Abstract
Rationale and objective:
Intra-dialytic hypotension (IDH) is a common complication at the initiation of hemodialysis (HD) therapy, is associated with greater mortality, and may be related to relatively rapid shifts in plasma osmolality. This study sought to evaluate the effect of an intervention to minimize intra-dialytic changes in plasma osmolality on the occurrence of IDH.
Study Design:
Double-blind single-center randomized controlled trial
Setting and Participants:
Individuals requiring initiation of HD for acute or chronic kidney disease
Intervention:
Mannitol 0.25g/kg/h versus a similar volume of 0.9% saline during the first three HD sessions.
Outcomes:
The primary endpoint was the average decline in systolic blood pressure (SBP). The secondary endpoint was the proportion of total sessions complicated by IDH (defined as a drop of ≥20 mmHg from the pre-HD SBP). Exploratory endpoints included biomarkers of cardiac and kidney injury.
Results:
A total of 52 patients were randomized and contributed to 156 study visits. There were no significant differences in the average SBP decline between the mannitol and placebo groups (15 ±11 vs. 19 ±16 mmHg; P=0.3). The proportion of total sessions complicated by IDH was lower in the mannitol group compared with placebo (25% vs. 43%), with a nominally lower risk of developing an episode of IDH (OR, 0.38; 95% CI, 0.14–1.00), though this finding was of borderline statistical significance (P=0.05). There were no consistent differences in cardiac and kidney injury biomarkers between treatment groups.
Limitations:
Modest sample size and number of events
Conclusion:
In this pilot RCT studying patients requiring initiation of HD, we found no difference in the absolute SBP decline between those who received mannitol and those who received saline. However, there were fewer overall IDH events and a nominally lower risk of dialysis sessions being complicated by IDH in the mannitol group. A larger multi-center randomized controlled trial is warranted.
Funding:
Government funding to an author (Dr Mc Causland is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511).
Trial Registration:
Registered at ClinicalTrials.gov with study number .
Keywords: Mannitol, Osmolality, intradialytic hypotension (IDH), hemodialysis, hemodynamic instability, dialysis disequilibrium, systolic blood pressure (SBP), end-stage renal disease (ESRD), acute kidney injury (AKI), dialysis initiation, kidney injury biomarker, randomized controlled trial (RCT)
Introduction
There were 120,688 incident cases of end-stage renal disease (ESRD) in the United Sates in 2014, of which 87% commenced hemodialysis (HD). Among patients receiving HD, mortality was greatest during the first three months, peaking at 382 deaths per 1,000 patient-years by the second month, and falling to 189 per 1,000 patient-years at the end of the first year. Cardiovascular disease remains the leading cause of death in this group.1
The initiation of HD is a period of marked variation from homeostatic norms. Rapid changes in osmolality and volume status may predispose to the development of adverse patient symptoms and hemodynamic instability, which may contribute to the features known as dialysis disequilibrium.2 Intra-dialytic hypotension (IDH) is common during this period,3 and has been associated with several detrimental sequelae, including loss of residual kidney function4 and greater mortality.3 Therefore, prevention of IDH may represent an important modifiable risk factor during the initiation of HD.
In a prior observational study, we leveraged practice differences between two large academic teaching hospitals with respect to the use of mannitol for the initial sessions of HD initiation, reporting a lower incidence of IDH in those who received mannitol.5 Building on this observation, we designed a randomized trial to assess the effect of mannitol on average intradialytic decline in systolic blood pressure (SBP) and the proportion of dialysis sessions complicated by IDH. As a pilot trial with modest sample size, the main goals of this project were to compare observed changes in parameters with those from prior observational studies and to assess overall protocol feasibility.
Methods
Study design and population
The study was designed as a double-blind, placebo-controlled, single-center, pilot randomized trial. It was registered at ClinicalTrials.gov (study number ). All participants provided written informed consent and the study was approved by the hospital IRB (approval number _____).
