Abstract
Individuals with amnestic mild cognitive impairment (aMCI) experience cognitive declines in learning and memory greater than expected for normal aging, and are at a high risk of dementia. We previously reported that sedentary aMCI patients exhibited neuroinflammation that correlated with brain amyloid beta (Aβ) burden, as determined by 18F-florbetapir positron emission tomography (PET). These aMCI patients enrolled in a one-year randomized control trial (AETMCI, ) to test the beneficial effects of 12 months of moderate-to-high intensity aerobic exercise training (AET) or stretching/toning (ST) control intervention on neurocognitive function. A subset of aMCI participants had PET imaging, cognitive testing, and immunophenotyping of cerebrospinal fluid (CSF) and peripheral blood after AET or ST interventions. As adaptive immune responses were similar between AET and ST groups, we combined AET/ST into a general ‘physical activity’ (PA) group and compared Aβ burden, cognitive function, and adaptive immune cell subsets to sedentary lifestyle before intervention. We found that PA-induced immunomodulation of CD4+ and CD8+ T cells in CSF correlated with changes in Aβ burden in brain regions associated with executive function. Furthermore, after PA, cognitive scores on tests of memory, processing speed, attention, verbal fluency, and executive function were associated with increased percent representation of circulating naïve B cells and CD8+ T cells. We review the literature on aMCI-related cognition and immune changes as they relate to exercise, and highlight how our preliminary data suggest a complex interplay between the adaptive immune system, physical activity, cognition, and Aβ burden in aMCI.
Keywords: amnestic mild cognitive impairment, physical activity, adaptive immunity, lymphocytes, PET amyloid imaging
INTRODUCTION
Individuals with amnestic mild cognitive impairment (aMCI) experience cognitive decline greater than expected for normal aging, and exhibit primary impairments in learning and memory (1). More than half of patients with aMCI exhibit increased amyloid beta (Aβ) deposition in the brain and progress to dementia (2, 3). Currently, there are no approved therapies to prevent and protect against aMCI (4), but a growing body of literature provides evidence for the beneficial effects of exercise interventions (5). Physical activity (PA) has been reported to reduce the incidence of aMCI, as well as improve cognitive function in multiple domains (6), including global cognition, attention, executive functioning, verbal fluency, and memory (7–11).
One mechanism that may explain the positive effects of PA in aMCI patients is exercise-induced alterations within the immune system. To date, only 3 studies have investigated whether exercise modulates inflammation in patients with MCI (12–14), primarily focusing on post-exercise serum concentrations of cytokines, small molecules secreted by leukocytes that impact the function and phenotype of other immune cells (12–14). While these studies utilized different exercise training regimens, they generally conclude that exercise increases anti-inflammatory cytokines while concomitantly reducing pro-inflammatory cytokine serum levels in patients with MCI (12–14). The one study that examined changes in leukocytes after exercise found circulating lymphocyte (i.e. adaptive immune cell) populations reduced by 28 weeks of strength training in MCI patients (12). Together, these studies suggest that exercise impacts the immune system of aMCI patients, but provide limited information about the effect of PA on particular immune cell subsets, specifically B and T cells, and how immune changes after PA may be related to cognitive performance.
Previously, we examined baseline data from an aMCI cohort enrolled in a one-year randomized control trial (AETMCI, ) to test the efficacy of 12 months of moderate-to-high intensity aerobic exercise training (AET) on cognition compared to a stretching/toning (ST) control group. Participants underwent AET that progressively increased in intensity for 26 weeks, at which point they underwent 4–5 exercise sessions weekly with 2–3 moderate intensity training sessions (75–85% of maximal heart rate) and 2 high intensity sessions (85–90% of maximal heart rate). ST cohorts received similar attention from, and interactions with, investigators and underwent weekly bouts of stretching and toning (<50% of maximal heart rate). We found that sedentary aMCI patients exhibited elevated immune cells in the CSF (15) that correlated with increased Aβ burden in the brain, as determined by 18F-florbetapir positron emission tomography (PET) (16). The analysis of adaptive immune cells within the AETMCI trial was only available during the final year of enrollment, and thus did not include the entire aMCI patient data set. Nevertheless, we sought to elucidate whether PA altered adaptive immune cell subsets in individuals with aMCI. Specifically, we examined how PA affected B and T cells in the blood and CSF, and whether immunomodulation was associated with changes in either cognitive function or brain Aβ burden. Collectively, our preliminary data suggested a complex interplay between the adaptive immune system, cognition, and Aβ burden in aMCI patients and support the hypothesis that PA could be a beneficial therapeutic intervention for patients with aMCI.
METHODS
Participant characteristics:
All subjects enrolled in this study (15–17) provided written informed consent approved by UT Southwestern (UTSW) Medical Center and Texas Health Presbyterian Hospital of Dallas, Texas Institutional Review Boards. Subjects enrolled in the AETMCI () clinical trial gave additional consent for lumbar puncture, blood draw, and PET imaging. The diagnosis of aMCI was based upon standard Petersen criteria (18), as modified from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) project (http://adni-info.org). Diagnostic criteria included a global Clinical Dementia Rating (CDR) scale of 0.5 in the memory category, objective memory loss as demonstrated by scores on the Logical Memory subtest of the Wechsler Memory Scale-Revised, and a score between 24 and 30 on the Mini-Mental State Exam (MMSE).
Participants were randomized into either a moderate to high-intensity aerobic exercise training (AET) or active stretching/toning (ST) assignment. Exclusion criteria included a diagnosis of AD or other types of dementia or a diagnosis of a major neurological, vascular, or psychiatric disorder. Participants with a history of regular exercise in the last 2 years, body mass index ≥35 kg/m2, sleep disorders including clinically diagnosed or self-reported sleep apnea, uncontrolled hypertension, diabetes, and a history of smoking within the past 2 years were excluded from the study. Patients who spent >90 minutes of moderate-to-high intensity PA, as assessed by 1 week of monitoring with an Actial accelerometer (Actical, Philips Respironics, USA), were also excluded. Further information about the inclusion/exclusion criteria is located at ClinicalTrials.gov: and in previous publications (16, 17).
Because we found no difference in lymphocyte populations in the blood and CSF between patients that underwent ST and AET, ST and AET cohorts were pooled into a PA group (Table 1). Nineteen subjects at baseline, and 18 subjects after PA, had blood draws. Fourteen subjects at baseline, and 13 subjects after PA, had lumbar punctures. Sixteen subjects at baseline, and 17 subjects after PA, underwent both cognitive testing and immunophenotyping in the blood. Only 5 aMCI (2 from the ST group, 3 from the AET group) participants had pre- and post-PA sample collection of peripheral blood, and 3 subjects (1 from the ST group, 2 from the AET group) did not have 12-month sample collection, so 6 month samples were used. Forty-one patients underwent PET imaging at baseline, and 12 patients had post-PA imaging.
Table 1.
Demographics- cognitive testing and immunophenotyping cohort
| Pre-PA group (n=16) | Post-PA group (n=17) | Mann-Whitney (p value) | |
|---|---|---|---|
| Aqe (55–76) | 64.1 (6.0) | 65.4 (6.6) | 0.54 |
| Race (% Caucasian) | 87.5 | 88.2 | 0.66 |
| Gender (% Female) | 56.2 | 52.9 | 0.99 |
| Education (12–18) | 15.7 (2.4) | 16.0 (2.4) | 0.62 |
Mean (Standard deviation)
Aerobic Exercise Training (AET):
Exercise dose and intensity for the AET cohort was determined for each participant’s fitness level by assessing the peak oxygen uptake (VO2) and progressively increasing exercise intensity as individual’s adapted to previous weeks of AET. During the initiation phase of AET, participants underwent three 25–30 minute aerobic exercise sessions per week at an intensity of 75–85% maximal heart rate as measured during peak VO2 at baseline. By week 11 of the intervention, participants had three to four 30–35 minute exercise sessions weekly. On weeks in which participants performed 3 exercise sessions, participants also performed a high intensity exercise session of 30 minutes of brisk uphill walking (85–90% maximal heart rate). By week 26, participants underwent four to five 30–40 minute exercise sessions per week, including two high intensity sessions. During each session, participants had a 5 minute warm-up and 5 minute cool-down. Any form of aerobic exercise was permitted as long as appropriate dose and intensity of training was achieved, as assessed by heart rate during the exercise session by a heart rate monitor watch (Polar RS400, Polar Electro, USA). The AET protocol met the national standards for physical activity guidelines for older adults. Our previous studies found that AET significantly improves cardiorespiratory fitness in sedentary individuals over the age of 65 (19).
ST:
The control ST cohort underwent a stretching/toning regimen that focused on the upper arm and lower body with the same frequency and duration as described in the AET protocol above. At week 19, we introduced a second, more advanced set of full body stretching. At week 26, patients began a set of low resistance Theraband exercises focusing on strengthening the upper and lower body. Participants were required to keep their heart rate below 50% of maximal heart rate as measured by heart monitor watch during each ST session. By using our ST cohort as an active control group we ensured that participants received similar attention from, and interactions with, investigators as those randomized to the AET group.
Immune cell collection and analysis:
Biosample collections were performed through The University of Texas Southwestern Medical Center Alzheimer’s Disease Center using established protocols (15). Collection was generally performed during morning visits and processing within 60 minutes of collection. Peripheral blood mononuclear cells (PBMC) and cerebrospinal fluid (CSF) immune cells were isolated by centrifugal Ficoll-based separations previously described (16). CSF samples tinged red were excluded from further analysis. PBMCs and CSF cells were stained with fluorescent antibodies and data acquired via flow cytometry. Our multi-parameter antibody panel consisted of CD45 (APC-Cy7), CD4 (PECy7), CD8 (APC), CD19 (PerCPCy5.5 or Brilliant Violet 421), CD27 (FITC-A), and CD138 (PE-A) antibodies (BD Biosciences, San Jose, CA, USA). Five patients at baseline and 3 PA subjects (ST n=1, AET n=2) did not have CD3 staining. No live/dead stain was used as cells were rapidly processed after collection. Gating strategies were previously published (16). Flow cytometry data were analyzed (Flowjo; Tree Star) and normalized to the CD45+ cell gate to compare over time and across samples. CSF cell analysis included all cells collected, with no minimum cell number required to perform flow cytometry.
