Abstract
Objective.
Home-based exercise is an alternative exercise mode to a structured supervised program to improve symptoms in patients with peripheral artery disease (PAD), but little is known whether the slow paced and less intense home program also elicits changes in vascular and inflammatory biomarkers. In an exploratory analysis from a randomized-controlled trial, we compared changes in vascular and inflammatory biomarkers in patients with symptomatic PAD (typical and atypical of claudication) following a home-based exercise program, a supervised exercise program, and an attention-control group.
Methods.
A total of 114 patients were randomized into one of the three groups (n=38 per group). Two groups performed exercise interventions, consisting of home-based and supervised programs of intermittent walking to mild-to-moderate claudication pain for 12 weeks, whereas a third group performed light resistance training as a non-walking attention-control group. Before and after intervention, patients were characterized on treadmill performance, endothelial effects of circulating factors present in sera using a cell culture-based bioassay on primary human arterial endothelial cells, and were further evaluated on circulating vascular and inflammatory biomarkers.
Results.
Treadmill peak walking time increased (P=.008) in the two exercise groups but not in the control group (P>.05). Cultured endothelial cell apoptosis decreased after home-based exercise (P<.001) and supervised exercise (P=.007), and the change in the exercise groups combined was different than the control group (P=.005). For circulating biomarkers, increases were found in hydroxyl radical antioxidant capacity (HORAC) (P=.003) and vascular endothelial growth factor-A (VEGF-A) (P=.037), and decreases were observed in E-selectin (P=.007) and blood glucose (P=.012) after home-based exercise only. The changes in HORAC (P=.005), VEGF-A (P=.008), and E-selectin (P=.034) in the exercise groups combined were different than the control group.
Conclusions.
This exploratory analysis found that both home-based and supervised exercise programs are efficacious to decrease cultured endothelial cell apoptosis in patients with symptomatic PAD. Furthermore, a monitored home-based exercise program elicits additional vascular benefits by improving circulating markers of endogenous antioxidant capacity, angiogenesis, endothelium-derived inflammation, and blood glucose in patients with symptomatic PAD. The novel clinical significance is that important trends were found in this exploratory analysis that a contemporary home-based exercise program and a traditional supervised exercise program may favorably improve vascular and inflammatory biomarkers in addition to the well-described ambulatory improvements in symptomatic patients with PAD.
Table of Contents Summary
This prospective, randomized controlled trial of 114 patients found that home-based and supervised walking programs improved vascular and inflammatory biomarkers, as well as ambulation. This study suggests that home-based walking at low intensity may provide an alternative to a supervised program to improve inflammation and ambulation.
INTRODUCTION
Peripheral artery disease (PAD) is highly prevalent,1 costly,2 leads to poor quality of life,3 and is associated with a high rate of mortality.4 Between 40 and 75 percent of those with PAD experience symptomatic leg pain during ambulation that is either typical or atypical of classic claudication,5 resulting in disability. Patients with PAD also have greater cardiovascular burden, inflammation, and oxidative stress than healthy older adults.6-9 Inflammation and oxidative stress lead to accelerated myopathy by damaging mitochondrial electron transport chain function, thereby reducing energy production and increasing apoptosis and sarcopenia.10,11 Thus, biomarkers of inflammation and oxidative stress are associated with walking dysfunction measured during a maximal treadmill test,12 a 6-minute walk test,6,7 and a short gait test in symptomatic patients with PAD.13
Physical activity reduces oxidative stress and increases the ability to respond to oxidative challenges in older adults,14 suggesting that ischemic preconditioning may occur with repeated episodes of exercise.15 We previously found that higher levels of community-based daily ambulatory activity is associated with higher levels of circulating antioxidant capacity,16 lower levels of inflammation determined by high-sensitivity C-reactive protein (hsCRP),8 and no increase in oxidative stress markers8 in symptomatic patients with PAD. These data suggest that chronic exercise may improve anti-oxidant capacity and inflammation without negatively influencing oxidative stress in symptomatic patients with PAD.
Previous exercise training studies report that supervised exercise lowers circulating vascular and inflammatory biomarkers such as E-selectin,17 intercellular adhesion molecule (ICAM-1),17 and interleukin-6 (IL-6),18 and may19 or may not20 lower hsCRP. Recently, home-based exercise has been found to be an alternative mode of exercise to improve symptoms in patients with PAD.21-24 A monitored home-based exercise program has numerous advantages to a supervised program for both patients and clinical staff, as less time, effort, and resources are required to implement home-based exercise. However, minimal work has examined whether home-based exercise, which is done at slower pace and lower exercise intensity than supervised exercise,21,22 elicits changes in both vascular and inflammatory biomarkers, and whether these changes are different from supervised exercise programs. A direct assessment of home-based and supervised exercise programs compared to a control group has not been done on their effects on circulating antioxidant capacity, inflammation, and oxidative stress biomarkers, and on endothelial cell biomarkers.
Therefore, in an exploratory analysis from our randomized-controlled trial, we compared changes in vascular and inflammatory biomarkers in patients with symptomatic PAD following a home-based exercise program, a supervised exercise program, and an attention-control group who performed light resistance training exercise.
METHODS
Patients
Approval and Informed Consent.
The procedures of this study were approved by the institutional review board at the University of Oklahoma Health Sciences Center. Written informed consent was obtained from each patient at the beginning of investigation.
Recruitment.