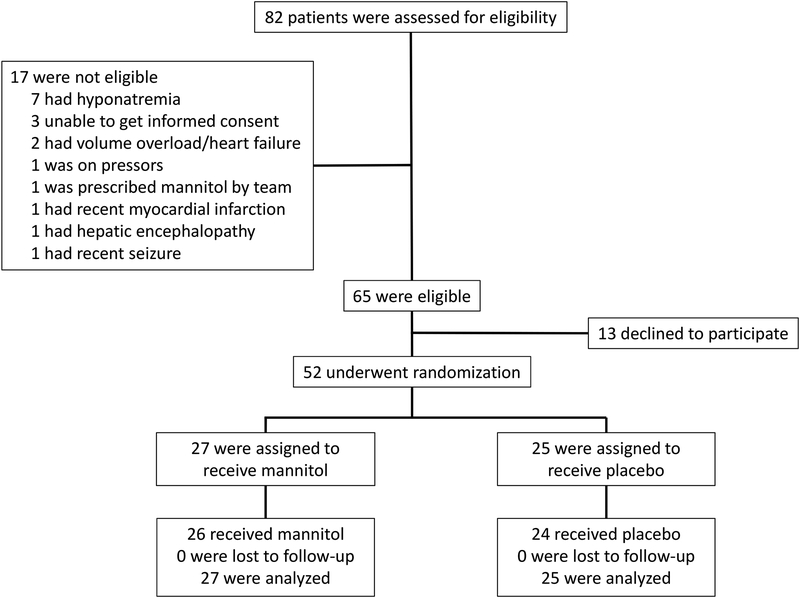
Adult patients with progressive chronic kidney disease (CKD) or acute kidney injury (AKI) that required the initiation of HD at Brigham and Women’s Hospital (BWH) were eligible for enrollment (Figure 1). Patients with AKI requiring HD were considered for inclusion based on prior experience with mannitol use in this group,5 as well as on the biological rationale that osmolality changes would also be relevant to hemodynamic stability in this setting. Patients with the following factors were excluded: hyponatremia (pre-dialysis sodium <130 mmol/L); acute myocardial infarction or stroke/seizure within the last seven days; severe volume overload; cardiac transplant; unstable ventricular arrhythmia; unstable angina; use of vasopressors or midodrine; pregnancy; inability to obtain informed consent prior to the first HD session. All patients received their first three HD sessions at BWH. The typical prescription for patients initiating HD at BWH is as follows: 1st session (HD1): blood flow (Qb) 200 mL/min, dialysate flow (Qd) 400 mL/min, session length 2 hours; HD2: Qb 300 mL/min, Qd 600 mL/min and session length 3 hours; HD3: Qb 400 mL/min, Qd 800 mL/min, session length 3.5–4 hours. A dialysate sodium concentration of 140 mmol/L was used for each treatment.
Figure 1.
Enrollment flow chart
Study procedures
Patients were randomly assigned in a 1:1 ratio to receive 20% mannitol (0.25g/kg/h; up to a maximum dose of 75g per session) or placebo (equivalent volume of 0.9% saline; 375 mL). Randomization was performed with the use of a computer-generated, permuted-block design, with a block size of four. Mannitol and saline were prepared immediately prior to infusion by the Investigational Drug Service at BWH and were delivered to the dialysis unit in opaque bags to ensure blinding of patients, providers, and study staff.
EDTA-anticoagulated plasma samples were collected immediately prior to and at the cessation of each HD session. Urine was collected immediately prior to each session. All samples were processed on-site, centrifuged, and stored in a −80°C freezer. Concentrations of cardiac troponin T (TnT) were measured in pre-HD samples using a high sensitivity (hs) TnT electrochemiluminescent immunoassay assay (Roche Diagnostics, Indianapolis, IN), with a lower limit of detection of 3 ng/L.6 Levels ≥15 ng/L for men and ≥10 ng/L for women were considered as the diagnostic cut points, or upper reference limit (URL), representing the 99th percentile in healthy individuals.7 Observed coefficient of variation (CV) was 2.0% at 28.8 ng/L and 1.3% at 2219 ng/L. Plasma NT-proBNP (N-terminal pro–brain natriuretic peptide) concentrations were measured from samples collected prior to HD1 and HD3 using a sandwich electrochemiluminescent immunoassay (proBNP II; Roche Diagnostics). The analytical range extends from 5 to 35,000 pg/mL; the observed CV was 2.1% at both 138 pg/mL and 4481 pg/mL. Plasma osmolality was measured by freezing point depression from samples collected pre- and post-dialysis from all three sessions. Where available, pre-HD plasma and urine levels of kidney injury biomarkers (monocyte chemoattractant protein 1 (MCP-1), human tumor necrosis factor receptors 1 and 2 (TNFr1 and TNFr2), kidney injury molecule 1 (KIM-1), interleukin 18 (Il-18) and soluble human urokinase plasminogen activator receptor (suPAR)) were measured using multiplex microbead-based ELISA on a Luminex platform. Plasma and urine samples were diluted five-fold using sample diluent buffer (0.1M HEPES, 0.1M NaCl, 0.1% TWEEN 20 and 1% BSA; pH 7.4; filter sterilized) and cocktail of serially diluted recombinant proteins (R&D Systems, Minneapolis, MN) were incubated with microbeads coupled with capture antibodies (R&D Systems) for 1 hour on an orbital shaker, washed three times with 100 ul phosphate buffered saline (PBS)-TWEEN buffer and incubated with corresponding secondary antibodies (RnD systems) for one hour. After incubation, plates were washed again with PBS-TWEEN buffer and incubated with streptavidin-phycoerythrin for another 15 minutes. Beads were washed again with PBS-TWEEN buffer and fluorescence was measured on a BioPlex 200 (Bio-Rad, Hercules, CA) analyzer. Values of each biomarker were interpolated using a standard curve generated from its corresponding parametric logistic regression model. Samples were measured in duplicates, with CV <15% across duplicates.
