Abstract
Although selective activation of the M1 muscarinic acetylcholine receptor (mAChR) subtype has been shown to improve cognitive function in animal models of neuropsychiatric disorders, recent evidence suggests that enhancing M4 mAChR function can also improve memory performance. Positive allosteric modulators (PAMs) targeting the M4 mAChR subtype have shown therapeutic potential for the treatment of multiple symptoms observed in schizophrenia, including positive and cognitive symptoms when assessed in acute pre-clinical dosing paradigms. Since the cholinergic system has been implicated in multiple stages of learning and memory, we evaluated the effects of repeated dosing with the highly selective M4 PAM VU0467154 on either acquisition and/or consolidation of learning and memory when dosed alone or after pharmacologic challenge with the N-methyl-D-aspartate subtype of glutamate receptors (NMDAR) antagonist MK-801. In animals, MK-801 challenge represents a well-documented model of NMDAR hypofunction that is thought to underlie some of the positive and cognitive symptoms observed in schizophrenia. In wildtype mice, 10-day, once-daily dosing of VU0467154 either prior to, or immediately after daily testing enhanced the rate of learning in a touchscreen visual pairwise discrimination task; these effects were absent in M4 mAChR knockout mice. Following a similar 10-day, once-daily dosing regimen of VU0467154, we also observed 1) improved acquisition of memory in a cue-mediated conditioned freezing paradigm, 2) attenuation of MK-801-induced disruptions in the acquisition of memory in a context-mediated conditioned freezing paradigm and 3) reversal of MK-801-induced hyperlocomotion. Comparable efficacy and plasma and brain concentrations of VU0467154 were observed after repeated dosing as those previously reported with an acute, single dose administration of this M4 PAM. Together, these studies are the first to demonstrate that cognitive enhancing and antipsychotic-like activity are not subject to the development of tolerance following repeated dosing with a selective M4 PAM in mice and further suggest that activation of M4 mAChRs may modulate both acquisition and con-solidation of memory functions.
Keywords: Positive allosteric modulator, VU0467154, Antipsychotic-like activity, Cognitive enhancement, M4 muscarinic acetylcholine receptor, MK-801
1. Introduction
Currently available antipsychotic drugs (APDs) provide limited therapeutic benefits for many of the complex symptom clusters associated with schizophrenia, particularly the cognitive impairments in attention, memory, and executive functions (APA, 2000; Barch and Ceaser, 2012; Nuechterlein et al., 2004). With overall functional outcome measures positively linked to cognitive function in patients with schizophrenia, there is a critical need to focus on the development of novel treatments to improve cognitive performance (Bobes et al., 2007; Green, 1996; Green et al., 2004). Of the five different subtypes of muscarinic acetylcholine receptors (mAChRs), termed M1-M5 (Bonner et al., 1987, 1988), accumulating evidence indicates that selective activators of M1 and/or M4 mAChRs may provide exciting alternative strategies for the treatment of core symptoms in schizophrenia, including cognitive impairments (Jones et al., 2012). For example, the M1/M4-preferring mAChR agonist xanomeline reduced the psychotic symptoms and improved some cognitive deficits in an early proof-of-concept trial in patients with schizophrenia (Shekhar et al., 2008). Unfortunately, xanomeline, similar to other nonselective mAChR agonists, failed in clinical development due to dose-limiting adverse side effects attributed to off-target activation of peripheral M2 and M3 mAChRs (McArthur et al., 2010).
More recently, our group and others have identified subtype-selective mAChR ligands that potentiate specific mAChR subtypes via binding to sites topographically distinct from the orthosteric binding site of acetylcholine (ACh), termed allosteric sites (Conn et al., 2009). These efforts have resulted in the discovery of several novel and highly selective M4 positive allosteric modulators (PAMs), including the optimized M4 PAM VU0467154 (Brady et al., 2008; Bubser et al., 2014; Byun et al., 2014; Shirey et al., 2008). VU0467154, like other M4 PAMs, does not activate M4 directly, but markedly potentiates the response of the rat M4 receptor to ACh with an EC50 value of 17.7 nM and induces a 68% maximal response when compared to the ECmax of ACh (AChmax) (Bubser et al., 2014). Relative to other M4 PAMs, VU0467154 also possesses enhanced rodent pharmacokinetic (PK) properties that allow more extensive characterization of M4-mediated mechanisms in rodent models predictive of antipsychotic-like activity and improved cognitive performance (Bubser et al., 2014). As previously reported, our selective M4 PAMs, including VU0467154, exhibit an APD-like profile in rodents comparable to the M1/M4-preferring agonist xanomeline without induction of adverse side effects (e.g., salivation, lacrimation, diarrhea) (Brady et al., 2008; Bubser et al., 2014; Byun et al., 2014).
Using the M4 PAM VU0467154, we recently demonstrated that selective potentiation of M4 signaling could also ameliorate the behavioral and associative learning impairments induced by the noncompetitive N-methyl-D-aspartate subtype of the glutamate receptor (NMDAR) antagonist MK-801 in wildtype, but not in M4 mAChR knockout (KO), mice (Bubser et al., 2014). Pharmacologic challenge with MK-801 represents a well validated preclinical model of NM-DAR hypofunction, which is thought to underlie many of the symptoms observed in schizophrenia patients (Anticevic et al., 2015; Blot et al., 2013; Coyle et al., 2012). In addition, VU0467154 reversed MK-801-induced increases in high frequency cortical gamma power similar to effects observed with the atypical APD clozapine (Gould et al., 2016). While collectively these findings are promising, all previous studies evaluating the effects of M4 PAMs on cognition were conducted using only acute, single dosing paradigms. To date, the potential for cognitive enhancing effects in more complex models of learning and memory and antipsychotic-like activity following repeated dosing of M4 PAMs have not been investigated.
The purpose of the present studies was to first evaluate the effects of 10-day, once-daily dosing of VU0467154, administered prior to each daily training session, on the acquisition of a touchscreen visual pairwise discrimination task, a preclinical model of associative learning and memory. Potential changes induced by M4 activation in both the rate of learning (increases in percent accuracy across 10 consecutive testing days) and acquisition of this task (the number of training days to achieve 80% accuracy) were measured in wildtype and M4 KO mice. Since the muscarinic cholinergic system has also been implicated in the stabilization or consolidation of memories over longer periods of time, which are often associated with sleep-dependent mechanisms (Gais and Born, 2004a, b; Hasselmo and Sarter, 2011), we next examined the effects of 10-day, once-daily dosing of VU0467154, when administered immediately after each daily session, on pairwise discrimination learning in wildtype mice. Finally, we evaluated the effects of VU0467154 after 10 days of once-daily dosing on previously reported acute effects in two preclinical models of hippocampal and non-hippocampal learning and memory functions and in a preclinical model predictive of APD-like activity. Specifically, we examined the ability of VU0467154 to 1) enhance the acquisition of memory using a cue-mediated conditioned freezing assay, 2) attenuate MK-801-induced disruptions in the acquisition of memory using a contextual conditioned freezing assay, and 3) attenuate MK-801-induced hyperlocomotion in wildtype mice. In addition, we measured brain and plasma concentrations of VU0467154 after once daily dosing to determine if potential changes in efficacy could be related to steady-state accumulation or altered metabolic clearance of VU0467154 through auto-induction of cytochrome P450s. Importantly, this 10-day, once-daily dosing regimen produced similar behavioral effects and plasma and brain concentrations as previously reported with a single acute dose of VU0467154.
