Abstract
Purpose of review:
Primary graft dysfunction is an acute lung injury syndrome occurring immediately following lung transplantation. This review aims to provide an overview of the current understanding of PGD, including epidemiology, immunology, clinical outcomes and management.
Recent findings:
Identification of donor and recipient factors allowing accurate prediction of PGD has been actively pursued. Improved understanding of the immunology underlying PGD has spurred interest in identifying relevant biomarkers. Work in PGD prediction, severity stratification and targeted therapies continue to make progress. Donor expansion strategies continue to be pursued with ex vivo lung perfusion playing a prominent role. While care of PGD remains supportive, ECMO has established a prominent role in the early aggressive management of severe PGD.
Summary:
A consensus definition of PGD has allowed marked advances in research and clinical care of affected patients. Future research will lead to reliable predictive tools, and targeted therapeutics of this important syndrome.
Keywords: Primary graft dysfunction, Lung Transplantation, ex-vivo lung perfusion, acute lung injury, ischemia-reperfusion injury
Definition and Diagnosis:
Primary graft dysfunction (PGD) is a syndrome of acute lung injury beginning immediately after allograft transplantation.1 This syndrome had initially been ambiguously defined and was known as ischemia-reperfusion injury, re-implantation response, reperfusion edema, early graft dysfunction, and post-transplant acute respiratory distress syndrome (ARDS), among many other similar names. This ambiguity led to confusion which prompted the development of the first consensus definition by the International Society of Heart and Lung Transplantation (ISHLT) in 2005.2 This initial working group was convened to create a definition with the express purpose of defining standardized criteria for PGD in order to accurately capture the spectrum of this syndrome and to facilitate future clinical, epidemiological and translational research.3 The 2005 definition graded disease severity based upon the presence of radiographic findings and the degree of hypoxemia as determined by PaO2/FiO2 (P/F) ratio.3 (TABLE 1) This initial definition was clinically validated with a number of epidemiologic studies demonstrating correlation with disease presence and severity.4–6
Table 1.
2005 ISHLT consensus definition and severity grading for PGD and 2016 update.1
| PGD stage | P/F ratio (mmHg) | Chest radiography | Updates from 2016 Consensus Group |
|---|---|---|---|
| 0 | >300 | Normal | Any P/F ratio |
| 1 | >300 | Diffuse allograft infiltration/pulmonary oedema | No changes |
| 2 | 200–300 | Diffuse allograft infiltration/pulmonary oedema | No changes |
| 3 | <200 | Diffuse allograft infiltration/pulmonary oedema | No changes |
ISHLT, the International Society for Heart and Lung Transplantation; PGD, primary graft dysfunction; P/F, PaO2/FiO2.
As transplant medicine and critical care practice7 have evolved, questions with the PGD criteria have arisen. In 2016, an iterative update of the PGD guidelines was released (TABLE 1). These guidelines stressed the importance of radiographic changes of bilateral alveolar infiltrates in addition to hypoxemia when assigning a severity grade. Diagnosis of PGD requires the exclusion of confounding clinical factors. Potential confounding diagnoses include cardiogenic edema, vascular anastomotic complications, infectious or mechanical airway issues and extra-pulmonary thoracic complications.1
Assessment of lung function begins immediately after reperfusion of the last allograft and is generally measured upon ICU admission. This is considered T0 provided this is within a window of 6 hours from reperfusion. Subsequent grades are measured at 24 hour intervals beginning at T24 and ending at T72. The P/F ratio defines the severity grade for each time point, provided there are appropriate chest radiograph abnormalities.1 Because the transport of patients from the operating room can sometimes lead to derecruitment of the lung, or other rapidly reversible airway pathology which can confound initial PGD assessment, recent evidence suggests later time points may more meaningfully capture short- and long-term clinical outcomes.8,9
The 2016 consensus definition further addressed nuances of PGD measurement. Patients who are not receiving invasive mechanical ventilation should still be graded similar to those receiving invasive ventilation.1 Further, the use of pulmonary specific vasodilators should not affect grading of PGD.1 Finally, the ISHLT consensus definition continues to recommend grading patients receiving extra-corporeal life support (ECLS) as PGD grade 3, provided they have appropriate radiographic abnormalities.1
A recent study by Cantu et al analyzed the construct validity of the 2016 updated PGD definition.10 They demonstrated no difference in mortality between no (grade 0) and mild (grade 1) PGD, raising the question whether grades with P/F ratios >300 should still be considered separately. On the other extreme, addition of a very severe category (P/F ratio <100) did not improve the ability to predict mortality in severe (grade 3) PGD. Additionally, they demonstrated improved mortality discrimination using later individual (T48 or T72) or interval grading (T48–72). Because of concerns raised regarding the effects of single versus double lung transplantation on mortality discrimination, the group evaluated this and found procedure type to have minimal impact. Lastly, while ventilator status had little impact on PGD discrimination, reclassification of patients with severe PGD to no PGD if they were not on mechanical ventilated resulted in a group of patients with intermediate mortality risk between no PGD and severe PGD. This suggests that the P/F ratio should still be used in patients not on mechanical ventilation supporting the revised 2016 definition10.
Despite the progress made to date, a number of issues remain to be addressed in future versions of the PGD definition. These include the impact of ECLS and ex-vivo lung conditioning and the impact of pharmacologic agents used to improve P/F ratio when grading PGD.
Epidemiology and Outcomes – Clinical Significance
Transplant practitioners believe that PGD is an unavoidable potential complication in a lung transplant practice11 and that it has a significant impact on clinical outcomes. Standardization of PGD definition in 2005 allowed for improved outcome monitoring12, as well as assessment of short- and long-term morbidity and mortality.
