Abstract
Data regarding the association between subclinical thyroid dysfunction and clinical outcomes in ischemic stroke patients with intravenous thrombolysis (IVT) are limited. We aimed to investigate the predictive value of subclinical thyroid dysfunction in END, functional outcome and mortality at 3 months among IVT patients. We prospectively recruited 563 IVT patients from 5 stroke centers in China. Thyroid function status was classified as subclinical hypothyroidism, subclinical hyperthyroidism (SHyper) and euthyroidism. The primary outcome was END, defined as ≥ 4 point in the NIHSS score within 24 h after IVT. Secondary outcomes included 3-month functional outcome and mortality. Of the 563 participants, END occurred in 14.7%, poor outcome in 50.8%, and mortality in 9.4%. SHyper was an independent predictor of END [odd ratio (OR), 4.35; 95% confidence interval [CI], 1.86–9.68, P = 0.003], 3-month poor outcome (OR, 3.24; 95% CI, 1.43–7.33, P = 0.005) and mortality [hazard ratio, 2.78; 95% CI, 1.55–5.36, P = 0.003]. Subgroup analysis showed that there was no significant relationship between SHyper and clinical outcomes in IVT patients with endovascular therapy. In summary, SHyper is associated with increased risk of END, and poor outcome and mortality at 3 months in IVT patients without endovascular therapy.
Keywords: subclinical hypothyroidism, subclinical hyperthyroidism, ischemic stroke, thrombolysis, early neurological deterioration
INTRODUCTION
Intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator is an approved medical reperfusion treatment in acute ischemic stroke patients [1]. Recent studies revealed that a substantial fraction of patients cannot virtually recover but experienced early worsening following IVT, which were termed as early neurological deterioration (END) [2]. In fact, END may be accountable for living in dependence and even death, which were not surprisingly found in about half of the stroke survivors, despite IVT [3, 4]. Besides, older age, hyperglycemia, stroke severity, infarct volume, and vascular occlusion have been reported to be associated with a potentially increased risk of poor functional outcome after IVT [3, 5]. However, clinical outcomes are not easily foreseen at the initiation of the therapy as most of the current clinical-radiological risk factors are non-specifc (e.g., advanced age, stroke severity, symptomatic intracerebral hemorrhage, etc.). Therefore, it is essential to detect a potentially useful biomarker, which is involved in the underlying pathophysiological pathway and confers significant predictive value regarding therapy outcomes after IVT.
Subclinical thyroid dysfunction is a common endocrine condition among general population, including a prevalence reaching up to 15% for subclinical hypothyroidism (SHypo), and 12% for subclinical hyperthyroidism (SHyper) [6–9]. The cardio-cerebral vascular system is one of the major targets of thyroid hormones [6, 10, 11]. SHypo has been displayed to propagate vascular risk factors, such as hyperlipidemia [12], metabolic syndrome [13] and vascular stiffness [14]. SHyper has been proved to promote vascular damage by numerous ways, including facilitating hypertension, maintaining hypercoagulable state and causing endothelial dysfunction [15, 16]. Intriguingly, researchers found that ischemic stroke patients with SHypo or SHyper exhibited totally opposite outcomes [17, 18]. SHypo has been reported to be associated with favorable prognosis, while SHyper is related to poor outcomes.
IVT for acute ischemic stroke is often associated with hemorrhagic complications, such as symptomatic or asymptomatic intracerebral hemorrhage, mucosa bleedings and ecchymosis [19]. Thyroid diseases associated with thrombolysis are extremely rare. However, cases of thyroid hemorrhage have been reported [20, 21]. Besides, rt-PA can liberate bradykinin, causing vasodilation and capillary leakage in thyroid [22]. Recently, a case of transient thyroid edema has been confirmed following intravenous thrombolysis for acute ischemic stroke [23]. Thus, we hypothesized that IVT treatment may change the roles of thyroid function in predicting the clinical outcomes of ischemic stroke patients. Several cross-sectional studies have validated the predictive effects of thyroid function on prognosis in ischemic stroke patients [17, 18, 24], while there are yet no data on the prognostic value of subclinical thyroid dysfunction patterns in ischemic stroke patients treated with IVT. Herein, we aimed performed this prospective cohort to identify whether a potential prognostic value was existed in thyroid function in relation to short- and long-term outcomes of IVT patients and whether different dysfunction patterns would possess distinct prognostic values.
RESULTS
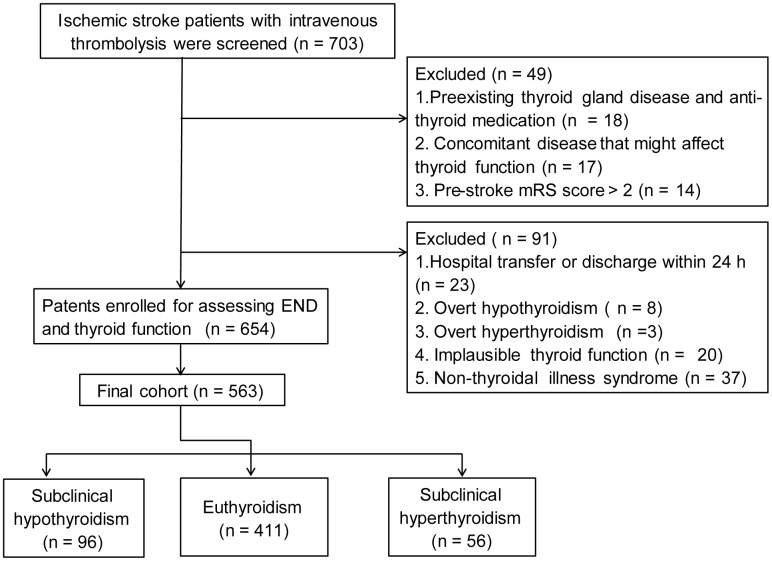
Finally, 563 ischemic stroke patients (mean age, 67.0 ± 11.8 years; 58.4% male) with thrombolytic therapy were recruited. Flow chart of patient inclusion was present in Figure 1. The baseline characteristics between patients included and patients excluded were shown in Table 1.
Figure 1.
Flow chart of patient inclusion.
