Abstract
All living cells are covered with a dense “sugar-coat” of carbohydrate chains (glycans) conjugated to proteins and lipids. The cell surface glycome is determined by a non-template driven process related to the collection of enzymes that assemble glycans in a sequential manner. In mammals, many of these glycans are topped with sialic acids (Sia), a large family of acidic sugars. The “Sialome” is highly diverse owing to various Sia types, linkage to underlying glycans, range of carriers, and complex spatial organization. Presented at the front of cells, Sia play a major role in immunity and recognition of “self” versus “non-self,” largely mediated by the siglecs family of Sia-binding host receptors. Albeit many mammalian pathogens have evolved to hijack this recognition system to avoid host immune attack, presenting a fascinating host-pathogen evolutionary arms race. Similarly, cancer cells exploit Sia for their own survival and propagation. As part of this ongoing fitness, humans lost the ability to synthesize the Sia type N-glycolylneuraminic acid (Neu5Gc), in contrast to other mammals. While this loss had provided an advantage against certain pathogens, humans are continuously exposed to Neu5Gc through mammalian-derived diet (eg, red meat), consequently generating a complex immune response against it. Circulating anti-Neu5Gc antibodies together with Neu5Gc on some human tissues mediate chronic inflammation “xenosialitis” that exacerbate various human diseases (eg, cancer and atherosclerosis). Similarly, Neu5Gc-containing xenografts are exposed to human anti-Neu5Gc antibodies with implications to sustainability. This review aimed to provide a glimpse into the evolution of Sia and their implications to xenotransplantation.
Keywords: anti-carbohydrate antibodies, N-acetylneuraminic acid, N-glycolylneuraminic acid, sialic acid, xenotransplantation
1. Introduction
All cells in nature have a dense and complex outer cover known as “glycocalyx” that is composed of carbohydrates, one of the major building blocks of cells. Glycolipids, membrane-embedded or secreted glycoproteins, and glycoproteins in the extracellular matrix are also abundantly enriched with glycans (collectively referred to as glycoconjugates). This ubiquitous nature of glycan expression suggests that the “glycome,” the entire glycan composition in a cell or an organism, is essential for the maintenance of life similar to the genome, transcriptome, proteome, and lipidome.1,2 Unlike the synthesis of DNA, RNA, or proteins, glycosylation is a non-template-driven process that rely on a collection of enzymes, each responsible for the addition of one sugar unit at a time, in a sequential manner.3,4 Thus, rather than the genetic code, it is the glycosylation metabolic pathways–the collection of glycosyltrasferases, glycosidases, sugar transporters, and available monosaccharides–that determine the composition of the cellular glycome.3,4 Glycan complexity is the highest among the other macromolecules in cells (e.g, DNA, RNA, proteins) owing to the large number of different monosaccharides, the diverse linkages between the different sugar units, their enormous combinatorial composition, and the various carrier molecules.5–7 In addition, variability in the occupancy of the diverse glycosylation sites within a given protein (macroheterogeneity) or variation in the glycan structures within the same glycosylation site (microheterogeneity) add further complexity.8 Thus, a protein with two potential glycosylation sites and 10 possible different glycans in each site could potentially generate 120 different glycoforms of the protein. This glyco-heterogeneity may affect the protein’s fundamental biological properties thereby contributing to its functional diversity,8–10 as clearly demonstrated for glycosylated antibodies.10–14 A major factor that contribute to the structural and functional diversity of glycoconjugates is their sialic acids capping.15–17 Here we discuss diverse aspects of sialic acids biology and their implications on human-specific immune responses during health, disease, and xenotransplantation.
2. Structural Diversity of the Sialome
Nonulosonic acids (NulO) are diverse nine-carbon backbone α-keto acidic carbohydrates distributed among the three domains of life–Bacteria, Archea, and Eukarya. Sialic acids (Sia) encompass an evolutionary and structurally related subgroup of NulO. Sia are prominently expressed at the Deuterostome lineage (e.g, vertebrates and some advanced invertebrates, such as echinoderms) and their related pathogenic microbes. Although Sia have been found in some protostomes (i.e, drosophila, octopus, and squid), they are mostly absent in invertebrates as well as in plants.18
