Abstract
Purpose
This study investigated whether size at birth and infant growth were associated with age of indicators of pubertal development in boys and girls. We hypothesized that restricted fetal growth and accelerated infant growth lead to earlier pubertal age.
Patients and methods
In total, 15,822 boys and girls answered questionnaires half-yearly with information on pubertal development: age at menarche, first ejaculation, voice break, Tanner stages, axillary hair, and acne. Birth weight and gestational age were used to calculate birth weight Z-scores. Changes in infant weight Z-score from 0 to 5, 5 to 12, and 0 to 12 months were estimated. We estimated the mean monthly difference in timing of puberty between children born small-for-gestational age (SGA) and large-for-gestational age (LGA) with children born appropriate-for-gestational age (AGA) as reference. We further investigated whether increasing infant weight Z-scores were associated with age at attaining indicators of pubertal development.
Results
Girls born SGA reached all pubertal markers at an earlier mean age than girls born AGA, as indicated by mean age differences below zero (eg, age at menarche: −2.3 months, 95% CI: −3.4, −1.2), except for breast development. Girls born LGA reached pubertal markers later than girls born AGA (eg, age at menarche: 1.7 months, 95% CI 0.5, 2.9). Boys born SGA and LGA achieved puberty earlier than boys born AGA, though with CIs crossing zero (eg, age at voice break for SGA: −0.7 months, 95% CI −2.1, 0.7 and for LGA: −0.7 months, 95% CI −2.1, 0.8). A 1-unit increase in weight Z-score from 0 to 12 months was associated with a mean age difference of −1.7 to −0.3 months for pubertal development in both sexes.
Conclusion
Small size at birth and rapid infant growth were associated with early pubertal age, most consistent and pronounced in girls.
Keywords: puberty, pubertal development, tanner stages, birth weight
Introduction
Pubertal development is controlled by the hypothalamic–pituitary–gonadal axis (HPG axis). The axis is developed early in fetal life and is active throughout pregnancy and within the first year of life (the minipuberty).1,2 Hereafter, it remains in a quiescent stage until reactivation at the onset of puberty. Consequently, the HPG axis could potentially be programmed by both prenatal and early postnatal exposures. Exposure to a deleterious intrauterine environment may result in fetal growth restriction (FGR). FGR has been suggested to impact epigenetic programming,3,4 which may affect the development of the HPG axis and its reawakening at puberty. Furthermore, most FGR children show catch-up growth during infancy, which may affect the HPG axis and pubertal development by increasing sex-hormone bioavailability through changes in fat metabolism and insulin resistance.5,6
Previous studies on the association between fetal growth and pubertal development have mainly investigated age of menarche in girls.7–24 The majority found that low birth weight or small-for-gestational age (SGA) was associated with earlier age at menarche. Some studies used Tanner stages or onset of the growth spurt as markers of female puberty with less consistent findings.8,15,21,23,25,26 In boys, previous studies have investigated Tanner stages8,25–27 and onset of pubertal growth spurt,21,23 but data are too sparse to conclude on the direction or magnitude of association. In this large cohort study, we investigated several indicators of pubertal timing in both sexes, using longitudinally collected data.
We hypothesized that FGR leads to earlier pubertal development, especially in children with catch-up growth. Therefore, we investigated the associations between birth-size-for-gestational age, infant growth, and pubertal development.
Materials and methods
Participants and setting
This study is based on the Puberty Cohort, a sub-cohort of the Danish National Birth Cohort (DNBC). The DNBC enrolled pregnant women from 1996 to 2002 and holds data on approximately 92,000 women and their approximately 100,000 children. The majority of women in the cohort were Caucasian, reflecting the Danish population at the time. The women gave information on lifestyle and health-related factors through computer-assisted telephone interviews carried out twice during pregnancy (gestational weeks 17 and 32) and when their child was 6 and 18 months. Additional questionnaires were completed as the children turned 7 and 11 years.28
The ongoing Puberty Cohort was established in August 2012. A total of 56,641 singletons born from 2000 to 2003, whose mothers participated in the first DNBC interview and had not died or withdrawn from the DNBC by May 2012, were eligible for being sampled to the Puberty Cohort. In total, a subsample of 22,439 children among the 56,641 children born into the DNBC between 2000 and 2003 were sampled and invited by letter or email to give information through web-based questionnaires about their pubertal development half-yearly from the age of 11.5 years until full sexual maturation (defined as Tanner stage 5 for breast or genital development and pubic hair development) or turning 18 years old, whichever came first.
A total of 14,759 (66%) of the 22,439 sampled children gave information on pubertal development in the Puberty Cohort. Furthermore, all children in the DNBC were invited to give information on puberty at the 11-year follow-up, and 10,688 (48%) of the children sampled for invitation to the Puberty Cohort gave information through this questionnaire. Altogether, 15,822 children provided information on pubertal development at least once when combining these two sources of data (participation rate: 71%). Children without any information on pubertal development were considered nonparticipants (loss to follow-up).