2. Materials and methods
2.1. Subjects
All studies were conducted in 8–12 week old adult male wildtype and M4 KO mice with the same genetic background (C57BL/6NTac) and group-housed under a 12/12 h light-dark cycle with water available ad libitum. For all touchscreen cognition studies (but not conditioned freezing studies), mice were gradually food restricted and maintained at ~85% free-feeding weight prior to initiation of training. Pairwise discrimination sessions occurred during the first 6 h of the light phase. All animal experiments were approved by the Vanderbilt University Animal Care and Use Committee and experimental procedures conformed to guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
2.2. Assessment of repeated (10 days) dosing of VU0467154 prior to daily testing on rate of learning and acquisition of a pairwise discrimination task in wildtype mice
2.2.1. Touchscreen training
Mice were trained in operant chambers (Lafayette Instruments, Lafayette, IN) to respond to stimuli presented on a computer screen by breaking an infrared beam in close proximity to the stimuli (e.g. a nosepoke) according to convention (for detailed methods see Bubser et al., 2014; Gould et al., 2015; Horner et al., 2013). Throughout training and testing, a mask was placed over the touchscreen such that responses could only be made in one of two (2×2 inch) windows on the screen. In brief, mice were trained over the course of 7–10 days to initiate each trial by emitting a response via a nose-poke in a receptacle located on the wall opposite the touchscreen. After initiation of each trial, mice were required to nose-poke on a stimulus appearing in one of the response windows, and then collect a liquid reward (33% diluted Ensure; 30 μL delivered via a peristaltic pump) from the receptacle located on the opposite wall from the touchscreen. Trial availability was signaled by illumination of a light within the receptacle, with a 5 s inter-trial interval. Incorrect responses (nose-poke on a window without a stimulus) were signaled by extinguishing the houselight for 5 s. Once mice reliably tracked and responded on the stimuli (>80% accuracy), they were assigned to treatment groups counterbalanced across the following variables based on the last day of training before initiation of the pairwise discrimination task: weight, accuracy, session length, correct response latency (average duration of time from trial initiation to a registered nose poke on the stimulus) and reinforcer retrieval latency (average duration of time to make a head entry into the reward receptacle following a correct response).
2.2.2. Pairwise discrimination task
Following training, mice were exposed to 10 consecutive daily cognition sessions in which two stimuli were pseudo-randomly presented on the left and right windows of the screen. Responding on one stimulus (S+, “fan”) resulted in delivery of a liquid reward followed by a 5 s intertrial interval (ITI); responding on the incorrect stimulus (S-, “marbles”) terminated the trial, extinguished the house light, and initiated the 5 s ITI before the house light illuminated again to signal the next trial. “Correction trials” were not implemented following incorrect responses. Total sessions lasted 60 trials or a maximum of 60 min. For pairwise discrimination studies, effects of dose (Study 2.2: Vehicle, 0.3, 1, 3, 10 mg/kg VU0467154; Study 2.3: Vehicle, 3 mg/kg VU0467154), or the time of dosing (Study 2.4: Vehicle compared to 1 mg/kg VU0467154 administered prior to or post-test sessions) were determined by a Two-Way ANOVA followed by a Holm-Sidak test comparing treatment to vehicle groups (Study 2.2 and 2.3) or comparing two groups to each other, respectively.
Starting on Day 1 after completing training, wildtype mice were administered vehicle (10% Tween 80, 90% water, v/v; 10 mL/kg) or a dose of VU0467154 (0.3, 1, 3 or 10 mg/kg; 10 mL/kg) via intraperitoneal (IP) injection 60 min prior to the start of each discrimination session for 10 consecutive days.
To assess effects of repeated dosing of VU0467154 on plasma and brain levels, a subset of mice were administered vehicle or VU0467154 (1, 3, 10 mg/kg; IP) once daily for 4 additional days after the last cognition session (total 14 days of daily dosing). 2.5 h following administration on day 14, mice were anesthetized with isoflurane, decapitated and trunk blood was collected and stored on ice in EDTA-coated blood collection tubes until centrifuged (10 min, 1623 rcf, 4 °C) to obtain plasma. Brains were extracted, briefly rinsed with phosphate buffered saline and flash frozen on dry ice. Plasma and whole brain samples were stored at −80 °C until analysis. Total plasma and brain concentrations of VU0467154 were quantified via LC-MS/MS methods as previously described (Bubser et al., 2014). Collection time points and procedures were identical to prior studies in order to compare present data with previously published data following acute administration (Bubser et al., 2014). Calculated unbound plasma and brain concentrations were determined based on in vitro mouse plasma (0.022) and brain (0.014) fraction unbound (fu) values (Bubser et al., 2014). Data were calculated as mean ± standard deviation (SD; n = 4–5/dose).
2.3. Assessment of the effects of repeated (10 days) dosing of VU0467154 on rate of learning and acquisition of a pairwise discrimination task in M4 KO mice
To confirm that the cognitive enhancing effects of VU0467154 in the visual pairwise discrimination task were mediated through potentiation of M4 mAChRs, separate groups of M4 KO mice were administered vehicle or 3 mg/kg VU0467154. We have previously reported cognitive impairments in M4 mAChR KO mice (Bubser et al., 2014). Therefore, to increase the sensitivity of this experiment, we selected the highest dose of VU0467154 that produced cognitive enhancing effects in wildtype mice (3 mg/kg VU0467154), which as dosed 60 min prior to each of 10 consecutive pairwise discrimination training days. Compound formulation/administration, data collection and analyses were conducted as previously outlined.
2.4. Assessment of the effects of repeated (10 days) dosing of VU0467154 on acquisition versus consolidation of a pairwise discrimination task in wildtype mice
To determine whether the cognitive enhancing effects of VU0467154 were mediated by enhanced acquisition or consolidation of memory functions in the pairwise discrimination task, wildtype mice were trained as above and then randomized into the treatment groups, and administered either vehicle (10% Tween 80, 90% water, v/v; IP, 10 mL/kg) or a dose of 1 mg/kg VU0467154 (IP, 10 mL/kg) 60 min prior to the start or immediately upon completion of each pairwise discrimination training session for 10 consecutive days. The 1 mg/kg dose was selected based on the pharmacokinetic profile of VU0467154 and because it was the lowest dose shown to improve cognitive performance based on results from Experiment 2. In previous mouse pharmacokinetic studies (Bubser et al., 2014), VU0467154 exhibited an elimination half-life of 4.7 h following a single 1 mg/kg IP administration. Thus, in the present studies, elimination of >95% of the administered 1 mg/kg dose should have occurred by 24 h (i.e., ≥5 half-lives). Because the percent accuracy between vehicle-treated groups dosed before or after each session was not different, all vehicle data have been combined and reported together (see Supplemental Fig. 1).
To further determine if the pharmacodynamic effects of VU0467154 were altered by repeated dosing using a 10-day, once-daily dosing regimen, we examined the ability of VU0467154 to 1) enhance acquisition of memory using a cue-mediated conditioned freezing assay, 2) attenuate MK-801-induced disruptions in the acquisition of memory using a contextual conditioned freezing assay, and 3) attenuate MK-801-induced hyperlocomotion. Importantly, the selection of these three assays and doses of VU0467154 were based on previously published full dose-response evaluations of the acute effects of VU0467154 in the same assays (see Bubser et al., 2014).
