Abstract
Purpose
Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae bacteria causes nosocomial infections worldwide. However, KPC-producing K. pneumoniae outbreak has never been reported in Shandong Province, China. The purpose of our study was to elucidate the epidemiological and drug resistance mechanisms of KPC-producing K. pneumoniae strains collected from a large teaching hospital in Shandong during the outbreak. Moreover, we attempted to characterize the genetic environment and phylogenetic analysis of blaKPC-2 in outbreak isolates.
Methods
We monitored a 64-day outbreak of infection in a general hospital in Shandong Province, and the bacteria causing the infection were all ST11-type K. pneumoniae. The genotype correlation of KPC-producing K. pneumoniae isolates was assessed by whole-genome sequencing (WGS) phylogenetic analysis. Subsequent studies included antibiotic susceptibility testing, multilocus sequence typing (MLST) and S1-pulsed-field gel electrophoresis (S1-PFGE), Southern blot hybridization.
Results
From February 1, 2018 to April 5, 2018, 14 KPC-producing K. pneumoniae isolates from different wards were collected. All 14 isolates were resistant to carbapenems and carried the extended-spectrum β-lactamase (ESBL) gene as well as fosA, and sul genes. Whole-genome analysis showed that all 14 the outbreak isolates were all ST11 type. The blaKPC-2 carrying plasmids were all belong to IncFIIK2 type, and the size ranged from 94 kb to 368 kb.
Conclusion
As far as we know, this report first describes the genomics characterization of KPC-2-producing K. pneumoniae outbreak isolates from Shandong Province, China. In our study, these isolates appeared to be cloned, and ST11 K. pneumoniae was the major clone caused the outbreak. Therefore, routine surveillance of such strains in this region is urgently warranted.
Keywords: Klebsiella pneumoniae, ST11, whole-genome sequencing, SNP, outbreak, IncFIIK2
Introduction
Bacterial resistance can reduce the effectiveness of antibiotics and increase the difficulty of treating infectious diseases, becoming a major problem affecting global public health.1 Carbapenems can be used to treat infections caused by various extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae isolates. However, due to the irrational use of carbapenems, the emergence of carbapenem-resistant Enterobacteriaceae (CRE) has caused difficulties in clinical work.2 KPC enzymes hydrolyze carbapenems, not only that, but it is also the most important enzyme among class A carbapenemases.3 Infections caused by KPC-producing organisms are associated with high mortality rates up to 51%, which poses a huge challenge for clinical diagnosis and treatment.4,5
Klebsiellapneumoniae is a gram-negative pathogen and is the most common cause of hospital-acquired and community-acquired infections.6,7 It has been reported that the widespread of carbapenem-resistant K. pneumoniae (CRKP) is caused by horizontal transfer of mobile elements such as plasmids and insertion sequences.8 According to the Carbapenem-Resistant Enterobacteriaceae Network, pneumonia and bloodstream infections caused by carbapenem-resistant K. pneumoniae have a higher mortality rate. Carbapenem-resistant K. pneumoniae nonbacteremic infections can result in a 24.3% mortality rate.9
KPC-producing K. pneumoniae, which can lead to outbreaks of serious diseases globally,10,11 are rarely reported in Shandong Province, China. In this study, we identified 14 clinical K. pneumoniae strains carrying blaKPC-2, all of which belong to ST11. The aim of our study was to elucidate the epidemiological and drug resistance mechanisms of KPC-producing K. pneumoniae strains collected from a large hospital in Shandong during the outbreak. Moreover, we attempted to characterize the genetic environment and phylogenetic analysis of blaKPC-2 in outbreak isolates.
Materials And Methods
Sample Collection
From February 1, 2018 to April 5, 2018, we have collected strains producing carbapenemase from the laboratory of a large teaching hospital in Jinan, Shandong Province. Bacterial identification was conducted with both matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS) (Bruker Daltonik GmbH, Bremen, Germany). Identification of carbapenemase genes (blaKPC, blaNDM, blaOXA-48, blaVIM and blaIMP) using PCR and Sanger sequencing.5
Antibiotic Susceptibility Testing
Determine the minimum inhibitory concentrations (MICs) of antibiotics (Dalian Meilun Biotech Co., Ltd, Dalian, China) using agar dilution method: amikacin, aztreonam, cefotaxime, cefpirome, ciprofloxacin, ceftazidime, gentamicin, piperacillin-tazobactam, imipenem, meropenem, tobramycin, amoxicillin-clavulanic acid, chloramphenicol and fosfomycin.12 Tigecycline and polymyxin were determined by the broth microdilution method. Results were interpreted using the CLSI standards (https://clsi.org). Controls were performed using Escherichia coli ATCC 25922 and K. pneumoniae ATCC 700603.