PET imaging:
aMCI patients received a bolus of 10-mCi 18F-florbetapir 30-min prior to positioning in a Siemens ECAT HR PET scanner for a 10-min emission and 10-min transmission scan, as previously described in detail (16). Fifty minutes after tracer injection, a 5-min PET emission scan and a 7-min transmission scan were acquired. Every PET image was normalized spatially to 18F-florbetapir uptake template using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) and MATLAB scripts (Mathworks Inc., Natick, MA) and inspected for quality. Standardized uptake value ratio (SUVR) was computed and compared to mean cerebellar uptake as a brain reference. The mean cortical SUVR was the average of: posterior and anterior cingulate, precuneus, temporal, dorso-lateral prefrontal, orbitofrontal, parietal, and occipital SUVRs (17).
Neurocognitive tests:
aMCI diagnosis was based on Petersen criteria (18), as modified from Alzheimer’s Disease Neuroimaging Initiative (ADNI) project (http://adniinfo.org). Cognitive testing (Table 2) included well validated measures of attention/concentration (Digit Span Forward and Backwards), processing speed (Trails A), memory (California Verbal Learning Test; CVLT), Wechsler Memory Scale-Logical Memory Immediate Recall); LMIR verbal fluency (Letter Fluency, Semantic Fluency); LMIR, and executive functioning (Trails B, Stroop Color Word Interference Test). All tests but LMIR (raw score) and CVLT (t-score) and with all cognitive function tests, higher scores reflect better cognitive function.
Table 2.
Summary of cognitive scores by group
| Pre-PA group (n=16) | Post-PA group (n=17) | Mann-Whitney (p value) | |||
|---|---|---|---|---|---|
| Cognitive tests by domain | Mean | SD | Mean | SD | |
| Attention and Concentration | |||||
| Digit Span Forward | 8.07 | 2.58 | 8.35 | 2.37 | 0.786 |
| Digit Span Backwards | 6.2 | 2.51 | 6.65 | 2.45 | 0.565 |
| Processing Speed | |||||
| Trails A | 11.94 | 2.14 | 13.38 | 1.85 | 0.019* |
| WAIS-R Coding | 11.5 | 2.42 | 12.06 | 2.32 | 0.579 |
| Memory | |||||
| Logical Memory Immediate Recall | 11.88 | 1.36 | 12.76 | 2.44 | 0.266 |
| CVLT Total (t-score) | 49.19 | 9.88 | 49.35 | 12.48 | 0.715 |
| Verbal Fluency | |||||
| Letter Fluency | 10.25 | 1.92 | 12.13 | 3.56 | 0.145 |
| Semantic Fluency | 9.88 | 3.42 | 12.73 | 3.52 | 0.043* |
| Executive Function | |||||
| Trails B | 11.69 | 2.06 | 12.06 | 2.25 | 0.672 |
| Color Word Interference Test | 10.47 | 1.88 | 11.13 | 2.42 | 0.238 |
CVLT, California Verbal Learning Test;
p<0.05 with significant values bolded
Statistical analysis:
All data are reported as mean ± standard deviation, with statistical significance set a priori at p<0.05 for all tests and trending values were defined as p≤0.06. Kruskal-Wallis tests were performed to compare immune populations between baseline, AET, and ST cohorts. Mann-Whitney tests were performed to compare the baseline and the overall PA sample (composed of both AET and ST groups) and to compare age, education level, CDR, and cognitive results between groups as appropriate. Fisher’s Exact tests were performed to see if sex or race differed between groups. Linear regressions were performed to examine the relationships between adaptive immune populations, brain Aβ burden, and cognitive domains. Multiple comparison correction was not performed for this exploratory study and all statistical analyses were performed using GraphPad Prism (La Jolla, CA).
RESULTS
Physical activity does not modulate frequency of B and T cells in aMCI patients
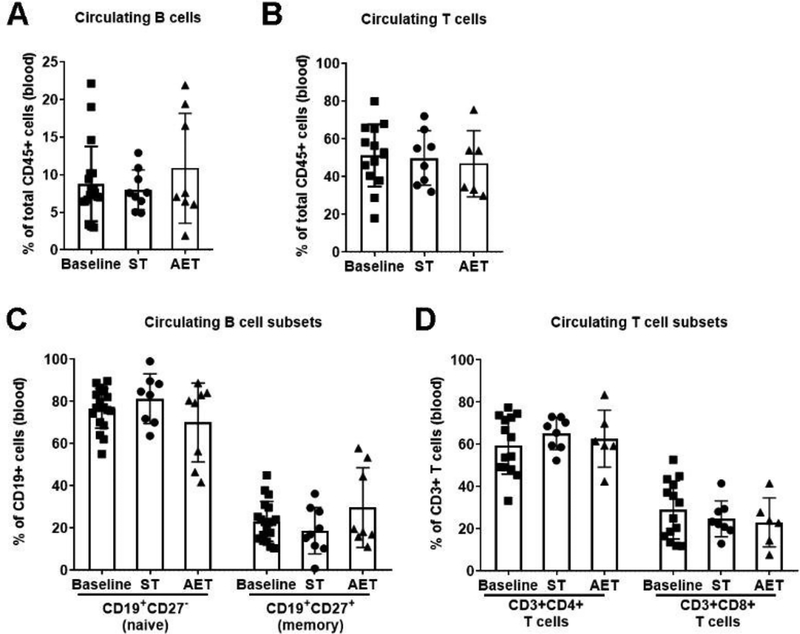
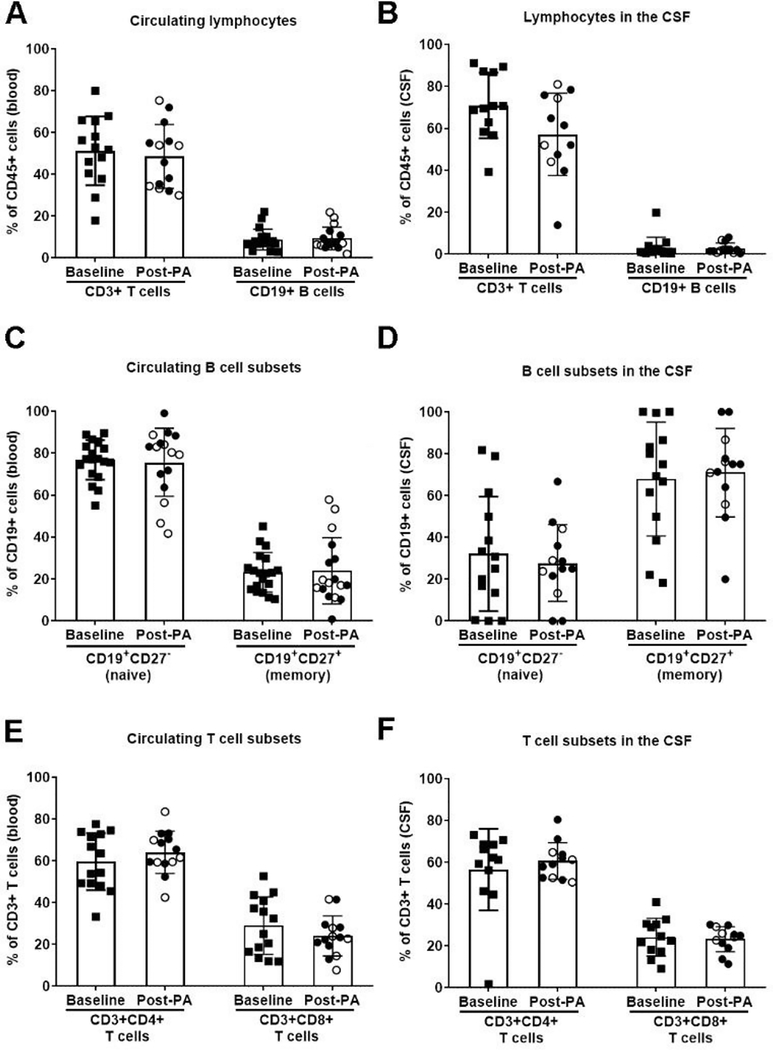
To determine if PA impacted adaptive immunity in the periphery and/or central nervous system (CNS), we analyzed B and T cell subsets in the blood and CSF isolated from a subset of aMCI patients at baseline (n=19) and subsets of aMCI patients after either AET (n=8) or ST (n=9) intervention. Overall, CD19+ B cells and CD3+ T cells in the CSF (data not graphed) and blood (Fig. 1A–B) did not differ between interventions. Furthermore, there was no difference in any circulating B or T cell subset, including naïve B cells, memory B cells, CD4+ T cells, and CD8+ T cells (Fig. 1C–D). Given no observable differences in the distribution of B and T cells in the blood and CSF, AET and ST cohorts were pooled. After PA, B and T cells (and their respective subsets) did not differ from baseline in either CSF or blood (Fig. 2). Our preliminary data from this pilot sample of aMCI participants suggests that the distribution of adaptive immune cells in the CSF and blood do not change after an extended period of PA.
Figure 1. Aerobic exercise training and stretching/toning exert minimal effects on adaptive immune cell populations in aMCI patients.
General (A) B cell (CD19+) and (B) T cell (CD3+) populations in the blood do not differ between sedentary baseline (squares; n=19) and individuals in the stretching/toning (ST; circles; n=9) and aerobic exercise training (AET; triangles; n=8) interventions. There is also no difference for circulating (C) B cell subsets (baseline, n=19; ST, n=9; AET, n=8) and (D) T cell subsets in the blood. 3 individuals were excluded from overall T cell and T cell subset quantification due to insufficient CD3+ staining.
Figure 2. Physical activity does not alter adaptive immune profiles in aMCI patients.
General T cell (CD3+) and B cell (CD19+) populations in (A) blood or (B) cerebrospinal fluid (CSF) do not differ between sedentary baseline (squares) and physical activity (PA) groups, including individuals in the stretching/toning (closed circles) and aerobic exercise training (open circles) interventions. There is also no difference for B cell subsets in the (C) blood and (D) CSF, as well as T cell subsets in the (E) blood and (F) CSF..
