on HRCT in both populations, a reduced DLCO in 17%, and a restrictive pattern on PFTs in 8% of the RA population, lower than the results of our study. Therefore, a prospective study in a larger cohort population should be performed to clarify the true prevalence of RA-ILD.
We revealed that the UIP pattern is the most frequent HRCT finding, followed by NSIP and OP. These data corroborate what previously described literature.[32,34] Recent data showed that the UIP pattern has the worst prognosis, with a median survival of 8.27 years, similar to that of idiopatic pulmonary fibrosis (IPF), and UIP had the worst response to treatment compared to non-UIP patterns.[32] Jacob et al[34] showed that the presence of “honeycombing”—the typical feature of the UIP pattern—is the best predictive parameter of mortality in RA-ILD patients. They also demonstrated that both CoVR and CaM, applied to HRCT images, can predict mortality. These data underline the need to diagnose and characterize RA-ILD as early as possible to define a personalized therapeutic approach and predict mortality.[35]
Secondly, our study results portray a specific profile of patients with RA at high risk of developing a significant ILD: they are generally over 65 years old and over 65 years of age at RA onset, have been or are smokers, and have a high serum ACPA titer. There were no significant differences in sex, duration and activity of the disease, or in current treatment between patients with RA-ILD and patients without ILD. Other studies identified old age and late onset as risk factors for RA-ILD. Song et al[36] identified increased age, late onset of disease, male sex, and high RF titer as risk factors for RA-ILD. In another study, an age above 65 years increased the risk of ILD by 4 times.[37] These results are similar, and the differences recorded may depend on the selection error.
The relationship between tobacco exposure, ACPA positivity, and RA is well-known: smoking demonstrated to increase the risk of ILD,[38] of the extra-articular manifestations,[7] and the presence of ACPA in the respiratory tract and serum of smokers appears before arthritis,[39] emphasizing the role of the lung as the initial site of the pathogenesis of ACPA-positive RA.[40] Wang and Du[41] found that older age, being older at the onset of RA, ACPA titer, and smoking habit were all associated independently with a diagnosis of RA-ILD in multivariate analysis. Similar results were derived from our multivariate regression analysis, highlighting the correlation between age, ACPA titer, age at RA onset, smoking, and the CaM score.
As far as sex and ILD are concerned, the data are not exhaustive; in fact, some authors found a positive correlation between male sex and RA-ILD,[2,36] whereas others did not found such a correlation as in our cohort.[31,37] This fact could be explained by the increase in the smoking rate in women, influencing the prevalence of RA-ILD in the male group.[42] As a result, although overall RA mortality appears to be decreasing, RA-ILD mortality appears to be increasing, particularly in women and older age groups.[43]
Another important clinical aspect is the correlation between RA-ILD and articular disease. Our results revealed that the EORA group did not show a higher disease activity than the YORA group, but patients with RA-ILD showed a higher disability. Pérez-Dórame et al[44] found in a RA-ILD cohort of 64 patients, a positive correlation between RA disease activity and the HRCT “ground glass score,” using a CoVR score, at the beginning of the study and also after a treatment with high-dose steroids. They found no correlation between disease activity and pulmonary fibrosis score, as our data also confirmed. Bongartz et al,[10] in a larger retrospective study, found a significant correlation between the functional musculoskeletal status and the prevalence of RA-ILD.
Natalini et al[45] studied the determinants of quality of life in RA-ILD and IPF, showing that the RA-ILD group had a more impaired quality of life than IPF patients, mainly due to joint pain, stiffness, and fatigue. Youssef et al[46] investigated the value of respiratory symptoms and disease features in predicting RA lung disease, finding that dyspnea was the most frequent symptom (61.1%), followed by cough (52.8%), and that was also the only parameter that could predict a restrictive HRCT pattern. We showed that both HAQ-DI and PMCDs are positively correlated with HRCT scores, in particular, with the CoVR extension and severity score, and with the percentage of fibrosis in CaM quantification. These results underline the importance of assessing respiratory symptoms and quality of life in clinical practice as new elements for determining predictive data for RA-ILD.