Birth weight and infant growth
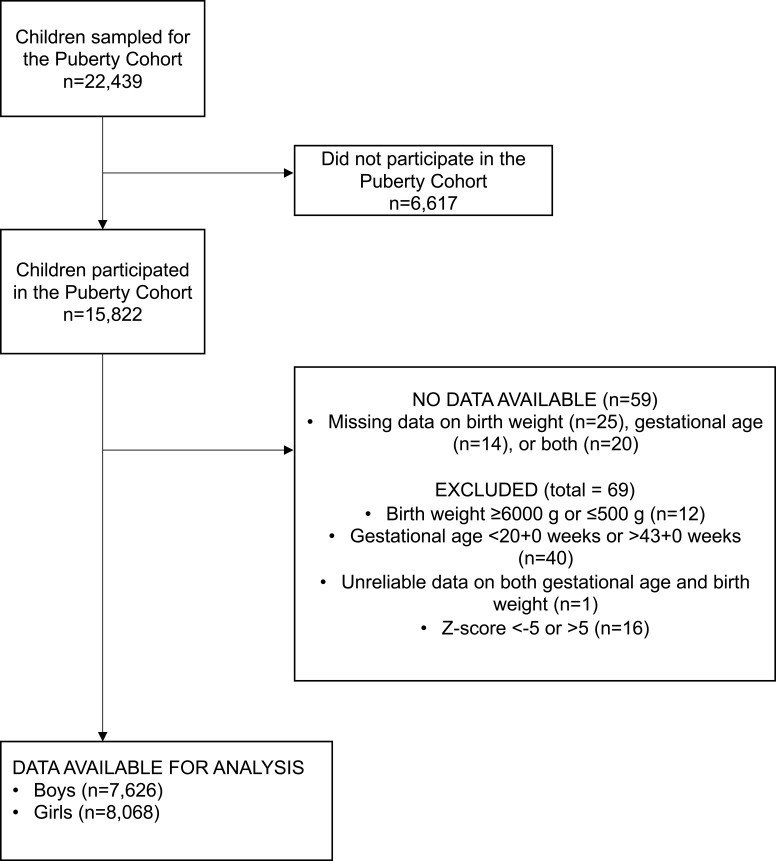
Gestational age and birth weight were obtained from The Danish Medical Birth Registry.30 Size at birth was computed as birth weight Z-scores using the reference material developed by Marsal et al.31 Birth weight Z-scores are the number of standard deviations the birth weight deviates from the expected birth weight based on sex and gestational age. We excluded children with missing information on birth weight or gestational age (n=59) as well as those with birth weight <500 g or >6000 g, gestational age <20+0 weeks or >43+0 weeks, or birth weight Z-score <-5 or >5 (n=69). We were able to calculate birth weight Z-scores on 15,694 of the children with information on pubertal development (Figure 1). Children were categorized as SGA (birth weight Z-score below the 10th percentile, n=2056), appropriate-for-gestational age (AGA, birth weight Z-score between the 10th and 90th percentile, n=11,702), or large-for-gestational age (LGA, birth weight Z-score above 90th percentile, n=1936).
Figure 1.
Flowchart of the study population after invitation to participate in the Puberty Cohort.
The weight of children at 5 and 12 months of age was routinely measured in the Danish postnatal health examinations. These weights were reported by the mother in the computer-assisted telephone interview in the DNBC at 18 months postpartum. Data were considered unreliable and recoded to missing if the age at 5-month examination was listed as <3 months or >7 months in the registry (n=171) or the age at 12-month examination was listed as <10 months or >15 months (n=181). Analyses on infant growth were restricted to children born at term (37+0 to 42+0 weeks) with information on puberty (n=13,262). To evaluate infant growth, we calculated weight Z-scores, using the United Kingdom World Health Organization (UK-WHO) Term Growth Charts at birth, 5 months, and 12 months.32 Z-scores <-5 or >5 were considered unreliable and recoded to missing (n=8). The individual weight Z-scores at 0, 5, and 12 months were used to calculate the change in Z-score from 0 to 5 months (n=8897), 5 to 12 months (n=7886), and 0 to 12 months (n=8312).
Puberty
Information on pubertal development was collected through a web-based, translated version of the questionnaire used in the British Avon Longitudinal Study of Parents and Children.27 The Danish questionnaire is available at www.bsig.dk. It included information on axillary hair (yes/no), acne (yes/no), pubic hair and breast or genital growth, first menstrual period in girls (yes/no, age: year and month), first ejaculation in boys (yes/no, age: year and month), voice break in boys (yes – sometimes, yes – definitive changes, no, do not know), as well as height and weight (cm, kg). The Tanner scale was used to measure the stage of breast, genital, and pubic hair growth,33,34 and explanatory text with pictures was included to improve the reporting. Children were encouraged to respond to the questionnaires themselves at all times.
Covariates
Using directed acyclic graphs,35 we identified and included the following potential confounders in all analyses: pre-pregnancy body mass index (BMI), maternal smoking in the first trimester, highest educational class of parents, parity, and maternal age of menarche. The potential confounders were categorized as seen in Table 1. Parity was obtained from The Danish Medical Birth Registry30 and highest educational class of parents from Statistic Denmark. Information on the remaining potential confounders was obtained from the DNBC.
Table 1.
Baseline characteristics of the children in the Puberty Cohort (N=15,694), Denmark, 2012–2017. Children are categorized as SGA, AGA, or LGA
| Size for gestational age | Missing | |||||||
|---|---|---|---|---|---|---|---|---|
| SGA | AGA | LGA | ||||||
| n | % | n | % | n | % | n | % | |
| Total | 2056 | 13.1 | 11,702 | 74.6 | 1936 | 12.3 | ||
| Child characteristics | ||||||||
| Sex | 0 | 0.0 | ||||||
| Male | 1007 | 49.0 | 5636 | 48.2 | 983 | 50.8 | ||
| Gestational age | 0 | 0.0 | ||||||
| <37 weeks | 220 | 10.7 | 718 | 6.1 | 164 | 8.5 | ||
| ≥37 weeks | 1836 | 89.3 | 10,984 | 93.9 | 1772 | 91.5 | ||
| Birth weight (SD)a | 2056 | 2738 (454) | 11,702 | 3543 (440) | 1936 | 4283 (459) | 0 | 0.0 |
| Weight at 5 months (SD)a | 1406 | 7035 (911) | 8179 | 7738 (929) | 1396 | 8319 (1043) | 4713 | 30.0 |
| Weight at 12 months (SD)a | 1332 | 9482 (1102) | 7749 | 10,202 (1132) | 1325 | 10,825 (1321) | 5288 | 33.7 |
| Maternal characteristics | ||||||||
| Maternal pre-pregnancy BMI | 214 | 1.4 | ||||||
| <18.5 | 207 | 10.2 | 797 | 6.9 | 42 | 2.2 | ||
| 18.5–<25 | 1318 | 64.8 | 7251 | 62.8 | 1012 | 53.0 | ||
| ≥25–<30 | 360 | 17.7 | 2412 | 20.9 | 508 | 26.6 | ||
| 30+ | 149 | 7.3 | 1078 | 9.3 | 346 | 18.1 | ||
| Smoking during first trimester | 53 | 0.3 | ||||||
| Nonsmoker | 1247 | 61.0 | 8466 | 72.6 | 1531 | 79.2 | ||
| 1–<10 cigarettes per day | 592 | 29.0 | 2560 | 22.0 | 340 | 17.6 | ||
| ≥10 cigarettes per day | 205 | 10.0 | 639 | 5.5 | 61 | 3.2 | ||
| Parity | 0 | 0.0 | ||||||
| First child | 1354 | 65.9 | 5903 | 50.4 | 653 | 33.7 | ||
| Second or later child | 702 | 34.1 | 5799 | 49.6 | 1283 | 66.3 | ||
| Maternal age at menarche | 121 | 0.8 | ||||||
| Earlier than peers | 495 | 24.3 | 2940 | 25.3 | 546 | 28.4 | ||
| Same as peers | 1183 | 58.0 | 6657 | 57.3 | 1072 | 55.8 | ||
| Later than peers | 362 | 17.8 | 2015 | 17.4 | 303 | 15.8 | ||
| Highest educational class of parents | 31 | 0.2 | ||||||
| High-grade professional | 453 | 22.1 | 2741 | 23.5 | 463 | 23.9 | ||
| Low-grade professional | 605 | 29.5 | 3888 | 33.3 | 667 | 34.5 | ||
| Skilled worker | 620 | 30.2 | 3155 | 27.0 | 535 | 27.6 | ||
| Unskilled worker | 305 | 14.9 | 1596 | 13.7 | 234 | 12.1 | ||
| Students | 47 | 2.3 | 232 | 2.0 | 31 | 1.6 | ||
| Economically inactive | 23 | 1.1 | 62 | 0.5 | 6 | 0.3 | ||
Notes: aMean values in gram (SD) presented instead of column %.
