Abstract
Aim:
Effects of azithromycin on asthma reported in clinical trials are less consistent. We aimed to further clarify the efficacy and safety of azithromycin in treatment of asthma.
Methods:
The protocol registration number was CRD42017074318 (http://www.crd.york.ac.uk/Prospero). We searched PubMed, EMBASE, Cochrane databases, China National Knowledge Internet (CNKI), and Wanfang databases for the randomized controlled trials (RCTs) with prolonged treatment of azithromycin for more than 3 weeks. Random-effects or fixed-effects model was applied to calculate risk ratio (RR) and mean difference (MD) for dichotomous and continuous data respectively.
Results:
A total of eight studies were included for analysis. The pooled result of adjunctive azithromycin therapy in asthma showed a small, but statistically significant increase in forced expiratory volume in one second (FEV1) (MD = 0.06, 95% confidence interval [CI]: 0.01–0.12, P = .02), but no significant differences in exacerbation frequency (MD = −0.42, 95%CI: −1.13 to 0.30, P = .25) and peak expiratory flow (PEF) (MD = 0.20, 95% CI: −0.05 to 0.44, P = .12), fractional exhaled nitric oxide (FeNO) (MD = 4.12, 95% CI: −2.06 to 10.30, P = .19), asthma quality of life questionnaire (AQLQ) (MD: 0.05, 95% CI: −0.17 to 0.28, P = .65), asthma control questionnaire (ACQ) (MD: −0.03, 95% CI: −0.21 to 0.15, P = .75). The subgroup analysis revealed that azithromycin could decrease FeNO among Asian asthma (MD = 15.04, 95% CI: 6.18–23.90, P = .0009).
Conclusions:
Add-on therapy of azithromycin in asthma patients could improve the FEV1, but failed to improve asthma exacerbations, PEF, ACQ, AQLQ, and FeNO. Subgroup analysis indicated that azithromycin could improve FeNO in Asian group asthmatics.
Keywords: asthma, azithromycin, exacerbation, meta-analysis
1. Introduction
Asthma is a common chronic airway disease that affects more than 300 million people worldwide. It is reported that major asthma-related morbidity, mortality, and health care costs are caused by acute exacerbations.[1] Asthma exacerbations can occur during the maintenance treatment with inhaled corticosteroids and long-acting bronchodilators, which suggests the patients with uncontrolled persistent asthma might need additional treatment options.[2] When monoclonal antibodies such as anti-immunoglobulin(Ig)E and anti-interleukin(IL)5 have been demonstrated to be efficacious in eosinophilic asthma, there is still no effective treatment for neutrophil asthma.[3,4] Besides, the high costs of monoclonal antibodies also preclude widespread use in many asthmatics. Therefore, effective, economical, and safe add-on therapies in patients with poorly controlled asthma are needed.
Azithromycin, a second-generation macrolide, is a commonly used antibiotic with a broad antibacterial spectrum and has received increasing attention in recent years because of its additional effects on host-defense reactions and human chronic diseases.[5] For patients with asthma, azithromycin has been investigated in several clinical trials. However, their effects on asthma are less consistent. Hence, we conducted a meta-analysis of randomized controlled trials (RCTs) to assess the efficacy and safety of azithromycin in patients with persistent asthma.
2. Materials and methods
We prepared this article according to the PRISMA guidelines for systematic reviews and meta-analysis.[6] The protocol was registered in an international database of prospectively registered systematic reviews named as PROSPERO (http://www.crd.york.ac.uk/PROSPERO, registration number: CRD42017074318) before the search of eligible trials. A pilot of the study was previously carried out to adjust the search strategy.
2.1. Search strategy and sources
We conducted a comprehensive search in PubMed, Embase, the Cochrane Central Register of Controlled Trials, China National Knowledge Internet, and Wanfang databases using “azithromycin or macrolide” and “asthma or bronchial asthma” and covered all articles published until November 2018. In addition, we screened the reference lists of the papers identified through the databases to avoid missing any potential studies.
2.2. Inclusion and exclusion criteria
Original studies were eligible for inclusion if they met the following criteria:
-
1.
randomized controlled studies;
-
2.
prolonged treatment (three or more weeks) with azithromycin or placebo in adult patients (age > 18 years) with asthma;
-
3.
data extractable for systematic review and meta-analysis regardless of the publication type.
-
4.
We excluded non-randomized clinical trials with ineligible interventions or outcomes.