Other studies revealed a correlation between PFTs and HRCT damage.[10,47] Leonel et al[47] found that a forced expiratory volume in the first second below 81.7 had a sensitivity of 59% and a specificity of 83% to predict HRCT alterations in RA patients, but they did not find such a correlation with DLCO, as we did. Gochuico et al[15] demonstrated that the predicted percentage of DLCO was statistically lower in RA-ILD than in RA non-ILD. Zamora-Legoff et al[35] studied the clinical course of RA-ILD in a large single-center cohort. They found that a low value of both DLCO and FVC at the time of ILD diagnosis increases the risk of ILD progression, and higher rates of change of these parameters in the first 6 months increase the risk of severe ILD, requiring supplemental oxygen in 33% of cases within 5 years of diagnosis.
The present study contains some limitations. First, the study is retrospective and the processing of the results comes from the data collected by our database, which suffers from selection errors. Secondly, a larger sample could reach stronger conclusions and a multicenter study with central imaging review could be planned. Finally, we have limited our analysis of possible causative factors to routine biochemistry for RA patients and their clinical history. Exploratory studies should be carried out to discover new potential markers of ILD in RA patients.
5. Conclusions
Our results offer clinicians some practical points. The need to diagnose RA-ILD before a symptomatic phase becomes a priority, as early treatment in patients with RA can improve quality of life and performance status. To improve the ability to diagnose RA-ILD, a screening tool with a higher sensitivity than those we currently use should be implemented. The introduction of a composite screening method could be considered, including known risk factors (age and age at onset of RA over 65, smoking habit), clinical (HAQ-DI, PCMD), laboratory (ACPA), and instrumental data (DLco) to select patients to be studied with an HRCT scan. In fact, CaM scoring provides clinicians an easy-to-use and repeatable tool for assessing presence and monitoring ILD, making a prognosis and choosing the most appropriate treatment.
Author contributions
Conceptualization: Fausto Salaffi, Marina Carotti, Andrea Giovagnoni.
Data curation: Fausto Salaffi.
Formal analysis: Fausto Salaffi, Marco Di Carlo.
Investigation: Fausto Salaffi, Marika Tardella, Marco Di Carlo, Marina Carotti.
Project administration: Fausto Salaffi.
Supervision: Fausto Salaffi, Andrea Giovagnoni.
Writing – original draft: Fausto Salaffi, Marika Tardella, Marco Di Carlo.
Writing – review & editing: Marika Tardella, Marco Di Carlo, Marina Carotti, Andrea Giovagnoni.
Conceptualization: Fausto Salaffi, Marina Carotti, Andrea Giovagnoni.
Data curation: Fausto Salaffi, Marina Carotti, Marco Di Carlo, Marika Tardella.
Formal analysis: Fausto Salaffi.
Investigation: Fausto Salaffi, Marina Carotti, Marco Di Carlo, Marika Tardella.
Methodology: Fausto Salaffi.
Resources: Marina Carotti, Marika Tardella.
Supervision: Fausto Salaffi, Andrea Giovagnoni.
Writing – original draft: Fausto Salaffi, Marina Carotti, Marco Di Carlo, Marika Tardella.
Writing – review & editing: Andrea Giovagnoni.
Marika Tardella orcid: 0000-0003-4764-7197.
Footnotes
Abbreviations: ACPA = anti-citrullinated protein antibodies, AUC-ROC curve = area under the receiving-operating characteristic curve, BDI = Borg Dyspnea Index, CaM = computer-aided method, CoVR = conventional visual reader-based score, CRP = C-reactive protein, DAD = diffuse alveolar damage, DLCO = carbon monoxide diffusion capacity, DMARDs = disease-modifying anti-rheumatic drugs, EORA = elderly-onset rheumatoid arthritis, ESR = erythrocyte sedimentation rate; FVC = forced vital capacity, HAQ-DI = Health Assessment Questionnaire—Disability Index, HRCT = high-resolution computed tomography, ICC = intraclass correlation coefficient, ILD = interstitial lung disease, IPF = interstitial pulmonary fibrosis, NSIP = nonspecific interstitial pneumonia, OP = organizing pneumonia, PaGH = patient general health status, PCMD = patient-centred measures of dyspnea, PFTs = pulmonary function tests, RA = rheumatoid arthritis, SD = standard deviation, SJC = swollen joint count, TJC = tender joint count, UIP = usual interstitial pneumonia, VAS = visual analog scale, YORA = young-onset rheumatoid arthritis.