2.4.1. Assessment of the effects of acute (1 day) versus repeated (10 days) dosing ofVU0467154 on the acquisition of memory using a cue-dependent conditioned freezing paradigm
Separate groups of wildtype mice were administered vehicle once daily (n = 42; 10% Tween 80, 90% water, v/v; 10 mL/kg) or a dose of 3 (n = 14) or 10 (n = 16) mg/kg VU0467154 (10 mL/kg) via IP injection for 9 consecutive days. On Day 10 (day of 1 of conditioned freezing paradigm), vehicle treated mice were randomly separated into 3 groups and were administered vehicle (n = 16), 3 (n = 13) or 10 (n= 13) mg/kg VU0467154 (acute dose group). The groups that received 3 or 10 mg/kg VU0467154 for 9 days also were administered the same dose on Day 10 (repeated dosing groups). Effects of acute (1 day) versus repeated administration (10 days) of VU0467154 on cue-mediated conditioned freezing were examined using the following paradigm: Studies were conducted using conditioning chambers in sound attenuating cubicles equipped with a stainless steel grid floor for shock delivery, 1 mL of 10% vanilla extract as an odor cue, and a video camera for recording freezing behavior as previously described (MedAssociates, Allentown, NJ) (see (Lebois et al., 2010)). On the conditioning day (day 10 of dosing), mice were habituated for 1 h in an anteroom. Mice were then administered their respective treatment dose (vehicle, 3 or 10 mg/kg VU0467154 IP) 1 h prior to conditioning. After the 1 h pretreatment period elapsed, mice were placed into the conditioning chamber that was scented with 1.0 mL 10% vanilla extract and illuminated with a white house light and exposed to the following 8-min session: 90 s habituation followed by four 30 s tone presentations (85 dB, 2500 Hz) co-terminating with a shock (0.7 mA, 1 s) with an interstimulus interval of 60 s, followed by a 90-s interval without stimuli. Approximately 24 h after conditioning, mice were returned to the anteroom where they were habituated under infrared light for 60 min. The test room and chamber were also illuminated by an infrared light only. The context of the chamber was altered with the addition of a white Plexiglass floor on top of the shock grid, a black teepee to alter the shape/size of the chamber, and a 0.5 mL 10% eucalyptus oil odor cue. Mice were presented with the identical testing paradigm as on conditioning day (4 tones) but without the shock stimuli. Freezing behavior, defined as motionless posture, excluding respiratory movements, was measured in the absence of any shock stimuli for 8 min. Data are presented as means ± S.E.M. and analyzed by one-way ANOVA followed by Holm-Sidak’s test comparing all groups to the group treated with Vehicle for 10 days.
2.4.2. Assessment of the effects of repeated (10 days) dosing of VU0467154 on MK-801-induced-disruptions in the acquisition of memory in the contextual conditioned freezing task
Studies were conducted using a similar 10-day once-daily dosing paradigm and identical conditioning chambers as those described for cue-mediated conditioned freezing studies. Separate groups of wildtype mice were administered once-daily vehicle (n = 29; 10% Tween 80, 90% water, v/v; 10 mL/kg) or a dose of 3 (n = 13) or 10 (n = 26) mg/kg VU0467154 (10 mL/kg) via IP injection for 10 consecutive days; respective doses on day 10 (day of 1 of conditioned freezing paradigm) were administered 1 h prior to the conditioning procedure (see below). On day 10, 30 min prior to the conditioning training approximately half of the mice that were administered vehicle (n = 15) and the other half were administered 10 mg/kg (n = 16) (and all that were administered 3 mg/kg) VU0467154 were administered 0.1 mg/kg MK-801 (IP), a dose that was previously shown to disrupt contextual conditioned freezing (Bubser et al., 2014). For respective control groups, the other half of mice that received vehicle or 10 mg/kg VU0467154 for 10 consecutive days were administered saline 30 min prior to conditioning. Effects of repeated administration (10 days) of VU0467154 on context-mediated conditioned freezing were examined using the following paradigm: After a 2-min habituation period in the conditioning chamber scented with 1.0 mL of 10% Vanilla extract and illuminated by a white house light, four presentations of an unconditioned stimulus (0.7 mA 1-s footshock; 89-s intertrial interval) were delivered followed by a 90-s interval (8-min total). Approximately 24 h after conditioning, mice were exposed to the same conditioning context (identical conditioning chamber and odor cue) under drug-free conditions and freezing behavior was determined. Data are presented as means ± S.E.M. and analyzed by one-way ANOVA followed by Holm-Sidak’s test comparing all groups to the group treated with Vehicle for 10 days and then administered MK-801 acutely on day 10.
2.4.3. Assessment of the effects of acute (1 day) versus repeated (10 days) dosing of VU0467154 on MK-801-induced hyperlocomotion
Separate groups of mice were administered once-daily vehicle (n = 23; 10% Tween 80, 90% water, v/v; 10 mL/kg) or a dose of 10 mg/kg VU0467154 (n = 12; 10 mL/kg) via IP injection for 9 consecutive days. On Day 10, half of the mice in the vehicle group were administered vehicle (n = 11) and the other half was administered 10 mg/kg VU0467154 (acute dose group). The group that received 10 mg/kg VU0467154 for 9 days also was administered 10 mg/kg VU0467154 on Day 10 (repeated dosing group). Effects of acute (1 day) versus repeated administration (10 days) of VU0467154 on MK-801-induced hyperlocomotion were examined using the following paradigm: Open field activity was tested using an open field system (OFA-510, MedAssociates, St. Albans, VT) with three 16 × 16 arrays of infrared photobeams as described previously (Bubser et al., 2014; Byun et al., 2014). Wildtype mice were habituated for 90 min in the open field before being injected with vehicle or 10 mg/kg VU0467154; 30 min later 0.3 mg/kg MK-801 (in 0.9% saline; 10 mL/kg, IP) was administered, and locomotor activity was recorded for an additional 120 min. Doses of VU0467154 and Mk-801 were selected based on previously published full dose-response evaluations (Bubser et al., 2014). Specifically, given that tolerance is most likely to occur following repeated exposure to higher concentrations of a given test compound, we chose to administer the top dose tested I the pairwise discrimination test (10 mg/kg VU0467154) once daily for 10 days to determine whether tolerance would develop to the acute pharmacodynamic effects of VU0467154. The time course of drug-induced changes in ambulation was expressed as total distance travelled (cm)/5 min over the entire 240-min session, as well as total distance travelled in the 120 min following administration of MK-801. Total activity data (means ± S.E.M.) were analyzed by a one-way ANOVA and post hoc comparisons were made by a Holm-Sidak test.
3. Results
3.1. Assessment of repeated (10 days) dosing of VU0467154 prior to daily testing on rate of learning and acquisition of a pairwise discrimination task in wildtype mice
As shown in Fig. 1A, on day 1 percent accuracy for all groups was near 50% (chance). For the overall data analysis, 4 of the total 59 mice tested were excluded; 2 mice that completed <20 trials per day and mice that did not improve above 55% accuracy across the 10 days. Over the 10-day dosing period, there was a significant increase in percent accuracy (F9,450 = 71.48; p < 0.0001), and a significant interaction between day and group (F36,450 = 1.92; p < 0.01), but not a significant effect of dose (F4,50 = 2.52; p = 0.053). Holm-Sidak post-hoc analysis revealed that mice treated with 1 and 3 mg/kg VU0467154 demonstrated a significant improvement in rate of learning, as shown by significantly higher percent accuracy on day 5 (1 mg/kg) and day 6(1 and 3 mg/kg), compared to the vehicle-treated wildtype mice (all p < 0.05). Daily IP administration of 0.3 and 10 mg/kg VU467154 did not improve rate of learning in wildtype mice compared to respective vehicle-treated mice, demonstrating a classic inverted-U-shaped dose-effect curve (see below).
Fig. 1.
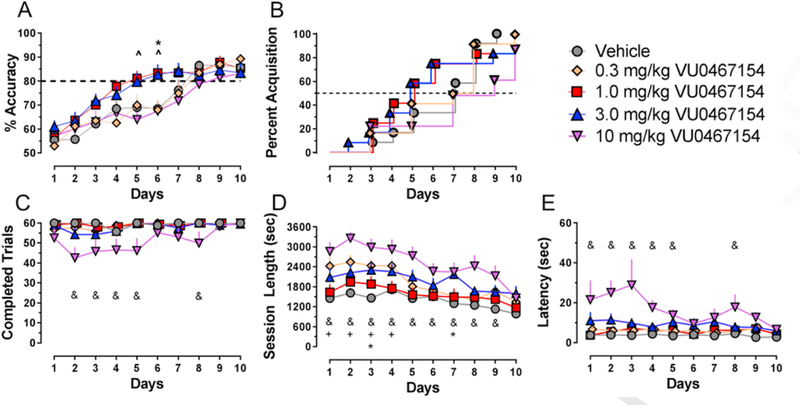
VU0467154 dose-dependently improves rate of acquisition of a pairwise discrimination task in mice when administered 60 min prior to each daily test session. A) Percent accuracy across the first 10 days of learning a pairwise discrimination (PWD) task. B) Percent of mice that reached acquisition criteria (>80% accuracy) across the 10 days of learning a PWD task. C) Total number of completed trials, D) session length, and E) average latency to respond for all correct trials, reward across 10 days of learning a PWD task; n = 10–12 mice/group; +, 0.3 mg/kg; ^, 1 mg/kg; *, 3 mg/kg; &, 10 mg/kg, p < 0.05 compared to vehicle-treated mice on the same day. All data shown as group average (±SEM).