Table 1. Baseline characteristics of the included and excluded patients.
| Variable | Study population (n = 563) | Excluded subjects (n = 140) | P |
| Age, year | 67.0 ± 11.8 | 68.2 ± 10.2 | 0.270 |
| Male,% | 329 (58.4) | 92 (65.7) | 0.124 |
| Hypertension,% | 390 (69.3) | 105 (75.0) | 0.184 |
| Diabetes mellitus,% | 151 (26.8) | 38 (27.1) | 0.939 |
| Hyperlipidemia,% | 72 (12.8) | 19 (13.5) | 0.507 |
| Coronary heart disease,% | 99 (17.6) | 28 (19.9) | 0.536 |
| Smoking,% | 207 (37.0) | 59 (42.1) | 0.265 |
After admission, SHypo, SHyper and euthyroidism were found in 96 (17.1%), 56 (9.9%) and 411 (73.0%) patients, respectively. END was observed in 83 (14.7%) patients. Of the 563 patients, 50.8% developed poor outcome, and 9.4% died at 3 months. Compared with euthyroidism, patients with SHypo had a higher prevalence of diabetes and coronary heart disease, higher triglyceride levels, but a lower baseline NIHSS score. Furthermore, hypertension, END, poor outcome and mortality at 3 months were more common in patients with SHyper compared with patients with euthyroidism (Table 2).
Table 2. Baseline characteristics of the study population stratified by the status of subclinical thyroid function.
| Variable | Euthyroidism group (n = 411) | SHyper group (n = 56) | P* | SHypo group (n = 96) | P# |
| Demographics data | |||||
| Age, year | 67.3 ± 11.7 | 65.1 ± 11.2 | 0.207 | 66.8 ± 12.9 | 0.691 |
| Male, % | 247 (60.1) | 33 (58.9) | 0.867 | 49 (51.0) | 0.105 |
| Vascular risk factors, % | |||||
| Hypertension | 283 (68.9) | 48 (85.7) | 0.009 | 59 (61.5) | 0.164 |
| Diabetes mellitus | 105 (25.5) | 12 (21.4) | 0.505 | 34 (35.4) | 0.049 |
| Hyperlipidemia | 50 (12.2) | 5 (9.1) | 0.477 | 17 (17.7) | 0.155 |
| Coronary heart disease | 67 (16.3) | 8 (14.5) | 0.739 | 24 (25.0) | 0.046 |
| Atrial fibrillation | 115 (28.0) | 20 (35.7) | 0.231 | 26 (27.1) | 0.860 |
| Smoking | 157 (39.0) | 19 (36.5) | 0.806 | 31 (32.3) | 0.273 |
| Clinical data | |||||
| Previous antiplatelet, % | 85 (20.7) | 13 (24.5) | 0.524 | 17 (17.7) | 0.506 |
| Previous statin, % | 34 (8.3) | 7 (12.5) | 0.294 | 10 (10.4) | 0.502 |
| Systolic blood pressure, mmHg | 136.8 ± 20.9 | 137.5 ± 24.8 | 0.760 | 136.2 ± 21.3 | 0.892 |
| Diastolic blood pressure, mmHg | 88.1 ± 14.8 | 85.4 ± 14.5 | 0.217 | 86.2 ± 12.8 | 0.252 |
| Body mass index, kg/m2 | 24.1 ± 3.2 | 24.5 ± 3.8 | 0.366 | 24.4 ± 3.2 | 0.451 |
| Onset to blood drawing time, h | 11.0 (6.0, 17.0) | 11.0 (6.0, 16.0) | 0.592 | 10.0 (6.0, 15.0) | 0.277 |
| Baseline NIHSS, score | 8.0 (4.0, 13.0) | 11.0 (4.0, 19.0) | 0.076 | 5.0 (3.0, 11.0) | 0.010 |
| Onset to treatment time, minutes | 144.8 ± 61.4 | 148.7 ± 69.0 | 0.657 | 142.6 ± 65.0 | 0.767 |
| Imaging data | |||||
| ASPECTS at admission | 9.0 (9.0, 10.0) | 9.0 (9.0, 10.0) | 0.616 | 9.0 (9.0, 10.0) | 0.793 |
| ASPECTS at 24 h | 8.0 (6.0, 9.0) | 7.0 (5.0, 9.0) | 0.061 | 8.0 (6.0, 9.0) | 0.384 |
| Vascular occlusion, %† | 178 (46.6) | 30 (56.6) | 0.183 | 47 (51.1) | 0.465 |
| sICH, % | 22 (5.4) | 6 (10.7) | 0.113 | 2 (2.1) | 0.174 |
| Lesion location, % | 0.467 | 0.815 | |||
| Frontal lobe | 98 (23.8) | 19 (33.9) | 19 (19.8) | ||
| Parietal lobe | 40 (9.7) | 4 (7.1) | 7 (7.3) | ||
| Basal ganglia | 118 (28.7) | 16 (28.6) | 31 (32.3) | ||
| Posterior fossa | 76 (18.5) | 10 (17.9) | 19 (19.8) | ||
| Other | 79 (19.2) | 7 (12.5) | 20 (20.8) | ||
| Stroke subtype, % | 0.116 | 0.364 | |||
| Large artery atherosclerosis | 161 (39.2) | 29 (51.8) | 41 (42.7) | ||
| Cardioembolism | 107 (26.0) | 14 (25.0) | 20 (20.8) | ||
| Small vessel occlusion | 88 (21.4) | 11 (19.6) | 26 (27.1) | ||
| Others | 55 (13.4) | 2 (3.6) | 9 (9.4) | ||
| Clinical outcomes, % | |||||
| Early neurological deterioration | 56 (13.6) | 19 (33.9) | 0.001 | 8 (8.3) | 0.160 |
| Unfavorable outcome at 3 months | 205 (49.9) | 40 (71.4) | 0.003 | 41 (42.7) | 0.206 |
| Mortality at 3 months | 33 (8.0) | 16 (28.6) | 0.001 | 4 (4.2) | 0.190 |
| Thyroid function tests | |||||
| TSH, mIU/L | 1.3 (0.9, 1.7) | 0.3 (0.2, 0.4) | 0.001 | 3.4 (2.8, 4.1) | 0.001 |
| Free T3, pmol/L | 4.8 ± 0.9 | 4.9 ± 0.9 | 0.603 | 4.8 ± 0.9 | 0.944 |
| Free T4, pmol/L | 14.6 ± 2.8 | 14.6 ± 2.7 | 0.735 | 14.1 ± 2.7 | 0.154 |
| Laboratory findings | |||||
| TC, mmol/L | 4.4 ± 1.1 | 4.4 ± 1.0 | 0.911 | 4.6 ± 1.2 | 0.278 |
| TG, mmol/L | 1.2 (0.9, 1.8) | 1.1 (0.8, 1.8) | 0.675 | 1.4 (1.1, 2.3) | 0.018 |
| LDL, mmol/L | 2.6 (2.1, 3.3) | 2.8 (2.1, 3.3) | 0.512 | 2.6 (2.1, 3.2) | 0.972 |
| HDL, mmol/L | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.802 | 1.2 ± 0.4 | 0.742 |
| FGB, mmol/L | 6.3 ± 2.2 | 6.4 ± 2.2 | 0.703 | 6.5 ± 2.8 | 0.464 |
| Homocysteine, umol/L | 15.7 ± 9.2 | 16.1 ± 7.4 | 0.795 | 15.2 ± 8.0 | 0.586 |
| Hs-CRP, mg/L | 5.7 (2.3, 12.3) | 8.4 (2.0, 11.6) | 0.388 | 5.0 (2.0, 12.5) | 0.343 |
Abbreviations: ASPECTS: alberta stroke program early CT score; FGB: fasting blood glucose; HDL: high density lipoprotein; Hs-CRP: high-sensitivity C-reactive protein; LDL: low density lipoprotein; NIHSS: national institutes of health stroke scale; SHyper: subclinical hyperthyroidism; SHypo: subclinical hypothyroidism; sICH: symptomatic intracranial hemorrhage; TC: total cholesterol; TG: triglyceride; TSH: thyroid stimulating hormone.