2.3. Data extraction and quality assessment
Two authors (Xiaohu Wang and Dan Wang) independently extracted related data in a blinded fashion from eligible studies based on the predefined criteria, which included the characteristics of the trials, interventions, and outcomes. No language and time restriction were imposed in this meta-analysis. Any disagreements were resolved by consensus with a third author (Chuntao Liu) when necessary. Figure 1 summarized the study selection process. The main outcomes were rates of asthma exacerbation, lung function (forced expiratory volume in one second [FEV1], peak expiratory flow [PEF]), fractional exhaled nitric oxide (FeNO), Asthma Control Questionnaire (ACQ), asthma quality of life questionnaire (AQLQ), resistance to azithromycin, and side effects. The risk of bias was assessed using Cochrane-recommended tools,[7] including:
Figure 1.
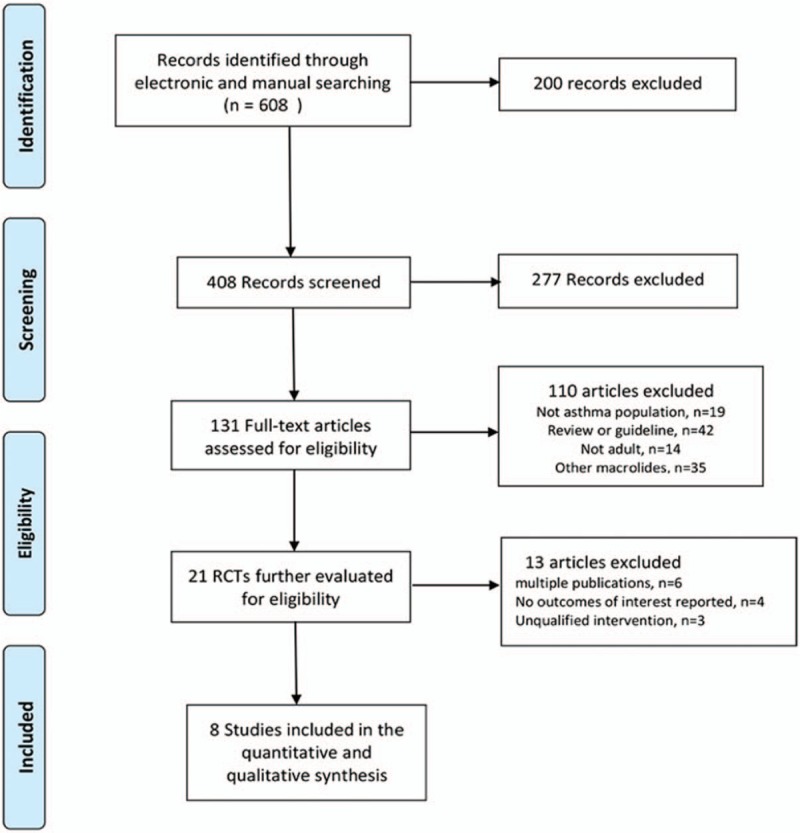
Flow chart of study identification, inclusion, and exclusion.
-
1.
adequate sequence generation;
-
2.
allocation concealment;
-
3.
blinding;
-
4.
incomplete outcome data addressed;
-
5.
free of selective reporting; and
-
6.
free of other bias.
2.4. Statistical analyses
All analyses were performed with Review Manager (Version 5.3, The Cochrane Collaboration). P < .05 was considered statistically significant. Risk ratio (RR) and 95% confidence interval (CIs) were used to analyze dichotomous data, and mean difference (MD) and 95% CI were used for continuous data. We assessed the statistical heterogeneity among trials, which was defined as P ≤ .1 and I2 ≥ 50% in χ2 and I2 test, respectively.[8] Fixed-effects models were used when no statistical heterogeneity was detected. Otherwise, random-effects models were used.[9] Additionally, we performed a subgroup analysis to evaluate the ethnicity-specific effects.
3. Results
3.1. Study description
A total of 608 studies were initially identified. among which 279 were from PubMed, 59 were from EMBASE, 71 were from Cochrane Library, 56 were from CNKI and 143 were from Wanfang databases. After removal of duplicates, from them, 21 potentially eligible RCTs were identified. Then, 13 studies were excluded for multiple publications, no outcomes of interest reported and unqualified intervention. Finally, eight studies[10–17] were included in the meta-analysis. A total of 855 asthma patients were recruited to these eight trials: 452 were randomized to the azithromycin group and 403 to the control group. The mean age ranged from 18 to 60 years old, the proportion of male subject varied from 32% to 80%, and the treatment duration was between 6 weeks to 12 months. Patients were permitted to take standard asthma medications including inhaled corticosteroids plus a long-acting bronchodilator, and the use of salbutamol if necessary. Baseline characteristics of the patients enrolled were described in Table 1. Details of patients’ characteristics, intervention strategies, and outcomes were summarized in Table 2, and A summary of the ‘Risk of bias’ assessment was presented in Figure 2.