Abbreviations: AGA, appropriate-for-gestational age; BMI, body mass index; LGA, large-for-gestational age; SGA, small-for-gestational age.
Statistical analyses
STATA 15.1 MP software (Statacorp, College Station, TX, USA) was used to conduct the analyses. Since the participants responded to questionnaires every 6 months, the data on outcomes were either left, right, or interval censored. The outcome was left-censored if a pubertal stage was attained before the first completed questionnaire, interval-censored if the pubertal stage was attained between two questionnaires, or right-censored if the pubertal stage was not attained by the last questionnaire. We used a multivariable regression model for interval-censored time-to-event data based on the normal distribution since age at puberty has been found to be normally distributed in healthy populations.36 To check the assumption of normally distributed residuals, the residuals were visually inspected in R (x64 3.3.1) by comparing the cumulative incidence function based on the Turnbull estimator to the cumulative incidence function based on the normal distribution (data not shown).
In the main analyses, we first estimated the difference in mean age (in months) in reaching the different indicators of pubertal development when comparing SGA and LGA with AGA (without accounting for infant growth).
Second, to examine the impact of infant growth on timing of pubertal development without considering size at birth, the changes in Z-scores from 0 to 5 months, 5 to 12 months, and 0 to 12 months were used to obtain the difference in mean age (in months) at attaining a pubertal milestone per 1-unit increase in Z-scores.
Furthermore, we performed the following subanalyses: First, the analysis comparing SGA to AGA was stratified on being born either preterm (gestational age <37+0 weeks) or at term (gestational age 37+0 to 41+6 weeks) to evaluate potential effect modification by preterm birth. Second, to look for trends, we assessed the association with birth weight Z-scores as a continuous variable to obtain the difference in mean age (in months) at attaining a pubertal milestone per 1-unit increase in birth weight Z-score. Third, to assess potential effect modification by birth weight Z-score on the association between infant growth and pubertal development, we repeated the analysis stratified on the birth weight Z-score being either below or above the 33-percentile.
To increase statistical efficiency, participants in the Puberty Cohort were sampled from 12 subgroups, based on pregnancy or childhood exposures, hypothesized to affect pubertal timing, including SGA. Additionally, a reference group of 8000 children was randomly sampled. In total, 22,439 children were sampled and invited for participation. Sampling weights were used to account for the sampling procedure.29 Robust standard errors were used to account for the use of sampling weights and clustering of siblings.
The study was approved by the Steering Committee of the DNBC (2017–06). Data in DNBC and The Puberty Cohort were registered by the Danish Data Protection Agency (journal number 2012-41-0379 and 2015-57-0002). When the DNBC was established, the Committee on Biomedical Research Ethics approved the data collection ((KF) 01-471/94). Written consent was obtained from all women at recruitment.
Results
Study population
15,694 children were included in the main analyses. In our cohort, 13.1% were born SGA, 74.6% were born AGA, and 12.3% were born LGA. Compared to children born AGA, SGA children were more likely born preterm, first born, to have a mother with pre-pregnancy BMI <18.5 kg/m2, and to have a mother that smoked ≥10 cigarettes a day during the first trimester (Table 1). Compared to children born AGA, LGA children were more likely to be born second or later, to have a nonsmoking mother, and to have a mother with a larger pre-pregnancy BMI (Table 1).
Size at birth and puberty
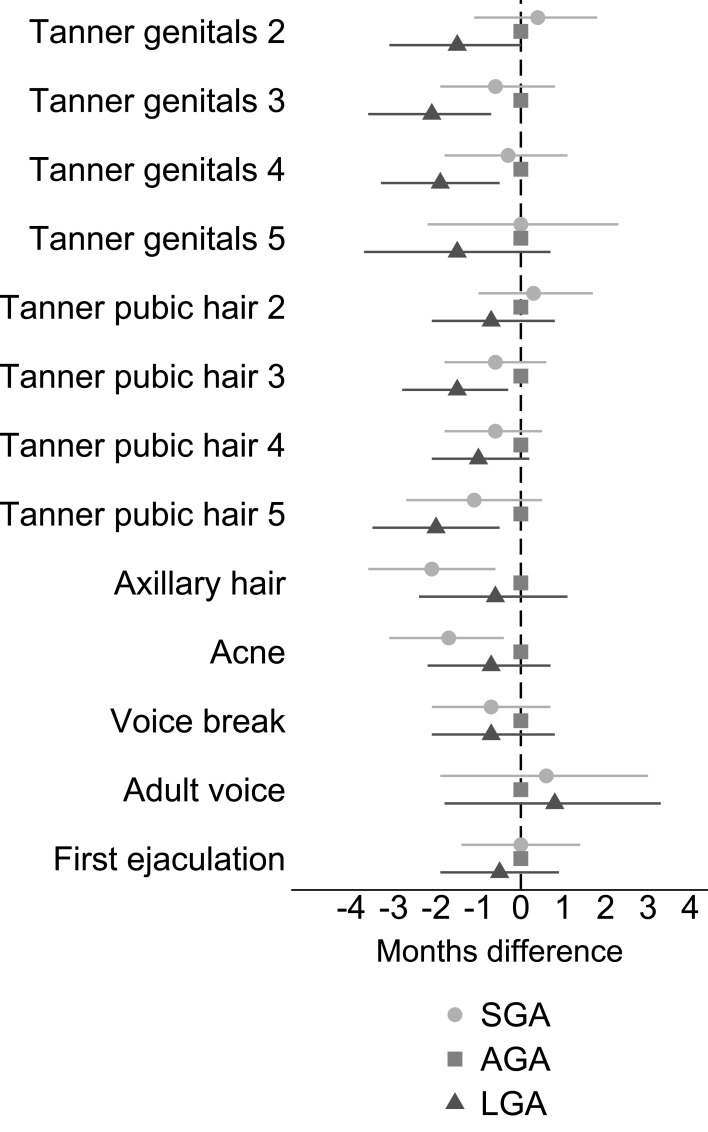
As presented in Figure 2 and Table S1, boys born SGA achieved earlier puberty than boys born AGA, though with CIs overlapping zero (eg, Tanner genital stage 3: −0.6 months, 95% CI: −1.9, 0.8 and Tanner pubic hair stage 3: −0.6 months, 95% CI: −1.8, 0.6). Boys born LGA also reached pubertal milestones earlier than boys born AGA, with greater mean age differences than SGA (eg, Tanner genital stage 3: −2.1 months, 95% CI: −3.6, −0.7 and Tanner pubic hair stage 3: −1.5 months, 95% CI: −2.8, −0.3). Still, CIs overlapped zero for most of the milestones.