Plasmid Characterization And Conjugation Assay
The plasmid was characterized by S1-PFGE, and the location of blaKPC was identified by Southern hybridization with digoxigenin-labelled blaKPC probe using the DIG-High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics). Plasmid conjugation experiments by mating with E. coli J53 as a recipient strain. Next, the transconjugants were cultured on agar (OXOID, Hampshire, UK) medium supplemented with 200 mg/L sodium azide and 2 mg/L meropenem. Finally, MALDI-TOFMS was identified for transconjugants, and blaKPC was tested by PCR to ensure that the plasmid was successfully transferred to the recipient strain.
Whole-Genome Sequencing
Total DNA was obtained using an OMEGA Bacterial DNA Kit (Omega Bio-tek, Norcross, USA), followed by sequencing using Illumina HiSeq 4000-PE150 platform (Illumina, San Diego, CA, USA). We created genome sequence for 14 clinical K. pneumoniae isolates using SPAdes 3.11 by combining our Illumina sequencing reads. In addition, an online tool (http://www.genomicepidemiology.org/) was used to detect the acquired antimicrobial resistance genes in 14 isolates. The whole-genome sequences of the 14 isolates were deposited in GenBank under the following accession numbers: VJNW00000000-VJOJ00000000. The bacterial genome was annotated using the RAST server (http://rast.nmpdr.org/) and the transposon and IS elements were identified using the ISFinder database (https://www-is.biotoul.fr/). The genetic environment surrounding the carbe-genes were annotated using Easyfig 2.2.3. The presence of the virulence gene was identified by aligning the sequences of virulence factor from the database (http://www.mgc.ac.cn/VFs/). The gene sequence was uploaded to the PubMLST database (http://pubmlst.org/) to determine the ST type of the isolate.
Phylogenetic Reconstruction And Analysis
Identification of core genomic single nucleotide polymorphisms SNPs on WGS data for 14 isolates was conducted by using the kSNP program.13 kSNP is a program based on the k-mer analysis. Kchooser is used to evaluate the optimal value of k-mer before kSNP is run. After the run of kSNP program, the output file was used for further analysis.14 The maximum likelihood tree of the core SNP matrix output of kSNP was generated by using iTOL (https://itol.embl.de/).
Results
Detection Of KPC-Producing K. Pneumoniae From Clinical Samples
The study population included all patients in the hospital, and the case patients were specimens producing carbapenem-resistant K. pneumoniae. Patients who were isolated from the first carbapenem-resistant K. pneumoniae were considered to be the source of transmission, and then carbapenem-resistant K. pneumoniae was continuously collected. The outbreak lasted for 64 days. A total of 14 carbapenem-resistant K. pneumoniae were collected from sputum (8/14), urine (5/14)and pus (1/14). After the bacteria were cultured and purified, single colonies were selected from the culture plates and identified with MALDI-TOF-MS. The isolates were recovered from the selected medium for PCR and sequencing, and the blaKPC gene was identified in all isolates, no other carbapenemase encoding genes were detected.
Clinical Characteristics Of 14 Carbapenem-Resistant Isolates
Clinical characteristics of 14 carbapenem-resistant isolates were summarized in Table S1. In this study, isolates were defined as nonduplicated strains only if they were isolated from different patients. Among them, there are 5 females and 9 males, aged between 45 and 88 years old. The patients came from 4 different wards, including 10 from intensive care unit (ICU), 2 from respiratory ward, 1 from neurosurgical ward and 1 from recovery unit. Ten of them had a history of infectious diseases.