Prior to the initial consensus definition, heterogeneity in definitions led to a broad range of PGD incidence, ranging from 15–57%.12 Single and multi-center cohorts have reported their respective incidences of PGD, however the ISHLT reports an incidence of ~30% early after transplant and 15–20% grade 3 PGD present at 72 hours.12,13
PGD is associated with both short- and long-term mortality post-transplant. Thirty and 90-day mortality has been shown to increase with worsening PGD severity.8,9,14 A large, multi-center, prospective cohort demonstrated a 23% 90-day and 34% 1-year mortality for patients with PGD 3, as compared with 5% and 11% respectively in those without PGD.15 In a 1000 patient cohort, Kreisel et al demonstrated the impact of PGD on overall mortality. Patients with PGD had 1, 5 and 10 year survival rates of 72.8, 43.9 and 18.7%, compared to 87.1, 59.8 and 35.7% survival in non-afflicted recipients.16 Patients with a phenotype of persistent severe PGD – characterized by PGD grade 3 at T0 and persisting to T72 - have worse 90-day mortality when compared to peers with resolving graft dysfunction.8
PGD has also been shown to be a risk factor for the development of bronchiolitis obliterans syndrome (BOS), a form of chronic lung allograft dysfunction (CLAD). CLAD is the most common cause of long-term mortality in lung transplant recipients, and a major driver of morbidity and decreased quality of life.17 Daud et al demonstrated an increased risk of BOS with increasing PGD severity, up to a relative risk of 2.53 for patients with grade 3 PGD.5
Few studies report the impact of PGD on patient function or quality of life, though one can infer a decreased quality of life based upon the known relationship between PGD and BOS,5 and between BOS and decreased quality of life.18 In terms of functional outcomes, patients with PGD have been shown to have a lower mean forced expiratory volume in 1 second (FEV-1)4, and to have shorter median walk distances on six minute walk tests (6MWT) performed 1-year post transplant when compared to transplant recipients not suffering PGD.19
Risk Factors:
Risk factors for PGD can be divided into those inherent to the donor or to the recipient, and those related to technical aspects of organ procurement, preservation and implantation. (Figure 1) A complex interaction likely exists amongst the many PGD risk factors. An improving understanding of the role of innate and adaptive immunology in the pathophysiology of PGD is emerging, and providing exciting new insights into PGD and novel targets for management. An understanding of these factors allows the transplant team to gauge the degree of risk of PGD in a recipient, and adjust their management appropriately.
Figure 115:
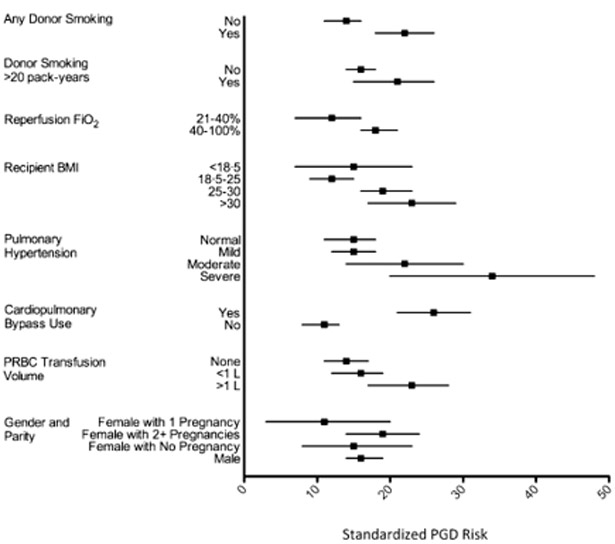
Standardized risk factors for grade 3 PGD. Dots represent the point estimate for adjusted standardized risk from a logistic regression equation. Bar represents 95% confidence intervals. These risks reflect risk if all variables were kept stable, except for the variable of interest.
Donor Risk Factors:
Less than 10% of potential donor lungs meet ideal criteria; therefore, almost all donor lungs have some degree of injury, typically related to the mechanism of death. These may include edema, trauma, aspiration or pulmonary emboli, among other injuries.20,21 Because of increasing numbers of patients awaiting transplant and limited numbers of ideal organs for transplant the concept of extended criteria donors has evolved to allow for the use of organs not meeting traditional ideal criteria.22 Though most would agree that use of these types of donors is safe, patient centered outcomes have been mixed.23,24
Because of these factors, concerted effort has been made to improve donor management strategies to optimize donor use and improve donor quality. Hemodynamic resuscitation with the maintenance of euvolemia and vasopressor support as indicated, and hormonal resuscitation with glucocorticoids are basic tenets of supportive care.25 Low tidal volumes and a higher positive end-expiratory pressure (PEEP) strategy have been shown to increase organ procurement.26 Implementation of an aggressive lung donor management protocol with low tidal volumes, PEEP of 8–10cm H2O, hourly recruitment maneuvers, attention to cardiac performance, maintenance of a low extravascular lung water index, and bronchoscopy to evaluate areas of radiographic abnormalities more than doubled the number of lung donors, with no change in the rate of severe PGD.27
Despite all the work to improve donor quality, there are some factors that cannot be changed with appropriate donor management. Donor cigarette exposure (>20 pack years) has been identified as a major risk factor for PGD; however, it is not clear how the intensity of exposure, or timing of last use affects this risk.15,28,29 Donor traumatic brain injury causing death has been shown to increase risk of PGD in a UNOS database analysis,30 although this association was not borne out in multi-center analyses.15,31 A series of 60 donations following circulatory determination of death (DCD) showed increased risk and severity of PGD, with a greater need for ECLS when compared to propensity matched donations after brain death (DBD).32 However, an Australian series of 70 Maastricht category III DCD donors showed similar incidence of grade 3 PGD and similar 1 and 5 year survival to DBD donors.33 A 2015 meta-analysis of 5 studies likewise found no significant difference between DBD and DCD donors.34 The use of extended criteria donors (ECD) appears to increase risk of PGD, though the emerging use of ex-vivo lung perfusion (EVLP) may mitigate much of this risk.24,35–38 Additional risk factors include increased donor age - although a threshold age has not been fully elucidated14,39,40 - and heavy donor alcohol abuse.41,42 There is an unclear association between donor race and donor sex on risk of PGD.15 Older studies initially found these factors to increase the risk of PGD, however more recent data has not borne out this association.12
Recipient risk factors:
Recipient risk factors are for the most part fixed though they can contribute significantly to the PGD risk assessment. For example, the recipient’s primary lung disease is an important contributor to the development of PGD. Recipients with IPF, sarcoidosis and idiopathic pulmonary arterial hypertension (IPAH) are more likely to develop PGD than those patients transplanted for COPD or cystic fibrosis.15,40,43 Additionally, increasing mean pulmonary artery pressure (mPAP) as occurs with increasing native lung disease severity has been related to increasing risk of PGD,15,43,44 with a 30% increased risk of PGD per 10 mmHg increase in the mean PA pressure (Figure 2). Increased right atrial pressure, pulmonary vascular resistance and left ventricular diastolic dysfunction have also been shown to increase risk of PGD.45,46 It must be pointed out that modifiable factors should be addressed and can potentially mitigate PGD risk. Of these modifiable factors recipient adiposity, as measured by body mass index or by levels of the protein leptin, has been associated with increased PGD risk 15,47,48 but active measures of weight reduction may mitigate this risk. Along these same lines, objective measures of frailty should be addressed and improvement in these measures may also reduce mortality risk.