*Univariate analysis between SHyper group and euthyroidism group;
#Univariate analysis between SHypo group and euthyroidism group;
†Data available for 525 patients.
Table 3 demonstrated the baseline data of the study population according to clinical outcomes. Patients with END were older, had higher prevalence of endovascular therapy, vascular occlusion, sICH, large artery atherosclerosis and SHyper, and had higher baseline NIHSS score, onset to treatment time, fasting blood glucose and homocysteine levels. The ASPECTS score at 24 h was lower in patients with END than those without. Furthermore, endovascular therapy, vascular occlusion, sICH, and SHyper were more frequent in patients with 3-month poor outcome and mortality.
Table 3. Baseline characteristics of the study population stratified by the clinical outcomes in IVT patients.
| Variable | END | P | Poor outcome at 3 months | P | Mortality at 3 months | P | |||
| Yes (n = 83) | No (n = 480) | Yes (n = 286) | No (n = 277) | Yes (n = 53) | No (n = 510) | ||||
| Demographics data | |||||||||
| Age, year | 69.1 ± 9.6 | 66.2 ± 11.8 | 0.007 | 67.5 ± 11.8 | 65.7 ± 11.2 | 0.055 | 68.7 ± 12.0 | 66.4 ± 11.5 | 0.173 |
| Male, % | 44 (53.0) | 285 (59.4) | 0.277 | 122 (42.7) | 112 (40.4) | 0.592 | 27 (50.9) | 302 (59.2) | 0.245 |
| Vascular risk factors, % | |||||||||
| Hypertension | 60 (72.3) | 330 (68.8) | 0.519 | 94 (32.9) | 79 (28.5) | 0.264 | 39 (73.6) | 351 (68.8) | 0.475 |
| Diabetes mellitus | 26 (31.3) | 125 (26.0) | 0.316 | 71 (24.8) | 80 (28.9) | 0.278 | 13 (24.5) | 138 (27.1) | 0.692 |
| Hyperlipidemia | 10 (12.2) | 62 (12.9) | 0.857 | 41 (14.3) | 31 (11.2) | 0.271 | 9 (17.0) | 63 (12.4) | 0.340 |
| Coronary heart disease | 18 (21.7) | 81 (16.9) | 0.292 | 51 (17.9) | 48 (17.3) | 0.860 | 8 (15.1) | 91 (17.9) | 0.613 |
| Atrial fibrillation | 19 (22.9) | 142 (29.6) | 0.213 | 90 (31.5) | 71 (25.6) | 0.125 | 25 (47.2) | 136 (26.7) | 0.098 |
| Smoking | 32 (39.0) | 176 (37.0) | 0.723 | 113 (39.5) | 95 (34.9) | 0.263 | 17 (32.1) | 191 (37.8) | 0.410 |
| Clinical data | |||||||||
| Previous antiplatelet, % | 21 (25.9) | 94 (19.7) | 0.197 | 53 (18.5) | 62 (22.8) | 0.232 | 11 (20.8) | 104 (20.6) | 0.972 |
| Previous statin, % | 6 (7.2) | 45 (9.4) | 0.529 | 25 (8.7) | 26 (9.4) | 0.790 | 5 (9.4) | 46 (9.0) | 0.904 |
| SBP, mmHg | 137.7 ± 18.8 | 136.7 ± 17.5 | 0.639 | 136.9 ± 18.5 | 136.9 ± 16.9 | 0.873 | 138.3 ± 19.4 | 136.8 ± 17.5 | 0.524 |
| DBP, mmHg | 87.7 ± 11.6 | 86.2 ± 11.9 | 0.215 | 86.8 ± 12.9 | 85.7 ± 10.9 | 0.284 | 87.8 ± 14.6 | 86.1 ± 11.6 | 0.316 |
| Body mass index, kg/m2 | 23.8 ± 2.8 | 24.2 ± 3.3 | 0.322 | 24.2 ± 3.2 | 24.1 ± 3.3 | 0.675 | 24.1 ± 4.1 | 24.2 ± 3.1 | 0.737 |
| Baseline NIHSS, score | 14.0 (6.0, 19.0) | 7.0 (4.0, 12.0) | 0.001 | 10.0 (5.0, 16.0) | 5.0 (3.0, 10.0) | 0.001 | 16.0 (10.0, 20.0) | 7.0 (4.0, 12.0) | 0.001 |
| OTT, minutes | 158.0 ± 63.1 | 142.8 ± 62.3 | 0.047 | 164.5 ± 59 | 124.9 ± 60.2 | 0.002 | 158.5 ± 57.9 | 143.4 ± 62.8 | 0.095 |
| Endovascular therapy, % | 21 (25.3) | 64 (13.3) | 0.005 | 71 (24.8) | 14 (5.1) | 0.001 | 17 (32.1) | 68 (13.3) | 0.001 |
| Center, % | 0.389 | 0.234 | 0.156 | ||||||
| Center 1 | 42 (50.6) | 197 (41.0) | 129 (45.1) | 110 (39.7) | 19 (35.8) | 220 (43.1) | |||
| Center 2 | 9 (10.8) | 85 (17.7) | 49 (17.1) | 45 (16.2) | 6 (11.3) | 88 (17.3) | |||
| Center 3 | 12 (14.5) | 62 (12.9) | 41 (14.3) | 33 (11.9) | 11 (20.8) | 63 (12.4) | |||