How to cite this article: Salaffi F, Carotti M, Di Carlo M, Tardella M, Giovagnoni A. High resolution computed tomography of the lung in patients with rheumatoid arthritis. Medicine. 2019;98:38(e17088).
The authors have no funding and conflicts of interest to disclose.
References
- [1].Salaffi F, De Angelis R, Grassi W. MArche Pain Prevalence; INvestigation Group (MAPPING) study. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The MAPPING study. Clin Exp Rheumatol 2005;23:819–28. [PubMed] [Google Scholar]
- [2].Turesson C, O’Fallon WM, Crowson CS, et al. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis 2003;62:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carotti M, Salaffi F, Manganelli P, et al. The subclinical involvement of the lung in rheumatoid arthritis: evaluation by high-resolution computed tomography. Reumatismo 2001;53:280–8. [DOI] [PubMed] [Google Scholar]
- [4].Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc 2007;4:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richman NC, Yazdany J, Graf J, et al. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore) 2013;92:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of co-morbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford) 2013;52:99–110. [DOI] [PubMed] [Google Scholar]
- [7].Zou YQ, Li YS, Ding XN, et al. The clinical significance of HRCT in evaluation of patients with rheumatoid arthritis-associated interstitial lung disease: a report from China. Rheumatol Intern 2012;32:669–73. [DOI] [PubMed] [Google Scholar]
- [8].Carmona L, Gonzalez-Alvaro I, Balsa A, et al. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis 2003;62:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sihvonen S, Korpela M, Laippala P, et al. Death rates and causes of death in patients with rheumatoid arthritis: a population-based study. Scand J Rheumatol 2004;33:221–7. [DOI] [PubMed] [Google Scholar]
- [10].Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010;62:1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim D, Cho SK, Choi CB, et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol Int 2017;37:1735–45. [DOI] [PubMed] [Google Scholar]
- [12].Weyand CM, Schmidt D, Wagner U, et al. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum 1998;41:817–22. [DOI] [PubMed] [Google Scholar]
- [13].Linn-Rasker SP, van der Helm-van Mil AHM, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis 2006;65:366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kelly CA, Saravanan V, Nisar M, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristic: a large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–82. [DOI] [PubMed] [Google Scholar]
- [15].Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 2008;168:159–66. [DOI] [PubMed] [Google Scholar]
- [16].Nurmia HM, Kettunenc HP, Suorantac SK, et al. Several high-resolution computed tomography findings associate with survival and clinical features in rheumatoid arthritis-associated interstitial lung disease. Respir Med 2018;134:24–30. [DOI] [PubMed] [Google Scholar]
- [17].Salaffi F, Carotti M, Di Donato E, et al. Computer-aided tomographic analysis of interstitial lung disease (ILD) in patients with systemic sclerosis (SSc). Correlation with pulmonary physiologic tests and patient-centred measures of perceived dyspnea and functional disability. PLoS One 2016;11:e0149240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Salaffi F, Carotti M, Bosello S, et al. Computer-aided quantification of interstitial lung disease from high resolution computed tomography images in systemic sclerosis: correlation with visual reader-based score and physiologic tests. Biomed Res Int 2015;2015:834262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ariani A, Carotti M, Gutierrez M, et al. Utility of an open-source DICOM viewer software (OsiriX) to assess pulmonary fibrosis in systemic sclerosis: preliminary results. Rheumatol Int 2014;34:511–6. [DOI] [PubMed] [Google Scholar]
- [20].Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- [21].Deal CL, Meenan RF, Goldenberg DL, et al. The clinical features of elderly-onset rheumatoid arthritis: a comparison with younger-onset disease of similar duration. Arthritis Rheum 1985;28:987–94. [DOI] [PubMed] [Google Scholar]
- [22].Bilgici A, Ulusoy H, Kuru O, et al. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 2005;25:429–35. [DOI] [PubMed] [Google Scholar]