Table 1.
Characteristics of included studies in the present study.
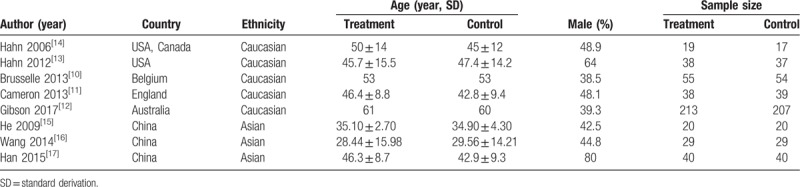
Table 2.
Details of each enrolled study.
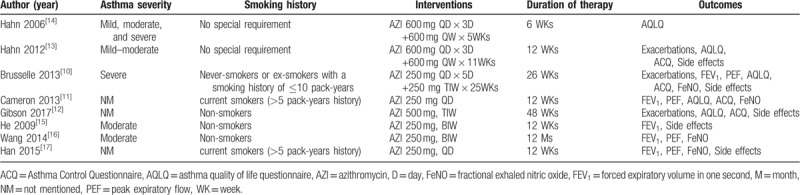
Figure 2.
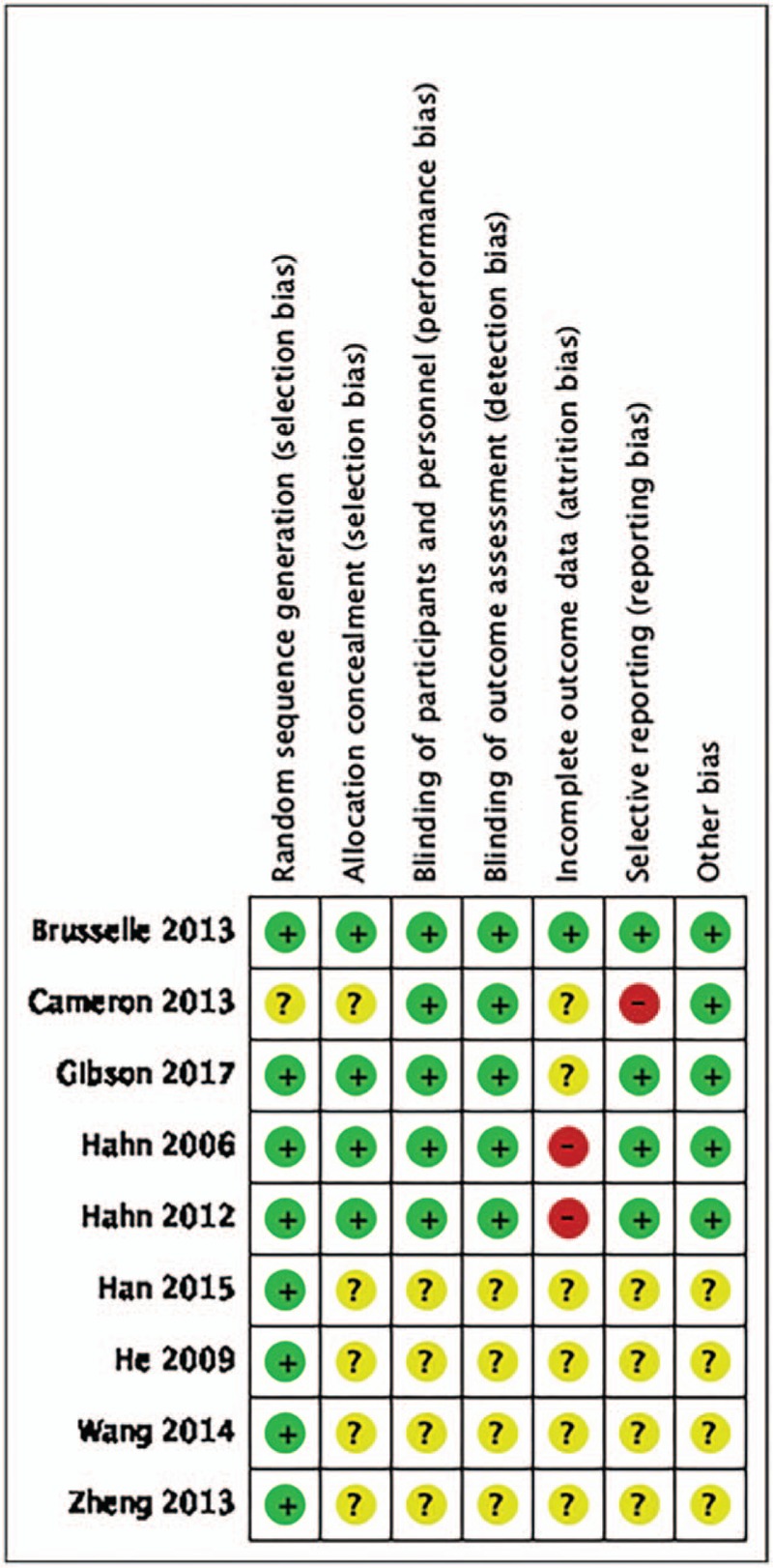
Risk of bias assessment summary.