Figure 245:
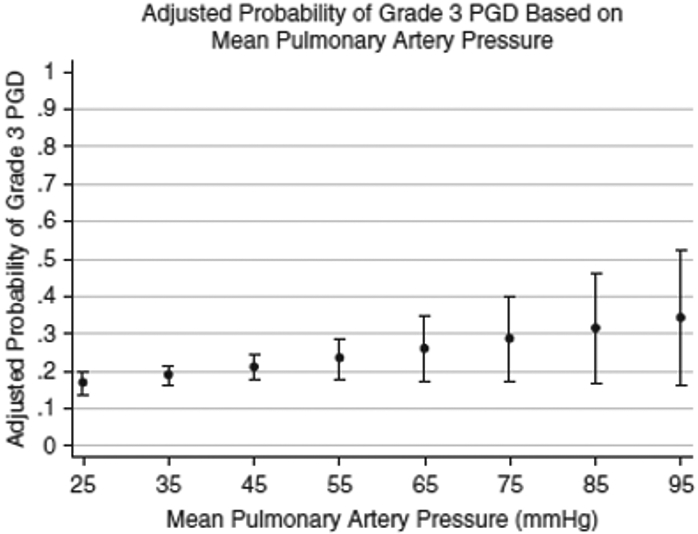
Adjusted probability of grade 3 PGD based on recipient mean pulmonary artery pressure.
Operative Considerations:
The surgical contribution to PGD risk begins at donor procurement. Most of the data supporting the approach to organ preservation comes from animal models. The standard approach uses a low potassium, acellular preservation fluid, with antegrade and retrograde flushing of the pulmonary vasculature, FiO2 of 30–50%, maintenance of TLC near 50% and hypothermic organ preservation at 4–8 degrees Celsius.20,49 Many centers add prostaglandin as an anti-inflammatory, and heparin as an anti-thrombotic to preservation fluid.49 Standardized assessment and procurement practices can play a significant role in PGD risk and with the introduction of (EVLP) expanded donor assessment and potential therapies may further mitigate PGD risks.
Ex vivo organ perfusion was first successfully applied in 1935, but has found renewed interest in the past 20 years,50,51 coincident with efforts to increase the available donor pool including ECD and DCD donations.52 EVLP allows for thorough evaluation and reconditioning of marginal or sub-optimal candidate organs (Figure 3). Centers using EVLP have had increases in transplantation volume up to 33%.53 The largest cohort of lung allograft recipients following use of EVLP failed to demonstrate a statistically significant decrease in the incidence of grade 3 PGD at 72 hours when compared to controls.54 Further, retrospective analysis has shown there to be no difference in functional or patient centered outcomes such as peak FEV1, change in 6-MWD, episodes of acute or chronic rejection, and allograft survival when compared to conventionally evaluated allografts.55 Though many metrics are not significantly different from conventional transplants, it must be kept in mind that many of these organs would never have been transplanted without EVLP and equivalence is remarkable.
Figure 351:
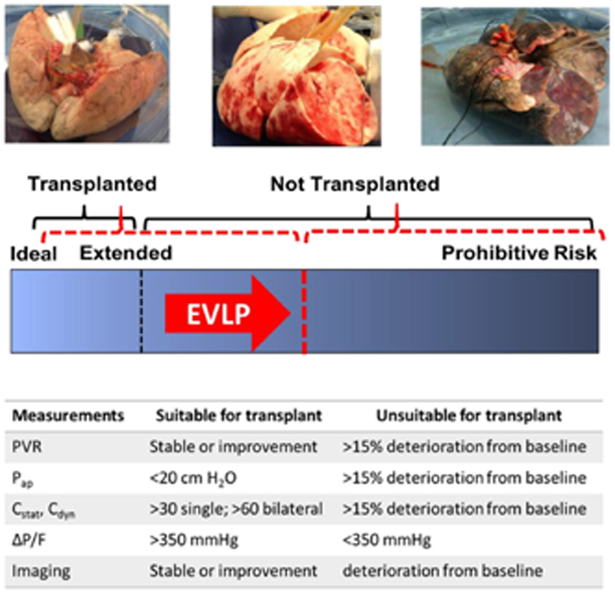
Conceptual framework of EVLP. EVLP extends the time to evaluate and recondition lungs, with the intention of increasing the availability of previously marginal lungs. Common variables used in the evaluation of donor lungs are displayed, though may vary by institution.
PVR = pulmonary vascular resistance; Pap = Peak airway pressure; Cstat = Static compliance; Cdyn = Dynamic compliance; P/F = PaO2/FiO2 ratio
Once donor assessment and procurement have been completed, the conduct of the operation and further technical aspects of the procedure may significantly impact PGD risk. It is worthwhile to consider which of these factors are modifiable and which are fixed while developing a treatment plan for lung transplant recipients. Appropriate size matching between donor and recipient has repeatedly been demonstrated to affect incidence of PGD. Retrospective reports and a meta-analysis have demonstrated increased risk of PGD with undersized lungs and increased survival following transplant of oversized lungs.20,56,57 Decisions on transplant type are generally made during listing. However, if circumstances arise where the decision is made at the time of procurement, in our center’s experience, PGD risk is not dependent on transplant type in isolation. The data to support our position is conflicting with a multi-center prospective cohort of 1,255 patients identified single lung transplantation as a risk factor for severe PGD,15 while a contemporaneous meta-analysis and a recent retrospective cohort did not support this association.43,45 Additionally, while ischemic time has been postulated as a risk factor for PGD, a retrospective review of 10,255 patients in the UNOS database did not reveal an association between prolonged total graft ischemic times and PGD or survival.58 Likewise, the 2017 ISHLT registry report focusing on allograft ischemic time was unable to draw any conclusions of the effect of ischemic time on PGD.59 While most data focuses on cold ischemic time, warm ischemic time may be more clinically meaningful, though it is harder to quantify.