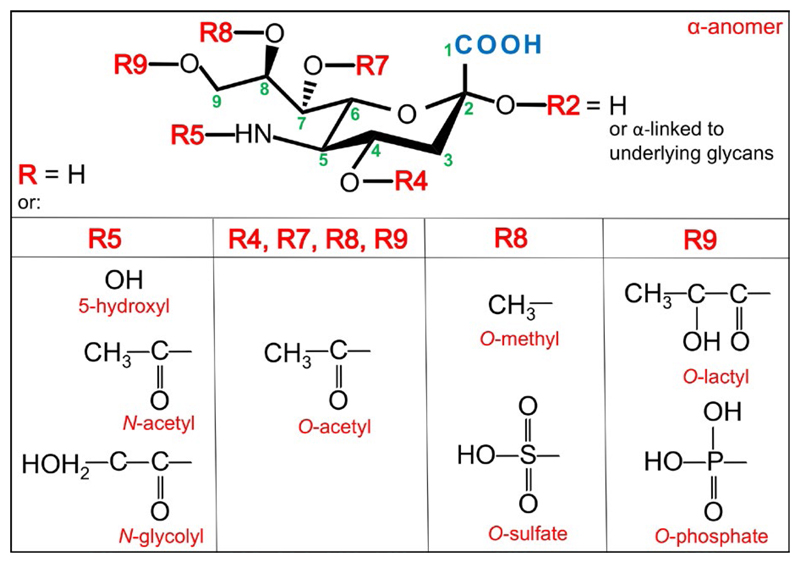
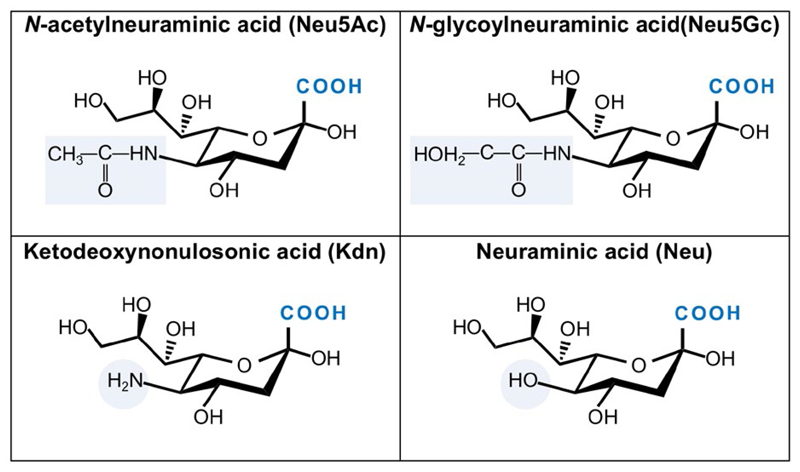
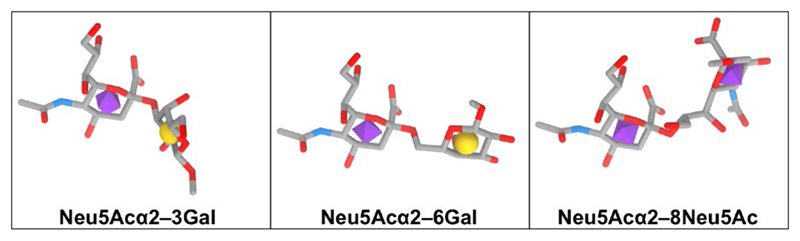
There are over 50 Sia monosaccharide derivatives, all containing characteristic three functional groups: a carboxylate (C–1) attached to the C–2 anomeric carbon that carries a carbonyl in the Sia open chain form (i.e. α-keto), a glycerol-like 3-carbon side chain (C–7, C–8, C–9) protruding out of the 6-carbon ring of a cyclic Sia, and an acylated amino group (Figure 1). Sia can be further diversified by various substitutions with acetyl, glycolyl, methyl, lactyl, or sulfate (Figure 1).18–21 The two most common Sia forms in mammals are N-acetylneuraminic acid (Neu5Ac; molecular weight 309 g/mol; pKa = 2.6) and N-glycolylneuraminic acid (Neu5Gc; molecular weight 325 g/mol; pKa = 2.92).20Ketodeoxynonulosonic acid (Kdn) with a hydroxyl at C–5 appears less abundant in mammals, whereas neuraminic acid (Neu) with a free amine at C–5 is rare in nature (Figure 2).20,22 To generate a glycosidic linkage with underlying carbohydrates, Sia is first activated with the nucleotide donor cytidine 5′-monophosphate; then, the CMP-Sia is transferred to diverse glycan acceptors in the Golgi. Uniquely, this Sia activation process occurs in the nucleus (in contrast to all other nucleotide sugars that are made in the cytoplasm) and then CPM-Sia returns to the cytosol and transported into the Golgi by a CMP antiporter.21,23–26 Of note, free Sia and CMP-Sia acquire the β-anomer configuration; however, upon conjugation to other carbohydrates Sia always becomes an α-anomer (R2 group on the anomeric C–2 is equatorial down and the carboxyl orientation up). Thus, Sia is alpha-linked through its C–2 position to galactose (at C–3/4/6; Siaα2–3/4/6Gal), N-acetylgalactosamine (at C–6; Siaα2–6GalNac), N-acetylglucosamine (at C–4/6; Siaα2–4/6GlcNAc), or to another Sia (at C–8/9; Siaα2–8/9Sia; Figure 3).20,27 Hence both the Sia type and the linkage to underlying glycans contribute to the diversity of Sia-containing glycans (sialoglycans).
Figure 1.
Structure and diversity of sialic acids. There are over 50 Sia monosaccharide derivatives. All Sia contain characteristic carboxylate group at C–1 that is attached to the C–2 anomeric carbon, a glycerol-like side chain (C–7, C–8, C–9) protruding out of the 6-carbon ring of a cyclic Sia, and an acylated amino group. Sia can be further diversified by various substitutions with acetyl, glycolyl, methyl, lactyl or sulfate, as depicted
Figure 2.
Common sialic acid forms. Four primary core Sia structures are depicted. Neu5Ac and Neu5Gc are the two most common Sia in mammals. Kdn with a hydroxyl at C–5 is less abundant in mammals, while Neu with a free amine at C–5 is rare in nature
Figure 3.
Diversity in sialic acid linkage to underlying glycans. Sia is alpha-linked through its C–2 position to galactose (at C–3/4/6; Siaα2–3/4/6Gal), N-acetylgalactosamine (at C–6; Siaα2–6GalNac), N-acetylglucosamine (at C–4/6; Siaα2–4/6GlcNAc), or to another Sia (at C–8/9; Siaα2–8/9Sia)
In vertebrates, Sia are normally found at the outermost position of glycans or extended beyond the terminal Sia with additional Sia units.28,29 Yet, in some bacteria, Sia can be found as an internal-glycan component in repeating units of the polysaccharide.18 Sialoglycans can be secreted from cells or found conjugated to glycosphingolipids and proteins that are secreted or embedded in the cell surface.20 Sialic acid containing glycosphingolipids (gangliosides) share the common core structure Galβ1–4Glc–βCer extended into a large collection of different gangliosides (>20).30 Various sialoglycans are covalently attached to proteins by a glycosidic bond most commonly to serine/threonine residues (O-glycans on Ser/Thr) or to asparagine residues (N-glycans on Asn-X-Ser/Thr; X≠proline).31,32 The most common O-glycan class starts with conjugation of GalNAc, then extended into one of four glycan core structures that can be further elongated. Mucin proteins are abundantly populated with hundreds of heterogeneous such O-GalNAc glycans.31 On the other hand, N-glycans contain the common core Man3GlcNAc2Asn that is commonly extended into oligomannose, complex, or hybrid types. Complex N-glycans have up to six branches further elongated with Galβ1-4GlcNAc (LacNAc) repeats.32 Each of these elongated O-GalNAcglycans core structures or N-glycans antennae can be capped with Sia in diverse linkages (Siaα2–3/6/8).31,32 Hence, in addition to the Sia type and linkage, the composition of the underlying carbohydrates, their type (N/O-glycans), and their carrier (protein/glycosphingolipid), all contribute the enormous diversity of sialic acid containing glycans (sialoglycans).