3.2. Clinical outcomes
3.2.1. Exacerbation frequency
Three trials[10,12,13] reported asthma exacerbations after treatment of azithromycin, but only two trials[10,12] with 529 patients could be included in our meta-analysis. The random-effects model was used due to significant heterogeneity across studies (I2 = 87%, P = .005). The pooled result did not show any benefit of azithromycin over placebo on exacerbation frequency (MD = −0.42, 95%CI: −1.13 to 0.30, P = .25, Fig. 3). Another trial[13] reported no significant differences in the rate of exacerbation between azithromycin group and control group, however, data were not proper for extraction.
Figure 3.
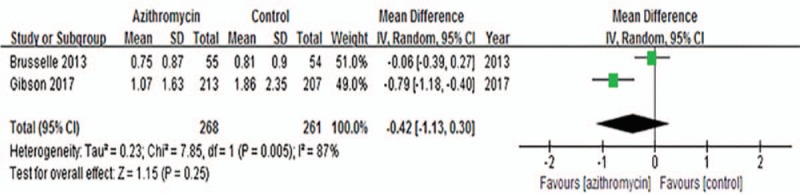
Effects of azithromycin on asthma exacerbations.
3.2.2. FEV1
Five trials[10,11,15–17] with 364 subjects were pooled in our meta-analysis, and fixed-effects model was used based on the low heterogeneity across studies (I2 = 39%, P = .16). We found that the addition of azithromycin in asthma patients slightly increased FEV1 (MD: 0.06, 95% CI: 0.01–0.12, P = .02, Fig. 4).
Figure 4.
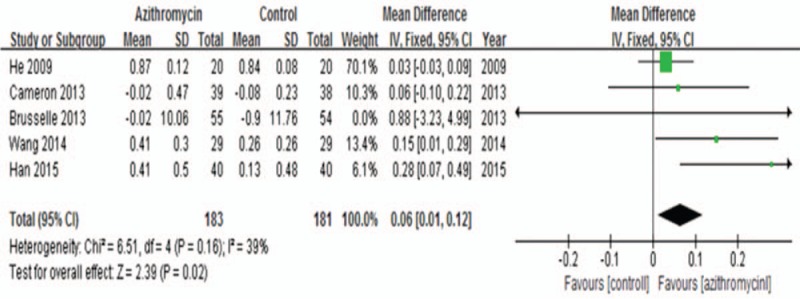
Effects of azithromycin on FEV1 (L).
3.2.3. PEF
Significant heterogeneity was detected across studies[10,11,16,17] (I2 = 53%, P = .09), and the random-effects model showed no significant difference in PEF between azithromycin and placebo treatment (MD = 0.20; 95% CI: −0.05 to 0.44, P = .12, Fig. 5). Similarly, the ethnicity subgroup analysis also demonstrated that azithromycin therapy could not improve PEF in Caucasian (MD = 0.05; 95% CI: −0.21 to 0.32, P = .70) or Asian (MD = 0.45, 95% CI: −0.26 to 1.15, P = .22) patients.
Figure 5.
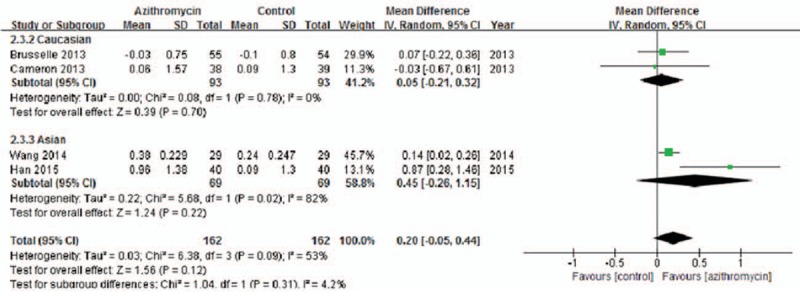
Effects of azithromycin on PEF (L/min).