Cardiopulmonary bypass (CPB) has consistently been associated with a higher risk of PGD, when compared to no CPB or other forms of mechanical circulatory support.15,43,60–65 Questions remain whether this increased risk is an effect inherent to CPB, or a marker of greater recipient illness. For this reason, we rarely use CPB and if support is required preferentially use extracorporeal membrane oxygenation (ECMO) to mitigate the risk of support. Similar to CPB, transfusion is generally a surrogate for technically difficult transplants and while increased blood product use is associated with increased PGD risk, it is generally not modifiable.15,43,66 Finally, in one prospective study of 1,255 lung transplant recipients, higher FiO2 at time of allograft reperfusion (i.e. >0.4) was strongly linked to development of severe PGD.15 For this reason, we routinely reperfuse with an Fio2 less than 40%. We also utilize a controlled reperfusion strategy for the first 10 minutes in order to reduce perfusion pressure in the implant, consistent with reported animal and clinical studies.20 Further, we limit tidal volumes and utilize higher peep20 and have modified our initial lung perfusate to attenuate the inflammatory response67.
Prediction:
The consensus definition of PGD has allowed for the use of PGD as a research endpoint. The ability to predict the risk of PGD can help the surgical teams decide whether a high risk patient is better served by remaining on the wait-list, or by taking the available donor organ. The ability to reliably predict development of PGD would enable researchers and clinicians to pre-empt this disease with novel therapeutic interventions.11 While there is currently no definitive predictive model for PGD, recent trials have looked at a variety of clinical risk factors, as well as biomarkers and physiologic measures to stratify risk.
Few trials have looked at objectively stratifying the risk of donor organs. Oto et al proposed a simple donor scoring system, using donor age, smoking history, chest radiograph findings, presence of secretions and arterial blood gas analysis. Their donor score was correlated with post-transplant PGD severity, P/F ratio, duration of intubation and length of ICU stay. This score remains to be prospectively validated.68 The University of Minnesota used a survey based consensus process to weight the importance of 17 clinical factors and developed a scoring application to rate donor lung quality. Their score identified likelihood of allograft acceptance with a sensitivity of 89% and a specificity of 55%. Though there was no association of their score with PGD or survival, they found that using their score increased the number of accepted donor offers and transplants performed, with no changes in transplantation outcomes.69
Shah et al used a multi-center prospective cohort of 1,255 patients from the LTOG group to identify low risk and high risk recipients using clinical variables. Adding a history of donor smoking significantly increased risk of PGD in high risk, but not low risk recipients.48 Porteus et al prospectively explored the use of a predictive model for severe PGD in patients with primary or secondary pulmonary hypertension. They found that a prognostic model including donor tobacco exposure, donor BMI, recipient female sex, recipient obesity, and higher recipient mPAP had a positive predictive value of 28.2%, and a negative predictive value of 83.3%.45
Diamond et al have shown that elevated post-transplant levels of the acute phase reactant long pentraxin-3 are associated with increased risk of PGD.70 Hashimoto et al demonstrated that markers of cellular inflammation and death including M30, M65 and HMGB-1 measured in ex-vivo lung perfusate were significantly higher in patients who developed severe PGD. They also demonstrated that all allografts undergo a degree of cellular death during EVLP, and that lungs ultimately rejected for transplantation had higher rates of increase of these markers over time.71 Pottecher et al showed that increased intra-operative recipient extravascular lung water (EVLW) and soluble receptor for advanced glycation end-products (RAGE) – a marker of pneumocyte epithelial injury - were predictive of severe PGD.72
Immunologic:
While numerous clinical risk factors predisposing allograft recipients to the development of PGD have been identified, ongoing research has begun to elucidate the cellular pathophysiology of PGD. Pulmonary endothelium and epithelium as well as both the innate and adaptive immune responses have been implicated in the ischemia reperfusion process. Ongoing research into this field has provided new biomarkers for predicting and grading PGD and novel therapeutic targets have been elucidated.
Ischemia-reperfusion injury (IRI) is central to the pathogenesis of PGD.73,74 IRI results from the damage of ischemia, generation of reactive oxygen species (ROS) at reperfusion, and subsequent activation of the damage-amplifying pro-inflammatory cascade. Experimental and clinical studies suggest that PGD develops in a biphasic pattern.75–77 The early phase is dominated by resident macrophages and lymphocytes in the donor lungs. In the later phase, development of PGD appears to result from influx of recipient neutrophils and lymphocytes enhancing inflammatory responses.
Downstream effectors such as tumor necrosis factor (TNF)-α, interleukin (IL)-17 and other mediators of leukocyte, neutrophil and innate immune components have been implicated in the recipient response.78 A study using bronchoalveolar lavage (BAL) and tissue biopsy samples showed increased levels of IL-8, a potent neutrophil chemoattractant, to correlate with PGD severity and mortality in a dose-dependent manner. The presence and activation of neutrophils in the pulmonary interstitium and airways is a major hallmark of PGD. When activated, neutrophils release reactive oxygen species, catalytic proteases and enzymes that disrupt cellular function, barrier integrity and induce local cellular death, and release cytokines which perpetuate the inflammatory response.79–81
Damage to airway epithelial cells results in the release of signals alerting and activating the innate immune system.82–84 Pulmonary vascular endothelium is also affected by the ischemia reperfusion process. Interruption of blood flow, revascularization, and flow related shear stress across endothelium during reperfusion are major events leading to endothelial activation,85 which further incites the innate immune system response.