Another layer of complexity stems from diverse population of glycosylation sites within a given protein (macroheterogeneity and microheterogeneity), and the three-dimensional organization of sialoglycoconjugates on cell surfaces. Occasionally, sialo-glycoproteins and gangliosides become clustered into micro-domains on cell surfaces to generated “clustered saccharide patches” that could mediate differential biological functional events than scattered sialoglycoconjugates.33 The overall immense structural diversity of the “sialome” had been compared to that of the forest canopy, with diverse leaves and flowers (variety of Sia), stems (Sia-linkages), branches (sialoglycans), trees (sialoglycoconjugates), and their spatial organization in the forest (saccharide patches).34 This structural complexity plays an important role in mediating numerous biological functions encoded by the sialome as a whole.34
3. General Biological Roles of Sialic Acids
Sialic acids have numerous biological roles.28,29,34–39 First and foremost, Sia have major biophysical effects on cells given the magnitude and breadth of their occurrence. Sia mediate repulsion between cells in light of their negative charge and ubiquitous expression on vertebrate cell surfaces (~100 mM local concentration40), thereby affecting the biophysical properties of cellular interactions.35,38 Accordingly, expression of polysialic acids on certain brain proteins is critical for neuronal development, morphogenesis, and functionality,41–43 while their expression on leukocytes modulates cellular interactions and immune responses.29,44–47
Presented at the outermost position of cell surface glycans, Sia are perfectly positioned to facilitate various immune recognition events mediated by responsive receptors of the host cells or those of pathogenic invaders.9,38,39 In fact, Sia have been suggested to operate as “self-associated molecular patterns” (SAMPS) responding to “self-pattern recognition receptors” (SPRR).48 Accordingly, some blood groups present antigenic variations of sialylated glycans contributing to allogenic differences between populations of the same species (e.g, MN blood groups in humans, and A/B blood groups in cats).35 The Sia “cloak” also serves as a biological mask to cover potentially antigenic glycoconjugates (i.e, cryptoantigens), or to block interactions between certain host receptors and the exposed underlying glycans.35,38,39 For example, removal of Sia by endogenous sialidases may uncover terminal galactose that would promote clearance mechanisms by galectin through its cross-linking of surface molecules, or by binding to liver asialoglycoprotein receptors.39,49,50 Inversely, addition of capping Sia residues to glycoproteins effectively increase their half-life,36,51,52 or change their mode of action.13,14
4. Receptor-Mediated Biological Roles of Sialic Acids
Binding of Sia by their cognate receptors mediate molecular recognition used by organisms to identify and decode the biological information that exist in the sialome.29,35 There are several Sia-binding lectins that function as SPRR, the most common of which are factor H, selectins, L1-CAM, and siglecs (sialic-acid-binding immunoglobulin-like lectins).27 The serum protein factor H binds to sialylated SAMPs on cell surfaces thereby blocking activation of the alternative complement pathway.48,53 Modification of Sia by 9-O-acetyl blocks the binding of factor H and abrogates its function as a negative regulator.27 The selectins are involved in cell-trafficking mediated by a cell–cell adhesion system, mainly between leukocytes and endothelial cells, but also occur between other cell types.29,54–56 Each selectin isoform binds a particular glycan ligand, such as sialyl-Lewis-X/A, respectively, recognized by E- and P-selectin on endothelium and platelets, and 6-sulpho- sialyl-Lewis-X by L-selectin on leukocytes.35,57 This recognition system initiates the tethering and rolling of circulating leukocytes on endothelial surfaces, facilitating their transmigration and extravasation into the surrounding tissues.29,35 Another receptor-mediated Sia recognition involves the siglecs family of I-type lectin receptors, that are abundantly expressed on various immune cells. Siglecs have N-terminal V-set Ig-like domain that recognize Sia patterns as “self” and often have immunoreceptor tyrosine-based inhibition motifs (ITIMs) within their cytosolic tails. Consequently, siglec binding to Sia deliver inhibitory signals to dampen immune cell activation.56,58,59 Yet, some siglecs have rather activating roles through their association with DAP12 adaptor containing immunoreceptor tyrosine-based activation motif (ITAM).60 Hence, Sia recognition by host receptors is a powerful tool for “self” vs “non-self” discrimination that is translated into appropriate immune responses.
5. Pathogen-Related Biological Roles of Sialic Acids
The sialome plays an important role in host–pathogen interactions. Some pathogens exploit this Sia-recognition system to evade host immune responses using diverse molecular mimicry strategies to cloak themselves with Sia.29,61 Microbial “self-like” Sia-coats dampen the host immune responses against these invaders by engaging inhibitory siglecs, by factor H binding to inhibit complement killing, and by reducing their immunogenicity to avoid humoral responses.35 In addition, various pathogenic protozoa, viruses, bacteria, and toxins use host sialylated-structures as receptors to mediate their invasion into cells.20,29,35,62 To avoid infection, host organisms developed molecular decoys in the form of heavily sialylated moieties (e.g, glycoproteins in plasma and extracellular fluids, and the heavily sialylated erythrocytes in the blood) that trap pathogens and keep them away from their target on host cell surfaces.35,63 However, pathogens have another host evasion strategy that use microbial sialidases to avoid such sialylated-decoys, by removing SAMPs to expose new attachment sites on host cells, and concurrently provide carbohydrate food source for the invading pathogens.29,35 Thus, host–pathogen sialome interactions mediate a fitness race to expedite “self” vs “non-self” immune responses orchestrating invasion or evasion strategies.
6. Sialome-Siglec Mediated Evolutionary Arms-Race
The siglec family of Sia-binding cell surface adhesion receptors provide an excellent evidence for the evolutionary arms-race between mammals and their pathogenic microbes. Siglecs function via phosphotyrosine-dependent signaling pathways to mediate immunoregulation.60,64–68 While some siglecs are highly conserved in vertebrates and share similar Sia-ligand binding preferences between species, the CD33-related siglecs of inhibitory or activating receptors show obvious inter-species differences and vary in their Sia-ligands specificity.60,65,66 The CD33-related siglecs encoding genes are clustered in a specific genomic location (on chromosome 19q in humans) and show high genetic diversification encompassing multiple mechanisms, that include expansions of gene subsets, gene deletions, pseudogenization, gene-conversion, exon shuffling, and increased amino acid substitutions in the Sia-binding domain.65,69,70 These changes are specific to the CD33-related siglecs encoding genes and are in contrast to the adjacent and conserved kallikrein-like genes.65,69
In addition to their high genetic diversification, there is evidence that the CD33-related siglecs also demonstrate functional evolutionary diversity.71 Analysis of closely related orthologs of CD33-related siglecs showed marked quantitative and qualitative inter-species differences in binding preferences of various sialoglycans and sialylated pathogens.71 In particular, human siglecs revealed higher preference towards Neu5Ac-containing glycans, while the siglecs of chimpanzees and baboons were more reactive against Neu5Gc-containing glycans.71 This Neu5Ac-preference functional diversity in humans compensates for the change in landscape from endogenous Neu5Gc-glycans into Neu5Ac-glycans. Human-specific loss of Neu5Gc is the result of an irreversible mutation in the human gene encoding CMP-Neu5Ac hydroxylase (CMAH) that is responsible for Neu5Gc synthesis in other mammals.72,73 Altogether, diversification of CD33-related siglecs in repertoire, sequence, and binding preferences is driven both in response to variation in expression of Sia-containing ligands and by direct competition against pathogens.65,66,74 Likewise, diversification in Sia can also drive a shift in pathogen binding preferences affecting its host selection with implications on evolution.