To examine effects of VU0467154 on acquisition, the percentage of each animals per day in each treatment group that acquired the test (>80% accuracy) was plotted as a survival curve. As shown in Fig. 1B and 50% and 80% of the mice treated with 1 and 3 mg/kg VU0467154 acquired this task in 5 and 6 days respectively, compared to 7 and 8 days for vehicle-treated mice.
We also examined the number of completed trials, overall session length, average response latency on correct trials and average reinforcer retrieval latency to provide measures of motor function or motivation to respond that may indirectly influence learning. As shown in Fig. 1C, there was a significant effect of dose (F4,499 = 18.96, p< 0.0001) and day (F9,499 = 2.70, p<0.01) but not an interaction (F36,499 = 0.98, p > 0.05) on number of trials completed. Mice receiving 10 mg/kg VU0467154 completed fewer trials compared to vehicle-treated mice on days 2–5 and day 8; p < 0.05). As shown in Fig. 1D, there was a significant effect of dose (F4,499 = 43.63, p < 0.0001) and day (F9,499 = 12.34, p< 0.0001) but not an interaction (F36,499 = 0.92, p > 0.05) on session length such that treatment with VU0467154 increased total session length. Mice receiving VU0467154 took longer to complete each session compared to vehicle-treated mice on the following days; 0.3 mg/kg, days 1–4; 3.0 mg/kg, days 3 and 7; 10 mg/kg, days 1–9; all p < 0.05). There was also a significant effect of dose (F4,498 = 29.07, p< 0.0001) but not day (F9,498 = 1.88, p > 0.05) nor an interaction (F36,498 = 1.05, p > 0.05) on correct response latency (Fig. 1E), such that 10 mg/kg VU0467154 significantly increased latency (days 1–5 and 8 significantly different from vehicle-treated mice; p < 0.05). Similarly, there was a significant effect of dose (F4,496 = 7.61, p< 0.0001) but not day (F9,496 = 1.73, p > 0.05) nor an interaction (F36,496 = 0.68, p > 0.05) on reinforcer retrieval latency such that 10 mg/kg increased latency on days 3 and 4, while lower doses did not affect retrieval latency (data not shown). In general, effects were dose-dependent and driven by the 10 mg/kg treatment group, suggesting that, at the highest dose tested, nonselective disruptive effects on motor output or motivation may contribute to lack of cognitive enhancing effects; however, in all cases these effects dissipated across the 10 days.
These studies are the first to report effects of repeated administration of an M4 PAM on measures of cognition. To confirm that effects on cognition were not influenced by pharmacokinetic factors (e.g. cytochrome P450 auto-induction or steady-state accumulation), a subset of mice from this pairwise discrimination study was administered VU0467154 for an additional 4 days (total 14 days of dosing), followed by measurement of plasma and brain levels of VU0467154 2.5 h after the last dose of VU0467154 was administered. As shown in Table 1, repeated administration of 1, 3, and 10 mg/kg VU0467154 produced 1.2, 2.2, and 4.2 nM calculated unbound brain concentrations. The 2.5 h post-final dosing time point was chosen to correspond with the previously reported time point for assessment of plasma and brain concentrations following a single administration of 1, 3, and 10 mg/kg VU0467154 in mice, which resulted in 1.0, 2.2, and 5.6 nM calculated unbound brain concentrations (Bubser et al., 2014). Comparing the log-transformed molar concentrations for the present pharmacokinetic data with our previously published acute treatment data, the difference between acute and repeated administration for 10 mg/kg VU0467154 revealed a minimal difference (~2%) between unbound brain concentrations. Thus, once daily dosing produced plasma/ brain concentrations similar to those observed from a single dose of VU0467154, consistent with the once daily dosing interval (24 h) encompassing ≥5 half-lives, and thereby precluding steady-state accumulation.
Table 1.
In vivo plasma and brain concentrations following once daily dosing of VU0467154 for 10 days. Arithmetic mean (±SD) of total and calculated unbound plasma and brain concentrations of VU0467154 in wildtype mice 2.5 h after intraperitoneal administration of 1, 3 or 10 mg/kg VU0467154 once daily for 14 days; n = 4–5/group. Kp, Kp,uu, total and calculated unbound brain/plasma partition coefficients. Mouse plasma and brain unbound fractions, 0.022 and 0.014, respectively.
| 1 mg/kg | 3 mg/kg | 10 mg/kg | ||||
|---|---|---|---|---|---|---|
| total | unbound | total | unbound | total | unbound | |
| plasma [nM] | 383.8 (268.8) | 8.4 | 1239.7 | 27.3 | 3034.3 | 66.8 |
| (5.9) | (539.0) | (11.9) | (1350.8) | (29.7) | ||
| brain [nM] | 84.3 | 1.2 | 159.1 | 2.2 | 304.8 | 4.2 |
| (0.5) | (0.01) | (62.4) | (0.9) | (145.5) | (2.0) | |
| Kp | 0.22 | 0.13 | 0.10 | |||
| Kp,uu | 0.14 | 0.08 | 0.06 | |||
3.2. Assessment of the effects of repeated (10 days) dosing of VU0467154 on rate of learning and acquisition of a pairwise discrimination task in M4 KO mice
To determine whether enhancement of the rate of learning a pairwise discrimination task by VU0467154 was mediated through potentiation of M4 mAChRs, we administered vehicle or 3 mg/kg VU0467154 60 min before test sessions for 10 consecutive days in M4 KO mice. As shown in Fig. 2A, over the 10-day period, there was a significant increase in percent accuracy (F9,135 = 16.76; p< 0.0001), but no effect of VU0467154 treatment (F1,15 = 1.77; p > 0.05), nor a time × treatment interaction (F9,135 = 0.31; p > 0.05). There were no days on which VU0467154-treated mice differed from vehicle-treated mice (all p > 0.05). As shown in Fig. 2B, by day 10, 30% of the vehicle-treated mice and 60% of the VU0467154-treated mice had acquired the discrimination. 2 of the total 19 mice that did not improve above 55% accuracy across the 10 days were excluded from overall data analysis. There was a significant effect of day (F9,135 = 15.79, p < 0.0001), but not treatment group (F1,15 = 0.15, p > 0.05), nor an interaction (F9,135 = 0.46, p > 0.05) on session length (Fig. 2C). In addition, there were significant effects of treatment group (F1,149 = 11.32, p< 0.001) and day (F9,149 = 2.19, p<0.05), but no interaction (F9,149 = 0.38, p > 0.05) on correct response latency (Fig. 2D); both measures decreased across sessions and post-hoc tests did not reveal specific days on which the treatment groups (Vehicle or 3 mg/kg VU0467154) were significantly different from each other. There were no differences in number of trials completed or reinforcer retrieval latency between treatment groups (not shown).
Fig. 2.