Donor and recipient immune status can influence the development of PGD. Cantu et al showed that donor NOX3 polymorphisms were associated with increased risk of PGD.86 Machuca et al showed increased inflammatory cytokines in donor lungs were associated with higher risk of PGD.87 Studies of genetic determinants for development of severe PGD have pointed to genes in inflammasome-mediated innate immune pathways, and to genes in oxidant stress regulatory pathways. 74,86,88,89
Treatment:
Despite remarkable advances in our understanding of PGD, no definitive therapeutic options currently exist, instead supportive care predominates. Given the similarities between PGD and ARDS,7 many practitioners treat the two entities in a similar manner.13,90 Important elements of caring for these patients include lung protective ventilation strategies, early mobility, and attention to volume status.91 Notable dissimilarities include avoidance of proning, less use of paralysis, and early preferential use of ECMO. The role of pulmonary vasodilators for prophylaxis of PGD in lung transplantation remains unclear, though as in ARDS, they may be used as salvage therapy for refractory hypoxemia.90,91
Both veno-venous and veno-arterial ECMO have been used successfully for cases of severe PGD following transplantation. Though initial outcomes were modest,92 advances in ECMO technology and patient care have since led to improvements in outcomes.93 A UNOS registry review of 107 patients requiring post-transplant ECMO shows a 62.2% six-month survival. Survival was even greater at 86.7% for patients not requiring hemodialysis.94 Notably, allograft function has been shown to be worse in those requiring ECMO, though this is confounded by disease severity.93
Retransplantation for recipients with severe PGD is rarely performed due to poor patient survival and the ethical quandary of allocating a scarce resource to a patient with likely poor outcomes.95,96
Future directions:
With improved understanding of the epidemiology and pathophysiology of PGD, new avenues for prophylaxis and treatment of PGD are being explored.
Since the first use of EVLP,50 this platform has established itself as a viable model for the evaluation and reconditioning of borderline allografts.97 New approaches seek to build on the success of this model. Such approaches include provision of antibiotics during EVLP, extending the time for evaluation and reconditioning of lungs, and the use of a mobile organ perfusion system to limit cold ischemia exposure.98–100 Others have sought to find the optimal storage solution, while current research in animal models is seeking to develop targeted therapies to attenuate cellular injury incurred during the ischemia-reperfusion sequence.51,101–106
Dysregulation of the surfactant protein has been demonstrated in human and animal models of lung transplantation, causing decreased compliance and increased atelectasis and hypoxemia.20 Exogenous surfactant instilled via bronchoscopy has shown early promise as a prophylactic or therapeutic intervention of PGD. When instilled into allografts prior to retrieval, recipients ultimately had higher pulmonary function in the month following transplantation.107 In a separate prospective study, when surfactant was instilled following bronchial anastomosis, recipients demonstrated better short-term pulmonary outcomes, and improved PGD severity when compared to controls.108 Therapeutic trials of established PGD are thus far limited to a few case studies.
Other novel therapeutic avenues include mediation of the complement cascade with active complement cascade inhibition, platelet activating factor inhibition, decreasing activation of platelets via alternative mechanisms20, and manipulation of epithelial cell function.109 The potential of stem cells to inhibit inflammation, improve tissue oxygenation and repair is tantalizing. A number of trials using stem cells in ARDS are ongoing or nearing completion. If the results prove promising, it seems likely that their use would be explored in lung transplant as well.110–112
Conclusion:
The impact of the ISHLT consensus definition of PGD cannot be understated. A common definition has allowed for improved understanding of the epidemiology, risk factors and outcomes of PGD. Further, a consensus definition allows for PGD to be an endpoint in research and clinical trials. This has begun to pave a pathway towards developing more reliable predictive models and therapeutic interventions geared toward the underlying pathophysiology, with the aim of improving the short- and long-term outcomes of lung transplant recipients.
References
- 1.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097–1103. [DOI] [PubMed] [Google Scholar]; Consensus definition of primary graft dysfunction, providing rationale for updated definition, detailing validity of 2005 definition, and providing operational considerations for defining PGD.
- 2.Christie JD, Van Raemdonck D, de Perrot M, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: introduction and methods. J Heart Lung Transplant. 2005;24(10):1451–1453. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24(10):1454–1459. [DOI] [PubMed] [Google Scholar]
- 4.Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26(10):1004–1011. [DOI] [PubMed] [Google Scholar]
- 5.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. [DOI] [PubMed] [Google Scholar]
- 6.Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25(4):371–378. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. [DOI] [PubMed] [Google Scholar]
- 8.Shah RJ, Diamond JM, Cantu E, et al. Latent class analysis identifies distinct phenotypes of primary graft dysfunction after lung transplantation. Chest. 2013;144(2):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multicenter analysis looking at patients with grade 3 PGD, restrospectively differentiates amongst various phenotypes, suggesting differing mechanisms and outcomes for each.
- 9.Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantu E, Diamond JM, Suzuki Y, et al. Quantitative Evidence for Revising the Definition of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med. 2018;197(2):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]; An analysis of the validity of the 2016 updated PGD definition, considers outcomes in relation to PGD grades, effect of single vs. bilateral transplantation, effect of mechanical ventilation on outcomes.
- 11.Diamond JM, Shah RJ, Cantu E 3rd, Porteous MK, Christie JD. Survey of Lung Transplant Community’s Views on Primary Graft Dysfunction. Am J Transplant. 2016;16(2):724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond JM, Arcasoy S, Kennedy CC, et al. Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, part II: Epidemiology, risk factors, and outcomes-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1104–1113. [DOI] [PubMed] [Google Scholar]
- 13.Shah RJ, Diamond JM. Primary Graft Dysfunction (PGD) Following Lung Transplantation. Semin Respir Crit Care Med. 2018;39(2):148–154. [DOI] [PubMed] [Google Scholar]
- 14.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131(1):73–80. [DOI] [PubMed] [Google Scholar]
- 15.Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]; Multicenter, prospective cohort identifying risk factors for severe PGD, risk of PGD for each variable, and differential outcomes based upon transplant center.