Patients who were not currently exercising were recruited from vascular laboratories and vascular clinics from the university for possible enrollment into exercise rehabilitation programs to treat leg pain secondary to PAD.21,22
Medical Screening through History and Physical Examination
Patients were evaluated in the morning at the Clinical Research Center.22 Patients arrived fasted, but were permitted to take their usual medications. Patients were evaluated with a medical history and physical examination in which demographic information, height, weight, waist circumference, cardiovascular risk factors, co-morbid conditions, claudication history, ankle/brachial index (ABI), and a list of current medications were obtained. Blood samples were drawn by venipuncture from an antecubital vein and were analyzed on routine clinical panels. Additional samples were obtained, and the serum was stored at −80°C for subsequent batched analyses in duplicate of circulating and cultured endothelial cell biomarkers. In the event of an acute illness on days of baseline and post-testing evaluation, patients had their study visit rescheduled due to the possible influence that illness could have on study results, particularly with the blood biomarker analyses.
Inclusion and Exclusion Criteria.
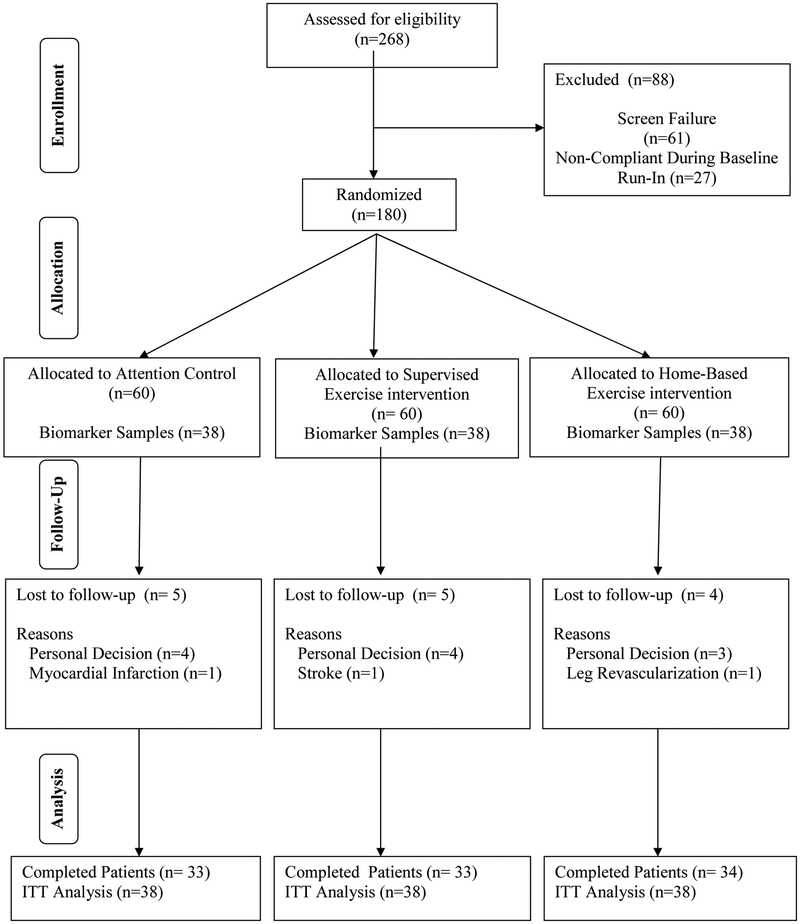
According to inclusion and exclusion criteria previously listed and described in detail for this trial,22 patients with symptomatic PAD were examined and randomized into this study. A total of 180 patients were randomized in this clinical trial, in which 60 patients were assigned into each of the three groups, as detailed in our original report22 and shown in Figure 1. Due to initial budgetary constraints, the additional biomarker samples included in the current investigation were only obtained from the final 114 randomized patients, consisting of the final 38 patients randomized into each group (Figure 1). All biomarkers were selected during study design, and were planned for exploratory analyses after the primary outcome analyses. A computer-generated random allocation sequence was created by the biostatistician at the beginning of the study using the NCSS statistical package (Kaysville, UT). The allocation sequence was concealed from the research staff enrolling and assessing patients. At completion of baseline testing, patients were randomized to one of three groups within blocks to assure that no more than two sequential subjects were assigned to the same group.
Figure 1.
Consolidated standards of reporting trials (CONSORT) flow diagram of patients through the trial.
Intervention and Control Groups
Home-Based Exercise Rehabilitation Program.
The home-based exercise program consisted of three months of intermittent walking to mild-to-moderate claudication pain at least three days per week at a self-selected pace, in which exercise session duration was progressively increased with the aid of a step activity monitor.21,22 Patients wore the step activity monitor during each exercise session, and returned the monitor and a logbook to the research staff at the end of week 1, 4, 8, and 12.22 During these brief 15-minute meetings, monitor data was downloaded, results were reviewed and sessions were recorded, and feedback was provided for the subsequent month of training.
Supervised Exercise Rehabilitation Program.
Exercise sessions in our supervised, on-site, treadmill exercise program were performed while also wearing a step activity monitor.21,22 The supervised program consisted of three months of intermittent treadmill walking to mild-to-moderate claudication pain three days per week in which exercise session duration was progressively increased under the direct on-site supervision of the research staff.21,22
Attention-Control, Light Resistance Program.