Figure 2.
Adjusted difference (with 95% CI) in reaching pubertal milestones when comparing SGA and LGA boys to boys born AGA.
Notes: Adjusted for parity, maternal age at menarche, maternal pre-pregnancy BMI, maternal smoking during the first trimester, and highest educational class of parents. Exact estimates are provided in Table S1.
Abbreviations: AGA, appropriate-for-gestational age; BMI, body mass index; LGA, large-for-gestational age; SGA, small-for-gestational age.
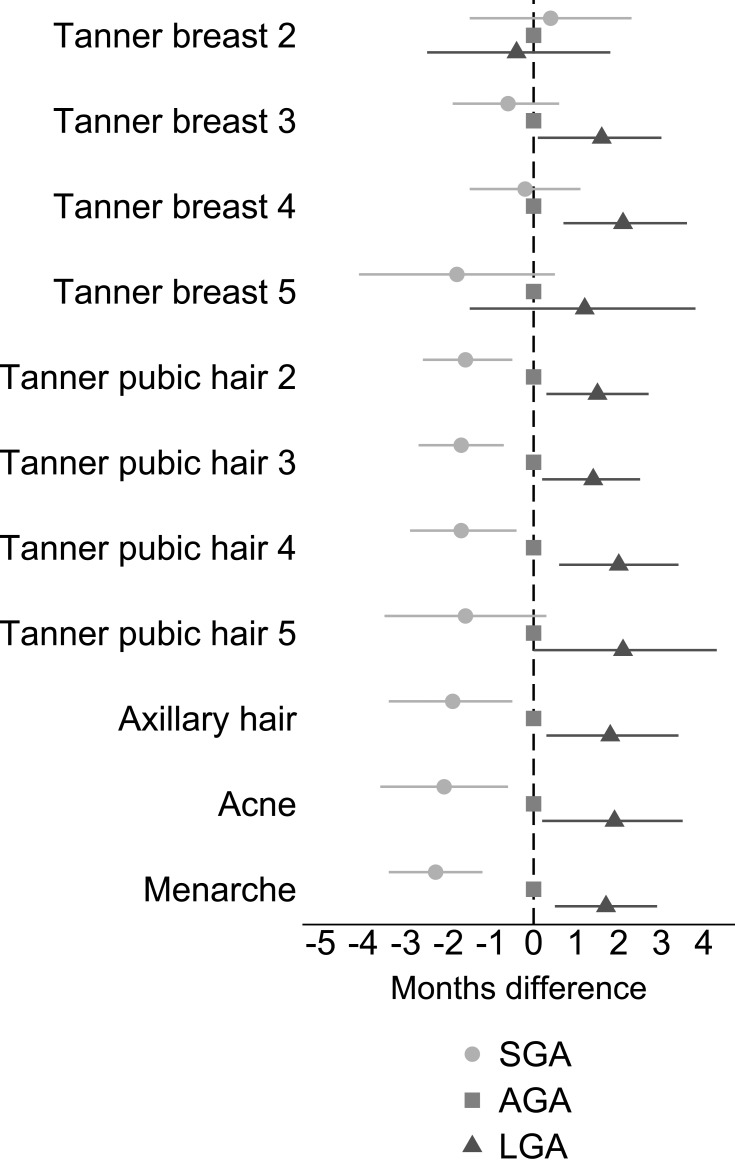
As presented in Figure 3 and Table S1, girls born SGA reached pubertal stages of pubic hair development, axillary hair development, acne, and menarche 1.6 to 2.3 months earlier than AGA girls (eg, Tanner pubic hair stage 2: −1.6 months, 95% CI: −2.6, −0.5 and age at menarche: −2.3 months, 95% CI: −3.4, −1.2). We found no difference in age of reaching breast stages 2, 3, and 4. LGA girls had later puberty in the order of 1.2 to 2.1 months compared to AGA, except breast stage 2 and 5 and pubic hair stage 5 (eg, Tanner pubic hair stage 2: 1.5 months, 95% CI: 0.3, 2.7 and age at menarche: 1.7 months, 95% CI: 0.5, 2.9).
Figure 3.
Adjusted difference (with 95% CI) in reaching pubertal milestones when comparing SGA and LGA girls to girls born AGA.
Notes: Adjusted for parity, maternal age at menarche, maternal pre-pregnancy BMI, maternal smoking during the first trimester, and highest educational class of parents. Exact estimates are provided in Table S1.
Abbreviations: AGA, appropriate-for-gestational age; BMI, body mass index; LGA, large-for-gestational age; SGA, small-for-gestational age.
When stratifying on gestational age, the association between SGA and age at pubertal development was accentuated for most pubertal milestones among preterm, but CIs were wide and overlapping zero (Table S2).
Increasing birth weight Z-score was associated with later puberty in all pubertal outcomes in girls only (except for breast stage 2) in the order of 0.6 to 1 months per 1-unit increase in Z-score (Table 2).
Table 2.