| Center 4 | 11 (13.3) | 77 (16.0) | 37 (12.9) | 51 (18.4) | 7 (13.2) | 81 (15.9) | |||
| Center 5 | 9 (10.8) | 59 (12.3) | 30 (10.5) | 38 (13.7) | 10 (18.9) | 58 (11.4) | |||
| Imaging data | |||||||||
| ASPECTS at Admission | 9.0 (9.0, 10.0) | 9.0 (9.0, 10.0) | 0.167 | 9.0 (9.0, 10.0) | 10.0 (9.0, 10.0) | 0.205 | 9.0 (9.0, 10.0) | 9.0 (9.0, 10.0) | 0.137 |
| ASPECTS at 24 h | 6.0 (5.0, 7.0) | 8.0 (6.0, 9.0) | 0.001 | 6.0 (5.0, 8.0) | 9.0 (7.0, 9.0) | 0.001 | 6.0 (5.0, 7.0) | 8.0 (6.0, 9.0) | 0.001 |
| Vascular occlusion, %† | 47 (64.4) | 208 (46.2) | 0.004 | 150 (56.8) | 105 (40.2) | 0.002 | 35 (66.0) | 220 (46.0) | 0.001 |
| sICH, n (%) | 22 (26.5) | 8 (1.7) | 0.001 | 22 (7.7) | 8 (2.9) | 0.012 | 16 (30.2) | 14 (2.7) | 0.001 |
| Ischemic area, % | 0.689 | 0.907 | 0.641 | ||||||
| Frontal lobe | 20 (24.1) | 116 (24.2) | 72 (25.2) | 64 (23.1) | 14 (26.4) | 122 (23.9) | |||
| Parietal lobe | 8 (9.6) | 43 (9.0) | 26 (9.1) | 25 (9.0) | 7 (13.2) | 44 (8.6) | |||
| Basal ganglia | 23 (27.7) | 242 (29.6) | 84 (29.4) | 81 (29.2) | 14 (26.4) | 151 (29.6) | |||
| Posterior fossa | 15 (18.1) | 90 (18.8) | 49 (17.1) | 56 (20.2) | 11 (20.8) | 94 (18.4) | |||
| Other | 17 (20.5) | 89 (18.5) | 55 (19.2) | 51 (18.4) | 7 (13.2) | 99 (19.4) | |||
| Stroke subtype, % | 0.014 | 0.056 | 0.072 | ||||||
| Large artery atherosclerosis | 46 (55.4) | 185 (38.5) | 122 (42.7) | 109 (39.4) | 23 (43.4) | 208 (40.8) | |||
| Cardioembolism | 21 (25.3) | 120 (25.0) | 78 (27.3) | 63 (22.7) | 19 (35.8) | 122 (23.9) | |||
| Small vessel occlusion | 7 (8.4) | 118 (24.6) | 50 (17.5) | 75 (27.1) | 5 (9.4) | 120 (23.5) | |||
| Others | 9 (10.8) | 57 (11.9) | 36 (12.6) | 30 (10.8) | 6 (11.3) | 60 (11.8) | |||
| Thyroid function status | 0.007 | 0.002 | 0.002 | ||||||
| Euthyroidism | 56 (67.5) | 355 (74.0) | 205 (71.7) | 206 (74.4) | 33 (62.3) | 378 (74.1) | |||
| SHyper | 19 (22.9) | 37 (7.7) | 40 (14.0) | 16 (5.8) | 16 (30.2) | 40 (7.8) | |||
| SHypo | 8 (9.6) | 88 (18.3) | 41 (14.3) | 55 (19.9) | 4 (7.5) | 92 (18.0) | |||
| Laboratory findings | |||||||||
| TC, mmol/L | 4.5 ± 1.0 | 4.4 ± 1.1 | 0.636 | 4.4 ± 1.1 | 4.5 ± 1.2 | 0.261 | 4.2 ± 0.9 | 4.3 ± 1.1 | 0.124 |
| TG, mmol/L | 1.4 (0.9, 1.9) | 1.4 (1.0, 2.0) | 0.528 | 1.3 (0.9, 2.0) | 1.5 (1.0, 2.0) | 0.312 | 1.5 (0.8, 2.2) | 1.4 (1.0, 2.0) | 0.942 |
| LDL, mmol/L | 2.7 (2.2, 3.3) | 2.6 (2.1, 3.2) | 0.174 | 2.7 (2.1, 3.2) | 2.5 (2.1, 3.1) | 0.197 | 2.5 (2.1, 3.1) | 2.6 (2.1, 3.2) | 0.524 |
| HDL, mmol/L | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.304 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.544 | 1.2 ± 0.2 | 1.2 ± 0.3 | 0.847 |
| FGB, mmol/L | 7.1 ± 2.6 | 6.1 ± 2.3 | 0.003 | 6.4 ± 2.5 | 6.3 ± 2.3 | 0.507 | 6.8 ± 3.1 | 6.2 ± 2.3 | 0.105 |
| Homocysteine, umol/L | 18.1 ± 9.5 | 15.2 ± 8.7 | 0.011 | 16.8 ± 8.8 | 15.8 ± 8.7 | 0.003 | 16.0 ± 6.8 | 15.6 ± 9.1 | 0.695 |
| Hs-CRP, mg/L | 7.9 (2.4, 16.7) | 5.1 (2.3, 12.2) | 0.102 | 5.1 (2.1, 11.4) | 6.0 (2.8, 12.6) | 0.384 | 10.0 (2.9, 17.8) | 5.3 (2.2, 11.0) | 0.074 |
Abbreviations: ASPECTS: alberta stroke program early CT score; FGB: fasting blood glucose; HDL: high density lipoprotein; Hs-CRP: high-sensitivity C-reactive protein; LDL: low density lipoprotein; NIHSS: national institutes of health stroke scale; OTT: onset to treatment time; SHyper: subclinical hyperthyroidism; SHypo: subclinical hypothyroidism; sICH: symptomatic intracranial hemorrhage; TC: total cholesterol; TG: triglyceride.