Light resistance training was performed 3 times per week, without any walking exercise, using a Pro-Form Fusion 6.0 LX weight system (ICON Health and Fitness, Inc, Logan, UT).22 Resistance training consisted of performing both upper and lower extremity exercises. One set of 15 repetitions was performed for each exercise. If the resistance from the exercise machine could not be lifted, resistance bands were used instead. Each light resistance exercise session was performed under the direct on-site supervision of the research staff (i.e., attention).
Tests and Outcome Measurements
Graded Maximal Treadmill Test.
Patients performed a graded treadmill protocol to determine study eligibility, as well as to obtain outcome measures related to exercise performance.25 The claudication onset time (COT), defined as the walking time at which the patients first experienced pain, and the peak walking time (PWT), defined as the walking time at which ambulation could not continue due to maximal pain, were both recorded to quantify the severity of claudication. Calf muscle hemoglobin oxygen saturation (StO2) was also measured during the treadmill test using a continuous-wave, NIRS unit.27 The time taken to reach the minimum calf StO2 value during exercise was assessed.
6-Minute Walk Test.
On a separate day, typically within one week from the treadmill test, patients performed an over-ground, 6-minute walk test in which two cones were placed 100 feet apart in a marked corridor, as previously described.28 The total distance walked was recorded.
Endothelial Cell Culture Bioassay.
We used a cell culture-based bioassay approach utilizing cultured primary human arterial endothelial cells to characterize the endothelial effects of circulating factors present in the sera, as previously described.29
Apoptosis Assay.
Cultured endothelial cells were treated for 24 h with sera of patients to determine whether circulating factors in the sera exerted pro-apoptotic effects. Apoptotic cell death was assessed by caspase activities using Caspase-Glo 3/7 assay kit (Promega, Madison, WI).29
Cellular Oxidative Stress Production.
To assess cellular oxidative stress induced by factors present in the sera, hydrogen peroxide production in detector endothelial cells was measured fluorometrically using the Amplex Red/horseradish peroxidase assay.29
Transient Transfection, nuclear factor K-Light-Chain-Enhancer of Activated B (NF-κB) Cultured Endothelial Cells Reporter Gene Assay.
To assess cellular pro-inflammatory effects induced by factors in the sera, transcriptional activity of NF-kB was tested in serum-treated detector endothelial cells by a reporter gene assay.29
Circulating biomarkers of antioxidant capacity and inflammation
Serum Antioxidant Capacity.
Hydroxyl Radical Antioxidant Capacity (HORAC) was measured using the OxiSelect HORAC Activity Assay (Cell Biolabs Inc., San Diego, CA) to determine the capacity of antioxidant enzymes and other redox molecules to counterbalance the deleterious effects of oxidative stress.29
Circulating inflammatory and vascular biomarkers.29
A Milliplex Human Adipokine Magnetic Bead Kit was used for determining tumor necrosis factor alpha (TNF α), interleukin-1b (IL-1b), and IL-6. A Milliplex Human Cardiovascular Disease Panel 1 Kit was used for E-selectin. A Milliplex Human Apolipoprotein Kit was used for apolipoprotein B (ApoB). The Millipore kits were purchased from EMD Millipore, Billerica, MA. Affymetrix Procarta Immunoassay was used to detect vascular endothelial growth factor-A (VEGF-A). These assays were performed according to manufacturer’s protocols by a Bio-Plex 200 System, Bio Rad, CA). Sample protein content was determined for normalization purposes by a spectrophotometric quantification method using BCA reagent (Pierce Chemical Co., Rockford, IL).
Oxidized LDL.
Plasma oxidized LDL was measured by immunoassay (Mercodia, Uppsala, Sweden) according to the manufacturer’s protocol.8
Statistical Analyses
Measurement variables in table I were summarized as means and standard deviations and groups were compared with a one way ANOVA. Dichotomized variables were summarized as percent with characteristic. Groups were compared using a 2 by 3 Chi Square test. Only the prevalence of cerebral vascular accident and chronic kidney disease variables indicated a significant difference among groups, while none of the remaining variables were different. These were used as covariates in group comparisons in subsequent analyses. Changes from baseline (Deltas) were computed as post-test value minus pre-test value for all variables. The missing values routine of NCSS Computer Package was used to impute missing values to produce complete Delta sets for Intent to Treat (ITT) comparison. For statistical purposes the study is viewed as a one way design with three groups and with change scores as the response variables. Changes were selected as the response variable because this metric addresses the research questions.
Table I.