7. Sialome-Pathogen Mediated Evolutionary Arms-Race
The two most abundant Sia on mammalian cell surfaces are Neu5Ac and Neu5Gc. They differ by a single oxygen atom in their C–5 position. Neu5Ac carries the hydrophobic N-acetyl group, while the hydroxylated Neu5Gc form carries rather a hydrophilic N-glycolyl group (Figure 2). These moieties contribute to sialoglycans-specific functions and recognition by various receptors and pathogens.20,37 In fact, many pathogens use Sia as their point of entry to initiate host infection,20,75 and some have a clear preference for Neu5Gc-containing glycans.20,76–80
Neu5Ac is the major Sia in humans due to a human-specific inactivation of the CMAH gene, and hence inability to convert CMP-Neu5Ac into CMP-Neu5Gc.73,81,82 The CMAH inactivation occurred ~3 million years ago in the period just before the appearance of Homo (coinciding with the transition from the genus Australopithecus to genus Homo).83 It was postulated that the loss of Neu5Gc in humans ensued an adaptation to the changing environment, and likely provided an evolutionary advantage protecting against Neu5Gc-preferring non-human animal pathogens.82 The pathogenic malaria parasites provide an example of such adaptive changes in Sia recognition molecules, specifically responding to the Neu5Gc-loss in humans.75,78,84,85
Malaria is a mosquito-borne disease mediated by the Plasmodium parasite and a major cause of death worldwide.86 Plasmodium falciparum, one of the predominant species and causative agent of malignant malaria, exploits host-Sia for invasion through binding of the parasite dominant invasion receptor EBA-175 (Erythrocyte Binding Antigen 175) to Sia-containing glycophorin A on human red blood cells. Interestingly, the closely related chimpanzee parasite P. reichenowi cannot infect humans, and reciprocally the human parasite P. falciparum cannot infect chimpanzees.75,78,87 The basis for this species specificity relates to diversity in Sia-recognition mode of entry, in which the EBA-175 of the human malaria parasite prefers host Neu5Ac-glycans, while the chimpanzee parasite prefers host Neu5Gc-glycans, suggesting the parasites had co-evolved with their reciprocal hosts.78,88 Evidently, all known P. falciparum strains originated from P. reichenowi, probably by a single host transfer as early as 2-3 million years ago.84 Hence, two critical mutations supported deviation of the human malaria parasite: mutation in the human CMAH gene and then another mutation in the EBA-175 of P. falciparum lineage.84,85,87 In evolutionary perspective, elimination of Neu5Gc in human ancestors likely provided an advantage escaping from a common ape malaria, but then humans became vulnerable to a new variant that had evolved to prefer Neu5Ac-glycans on human red blood cells, rendering it even more pathogenically violent in its new host mediating malignant malaria.84,85,87
Salmonella Typhi provides another example of a pathogen that had evolutionary adapted to Neu5Gc-loss in humans.79,80 This is a bacterial pathogen causing typhoid fever exclusively in humans.79,89,90 The disease is manifested by the bacterial virulence factor, typhoid toxin, that is essential for development of typhoid fever symptoms.89,90 Typhoid toxin has a unique A2B5 structure, composed of two enzymatic heteromeric A subunits combined with a homopentameric B subunit that binds specifically to Neu5Ac-containing glycoproteins.80,91 It was found that the toxin is cytotoxic to cells expressing surface Neu5Ac-glycans but not to those expressing Neu5Gc-glycans. Likewise, although S.Typhi can replicate in chimpanzees, they do not develop typical disease symptoms.79,92 The reduced virulence is due to the predominant expression of Neu5Gc-glycoproteins that abrogate toxin binding to chimpanzee tissues.93 In contrast to S. Typhi, the serovar S. Typhimurium, that cause “food poisoning” disease, encodes an evolutionarily related AB5 toxin that can bind both Neu5Ac/Neu5Gc-containing glycoproteins and demonstrates a broad host specificity not limited to humans.80 Further studies revealed that the virulence factor evolution involved horizontal gene exchange within the same bacterial species, to exquisitely adapt to the Neu5Ac-expressing human host and the emergence of a powerful human-specific toxin.80
8. The “Red Queen” Effect in Evolutionary Arms-Race of Sialic Acid Biology
The collective evolutionary consequences of selection pressure on co-evolving organisms is termed the Red Queen Effect,2,94,95 and it is particularly demonstrated by sialic acid-containing glycans and their associated determinants, as described above. In this scenario, host sialoglycans change over time, struggling to outrace their pathogens that frequently use glycans to infect host cells, and that are even more rapidly evolving. Reciprocally, pathogens modify their own glycans to more closely resemble the host glycans, thereby evading host recognition. Such pathogen-mediated selection processes modify host glycan cloak for evasion; however, host sialoglycans are also critical SAMP components, especially in discriminating “self”/”non-self,” therefore their evolutionary altered expression patterns must not compromise the host own survival.2,6,63 Other changes involve host receptors that recognize SAMPs (e.g, siglecs), referred to as secondary Red Queen Effects.1,68,96 Overall, Neu5Gc loss in humans and its evolutionary outcomes, on both humans and their related pathogens, provide an excellent example of the Red Queen Effect concept.