†Data available for 525 patients.
Center 1: Nanjing First Hospital; Center 2: Mianyang Central Hospital; Center3: The First people’s Hospital of Yulin; Center 4: The Third People’s Hospital of Nantong. Center 5: Jiangsu Provincial Second Chinese Medicine Hospital.
Univariate regression analysis demonstrated that SHyper was associated with increased risk of END [odd ratio (OR), 3.26; 95% confidence interval [CI], 1.75–6.06, P = 0.001], 3-month poor outcome (OR, 2.51; 95% CI, 1.36–4.63; P = 0.003) and mortality [hazard ratio, 3.98; 95% CI, 2.24–7.43, P = 0.001]. Furthermore, after adjusting for age, sex, cardiovascular risk factors, center and variables with P < 0.05 in univariate analysis, this association did not significantly attenuate. However, no association was found in SHypo with clinical outcomes after IVT (Table 4).
Table 4. Unadjusted and adjusted regression analysis for clinical outcomes in IVT patients.
| OR (95%CI) for END | P | OR (95%CI) for poor outcome at 3 months | P | HR (95%CI) for mortality at 3 months | P | |
| Unadjusted model | ||||||
| SHyper vs Euthyroidism | 3.26 (1.75–6.06) | 0.001 | 2.51 (1.36–4.63) | 0.003 | 3.98 (2.24–7.43) | 0.001 |
| SHypo vs Euthyroidism | 0.58 (0.27–1.25) | 0.164 | 0.75 (0.48–1.17) | 0.207 | 0.51 (0.18–1.44) | 0.199 |
| Model 1 | ||||||
| SHyper vs Euthyroidism | 3.96 (2.06–7.68) | 0.001 | 3.14 (1.66–5.92) | 0.001 | 3.92 (2.13–7.24) | 0.001 |
| SHypo vs Euthyroidism | 0.56 (0.25–1.23) | 0.146 | 0.72 (0.46–1.15) | 0.167 | 0.48 (0.17–1.36) | 0.166 |
| Model 2 | ||||||
| SHyper vs Euthyroidism | 4.35 (1.86–9.68) | 0.003 | 3.24 (1.43–7.33) | 0.005 | 2.78 (1.55–5.36) | 0.003 |
| SHypo vs Euthyroidism | 0.78 (0.30–2.01) | 0.601 | 0.58 (0.30–1.12) | 0.104 | 0.47 (0.14–1.75) | 0.225 |
Abbreviations: CI: confidence interval; END: Early neurological deterioration; HR: hazard ratio; OR: odd ratio.
Eighty-five (15.1%) patients received endovascular therapy after admission. Results of subgroup analysis according to the IVT patients with or without endovascular therapy were listed in Table 5. For the patients receiving IVT followed by endovascular therapy, SHypo showed a trend for predicting 3-month poor outcome (adjusted OR, 0.27; 95% CI, 0.08–1.06; P = 0.057), but not for END and mortality. However, no association was found in SHyper with clinical outcomes among these patients.
Table 5. Subgroup analysis according to the IVT patients with and without endovascular therapy.
| Clinical outcomes | Patients with endovascular therapy (n = 85) | Patients without endovascular therapy (n = 478) | |||||
| Euthyroidism (n = 60) | SHyper (n = 12) | SHypo (n = 13) | Euthyroidism (n = 351) | SHyper (n = 44) | SHypo (n = 83) | ||
| Early neurological deterioration | |||||||
| Unadjusted OR (95%CI) | Reference | 1.34 (0.36–5.20) | 0.23 (0.03–1.91) | Reference | 4.02 (1.99–8.14)* | 0.72 (0.31–1.66) | |
| Adjusted OR (95%CI) | Reference | 1.17 (0.28–4.95) | 0.22 (0.03–1.96) | Reference | 4.72 (2.08–9.69)* | 1.08 (0.44–2.65) | |
| Poor outcome at 3 months | |||||||
| Unadjusted OR (95%CI) | Reference | 1.69 (0.19–9.94) | 0.25 (0.06–1.04) | Reference | 2.50 (1.30–4.83)* | 0.85 (0.30–4.83) | |
| Adjusted OR (95%CI) | Reference | 1.40 (0.15–9.33) | 0.27 (0.08–1.06) | Reference | 2.54 (1.21–5.33)* | 0.84 (0.49–1.45) | |
| Mortality at 3 months | |||||||
| Unadjusted HR (95%CI) | Reference | 2.75 (0.85–6.54) | 0.39 (0.04–2.27) | Reference | 4.29 (2.63–9.03)* | 0.83 (0.29–2.44) | |
| Adjusted HR (95%CI) | Reference | 1.77 (0.58–5.44) | 0.37 (0.06–2.31) | Reference | 3.99 (1.81–9.30)* | 0.89 (0.26–3.12) | |
Abbreviations: CI: confidence interval; OR: odd ratio; HR: hazard ratio; SHyper: subclinical hyperthyroidism; SHypo, subclinical hypothyroidism.
*P < 0.05
Adjusted model was controlled for age, sICH, ASPECTS score at 24 h and vascular occlusion.
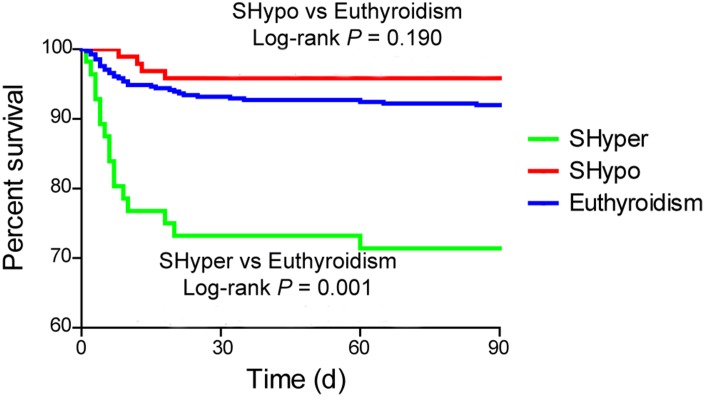
Kaplan-Meier curve revealed that mortality at 3 months was significantly higher in patients with SHyper when compared with euthyroidism. No association was detected in mortality between patients with SHypo and euthyroidism (Figure 2, log-rank test, P = 0.001).
Figure 2.
The Kaplan Meier curve for the cumulative 3-month survival rates according to the thyroid status. Log-rank test shows significant difference between patients with SHyper and euthyroidism.