B cells were associated with hippocampal Aβ burden
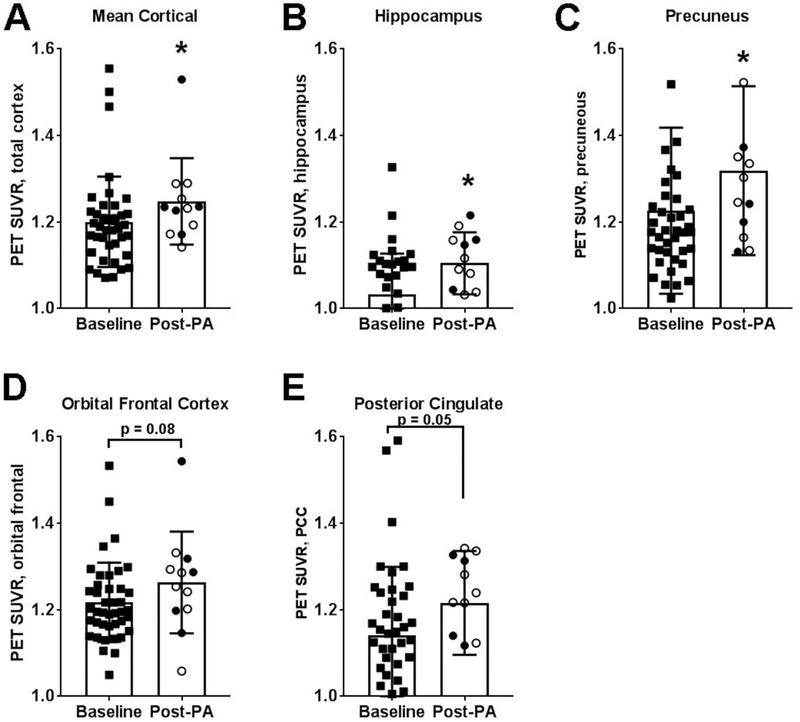
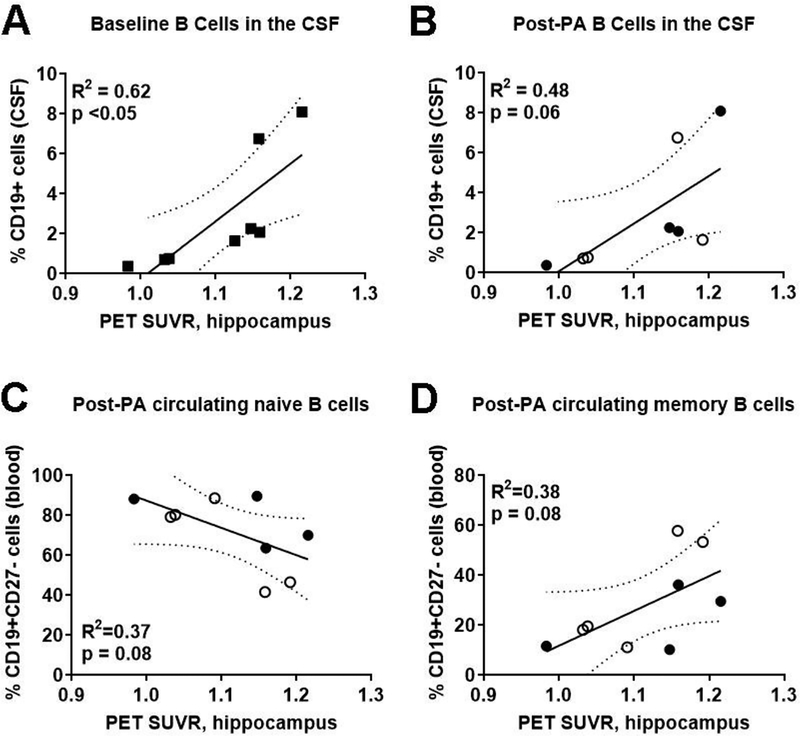
To understand the relationship between adaptive immunity and Aβ burden, we first examined whether PA altered Aβ burden in multiple regions of the brain. In aMCI patients, we identified a significant increase in mean cortical Aβ burden (p<0.05) and Aβ deposition in the hippocampus (p<0.05) and precuneus (p<0.05) post-PA (Fig. 3). There was also a trending increase in Aβ burden in the posterior cingulate (p=0.05; Fig. 3E). Next, we sought to determine if there were correlations between Aβ burden and overall B cell populations in the CSF and blood both before and after PA. For sedentary aMCI patients prior to PA, those with higher Aβ burden in the hippocampus exhibited higher B cell representation in the CSF (R2=0.62; p<0.05; n=8; Fig. 4A). This relationship persisted after PA; more B cells in the CSF were associated with greater hippocampal Aβ deposition (R2=0.48; p=0.06; Fig. 4B). There was no correlation between hippocampal Aβ deposition and overall B cells in the blood at baseline (R2=0.024, p=0.69) and after PA (R2=0.025, p=0.68).
Figure 3. Overall amyloid burden increased between sedentary baseline and after 6–12 month of physical activity.
Standardized uptake value ratio (SUVR) for PET imaging of 18F-florbetapir (amyloid burden) demonstrated a significant increase in Aβ load for (A) total brain, (B) hippocampus, and (C) precuneous cortices between sedentary baseline (squares; n=41) and physical activity (PA) groups, including individuals in the ST (closed circles; n=5) and aerobic exercise training (open circle; n=7) interventions. There were also trends for increased Aβ in the (D) orbital frontal and (E) posterior cingulate cortices. *p<0.05 vs Baseline.
Figure 4. Higher levels of CSF-localized B cells and circulating memory B cells associate with increased hippocampal Aβ deposition.
Standard ized uptake value ratio (SUVR) for PET imaging of 18F-florbetapir (amyloid burden) in the hippocampus associated with higher CD19+ B cells in the cerebrospinal fluid (CSF) for both (A) sedentary baseline (squares; n=8) and (B) physical activity (PA) groups, including individuals in the ST(closed circles; n=4) and AET (open circle; n=4) interventions. There were concomitant trends for (C) decreased circulating naïve B cells and (D) increased circulating memory B cells after PA.
Finally, we examined correlations between Aβ burden and particular subsets of B cells. At baseline and after PA, neither CSF-derived naïve nor memory B cell populations were associated with Aβ burden in the hippocampus (naïve-R2=0.31; memory-R2=0.33). Similarly, there were no correlations between either subset in the blood (naïve-R2=0.25; memory-R2=0.18) at baseline. After a one-year PA intervention, however, participants with increased hippocampal Aβ deposition had fewer naïve B cells (R2=0.37; p=0.08) and more memory B cells in the blood (R2=0.38; p=0.08), signifying a shift, albeit non-significant, in B cell phenotype (Fig. 4C–D). Other brain regions examined by PET (anterior and posterior cingulate, dorsolateral prefrontal cortex, frontal cortex, orbitofrontal cortex, precuneus, and temporal lobe) were not associated with changes in B cells or associated B cell subsets.
Peripheral B cell subsets differentially associated with cognitive test results
We examined whether there was a relationship between immune cell subsets and cognitive function, and if this differed after PA relative to baseline. First, we examined whether PA affected neurocognitive functioning in our pooled PA sample. Following PA, aMCI subjects performed similarly across most tests but showed slight but statistically significant improvements on tests of processing speed and semantic fluency (Table 2). The frequency of overall CD19+ B cells in the CSF did not correlate with any measures of cognitive function at either baseline or after PA. Circulating CD19+ B cells correlated with performance on only one measure of processing speed (Table 3), with fewer B cells in the blood associated with higher scores on the Trails A task (R2=0.45; p<0.01). However, the inverse was true after PA as a higher frequency of circulating B cells correlated with better performance (R2=0.30; p<0.05; data not graphed).
Table 3.