3.2.4. FeNO
Four trials[10,11,15,17] reported FeNO, but significant heterogeneity was noticed across studies (I2 = 80%, P = .002), which resulted in the use of random-effects model. No significant difference was found between azithromycin and control group (MD = 4.12, 95% CI: −2.06 to 10.30, P = .19, Fig. 6), however, the ethnicity subgroup analysis showed a significant decrease of FeNO after long-term azithromycin therapy in Asian patients (MD = 15.04, 95% CI: 6.18–23.90, P = .0009) rather than Caucasian patients (MD = −1.20, 95% CI: −3.08 to 0.67, P = .21).
Figure 6.
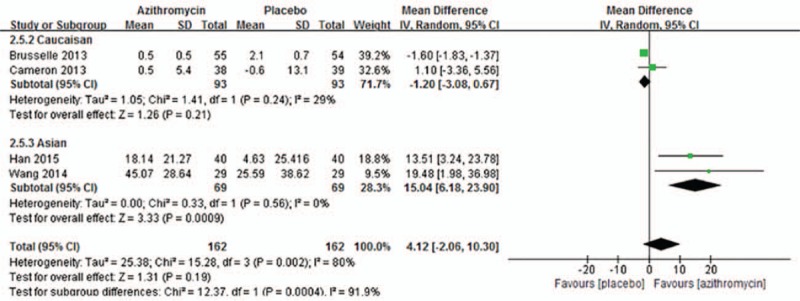
Effects of azithromycin on FeNO (ppb).
3.2.5. ACQ and AQLQ
Both ACQ and AQLQ can be used for evaluating asthma symptom. Four trials[10–13] were included for evaluating ACQ. Due to significant heterogeneity across studies (I2 = 51%, P = .10), the random-effects model was used. Generally, the pooled result showed that azithromycin administration had no impact on ACQ (MD = −0.03; 95% CI: −0.21 to 0.15, P = .75, Fig. 7). Figure 8 present five studies[10–14] included in the analysis of the effects of azithromycin on AQLQ in asthma patients. The random-effects model was used due to heterogeneity across studies (I2 = 57%, P = .06). The overall result indicated the addition of azithromycin therapy in asthma patients had no statistical significance on AQLQ (MD = 0.05, 95% CI: −0.17 to 0.28, P = .65, Fig. 8).
Figure 7.
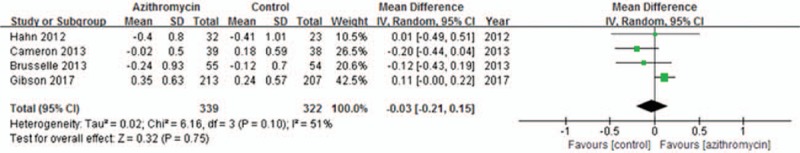
Effects of azithromycin on ACQ.
Figure 8.
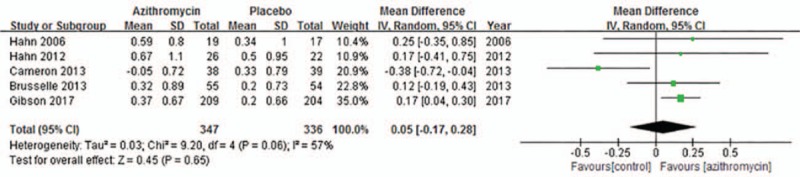
Effects of azithromycin on AQLQ.
3.2.6. Resistance to azithromycin
Two studies[10,12] reported azithromycin related antimicrobial resistance, but data was insufficient for pooled analysis. Brusselle et al[10] reported long-term treatment with azithromycin was associated with an increased proportion of macrolide-resistant oropharyngeal Streptococci, while Gibson et al[12] did not detect any significant difference between the two treatment groups.
3.2.7. Side-effects
Gastrointestinal side-effects such as nausea, diarrhoea, and abdominal pain were the main reported adverse events, and the related data extracted from three studies[10,12,13] showed no differences between azithromycin and placebo treatment (RR = 1.42; 95% CI: 0.97–2.09, P = .07, Fig. 9). Other side effects, such as rash, allergic reaction, vertigo, and headache were also reported similarly between the two groups, but it was not feasible to conduct a meta-analysis. Additionally, Gibson et al[12] and Brusselle et al[10] found a higher incidence of ‘QTc prolongation’ and ‘elevated liver function tests’ in azithromycin group, respectively.