DISCUSSION
In this large prospective cohort study of 563 ischemic stroke patients underwent IVT, we demonstrated that SHyper may increase the risk of END, poor outcome and mortality at 3 months in IVT patients without endovascular therapy.
The incidence of 14.7% for END in our study is similar to two recently published series of IVT-treated patients sharing the same END definition [25, 26]. Several risk factors have been postulated to lead to thrombolysis END, such as high blood glucose, hemorrhagic transformation, thrombus extension, and persistent arterial occlusion [25–27]. Data is scarce regarding the effect of neuroendocrine factors on mediating clinical outcomes after IVT. It is now believed that thyroid dysfunction is a potential mediator of the presence and outcome of cerebrovascular diseases. SHypo has been reported to be associated with favorable prognosis, while SHyper related to poor outcomes [17, 18]. Several other studies demonstrate that SHypo and elevated serum TSH level were correlated to increased risk of atherosclerosis [28, 29]. Cases of severe SHypo showed obvious exacerbation of carotid atherosclerosis [28]. In contrast, Cikim et al. found that SHypo was not associated with carotid atherosclerosis, but rather with decreased carotid artery intima-media thickness [30]. Interestingly, our present data did not show a remarkable association of SHypo with large artery atherosclerotic ischemic stroke and vascular occlusion. Some of these discrepancies might be explained at least in part by differences concerning the study population and methodology, especially the assessment of subclinical thyroid dysfunction.
More recently, high TSH levels were demonstrated to be independently correlated with a decreased risk of NIHSS score ≥ 5 at admission (prevalence proportion ratios, 0.62; 95% CI, 0.41–0.94, P = 0.024 for 3rdtertile vs. 1st tertile) [31]. In addition, patients with high TSH levels may have a better functional outcome at discharge [31]. To our best knowledge, this is the first prospective study that assessed subclinical thyroid dysfunction pattern specifically in relation to clinical outcomes in IVT patients and found that SHyper may increase the risk of END and worse outcome at 3 months. Consistent with our hypothesis, the multivariable logistic regression analysis revealed an independent association between SHyper and END even after adjusting for age, NIHSS score, stroke subtypes, onset to treatment time, sICH, fasting blood glucose levels, homocysteine levels, ASPECTS score at 24 h after thrombolysis and vascular occlusion. There are several mechanisms that might explain for the negative effects of SHyper on worse outcome in patients receiving IVT. Firstly, elevated concentrations of thyroid hormones are associated with an increase in metabolic rate and hyperactive condition in neural tissues, which would be susceptible to the amplitude of neurological damage after ischemia-reperfusion [32]. Secondly, SHyper can cause hypercoagulable state [16, 33], negatively affecting the utility of IVT. Also, pro-inflammatory in SHyper is prone to result in endothelial dysfunction [16], an important pathogenetic role in the progression of stroke and unfavorable outcomes.
In the subgroup analysis of patients treated with IVT followed by endovascular therapy, SHypo showed a protective effect on functional outcome at 3 months. A reduced response under condition of SHypo has been viewed as a protective preconditioning before stroke [7, 17, 24]. Additionally, long-lasting SHypo inducing atherosclerosis in cerebral vessels may also contribute to the development of collateral vessels, which has been widely accepted to be associated with better clinical outcome after endovascular therapy [34]. However, we could not establish an association of SHyper with worse outcome in these patients. These results should be extended and confirmed in future studies.
The strengths of our study include its prospective design and multiple-centers with large sample size. Also, there are limitations to this study that should be considered when interpreting the results. Firstly, some studies have shown the association of neuro-imaging markers such as thrombus extension, persistent arterial occlusion and vascular recanalization with END. However, these markers were not measured in this study, and we did not exclude effects of these markers on END. Also, we cannot totally exclude the potential effect of other undetected factors such as drug, stress induced by stroke, disease status and genetic predisposition on the level of thyroid function. Secondly, FT3, FT4, and TSH concentrations were measured only once at admission. A serial test would also be useful in verifying the role of thyroid function as a predisposing factor on acute stroke outcomes. Finally, several patients transfer to other hospitals or discharge early, presumably because of severe neurological deficit. Exclusion of such patients might have underestimated the prevalence of END and poor outcome.
In conclusion, this study illustrates that SHyper might be associated with increased risk of END, 3-month poor outcome and mortality in IVT patients without endovascular therapy. The potential benefits of screening and intervention of thyroid function on early clinical recovery require further exploration in ischemic patients underwent IVT.
MATERIALS AND METHODS
Study population
From January 2017 to March 2018, we consecutively recruited acute ischemic stroke patients underwent IVT within 4.5 h of symptom onset at 5 stroke centers in China (Nanjing First Hospital, Mianyang Central Hospital, The First people’s Hospital of Yulin, The Third People’s Hospital of Nantong, and Jiangsu Provincial Second Chinese Medicine Hospital). Patients treated with a bridging therapy consisting of IVT followed by endovascular therapy were also included. Exclusion criteria were as follows: (1) hospital transfer or discharged within 24 h; (2) pre-stroke modified Rankin Scale (mRS) score > 2; (3) pre-existing thyroid glands diseases, anti-thyroid medications, or diseases that might affect thyroid function [35, 36]; (3) defined as any of the following thyroid function alterations after admission: implausible thyroid function, biochemically defined overt thyroid disease, and non-thyroidal illness syndrome [37]. All participants signed a written consent form, and the study protocol was approved by the Institutional Review Board at each hospital.
Clinical data collection and assessment criteria
Data collection was performed by neurologists with experience in stroke care using a standardized case report form. We recorded baseline characteristics, including demographics, cardiovascular risk factors (including hypertension, diabetes, hyperlipidemia, smoking, drinking, and atrial fibrillation) and previous medication. Moreover, body mass index, blood pressure, onset to-treatment time, imaging data, and stroke subtype were also recorded. In this study, symptomatic intracerebral hemorrhage (sICH) was defined according to the European Cooperative Acute Stroke Study II (ECASS-II) trial [38]. All computed tomography images at admission and 24 h after IVT were calculated for the Alberta Stroke Program Early CT Scores (ASPECTS) [39]. Computed tomography angiography, magnetic resonance angiography or digital subtraction angiography was performed to identify the affected vessel. Stroke subtype was classified according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria [40].