9. Neu5Gc-Glycans are Immunogenic in Humans
In addition to the evolutionary perspective, Neu5Gc has other consequences on human health.97–106 Despite the fact that humans lost the ability to synthesize Neu5Gc, small amounts can be found on human epithelial and endothelial cells.98,105,107,108 Neu5Gc expression on human cells originates from dietary consumption of mammalian-derived food item (red meat and dairy products), as CMAH remained active in most other mammals.108–110 Metabolic incorporation of Neu5Gc into human cells occurs through micropinocytosis; then, it is assembled into diverse glycans within the cells, that are finally delivered to the cell surface.108–110 While the human cellular machinery refers to the non-human Neu5Gc as if it was the human Sia Neu5Ac, the immune system does not tolerate even the minor oxygen difference between these two Sia and responds against Neu5Gc-glycans as foreign invaders.111,112 Neu5Gc enters human cells like a Trojan horse, assembled as “self,” but recognized as “non-self” as soon as exposed on the cell surface, and therefore is commonly referred to as xeno-autoantigen.99,111 Given the diversity of Sia and sialoglycans, incorporation of dietary Neu5Gc results in a highly diverse collection of Neu5Gc-containing glycans due to the myriad forms of Neu5Gc-modifications, various linkages, different underlying glycans, carrier molecule, and spatial organization. Consequently, all humans have circulating antibodies that reflect this diversity by recognizing a wide collection of Neu5Gc-containing glycoproteins and gangliosides.99,105,111–113 Anti-Neu5Gc antibodies class-switch into diverse Ig isotypes (IgA, IgG, IgM),111 and go through affinity maturation.114 It had been shown that anti-Neu5Gc IgG and IgM appear already in infants, coinciding with exposure to dietary Neu5Gc, and their inception could potentially be mediated by a human-specific commensal/pathogen (i.e, non-typeable Haemophilus influenza; NTHi) that incorporate Neu5Gc into its cell surface lipooligosaccharides thus becoming immunogenic.115
10. Consequences of Co-Expression of Neu5Gc on Human Cells and Anti-Neu5Gc Antibodies
The diverse collection of circulating polyclonal anti-Neu5Gc antibodies in human sera encounter diverse Neu5Gc-glycans on human tissues, thereby mediating an attack on these foreign entities that are presented in the context of “self.”97,99 While Neu5Gc can be incorporated into healthy tissues, it accumulates on cancer cells, especially those of epithelial origin.98,105,107,108 However, glycosylation patterns are different between healthy and cancer cells due to changes in the glycosylation pathways leading to expression of tumor-associated carbohydrate antigens.116–118 Therefore, incorporation of Neu5Gc into cancer cells generates a collection tumor-associated carbohydrate neoantigens perceived as foreign by the host.117 It had been shown that anti-Neu5Gc antibodies have dualistic and opposing roles in cancer, whereas a low dose stimulate tumor progression mediated by chronic inflammation,108,119 while higher doses can eradicate cancer cells.113,120 Furthermore, even a twofold difference can shift anti-Neu5Gc response from cancer stimulating into inhibiting,121 reflecting the general concept of hormesis,122 in which both the quantity and quality of antibody determine the outcome of response, but in an inverse fashion with the higher dose beneficial to the host.102,123
As in cancer cells, circulating anti-Neu5Gc antibodies also encounter Neu5Gc in the context of normal cells where they collide to mediate chronic inflammation, collectively denoted as “xenosialitis.”98 Xenosialitis in the context of cancer fit the known increased carcinoma risk associate with red meat consumption.98,120,124,125 Likewise, accumulation of Neu5Gc in atherogenic blood vessels exacerbate atherosclerotic cardiovascular disease mediated by xenosialitis98,126 and is found in line with the increased risk of cardiovascular disease that is associated with consumption of red and processed meat.127 Furthermore, it had been suggested that Neu5Gc and anti-Neu5Gc antibodies could also participate in other chronic inflammation-mediated diseases, including autoimmunity.97,125,128,129
11. Consequences of Neu5Gc and Anti-Neu5Gc Antibodies in Xenotransplantation
While it was well-known that humans lack intrinsic Neu5Gc expression, its immunogenic potential in xenotransplantation was largely underrated for decades, because it was assumed that anti-Neu5Gc antibodies are completely absent in normal healthy individuals, making it irrelevant in the context of xenotransplantation. In addition, it was considered that anti-Neu5Gc antibodies are being elicited in serum only in immune-activated conditions as in serum sickness.72,105 This notion had changed once conclusively demonstrating that healthy human sera contain anti-Neu5Gc antibodies, and that these represent the majority of the non-anti-Gal antibodies limiting xenotransplantation,111,112 suggesting the potential impact of Neu5Gc in xenotransplantation rejection risk.130 The αGal xenoantigen has long been considered the major xenoantigen limiting xenotransplantation.131–133 Similar to Neu5Gc, αGal cannot be synthesized by humans due do a loss-of-function mutation in the GGTA1 gene, and all humans carry anti-Gal IgA, IgG, or IgM antibodies.131However, while αGal is not found on any human tissue, Neu5Gc is constantly acquired by human diet and incorporates into human cells, resulting in a highly diverse anti-Neu5Gc antibodies response that vary between individuals. Another major difference is in their antigenic complexity: while αGal represents a single carbohydrate xenogenic antigen (Galα1-3–Galβ1-4–GlcNAc-R), there are multiple Neu5Gc-glycans on glycoproteins and glycolipids.130 These differences suggest that Neu5Gc complexity is an important factor to consider in xenotransplantation.
Besides the dietary exposure to Neu5Gc, humans can also encounter Neu5Gc that is expressed on exogenous glycosylated bio-therapeutics,106,134–136 biodevices, or xenografts,104,137–142 that are given to human patients. In this case, circulating anti-Neu5Gc antibodies could reduce efficacy of therapy,134–136 elicit an increased and diversified anti-Neu5Gc response that can remain long-lasting,143,144 or possibly mediate xenograft failure or rejection.104,136,142,145 Currently, it seems that both the αGal and Neu5Gc-glycans limit the potential of xenotransplantation, as recently reviewed elsewhere,103,104,146–148 and generation of double-knockout animals deficient in both the CMAH and GGTA1 genes seems to be promising.139,147,149–151
12. Summary
The sialome, comprising of myriad forms of Sia, sialoglycans, and sialoglycoconjugates, play a significant role in immune recognition and responses in humans. Sia are highly diverse and are important for various host intrinsic functions as well as escape from pathogens, in an ongoing evolutionary arms-race. They are recognized by various receptors that decode the sugar code into actionable pathways. Neu5Gc is uniquely not synthesized in humans, yet can be acquired through the diet then presented on various human tissues. Due to the presence of anti-Neu5Gc antibodies in circulation, Neu5Gc poses threat to immune homeostasis as it triggers immune response, resulting in “xenosialitis” exacerbating atherosclerosis and cancer. Apart from the common xenoantigen αGal, Neu5Gc has been recognized as a major carbohydrate epitope in xenotransplantation. The role of Neu5Gc in mediating immune responses in xenotransplantation needs to be addressed with due importance.
Acknowledgments
This work was supported by the 7th Framework Program FP7-Health-2013-INNOVATION-1-603049 of the European Commission and the European Union H2020 Program grants ERC-2016-STG-716220 (to V.P-K). We greatly appreciate the assistance of Oliver C. Grant from the Complex Carbohydrate Research Center, University of Georgia, Athens 30606, GA, USA in generating Figure 3.
Funding information
European Commission, Grant/Award Numbers: FP7-603049 and ERC-2016-STG-716220
Footnotes
Conflict of Interest
None declared.