Figure 9.
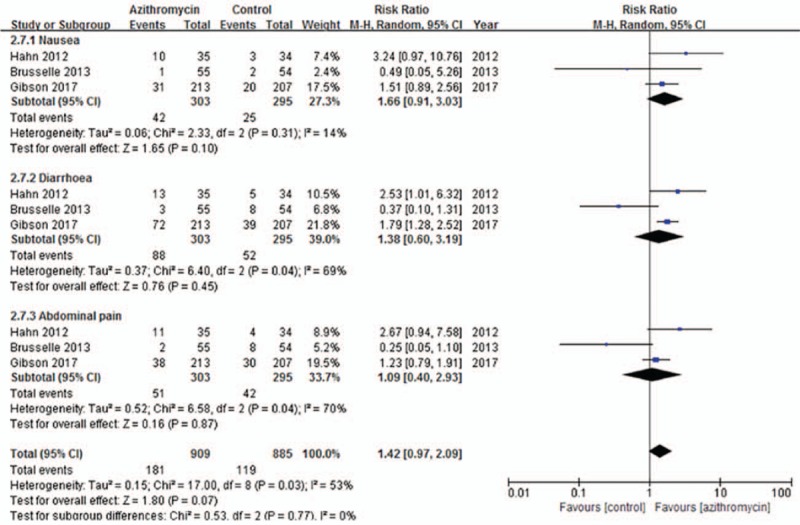
Effects of azithromycin on side effects.
4. Discussion
Asthma exacerbation is a major cause of disease morbidity, increase in health care costs, and, in some patients, greater progressive loss of lung function.[18] Asthma exacerbations always can be controlled by inhaled corticosteroid (ICS) or ICS combined with long-acting β2-adrenergic agonists, but in some cases, which correspond to severe asthma or refractory asthma, the symptoms are refractory to these standard treatments, and often lead to emergency visit or hospitalization. Given the important role of airway inflammation in asthma, the prompt initiation of an anti-inflammatory agent is the mainstay of therapy.[19] Moreover, it has been reported that chronic infection of the lower respiratory tract with Mycoplasma pneumonia and/or Chlamydia pneumonia may also be an important contributor to the pathophysiology of acute exacerbations.[20] Therefore, novel drugs that contain both anti-inflammatory and anti-infective effects are warranted and may be an alternative option in treating refractory asthma.
Azithromycin, containing a macrocyclic 15-membered lactone ring with excellent tissue penetration and antimicrobial activity against a broad range of Gram-positive and Gram-negative bacteria,[21] is widely used in clinical settings. During recent years, besides the antimicrobial effects, its anti-inflammatory and immunomodulatory effects have been increasingly recognized. These effects including but not limited to inhibition of pro-inflammatory pathways, modulation of macrophages and monocyte function and phenotype, anti-neutrophilic inflammation effects, potential inhibition of Th2 immune response and potential direct and indirect antiviral properties.[22] The mouse model has demonstrated the potential value of azithromycin in the treatment of chronic respiratory diseases due to the non-anti-infection effects,[23] and long-term use of macrolides has also been found to be effective for patients with diffuse panbronchiolitis,[24] bronchiectasis,[25] cystic fibrosis,[26] and chronic obstructive pulmonary disease (COPD).[27] Additionally, some macrolides are reported to be able to alter steroid metabolism, suggesting that these agents might also function as steroid-sparing agents[28] and may be used to treat steroid-insensitive asthma. Other possible mechanisms which intrigue the potential applications of azithromycin in asthma treatment includes:
-
1.
azithromycin can reduce airway epithelial cell apoptosis by improving the imbalance of Bax/Bcl-2 ratio and inhibiting Caspase-3 level in airway epithelium, and therefore restore epithelium integrity[29];
-
2.
azithromycin may be tackle VEGF-mediated remodeling of bronchial smooth muscles via p38 MAPK pathway[30]; and
-
3.
azithromycin dose-dependently augmented viral-induced interferon expression in asthmatic bronchial epithelial cells, which decreases the viral load and viral-induced asthma exacerbations.[31]
Although there are systematic reviews about macrolide for chronic asthma published previously,[32,33] special meta-analysis for azithromycin in asthma has not been reported. In our meta-analysis, in terms of lung functions and asthma symptom control, a similar pattern was found in our study as reported in the 2015 Cochrane Report[33] that add-on therapy of azithromycin improved FEV1 but not PEF, ACQ, AQLQ in asthma patients. However, another meta-analysis[32] published in 2013 reported that prolonged macrolides use could not improve FEV1, but can improve PEF and quality-of-life (QOL). There are several possible reasons for the different results between Reiter's and our study:
-
1.