Antibiotic Resistance Profiles Of 14 Carbapenem-Resistant Isolates
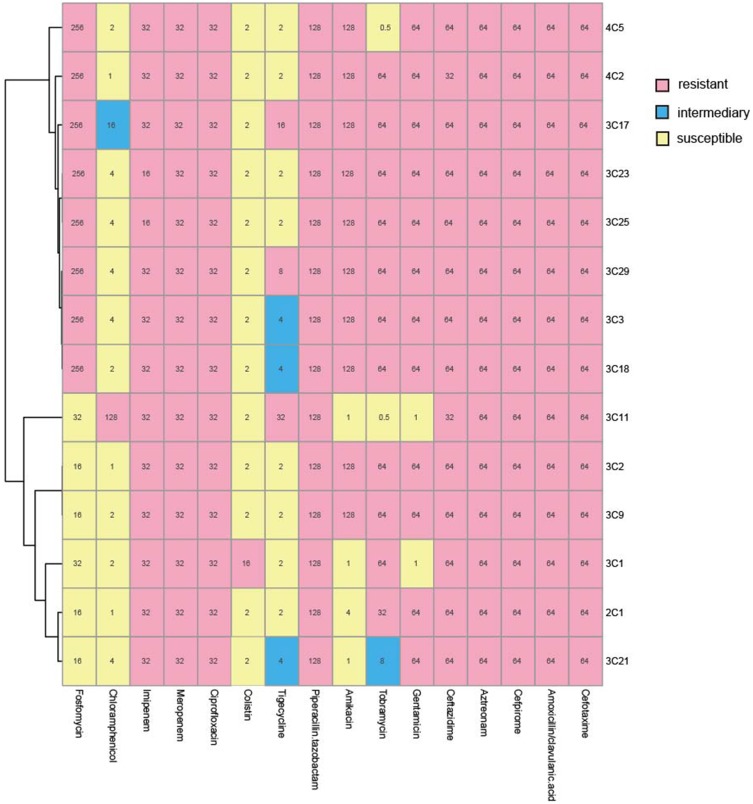
As shown in Figure 1, the antibiotic susceptibility test showed that all 14 isolates were resistant to piperacillin tazobactam, cefotaxime, ceftazidime, cefprozil, aztreonam, imipenem and meropenem, ciprofloxacin. Gentamicin (14%), tobramycin (14%) and amikacin (28%) have low sensitivity. Sensitivity to chloramphenicol (93%) and tigecycline (78%) was higher. Moreover, the experimental results showed that all 14 isolates were multi-drug resistant.
Figure 1.
MICs were determined by agar dilution methods for all antibiotics except for colistin and tigecycline, for which broth microdilution was used. Results were interpreted using the CLSI guidelines. Pink indicates resistance, blue indicates mediation, and light yellow indicates sensitivity.
Antimicrobial Resistance Genes
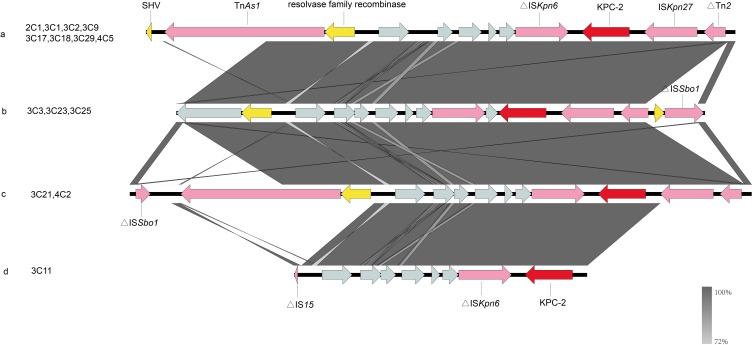
We present the data for the antimicrobial resistance genes in Table 1. The results of the analysis indicated that all isolates carried blaKPC-2. Figure 2 shows the genetic environment surrounding the blaKPC-2. There are four distinct genetic environments in the isolates carrying blaKPC-2 in this study. Thirteen isolates (92%) carried blaCTX-M-65, 12 isolates (85%) carried blaSHV-11and 9 isolates carried blaTEM-1B (64%). All isolates carried fosA, and sul- genes, encoding fosfomycin- and sulphonamide-, resistance. Moreover, a high prevalence of tetracycline resistance gene (57% isolates) was also observed. In addition, 14 isolates also carry resistance genes such as oqxA, oqxB, mphA, tetA and tetM (Table 1).