Data related to demographics, comorbid conditions, medication use, and laboratory values (sodium, potassium, bicarbonate, blood urea nitrogen, creatinine, glucose, calcium and hemoglobin) were collected prior to the first randomized treatment session. At each study visit, information was gathered regarding the dialysate prescription (blood flow, dialysate flow, session length, ultrafiltration volume), BP measurements, pre-dialysis laboratory measurements, adverse events since last interview, and the concomitant use of other medications. The principal investigator assumed responsibility for data and safety monitoring, as approved by the hospital IRB.
Evaluation of Outcomes
The primary endpoint was defined as the intra-dialytic decline in systolic blood pressure (in mmHg), calculated as the pre-dialysis SBP minus the lowest intra-dialytic SBP. The secondary endpoint was defined as the occurrence of IDH (any decline of ≥20 mmHg from the pre-HD SBP versus not). In exploratory analyses, the number of sessions per participant complicated by IDH was also considered (i.e. zero, one, two or three sessions), as well as alternative definitions of IDH (SBP decline ≥25 mmHg or ≥30 mmHg). For relevant biomarkers of interest, we calculated the difference in pre-HD concentrations between HD1 and HD3, according to the randomized treatment assignment. From the observed difference in SBP decline from our prior observational study,5 assuming a standard deviation of 14 mmHg, a sample size of 50 participants would allow us to detect a difference in mean SBP decline of 11 mmHg with 80% power, with alpha=0.05.
Statistical Analyses
Continuous variables were examined graphically and recorded as means (± standard deviations) for normally distributed data, or medians (with interquartile ranges) for non-normally distributed data. Comparisons were made using t tests or Wilcoxon rank sum tests as appropriate. Categorical variables were examined by frequency distribution and recorded as proportions. Comparisons were made using the χ2 test.
To account for within-person correlations, the difference in the SBP decline between those who received mannitol and those who received placebo was analyzed by fitting repeated measures mixed-effects model that used patient ID as a random effect. Initially unadjusted models were fit according to the intention-to-treat principle (mannitol versus placebo). Subsequently, recognizing that pre-SBP is a major determinant of the intra-dialytic decline in SBP and that there were baseline differences in SUN, sensitivity analyses were performed that adjusted for these variables. For the secondary endpoint, the difference in the total proportion of sessions complicated by at least one episode of IDH was assessed by the χ2 test. Subsequently, mixed-effects logistic regression models were fit to account for within-person correlations. Finally, in exploratory analyses, ordinal logistic regression models were fit to determine the effect of mannitol vs. placebo on the occurrence of IDH during repeated HD sessions for an individual participant (i.e. none, one, two or all three HD study sessions).
Urine biomarkers were reported as ratios with urine creatinine. Urine biomarker-creatinine ratio and plasma biomarker measurements were log-transformed for further analyses. Linear regression models were fit to determine the effect of mannitol vs. placebo on the difference in pre-HD biomarkers between HD1 and HD3, adjusted for the pre-HD1 concentration.
Nominal two-sided P values of <0.05 were considered statistically significant. All analyses were performed using STATA 14SE (College Station, Tex., USA).
Results
Baseline Characteristics
We enrolled a total of 52 participants requiring initiation of HD between August 2012 and March 2016 (Figure 1). The average age was 56 ±16 years and 50% were women. Overall, 87% of patients were initiated on HD for progression from earlier stages of CKD to ESRD and 46% had diabetes mellitus. Overall, the groups were similar, except for pre-dialysis SUN (and consequently plasma osmolality), which was higher in the participants assigned to the placebo arm (Table 1 and Table S1). The majority of HD sessions (77%) occurred on sequential days, while 22% had one day between sessions. There were no differences in inter-dialytic interval according to treatment assignment (P=0.3).
Table 1.