Linear regression of adaptive lymphocyte populations vs. neurocognitive outcomes
| CD3+ T cells | CD4+ T cells | CD8+ T cells | CD19+ B cells | Naïve | Memory | CD3+ T cells | CD4+ T cells | CD8+ T cells | CD19+ B cells | Naïve | Memory | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attention and Concentration | |||||||||||||
| Digit Forward | 0.00 | 0.21 | 0.29 | 0.00 | 0.00 | 0.00 | 0.02 | 0.05 | 0.11 | 0.02 | ↓0.48** | ↑O.48** | |
| 0.14 | 0.00 | 0.00 | 0.02 | ↑0.27* | ↓0.25* | 0.00 | 0.06 | 0.01 | 0.05 | 0.12 | 0.12 | ||
| Digit | 0.02 | 0.01 | 0.01 | 0.00 | 0.02 | 0.02 | 0.00 | 0.04 | 0.01 | 0.03 | 0.05 | 0.04 | |
| Backwards | 0.00 | 0.03 | 0.00 | 0.00 | ↑0.25* | ↓0.25* | 0.00 | 0.12 | 0.12 | 0.02 | 0.00 | 0.01 | |
| Processing Speed | |||||||||||||
| Trails A | 0.11 | 0.01 | 0.00 | ↓0.45** | 0.16 | 0.16 | 0.00 | 0.04 | 0.04 | 0.00 | 0.09 | 0.07 | |
| 0.32 | 0.14 | ↓0.35* | ↑0.30* | 0.10 | 0.12 | 0.23 | 0.09 | 0.28 | 0.33 | 0.02 | 0.01 | ||
| WAIS-R | 0.07 | 0.11 | 0.10 | 0.05 | 0.08 | 0.09 | 0.00 | 0.05 | 0.05 | 0.12 | 0.01 | 0.02 | |
| Coding | 0.13 | 0.19 | 0.27 | 0.07 | 0.21 | 0.23 | 0.28 | 0.00 | 0.01 | 0.11 | 0.03 | 0.05 | |
| Memory | |||||||||||||
| LMIR | ↓0.40* | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.31 | 0.03 | 0.03 | 0.04 | 0.01 | 0.01 | |
| 0.07 | 0.05 | 0.02 | 0.00 | 0.03 | 0.03 | 0.06 | 0.00 | 0.05 | 0.14 | 0.01 | 0.01 | ||
| CVLT Total (t- | 0.01 | 0.13 | 0.06 | 0.15 | 0.00 | 0.00 | 0.01 | 0.12 | 0.00 | 0.05 | 0.03 | 0.02 | |
| score) | ↓0.36* | 0.02 | 0.00 | 0.01 | 0.11 | 0.10 | 0.08 | 0.12 | 0.08 | 0.03 | 0.00 | 0.00 | |
| Verbal Fluency | |||||||||||||
| Letter Fluency | 0.13 | ↓0.47** | ↑0.53** | 0.07 | 0.05 | 0.05 | 0.05 | 0.14 | 0.12 | 0.11 | ↓0.40* | ↑0.41* | |
| 0.28 | 0.10 | 0.01 | 0.02 | 0.04 | 0.03 | 0.01 | 0.00 | 0.15 | 0.00 | 0.12 | 0.08 | ||
| Semantic | 0.04 | ↓0.51** | ↑0.59** | 0.16 | 0.02 | 0.03 | 0.10 | 0.04 | 0.00 | 0.03 | 0.06 | 0.04 | |
| Fluency | ↓0.33* | 0.01 | 0.07 | 0.20 | 0.12 | 0.12 | 0.14 | 0.00 | 0.11 | 0.30 | 0.04 | 0.04 | |
| Executive Function | |||||||||||||
| Trails B | 0.13 | 0.01 | 0.01 | 0.01 | 0.01 | 0.04 | 0.03 | 0.09 | 0.06 | 0.00 | 0.22 | 0.21 | |
| 0.16 | ↑0.43* | ↓0.41* | 0.04 | 0.11 | 0.12 | 0.20 | 0.02 | 0.00 | 0.01 | 0.09 | 0.11 | ||
| CWIT | 0.13 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.03 | 0.00 | 0.03 | 0.07 | 0.02 | 0.02 | |
| 0.11 | 0.19 | 0.22 | 0.06 | 0.06 | 0.06 | ↑0.39* | 0.03 | 0.00 | 0.02 | 0.03 | 0.05 | ||
CWIT: Color Word Interference Test; LMIR, Logical memory Immediate Recall; up arrows indicate positive correlation, down arrows indicate negative correlation;
p<0.05;
p<0.01
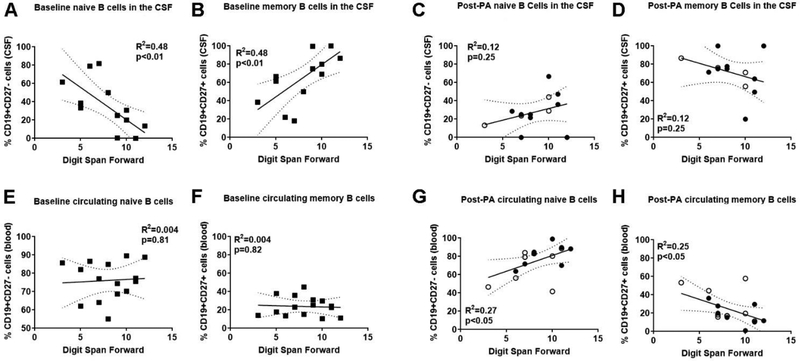
Subset-specific relationships between naïve and memory B cells and neurocognitive functioning were also examined. First, fewer naïve B cells (R2=0.48; p<0.01) and more memory B cells (R2=0.48; p<0.01) in the CSF associated with higher scores on measures of attention and concentration (Fig. 5A–B), as well as verbal fluency (Table 3) at baseline. After PA, all correlation between CSF-localized B cell subsets and performance on any neurocognitive task was lost (Fig. 5C–D). In the blood, baseline levels of circulating B cell subsets did not correlate with any measure of cognitive function (Fig. 5 E–F; Table 3). However, after PA, attention and concentration scores correlated with the frequency of B cell subsets in the blood. Specifically, a higher frequency of naïve B cells (R2=0.27; p<0.05) and lower frequency of memory B cells in the blood (R2=0.25; p<0.05) associated with higher attention and concentration scores (Fig. 5 G–H). Together, this shows that naïve and memory B cells and cognitive correlates were minimal and varied based on the tissue and time point examined.
Figure 5. Shifts from naïve to memory B cell populations reflect neurocognitive function.
(A-D) Sedentary baseline (squares; n=15) aMCI subjects with better attention and concentrations (i.e. higher numbers, digital span forward test) exhibit (A) lower naïve B cells and (B) more memory B cells in the cerebrospinal fluid (CSF). This relationship in the CSF is lost in post-physical activity (PA) groups for (C) naïve and (D) memory B cells. Individuals in the ST (closed circles; n=9) and AET (open circles; n=4) interventions are identified. (E-H) Circulating B cell populations in the same subjects show no correlation at baseline for (E) naïve and (F) memory B cell populations. However, post-PA groups exhibit (G) higher naïve B cells and (H) lower circulating memory B cells with improved attention and concentration.
CSF-localized CD4+ T cells decrease with higher brain Aβ deposition after physical activity
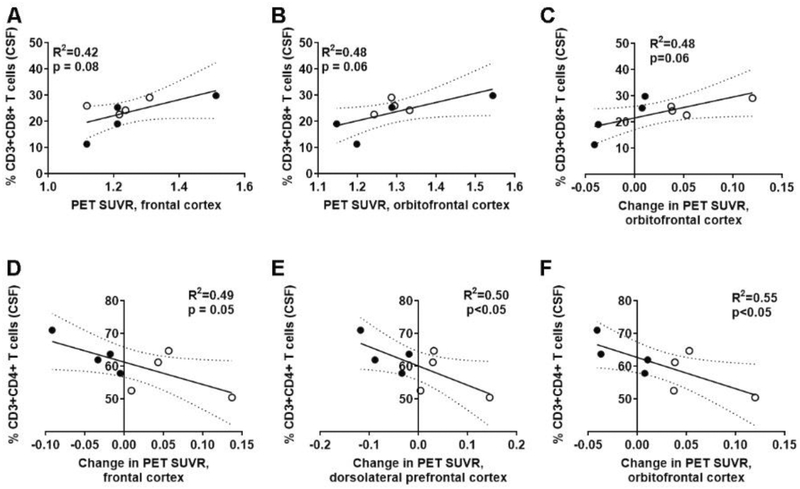
Again, we investigated both the amyloid imaging at either baseline or post-PA, as well as the change of amyloid deposition over one year within subjects with both baseline and post-PA PET imaging. Investigation into CSF-derived T cell subsets and PET imaging showed that at baseline, there were no correlations with Aβ burden lymphocyte profiles. After one year of PA, however, there was a trend for higher frequencies of CD8+ T cells in the CSF to be associated with increased Aβ burden in the orbitofrontal cortices (R2=0.48; p=0.06; Fig. 6B). Furthermore, a greater number of CD8+ T cells in the CSF correlated with an increase in Aβ burden in the orbitofrontal cortex over time (R2=0.48; p=0.06; Fig. 6C), although this did not reach statistical significance. Peripheral CD8+ T cells did not correlate with either Aβ deposition or the change in deposition over the one year for the aMCI patients with pre-post PET imaging and post-PA immunophenotyping. CSF-localized CD4+ T cells were only associated with changes in Aβ burden over time. Specifically, decreased frequency of CD4+ T cells in the CSF correlated with the increased Aβ deposition in brain regions associated with executive functioning (20), namely the frontal cortex (R2=0.49; p=0.05; n=8; Fig. 6D), dorsolateral prefrontal cortex (R2=0.50; p<0.05; Fig. 6E), and orbitofrontal cortex (R2=0.55; p<0.05; Fig. 6F). Peripheral circulating CD4+ T cells were not associated with changes in any of these regions. Together, this shows a CSF-specific relationship between T cell subsets and Aβ deposition, similar to our previous results at baseline (16), and confirms that PA does not affect this relationship.
Figure 6. Higher levels of CSF-localized CD8 T cells, and lower CD4 T cells associate with increased Aβ deposition after physical activity.
(A-C) Standardized uptake value ratio (SUVR) for PET imaging of 18F-florbetapir (amyloid burden) associated higher CD8+ T cells in the cerebrospinal fluid (CSF) in both (A) frontal and (B) orbitofrontal cortices after PA. Individuals in the ST (closed circles; n=4) and AET (open circles; n=4) interventions are identified. (C) Increased CD8 T cells also associated with increased Aβ deposition. (D-F) There were concomitant decreases for CD4 T cells in (D) frontal, (E) dorsolateral prefrontal, and (F) orbitofrontal cortices with increased Aβ deposition when comparing baseline and post-PA PET imaging.
Improved cognitive function after physical activity associated with increased circulating CD4+ T cells
Given the association with CD4+ and CD8+ T cells with Aβ burden in regions associated with executive function, we sought to examine if executive functioning, or other cognitive domains, were affected by the frequency of T cells in the CSF and blood. At baseline, CD3+ T cells in the CSF did not associated with cognitive function. After PA, higher levels of CSF-derived T cells associated with better scores on one measure of executive function (Table 3), however CD3 is a pan-T cell marker and neither the CD4+ nor CD8+ subpopulations in the CSF reflected this correlation (Table 3). Baseline circulating levels of CD3+ T cells in the blood associated with higher Logical Memory scores (Table 3). After PA, fewer CD3+ T cells in the periphery were associated with higher scores on memory verbal fluency tasks (Table 3).
Like B cells after PA, CSF-derived CD4+ and CD8+ T cell subsets did not associate with cognitive outcomes, but instead effects were limited to the circulating lymphocyte populations. In stark contrast to the complete lack of correlation at sedentary baseline (R2=0.009; p=0.77; Fig. 7A, Table 3), higher levels of circulating CD4+ T cells associated with improved executive function after PA (R2=0.43; p<0.05; Fig. 7B). Conversely, the frequency of CD8+ T cells, which also had no correlation with executive functioning or processing speed at baseline (R2=0.009; Fig. 7C), declined in the blood of subjects displaying higher executive functioning and processing speed scores (R2=0.41; p<0.05; Fig. 7D) after PA. Together, these data indicate that peripheral CD4+ and CD8+ T cells differentially associate with neurocognitive functioning in aMCI patients after PA.
Figure 7. Circulating T cell populations reflect neurocognitive function after long-term physical activity.