Orcid
Vered Padler-Karavani http://orcid.org/0000-0002-4761-3571
References
- 1.Varki A. Evolutionary forces shaping the Golgi glycosylation machinery: why cell surface glycans are universal to living cells. Cold Spring Harb Perspect Biol. 2011;3:a005462. doi: 10.1101/cshperspect.a005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Rini JM, Esko JD. Glycosyltransferases and glycan-processing enzymes. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 4.Henrissat B, Surolia A, Stanley P. A genomic view of glycobiology. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 5.Turnbull JE, Field RA. Emerging glycomics technologies. Nat Chem Biol. 2007;3:74–77. doi: 10.1038/nchembio0207-74. [DOI] [PubMed] [Google Scholar]
- 6.Gagneux P, Aebi M, Varki A. Evolution of glycan diversity. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 7.Seeberger PH. Monosaccharide diversity. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 8.An HJ, Froehlich JW, Lebrilla CB. Determination of glycosylation sites and site-specific heterogeneity in glycoproteins. Curr Opin Chem Biol. 2009;13:421–426. doi: 10.1016/j.cbpa.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varki A, Gagneux P. Biological functions of glycans. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 10.Jennewein MF, Alter G. The immunoregulatory roles of antibody glycosylation. Trends Immunol. 2017;38:358–372. doi: 10.1016/j.it.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lu LL, Suscovich TJ, Fortune SM, et al. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 13.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl. 1):S9–S14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 14.Anthony RM, Kobayashi T, Wermeling F, et al. Intravenous gamma-globulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raju TS, Lang SE. Diversity in structure and functions of antibody sialylation in the Fc. Curr Opin Biotechnol. 2014;30:147–152. doi: 10.1016/j.copbio.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Bournazos S, Ravetch JV. Fcγ receptor function and the design of vaccination strategies. Immunity. 2017;47:224–233. doi: 10.1016/j.immuni.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Chung CY, Chough S, et al. Antibody glycoengineering strategies in mammalian cells. Biotechnol Bioeng. 2018;115:1378–1393. doi: 10.1002/bit.26567. [DOI] [PubMed] [Google Scholar]
- 18.Varki A, Schnaar RL, Schauer R. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 19.Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- 20.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 21.Isa P, Arias CF, Lopez S. Role of sialic acids in rotavirus infection. Glycoconj J. 2006;23:27–37. doi: 10.1007/s10719-006-5435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue S, Kitajima K. KDN (deaminated neuraminic acid): dreamful past and exciting future of the newest member of the sialic acid family. Glycoconj J. 2006;23:277–290. doi: 10.1007/s10719-006-6484-y. [DOI] [PubMed] [Google Scholar]
- 23.Kean EL. Sialic acid activation. Glycobiology. 1991;1:441–447. doi: 10.1093/glycob/1.5.441. [DOI] [PubMed] [Google Scholar]
- 24.Munster-Kuhnel AK, Tiralongo J, Krapp S, et al. Structure and function of vertebrate CMP-sialic acid synthetases. Glycobiology. 2004;14:43R–51R. doi: 10.1093/glycob/cwh113. [DOI] [PubMed] [Google Scholar]
- 25.Altheide TK, Hayakawa T, Mikkelsen TS, et al. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: evidence for two modes of rapid evolution. J Biol Chem. 2006;281:25689–25702. doi: 10.1074/jbc.M604221200. [DOI] [PubMed] [Google Scholar]
- 26.Sellmeier M, Weinhold B, Münster-Kühnel A. CMP-sialic acid synthetase: the point of constriction in the sialylation pathway. Top Curr Chem. 2015;366:139–167. doi: 10.1007/128_2013_477. [DOI] [PubMed] [Google Scholar]
- 27.Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- 28.Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–857. doi: 10.1038/labinvest.3700656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nat Rev Immunol. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnaar RL, Kinoshita T. Glycosphingolipids. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 31.Brockhausen I, Stanley P. O-GalNAc glycans. In: Varki A, Cummings RD, Esko JD, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 32.Stanley P, Taniguchi N, Aebi M. N-Glycans. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 33.Cohen M, Varki A. Modulation of glycan recognition by clustered saccharide patches. Int Rev Cell Mol Biol. 2014;308:75–125. doi: 10.1016/B978-0-12-800097-7.00003-8. [DOI] [PubMed] [Google Scholar]
- 34.Cohen M, Varki A. The sialome-far more than the sum of its parts. OMICS. 2010;14:455–464. doi: 10.1089/omi.2009.0148. [DOI] [PubMed] [Google Scholar]
- 35.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne B, Donohoe GG, O’kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today. 2007;12:319–326. doi: 10.1016/j.drudis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. Int Rev Cytol. 1997;175:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins BE, Blixt O, Desieno AR, et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci USA. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 42.Bonfanti L, Theodosis DT. Polysialic acid and activity-dependent synapse remodeling. Cell Adh Migr. 2009;3:43–50. doi: 10.4161/cam.3.1.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Troy FA. Polysialylation: from bacteria to brains. Glycobiology. 1992;2:5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Drake PM, Nathan JK, Stock CM, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181:6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatos NM, Zhang L, Jokilammi A, et al. Changes in polysialic acid expression on myeloid cells during differentiation and recruitment to sites of inflammation: role in phagocytosis. Glycobiology. 2014;24:864–879. doi: 10.1093/glycob/cwu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curreli S, Arany Z, Gerardy-Schahn R, et al. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- 47.Avril T, North SJ, Haslam SM, et al. Probing the cis interactions of the inhibitory receptor Siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. J Leukoc Biol. 2006;80:787–796. doi: 10.1189/jlb.1005559. [DOI] [PubMed] [Google Scholar]
- 48.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology. 2011;21:1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss P, Ashwell G. The asialoglycoprotein receptor: properties and modulation by ligand. Prog Clin Biol Res. 1989;300:169–184. [PubMed] [Google Scholar]
- 50.Elward K, Gasque K. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes AI, Gregoriadis G. The effect of polysialylation on the immunogenicity and antigenicity of asparaginase: implication in its pharmacokinetics. Int J Pharm. 2001;217:215–224. doi: 10.1016/s0378-5173(01)00603-2. [DOI] [PubMed] [Google Scholar]
- 52.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Br J Cancer. 2001;84(Suppl 1):3–10. doi: 10.1054/bjoc.2001.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaum BS. The lectin self of complement factor H. Curr Opin Struct Biol. 2017;44:111–118. doi: 10.1016/j.sbi.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Mcever RP. Selectins: novel receptors that mediate leukocyte adhesion during inflammation. Thromb Haemost. 1991;65:223–228. [PubMed] [Google Scholar]
- 55.Lowe JB. Glycosyltransferases and glycan structures contributing to the adhesive activities of L-, E- and P-selectin counter-receptors. Biochem Soc Symp. 2002;69:33–45. doi: 10.1042/bss0690033. [DOI] [PubMed] [Google Scholar]
- 56.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2015;135:598–608. doi: 10.1016/j.jaci.2014.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings RD, Smith DF. The selectin family of carbohydrate-binding proteins: structure and importance of carbohydrate ligands for cell adhesion. BioEssays. 1992;14:849–856. doi: 10.1002/bies.950141210. [DOI] [PubMed] [Google Scholar]