Table 1.
Antibiotic Resistance Genes, STs, Plasmid Type And Plasmid Size Of KPC-Producing K. Pneumoniae Isolates
| Isolate | Source | Antibiotic Resistance Genes | MLST | Plasmid Type | Plasmid Size |
|---|---|---|---|---|---|
| 2C1 | Sputum | blaCTX-M-65, fosA6, sul2, aph(3’’)-Ib, aph(6)-Id, blaSHV, blaCTX-M-3, qnrS1, sul1, aph(3’)-Ia, qnrB2, aac(3)-Iid, mph(A), aadA16, dfrA27, ARR-3, aac(6’)Ib-cr | ST11 | IncFⅡK2 | 94 kb |
| 3C1 | Sputum | dfrA1, ant(3’’)-Ia, erm(42), ant(3’’)-Ia, sul1, sul2, blaSHV-11, fosA6 | ST11 | IncFⅡK2 | 94 kb |
| 3C2 | Sputum | rmtB, blaTEM-1B, blaSHV-11, blaCTX-M-65, fosA6, ant(3’’)-Ia, sul1 | ST11 | IncFⅡK2 | 140 kb |
| 3C3 | Sputum | blaCTX-M-65, oqxA, oqxB, blaTEM-1B, rmtB, blaSHV-11, fosA3, fosA6, sul1, ant(3’’)-Ia, blaCTX-M-15 | ST11 | IncFⅡK2 | 140 kb |
| 3C9 | Urine | blaSHV-11, fosA6, ant(3’’)-Ia, sul1, rmtB, blaTEM-1B, blaCTX-M-65 | ST11 | IncFⅡK2 | 138.9 kb |
| 3C11 | Sputum | mph(A), aph(6)-Id, aph(3’’)-Ib, sul2, tet(A), fosA6, catA2, blaCTX-M-65, dfrA12, aadA2, sul1, blaSHV-11, blaTEM-1B, rmtB, aph(3’)-Ia | ST11 | IncFⅡK2 | 336.5 kb |
| 3C17 | Urine | erm(42), ant(3’’)-Ia, sul1, blaCMY-2, fosA3, blaCTX-M-65, blaTEM-1B, rmtB, dfrA1, sul2, fosA6 | ST11 | IncFⅡK2 | 160 kb |
| 3C18 | Sputum | blaSHV-12, ant(3’’)-Ia, sul1, fosA6, rmtB, blaTEM-1B, blaCTX-M-65 | ST11 | IncFⅡK2 | 135 kb |
| 3C21 | Urine | sul1, aadA2, dfrA12, blaSHV-11, blaCTX-M-65, aac(3)-Iid, fosA6 | ST11 | IncFⅡK2 | 140 kb |
| 3C23 | Sputum | fosA3, sul1, aac(6’)Ib-cr, ARR-3, dfrA27, aadA16, aph(3’’)-Ib, aph(6)-Id, blaCTX-M-65, rmtB, sul2, aadA2, blaTEM-141, blaSHV-11, fosA6, oqxB, oqxA | ST11 | IncFⅡK2 | 368 kb |
| 3C25 | Sputum | aadA2, blaTEM-141, sul1, blaCTX-M-15, aac(6’)Ib-cr, ARR-3, dfrA27, aadA16, aph(6)-Id, aph(3’’)-Ib, rmtB, sul2, blaCTX-M-65, oqxA, oqxB, blaSHV-11, fosA3 | ST11 | IncFⅡK2 | 140 kb |
| 3C29 | Urine | blaTEM-1B, rmtB, blaCTX-M-65, ant(3’’)-Ia, sul1, blaSHV-11, fosA6 | ST11 | IncFⅡK2 | 150 kb |
| 4C2 | Urine | fosA3, fosA6, dfrA14, sul2, aph(3’’)-Ib, aph(6)-Id, qnrS1, sul1, aadA2, blaCTX-M-65, rmtB, blaTEM-1B, blaSHV-11 | ST11 | IncFⅡK2 | 94 kb |
| 4C5 | Pus | blaSHV-11, rmtB, blaTEM-1B, sul1, ant(3’’)-Ia, blaCTX-M-65, fosA6, fosA3 | ST11 | IncFⅡK2 | 150 kb |
Figure 2.