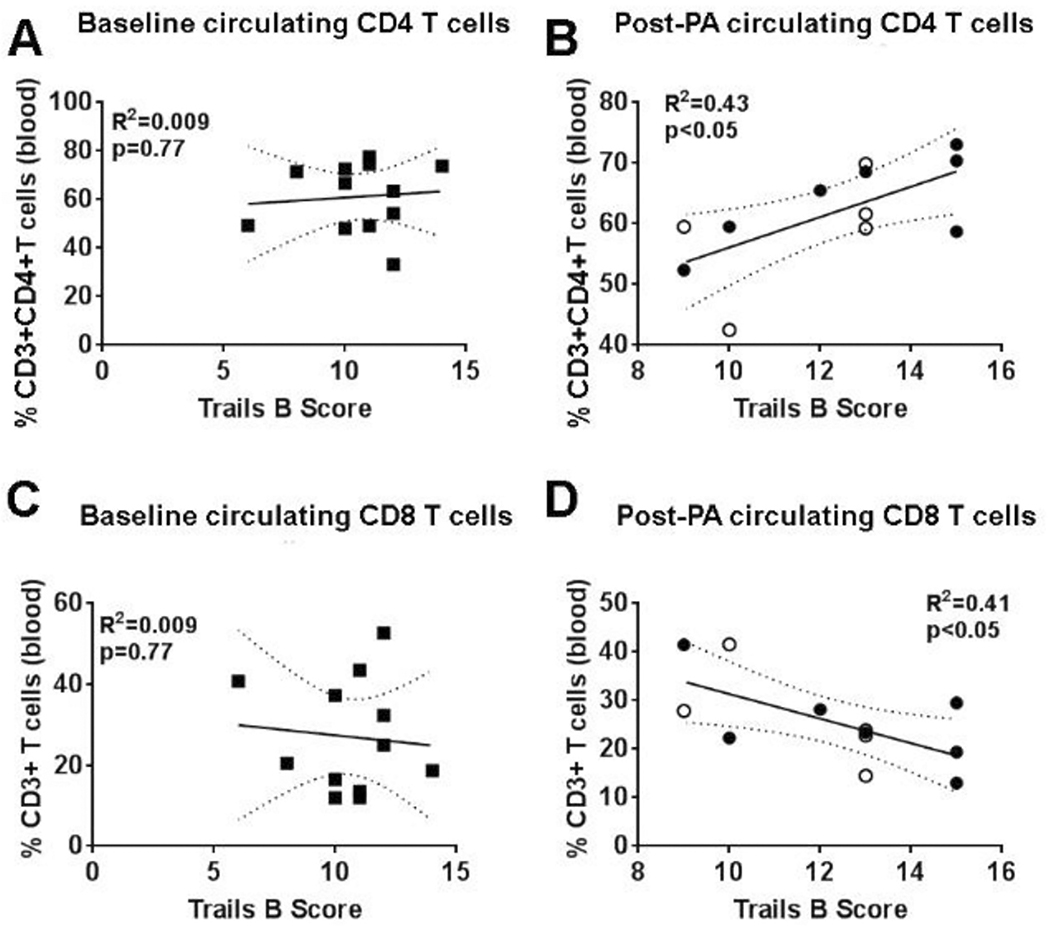
(A) Sedentary baseline (squares; n=12) aMCI subjects with better executive function (i.e. higher numbers = better cognitive function, Trails B) exhibit no trend for either circulating (A) CD4+ T cells or (C) CD8+ T cells. Physical activity (PA) group, including individuals in the stretching/toning (closed circles; n=7) and aerobic exercise training (open circles; n=5) interventions exhibit opposite patterns for T cell subsets in the circulation, with improved executive function associated with more (B) CD4+ T cells and fewer (D) CD8+ T cells in the blood.
DISCUSSION
Defining MCI
As mentioned above, MCI is a neurocognitive disorder distinguished by a cognitive decline greater than typically expected for normal aging. Individuals with MCI are still able to perform all of their daily activities, but they may take longer and/or use adaptive strategies (e.g. memory aids) to successfully complete their day-to-day tasks (21). Based on which cognitive domains are affected, individuals with MCI will be classified as aMCI or non-amnestic MCI (naMCI). aMCI refers to cognitive impairment solely in the domain of learning and memory, including difficulty in the retrieval of recently stored information. Conversely, naMCI refers to impairments in one or more cognitive domains, excluding learning and memory. Approximately 5–10% of individuals with MCI progress to dementia annually (22, 23). Furthermore, those with a diagnosis of naMCI are more likely to progress toward other dementias, such as Lewy body dementia, vascular dementia, or frontotemporal dementia, whereas patients with aMCI are at higher risk of developing AD (21, 24–26). Known risk factors for MCI include age, male sex, presence of APOε4 allele, and family history of cognitive impairment (27–30). Chronic conditions including hypertension, hyper-lipidemia, coronary artery disease, stroke, osteoarthritis, chronic obstructive pulmonary disease, depression, traumatic brain injury, and diabetes mellitus have been identified as risk factors for MCI (31–35). Sedentary behavior, both cognitively and physically, is also a proposed risk factor for MCI (36, 37), underscoring the importance of exercise as a potential therapeutic intervention for MCI patients.
Immune alterations in MCI patients
A wide range of mechanisms contribute to MCI pathology. These include structural changes in the brain, accumulation of Aβ and neurofibrillary tangles, declining neuroplasticity, dysfunctional cholinergic and serotonergic systems, and increased oxidative stress (38). In addition to these mechanisms, recently we and others reported immune changes in the periphery and CNS of aMCI subjects (15, 16, 39). As healthy individuals age, leukocytes become immunosenes-cent (40–42) and B and T cell function declines (40). B and T cells are two components of the adaptive immune system. B cells serve two primary functions: secretion of antibodies and antigen presentation (43). After encountering antigens, naïve B cells become short-lived plasmablasts or memory B cells (43). Memory B cells produce very specific antibodies upon re-exposure to a familiar antigen that induced their formation. B cells can present antigens to other cells including CD4+ T cells (i.e. helper T cells) that recognize antigens and secrete large quantities of cytokines (43). CD8+ T cells, also known as cytotoxic T cells, recognize particular antigens and induce apoptosis in foreign cells (43). Naïve B and T cells decline in the periphery with age, reducing antibody production and responses to mitogen stimulation, respectively, with a simultaneous increase in anergic memory T and B cells (40, 44). Furthermore, a low-grade systemic inflammation develops with age, leading to greater secretion of pro-inflammatory cytokines and decreased anti-inflammatory regulatory immune cells (42, 44). MCI patients present with greater immunosenescence and inflammation, likely contributing to disease pathogenesis and progression toward dementia (45).
While the data are not conclusive, there is significant evidence suggesting that pro-inflammatory cytokines are increased in MCI (46). Some studies report higher serum/plasma levels of inflammatory cytokines (e.g. IFN-γ, TNF-α) and chemokines (46). In the CSF, fewer cytokines and chemokines have been examined, but MCP-1 (i.e. CCL2), a chemokine that promotes leukocyte recruitment, was increased in the CSF of MCI patients while TGF-β, an anti-inflammatory cytokine, was downregulated in the CSF (46). Interestingly, some inflammatory factors also associate with the progression of MCI to AD. High levels of soluble CD40 (sCD40) at baseline correlate with the risk of progression from MCI to AD over the course of 4–7 years (47). In both MCI-AD and control cases, higher levels of plasma sCD40 were associated with lower MMSE scores, representing global cognitive decline (47). sCD40 interferes with B cell-T cell interactions and can suppress the development of memory B cells, which could contribute to the dysregulation of the ratio of naïve to memory B cells that we see in our patients (48, 49). Another promising predictor of progression from MCI to AD is the soluble TNF-α receptor, sTNFR (50). MCI patients that progressed to AD had higher levels of sTNFR1 than patients that did not progress (50). While further research is needed, it is possible that alterations in sCD40 and sTNFR1 may be useful immunological biomarkers to determine if an MCI patient is likely to progress to AD, which could allow for early interventions such as exercise.
In addition to cytokines, researchers examined whether leukocyte populations are altered in MCI patients. Differences in T cell profiles in MCI patients include diminished CD3+ T cells in the blood of MCI patients relative to healthy controls (51). In the CSF, activated CD4+ T cells were increased in both patients with MCI and mild AD relative to healthy controls, while activation of CD8+ T cells was associated with parahippocampal structural damage and cognitive deficits (52). In our sedentary cohort, we found that Aβ burden was negatively associated with CSF-derived CD4+ T cell populations, with fewer CD4+ T cells correlating with greater Aβ deposition over time (53). Therefore, increasing CD4+ T cells in MCI patients may be playing a beneficial role in the CNS, particularly as aMCI patients have more anti-inflammatory regulatory T cells (Tregs) (54, 55). Unfortunately, our immune panel did not specifically identify Tregs, but this suggests that decreases of potentially beneficial CD4+ T cells, and concomitant increases of potentially pathogenic CD8+ T cells, contribute to aMCI pathology and deterioration of cognitive functioning. Further research is necessary to improve our understanding of the functional role of these T cell subsets in aMCI patients and during the progression to AD.
Less is known about how B lymphocytes affect, alter, and/or support cognition. Magaki et al. reported that overall B cells in the periphery did not significantly differ between aMCI and healthy controls (51). However, in our prior study we found subset-specific changes in B cell populations in sedentary aMCI patients as naïve B cells were decreased and memory B cells were increased in APOε4 carriers with aMCI (16), who are at a significantly greater risk of developing AD (53). Given that the switch from naïve to memory phenotype is observed in healthy elderly individuals (40), our findings suggest that aMCI-induced immune changes are potentially a more severe form of immunosenescence that naturally occurs with aging.
Aβ and the adaptive immune response during MCI
One of the hallmark features used to predict the progression from MCI to AD is the accumulation of Aβ in the brain (2, 3). Aβ deposition is believed to trigger the cascade of events leading to brain atrophy and synaptic dysfunction characteristic of AD (2, 3). In response to Aβ deposition, leukocyte function and phenotype may also be altered. Based on our small cohort of aMCI patients, we found preliminary evidence of a significant relationship between Aβ burden and different lymphocyte populations in aMCI. First, we found that brain Aβ deposition increased in several brain regions over one year despite initiation of PA. These are predictable results given that these regions have previously been identified as sites of early Aβ deposition (56). In MCI patients, greater accumulation of Aβ in the posterior cingulate over the course of 2 years associated with more severe memory impairments and higher rates of progression to AD (57, 58). It is possible, therefore, that the Aβ deposition we observed in those regions was a product of the natural progression of aMCI over time and was not impacted by PA. However, the specific hippocampal Aβ deposition we observed has been less thoroughly characterized in aMCI patients. Greater hippocampal atrophy and hyperexcitability is associated with worse cognitive decline and progression to AD (59) and thus hippocampal Aβ may be a potentially interesting therapeutic target.