- 58.Varki A, Schnaar RL, Crocker PR. I-Type Lectins. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [PubMed] [Google Scholar]
- 59.Brown GD, Crocker PR. Lectin receptors expressed on myeloid cells. Microbiol Spectr. 2016;4:1–26. doi: 10.1128/microbiolspec.MCHD-0036-2016. [DOI] [PubMed] [Google Scholar]
- 60.Pillai S, Netravali IA, Cariappa A, et al. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vimr E, Lichtensteiger C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 2002;10:254–257. doi: 10.1016/s0966-842x(02)02361-2. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann F, Tiralongo E, Tiralongo J. Sialic acid-specific lectins: occurrence, specificity and function. Cell Mol Life Sci. 2006;63:1331–1354. doi: 10.1007/s00018-005-5589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 64.Crocker PR, Mcmillan SJ, Richards HE. CD33-related siglecs as potential modulators of inflammatory responses. Ann N Y Acad Sci. 2012;1253:102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- 65.Angata T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol Divers. 2006;10:555–566. doi: 10.1007/s11030-006-9029-1. [DOI] [PubMed] [Google Scholar]
- 66.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 67.Von Gunten S, Bochner BS. Basic and clinical immunology of Siglecs. Ann N Y Acad Sci. 2008;1143:61–82. doi: 10.1196/annals.1443.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 69.Angata T, Margulies EH, Green ED, et al. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J Biol Chem. 2001;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- 71.Padler-Karavani V, Hurtado-Ziola N, Chang YC, et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J. 2013;28:1280–1293. doi: 10.1096/fj.13-241497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varki A. N-glycolylneuraminic acid deficiency in humans. Biochimie. 2001;83:615–622. doi: 10.1016/s0300-9084(01)01309-8. [DOI] [PubMed] [Google Scholar]
- 73.Varki A. Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and implications for hominid evolution. Am J Phys Anthropol. 2001;(Suppl. 33):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonnenburg JL, Altheide TK, Varki A. A uniquely human consequence of domain-specific functional adaptation in a sialic acid-binding receptor. Glycobiology. 2004;14:339–346. doi: 10.1093/glycob/cwh039. [DOI] [PubMed] [Google Scholar]
- 75.Abercrombie M, Ambrose EJ. The surface properties of cancer cells: a review. Cancer Res. 1962;22:525–548. [PubMed] [Google Scholar]
- 76.Smit H, Gaastra W, Kamerling JP, et al. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984;46:578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campanero-Rhodes MA, Smith A, Chai W, et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81:12846–12858. doi: 10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin MJ, Rayner JC, Gagneux P, et al. Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc Natl Acad Sci USA. 2005;102:12819–12824. doi: 10.1073/pnas.0503819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng L, Song J, Gao X, et al. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell. 2014;159:1290–1299. doi: 10.1016/j.cell.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao X, Deng L, Stack G, et al. Evolution of host adaptation in the Salmonella typhoid toxin. Nat Microbiol. 2017;2:1592–1599. doi: 10.1038/s41564-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chou HH, Takematsu H, Diaz S, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayakawa T, Satta Y, Gagneux P, et al. Alu-mediated inactivation of the human CMP- N-acetylneuraminic acid hydroxylase gene. Proc Natl Acad Sci USA. 2001;98:11399–11404. doi: 10.1073/pnas.191268198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chou HH, Hayakawa T, Diaz S, et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rich SM, Leendertz FH, Xu G, et al. The origin of malignant malaria. Proc Natl Acad Sci USA. 2009;106:14902–14907. doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varki A, Gagneux P. Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery? Proc Natl Acad Sci USA. 2009;106:14739–14740. doi: 10.1073/pnas.0908196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ashley EA, Pyae Phyo A, Woodrow CJ. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 87.Rayner JC, Liu W, Peeters M, et al. A plethora of Plasmodium species in wild apes: a source of human infection. Trends Parasitol. 2011;27:222–229. doi: 10.1016/j.pt.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varki A, Freeze HH, Gagneux P. Evolution of glycan diversity. In: Varki A, Cummings RD, Esko JD, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [Google Scholar]
- 89.Parry CM, Hien TT, Dougan G, et al. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 90.Raffatellu M, Wilson RP, Winter SE, et al. Clinical pathogenesis of typhoid fever. J Infect Dev Ctries. 2008;2:260–266. doi: 10.3855/jidc.219. [DOI] [PubMed] [Google Scholar]
- 91.Song J, Gao X, Galán JE. Structure and function of the Salmonella Typhi chimaeric A(2)B(5) typhoid toxin. Nature. 2013;499:350–354. doi: 10.1038/nature12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edsall G, Gaines S, Landy M, et al. Studies on infection and immunity in experimental typhoid fever. I. Typhoid fever in chimpanzees orally infected with Salmonella typhosa. J Exp Med. 1960;112:143–166. doi: 10.1084/jem.112.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varki NM, Strobert E, Dick EJ, et al. Biomedical differences between human and nonhuman hominids: potential roles for uniquely human aspects of Sialic acid biology. Annu Rev Pathol. 2011;6:365–393. doi: 10.1146/annurev-pathol-011110-130315. [DOI] [PubMed] [Google Scholar]
- 94.Van Valen L. Molecular evolution as predicted by natural selection. J Mol Evol. 1974;3:89–101. doi: 10.1007/BF01796554. [DOI] [PubMed] [Google Scholar]
- 95.Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc Natl Acad Sci USA. 1990;87:3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 97.Varki A. Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology. J Autoimmun. 2017;83:134–142. doi: 10.1016/j.jaut.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 98.Samraj AN, Läubli H, Varki N, et al. Involvement of a non-human sialic Acid in human cancer. Front Oncol. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Okerblom J, Varki A. Biochemical, cellular, physiological, and pathological consequences of human loss of N-glycolylneuraminic acid. ChemBioChem. 2017;18:1155–1171. doi: 10.1002/cbic.201700077. [DOI] [PubMed] [Google Scholar]
- 100.French BM, Sendil S, Pierson RN, et al. The role of sialic acids in the immune recognition of xenografts. Xenotransplantation. 2017;24:e12345. doi: 10.1111/xen.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Apostolovic D, Tran TA, Starkhammar M, et al. The red meat allergy syndrome in Sweden. Allergo J Int. 2016;25:49–54. doi: 10.1007/s40629-016-0098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearce OM, Läubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111–128. doi: 10.1093/glycob/cwv097. [DOI] [PubMed] [Google Scholar]
- 103.Byrne GW, Mcgregor CGA, Breimer ME. Recent investigations into pig antigen and anti-pig antibody expression. Int J Surg. 2015;23:223–228. doi: 10.1016/j.ijsu.2015.07.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salama A, Evanno G, Harb J, et al. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85–94. doi: 10.1111/xen.12142. [DOI] [PubMed] [Google Scholar]
- 105.Amon R, Reuven EM, Leviatan Ben-Arye S, et al. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115–122. doi: 10.1016/j.carres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 106.Ghaderi D, Zhang M, Hurtado-Ziola N, et al. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147–175. doi: 10.5661/bger-28-147. [DOI] [PubMed] [Google Scholar]