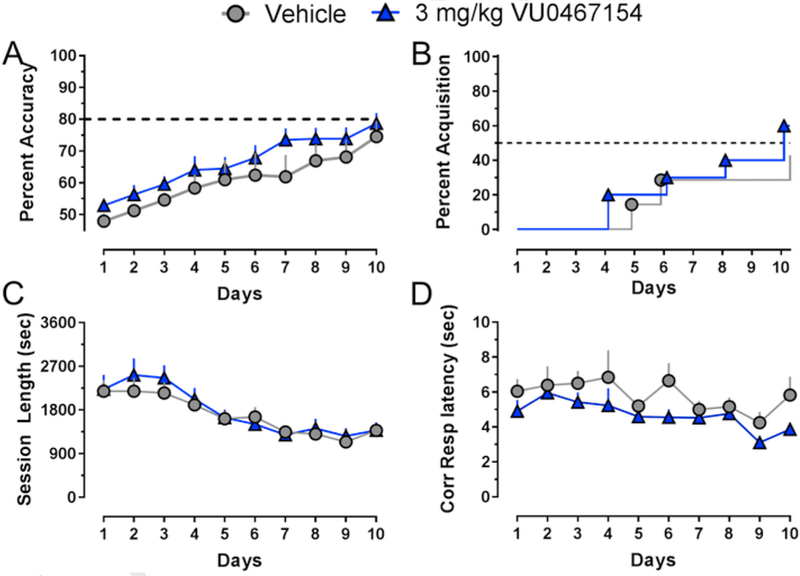
VU0467154 does not improve rate of acquisition of a pairwise discrimination task in M4 KO mice when administered 60 min prior to each daily test session. A) Percent accuracy across the first 10 days of learning a pairwise discrimination (PWD) task. B) Percent of mice that acquired (>80% accuracy) across the 10 days of learning a PWD task. C) Average total session length, D) average latency to respond for all correct trials across 10 days of learning a PWD task; n = 7–10 mice/group. All data shown as group average (±SEM).
3.3. Assessment of the effects of repeated (10 days) dosing of VU0467154 on acquisition versus consolidation of a pairwise discrimination task in wildtype mice
To determine whether enhancement of the rate of learning a pair-wise discrimination task by VU0467154 was through acquisition or consolidation of memory, we administered 1 mg/kg VU0467154 60 min before or immediately after each session for 10 consecutive days. As shown in Fig. 3A, over the 10-day period, there was a significant increase in percent accuracy over time (F9,209 = 25.95; p < 0.0001), and a significant effect of treatment group (F2,209 = 17.65; p< 0.0001), but no significant interaction (F18,209 = 0.66; p> 0.05). Holm-Sidak post-hoc analysis revealed that mice treated with 1 mg/kg VU0467154 before daily sessions demonstrated a significant improvement in rate of learning, as shown by significantly higher percent accuracy on days 2–5. Mice treated with 1 mg/kg VU0467154 after each session demonstrated a significant improvement on day 3, compared to the vehicle-treated wildtype mice (all p < 0.05); there were no significant differences between groups treated with VU0467154. As shown in Fig. 3B and 50% of mice dosed with VU0467154 before and after daily sessions acquired the discrimination on days 3 and 4, respectively, compared to 6 days in the vehicle-treated group.
Fig. 3.
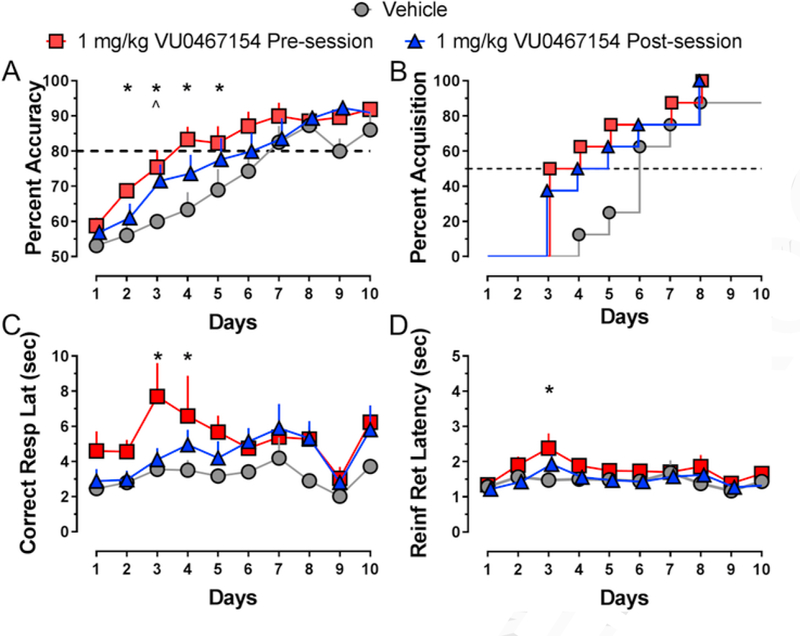
VU0467154 improves rate of acquisition of a pairwise discrimination task in wildtype mice (A, B) when administered 60 min prior to (red squares) or immediately following (blue triangles) each daily test session. A) Percent accuracy across the first 10 days of learning a pairwise discrimination (PWD) task. B) Percent of mice that acquired (>80% accuracy) across the 10 days of learning a PWD task. C) Average latency to respond for all correct trials, D) and average latency to retrieve the reinforcer after each correct trial across 10 days of learning a PWD task; n = 8 mice/group; *, 1 mg/kg pre-session; ^, 1 mg/kg post-session, p < 0.05 compared to vehicle-treated mice on the same day. All data shown as group average (±SEM).
There was a significant effect of treatment group (F2,205 = 13.64, p< 0.0001) and day (F9,205 = 2.76, p<0.01), but no interaction (F18,205 = 0.55, p > 0.05) on correct response latency (Fig. 3C). Correct response latency was higher in mice treated with VU0467154 before the session on day 3 and 4 compared to vehicle-treated groups (p < 0.05). There was a significant effect of treatment group (F2,204 = 8.83, p < 0.001) and day (F9,204 = 3.10, p < 0.05) but not an interaction (F18,204 = 0.55, p>0.05) on reinforcer retrieval latency (Fig. 3D). Reinforcer retrieval latency was higher on day 3 in mice administered VU0467154 before the session compared to vehicle (p < 0.05). However, similar to our previous data (Fig. 1), significant effects on latency were driven by decreases across the 10-day period. There were no differences in number of trials completed or overall session length between treatment groups.
3.3.1. Assessment of the effects of acute (1 day) versus repeated (10 days) dosing of VU0467154 on the acquisition of memory using a cue-dependent conditioned freezing paradigm
Of the 72 mice that completed the study, 9 were excluded from statistical analysis that demonstrated <20% freezing on the test day (3 from the 10-day repeated Vehicle treatment group, 2 from each of the 10-day repeated 3 and 10 mg/kg VU0467154 treatment group, and 1 mouse each from the acute 3 and 10 mg/kg VU0467154 treatment group). As shown in Fig. 4B, there was a significant main effect of treatment group (F4,56 = 2.67, p < 0.05). Both the group that was administered 10 mg/kg VU0467154 only on Day 10 (acute group) and the group that was administered 10 mg/kg VU0467154 once-daily for 10 days were significantly different from the group of mice that were administered vehicle for 10 days (both p < 0.05).
Fig. 4.