In our study, we found significant correlations between B and T cells and brain Aβ burden during aMCI. CSF-localized CD8+ T cells positively correlated with Aβ beta burden in the orbitofrontal cortex after PA. Conversely, CD4+ T cells decreased in aMCI patients that had greater accumulation of Aβ over time in the frontal cortex, dorsolateral prefrontal cortex, and orbitofrontal cortex. This inverse relationship between CD4+ and CD8+ T cells in the CSF suggests that these cell types may be playing different roles in response to Aβ in the brain. In AD patients, T cells, in particular CD8+ T cells, increase in the extravascular space in the hippocampus and midfrontal gyrus (60). Similarly, we found that there was an increase in CD8+ T cells in the CSF of MCI patients with higher Aβ burden in frontal cortices, suggesting that CD8+ T cells are pathogenic or elevated in response to the pathology. Previous work found that CD8+ T cell influx into the extravascular space associated with tau pathology but not Aβ burden (60), thus future studies should determine possible mechanisms of action for cytotoxic T cells in the development of dementia.
Our data also suggest that CD4+ T cells have the opposite relationship to Aβ deposition compared to CD8+ T cells. We found that as Aβ burden declined, there were a greater number of CD4+ T cells in the CSF. In a murine model of AD, injections of Aβ-specific CD4+ T cells injected in the CSF migrate into the brain where they target Aβ plaques in the hippocampus and cortex, increase phagocytosis of Aβ by innate immune cells, and stimulate neurogenesis (61). Another study demonstrated that injecting Aβ-specific CD4+ T cells into the blood decreases Aβ burden and improves behavioral function in mice (62). In patients exhibiting cognitive impairment, higher levels of CD4+ Tregs capable of producing anti-inflammatory cytokines correlated with lower levels of amyloidopathy (63). Thus, the recruitment of CD4+ T cells with increased PA may reflect an activation of an adaptive immune-mediated response to counter progression of the disease.
B cell response to Aβ burden is not as well defined as that of T cells. In aMCI sedentary subjects, decreased overall B cells in the periphery were associated with greater Aβ burden in the precuneus (53), whereas in this study increased CSF-derived B cells were associated with greater Aβ burden in the hippocampus both before and after PA. Both of these suggest a movement from the periphery into the CSF with increased Aβ deposition (or conversely a loss of amyloid clearance (64)). Memory B cells may be a particularly important B cell subset; memory B cells isolated from the blood of mild AD patients have increased expression of CCR5, a pro-inflammatory chemokine receptor that enhances recruitment of lymphocytes to sites of inflammation, such as Aβ plaques (65). At baseline and after PA, increased peripheral memory B cells and decreased naïve B cells associated with greater hippocampal Aβ burden. We also previously found that CSF-derived memory B cells increased as Aβ burden increased in sedentary aMCI patients (53). Further investigations are required to determine the protective or pathogenic potential of B cell subsets in the progression of Aβ deposition.
Cognition and adaptive immune responses
In addition to the accumulation of Aβ and learning and memory deficits, individuals with aMCI may exhibit deficits in other cognitive domains. Inflammation in the CNS and periphery is associated with worse cognition in healthy individuals, particularly in the areas of learning, memory and attention, while elevated peripheral cytokine levels in the blood are related to impaired cognitive performance (66). Additionally, lymphocyte subsets have been associated with neurocognitive functioning (67). In healthy elderly, better cognitive function is associated with lower numbers of CD4+ T cells and higher numbers of naïve CD8+ T cells and B cells. Even when variables such as age, mood, and length of education were controlled for, lower levels of circulating effector CD4+ T cells and higher levels of B cells correlated with better performance on measures of executive function and memory (67).
Previously it has been shown in patients with MCI that T cells were associated with changes in cognitive functioning (52). In particular, CSF-localized, activated CD8+ T cells were negatively associated with learning and visuospatial skills, but did not correlate with global cognition, verbal memory, confrontation naming, verbal fluency or executive functioning (52). We also found evidence suggesting that CD8+ T cells were related to cognitive functioning. After PA, the frequency of CD8+ T cells in the blood decreased, which was associated with higher scores on tests of processing speed and executive function, though a movement into the CSF from the periphery was not reflected in our data set and may be limited by the small sample size. Lueg et al. reported that activated CD4+ T cells in the CSF and blood were not associated with impairments in any cognitive domain in MCI patients (52). However, we found that after PA, increased CD4+ T cells correlated with better executive functioning, suggesting that PA may drive relationships between the peripheral immune response and cognition not identified in prior studies.
Currently, little is known about B cells and cognition in individuals with aMCI. At baseline, we found predominantly changes in CSF-derived naïve and memory B cells in sedentary aMCI patients. Fewer naïve B cells and more memory B cells in the CSF were associated with improved attention and concentration and verbal fluency. After PA, however, cognition was only associated with changes in B cell subsets in the periphery. As peripheral naïve B cells increased and memory B cells decreased, individuals with aMCI had higher scores on measures of attention and concentration, and executive function. This recapitulates studies examining B cell subsets and global cognition in AD; naïve B cells in the blood were positively correlated with global cognition scores, while memory B cells were negatively correlated with global cognition scores (65).
Overall, our findings indicate that prior to PA in sedentary aMCI patients, correlations between B cell subsets and cognition are limited to the CSF while T cell correlations are limited to the blood. However, after PA, correlations between cognition and both B cells and T cells were found exclusively in the periphery. We also found that increased naïve B cells and CD4+ T cells, and decreased memory B cells and CD8+ T cells, were associated with better neurocognitive performance. This replicates our findings that identified increased naïve B cell and decreased memory B cell populations in the blood associated with less Aβ deposition in the hippocampus. Furthermore, our findings suggest that T cell subsets may be particularly associated with aspects of executive functioning. Specifically, decreased CD4+ T cells and increased CD8+ T cells in the CSF were associated with increased Aβ in the frontal cortex, dorsolateral prefrontal cortex, and the orbitofrontal cortex, all regions associated with executive function. Interestingly, although naïve and memory B cells were associated with changes in Aβ deposition in the hippocampus, none of the memory tasks showed significant associations between overall B cells and B cell subsets. On the other hand, T cell subsets were correlated with both Aβ burden in cortical regions associated with executive function and clinical measures of executive function, suggesting a closer association between executive functioning and T cell subsets. This highlights the need to look at long-term cognitive decline and these adaptive immune cell subsets, with additional pre-clinical mechanistic studies to understand the complex neuro-immune response.
Exercise interventions in MCI patients
Exercise is a lifestyle factor that can improve cognition in normal subjects, but more recently, exercise and PA have also been examined in individuals with MCI. Several systematic reviews and meta-analyses look at observational studies and randomized control trials in MCI and provide evidence suggesting an exercise benefit for incidence of MCI (6). A large multi-national epidemiological study examined the frequency of MCI in individuals that met the weekly PA recommendation (i.e. 150 minutes of moderate-to-vigorous PA), compared to those that did not, and found that failing to meet the weekly PA recommendation was associated with increased risk of MCI (68). Similarly, another study found that any frequency of moderate exercise, as opposed to light or vigorous exercise, significantly reduced the odds of developing MCI (37). Several studies report improvements in global cognition after PA for aMCI patients (7, 9, 10, 69), including an association between physically active individuals and better scores on a global screening measure (70). Some also show evidence for improvements in attention (7), executive functioning (7, 10), verbal fluency (11), and memory (7, 9, 10). It should also be noted that some of these results do not reflect clinically meaningful changes in cognition (11), and/or inconclusive results (71).
The aforementioned studies relied on self-reported exercise data, though some used actigraphy data to categorize the frequency and intensity of exercise for aMCI patients. Healthy older individuals with low levels of daily PA (as measured by actigraphy) had more than a 2-fold risk of developing AD relative to physically active individuals (72). Another actigraph study found that increased levels of PA were associated with higher cognitive scores, but only in subjects who were cognitively normal, while one MCI investigation did not find any association between PA and improved cognitive performance (73). Falck et al. suggested this may be due to elevated rates of sedentary behavior in the MCI cohort (73) that may also influence the magnitude of effects of any PA on these patients, be it vigorous (i.e. AET) or mild (i.e. ST) interventions.
Overview of exercise-induced alterations to adaptive immunity
During and immediately after acute exercise, lymphocytes are mobilized in the blood (lymphocytosis) in an intensity-dependent manner (74, 75). Several mechanisms have been proposed to explain lymphocytosis following exercise. Exercise increases sympathetic activity, leading to greater concentrations of epinephrine and norepinephrine, which in turn stimulate lymphocyte proliferation, differentiation, and cytokine secretion (74). Increased catecholamine release acutely following exercise mobilizes adaptive immune cells through interactions with β-adrenoceptors expressed on lymphocyte surface (75). The largest changes occur in CD8+ T cells, which have high β2-adrenergic receptor expression on their surface, leading to preferential mobilization (75). However, CD4+ T cells and B cells also express β2-adrenergic receptors and undergo lymphocytosis after exercise, albeit to a lesser extent. Exercise preferentially mobilizes subsets of T cells, including a subset of anti-inflammatory Tregs (76–78). There are also subset-specific changes in B cells during exercise, with the greatest increase in immature B cells, followed by memory B cells, and then naïve B cells (77). These data indicate that lymphocyte subpopulations are differentially impacted during exercise and thus may play different roles in post-exercise immune function.