The genetic environment of the blaKPC-2 gene in K. pneumoniae was isolated from clinical sources. The arrows represent the direction of transcription. The red open reading frame (ORF) indicates the blaKPC-2 gene, the pink ORF indicates the mobile element, the yellow ORF indicates other resistance genes or enzymes and the gray ORF indicates other genes or genes of unknown function. (A) The genetic environment of blaKPC-2 is similar in isolates 2C1, 3C1, 3C2, 3C9, 3C17, 3C18, 3C29 and 4C5. (B) The isolates 3C3, 3C23 and 3C25 all carry blaKPC-2 and share the identical genetic environment surrounding the same gene. (C) The isolate 3C21 and the isolate 4C2 have the same genetic environment. (D) The genetic environment of the isolate 3C11 carrying the blaKPC-2 gene.
Molecular Characteristics Of 14 Clinical Isolates
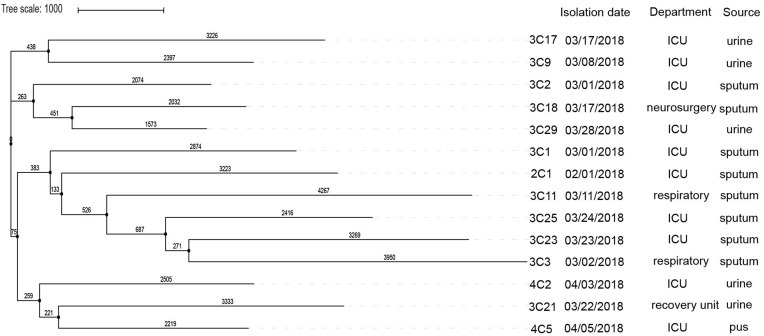
MLST analysis found that 14 clinically derived carbapenemase-producing K. pneumoniae were all ST11. S1-PFGE and Southern blot analysis demonstrated that the plasmid size carrying blaKPC-2 ranged from 94 kb to 368 kb (Table 1, Figure S1). By comparison of the plasmid sequences, 14 isolates belonged to the IncFIIK2 type plasmid. The results of the conjugation experiments showed that only the plasmid of the isolate 3C25 was successfully transferred to the E. coli J53. The virulence-related genes detected in 14 isolates included ybtX (100%, 14/14), mrk (93%, 13/14), fim (93%, 13/14), entB (93%, 13/). 14), entC (93%, 13/14), entF (93%, 13/14), irp1 (93%, 13/14), ybtE (0.7%, 1/14), ybtP (0.7%, 1/14) and entD (0.7%, 1/14) (Figure S2). Phylogenetic analysis based on k-mer algorithm shows that 3C9 and 3C17, 3C18 and 3C29, 3C3 and 3C23 have certain phylogenetic relationships (Figure 3). Moreover, their blaKPC-2 gene has the same genetic environment (Figure 2).
Figure 3.
SNP analysis of KPC-2-producing K. pneumoniae isolates, performed using kSNP. The maximum likelihood analysis of the core SNP matrix output for kSNP is performed in iTOL.