- [23].Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [24].Fries JF, Spitz P, Kraines RG, et al. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- [25].Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377–81. [PubMed] [Google Scholar]
- [26].Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- [27].Travis WD, Costabel U, Hansell DM, et al. An Official American Thoracic Society/European Respiratory Society Statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Warrick JH, Bhalla M, Schabel SI, et al. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520–8. [PubMed] [Google Scholar]
- [29].Diot E, Boissinot E, Asquier E, et al. Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest 1998;114:1623–9. [DOI] [PubMed] [Google Scholar]
- [30].Shin KE, Chung MJ, Jung MP, et al. Quantitative computed tomographic indexes in diffuse interstitial lung disease: correlation with physiologic tests and computed tomography visual scores. J Comput Assist Tomogr 2011;35:266–71. [DOI] [PubMed] [Google Scholar]
- [31].Johnson C, Giles JT, Bathon J, et al. Smoking and subclinical ILD in RA versus the multi-ethnic study of atherosclerosis. PLoS One 2016;11:e0153024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yunt ZX, Chung JH, Hobbs S, et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: relationship to survival. Respir Med 2017;126:100–4. [DOI] [PubMed] [Google Scholar]
- [33].Koduri G, Norton S, Young A, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 2010;49:1483–9. [DOI] [PubMed] [Google Scholar]
- [34].Jacob J, Hirani N, van Moorsel CHM, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 2019;53: doi: 10.1183/13993003.00869-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zamora-Legoff JA, Krause ML, Crowson CS, et al. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Song ST, Kim SS, Kim JY, et al. Association of single nucleotide polymorphisms of PADI4 and HLA-DRB1 alleles with susceptibility to rheumatoid arthritis-related lung diseases. Lung 2016;194:745–53. [DOI] [PubMed] [Google Scholar]
- [37].Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med 2012;106:1591–9. [DOI] [PubMed] [Google Scholar]
- [38].Bergström U, Jacobsson LT, Nilsson JÅ, et al. Pulmonary dysfunction, smoking, socio-economic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford) 2011;50:2005–13. [DOI] [PubMed] [Google Scholar]
- [39].Yahya A, Bengtsson C, Lai TC, et al. Smoking is associated with an increased risk of developing ACPA-positive but not ACPA-negative rheumatoid arthritis in Asian populations: evidence from the Malaysian MyEIRA case-control study. Mod Rheumatol 2012;22:524–31. [DOI] [PubMed] [Google Scholar]
- [40].Perry E, Kelly C, Eggleton P, et al. The lung in ACPA-positive rheumatoid arthritis: an initiating site of injury? Rheumatology (Oxford) 2014;53:1940–50. [DOI] [PubMed] [Google Scholar]
- [41].Wang JX, Du CG. A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit 2015;21:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Minutillo A, Pacifici R, Scaravelli G, et al. Gender disparity in addiction: an Italian epidemiological sketch. Ann Ist Super Sanita 2016;52:176–83. [DOI] [PubMed] [Google Scholar]
- [43].Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med 2011;183:372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pérez-Dórame R, Mejíaa M, Mateos-Toledoa H, et al. Rheumatoid arthritis-associated interstitial lung disease: lung inflammation evaluated with high resolution computed tomography scan is correlated to rheumatoid arthritis disease activity. Reumatol Clin 2015;11:12–6. [DOI] [PubMed] [Google Scholar]
- [45].Natalini JG, Swigris JJ, Morisset J, et al. Understanding the determinants of health-related quality of life in rheumatoid arthritis-associated interstitial lung disease. Respiratory Medicine 2017;127:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Youssef AA, Machaly SA, El-Dosoky ME, et al. Respiratory symptoms in rheumatoid arthritis: relation to pulmonary abnormalities detected by high-resolution CT and pulmonary functional testing. Rheumatol Int 2012;32:1985–95. [DOI] [PubMed] [Google Scholar]
- [47].Leonel D, Lucia C, Martha-Alicia H, et al. Pulmonary function test: its correlation with pulmonary high-resolution computed tomography in patients with rheumatoid arthritis. Rheumatol Int 2012;32:2111–6. [DOI] [PubMed] [Google Scholar]