Within 1–2 hours post-exercise, however, there is a transient decline in circulating adaptive immune cells (lymphopenia) in the blood. Lymphopenia can be attributed to two major factors: apoptosis and lymphocyte redistribution (79). High catecholamine levels and greater oxidative stress induce lymphocyte apoptosis, though this mechanism accounts for less than 10% of lymphopenia observed after exercise (79). Lymphopenia is predominantly secondary to a redistribution of lymphocytes, particularly T cells, throughout the body. A study using cell fluorescent trafficking in mice found that CD3+ T cells preferentially move from the spleen to lymphoid and nonlymphoid organs, including Peyer’s patch, lungs, and bone marrow after exercise (74). Exercise also increased memory CD8+ T cells expressing receptors that promote trafficking to peripheral organs, and these cells exhibit strong effector functions (80). However, outside of the aforementioned study showing a preferential increase in circulating immature B cells, little is known about B cell trafficking or apoptosis after exercise, making them a promising subject for future research. Thus, exercise may enhance immune function by promoting the redistribution of adaptive immune cells to detect antigens in remote tissues to perform secondary effector functions.
In healthy elderly adults, lifelong exercise alters baseline levels of circulating adaptive immune cells. Duggal et al. recently compared immune profiles in the blood of healthy sedentary young adults, sedentary older adults (55–79 years), and older lifelong cyclists to determine how T and B cell levels change in the blood of older individuals (81). Physically inactive older individuals had fewer T cells in their blood. However, in master cyclists, circulating T cell levels were comparable to young healthy donors. Researchers also found that sedentary older adults had more unswitched memory B cells in the blood not found in master cyclists. Together, these data suggest that age affects B and T cell profiles, but a lifetime of physical activity can preserve a “young” immune phenotype in the blood (81).
Finally, it should also be noted that an excess of anti-inflammatory regulatory immune cells can lead to immunosuppression after strenuous exercise and/or prolonged training. Marathon-trained runners had a higher percentage of inter-leukin (IL)-10+ and TGF-β+ CD4+ T cells in the blood than untrained controls (82). Furthermore, high intensity exhaustive exercise results in prolonged lymphopenia, impairs T cell function, and decreases production of secretory IgA, an antibody produced by B cells (83). While this immunosuppression increases the risk of upper respiratory infections in elite athletes, the immunosuppressive effect of exercise could also counteract the systemic inflammation that naturally occurs in aging MCI patients (84, 85).
Exercise interventions and adaptive immune responses in MCI patients
Alterations to immunity may be one mechanism by which exercise interventions benefit MCI patients. Several studies examined the effect of exercise training on immunity, particularly cytokines, in MCI patients. Serum levels of the anti-inflammatory cytokine, IL-10 and the neuroprotective growth factor, brain derived growth factor (BDNF), increase after 28 weeks of strength training and 16 weeks of multimodal physical training, respectively (12, 13). As shown by Stigger et al. in a recent meta-analysis, PA also significantly decreases the pro-inflammatory cytokines, TNF- α and IL-6 (13, 86). IL-6, which functions as both a cytokine and myokine (muscle-derived cytokine), increases immediately after a high bout of intense cycling. Multimodal physical training reduced serum levels of IL-6 and high intensity exercise training reduced IL-6 levels at rest (13, 14). Sixteen weeks of multimodal training for MCI patients found that declining levels of IL-6 and TNF-α, as well as an increased BDNF, was associated with improved cognition (13).
Only one MCI study examined changes in overall immune cell populations, with findings indicating that strength training reduced leukocyte and lymphocyte populations compared to baseline levels, but this study did not specifically examine either B or T cell subsets (12). Chupel et al. also showed that 28 weeks of strength training reduced leukocyte counts, particularly lymphocytes, relative to baseline in MCI patients, but not healthy controls (12). We did not see an effect in overall lymphocyte populations after 1-year of AET compared to ST interventions in our aMCI sample. Instead, we found that increased PA in previously sedentary individuals altered the adaptive immune responses to Aβ deposition in the brain and cognitive function, though the complexity of the responses suggest several mechanisms working in synergy during aMCI.
Study limitations
While our study provides preliminary data suggesting PA-induced immunomodulation of adaptive immune cell subsets were associated with Aβ deposition and subtle changes in cognition, only a small subset of participants in the clinical trial completed immunophenotyping, PET imaging, and cognitive testing. For example, only 5 subjects had both pre- and post-PA blood and CSF collection, so we could not analyze adaptive immune changes utilizing a within-subject design. This limited our analysis to two different groups of subjects, those at sedentary baseline and those that had post-PA blood draws and lumbar punctures. Future studies should examine immunomodulation by PA in a within-subject manner to look at how immune populations change within a particular individual to determine if baseline levels of lymphocytes impact the effect of PA on the immune system, as well as neurocognitive functioning and Aβ deposition. Because we observed no differences in the immunophenotypes in our smaller cohorts of participants, we concluded that AET and ST groups could be pooled into one larger PA cohort. We utilized this larger PA cohort when assessing the relationship between immunity and cognition, as well as immunity and Aβ deposition. We cannot exclude the possibility that by combining these cohorts, we could be obscuring the true relationship between cognition and adaptive immunity. Thus, our results only provide preliminary evidence of immune-mediated changes in neurocognitive functioning and Aβ burden after PA in patients suffering from aMCI, and may underestimate or overestimate the true effect of PA on immunity, cognition, and Aβ burden. Our conclusions should be interpreted with caution and future studies should confirm our findings in larger cohorts of aMCI patients.
CONCLUSION
Our study is unique in that it is the first to relate changes in B cell and T cell subsets to neurocognitive function and Aβ deposition in the aMCI brain after one year of PA. Preliminary data from our small cohort of aMCI patients suggest several key ways that PA may impact cognitive functioning and Aβ in aMCI. First, increased Aβ load in the hippocampus was associated with fewer naïve B cells and more memory B cells in the blood. However, there was no relationship between performance on memory tasks and B cell subsets in either the blood or CSF after PA. Instead, increased memory B cells and diminished naïve B cells were associated with lower attention and concentration scores. Second, CSF-derived CD4+ T cells decreased, and CD8+ T cells increased, with more Aβ deposition in brain regions associated with executive function (20). Furthermore, our data showed that aMCI patients with lower executive function scores had fewer CD4+ T cells and more CD8+ T cells in the blood. This suggests that PA-induced global alterations in T cell subsets may be associated with both the harmful accumulation of Aβ and associated changes in neurocognitive functioning. Finally, we found that after PA, there was an inverse relationship between memory/naïve B cells and CD4+/CD8+ T cells. Overall, increased peripheral naïve B cells and CD4+ T cells are associated with better cognitive function in aMCI patients, while memory B cells and CD8+ T cells are associated with greater pathology. Our findings suggest a complex interplay between physical activity, cognition, brain Aβ burden, and adaptive immunity. Larger clinical studies should aim to validate our findings and determine what mechanisms underlie PA-induced immunomodulation and its impact on clinical outcomes in MCI patients.
ACKNOWLEDGMENTS
We thank the patients who consented to participate in this study. We also thank the UT Southwestern Alzheimer’s Disease Center, the NIH/NIA (P30AG12300–21), the NIH/NIAID (T32AI005284–38), the UTSWMC flow cytometry core, and the CONQUER biorepository staff for technical support. The 18F-florbetapir PET radiotracer was provided to the study by Avid Radiopharmaceuticals.
REFERENCES
- 1.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 2.Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Nagren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):75–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waragai M, Okamura N, Furukawa K, Tashiro M, Furumoto S, Funaki Y, et al. Comparison study of amyloid PET and voxel-based morphometry analysis in mild cognitive impairment and Alzheimer’s disease. J Neurol Sci. 2009;285(1–2):100–8. [DOI] [PubMed] [Google Scholar]
- 4.Corey-Bloom J Treatment trials in aging and mild cognitive impairment. Curr Top Behav Neurosci. 2012;10:347–56. [DOI] [PubMed] [Google Scholar]
- 5.Strohle A, Schmidt DK, Schultz F, Fricke N, Staden T, Hellweg R, et al. Drug and Exercise Treatment of Alzheimer Disease and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Effects on Cognition in Randomized Controlled Trials. Am J Geriatr Psychiatry. 2015;23(12):1234–49. [DOI] [PubMed] [Google Scholar]
- 6.Etgen T, Sander D, Bickel H, Forstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Arztebl Int. 2011;108(44):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohman H, Savikko N, Strandberg TE, Pitkala KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord. 2014;38(5–6):347–65. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Yu JT, Wang HF, Tan CC, Meng XF, Tan L. Nonpharmacological interventions for patients with mild cognitive impairment: a meta-analysis of randomized controlled trials of cognition-based and exercise interventions. J Alzheimers Dis. 2014;42(2):663–78. [DOI] [PubMed] [Google Scholar]
- 9.Zheng G, Xia R, Zhou W, Tao J, Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016. [DOI] [PubMed] [Google Scholar]
- 10.Cammisuli DM, Innocenti A, Franzoni F, Pruneti C. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch Ital Biol. 2017;155(1–2):54–62. [DOI] [PubMed] [Google Scholar]
- 11.Gates N, Fiatarone Singh MA, Sachdev PS, Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry. 2013;21(11):1086–97. [DOI] [PubMed] [Google Scholar]
- 12.Chupel MU, Direito F, Furtado GE, Minuzzi LG, Pedrosa FM, Colado JC, et al. Strength Training Decreases Inflammation and Increases Cognition and Physical Fitness in Older Women with Cognitive Impairment. Front Physiol. 2017;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimento CM, Pereira JR, de Andrade LP, Garuffi M, Talib LL, Forlenza OV, et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799–805. [DOI] [PubMed] [Google Scholar]
- 14.Lotta Bengtsson Lindberg MW; Vestberg Susanna; Helene Jacobs-son; Wisen Anita Exercise-induced Release of Cytokines/Myokines in a Single Exercise Test before and after a Training Intervention in Patients with Mild Cognitive Impairment. International Jounral of Physical Therapy & Rehabilitation. 2017;3(138). [Google Scholar]