Thyroid function tests
Blood samples were obtained from all patients within 24h after onset, and collected in chemistry test tubes. After centrifugation, serum samples were separated, and kept frozen at −80 °C for later analysis. Free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH) levels were determined for each patient by electrochemiluminescence immunoassay (Abbott Architect i2000, Abbott Diagnostics, Abbott Park, IL, USA). In accordance with previous studies [9, 18], levels of thyroid function were defined as SHyper [0.1 < TSH < 0.45 mIU/L], and SHypo (TSH: 2.50–19.99 mIU/L) with thyroid function test results. Patients with serum TSH levels between 0.45–2.49 mIU/L were considered as control group. Reference ranges for FT4 were 11.58–23.16pmol/L and for FT3 were 3.54–6.46pmol/L.
Clinical outcomes measurement
The primary outcome was END, which was defined as an increment of at least 4 point in total NIHSS score between admission and within 24 h after IVT (2). During a follow-up at 3 months after the index stroke, all patients were assessed for clinical outcome using the modified Rankin Scale (mRS) by neurologists who were blinded to clinical data. The following secondary outcomes were recorded: (1) functional outcome: categorized as good (mRS score of 0–2) and poor (mRS score of 3–6); (2) mortality.
Statistical analysis
All statistical analysis was done with SPSS software, version 23.0 (SPSS Inc., Chicago, IL). Continuous variables were summarized as mean ± standard deviations (SD) or medians with interquartile ranges (IQR) and compared by Student's t test or Mann-Whitney U test as appropriate. Categorical variables were expressed as percentages and analyzed with the chi-square test or Fisher’s exact test, as appropriate. Binary logistic regression analysis was designed to describe adjusted estimates of the association of subclinical thyroid dysfunction with END and poor outcome at 3 months. Cox’s proportional hazard regression analysis was performed to estimate the hazard ratio for mortality. All regression analyses were first adjusted for age, sex, cardiovascular risk factors and center (Model 1), and additionally adjusted for variables with P < 0.05 in univariate analysis (Model 2). We also used Kaplan-Meier curve to calculate 3-month survival probabilities. In all analyses, P < 0.05 was considered as statistically significant.
ACKNOWLEDGMENTS
We express our gratitude to all the researchers and patients who participated in this study.
Footnotes
CONFLICTS OF INTEREST: The authors declare no actual or potential conflicts of interest.
FUNDING: This work was supported by grants from the Clinical Research Innovation Plan of Shanghai General Hospital (No.CTCCR-2018BP05).
REFERENCES
- 1.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, et al. , and Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384:1929–35. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seners P, Turc G, Tisserand M, Legrand L, Labeyrie MA, Calvet D, Meder JF, Mas JL, Oppenheim C, Baron JC. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke. 2014; 45:2004–09. 10.1161/STROKEAHA.114.005426 [DOI] [PubMed] [Google Scholar]
- 3.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Dávalos A, Erilä T, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Köhrmann M, et al. , and Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy Investigators. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-MOnitoring STudy (SITS-MOST). Stroke. 2008; 39:3316–22. 10.1161/STROKEAHA.107.510768 [DOI] [PubMed] [Google Scholar]
- 4.Choi JC, Lee JS, Park TH, Cho YJ, Park JM, Kang K, Lee KB, Lee SJ, Kim JG, Lee J, Park MS, Choi KH, Kim JT, et al. Prestroke Antiplatelet Effect on Symptomatic Intracranial Hemorrhage and Functional Outcome in Intravenous Thrombolysis. J Stroke. 2016; 18:344–51. 10.5853/jos.2016.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tóth NK, Székely EG, Czuriga-Kovács KR, Sarkady F, Nagy O, Lánczi LI, Berényi E, Fekete K, Fekete I, Csiba L, Bagoly Z. Elevated Factor VIII and von Willebrand Factor Levels Predict Unfavorable Outcome in Stroke Patients Treated with Intravenous Thrombolysis. Front Neurol. 2018; 8:721. 10.3389/fneur.2017.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collet TH, Gussekloo J, Bauer DC, den Elzen WP, Cappola AR, Balmer P, Iervasi G, Åsvold BO, Sgarbi JA, Völzke H, Gencer B, Maciel RM, Molinaro S, et al. , and Thyroid Studies Collaboration. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012; 172:799–809. 10.1001/archinternmed.2012.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008; 29:76–131. 10.1210/er.2006-0043 [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012; 379:1142–54. 10.1016/S0140-6736(11)60276-6 [DOI] [PubMed] [Google Scholar]
- 9.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006; 295:1033–41. 10.1001/jama.295.9.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squizzato A, Gerdes VE, Brandjes DP, Büller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke. 2005; 36:2302–10. 10.1161/01.STR.0000181772.78492.07 [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Xie Y, Ding C, Xiao J, Tang Y, Jiang X, Shan H, Lin Y, Zhu Y, Li C, Hu D, Ling Z, Xu G, Sheng L. Subclinical hypothyroidism and risk of cerebral small vessel disease: A hospital-based observational study. Clin Endocrinol (Oxf). 2017; 87:581–86. 10.1111/cen.13383 [DOI] [PubMed] [Google Scholar]
- 12.Humerah S, Siddiqui A, Khan HF. Pattern of Altered Lipid Profile in Patients with Subclinical and Clinical Hypothyroidism and its Correlation with Body Mass Index. J Coll Physicians Surg Pak. 2016; 26:463–66. [PubMed] [Google Scholar]
- 13.Eftekharzadeh A, Khamseh ME, Farshchi A, Malek M. The Association Between Subclinical Hypothyroidism and Metabolic Syndrome as Defined by the ATP III Criteria. Metab Syndr Relat Disord. 2016; 14:137–44. 10.1089/met.2015.0065 [DOI] [PubMed] [Google Scholar]
- 14.Owen PJ, Rajiv C, Vinereanu D, Mathew T, Fraser AG, Lazarus JH. Subclinical hypothyroidism, arterial stiffness, and myocardial reserve. J Clin Endocrinol Metab. 2006; 91:2126–32. 10.1210/jc.2005-2108 [DOI] [PubMed] [Google Scholar]