Mean age difference (in months) at attaining pubertal milestone for each 1-unit increase in birth weight Z-score
| Pubertal milestone | na | Unadjusted age difference | Adjusted age difference (95% CI)b |
|---|---|---|---|
| Boys | |||
| Tanner genital stage 2 | 6927 | −0.4 | −0.5 (−0.9–0.0) |
| Tanner genital stage 3 | 6927 | −0.4 | −0.4 (−0.8–0.1) |
| Tanner genital stage 4 | 6927 | −0.3 | −0.3 (−0.8–0.1) |
| Tanner genital stage 5 | 6927 | −0.6 | −0.6 (−1.3–0.0) |
| Tanner pubic hair stage 2 | 6931 | −0.2 | −0.2 (−0.6–0.3) |
| Tanner pubic hair stage 3 | 6931 | −0.3 | −0.2 (−0.6–0.2) |
| Tanner pubic hair stage 4 | 6931 | 0.0 | 0.0 (−0.4–0.3) |
| Tanner pubic hair stage 5 | 6931 | −0.2 | −0.2 (−0.7–0.3) |
| Axillary hair | 6936 | 0.4 | 0.5 (0.0–1.0) |
| Acne | 6936 | 0.6 | 0.4 (−0.1–0.8) |
| Voice break | 6742 | −0.1 | −0.2 (−0.7–0.2) |
| Adult voice | 6742 | 0.4 | 0.4 (−0.4–1.1) |
| First ejaculation | 6923 | −0.3 | −0.3 (−0.7–0.2) |
| Girls | |||
| Tanner breast stage 2 | 7348 | −0.4 | −0.2 (−0.8–0.4) |
| Tanner breast stage 3 | 7348 | 0.4 | 0.6 (0.2–1.0) |
| Tanner breast stage 4 | 7348 | 0.3 | 0.6 (0.2–1.0) |
| Tanner breast stage 5 | 7348 | 0.2 | 0.6 (−0.2–1.4) |
| Tanner pubic hair stage 2 | 7349 | 0.7 | 0.6 (0.3–1.0) |
| Tanner pubic hair stage 3 | 7349 | 0.7 | 0.8 (0.5–1.2) |
| Tanner pubic hair stage 4 | 7349 | 0.8 | 0.9 (0.5–1.4) |
| Tanner pubic hair stage 5 | 7349 | 0.8 | 1.0 (0.4–1.6) |
| Axillary hair | 7354 | 0.9 | 0.9 (0.5–1.4) |
| Acne | 7354 | 1.1 | 1.0 (0.5–1.5) |
| Menarche | 7346 | 0.7 | 0.9 (0.6–1.2) |
Notes: aNumber of observations in adjusted analyses. Numbers vary since some children did not provide information on all outcome variables. bAdjusted for parity, maternal age at menarche, maternal pre-pregnancy BMI, maternal smoking during first trimester, and highest educational class of parents.
Abbreviations: AGA, appropriate-for-gestational age; BMI, body mass index; LGA, large-for-gestational age; n, number; SGA, small-for-gestational age.
Infant growth and puberty
A 1-unit increase in Z-score from 0 to 12 months was associated with earlier puberty in both sexes (Table 3). When evaluating Z-score change in shorter time intervals, an increase in Z-score between 5 and 12 months was slightly stronger associated with age at puberty than an increase in Z-scores between 0 and 5 months.
Table 3.
Mean age difference (in months) at attaining pubertal milestone for each 1-unit increase in Z-score during the periods from 0 to 5 months, 5 to 12 months, and 0 to 12 months
| Pubertal milestone | Z-score change 0 to 5 months | Z-score change 5 to 12 months | Z-score change 0 to 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| na | Unadjusted age difference | Adjusted age difference (95% CI)b | na | Unadjusted age difference | Adjusted age difference (95% CI)b | na | Unadjusted age difference | Adjusted age difference (95% CI)b | |
| Boys | |||||||||
| Tanner genital stage 2 | 3871 | −0.3 | −0.4 (−1.0–0.3) | 3399 | −1.1 | −0.9 (−2.0–0.1) | 3580 | −0.7 | −0.8 (−1.4– −0.1) |
| Tanner genital stage 3 | 3871 | −0.4 | −0.5 (−1.1–0.1) | 3399 | −1.5 | −1.4 (−2.5– −0.4) | 3580 | −0.7 | −0.9 (−1.5– −0.3) |
| Tanner genital stage 4 | 3871 | −0.3 | −0.4 (−1.0–0.2) | 3399 | −1.4 | −1.3 (−2.3– −0.3) | 3580 | −0.7 | −0.7 (−1.3– −0.1) |
| Tanner genital stage 5 | 3871 | −0.8 | −0.8 (−1.7–0.1) | 3399 | −1.2 | −1.3 (−2.8–0.3) | 3580 | −1.1 | −1.0 (−2.0– −0.1) |
| Tanner pubic hair stage 2 | 3872 | 0.0 | −0.1 (−0.7–0.5) | 3400 | −1.0 | −0.9 (−1.9–0.1) | 3581 | −0.6 | −0.6 (−1.2–0.0) |
| Tanner pubic hair stage 3 | 3872 | −0.3 | −0.5 (−1.1–0.0) | 3400 | −1.3 | −1.1 (−2.0– −0.2) | 3581 | −0.8 | −0.9 (−1.5– −0.4) |
| Tanner pubic hair stage 4 | 3872 | −0.4 | −0.5 (−1.0–0.1) | 3400 | −1.1 | −0.9 (−1.8–0.0) | 3581 | −0.8 | −0.7 (−1.2– −0.2) |
| Tanner pubic hair stage 5 | 3872 | −0.9 | −1.0 (−1.6– −0.3) | 3400 | −1.2 | −1.1 (−2.2–0.0) | 3581 | −1.4 | −1.4 (−2.1– −0.8) |
| Axillary Hair | 3875 | −1.2 | −1.3 (−1.9– −0.6) | 3403 | −1.2 | −1.0 (−2.1–0.2) | 3584 | −1.5 | −1.6 (−2.2– −0.9) |
| Acne | 3875 | −0.7 | −0.7 (−1.3– −0.1) | 3403 | −1.2 | −0.9 (−1.9–0.1) | 3584 | −1.2 | −1.1 (−1.7– −0.4) |
| Voice Break | 3791 | −0.3 | −0.4 (−1.0–0.2) | 3335 | −1.4 | −1.2 (−2.3– −0.1) | 3506 | −1.1 | −1.1 (−1.7– −0.5) |
| Adult Voice | 3791 | −0.6 | −0.7 (−1.7–0.3) | 3335 | −1.1 | −0.6 (−2.4–1.2) | 3506 | −1.0 | −1.0 (−2.0–0.1) |
| First ejaculation | 3870 | 0.2 | 0.2 (−0.5–0.8) | 3398 | −0.8 | −0.8 (−1.9–0.2) | 3579 | −0.2 | −0.3 (−0.9–0.3) |
| Girls | |||||||||
| Tanner breast stage 2 | 4198 | −0.3 | −0.2 (−1.1–0.6) | 3755 | −1.0 | −1.0 (−2.5–0.6) | 3969 | −0.5 | −0.6 (−1.4–0.3) |
| Tanner breast stage 3 | 4198 | −0.5 | −0.5 (−1.1–0.1) | 3755 | −0.8 | −0.9 (−1.8–0.1) | 3969 | −0.7 | −0.8 (−1.3– −0.2) |
| Tanner breast stage 4 | 4198 | −0.5 | −0.6 (−1.2–0.0) | 3755 | −1.2 | −1.4 (−2.4– −0.3) | 3969 | −0.9 | −1.1 (−1.6– −0.5) |
| Tanner breast stage 5 | 4198 | −0.6 | −0.6 (−1.7–0.4) | 3755 | −1.8 | −2.2 (−4.0– −0.3) | 3969 | −1.0 | −1.3 (−2.3– −0.3) |
| Tanner pubic hair stage 2 | 4199 | −0.6 | −0.5 (−0.9–0.0) | 3756 | −0.9 | −0.9 (−1.7– −0.1) | 3970 | −0.9 | −0.8 (−1.3– −0.3) |
| Tanner pubic hair stage 3 | 4199 | −1.0 | −1.0 (−1.5– −0.6) | 3756 | −1.0 | −1.0 (−1.8– −0.3) | 3970 | −1.2 | −1.3 (−1.8– −0.8) |