- 107.Malykh YN, Schauer R, Shaw L. N-Glycolylneuraminic acid in human tumours. Biochimie. 2001;83:623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 108.Samraj AN, Pearce OM, Läubli H, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bardor M, Nguyen DH, Diaz S, et al. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 111.Padler-Karavani V, Yu H, Cao H, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 114.Lu Q, Padler-Karavani V, Yu H, et al. LC-MS analysis of polyclonal human anti-Neu5Gc xeno-autoantibodies immunoglobulin G Subclass and partial sequence using multistep intravenous immunoglobulin affinity purification and multienzymatic digestion. Anal Chem. 2012;84:2761–2768. doi: 10.1021/ac2030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taylor RE, Gregg CJ, Padler-Karavani V, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varki A, Kannagi R, Toole B, et al. Glycosylation changes in cancer. In: Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- 117.Padler-Karavani V. Aiming at the sweet side of cancer: aberrant glycosylation as possible target for personalized-medicine. Cancer Lett. 2014;352:102–112. doi: 10.1016/j.canlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 118.Schultz MJ, Swindall AF, Bellis SL. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012;31:501–518. doi: 10.1007/s10555-012-9359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hedlund M, Padler-Karavani V, Varki NM, et al. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci USA. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Padler-Karavani V, Hurtado-Ziola N, Pu M, et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011;71:3352–3363. doi: 10.1158/0008-5472.CAN-10-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pearce OM, Laubli H, Verhagen A, et al. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci USA. 2014;111:5998–6003. doi: 10.1073/pnas.1209067111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prehn RT. The initial immune reaction to a new tumor antigen is always stimulatory and probably necessary for the tumor’s growth. Clin Dev Immunol. 2010;2010:851728. doi: 10.1155/2010/851728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pearce OM, Laubli H, Bui J, et al. Hormesis in cancer immunology: does the quantity of an immune reactant matter? Oncoimmunology. 2014;3:e29312. doi: 10.4161/onci.29312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alisson-Silva F, Kawanishi K, Varki A. Human risk of diseases associated with red meat intake: analysis of current theories and proposed role for metabolic incorporation of a non-human sialic acid. Mol Aspects Med. 2016;51:16–30. doi: 10.1016/j.mam.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pham T, Gregg CJ, Karp F, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Soulillou JP. Missing links in multiple sclerosis etiology. A working connecting hypothesis. Med Hypotheses. 2013;80:509–516. doi: 10.1016/j.mehy.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 129.Montassier E, Berthelot L, Soulillou JP. Are the decrease in circulating anti-α1,3-Gal IgG and the lower content of galactosyl transferase A1 in the microbiota of patients with multiple sclerosis a novel environmental risk factor for the disease. Mol Immunol. 2018;93:162–165. doi: 10.1016/j.molimm.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 130.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galili U. Discovery of the natural anti-Gal antibody and its past and future relevance to medicine. Xenotransplantation. 2013;20:138–147. doi: 10.1111/xen.12034. [DOI] [PubMed] [Google Scholar]
- 132.Galili U. The alpha-gal epitope and the anti-Gal antibody in xeno-transplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 133.Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant. 2017;22:513–521. doi: 10.1097/MOT.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghaderi D, Taylor RE, Padler-Karavani V, et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28:863–867. doi: 10.1038/nbt.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salama A, Evanno G, Lim N, et al. Anti-Gal and anti-Neu5Gc responses in nonimmunosuppressed patients following treatment with rabbit anti-thymocyte polyclonal IgGs. Transplantation. 2017;101:2501–2507. doi: 10.1097/TP.0000000000001686. [DOI] [PubMed] [Google Scholar]
- 136.Couvrat-Desvergnes G, Salama A, Le Berre L, et al. Rabbit anti-thymocyte globulin-induced serum sickness disease and human kidney graft survival. J Clin Invest. 2015;125:4655–4665. doi: 10.1172/JCI82267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reuven EM, Leviatan Ben-Arye S, Marshanski T, et al. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation. 2016;23:381–392. doi: 10.1111/xen.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang R, Wang Y, Chen L, et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/β4GalNT2/CMAH. Acta Biomater. 2018;72:196–205. doi: 10.1016/j.actbio.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 139.Lee W, Long C, Ramsoondar J, et al. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration. Xenotransplantation. 2016;23:370–380. doi: 10.1111/xen.12254. [DOI] [PubMed] [Google Scholar]
- 140.Cohen D, Miyagawa Y, Mehra R, et al. Distribution of non-gal antigens in pig cornea: relevance to corneal xenotransplantation. Cornea. 2014;33:390–397. doi: 10.1097/ICO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 141.Manji RA, Lee W, Cooper DK. Xenograft bioprosthetic heart valves: past, present and future. Int J Surg. 2015;23(Pt B):280–284. doi: 10.1016/j.ijsu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 142.Galili U. Induced anti-non gal antibodies in human xenograft recipients [editorial] Transplantation. 2012;93:11–16. doi: 10.1097/TP.0b013e31823be870. [DOI] [PubMed] [Google Scholar]
- 143.Amon R, Ben-Arye SL, Engler L, et al. Glycan microarray reveal induced IgGs repertoire shift against a dietary carbohydrate in response to rabbit anti-human thymocyte therapy. Oncotarget. 2017;8:112236–112244. doi: 10.18632/oncotarget.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scobie L, Padler-Karavani V, Le Bas-Bernardet S, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mai HL, Treilhaud M, Ben-Arye SL, et al. Poor patient and graft outcome after induction treatment by antithymocyte globulin in recipients of a kidney graft after nonrenal organ transplantation. Transplant Direct. 2018;4:e357. doi: 10.1097/TXD.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miwa Y, Kobayashi T, Nagasaka T, et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11:247–253. doi: 10.1111/j.1399-3089.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 147.Wang ZY, Burlak C, Estrada JL, et al. Erythrocytes from GGTA1/CMAH knockout pigs: implications for xenotransfusion and testing in non-human primates. Xenotransplantation. 2014;21:376–384. doi: 10.1111/xen.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vadori M, Cozzi E. The immunological barriers to xenotransplantation. Tissue Antigens. 2015;86:239–253. doi: 10.1111/tan.12669. [DOI] [PubMed] [Google Scholar]
- 149.Gao H, Zhao C, Xiang X, et al. Production of α1,3-galactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene double-deficient pigs by CRISPR/Cas9 and handmade cloning. J Reprod Dev. 2017;63:17–26. doi: 10.1262/jrd.2016-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Burlak C, Paris LL, Lutz AJ, et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14:1895–1900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salama A, Mosser M, Lévêque X, et al. Neu5Gc and α1-3 GAL xenoantigen knockout does not affect glycemia homeostasis and insulin secretion in pigs. Diabetes. 2017;66:987–993. doi: 10.2337/db16-1060. [DOI] [PubMed] [Google Scholar]