- 15.Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, Michelangeli V. Subclinical thyroid dysfunction and blood pressure: a community-based study. Clin Endocrinol (Oxf). 2006; 65:486–91. 10.1111/j.1365-2265.2006.02619.x [DOI] [PubMed] [Google Scholar]
- 16.Popławska-Kita A, Siewko K, Telejko B, Modzelewska A, Myśliwiec J, Milewski R, Górska M, Szelachowska M. The changes in the endothelial function and haemostatic and inflammatory parameters in subclinical and overt hyperthyroidism. Int J Endocrinol. 2013; 2013:981638. 10.1155/2013/981638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhoundi FH, Ghorbani A, Soltani A, Meysamie A. Favorable functional outcomes in acute ischemic stroke patients with subclinical hypothyroidism. Neurology. 2011; 77:349–54. 10.1212/WNL.0b013e3182267ba0 [DOI] [PubMed] [Google Scholar]
- 18.Wollenweber FA, Zietemann V, Gschwendtner A, Opherk C, Dichgans M. Subclinical hyperthyroidism is a risk factor for poor functional outcome after ischemic stroke. Stroke. 2013; 44:1446–48. 10.1161/STROKEAHA.113.000833 [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, and ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 20.Sutter R, Bruder E, Weissenburg M, Balestra GM. Thyroid hemorrhage causing airway obstruction after intravenous thrombolysis for acute ischemic stroke. Neurocrit Care. 2013; 19:381–84. 10.1007/s12028-013-9889-z [DOI] [PubMed] [Google Scholar]
- 21.Chia PL. Thyroid hemorrhage after thrombolytic therapy for acute myocardial infarction. J Cardiovasc Med (Hagerstown). 2008; 9:935–36. 10.2459/JCM.0b013e3282ff82e8 [DOI] [PubMed] [Google Scholar]
- 22.Molinaro G, Gervais N, Adam A. Biochemical basis of angioedema associated with recombinant tissue plasminogen activator treatment: an in vitro experimental approach. Stroke. 2002; 33:1712–16. 10.1161/01.STR.0000017284.77838.87 [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Yan C, Xu W, Huang P. Acute diffuse and transient thyroid swelling after intravenous thrombolysis for acute ischemic stroke: A case report. Medicine (Baltimore). 2018; 97:e12149. 10.1097/MD.0000000000012149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek JH, Chung PW, Kim YB, Moon HS, Suh BC, Jin DK, Kim BM, Rhee EJ, Lee YT, Park KY. Favorable influence of subclinical hypothyroidism on the functional outcomes in stroke patients. Endocr J. 2010; 57:23–29. 10.1507/endocrj.K09E-206 [DOI] [PubMed] [Google Scholar]
- 25.Mori M, Naganuma M, Okada Y, Hasegawa Y, Shiokawa Y, Nakagawara J, Furui E, Kimura K, Yamagami H, Kario K, Okuda S, Koga M, Minematsu K, Toyoda K. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the Stroke Acute Management with Urgent Risk Factor Assessment and Improvement rt-PA Registry. Cerebrovasc Dis. 2012; 34:140–46. 10.1159/000339759 [DOI] [PubMed] [Google Scholar]
- 26.Saqqur M, Molina CA, Salam A, Siddiqui M, Ribo M, Uchino K, Calleja S, Garami Z, Khan K, Akhtar N, O’Rourke F, Shuaib A, Demchuk AM, Alexandrov AV, and CLOTBUST Investigators. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007; 38:69–74. 10.1161/01.STR.0000251800.01964.f6 [DOI] [PubMed] [Google Scholar]
- 27.Seners P, Hurford R, Tisserand M, Turc G, Legrand L, Naggara O, Mas JL, Oppenheim C, Baron JC. Is Unexplained Early Neurological Deterioration After Intravenous Thrombolysis Associated With Thrombus Extension? Stroke. 2017; 48:348–52. 10.1161/STROKEAHA.116.015414 [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness in patients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis. 2013; 227:18–25. 10.1016/j.atherosclerosis.2012.10.070 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Kim BK, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, Kwon MJ, Rampal S, Zhao D, Pastor-Barriuso R, Lima JA, Shin H, Guallar E. Thyroid hormones and coronary artery calcification in euthyroid men and women. Arterioscler Thromb Vasc Biol. 2014; 34:2128–34. 10.1161/ATVBAHA.114.303889 [DOI] [PubMed] [Google Scholar]
- 30.Cikim AS, Oflaz H, Ozbey N, Cikim K, Umman S, Meric M, Sencer E, Molvalilar S. Evaluation of endothelial function in subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid. 2004; 14:605–09. 10.1089/1050725041692891 [DOI] [PubMed] [Google Scholar]
- 31.Delpont B, Aboa-Eboulé C, Durier J, Petit JM, Daumas A, Legris N, Daubail B, Giroud M, Béjot Y. Associations between Thyroid Stimulating Hormone Levels and Both Severity and Early Outcome of Patients with Ischemic Stroke. Eur Neurol. 2016; 76:125–31. 10.1159/000449055 [DOI] [PubMed] [Google Scholar]
- 32.Rastogi L, Gupta S, Godbole MM. Pathophysiological basis for thyrotoxicosis as an aggravating factor in post-ischemic brain injury in rats. J Endocrinol. 2008; 196:335–41. 10.1677/JOE-07-0483 [DOI] [PubMed] [Google Scholar]
- 33.Erem C. Blood coagulation, fibrinolytic activity and lipid profile in subclinical thyroid disease: subclinical hyperthyroidism increases plasma factor X activity. Clin Endocrinol (Oxf). 2006; 64:323–29. 10.1111/j.1365-2265.2006.02464.x [DOI] [PubMed] [Google Scholar]
- 34.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, Jovin TG, Khatri P, von Kummer R, Sugg RM, Zaidat OO, Hussain SI, Goyal M, et al. , and IMS III Investigators. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014; 45:759–64. 10.1161/STROKEAHA.113.004072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mebis L, Debaveye Y, Visser T, Van den Berghe G. Changes within the thyroid axis during the course of critical illness. Endocrinol Metab Clin North Am. 2006; 35:807–21. 10.1016/j.ecl.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 36.Peeters R, Debaveye Y, Fliers E, Visser T. Changes within the thyroid axis during critical illness. Crit Care Clin. 2006; 22:41–55. 10.1016/j.ccc.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 37.Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: an update. J Endocrinol. 2010; 205:1–13. 10.1677/JOE-09-0412 [DOI] [PubMed] [Google Scholar]
- 38.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P, and Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998; 352:1245–51. 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 39.Aviv RI, Mandelcorn J, Chakraborty S, Gladstone D, Malham S, Tomlinson G, Fox AJ, Symons S. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol. 2007; 28:1975–80. 10.3174/ajnr.A0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]