| Tanner pubic hair stage 4 | 4199 | −1.0 | −1.0 (−1.6– −0.4) | 3756 | −1.0 | −1.1 (−2.1– −0.2) | 3970 | −1.3 | −1.3 (−1.9– −0.8) |
| Tanner pubic hair stage 5 | 4199 | −1.5 | −1.5 (−2.4– −0.7) | 3756 | −0.1 | −0.2 (−1.7–1.3) | 3970 | −1.5 | −1.6 (−2.5– −0.8) |
| Axillary hair | 4202 | −1.3 | −1.3 (−1.9– −0.6) | 3759 | −0.9 | −0.6 (−1.7–0.5) | 3973 | −1.7 | −1.7 (−2.3– −1.1) |
| Acne | 4202 | −0.8 | −0.6 (−1.3–0.1) | 3759 | −1.4 | −1.0 (−2.3–0.2) | 3973 | −1.3 | −1.1 (−1.8– −0.4) |
| Menarche | 4198 | −0.7 | −0.5 (−1.0– −0.1) | 3755 | −1.6 | −1.7 (−2.5– −0.9) | 3969 | −1.2 | −1.2 (−1.7– −0.7) |
Notes: aNumber of observations in adjusted analyses. Numbers vary due to different numbers of observations with measurements from birth, 5 months, and 12 months. bAdjusted for parity, maternal age at menarche, maternal pre-pregnancy BMI, maternal smoking during the first, trimester and highest educational class of parents.
Abbreviations: AGA, appropriate-for-gestational age; BMI, body mass index; LGA, large-for-gestational age; SGA, small-for-gestational age.
The subanalyses of infant growth at 0 to 12 months stratified by birth weight Z-score (above or below the 33-percentile) revealed no signs of effect modifications (Table S3).
Discussion
SGA boys and girls reached pubertal milestones earlier on average, except for Tanner breast stages in girls. Boys born LGA reached pubertal milestones earlier, while girls born LGA reached milestones later. A linear relationship between birth weight Z-score and age at pubertal milestones was only observed among girls. In both sexes, an increase in weight Z-score from 0 to 12 months was associated with earlier age at reaching pubertal milestones.
Age at puberty in girls has declined during the last century.37 Whether age at onset of male puberty has declined is less certain.38 Determinants of pubertal onset remain largely unknown, but prenatal exposures have been suggested to play a role. Some prenatal exposures may cause a suboptimal growth environment and result in FGR.39 FGR has been related to insulin resistance and compensatory hyperinsulinism later in life through rapid weight gain in early childhood, which could lead to increased sex-hormone bioavailability and ultimately advanced timing of puberty.5 Although we were not able to specifically measure FGR, we used SGA as a surrogate measure.
Former literature on SGA boys and pubertal timing is limited, but some studies are consistent with our findings; two studies found that low birth weight was associated with earlier onset of pubic hair development27 and earlier age at onset of pubertal growth spurt.23 In contrast to our findings, some studies have reported no association between size-for-gestational age and Tanner genital stage25,26 or onset of pubertal growth spurt.21 One study found no heterogeneity by sex, and therefore calculated a joint estimate for Tanner genital stage 2 and breast stage 2 in boys and girls.25 Since we found no association between SGA and Tanner breast stage 2 in girls, earlier puberty among the boys, as found in our study, may have vanished in their joint estimate. Three studies only included few boys in the analyses.21,23,26 We observed earlier age at all indicators of pubertal development among LGA boys, except adult voice, though not statistically significant in all estimates. Wang et al (2012) found similar results with a trend toward earlier genital and pubic hair development in boys with higher birth weight Z-score.8
We found that SGA girls reached pubic hair stages, axillary hair, acne, and menarche earlier than AGA girls. This is in line with former studies,9,15,21 although the results from one of these were not statistically significant.15 In our study, SGA girls did not reach breast stages earlier than AGA girls. Hui et al (2012) also found no association between being born SGA and age at Tanner breast stage 2,25 though the estimate was based on both boys (Tanner genital stage 2) and girls as described above. The lack of association in our study could be explained by misclassification of the breast stages,40 or it could be primarily pubic hair development that is affected by growth and not breast development. In this study, higher birth weight Z-score and being LGA was associated with older age at pubertal development in girls. Several studies are consistent with these findings.11,16,18–20,22 Opposing to our results, two studies found no association between birth weight and age at menarche,13,17 and two found a higher birth weight to be associated with earlier pubertal development in girls.8,12 These studies were smaller than ours, participants were different ethnicities,13 and information on menarche was collected retrospectively in one study.17
When comparing boys and girls, birth weight seems to be associated with pubertal age differently. From our data, it seems that being SGA is associated with earlier puberty in both sexes, while being born LGA is associated with pubertal age in opposite directions. Our data and the current literature do not provide any explanation for the sex differences observed, and it should be a subject of future studies.