Baseline clinical characteristics. Values are means (standard deviation) or percentage of patients
| Variables | Control Group (n = 38) |
Supervised Exercise Group (n = 38) |
Home-Based Exercise Group (n = 38) |
|---|---|---|---|
| Age (years) | 63 (8) | 64 (10) | 67 (11) |
| Weight (kg) | 84.5 (17.3) | 81.8 (21.0) | 82.7 (22.0) |
| Body Mass Index (kg/m2) | 29.4 (5.5) | 29.3 (6.8) | 28.7 (6.4) |
| Ankle/Brachial Index | 0.76 (0.21) | 0.69 (0.24) | 0.68 (0.28) |
| Sex (% Men) | 58 | 50 | 55 |
| Race (% Caucasian) | 50 | 45 | 58 |
| Current Smoking (% yes) | 45 | 40 | 34 |
| Hypertension (% yes) | 89 | 92 | 87 |
| Medication Use (% yes) | 87 | 87 | 84 |
| Dyslipidemia (% yes) | 92 | 92 | 97 |
| Medication Use (%) | 68 | 76 | 84 |
| Diabetes (% yes) | 42 | 55 | 34 |
| Medication Use (%) | 42 | 45 | 26 |
| Abdominal Obesity (% yes) | 58 | 55 | 55 |
| Metabolic Syndrome (% yes) | 84 | 84 | 84 |
| Obesity (% yes) | 45 | 37 | 42 |
| Lower Extremity Revascularization (% yes) | 39 | 39 | 39 |
| Coronary Artery Disease (% yes) | 29 | 32 | 34 |
| Myocardial Infarction (% yes) | 18 | 24 | 18 |
| Cerebrovascular Disease (% yes) | 11 | 18 | 32 |
| Cerebrovascular Accident (% yes) * | 5 | 18 | 32 |
| Chronic Kidney Disease (% yes) † | 19 | 16 | 45 |
| Chronic Obstructive Pulmonary Disease (% yes) | 21 | 32 | 18 |
| Dyspnea (% yes) | 58 | 58 | 53 |
| Arthritis (% yes) | 66 | 63 | 63 |
Different among groups (P < .05),
(P < .01).
The variables in Table II were summarized as means and standard deviations. Within each group Delta means were examined for difference from zero with one sample t-test, as changes in variables did not reveal any extreme departures from normal distribution. Comparisons of Delta means among groups were made by an ANCOVA followed by two orthogonal contrasts. For each variable an ITT analysis among groups was examined for the group main effect. Contrast 1 compared the two exercise groups. Contrast 2 compared the two exercise groups combined to the control group. Variables in Tables III and IV exhibited significant departures from normal distribution. Therefore, these were summarized as medians and interquartile ranges. Within each group Delta medians were examined for difference from zero with one sample Wilcoxon test. For each variable an ITT analysis among groups was examined for the group main effect using Kruskal-Wallis procedure. Note that no nonparametric analog of ANCOVA is available. Therefore, the main effect p values were not adjusted for covariates. However, since the Wilcoxon two sample test and Spearman correlations of variable with a dichotomous variable yields the same P-values, Partial Spearman correlations controlled for two covariates were used as a nonparametric analogue for Contrast 1 comparison of the two exercise groups. Similar procedure was used for analogue of Contrast 2 with the two exercise groups combined compared with controls. For the ITT main effect of this analysis, the range of difference for a one-way design for three groups with 38 subjects available per group was, for 80% power, between 0.62 and 0.72 standard deviations of within cell variation. Due to the exploratory nature of this investigation, these analyses are designed more as hypothesis generating rather than hypothesis confirming.
Table II.
Lipids and glucose metabolism measurements in patients in the control, supervised exercise, and home-based exercise groups. Values are means (standard deviation).
| Variables | Pre-Test | Post-Test | Mean Change Score |
Main Effect P values |
|---|---|---|---|---|
| Cholesterol (mg/dl) | ||||
| Control Group | 176 (44) | 178 (51) | 1 (28) | |
| Supervised Exercise Group | 166 (4l) | 166 (46) | 0 (24) | .607 |
| Home-Based Exercise Group | 162 (4l) | 160 (56) | −2 (44) | |
| HDL-C (mg/dl) | ||||
| Control Group | 45 (13) | 45 (14) | 0 (6) | |
| Supervised Exercise Group | 39 (9) | 39 (10) | −1 (6) | .793 |
| Home-Based Exercise Group | 46 (17) | 46 (19) | −0 (8) | |
| LDL-C (mg/dl) | ||||
| Control Group | 104 (41) | 99 (49) | −4 (38) | |
| Supervised Exercise Group | 99 (35) | 94 (47) | −5 (32) | .592 |
| Home-Based Exercise Group | 85 (31) | 78 (26) | −7 (33) | |
| LDL-C / HDL-C | ||||
| Control Group | 2.4 (1.0) | 2.3 (1.3) | −0.1 (1.1) | |
| Supervised Exercise Group | 2.7 (1.3) | 2.6 (1.6) | −0.1 (1.0) | .370 |
| Home-Based Exercise Group | 2.2 (1.3) | 2.0 (1.0) | −0.2 (0.9) | |
| Non-HDL-C (mg/dl) | ||||
| Control Group | 132 (41) | 134 (56) | 2 (31) | |
| Supervised Exercise Group | 127 (41) | 128 (46) | 0 (21) | .510 |
| Home-Based Exercise Group | 118 (46) | 116 (60) | −2 (42) | |
| Triglycerides (mg/dl) | ||||
| Control Group | 138 (152) | 154 (206) | 16 (68) | |
| Supervised Exercise Group | 145 (104) | 153 (105) | 8 (41) | .803 |
| Home-Based Exercise Group | 171 (199) | 229 (564) | 58 (376) | |
| Glucose (mg/L) | ||||
| Control Group | 111 (51) | 115 (44) | 4 (36) | |
| Supervised Exercise Group | 105 (32) | 110 (37) | 5 (38) | .041 |
| Home-Based Exercise Group | 100 (36) | 90 (35) | −10 (26) * † | |
| Insulin (mg/L) | ||||
| Control Group | 12.7 (13.6) | 12.1 (8.9) | −0.6 (11.7) | |
| Supervised Exercise Group | 17.6 (19.9) | 18.7 (19.5) | 1.2 (10.7) | .155 |
| Home-Based Exercise Group | 15.5 (21.6) | 19.7 (35.2) | 4.2 (17.6) | |
| HOMA-IR (mg/L) | ||||
| Control Group | 3.98 (6.07) | 3.84 (3.87) | −0.15 (5.78) | |
| Supervised Exercise Group | 5.40 (6.88) | 5.64 (5.73) | 0.24 (4.86) | .341 |
| Home-Based Exercise Group | 3.94 (5.24) | 5.05 (9.12) | 1.10 (5.57) |
Change from pre-test (P < .05).