A high-quality RCT[12] published recently and different ethnicities were included in our meta-analysis, and it was conducive to close to the real result.
-
2.
Different types of macrolides possessed various properties were included in Reiter's study and maybe differed in their effects on asthma exacerbations, and hence caused different outcome.
-
3.
The present study did not include children asthmatics because we were afraid of children's reaction to azithromycin may be unlike adults.
FeNO is regarded as a simple, non-invasive method for assessing asthma and it is recommended by the American Thoracic Society to facilitate asthma diagnosis and monitoring, as well as identifying steroid responsive individuals whose chronic respiratory symptoms may be due to asthma.[34] However, there was no previous meta of macrolides on asthma concerning this important outcome to our knowledge. For the first time, our study performed a meta-analysis of azithromycin in FeNO. Although the overall effect showed no significant improvement in FeNO, the subgroup analysis found that azithromycin could improve FeNO in Asian patients with asthma. The previous study[35] showed that higher FeNO values were found in healthy adults in China compared with other ethnicities. Hence we speculated that different races maybe have different reactions to azithromycin. Chinese perhaps more sensitive to azithromycin. However, the subgroup results should be interpreted with caution because of the limited sample size and potential bias inherent to subgroup analysis.
In our study, the safety analysis showed azithromycin did not significantly increase gastrointestinal side-effects in asthma patients compared to placebo, which indicates that treatment of azithromycin in patients with asthma is of good safety and tolerance. Microbial resistance is a worldwide problem and should be considered when any antibiotics used. Our systematic review showed a controversial outcome of azithromycin in microbial resistance, but the interpretation of our result should be careful due to the limited data available, thus it seems difficult to draw a convincing conclusion in whether long-term azithromycin administration could result in drug-resistant bacteria in asthma patients.
There are several limitations to the present study. First, significant heterogeneity was found in PEF, AQLQ, ACQ, and side effects, which might affect the accuracy of our result although random-effects model was used. Second, asthma severity and baseline characteristics of the patients varied among studies, which might impede the generalized use of azithromycin in all asthma patients. Third, the number of included studies and patient samples were relatively small, which might weaken the power of our study and disenable us to assess other risk factors that may modify the effects of azithromycin such as gender, environmental exposures, age, dose, and duration of therapy.
5. Conclusions
The present study showed that add-on therapy of azithromycin in asthma patients could improve the FEV1, but failed to improve asthma exacerbations, PEF, ACQ, AQLQ, and FeNO. Moreover, we found the subgroup analysis indicated that azithromycin could improve FeNO in Asian group asthmatics. Future studies, especially the multi-centered and well-designed RCTs, are warranted to further determine the effects of azithromycin in asthma, and asthma phenotypes can also be taken into account to figure out the potential azithromycin-sensitive subgroups.
Author contributions
Conceptualization: Xiaohu Wang, Chuntao Liu.
Data curation: Xiaohu Wang.
Formal analysis: Xiaohu Wang.
Investigation: Xiaohu Wang.
Methodology: Xiaohu Wang.
Software: Jian Luo.
Supervision: Xiaohu Wang, Jian Luo, Dan Wang, Bicui Liu.
Validation: Chuntao Liu.
Writing – original draft: Xiaohu Wang.
Writing – review & editing: Jian Luo, Chuntao Liu.
Footnotes
Abbreviations: ACQ = Asthma Control Questionnaire, AQLQ = asthma quality of life questionnaire, CI = confidence interval, COPD = chronic obstructive pulmonary disease, FeNO = fractional exhaled nitric oxide, FEV1 = forced expiratory volume in one second, ICS = inhaled corticosteroid, Ig = immunoglobulin, IL = interleukin, MD = mean difference, PEF = peak expiratory flow, QOL = quality-of-life, RCT = randomized controlled trial, RR = risk ratio.
How to cite this article: Wang X, Luo J, Wang D, Liu B, Liu C. The efficacy and safety of long-term add-on treatment of azithromycin in asthma. Medicine. 2019;98:38(e17190).
The authors have no conflicts of interest to disclose.
Competing interests: The authors declare that they have no competing interests.
Funding: Not applicable
Ethics approval and consents to participate: Not applicable. Ethical approval and consents were waived since this is a review article.