We found that an increase in weight Z-score from 0 to 12 months was associated with earlier puberty in both sexes. Rapid weight gain during early childhood has been associated with earlier puberty in former studies,8,12,14,15,23 but only two of these studies included boys.8,23 Especially the period from 5 to 12 months seemed important in the present study. We expected the strongest associations among infants born with the lowest birth weight Z-scores as these may be growth restricted and large infant growth would represent catch-up growth. Contrary to this expectation, size at birth did not modify the associations.
The major strength of this study is the large population with a relatively high participation and detailed information on pubertal development collected longitudinally.
A potential limitation is misclassification of self-reported variables. Data on birth weight and gestational age were collected from the birth registry, and data on weight at 5 and 12 months were self-reported by the mother at 18 months. It is unlikely that the misclassification is related to the pubertal timing of the children, as these data were collected before puberty, and therefore it is likely nondifferential. We used SGA as a proxy of FGR in this study. To identify the children that are truly growth restricted, one needs at least two measures of fetal size separated in time, eg, using ultrasound examination. Unfortunately, this information is rarely available. However, we believe that by using birth weight Z-scores, we capture most of the growth-restricted children in our analyses. In the subanalysis on infant growth, we had too low power to restrict the analyses to the SGA children and had to analyze the lowest third of birth weights. This might have affected our results since this group was less likely to be growth restricted than the true SGA group.
In a post-hoc exploratory analysis, we investigated whether the association between postnatal weight change in terms of change in weight Z-scores and pubertal timing was reasonably compatible with linearity. Specifically, we evaluated quadratic departure from linearity by including the change in Z-score between birth and 12 months as a second-order polynomial, and we found no statistically significant (P<0.05) quadratic departure from linearity, except for age at acne in girls. Thus, the association seemed to be reasonably compatible with linearity (data not shown).
Since data on puberty were collected longitudinally, recall bias is unlikely. Information on pubertal milestones was self-reported, which could introduce some nondifferential misclassification, since it is unlikely that the error of the reports depends on the children’s birth weight and weight gain. The collection of information started after some of the children entered pubertal stage 2, and hence a high proportion of early outcomes was left-censored. However, the estimates will be valid even in the presence of left-censoring as long as the pubertal milestones are normally distributed, which was supported by our model check. Children born SGA were less willing to participate in the cohort than children born AGA. Since only 29% chose not to participate, nonparticipation will most likely not bias the estimates considerably. Changes in weight Z-score after birth were only available in approximately 35% of all children in the Puberty Cohort, but since the mothers reported the weights before knowing the pubertal development of their child, the potential selection bias would likely be nondifferential. We were able to adjust for many important potential confounders, although the possibility of residual confounding from, eg, maternal or infant diet, cannot be ruled out.
Our data suggest that exposures in utero and infancy may affect pubertal development through prenatal and postnatal growth. Fetal life and infancy may provide a promising period of life where potential causes of earlier pubertal timing may exert their effect.
Conclusion
Our findings support the hypothesis that FGR measured by means of birth weight Z-score leads to earlier puberty in both sexes. Being born LGA was associated with later puberty in girls and earlier puberty in boys. Rapid weight gain in infancy was associated with earlier puberty in both sexes, with indications that the postnatal period from 5 to 12 months was of greater importance than the period from 0 to 5 months. This study indicates that both prenatal and postnatal growth affect pubertal development. Consequently, modifiable causes of altered prenatal and postnatal growth may provide explanations for the declining pubertal age.
Acknowledgment
This work was supported by the Danish Council for Independent Research [DFF 4183-00152 and 6166-00023]. The Danish Council for Independent Research was not involved in any stages from study design to submission of the paper.
The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation, and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation.
Follow-up of mothers and children has been supported by the Danish Medical Research Council (SSVF 0646, 271-08-0839/06-066023, O602-01042B, 0602-02738B), the Lundbeck Foundation (195/04, R100-A9193), The Innovation Fund Denmark 0603-00294B (09-067124), the Nordea Foundation (02-2013-2014), Aarhus Ideas (AU R9-A959-13-S804), University of Copenhagen Strategic Grant (IFSV 2012), and the Danish Council for Independent Research (DFF – 4183-00594 and DFF - 4183-00152).
Abbreviations
AGA, appropriate-for-gestational age; BMI, body mass index; DNBC, The Danish National Birth Cohort; FGR, fetal growth restriction; HPG axis, hypothalamic–pituitary–gonadal axis; LGA, large-for-gestational age; SGA, small-for-gestational age.
Data availability
The dataset analyzed in the study is not publicly available due to national data security legislation on sensitive personal data.