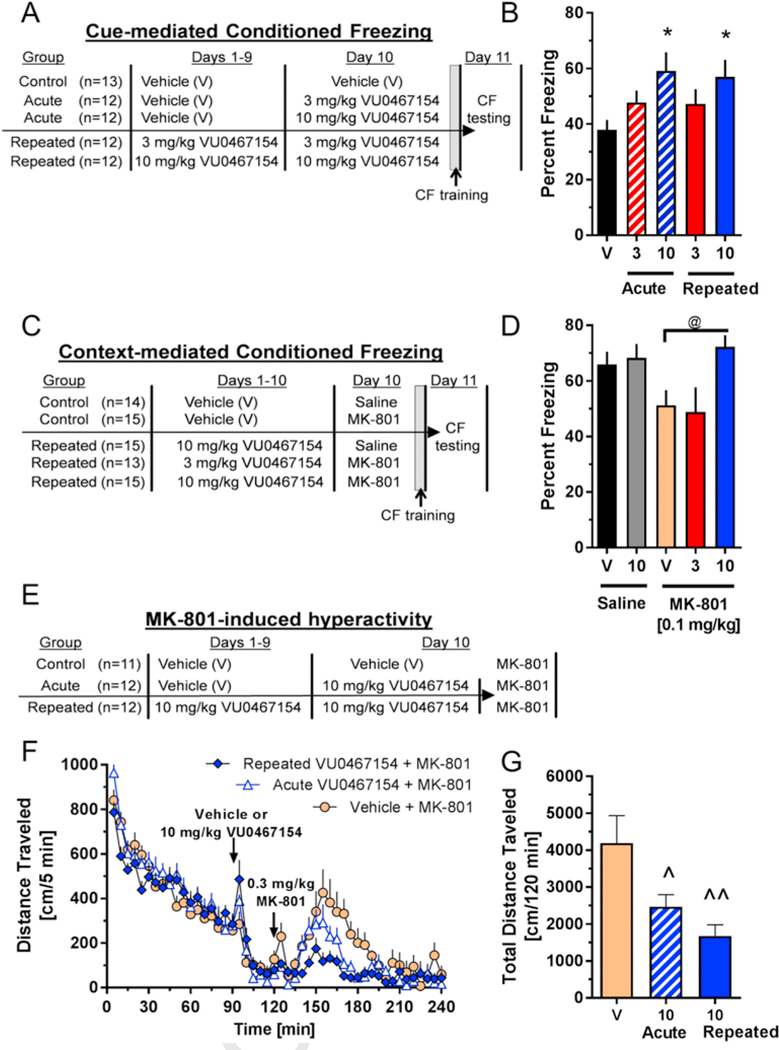
Tolerance does not develop to the behavioral effects of VU0467154 following 10-day, once daily dosing. (A,C,E) Schematics detailing treatment groups for examining the effects of 10-day, once-daily dosing of VU0467154 on A,B) cue-mediated conditioned freezing, C,D) MK-801-induced disruptions of context-mediated conditioned freezing, and E-G) MK-801-induced hyperlocomotion. B,D) Percent freezing during the 8-min session examined 24 h after training. F) Distance travelled in cm/5 min bins, and G) total distance travelled in the 120 min following MK-801 administration; n = 11–12/group per treatment group; *p < 0.05 compared to the group of mice that were administered vehicle for 10 days; @, p< 0.05 compared to the group of mice that were administered vehicle for 10 days and MK-801 acutely prior to conditioning; ^,p < 0.05, ^^ p< 0.01 compared to vehicle + MK-801 treatment group. All data shown as means (±SEM; n = 11–15/group).
3.3.2. Assessment of the effects of repeated (10 days) dosing of VU0467154 on MK-801-induced-disruptions of the acquisition of memory in the contextual conditioned freezing
As shown in Fig. 4D, there was a significant main effect of treatment group (F4,67 = 3.37, p < 0.05). The group of mice that was administered 10 mg/kg VU0467154 once-daily for 10 days and then administered 0.1 mg/kg MK-801 on Day 10 was significantly different from the group of vehicle-treated mice that was acutely administered MK-801 (p< 0.05).
3.3.3. Assessment of the effects of acute (1 day) versus repeated (10 days) dosing of VU0467154 on reversal of MK-801-induced hyperlocomotion
As shown in Fig. 4E/F, pretreatment with a dose of 10 mg/kg VU0467154 administered once (p< 0.05) or once daily for 10 days significantly (p < 0.01) attenuated MK-801-induced hyperlocomotion when summed across the 120 min period following MK-801 administration (F2,32 = 6.91, p<0.01); activity was not different between the acute and repeated VU0467154-treatment groups. Further analysis based on summed data in 30-min intervals (allowing for comparison of the onset and offset of MK-801’s effects) yielded similar results (e.g. both 10-day, once-daily dosing and single, acute dosing with VU0467154) attenuated MK-801’s effects yet were not different from each other; data not shown).
4. Discussion
Selective potentiation of M4 mAChRs represents an important novel mechanism for the potential treatment of multiple symptom clusters associated with schizophrenia, including positive symptoms and cognitive impairments. Given the dearth of M4-selective ligands with suitable pharmacokinetic properties for in vivo use until recently, all previous preclinical studies have focused on evaluations of efficacy using only acute dosing paradigms. The present studies provide the first demonstration that the acute cognitive enhancing and antipsychotic-like activity produced by selective activation of the M4 mAChR using an M4 PAM are not subject to the development of tolerance under a 10-day, once-daily dosing regimen in mice. Interestingly, these effects were observed in both hippocampal and non-hippocampal mediated cognitive task when administered alone or after pharmacologic challenge with MK-801. Moreover, these findings indicate a potential role for the modulation of M4 mAChRs in both the acquisition and consolidation of memory functions. Together, these studies extend accumulating evidence supporting future development of selective M4 PAMs for the treatment of cognitive deficits and positive symptoms observed in various neuropsychiatric disorders, such as schizophrenia.
Our current data show that repeated administration of the M4 PAM VU0467154 did not result in the development of tolerance to the behavioral effects observed across several preclinical models of cognition and antipsychotic-like activity relevant to schizophrenia. Although the development of tolerance for novel therapeutics represents an important potential source of failure in clinical trials, it is infrequently and/or insufficiently examined in preclinical studies (Bespalov et al., 2016). In general, there are three different types of tolerance that may affect perceived efficacy with a novel drug mechanism, including pharmacokinetic (altered metabolism or drug disposition), pharmacodynamic (altered response of the receptor system), and/or learned/behavioral tolerance (altered response within a specific drug-related context usually to overcome a detrimental effect on behavior) (Bespalov et al., 2016). With regard to pharmacokinetic tolerance, the brain and plasma concentrations (and brain:plasma distribution ratios) of VU0467154 after 14 consecutive days of dosing were comparable to previously reported levels after acute administration (see Table 3 from, Bubser et al., 2014). These pharmacokinetic findings are in agreement with previous results suggesting a lack of cytochrome P450 induction by VU0467154 that could increase metabolism/elimination and confirmed that little or no accumulation at steady-state would occur from a once daily IP administration schedule (Bubser et al., 2014). To assess possible induction of pharmacodynamic tolerance, we administered VU0467154 in the homecage for 9 days and then examined the ability of VU0467154 on the 10th day of dosing to acutely 1) enhance the acquisition of memory, 2) attenuate MK-801-induced disruptions in the acquisition of memory, and 3) attenuate MK-801-induced hyperlocomotion. Importantly, as shown in each of these experiments, we observed similar effects after repeated dosing as observed with a single, acute administration of VU0467154. Consistent with this approach, once-daily, repeated administration with the M4 PAM VU0467154 when initiated at presymptomatic ages in the YAC128 mouse model of Huntington’s Disease improved both motor and synaptic deficits after 5 months of daily dosing (Pancani et al., 2015). Based on the current and previous findings with repeated dosing of VU0467154, it will be important in future studies to further explore the potential for sustained efficacy and/or disease modification without the development of tolerance in more genetically relevant models of schizophrenia.