- 16.Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141(1):215–222. [DOI] [PubMed] [Google Scholar]
- 17.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44(6):1479–1503. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen KM, Groen H, van der Bij W, Erasmus ME, Koeter GH, TenVergert EM. The effect of bronchiolitis obliterans syndrome on health related quality of life. Clin Transplant. 2004;18(4):377–383. [DOI] [PubMed] [Google Scholar]
- 19.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127(1):161–165. [DOI] [PubMed] [Google Scholar]
- 20.Van Raemdonck D, Hartwig MG, Hertz MI, et al. Report of the ISHLT Working Group on primary lung graft dysfunction Part IV: Prevention and treatment: A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1121–1136. [DOI] [PubMed] [Google Scholar]
- 21.Dark JH. What’s new in pulmonary transplantation: Finding the right lung for every patient. J Thorac Cardiovasc Surg. 2016;151(2):315–316. [DOI] [PubMed] [Google Scholar]
- 22.Sommer W, Kuhn C, Tudorache I, et al. Extended criteria donor lungs and clinical outcome: results of an alternative allocation algorithm. J Heart Lung Transplant. 2013;32(11):1065–1072. [DOI] [PubMed] [Google Scholar]
- 23.Mulligan MJ, Sanchez PG, Evans CF, et al. The use of extended criteria donors decreases one-year survival in high-risk lung recipients: A review of the United Network of Organ Sharing Database. J Thorac Cardiovasc Surg. 2016;152(3):891–898 e892. [DOI] [PubMed] [Google Scholar]
- 24.Somers J, Ruttens D, Verleden SE, et al. A decade of extended-criteria lung donors in a single center: was it justified? Transpl Int. 2015;28(2):170–179. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff RM, Blosser S, Fulda GJ, et al. Management of the Potential Organ Donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med. 2015;43(6):1291–1325. [DOI] [PubMed] [Google Scholar]
- 26.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–2627. [DOI] [PubMed] [Google Scholar]
- 27.Minambres E, Coll E, Duerto J, et al. Effect of an intensive lung donor-management protocol on lung transplantation outcomes. J Heart Lung Transplant. 2014;33(2):178–184. [DOI] [PubMed] [Google Scholar]; Details a management protocol for potential lung donors. Analyzes impact on rate of organ acceptance, recipient survival and PGD after implementing their protocol
- 28.Bonser RS, Taylor R, Collett D, et al. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380(9843):747–755. [DOI] [PubMed] [Google Scholar]
- 29.Shigemura N, Toyoda Y, Bhama JK, et al. Donor smoking history and age in lung transplantation: a revisit. Transplantation. 2013;95(3):513–518. [DOI] [PubMed] [Google Scholar]
- 30.Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant. 2009;23(6):819–830. [DOI] [PubMed] [Google Scholar]
- 31.Porteous MK, Lee JC. Primary Graft Dysfunction After Lung Transplantation. Clin Chest Med. 2017;38(4):641–654. [DOI] [PubMed] [Google Scholar]
- 32.Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysisdagger. Eur J Cardiothorac Surg. 2016;49(1):46–53. [DOI] [PubMed] [Google Scholar]
- 33.Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant. 2012;12(9):2406–2413. [DOI] [PubMed] [Google Scholar]
- 34.Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant. 2015;34(5):675–684. [DOI] [PubMed] [Google Scholar]; A meta-analysis of 6 observational cohort studies, finding no difference in one-year mortality after lung transplant between DCD or DBD donors
- 35.Valenza F, Rosso L, Gatti S, et al. Extracorporeal lung perfusion and ventilation to improve donor lung function and increase the number of organs available for transplantation. Transplant Proc. 2012;44(7):1826–1829. [DOI] [PubMed] [Google Scholar]
- 36.Wallinder A, Ricksten SE, Hansson C, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2012;144(5):1222–1228. [DOI] [PubMed] [Google Scholar]
- 37.Moreno P, Alvarez A, Santos F, et al. Extended recipients but not extended donors are associated with poor outcomes following lung transplantation. Eur J Cardiothorac Surg. 2014;45(6):1040–1047. [DOI] [PubMed] [Google Scholar]
- 38.Zych B, Garcia Saez D, Sabashnikov A, et al. Lung transplantation from donors outside standard acceptability criteria--are they really marginal? Transpl Int. 2014;27(11):1183–1191. [DOI] [PubMed] [Google Scholar]
- 39.Baldwin MR, Peterson ER, Easthausen I, et al. Donor age and early graft failure after lung transplantation: a cohort study. Am J Transplant. 2013;13(10):2685–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. [DOI] [PubMed] [Google Scholar]
- 41.Lowery EM, Kuhlmann EA, Mahoney EL, Dilling DF, Kliethermes SA, Kovacs EJ. Heavy alcohol use in lung donors increases the risk for primary graft dysfunction. Alcohol Clin Exp Res. 2014;38(11):2853–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pelaez A, Mitchell PO, Shah NS, et al. The role of donor chronic alcohol abuse in the development of primary graft dysfunction in lung transplant recipients. Am J Med Sci. 2015;349(2):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang A, Studer S, Kawut SM, et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139(4):782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porteous MK, Lee JC, Lederer DJ, et al. Clinical Risk Factors and Prognostic Model for Primary Graft Dysfunction after Lung Transplantation in Patients with Pulmonary Hypertension. Ann Am Thorac Soc. 2017;14(10):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]; Uses a multi-center retrospective cohort to derive and then retrospectively validate a prognostic model for PGD, including donor, recipient and operative variables.
- 46.Porteous MK, Ky B, Kirkpatrick JN, et al. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med. 2016;193(12):1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184(9):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah RJ, Diamond JM, Cantu E, et al. Objective Estimates Improve Risk Stratification for Primary Graft Dysfunction after Lung Transplantation. Am J Transplant. 2015;15(8):2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Courtwright A, Cantu E. Evaluation and Management of the Potential Lung Donor. Clin Chest Med. 2017;38(4):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357(9259):825–829. [DOI] [PubMed] [Google Scholar]
- 51.Zhu B, Suzuki Y, DiSanto T, et al. Applications of Out of Body Lung Perfusion. Acad Radiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reeb J, Keshavjee S, Cypel M. Expanding the lung donor pool: advancements and emerging pathways. Curr Opin Organ Transplant. 2015;20(5):498–505. [DOI] [PubMed] [Google Scholar]
- 53.Popov AF, Sabashnikov A, Patil NP, et al. Ex vivo lung perfusion - state of the art in lung donor pool expansion. Med Sci Monit Basic Res. 2015;21:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144(5):1200–1206. [DOI] [PubMed] [Google Scholar]; Details the results of a large cohort of recpients of high-risk donor lungs following EVLP, demonstrating increased use of available allografts with no impact on outcomes.