Ethics approval and informed consent
The study was approved by the Steering Committee of the DNBC (2017-06). Data in DNBC and The Puberty Cohort were registered by the Danish Data Protection Agency (journal number 2012-41-0379 and 2015-57-0002). When the DNBC was established, the Committee on Biomedical Research Ethics approved the data collection ((KF) 01-471/94). Written consent was obtained from all women at recruitment.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57 Suppl 2(Suppl 2):2–14. doi: 10.1159/000058094 [DOI] [PubMed] [Google Scholar]
- 2.Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–264. doi: 10.1016/S2213-8587(15)00418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46(1):4–14. doi: 10.1111/j.1479-828X.2006.00506.x [DOI] [PubMed] [Google Scholar]
- 4.Roth CL, DiVall S. Consequences of early life programing by genetic and environmental influences: a synthesis regarding pubertal timing. Endocr Dev. 2016;29:134–152. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed ML, Ong KK, Dunger DB. Childhood obesity and the timing of puberty. Trends Endocrinol Metab. 2009;20(5):237–242. doi: 10.1016/j.tem.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Cho WK, Suh BK. Catch-up growth and catch-up fat in children born small for gestational age. Korean J Pediatr. 2016;59(1):1–7. doi: 10.3345/kjp.2016.59.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam CS, de Zegher F, Garnett SP, Baur LA, Cowell CT. Opposing influences of prenatal and postnatal growth on the timing of menarche. J Clin Endocrinol Metab. 2006;91(11):4369–4373. doi: 10.1210/jc.2006-0953 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Dinse GE, Rogan WJ. Birth weight, early weight gain and pubertal maturation: a longitudinal study. Pediatr Obes. 2012;7(2):101–109. doi: 10.1111/j.2047-6310.2011.00022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koziel S, Jankowska EA. Effect of low versus normal birthweight on menarche in 14-year-old polish girls. J Paediatr Child Health. 2002;38(3):268–271. [DOI] [PubMed] [Google Scholar]
- 10.Adair LS. Size at birth predicts age at menarche. Pediatrics. 2001;107(4):e59–e59. doi: 10.1542/peds.107.4.e59 [DOI] [PubMed] [Google Scholar]
- 11.Sloboda DM, Hart R, Doherty DA, Pennell CE, Hickey M. Age at menarche: influences of prenatal and postnatal growth. Int J Clin Endocrinol Metab. 2007;92(1):46–50. doi: 10.1210/jc.2006-1378 [DOI] [PubMed] [Google Scholar]
- 12.Terry MB, Ferris JS, Tehranifar P, Wei Y, Flom JD. Birth weight, postnatal growth, and age at menarche. Am J Epidemiol. 2009;170(1):72–79. doi: 10.1093/aje/kwp095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epplein M, Novotny R, Daida Y, Vijayadeva V, Onaka AT, Le Marchand L. Association of maternal and intrauterine characteristics with age at menarche in a multiethnic population in Hawaii. Cancer Cause Control. 2010;21(2):259–268. doi: 10.1007/s10552-009-9457-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos Silva I, De Stavola BL, Mann V, Kuh D, Hardy R, Wadsworth MEJ. Prenatal factors, childhood growth trajectories and age at menarche. Int J Epidemiol. 2002;31(2):405–412. doi: 10.1093/ije/31.2.405 [DOI] [PubMed] [Google Scholar]
- 15.Maisonet M, Christensen KY, Rubin C, et al. Role of prenatal characteristics and early growth on pubertal attainment of British girls. Pediatrics. 2010;126(3):e591–e600. doi: 10.1542/peds.2009-2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper C, Kuh D, Egger P, Wadsworth M, Barker D. Childhood growth and age at menarche. Br J Obstet Gynaecol. 1996;103(8):814–817. doi: 10.1111/j.1471-0528.1996.tb09879.x [DOI] [PubMed] [Google Scholar]
- 17.Blell M, Pollard TM, Pearce MS. Predictors of age at menarche in the newcastle thousand families study. J Biosoc Sci. 2008;40(4):563–575. doi: 10.1017/S0021932007002696 [DOI] [PubMed] [Google Scholar]
- 18.Behie AM, O’Donnell MH. Prenatal smoking and age at menarche: influence of the prenatal environment on the timing of puberty. Hum Reprod. 2015;30(4):957–962. doi: 10.1093/humrep/dev033 [DOI] [PubMed] [Google Scholar]
- 19.D’Aloisio AA, DeRoo LA, Baird DD, Weinberg CR, Sandler DP. Prenatal and infant exposures and age at menarche. Epidemiology. 2013;24(2):277–284. doi: 10.1097/EDE.0b013e31828062b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dossus L, Kvaskoff M, Bijon A, et al. Determinants of age at menarche and time to menstrual cycle regularity in the French E3N cohort. Ann Epidemiol. 2012;22(10):723–730. doi: 10.1016/j.annepidem.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 21.Persson I, Ahlsson F, Ewald U, et al. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am J Epidemiol. 1999;150(7):747–755. doi: 10.1093/oxfordjournals.aje.a010077 [DOI] [PubMed] [Google Scholar]
- 22.Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Determinants of age at menarche in the UK: analyses from the breakthrough generations study. Br J Cancer. 2010;103(11):1760–1764. doi: 10.1038/sj.bjc.6605978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karaolis-Danckert N, Buyken AE, Sonntag A, Kroke A. Birth and early life influences on the timing of puberty onset: results from the DONALD (DOrtmund nutritional and anthropometric longitudinally designed) study. Am J Clin Nutr. 2009;90(6):1559–1565. doi: 10.3945/ajcn.2009.28259 [DOI] [PubMed] [Google Scholar]
- 24.Romundstad PR, Vatten LJ, Nilsen TI, et al. Birth size in relation to age at menarche and adolescent body size: implications for breast cancer risk. Int J Cancer. 2003;105(3):400–403. doi: 10.1002/ijc.11103 [DOI] [PubMed] [Google Scholar]
- 25.Hui LL, Leung GM, Wong MY, Lam TH, Schooling CM. Small for gestational age and age at puberty: evidence from Hong Kong’s “Children of 1997” birth cohort. Am J Epidemiol. 2012;176(9):785–793. doi: 10.1093/aje/kws159 [DOI] [PubMed] [Google Scholar]
- 26.Veening MA, van Weissenbruch MM, Roord JJ, de Delemarre-van Waal HA. Pubertal development in children born small for gestational age. J Pediatr Endocrinol Metab. 2004;17(11):1497–1505. [DOI] [PubMed] [Google Scholar]
- 27.Monteilh C, Kieszak S, Flanders WD, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25(1):75–87. doi: 10.1111/j.1365-3016.2010.01168.x [DOI] [PubMed] [Google Scholar]
- 28.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. [DOI] [PubMed] [Google Scholar]
- 29.Brix N, Ernst A, Lauridsen LLB, et al. Maternal smoking during pregnancy and timing of puberty in sons and daughters: a population-based cohort study. Am J Epidemiol. 2019;188(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi: 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- 31.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85(7):843–848. doi: 10.1111/j.1651-2227.1996.tb14164.x [DOI] [PubMed] [Google Scholar]
- 32.Cole TJ, Williams AF, Wright CM, Group RGCE. Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol. 2011;38(1):7–11. doi: 10.3109/03014460.2011.544139 [DOI] [PubMed] [Google Scholar]
- 33.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 36.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693. doi: 10.1210/er.2002-0019 [DOI] [PubMed] [Google Scholar]
- 37.Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 2006;254–255:8–12. doi: 10.1016/j.mce.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 38.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D [DOI] [PubMed] [Google Scholar]
- 39.Nardozza LM, Caetano AC, Zamarian AC, et al. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017;295(5):1061–1077. doi: 10.1007/s00404-017-4341-9 [DOI] [PubMed] [Google Scholar]
- 40.Terry MB, Goldberg M, Schechter S, et al. Comparison of clinical, maternal, and self pubertal assessments: implications for health studies. Pediatrics. 2016;138(1):e20154571–e20154571. doi: 10.1542/peds.2015-4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed in the study is not publicly available due to national data security legislation on sensitive personal data.