Different than Supervised Exercise Group (P < .05) HDL-C = high density lipoprotein cholesterol, HOMA-IR = Homeostasis Model Assessment-Insulin Resistance, LDL-C = low density lipoprotein cholesterol.
Table III.
Measurements from cultured endothelial cells treated with sera in patients in the control, supervised exercise, and home-based exercise groups. Values are medians (interquartile ranges).
| Variables | Pre-Test | Post-Test | Median Change Score |
Main Effect P values |
|---|---|---|---|---|
| Apoptosis (AU) | ||||
| Control Group | 1.11 (0.32) | 1.20 (0.48) | 0.15 (0.52) ‡ | |
| Supervised Exercise Group | 1.05 (0.37) | 0.98 (0.70) | −0.20 (0.38) * | .016 |
| Home-Based Exercise Group | 1.11 (0.29) | 0.77 (0.57) | −0.26 (0.63) † | |
| Cellular Oxidative Stress (AU) | ||||
| Control Group | 27.63 (6.63) | 23.74 (7.67) | −0.96 (6.97) | |
| Supervised Exercise Group | 27.50 (5.26) | 23.92 (6.29) | −2.63 (5.74) * | .971 |
| Home-Based Exercise Group | 26.15 (9.51) | 24.65 (5.65) | −2.26 (6.38) * | |
| NF-κB activity (AU) | ||||
| Control Group | 1.18 (0.71) | 1.43 (0.94) | 0.37 (0.62) *§ | |
| Supervised Exercise Group | 1.61 (1.08) | 1.29 (0.69) | −0.15 (0.51) | < .001 |
| Home-Based Exercise Group | 1.11 (0.89) | 1.26 (1.19) | −0.29 (0.84) |
Change from pre-test (P < .01),
(P < .001).
Different than Exercise Groups (P < .01),
(P < .001).
NF-κB = nuclear factor K-Light-Chain-Enhancer of Activated B cells.
Table IV.
Circulating inflammatory, antioxidant capacity, and vascular biomarkers in patients in the control, supervised exercise, and home-based exercise groups. Values are medians (interquartile ranges).
| Variables | Pre-Test | Post-Test | Median Change Score |
Main Effect P values |
|---|---|---|---|---|
| TNF α (pg/ml) | ||||
| Control Group | 56.0 (31.0) | 51.0 (22.5) | −5.0 (30.5) | |
| Supervised Exercise Group | 56.5 (27.0) | 50.0 (21.5) | −12.5 (21.5) † | .269 |
| Home-Based Exercise Group | 46.5 (25.0) | 49.0 (35.0) | −7.5 (26.0) | |
| Interleukin-1b (pg/ml) | ||||
| Control Group | 15.0 (5.5) | 16.0 (5.0) | −1.0 (4.0) | |
| Supervised Exercise Group | 16.0 (4.0) | 14.0 (4.0) | −2.0 (4.0) | .325 |
| Home-Based Exercise Group | 15.0 (4.5) | 15.0 (5.0) | −1.0 (6.0) | |
| Interleukin-6 (pg/ml) | ||||
| Control Group | 23.0 (11.5) | 23.5 (13.5) | 3.0 (11.0) | |
| Supervised Exercise Group | 25.0 (17.0) | 21.5 (14.0) | −2.0 (13.5) | .754 |
| Home-Based Exercise Group | 23.0 (13.0) | 22.0 (9.5) | −2.0 (15.0) | |
| Oxidized LDL (U/L) | ||||
| Control Group | 70.8 (22.5) | 71.0 (39.3) | −2.2 (14.4) | |
| Supervised Exercise Group | 71.3 (42.2) | 66.3 (36.3) | −1.0 (13.5) | .108 |
| Home-Based Exercise Group | 63.0 (30.0) | 61.8 (27.3) | −0.9 (18.2) | |
| HORAC (AU) | ||||
| Control Group | 0.98 (0.35) | 0.94 (0.20) | −0.06 (0.30) § | |
| Supervised Exercise Group | 0.92 (0.17) | 0.95 (0.41) | 0.03 (0.40) | .005 |
| Home-Based Exercise Group | 0.95 (0.25) | 1.19 (0.40) | 0.15 (0.32) † | |
| E-selectin (pg/ml) | ||||
| Control Group | 43.0 (52.0) | 35.0 (67.0) | −1.5 (40.0) ‡ | |
| Supervised Exercise Group | 40.0 (40.5) | 45.0 (30.0) | −4.5 (20.5) | .040 |
| Home-Based Exercise Group | 36.0 (26.5) | 36.5 (23.0) | −8.0 (26.0) † | |
| VEGF-A (pg/ml) | ||||
| Control Group | 28.0 (24.0) | 24.0 (21.5) | −6.0 (18.0) § | |
| Supervised Exercise Group | 25.5 (19.5) | 31.0 (28.5) | 2.0 (29.0) | .003 |
| Home-Based Exercise Group | 26.0 (34.0) | 35.0 (59.5) | 9.0 (45.5) * | |
| Apolipoprotein B (ng/ml) | ||||
| Control Group | 67.0 (55.0) | 50.5 (51.0) | −15.5 (34.5) | |
| Supervised Exercise Group | 71.0 (51.0) | 47.0 (33.5) | −10.5 (28.0) * | .965 |
| Home-Based Exercise Group | 63.0 (69.0) | 41.0 (46.0) | −16.0 (49.5) * |
Change from pre-test (P < .05),
(P < .01).