Discussion
We reported the outbreak of ST11 KPC-producing K. pneumoniae for 2 months in a general hospital in Shandong Province, China. All 14 isolates belonged to ST11 and carried the KPC-2-encoded IncFIIK2 plasmid. ST11 KPC-producing K. pneumoniae isolates are very common in China.15 In Shandong Province, there are few reports about KPC-producing K. pneumoniae causing outbreaks in the teaching hospital. Previously, we report the discovery of a KPC-2-producing Raoultella ornithinolytica isolate from well water in rural Shandong, but the gene characteristics were not described in detail.16 In this study, we describe the genetic characteristics of blaKPC-2, similar to other parts of China, and can be mediated through different molecular mechanisms.17,18 Recent reports have shown a clear correlation between the K. pneumoniae ST11 and IncFII-like plasmid. This result indicates that the IncFII-like plasmid may promote the spread of the blaKPC gene in K. pneumoniae ST11 in China.19 In this work, 14 CRKP strains were isolated from a large teaching hospital in Shandong Province in two months. MIC results (Figure 1) demonstrated that 14 isolates exhibited multidrug resistance.20 All the 14 isolates were resistant to meropenem and imipenem, and the whole-genome sequencing results showed that all isolates carried blaKPC-2, indicating that the drug-resistant phenotype was consistent with the genotype. Moreover, we have also found other drug resistance genes, such as blaCTX-M-65 and blaTEM-1 blaSHV-11 encoding β-lactam resistance; mphA encoding macrolide resistance; aac(6’)Ib-cr and aph(3’)- Ia encodes aminoglycoside resistance; sul1, sul2 and sul3 encode sulfonamide resistance; oqxA and oqxB encode fluoroquinolone resistance; fosA encodes fosfomycin resistance; tetA and tetM encode tetracycline resistance; and dfrA1 encodes trimethoprim resistance.21 These findings illustrate the multi-drug resistant phenotype of these K. pneumoniae isolates.
It is very difficult to clarify the transmission events between patients based only on epidemiological data.22 Combining genetic information with clinical epidemiological information can explain the spread of outbreak strains. Based on phylogenetic analysis and SNP differences, we can divide the 14 isolates into three branches, each representing a single transmission event (Figure 3). Combined with patient inpatient department and SNP differences, the transmission of pathogens from one patient to another was demonstrated. For example, 3C3 and 3C23, 3C18 and 3C29 have a certain affinity with each other, but from different departments, not only that, they also have the same genetic environment. The 14 isolates in this study had shorter sampling intervals and the same plasmid carrying blaKPC-2, belonging to the same clone, and having a certain relationship with each other, indicating that the outbreak occurred in a short period of time.23 When an outbreak occurs, the patient and the environment should be disinfected immediately, and the patient should be given appropriate treatment, and if necessary, the patient should be isolated, which can effectively contain the outbreak.
By analyzing virulence genes, all isolates carry the mrk operon, encoding the genes for yersiniabactin (irp1, irp2, fyuA and ybtAEPQSTUX), however, the rmpA or rmpA2 genes were absent from all isolates (Figure S2), both of which encode a high mucus phenotype and serve as high mark of virulence. The mrk operon encodes type 3 fimbriae, a virulence factor prevalent in Streptococcus pneumoniae.24 Type 3 fimbriae can not only mediate biofilm formation but also enhance bacterial adhesion to medical devices. Moreover, type 3 fimbriae may be an important factor in the formation of biofilm-associated infections, which can enter the host and persist in the clinical environment.25,26 Yersiniabactin is an iron carrier that helps bacteria gain the ability to chelate iron from infected host cells.27 In this study, all isolates carried the yersiniabactin genes pose challenges for clinical treatment.
Previous investigations have documented the diversity of blaKPC-harboring plasmids, which include IncFII, IncN, IncL/M, IncR and ColE1 groups, ranging in size from 10 to 300 kb.28–31 IncF replicons can be divided into FIA, FIB, FIC and FII groups, wherein the IncFII plasmid family exists in various Enterobacter species and plays an important role in the spread of antibacterial resistance genes such as blaKPC.32,33 In addition, the FII replicons can be divided into different subtypes, including FIIY, FIIK and FIIS, generating a number of compatible variants for overcoming the incompatibility barrier with obtaining plasmids.34 In our study, all 14 isolates belonged to the IncFIIK2 type plasmid. IncFIIK2 was a common blaKPC-harboring plasmids, reported in the United States, Israel, the United Kingdom, Italy and Colombia.32 Unlike previous studies, plasmid sizes in this study ranged from 94 kb to 368 kb, which was relatively large. The results of the conjugation assay showed that the plasmid binding in this study was difficult and the binding efficiency was less than 10%. The results correlated not only with plasmid size, but also with the presence of mobile genetic elements.35Although the 14 isolates were all in the IncFIIK2 type plasmid, the size of the plasmid carrying the blaKPC-2 was different, indicating that the blaKPC-2 in the hospital may be of various origins and spread in hospitals for many years. It is necessary to conduct a long-term retrospective genomic study of KPC-producing K. pneumoniae throughout the hospital to elucidate the evolution of KPC-producing K. pneumoniae in the hospital.