- 55.Tikkanen JM, Cypel M, Machuca TN, et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J Heart Lung Transplant. 2015;34(4):547–556. [DOI] [PubMed] [Google Scholar]
- 56.Barnard JB, Davies O, Curry P, et al. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. 2013;32(9):849–860. [DOI] [PubMed] [Google Scholar]
- 57.Ganapathi AM, Mulvihill MS, Englum BR, et al. Transplant size mismatch in restrictive lung disease. Transpl Int. 2017;30(4):378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimm JC, Valero V 3rd, Kilic A, et al. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg. 2015;150(6):547–553. [DOI] [PubMed] [Google Scholar]
- 59.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1047–1059. [DOI] [PubMed] [Google Scholar]
- 60.Hoechter DJ, Shen YM, Kammerer T, et al. Extracorporeal Circulation During Lung Transplantation Procedures: A Meta-Analysis. ASAIO J. 2017;63(5):551–561. [DOI] [PubMed] [Google Scholar]
- 61.Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg. 2015;149(4):1152–1157. [DOI] [PubMed] [Google Scholar]
- 62.Nair P, Hoechter DJ, Buscher H, et al. Prospective observational study of hemostatic alterations during adult extracorporeal membrane oxygenation (ECMO) using point-of-care thromboelastometry and platelet aggregometry. J Cardiothorac Vasc Anesth. 2015;29(2):288–296. [DOI] [PubMed] [Google Scholar]
- 63.Biscotti M, Yang J, Sonett J, Bacchetta M. Comparison of extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg. 2014;148(5):2410–2415. [DOI] [PubMed] [Google Scholar]
- 64.Bermudez CA, Shiose A, Esper SA, et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg. 2014;98(6):1936–1942; discussion 1942–1933. [DOI] [PubMed] [Google Scholar]
- 65.Ius F, Kuehn C, Tudorache I, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012;144(6):1510–1516. [DOI] [PubMed] [Google Scholar]
- 66.Felten ML, Sinaceur M, Treilhaud M, et al. Factors associated with early graft dysfunction in cystic fibrosis patients receiving primary bilateral lung transplantation. Eur J Cardiothorac Surg. 2012;41(3):686–690. [DOI] [PubMed] [Google Scholar]
- 67.Schnickel GT, Ross DJ, Beygui R, et al. Modified reperfusion in clinical lung transplantation: the results of 100 consecutive cases. J Thorac Cardiovasc Surg. 2006;131(1):218–223. [DOI] [PubMed] [Google Scholar]
- 68.Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg. 2007;83(1):257–263. [DOI] [PubMed] [Google Scholar]
- 69.Loor G, Radosevich DM, Kelly RF, et al. The University of Minnesota Donor Lung Quality Index: A Consensus-Based Scoring Application Improves Donor Lung Use. Ann Thorac Surg. 2016;102(4):1156–1165. [DOI] [PubMed] [Google Scholar]
- 70.Diamond JM, Ramphal K, Porteous MK, et al. Association of long pentraxin-3 with pulmonary hypertension and primary graft dysfunction in lung transplant recipients. J Heart Lung Transplant. 2018;37(6):792–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashimoto K, Cypel M, Juvet S, et al. Higher M30 and high mobility group box 1 protein levels in ex vivo lung perfusate are associated with primary graft dysfunction after human lung transplantation. J Heart Lung Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 72.Pottecher J, Roche AC, Degot T, et al. Increased Extravascular Lung Water and Plasma Biomarkers of Acute Lung Injury Precede Oxygenation Impairment in Primary Graft Dysfunction After Lung Transplantation. Transplantation. 2017;101(1):112–121. [DOI] [PubMed] [Google Scholar]
- 73.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. American journal of respiratory and critical care medicine. 2003;167(4):490–511. [DOI] [PubMed] [Google Scholar]
- 74.Diamond JM, Wigfield CH. Role of innate immunity in primary graft dysfunction after lung transplantation. Curr Opin Organ Transplant. 2013;18(5):518–523. [DOI] [PubMed] [Google Scholar]
- 75.Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. The Journal of surgical research. 1995;58(6):713–718. [DOI] [PubMed] [Google Scholar]
- 76.Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Mediators of ischemia-reperfusion injury of rat lung. The American journal of pathology. 1997;150(5):1773–1784. [PMC free article] [PubMed] [Google Scholar]
- 77.Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. The Journal of thoracic and cardiovascular surgery. 2001;121(6):1069–1075. [DOI] [PubMed] [Google Scholar]
- 78.Sharma AK, Mulloy DP, Le LT, Laubach VE. NADPH oxidase mediates synergistic effects of IL-17 and TNF-alpha on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. American journal of physiology. Lung cellular and molecular physiology. 2014;306(1):L69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gelman AE, Fisher AJ, Huang HJ, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morrison MI, Pither TL, Fisher AJ. Pathophysiology and classification of primary graft dysfunction after lung transplantation. J Thorac Dis. 2017;9(10):4084–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Somers J, Ruttens D, Verleden SE, et al. Interleukin-17 receptor polymorphism predisposes to primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2015;34(7):941–949. [DOI] [PubMed] [Google Scholar]
- 83.Patel BV, Wilson MR, O’Dea KP, Takata M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol. 2013;190(8):4274–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharma AK, LaPar DJ, Stone ML, Zhao Y, Kron IL, Laubach VE. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am J Transplant. 2013;13(9):2255–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fang A, Studer S, Kawut SM, et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139(4):782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cantu E, Shah RJ, Lin W, et al. Oxidant stress regulatory genetic variation in recipients and donors contributes to risk of primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2015;149(2):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Machuca TN, Cypel M, Yeung JC, et al. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann Surg. 2015;261(3):591–597. [DOI] [PubMed] [Google Scholar]
- 88.Cantu E, Suzuki Y, Diamond JM, et al. Protein Quantitative Trait Loci Analysis Identifies Genetic Variation in the Innate Immune Regulator TOLLIP in Post-Lung Transplant Primary Graft Dysfunction Risk. Am J Transplant. 2016;16(3):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cantu E, Lederer DJ, Meyer K, et al. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am J Transplant. 2013;13(7):1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]; Novel study comparing genes present in the BAL fluid of donors and recipients. Amongst recipients with severe PGD eight gene sets were found to be upregulated. These genes implicate the innate immune system as a key mediator of severe PGD.