Baseline characteristics of participants according to randomized treatment assignment
| Characteristic | Placebo (n=27) | Mannitol (n=25) | Pa |
|---|---|---|---|
| Male sex(n, %) | 14 (52%) | 12 (48%) | 0.8 |
| Black (n, %) | 8 (31%) | 6 (24%) | 0.6 |
| Aqe (yrs) | 57.7 ± 14.7 | 53.4 ± 17.4 | 0.3 |
| Diabetes (n, %) | 13 (48%) | 11 (44%) | 0.8 |
| Heart Failure (n, %) | 9 (33%) | 7 (28%) | 0.7 |
| ESRD (n, %) | 22 (81%) | 23 (92%) | 0.3 |
| Catheter Access (n, %) | 16 (59%) | 15 (60%) | 0.9 |
| Pre-HD SBP (mmHq) | 144 ± 21 | 149 ± 26 | 0.5 |
| Pre-HD Weight (kg)b | 87.0 ± 28.5 | 73.9 ± 23.8 | 0.1 |
| UF Volume (L) | 0.5 [0.0, 1.0] | 0.4 [0.0–1.0] | 0.9 |
| Sodium (mmol/L) | 138.6 ± 3.9 | 139.2 ± 3.7 | 0.6 |
| Potassium (mmol/L) | 4.4 ± 0.7 | 4.2 ± 0.6 | 0.3 |
| Bicarbonate (mmol/L) | 20.6 ± 5.3 | 19.4 ± 4.0 | 0.4 |
| SUN (mg/dL) | 102 ± 32 | 73 ± 26 | 0.001 |
| Calcium (mg/dL) | 8.7 ± 1.1 | 8.6 ± 0.9 | 0.9 |
| Phosphorus (mg/dL) | 6.4 ± 2.0 | 6.0 ± 2.8 | 0.6 |
| Albumin (g/dL) | 3.4 ± 0.6 | 3.3 ± 0.8 | 0.6 |
| Hemoglobin (g/dL) | 8.4 ± 1.3 | 8.4 ± 1.4 | 0.9 |
| Glucose (mg/dL) | 104 [87, 127] | 102 [92, 116] | 0.7 |
| Creatinine (mg/dL) | 7.0 [5.3, 10.1] | 5.8 [4.8, 7.3] | 0.1 |
| ACE Inhibitor (n, %) | 5 (18.5%) | 3 (12%) | 0.5 |
| ARB (n,%) | 3 (11.1%) | 2 (8%) | 0.7 |
| Nitrates (n,%) | 2 (7.4%) | 3 (13%) | 0.5 |
| Beta Blockers (n,%) | 19 (70%) | 19 (76%) | 0.7 |
| Calcium Blockers (n,%) | 15 (56%) | 16 (64%) | 0.5 |
Values for continuous variables are given as mean ±standard deviation or median [interquartile range], values for categorical variables given as count (percentage).
Abbreviations: ESRD, end-stage renal disease, Pre-HD, pre-hemodialysis; SBP, systolic blood pressure; SUN, serum urea nitrogen; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
P values refer to a test for difference (t-test for normally distributed continuous variables; Wilcoxon Rank Sum for non-normally distributed continuous variables; and chi-squared test for categorical variables) according to randomized treatment assignment
there were 7 missing values for pre-HD weight
Effect of Mannitol versus placebo on SBP Changes During Hemodialysis
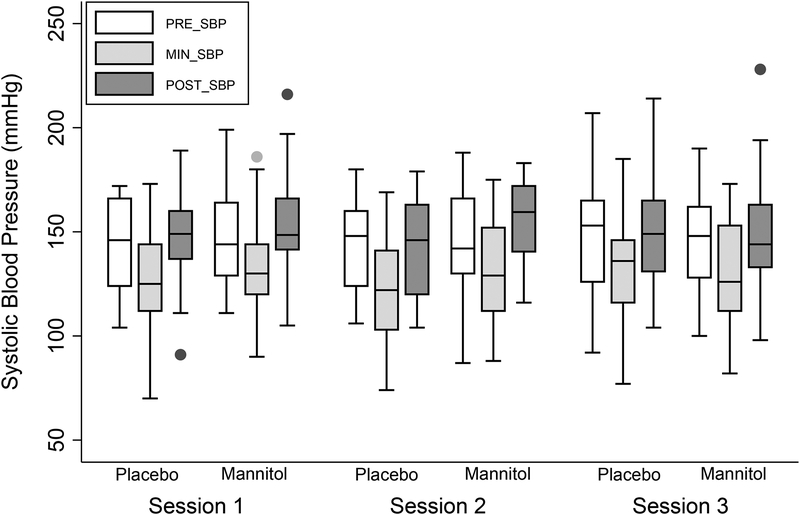
When averaged across all sessions, the mean pre-dialysis SBP was 146 ±25 mmHg, the mean nadir intradialytic SBP was 129 ±24 mmHg, and the mean post-HD SBP was 149 ±24mmHg. There were no significant differences in these parameters according to randomized treatment assignment (Figure 2).