In addition, we investigated the genetic environment surrounding blaKPC-2 (Figure 2) and the results suggest that mobile genetic elements may promote the transmission of the blaKPC-2 gene. Although the genetic structure of these bacteria is different, the genetic background of blaKPC-2 is relatively similar in all plasmids. The blaKPC-2 genes were located in the same genetic context, the insertion sequence ISKpn27 is located upstream, and ISKpn6 is located downstream, except for 3C11, which is the same as previously reported.36
Conclusion
We first reported an outbreak of ST11-type KPC-2-producing K. pneumoniae in a large hospital in Shandong Province in a short period of time, although it is prevalent in China. We presented the genomic characteristics of blaKPC-2 positive K. pneumoniae isolates through whole-genome sequencing. All 14 isolates carrying the blaKPC-2 gene and have four different types of genetic environments. All isolates carried the virulence genes pose challenges for clinical treatment. It is now necessary to carry out routine genomic monitoring of such plasmids to effectively curb the spread and spread of resistant bacteria in this area.
Acknowledgments
The authors would like to thank the participants, coordinators and administrators for their support during the study. This project was supported by the National Natural Science Foundation of China (41771499 and 81741098) and the Fundamental Research Funds of Shandong University (2018JC102).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.An S, Chen J, Wang Z, et al. Predominant characteristics of CTX-M-producing Klebsiella pneumoniae isolates from patients with lower respiratory tract infection in multiple medical centers in China. FEMS Microbiol Lett. 2012;332(2):137–145. doi: 10.1111/j.1574-6968.2012.02586.x [DOI] [PubMed] [Google Scholar]
- 2.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263–272. doi: 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 4.Giacobbe DR, Del Bono V, Trecarichi EM, et al. Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect. 2015;21(12):1106 e1101–1106 e1108. doi: 10.1016/j.cmi.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Zheng B, Zhang J, Ji J, et al. Emergence of Raoultella ornithinolytica coproducing IMP-4 and KPC-2 carbapenemases in China. Antimicrob Agents Chemother. 2015;59(11):7086–7089. doi: 10.1128/AAC.01363-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JC, Lee EJ, Lee JH, et al. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol Lett. 2012;331(1):17–24. doi: 10.1111/j.1574-6968.2012.02549.x [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Zhou K, Zheng B, et al. High prevalence of ESBL-producing Klebsiella pneumoniae causing community-onset infections in China. Front Microbiol. 2016;7:1830. doi: 10.3389/fmicb.2016.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temkin E, Adler A, Lerner A, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: biology, epidemiology, and management. Ann N Y Acad Sci. 2014;1323:22–42. doi: 10.1111/nyas.12537 [DOI] [PubMed] [Google Scholar]
- 9.Yawei Zhang QW, Yin Y, Chen H, et al. Epidemiology of carbapenem-resistant enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2):e01882–01817. doi: 10.1128/AAC.01882-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol. 2009;30(7):666–671. doi: 10.1086/598244 [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Wang X, Yu X, et al. First detection and genomics analysis of KPC-2-producing Citrobacter isolates from river sediments. Environ Pollut. 2018;235:931–937. doi: 10.1016/j.envpol.2017.12.084 [DOI] [PubMed] [Google Scholar]
- 13.Gardner SN, Slezak T, Hall BG. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics. 2015;31(17):2877–2878. doi: 10.1093/bioinformatics/btv271 [DOI] [PubMed] [Google Scholar]
- 14.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/bf01734359 [DOI] [PubMed] [Google Scholar]
- 15.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66(2):307–312. doi: 10.1093/jac/dkq431 [DOI] [PubMed] [Google Scholar]