Different than Exercise Groups (P < .05),
(P < .01)
HORAC = Hydroxyl Radical Antioxidant Capacity, LDL = Low Density Lipoprotein, TNF α = Tumor necrosis factor alpha, VEGF-A = Vascular Endothelial Growth Factor-A
RESULTS
Baseline Clinical Characteristics and Patient Flow
In Table I, none of the baseline characteristics, including cardiovascular risk factors and treatment with medications, were significantly different among the groups, except for chronic kidney disease (P=.004) and a history of cerebral vascular accident (P=.018), in which the home-based exercise group had the highest prevalence. Fourteen of the 114 patients did not complete the study, consisting of five from the control group, five from the supervised exercise group, and four from the home-based exercise group (Figure 1). Of these 14 patients, 11 discontinued due to personal decisions, and only three were discontinued due to an adverse event in which all were deemed unrelated to the three month exercise intervention.
Exercise Measures
During the interventions, the attention-control group completed 71% of the light-resistance training sessions, the supervised exercise group completed 86% of their walking sessions, and the home-based exercise group completed 70% of their walking sessions (data not shown). Significant differences were found in the adjusted change scores (mean±SEM) for COT in the attention-control, supervised exercise, and home-based exercise groups (47±34 sec, 213±33 sec, and 106±35 sec, respectively; P=.003), the adjusted change scores for PWT (28±34 sec, 208±34 sec, and 52±36 sec; P<.001), and the adjusted change scores for 6-minute walk distance (12±10 m, 22±10 m, and 72±10 m; P<.001). All change scores were significant in the two exercise groups (P=.009, P=.008, P=.016 for COT, PWT, and 6-minute walk distance, respectively) but not in the control group. The increases in COT (P=.029) and PWT (P=.002) were greater in the supervised exercise group than in the home-based exercise group, whereas the increase in 6-minute walk distance was greater in the home-based exercise group than in the supervised exercise group (P<.001). A significant difference was found in the adjusted change scores for the time to minimum calf StO2 during the treadmill test in the attention-control, supervised exercise, and home-based exercise groups (10±55 sec, 169±53 sec, and 125±46 sec, respectively; P=.002). The change scores were significant in the two exercise groups (P=.044) but not in the control group, and there was no difference between the two exercise groups.
Outcome Measures
In Table II, the change score for glucose was significantly different among the three groups. Glucose decreased in the home-based exercise group (P=.012), and this change was different than the change in the supervised exercise group (P=.020). None of the change scores for the remaining variables in Table II were significantly different among the three groups. In addition, the change scores were not significantly different among groups for fibrinolytic variables consisting of tPA activity, tPA antigen, PAI-1 activity, and fibrinogen (data not shown).
In Table III, the change score for endothelial cell apoptosis was significantly different among the three groups, as the median decreased in the home-based exercise group (P<.001) and in the supervised exercise group (P=.007), and these changes in apoptosis were different from the change score in the control group (P=.005). The change score for NF-κB activity was significantly different among the three groups (P<.001), as the median increased in the control group (P=.003), and this change score was significantly different than the two exercise groups (P<. 001), both of which did not experience a significant change. The change score for cellular oxidative stress was not significantly different among the three groups (P=.971), however the within-group median decreased in both exercise groups (P=.008).
In Table IV, the median change scores were significantly different among the three groups for HORAC, E-selectin, and VEGF-A. In the home-based exercise group, the median values increased for HORAC (P=.003) and VEGF-A (P=.037), and decreased for E-selectin (P=.007). Furthermore, these change scores were different between the control group compared to the two exercise groups combined for HORAC (P=.005), VEGF-A (P=.008), and E-selectin (P=.034). No significant group differences were seen for the remaining biomarkers.
DISCUSSION
A primary novel finding from this exploratory analysis was that both the home-based exercise and supervised exercise programs decreased cultured endothelial cell apoptosis. Additionally, the home-based exercise program elicited an increase in circulating antioxidant capacity (HORAC) and VEGF-A and a decrease in E-selectin and blood glucose.
Exercise-Mediated Changes in Cultured Endothelial Cells
PAD induces mitochondriopathy,10 which leads to increased production of oxidative stress within myocytes and damaging effects on muscle and other tissues.11 It has been proposed that repeated episodes of ischemia and reperfusion that occurs with acute bouts of walking by PAD patients, particularly at relatively high intensities, may worsen the oxidative stress and inflammatory responses.15 However, our results of reduced endothelial cell apoptosis after home-based and supervised exercise programs supports the notion that chronic cycles of ischemia and reperfusion from three months of exercise training elicits a preconditioning ischemic stimulus,15,30 resulting in an overall reduction in endothelial apoptosis.