- 90.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 91.Suzuki Y, Cantu E, Christie JD. Primary graft dysfunction. Semin Respir Crit Care Med. 2013;34(3):305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bermudez CA, Adusumilli PS, McCurry KR, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg. 2009;87(3):854–860. [DOI] [PubMed] [Google Scholar]
- 93.Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012;93(2):366–371. [DOI] [PubMed] [Google Scholar]
- 94.Mulvihill MS, Yerokun BA, Davis RP, Ranney DN, Daneshmand MA, Hartwig MG. Extracorporeal membrane oxygenation following lung transplantation: indications and survival. J Heart Lung Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 95.Osho AA, Castleberry AW, Snyder LD, et al. Differential outcomes with early and late repeat transplantation in the era of the lung allocation score. Ann Thorac Surg. 2014;98(6):1914–1920; discussion 1920–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]; UNOS registry analysis identifying risk factors for need for ECMO post transplant, and the outcomes amongst these patients
- 96.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report−−2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33(10):1009–1024. [DOI] [PubMed] [Google Scholar]
- 97.Reeb J, Cypel M. Ex vivo lung perfusion. Clin Transplant. 2016;30(3):183–194. [DOI] [PubMed] [Google Scholar]
- 98.Pharmacokinetics of Imipenem During Ex Vivo Lung Perfusion (EVLP). Accessed 18 Aug 2018 https://clinicaltrials.gov/ct2/show/NCT02670239?term=Pharmacokinetics+of+Imipenem+During+Ex+Vivo+Lung+Perfusion+%28EVLP%29&rank=1
- 99.Repair of Acute Respiratory Distress Syndrome by Stromal Cell Administration (REALIST). Accessed 18 Aug 2018 https://clinicaltrials.gov/ct2/show/NCT03042143?term=Repair+of+Acute+Respiratory+Distress+Syndrome+by+Stromal+Cell+Administration+%28REALIST%29.&rank=1 [DOI] [PMC free article] [PubMed]
- 100.Trial to Evaluate the Safety and Effectiveness of the Portable Organ Care System (OCS™) Lung System for Recruiting, Preserving and Assessing Non-Ideal Donor Lungs for Transplantation. Accessed 18 Aug 2018 https://clinicaltrials.gov/ct2/show/NCT03343535?term=Trial+to+Evaluate+the+Safety+and+Effectiveness+of+the+Portable+Organ+Care+System+%28OCS%E2%84%A2%29+Lung+System+for+Recruiting%2C+Preserving+and+Assessing+Non-Ideal+Donor+Lungs+for+Transplantation&rank=1
- 101.Mulloy DP, Sharma AK, Fernandez LG, et al. Adenosine A3 receptor activation attenuates lung ischemia-reperfusion injury. Ann Thorac Surg. 2013;95(5):1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehaffey JH, Charles EJ, Narahari AK, et al. Increasing circulating sphingosine-1-phosphate attenuates lung injury during ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2018;156(2):910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin H, Chen M, Tian F, et al. alpha1-Anti-trypsin improves function of porcine donor lungs during ex-vivo lung perfusion. J Heart Lung Transplant. 2018;37(5):656–666. [DOI] [PubMed] [Google Scholar]
- 104.Gotzfried J, Smirnova NF, Morrone C, et al. Preservation with alpha1-antitrypsin improves primary graft function of murine lung transplants. J Heart Lung Transplant. 2018;37(8):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao W, Zhao J, Kim H, et al. alpha1-Antitrypsin inhibits ischemia reperfusion-induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33(3):309–315. [DOI] [PubMed] [Google Scholar]
- 106.Charles EJ, Mehaffey JH, Sharma AK, et al. Lungs donated after circulatory death and prolonged warm ischemia are transplanted successfully after enhanced ex vivo lung perfusion using adenosine A2B receptor antagonism. J Thorac Cardiovasc Surg. 2017;154(5):1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Struber M, Fischer S, Niedermeyer J, et al. Effects of exogenous surfactant instillation in clinical lung transplantation: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2007;133(6):1620–1625. [DOI] [PubMed] [Google Scholar]
- 108.Amital A, Shitrit D, Raviv Y, et al. The use of surfactant in lung transplantation. Transplantation. 2008;86(11):1554–1559. [DOI] [PubMed] [Google Scholar]
- 109.Aigner C, Slama A, Barta M, et al. Treatment of primary graft dysfunction after lung transplantation with orally inhaled AP301: A prospective, randomized pilot study. J Heart Lung Transplant. 2017. [DOI] [PubMed] [Google Scholar]
- 110.Human Mesenchymal Stem Cells For Acute Respiratory Distress Syndrome (START). https://clinicaltrials.gov/ct2/show/NCT02097641?term=Human+Mesenchymal+Stem+Cells+For+Acute+Respiratory+Distress+Syndrome+%28START%29&rank=1
- 111.A Phase 1/2 Study to Assess MultiStem® Therapy in Acute Respiratory Distress Syndrome. Accessed 18 Aug 2018 https://clinicaltrials.gov/ct2/show/NCT02611609?term=A+Phase+1%2F2+Study+to+Assess+MultiStem%C2%AE+Therapy+in+Acute+Respiratory+Distress+Syndrome&rank=1
- 112.OCS™ Lung TOP Registry. Accessed 18 Aug 2018 https://clinicaltrials.gov/ct2/show/NCT03639025?cond=OCS%E2%84%A2+Lung+TOP+Registry&rank=1