The present studies provide the first demonstration of improve cognitive performance in a preclinical learning and memory task with once-daily dosing of an M4 PAM over multiple days. As shown in the dose-response determination (Fig. 1) and follow-up study (Fig. 3), VU0467154 significantly accelerated rates of learning and acquisition by 2–3 days in both experiments, demonstrating the reproducibility of a robust improvement on memory with once-daily repeated dosing. These improvements in cognitive performance were also absent in the M4 KO mice indicating that the effects of VU0467154 are mediated through actions at M4 mAChRs. By assessing alterations induced by VU0467154 over multiple days of the acquisition phase in the pairwise discrimination task, we controlled for several sources of variability often associated with acute cognition studies, including individual differences and fluctuations in baseline performance, or the need to introduce a cognitive disruptor to artificially increase the signal window to examine cognitive enhancing effects. Our data build on several previously reported studies that examined the acute effects of M4 mAChR potentiation on various memory tasks. For example, the M4 PAM VU0152100 enhanced acquisition of performance in an object recognition task following a 5 min delay, a classic preclinical model of working memory. We previously reported that the M4 PAM VU0467154 also acutely enhanced acquisition of cue-mediated conditioned freezing following a 24-hr delay and reversal of MK-801-induced acute deficits in the acquisition of contextual-mediated conditioned freezing tasks (Bubser et al., 2014), two well-validated animal models of long-term, non-hippocampal and hippocampal mediated memory functions, respectively.
The current studies are also the first to indicate that memory enhancing effects of M4 mAChR PAMs may be mediated in part through mechanisms of memory consolidation versus specific encoding or acquisition. While enhanced muscarinic cholinergic function is critical for acquisition of new memory formation, previous pre-clinical and clinical studies have demonstrated that decreased cholinergic tone is critical for memory consolidation, particularly sleep-dependent memory consolidation (Gais and Born, 2004a, b; Hasselmo and Sarter, 2011). For example, scopolamine, a nonselective mAChR antagonist, has shown differential effects on memory using a conditioned freezing paradigm depending on the time of dosing relative to the training conditions. When administered prior to training, scopolamine disrupts memory retention 24 h later (affecting acquisition of memory), yet when administered after the training period, facilitates memory retention, presumably through enhancing memory consolidation (Rasch et al., 2006; Winters et al., 2006).
Declarative memory, one of the most disrupted cognitive domains in schizophrenia (Green et al., 2000), is improved following slow wave-rich sleep compared to rapid eye movement-rich sleep in healthy subjects (Plihal and Born, 1997). Moreover, declarative memory is enhanced by increasing slow wave activity during slow wave sleep via transcranial direct current stimulation in patients with schizophrenia (Goder et al., 2013). Recently, we reported that, in healthy rats, VU0467154 dose-dependently increases the ratio of slow wave sleep to rapid eye movement sleep (Gould et al., 2016). Given the pharmacokinetic profile of VU0467154 in mouse (Tmax= 1 h, from IP suspension dose; elimination t1/2 = 4.7 h), mice receiving 1 mg/kg VU0467154 prior to each pairwise discrimination session would have peak concentrations of VU0467154 in the brain at the initiation of the session, and would still exhibit pharmacologically relevant concentrations of VU0467154 in the brain for several hours after the session. Mice are nocturnal and spend a large percentage of time sleeping during light periods. Since these studies were conducted during the light phase, mice presumably cycled in and out of sleep after each daily test session, while relevant concentrations of VU0467154 were present in the brain. In contrast, in mice dosed with VU0467154 immediately after the session, concentrations in the brain would not peak for ~1 h. Thus, the present effects may be affecting memory consolidation. Although recent studies have shown the acetylcholinesterase inhibitor donepezil can still enhance acquisition even when administered immediately after an object retrieval task in rats, these post-test effects on acquisition were concluded to have occurred only when relevant concentrations reached the brain with 10 min post training (Akkerman et al., 2016). Thus, VU0467154 may be affecting memory acquisition (during task performance) as well as consolidation (period directly after task performance thought to last ~1–6 h). In future studies, it will be important to further dissect the role of M4 mAChRs in memory acquisition and consolidation by studying the effects of an equipotent M4 PAM possessing a shorter elimination half-life but an otherwise similar disposition in mouse (not yet available), or by administering an M4 selective antagonist immediately after test sessions, to restrict M4 mAChR activation to the acquisition phase of the test.
Understanding the role of M4 mAChR potentiation in specific neural circuits underlying the cognitive enhancing effects in the pairwise discrimination and cue- and context-mediated conditioned freezing tasks holds potential promise for advancement of novel treatment approaches for the cognitive impairments in schizophrenia. Currently available typical and atypical APDs provide little, if any, therapeutic benefits for the cognitive deficits observed in individuals with schizophrenia, and may even further exacerbate cognitive disruption (Miyamoto et al., 2012; Price et al., 2014). The current validation of the cognitive enhancing effects of the M4 PAM mechanism across 3 distinct cognitive assays that are in part dependent on different brain regions (Sacchetti et al., 2002; Zhang et al., 2001) after administration alone or in combination with a pharmacological challenge using both Pavlovian conditioning and operant approaches under conditions of a 10-day, once-daily dosing regimen provides further support for the clinical development of M4 PAMs to address one or more of cognitive impairments observed in individuals with schizophrenia. Interestingly, M4 mAChRs are expressed throughout limbic and cortical brain regions known to be disrupted in individuals with schizophrenia, including the striatum, hippocampus, and cortex (Hersch and Levey, 1995; Levey et al., 1991; Rouse et al., 1999), and can function as autoreceptors in the striatum and midbrain (Tzavara et al., 2004; Zhang et al., 2002), as well as postsynaptic modulatory receptors in the striatum, neocortex, and hippocampus (Levey et al., 1991; Zang and Creese, 1997). Since efficacy was observed after 10-day, once daily administration of 10 mg/kg VU0467154 on enhancing the acquisition of memory in the cue-mediated conditioned freezing task and reversing both MK-801-induced impairments in the acquisition of a context-mediated conditioned freezing paradigm and induction of hyperactivity, these findings are consistent with the interpretation that selective M4 PAMs appear to provide a sufficient therapeutic index for potentially improving some of the positive and cognitive symptoms observed schizophrenia within the same dose range. However, based on the U-shaped dose response of the effects of VU0467154 in the acquisition of the visual pairwise discrimination task, it is also possible that for some aspects of cognition performance, lower doses of the M4 PAMs may be more optimal for boosting cognitive performance. Consistent with many cognition studies (Bentley et al., 2011), VU0467154 engendered an “inverted-U” dose response curve in the pairwise discrimination task, such that the top dose of 10 mg/kg VU0467154 actually decreased the number of trials completed and increased total session duration. This disruption in the pairwise discrimination may be attributed to one or more variables, including dose-limiting effects on general motor output or motivation, or simply reductions in the number of trials completed and subsequently chances for stimulus-reinforcement associations. Nevertheless, these effects of 10 mg/kg VU0467154 dissipated across the 10-day study in this assay suggesting a potential for learned/behavioral tolerance to the effect that was inhibiting maximum reinforcement. In contrast, the 10-day, once-daily administration of 10 mg/kg VU0467154 enhanced memory performance in the two conditioned freezing tasks when measured 24-hrs after the last dose of VU0467154 has been eliminated, suggesting that these effects are not attributed to motoric effects or altered motivation, possible effects that would hinder operant-based tasks. While the mechanism underlying these “inverted-U” effects remains unclear, recent studies suggest that there is a critical balance between modulation of phasic versus tonic cholinergic signaling for optimal signal detection and consolidation new acquired signals that appears to be both dose-dependent and brain region-specific manner (Howe et al., 2017; Sarter et al., 2016). Overall, this broader range of potential therapeutic utility (APD-like and cognitive enhancing effects) observed with the M4 PAM mechanism in the present studies may ultimately provide clinicians with an opportunity to titrate doses to treat multiple symptom clusters instead of titrating to maximizing therapeutic effects while minimizing adverse effects.