Exercise-Mediated Changes in Circulating Biomarkers
Another novel finding of this investigation was that the home-based exercise program increased circulating HORAC, indicating that the total antioxidant capacity was increased. This observation supports our previous report that baseline daily activity level was positively associated with HORAC in patients with symptomatic PAD,16 and suggests that ischemic preconditioning may play a role in the beneficial influences of habitual exercise on circulating antioxidant capacity.31 Furthermore, home-based exercise training resulted in an increase in circulating VEGF-A and a decrease in E-selectin and blood glucose, suggesting that home-based exercise elicited an increase in angiogenesis and decreases in endothelium-derived inflammation and insulin resistance. Our finding of exercise-mediated reduction in E-selectin supports a previous report that found E-selectin decreased in PAD patients following eight weeks of supervised exercise training.17 The effect of exercise on VEGF-A in patients with symptomatic PAD is inconsistent. Our findings of increased serum VEGF-A following home-based exercise differ from a recent non-randomized exercise trial that found serum VEGF-A did not change with a program of non-supervised exercise, but it was increased with a program of supervised exercise.32 However, an earlier report found skeletal muscle VEGF-A was decreased following 12 weeks of supervised exercise and remained unchanged after a home-based exercise program.33
Our observation of a reduction in blood glucose following a home-based exercise program is novel, and the finding that blood glucose does not change following supervised treadmill exercise supports prior work from our laboratory.34 Given that we have previously found elevated fasting glucose in patients with symptomatic PAD is associated with peripheral circulation, patient-perceived walking ability, health-related quality of life, and sedentary behavior,35,36 the reduction of blood glucose following home-based exercise has particular clinical significance for these patients. These results are supported by previous reports that diabetes impairs microcirculation,37 and metabolic syndrome impairs ABI and claudication distances in patients with PAD.38 In the current study, the fact that monitored home-based exercise training improved circulating markers of antioxidant capacity, angiogenesis, endothelium-derived inflammation, and blood glucose suggests that a home-based exercise program, typically done at lower exercise intensity than a traditional supervised treadmill training program, can be utilized to improve these biomarkers.
Limitations
Several study limitations exist. Patients were volunteers, and their participation may represent a self-selection bias (e.g., those most interested in their health were more likely to participate). A second limitation is that the study results may not be applicable to patients with either less severe (i.e., asymptomatic) or more severe (i.e., critical limb ischemia) PAD. Another limitation is that we used an indirect assay for the cultured endothelial cell data. Using this approach, the sera of patients was incubated with primary human endothelial cells, and thus was not the endothelial cells of each patient. A fourth limitation is that patients were not followed long-term after completing the exercise interventions, and it cannot be determined whether exercise-mediated changes in vascular and inflammatory biomarkers alter the progression of PAD or the observed declines in function.39,40 A final limitation was that due to the expense of the assay analyses, we only studied the biomarker data on the final 114 patients who were randomized into this study, and thus we may have had insufficient power to detect significant changes in some biomarkers. However, this was an exploratory analysis in which we found a number of significant changes in biomarkers with the exercise interventions, thereby providing a groundwork for future trials.
Conclusions and Clinical Implications
In conclusion, this exploratory analysis found that both home-based and supervised exercise programs are efficacious to decrease cultured endothelial cell apoptosis in patients with symptomatic PAD. Furthermore, a monitored home-based walking program improves circulating markers of endogenous antioxidant capacity, angiogenesis, endothelium-derived inflammation, and blood glucose in patients with symptomatic PAD. The novel clinical significance is that important trends were found in this exploratory analysis that a contemporary home-based exercise program and a traditional supervised exercise program may favorably improve vascular and inflammatory biomarkers in addition to the well-described ambulatory improvements in symptomatic patients with PAD.
ARTICLE HIGHLIGHTS.
Type of Research: Single-center, prospective, randomized controlled trial.
Key Findings: In 114 patients monitored home-based walking program and a supervised walking program both decreased cultured endothelial cell apoptosis in patients with symptomatic PAD. The home-based program further improved circulating markers of endogenous antioxidant capacity, angiogenesis, endothelium-derived inflammation, and blood glucose.
Take home Message: Participating in 3 months of a monitored home-based walking program, typically done at lower exercise intensity than a traditional supervised treadmill walking program, may favorably improve vascular and inflammatory biomarkers in addition to the well-described ambulatory improvements in symptomatic patients with PAD.