References
- [1].Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001;107:3–8. [DOI] [PubMed] [Google Scholar]
- [2].Calhoun WJ, Haselkorn T, Mink DR, et al. Clinical burden and predictors of asthma exacerbations in patients on guideline-based steps 4–6 asthma therapy in the TENOR cohort. J Allergy Clin Immunol Pract 2014;2:193–200. [DOI] [PubMed] [Google Scholar]
- [3].Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015;3:355–66. [DOI] [PubMed] [Google Scholar]
- [4].Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207. [DOI] [PubMed] [Google Scholar]
- [5].Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014;143:225–45. [DOI] [PubMed] [Google Scholar]
- [6].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [7].Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011; 2013. [Google Scholar]
- [8].Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206. [DOI] [PubMed] [Google Scholar]
- [9].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [10].Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013;68:322–9. [DOI] [PubMed] [Google Scholar]
- [11].Cameron EJ, Chaudhuri R, Mair F, et al. Randomised controlled trial of azithromycin in smokers with asthma. Eur Respir J 2013;42:1412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:659–68. [DOI] [PubMed] [Google Scholar]
- [13].Hahn DL, Grasmick M, Hetzel S, et al. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med 2012;25:442–59. [DOI] [PubMed] [Google Scholar]
- [14].Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials 2006;1:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He J, Zhu N, Chen XD. Clinical impacts of azithromycin on lung function and cytokines for athmatic patients (Chinese). Fudan Univ J Med Sci 2009;36:719–22. [Google Scholar]
- [16].Wang T, Pei FY, Song XP, et al. Clinical impacts of low-dose azithromycin on lung function and fraction of exhaled nitric oxide concentration in bronchial asthma. Med Innovat China 2014;11:122–4. [Google Scholar]
- [17].Han Y. The role of azithromycin in the treatment of patients with bronchial asthma. Sichuan Med J 2015;36:656–8. [Google Scholar]
- [18].Bai TR, Vonk JM, Postma DS, et al. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007;30:452–6. [DOI] [PubMed] [Google Scholar]
- [19].Williams SG, Schmidt DK, Redd SC, et al. Key clinical activities for quality asthma care. Recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003;52(Rr-6):1–8. [PubMed] [Google Scholar]
- [20].Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest 2007;132:1962–6. [DOI] [PubMed] [Google Scholar]
- [21].Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010;23:590–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med 2014;2:657–70. [DOI] [PubMed] [Google Scholar]
- [23].Beigelman A, Gunsten S, Mikols CL, et al. Azithromycin attenuates airway inflammation in a noninfectious mouse model of allergic asthma. Chest 2009;136:498–506. [DOI] [PubMed] [Google Scholar]
- [24].Nagai H, Shishido H, Yoneda R, et al. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 1991;58:145–9. [DOI] [PubMed] [Google Scholar]
- [25].Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251–9. [DOI] [PubMed] [Google Scholar]
- [26].Equi A, Balfour-Lynn IM, Bush A, et al. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 2002;360:978–84. [DOI] [PubMed] [Google Scholar]
- [27].Donath E, Chaudhry A, Hernandez-Aya LF, et al. A meta-analysis on the prophylactic use of macrolide antibiotics for the prevention of disease exacerbations in patients with Chronic Obstructive Pulmonary Disease. Respir Med 2013;107:1385–92. [DOI] [PubMed] [Google Scholar]
- [28].Szefler SJ, Rose JQ, Ellis EF, et al. The effect of troleandomycin on methylprednisolone elimination. J Allergy Clin Immunol 1980;66:447–51. [DOI] [PubMed] [Google Scholar]
- [29].Liu Y, Pu Y, Li D, et al. Azithromycin ameliorates airway remodeling via inhibiting airway epithelium apoptosis. Life Sci 2017;170:1–8. [DOI] [PubMed] [Google Scholar]
- [30].Willems-Widyastuti A, Vanaudenaerde BM, Vos R, et al. Azithromycin attenuates fibroblast growth factors induced vascular endothelial growth factor via p38(MAPK) signaling in human airway smooth muscle cells. Cell Biochem Biophys 2013;67:331–9. [DOI] [PubMed] [Google Scholar]
- [31].Menzel M, Akbarshahi H, Tufvesson E, et al. Azithromycin augments rhinovirus-induced IFNbeta via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget 2017;8:31601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy 2013;68:1040–9. [DOI] [PubMed] [Google Scholar]
- [33].Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev 2015;CD002997. [DOI] [PubMed] [Google Scholar]
- [34].Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Team FP. Multicenter study of FeNO normal values in healthy Chinese subjects. Chin J Gen Pract 2013;11:341–5. [Google Scholar]