In summary, the present findings provide compelling evidence that selective potentiation of M4 mAChRs enhances multiple aspects of learning and memory and antipsychotic-like activity with repeated administration over multiple days. Ongoing studies are currently investigating the impact of selective potentiation of M4 mAChRs by VU0467154 on additional cognitive domains, including attention and executive function that are also reported to be impaired in patients with schizophrenia. In addition, future studies will need to extend these findings to longer dosing regimens, compare the current once-daily dosing regimen to a dosing paradigm that provides 24-hr steady state exposure and determine whether the effects of M4 mAChR modulation are similar in males and females.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health National Institutes of Mental Health [Grants MH086601(CKJ), MH073676 (PJC), MH093366 (PJC) MH087965 (PJC)], AstraZeneca and a PhRMA Foundation postdoctoral fellowship grant in Pharmacology and Toxicology (RWG). We thank Weimin Peng, Josh Luffman, Zhuoyan Lu and Nathan Iyer for technical assistance. Studies were performed in part through the use of the Murine Neurobehavior Core laboratory at the Vanderbilt University Medical Center.
Abbreviations:
- mAChR
muscarinic acetylcholine receptor
- PAM
positive allosteric modulator
- NMDAR
N-methyl-D-aspartate subtype of the glutamate receptor
- LC-MS/MS
liquid chromatography tandem mass spectrometry
Footnotes
Financial disclosures
The authors declare the following competing financial interest(s): M.B., T.M.B., C.M.N., C.W.L., P.J.C., and C.K.J. received research/salary support from AstraZeneca and/or Bristol Myers Squibb. T.M.B., C.M.N., C.W.L., and P.J.C. are inventors on multiple composition of matter patents protecting allosteric modulators of GPCRs. M.W.W., N.J.B. and M.E.D. were employees of AstraZeneca when studies were conducted. The remaining authors declare no competing financial interests.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2017.07.013.
Uncited references
References
- Akkerman S, Blokland A, Prickaerts J, 2016. Possible overlapping time frames of acquisition and consolidation phases in object memory processes: a pharmacological approach. Learn Mem. 23, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Corlett PR, Cole MW, et al. , 2015. N-methyl-d-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol. Psychiatry 77, 569–580. [DOI] [PubMed] [Google Scholar]
- APA, 2000. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Washington, DC. [Google Scholar]
- Barch DM, Ceaser A, 2012. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 16, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ, 2011. Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog. Neurobiol. 94, 360–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Muller R, Relo AL, et al. , 2016. Drug tolerance: a known unknown in translational neuroscience. Trends Pharmacol. Sci. 37, 364–378. [DOI] [PubMed] [Google Scholar]
- Blot K, Bai J, Otani S, 2013. The effect of non-competitive NMDA receptor antagonist MK-801 on neuronal activity in rodent prefrontal cortex: an animal model for cognitive symptoms of schizophrenia. J. Physiol. Paris 107, 448–451. [DOI] [PubMed] [Google Scholar]
- Bobes J, Garcia-Portilla MP, Bascaran MT, et al. , 2007. Quality of life in schizophrenic patients. Dialogues Clin. Neurosci. 9, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, et al. , 1987. Identification of a family of muscarinic acetylcholine receptor genes. Science 237, 527–532. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Young AC, Brann MR, et al. , 1988. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron 1, 403–410. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, et al. , 2008. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J. Pharmacol. Exp. Ther. 327, 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubser M, Bridges TM, Dencker D, et al. , 2014. Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem. Neurosci. 5, 920–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun NE, Grannan M, Bubser M, et al. , 2014. Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology 39, 1578–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW, 2009. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, et al. , 2012. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb. Exp. Pharmacol. 267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Born J, 2004a. Declarative memory consolidation: mechanisms acting during human sleep. Learn Mem. 11, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Born J, 2004b. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 101, 2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway CR, Lebois EP, Shagarabi SL, et al. , 2014. Effects of selective activation of M1 and M4 muscarinic receptors on object recognition memory performance in rats. Pharmacology 93, 57–64. [DOI] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, et al. , 2013. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr. Res. 144, 153–154. [DOI] [PubMed] [Google Scholar]
- Gould RW, Dencker D, Grannan M, et al. , 2015. Role for the M1 muscarinic acetylcholine receptor in top-down cognitive processing using a touchscreen visual discrimination task in mice. ACS Chem. Neurosci. 6, 1683–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Nedelcovych MT, Gong X, et al. , 2016. State-dependent alterations in sleep/wake architecture elicited by the M4 PAM VU0467154-Relation to antipsychotic-like drug effects. Neuropharmacology 102, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, 1996. What are the functional consequences of neurocognitive deficits in schizophrenia?. Am. J. Psychiatry 153, 321–330. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, et al. , 2000. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff’?. Schizophr. Bull. 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK, 2004. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 72, 41–51. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M, 2011. Modes and models of forebrain cholinergic neuro-modulation of cognition. Neuropsychopharmacology 36, 52–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Levey AI, 1995. Diverse pre- and post-synaptic expression of m1-m4 muscarinic receptor proteins in neurons and afferents in the rat neostriatum. Life Sci. 56, 931–938. [DOI] [PubMed] [Google Scholar]
- Horner AE, Heath CJ, Hvoslef-Eide M, et al. , 2013. The touchscreen operant platform for testing learning and memory in rats and mice. Nat. Protoc. 8, 1961–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Gritton HJ, Lusk NA, et al. , 2017. Acetylcholine release in prefrontal cortex promotes gamma oscillations and theta-gamma coupling during cue detection. J. Neurosci. 37, 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CK, Byun N, Bubser M, 2012. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 37, 16–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois EP, Bridges TM, Lewis LM, et al. , 2010. Discovery and characterization of novel subtype-selective allosteric agonists for the investigation of M1 receptor function in the central nervous system. ACS Chem. Neurosci. 1, 104–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, et al. , 1991. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 11, 3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur RA, Gray J, Schreiber R, 2010. Cognitive effects of muscarinic M1 functional agonists in non-human primates and clinical trials. Curr. Opin. Investig. Drugs 11, 740–760. [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, et al. , 2012. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 17, 1206–1227. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, et al. , 2004. Identification of separable cognitive factors in schizophrenia. Schizophr. Res. 72, 29–39. [DOI] [PubMed] [Google Scholar]
- Pancani T, Foster DJ, Moehle MS, et al. , 2015. Allosteric activation of m4 muscarinic receptors improve behavioral and physiological alterations in early symptomatic YAC128 mice. Proc. Natl. Acad. Sci. U. S. A. 112 (45), 14078–14083, November 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plihal W, Born J, 1997. Effects of early and late nocturnal sleep on declarative and procedural memory. J. Cogn. Neurosci. 9, 534–547. [DOI] [PubMed] [Google Scholar]
- Price R, Salavati B, Graff-Guerrero A, et al. , 2014. Effects of antipsychotic D2 antagonists on long-term potentiation in animals and implications for human studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 54, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch BH, Born J, Gais S, 2006. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J. Cogn. Neurosci. 18, 793–802. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, et al. , 1999. Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci. 64, 501–509. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Baldi E, Lorenzini CA, et al. , 2002. Differential contribution of some cortical sites to the formation of memory traces supporting fear conditioning. Exp. Brain Res. 146, 223–232. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Berry AS, et al. , 2016. What do phasic cholinergic signals do?. Neurobiol. Learn Mem. 130, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Potter WZ, Lightfoot J, et al. , 2008. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Shirey JK, Xiang Z, Orton D, et al. , 2008. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat. Chem. Biol. 4, 42–50. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, et al. , 2004. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 18, 1410–1412. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ, 2006. Paradoxical facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. J. Neurosci. 26, 9520–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Z, Creese I, 1997. Differential regulation of expression of rat hippocampal muscarinic receptor subtypes following fimbria-fornix lesion. Biochem. Pharmacol. 53, 1379–1382. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J, 2001. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav. Brain Res. 126, 159–174. [DOI] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, et al. , 2002. Characterization of central inhibitory muscarinic autoreceptors by the use ofmuscarinic acetylcholine receptor knock-out mice. J. Neurosci. 22, 1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