Acknowledgments
Supported by grants from the National Institute on Aging (R01-AG-24296) and General Clinical Research Center (M01-RR-14467).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–15. [DOI] [PubMed] [Google Scholar]
- 3.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13:15–24. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–6. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, et al. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation. 2003;107:3191–8. [DOI] [PubMed] [Google Scholar]
- 7.McDermott MM, Liu K, Ferrucci L, Tian L, Guralnik JM, Green D, et al. Circulating blood markers and functional impairment in peripheral arterial disease. J Am Geriatr Soc.2008;56:1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner AW, Parker DE, Montgomery PS, Blevins SM, Teague AM, Casanegra AI. Monitored Daily Ambulatory Activity, Inflammation, and Oxidative Stress in Patients With Claudication. Angiology. 2013;65:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, et al. Greater endothelial apoptosis and oxidative stress in patients with peripheral artery disease. Int J Vasc Med. 2014;2014:160534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. VascEndovascularSurg. 2007;41:481–9. [DOI] [PubMed] [Google Scholar]
- 11.Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, et al. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. VascEndovascularSurg. 2008;42:101–12. [DOI] [PubMed] [Google Scholar]
- 12.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Ungvari Z, et al. Endothelial Cell Inflammation and Antioxidant Capacity are Associated With Exercise Performance and Microcirculation in Patients With Symptomatic Peripheral Artery Disease. Angiology. 2015;66:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner AW, Montgomery PS, Casanegra AI, Silva-Palacios F, Ungvari Z, Csiszar A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age. 2016;38:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traustadottir T, Davies SS, Su Y, Choi L, Brown-Borg HM, Roberts LJ 2nd, et al. Oxidative stress in older adults: effects of physical fitness. Age. 2012;34:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreozzi GM, Leone A, Laudani R, Deinite G, Martini R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int Angiol. 2007;26:12–7. [PubMed] [Google Scholar]
- 16.Gardner AW, Montgomery PS, Zhao YD, Silva-Palacios F, Ungvari Z, Csiszar A, et al. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J Vasc Surg. 2017;65:1762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saetre T, Enoksen E, Lyberg T, Stranden E, Jorgensen JJ, Sundhagen JO, et al. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E-selectin and ICAM-I in patients with peripheral arterial disease. Angiology. 2011;62:301–5. [DOI] [PubMed] [Google Scholar]
- 18.Nowak WN, Mika P, Nowobilski R, Kusinska K, Bukowska-Strakova K, Nizankowski R, et al. Exercise training in intermittent claudication: effects on antioxidant genes, inflammatory mediators and proangiogenic progenitor cells. Thrombosis and Haemostasis. 2012;108:824–31. [DOI] [PubMed] [Google Scholar]
- 19.Januszek R, Mika P, Konik A, Petriczek T, Nowobilski R, Nizankowski R. The effect of treadmill training on endothelial function and walking abilities in patients with peripheral arterial disease. J Cardiol. 2014;64:145–51. [DOI] [PubMed] [Google Scholar]
- 20.Mika P, Konik A, Januszek R, Petriczek T, Mika A, Nowobilski R, et al. Comparison of two treadmill training programs on walking ability and endothelial function in intermittent claudication. Int J Cardiol. 2013;168:838–42. [DOI] [PubMed] [Google Scholar]
- 21.Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3:e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Zhao L, Liu K, et al. Home-based walking exercise in peripheral artery disease: 12-month follow-up of the goals randomized trial.J Am Heart Assoc. 2014;3:e000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23:402–8. [PubMed] [Google Scholar]
- 26.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. [DOI] [PubMed] [Google Scholar]
- 27.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;48:644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–11. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AW, Parker DE, Montgomery PS, Sosnowska D, Casanegra AI, Esponda OL, et al. Impaired vascular endothelial growth factor A and inflammation in patients with peripheral artery disease. Angiology. 2014;65:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capecchi PL, Pasini FL, Cati G, Colafati M, Acciavatti A, Ceccatelli L, et al. Experimental model of short-time exercise-induced preconditioning in POAD patients. Angiology. 1997;48:469–80. [DOI] [PubMed] [Google Scholar]
- 31.Pickering AM, Vojtovich L, Tower J, KJ AD. Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radic Biol Med. 2013;55:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dopheide JF, Geissler P, Rubrech J, Trumpp A, Zeller GC, Daiber A, et al. Influence of exercise training on proangiogenic TIE-2 monocytes and circulating angiogenic cells in patients with peripheral arterial disease. Clin Res Cardiol. 2016;105:666–76. [DOI] [PubMed] [Google Scholar]
- 33.Jones WS, Duscha BD, Robbins JL, Duggan NN, Regensteiner JG, Kraus WE, et al. Alteration in angiogenic and anti-angiogenic forms of vascular endothelial growth factor-A in skeletal muscle of patients with intermittent claudication following exercise training. Vasc Med. 2012;17:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izquierdo-Porrera AM, Gardner AW, Powell CC, Katzel LI. Effects of exercise rehabilitation on cardiovascular risk factors in older patients with peripheral arterial occlusive disease. JVascSurg. 2000;31:670–7. [DOI] [PubMed] [Google Scholar]
- 35.Gardner AW, Montgomery PS. The effect of metabolic syndrome components on exercise performance in patients with intermittent claudication. J VascSurg. 2008;47:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farah BQ, Ritti-Dias RM, Montgomery PS, Casanegra AI, Silva-Palacios F, Gardner AW. Sedentary behavior is associated with impaired biomarkers in claudicants. Journal of Vascular Surgery. 2016;63:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohler ER III, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care. 2006;29:1856–9. [DOI] [PubMed] [Google Scholar]
- 38.Golledge J, Leicht A, Crowther RG, Clancy P, Spinks WL, Quigley F. Association of obesity and metabolic syndrome with the severity and outcome of intermittent claudication. JVascSurg. 2007;45:40–6. [DOI] [PubMed] [Google Scholar]
- 39.McDermott MM, Ferrucci L, Liu K, Criqui MH, Greenland P, Green D, et al. D-dimer and inflammatory markers as predictors of functional decline in men and women with and without peripheral arterial disease. J Am Geriatr Soc. 2005;53:1688–96. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MM, Liu K, Guralnik JM, Ferrucci L, Green D, Greenland P, et al. Functional decline in patients with and without peripheral arterial disease: predictive value of annual changes in levels of C-reactive protein and D-dimer. J Gerontol A Biol Sci Med Sci. 2006;61:374–9. [DOI] [PubMed] [Google Scholar]