- 16.Sun P, Bi Z, Nilsson M, et al. Occurrence of blaKPC-2, blaCTX-M, and mcr-1 in enterobacteriaceae from well water in rural China. Antimicrob Agents Chemother. 2017;61:4. doi: 10.1128/AAC.02569-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen P, Zhang Y, Li G, Jiang X. Characterization of the genetic environment of the blaKPC-2 gene among Klebsiella pneumoniae isolates from a Chinese Hospital. Braz J Infect Dis. 2016;20(4):384–388. doi: 10.1016/j.bjid.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Chen J, Lin D, Xu X, Cheng J, Sun C. Prevalence and drug resistance characteristics of carbapenem-resistant Enterobacteriaceae in Hangzhou, China. Front Med. 2017;12(2):182–188. doi: 10.1007/s11684-017-0529-4 [DOI] [PubMed] [Google Scholar]
- 19.Fu P, Tang Y, Li G, Yu L, Wang Y, Jiang X. Pandemic spread of blaKPC-2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII-like plasmids. Int J Antimicrob Agents. 2019. doi: 10.1016/j.ijantimicag.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 20.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 21.Zheng B, Lv T, Xu H, et al. Discovery and characterisation of an escherichia coli ST206 strain producing NDM-5 and MCR-1 from a patient with acute diarrhoea in China. Int J Antimicrob Agents. 2018;51(2):273–275. doi: 10.1016/j.ijantimicag.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):148ra116. doi: 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Wei Z, Wang Y, Hua X, Feng Y, Yu Y. Tracking a hospital outbreak of KPC-producing ST11 Klebsiella pneumoniae with whole genome sequencing. Clin Microbiol Infect. 2015;21(11):1001–1007. doi: 10.1016/j.cmi.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Mecsas MKPJ. Klebsiella pneumoniae: going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YJ, Liao HW, Wu CC, Peng HL. MrkF is a component of type 3 fimbriae in Klebsiella pneumoniae. Res Microbiol. 2009;160(1):71–79. doi: 10.1016/j.resmic.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 26.Struve C, Bojer M, Krogfelt KA. Identification of a conserved chromosomal region encoding Klebsiella pneumoniae type 1 and type 3 fimbriae and assessment of the role of fimbriae in pathogenicity. Infect Immun. 2009;77(11):5016–5024. doi: 10.1128/IAI.00585-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachman MA, Oyler JE, Burns SH, et al. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun. 2011;79(8):3309–3316. doi: 10.1128/IAI.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gootz TD, Lescoe MK, Dib-Hajj F, et al. Genetic organization of transposase regions surrounding blakpc carbapenemase genes on plasmids from klebsiella strains isolated in a New York City Hospital. Antimicrob Agents Chemother. 2009;53(5):1998–2004. doi: 10.1128/AAC.01355-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade LN, Curiao T, Ferreira JC, et al. Dissemination ofblaKPC-2by the spread of klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among enterobacteriaceae species in Brazil. Antimicrob Agents Chemother. 2011;55(7):3579–3583. doi: 10.1128/AAC.01783-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassis-Chikhani N, Frangeul L, Drieux L, et al. Complete nucleotide sequence of the first KPC-2- and SHV-12-encoding IncX plasmid, pKpS90, from Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57(1):618–620. doi: 10.1128/AAC.01712-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuzon G, Naas T, Truong H, et al. Worldwide diversity ofklebsiella pneumoniaethat produce β-LactamaseblaKPC-2Gene1. Emerg Infect Dis. 2010;16(9):1349–1356. doi: 10.3201/eid1609.091389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–696. doi: 10.1016/j.tim.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villa L, García-Fernández A, Fortini D, Carattoli A. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J Antimicrob Chemother. 2010;65(12):2518–2529. doi: 10.1093/jac/dkq347 [DOI] [PubMed] [Google Scholar]
- 34.Feng J, Yin Z, Zhao Q, et al. Genomic characterization of novel IncFII-type multidrug resistant plasmids p0716-KPC and p12181-KPC from Klebsiella pneumoniae. Sci Rep. 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Lopez R, Redondo S, Garcillan-Barcia MP, de la Cruz F. Towards a taxonomy of conjugative plasmids. Curr Opin Microbiol. 2017;38:106–113. doi: 10.1016/j.mib.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Liu L, McNally A, Zong Z. Coexistence of three blaKPC-2 genes on an IncF/IncR plasmid in ST11 Klebsiella pneumoniae. J Global Antimicrob Resist. 2019;17:90–93. doi: 10.1016/j.jgar.2018.11.017 [DOI] [PubMed] [Google Scholar]