- 15.Monson NL, Ireland SJ, Ligocki AJ, Chen D, Rounds WH, Li M, et al. Elevated CNS inflammation in patients with preclinical Alzheimer’s disease. J Cereb Blood Flow Metab. 2014;34(1):30–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stowe AM, Ireland SJ, Ortega SB, Chen D, Huebinger RM, Tarumi T, et al. Adaptive lymphocyte profiles correlate to brain Abeta burden in patients with mild cognitive impairment. Journal of neuroinflammation. 2017;14(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarumi T, Harris TS, Hill C, German Z, Riley J, Turner M, et al. Amyloid burden and sleep blood pressure in amnestic mild cognitive impairment. Neurology. 2015;85(22):1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58(12):1985–92. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, et al. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122(18):1797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan P, Raz N. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci Biobehav Rev. 2014;42:180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. [DOI] [PubMed] [Google Scholar]
- 22.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJ, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology. 2014;82(4):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119(4):252–65. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Bennett D. Mild cognitive impairment: is it Alzheimer’s disease or not? J Alzheimers Dis. 2005;7(3):241–5; discussion 55–62. [DOI] [PubMed] [Google Scholar]
- 26.Ferman TJ, Smith GE, Kantarci K, Boeve BF, Pankratz VS, Dickson DW, et al. Nonamnestic mild cognitive impairment progresses to dementia with Lewy bodies. Neurology. 2013;81(23):2032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green RC, Cupples LA, Go R, Benke KS, Edeki T, Griffith PA, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–36. [DOI] [PubMed] [Google Scholar]
- 30.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng TP, Feng L, Nyunt MS, Feng L, Gao Q, Lim ML, et al. Metabolic Syndrome and the Risk of Mild Cognitive Impairment and Progression to Dementia: Follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol. 2016;73(4):456–63. [DOI] [PubMed] [Google Scholar]
- 32.Vassilaki M, Aakre JA, Cha RH, Kremers WK, St Sauver JL, Mielke MM, et al. Multimorbidity and Risk of Mild Cognitive Impairment. J Am Geriatr Soc. 2015;63(9):1783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh B, Mielke MM, Parsaik AK, Cha RH, Roberts RO, Scanlon PD, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol. 2014;71(5):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171(5):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verghese J, LeValley A, Derby C, Kuslansky G, Katz M, Hall C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66(6):821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mufson EJ, Binder L, Counts SE, DeKosky ST, de Toledo-Morrell L, Ginsberg SD, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012;123(1):13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiology of aging. 2015;36(1):81–9. [DOI] [PubMed] [Google Scholar]
- 40.Biragyn A, Aliseychik M, Rogaev E. Potential importance of B cells in aging and aging-associated neurodegenerative diseases. Semin Immunopathol. 2017;39(3):283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costantini E, D’Angelo C, Reale M. The Role of Immunosenescence in Neurodegenerative Diseases. Mediators Inflamm. 2018;2018:6039171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol. 2017;8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvaraj UM, Poinsatte K, Torres V, Ortega SB, Stowe AM. Heterogeneity of B Cell Functions in Stroke-Related Risk, Prevention, Injury, and Repair. Neurotherapeutics. 2016;13(4):729–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age (Dordr). 2013;35(3):563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Benedetto S, Muller L, Wenger E, Duzel S, Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev. 2017;75:114–28. [DOI] [PubMed] [Google Scholar]
- 46.Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. 2014;50(2):534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchhave P, Janciauskiene S, Zetterberg H, Blennow K, Minthon L, Hansson O. Elevated plasma levels of soluble CD40 in incipient Alzheimer’s disease. Neurosci Lett. 2009;450(1):56–9. [DOI] [PubMed] [Google Scholar]
- 48.Contin C, Pitard V, Delmas Y, Pelletier N, Defrance T, Moreau JF, et al. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology. 2003;110(1):131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray D, Dullforce P, Jainandunsing S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J Exp Med. 1994;180(1):141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonca VA, Gattaz WF, et al. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2010;22(4):1305–11. [DOI] [PubMed] [Google Scholar]
- 51.Magaki S, Yellon SM, Mueller C, Kirsch WM. Immunopheno-types in the circulation of patients with mild cognitive impairment. J Psychiatr Res. 2008;42(3):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging. 2015;36(1):81–9. [DOI] [PubMed] [Google Scholar]
- 53.Stowe AM, Ireland SJ, Ortega SB, Chen D, Huebinger RM, Tarumi T, et al. Adaptive lymphocyte profiles correlate to brain Abeta burden in patients with mild cognitive impairment. J Neuroinflammation. 2017;14(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Calvo MG, et al. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2010;21(3):927–38. [DOI] [PubMed] [Google Scholar]
- 55.Le Page A, Garneau H, Dupuis G, Frost EH, Larbi A, Witkowski JM, et al. Differential Phenotypes of Myeloid-Derived Suppressor and T Regulatory Cells and Cytokine Levels in Amnestic Mild Cognitive Impairment Subjects Compared to Mild Alzheimer Diseased Patients. Front Immunol. 2017;8:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheff SW, Price DA, Ansari MA, Roberts KN, Schmitt FA, Ikonomovic MD, et al. Synaptic change in the posterior cingu-late gyrus in the progression of Alzheimer’s disease. J Alzheimers Dis. 2015;43(3):1073–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Small GW, Siddarth P, Kepe V, Ercoli LM, Burggren AC, Bookheimer SY, et al. Prediction of cognitive decline by positron emission tomography of brain amyloid and tau. Arch Neurol. 2012;69(2):215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koivunen J, Scheinin N, Virta JR, Aalto S, Vahlberg T, Nagren K, et al. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology. 2011;76(12):1085–90. [DOI] [PubMed] [Google Scholar]
- 59.Maruszak A, Thuret S. Why looking at the whole hippocampus is not enough-a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer’s disease diagnosis. Front Cell Neurosci. 2014;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merlini M, Kirabali T, Kulic L, Nitsch RM, Ferretti MT. Extravascular CD3+ T Cells in Brains of Alzheimer Disease Patients Correlate with Tau but Not with Amyloid Pathology: An Immunohistochemical Study. Neurodegener Dis. 2018;18(1):49–56. [DOI] [PubMed] [Google Scholar]
- 61.Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A. Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance. J Immunol. 2014;192(1):92–102. [DOI] [PubMed] [Google Scholar]
- 62.Cao C, Arendash GW, Dickson A, Mamcarz MB, Lin X, Ethell DW. Abeta-specific Th2 cells provide cognitive and pathological benefits to Alzheimer’s mice without infiltrating the CNS. Neurobiol Dis. 2009;34(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oberstein TJ, Taha L, Spitzer P, Hellstern J, Herrmann M, Kornhuber J, et al. Imbalance of Circulating Th17 and Regulatory T Cells in Alzheimer’s Disease: A Case Control Study. Front Immunol. 2018;9:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bulati M, Buffa S, Martorana A, Gervasi F, Camarda C, Azzarello DM, et al. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer’s disease patients and show a pro-inflammatory trafficking receptor phenotype. J Alzheimers Dis. 2015;44(4):1241–51. [DOI] [PubMed] [Google Scholar]
- 66.Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serre-Miranda C, Roque S, Santos NC, Portugal-Nunes C, Costa P, Palha JA, et al. Effector memory CD4(+) T cells are associated with cognitive performance in a senior population. Neurol Neuroimmunol Neuroinflamm. 2015;2(1):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vancampfort D, Stubbs B, Lara E, Vandenbulcke M, Swinnen N, Koyanagi A. Mild cognitive impairment and physical activity in the general population: Findings from six low- and middle-income countries. Exp Gerontol. 2017;100:100–5. [DOI] [PubMed] [Google Scholar]
- 69.Tan JP, Li N, Gao J, Wang LN, Zhao YM, Yu BC, et al. Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. J Alzheimers Dis. 2015;43(4):1403–12. [DOI] [PubMed] [Google Scholar]
- 70.Innocenti A, Cammisuli DM, Sgromo D, Franzoni F, Fusi J, Galetta F, et al. Lifestyle, Physical Activity and Cognitive Functions: the impact on the scores of Montreal Cognitive Assessment (MoCa). Arch Ital Biol. 2017;155(1–2):25–32. [DOI] [PubMed] [Google Scholar]
- 71.Song JY, Sohn JW, Jeong HW, Cheong HJ, Kim WJ, Kim MJ. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC infectious diseases. 2006;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falck RS, Landry GJ, Best JR, Davis JC, Chiu BK, Liu-Ambrose T. Cross-Sectional Relationships of Physical Activity and Sedentary Behavior With Cognitive Function in Older Adults With Probable Mild Cognitive Impairment. Phys Ther. 2017;97(10):975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kruger K, Lechtermann A, Fobker M, Volker K, Mooren FC. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. 2008;22(3):324–38. [DOI] [PubMed] [Google Scholar]
- 75.Graff RM, Kunz HE, Agha NH, Baker FL, Laughlin M, Bigley AB, et al. beta2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun. 2018. [DOI] [PubMed] [Google Scholar]
- 76.Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26(2):195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner JE, Spielmann G, Wadley AJ, Aldred S, Simpson RJ, Campbell JP. Exercise-induced B cell mobilisation: Preliminary evidence for an influx of immature cells into the bloodstream. Physiol Behav. 2016;164(Pt A):376–82. [DOI] [PubMed] [Google Scholar]
- 78.Wilson LD, Zaldivar FP, Schwindt CD, Wang-Rodriguez J, Cooper DM. Circulating T-regulatory cells, exercise and the elite adolescent swimmer. Pediatr Exerc Sci. 2009;21(3):305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campbell JP, Turner JE. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front Immunol. 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campbell JP, Riddell NE, Burns VE, Turner M, van Zanten JJ, Drayson MT, et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23(6):767–75. [DOI] [PubMed] [Google Scholar]
- 81.Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rehm K, Sunesara I, Marshall GD. Increased Circulating Anti-inflammatory Cells in Marathon-trained Runners. Int J Sports Med. 2015;36(10):832–6. [DOI] [PubMed] [Google Scholar]
- 83.Pedersen BK, Toft AD. Effects of exercise on lymphocytes and cytokines. Br J Sports Med. 2000;34(4):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shaw DM, Merien F, Braakhuis A, Dulson D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine. 2018;104:136–42. [DOI] [PubMed] [Google Scholar]
- 85.Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30(6):569–78. [DOI] [PubMed] [Google Scholar]
- 86.Stigger F, Marcolino MAZ, Portela KM, Plentz RDM. Effects of Exercise on Inflammatory, Oxidative and Neurotrophic Bio-markers on Cognitively Impaired Individuals Diagnosed with Dementia or Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PubMed] [Google Scholar]