Figure 2.
Systolic blood pressure measurements measured pre-dialysis (Pre-HD), lowest intra-dialysis (Nadir) and post-dialysis (Post-HD) at each HD session according to randomized treatment assignment.
The mean decline in SBP across all three HD sessions for those who assigned to placebo was 19 ±20 mmHg vs. 15 ±16 mmHg for those assigned to mannitol. From the unadjusted intention to treat analyses, mannitol use (vs. placebo) resulted in 3.9 mmHg less decline in SBP (P=0.3). When adjusted for the pre-HD SBP and SUN, the difference was 4.3 mmHg (P=0.3). There was no evidence for effect modification according to AKI (P-interaction=0.4).
Effect of Mannitol versus Placebo on Intra-Dialytic Hypotension
IDH developed during 43% of the placebo arm sessions (35 of 81 sessions) versus 25% of the mannitol arm sessions (19 of 75 sessions; P-difference=0.02). When analyzed to account for intra-patient correlation, the use of mannitol was associated with nominally lower odds of developing IDH (OR, 0.38; 95%CI, 0.14–1.00), although this was of borderline statistical significance (P=0.05). In unadjusted sensitivity analyses that considered increasing frequency of IDH events at a participant level (i.e. no IDH, IDH at one session, IDH at two sessions, or IDH at three sessions), mannitol resulted in nominally lower odds compared with placebo of repeated IDH (OR, 0.41; 95%CI, 0.15–1.13), although this did not reach the threshold for statistical significance (P=0.08). When adjusted for pre-dialysis SBP and SUN, similar effect estimates were noted (OR, 0.39; 95% CI, 0.12–1.24; P=0.1).
Similar patterns of association were found when alternative definitions of IDH were examined (Table S2).
High sensitivity Troponin T and NT-pro-BNP
The median baseline pre-dialysis hsTnT for all participants was 79 [IQR, 48 – 185] ng/L; the median baseline pre-HD NT-pro-BNP was 5,136 [IQR, 1,421 – 13,867] pg/mL. There were no significant differences between randomized groups at baseline, nor from the pre-HD measurements at HD2 or HD3. There were no significant differences in the change of pre-HD hsTnT or NT-pro-BNP concentrations between HD1 and HD3 according to the randomized treatment assignment (Table 3 and Table S2).
Table 3.
Difference in pre-HD Biomarkers According to Randomized Treatment Assignment
| Percent Change in Pre-HD Level from HD1 to HD3 | ||
|---|---|---|
| Ratio of Mannitol vs Placebo | P* | |
| Plasma SUN (n=49) | 4% (−15% to 26%) | 0.7 |
| Plasma Osmolality (n=49) | 1% (−1% to 3%) | 0.5 |
| Plasma hsTnT (n=49) | 1% (−15% to 21%) | 0.9 |
| Plasma NTproBNP (n=40) | 4% (−29% to 29%) | 0.8 |
Effect estimates are calculated as the difference in log-transformed biomarkers between HD3 and HD1, adjusted for baseline pre-HD1 biomarker concentrations.
P values are not adjusted for multiple comparisons.
Exploratory analyses of kidney injury biomarkers
Urine and plasma biomarkers of kidney injury were available in a subset of participants (the last 32 enrolled in the trial). At baseline there were no differences in median biomarker concentrations according to the randomized treatment assignment, although most biomarkers appeared to be marginally higher in those who randomized to the mannitol arm (Table S3). In general, kidney injury biomarkers appeared to increase from session to session in both the mannitol and placebo groups. There was no consistent pattern in relation to the difference in biomarkers between HD1 and HD3 in the reported estimates for mannitol versus placebo (Table S4).
Adverse Events
The total number of participants who developed adverse events was 18 (66.7%) in the placebo group and 17 (68%) in the mannitol group. The most common adverse events were hypertension (SBP>180 mmHg) and nausea (Table S5).
Discussion
In this placebo-controlled, double-blinded, pilot RCT of patients initiating HD, we report that the use of mannitol (versus placebo) resulted in no significant difference in decline of intra-dialytic SBP. There was a nominally lower frequency of IDH episodes, although this did not reach statistical significance. Mannitol appeared safe, with no difference in cardiac biomarkers of adverse events between the two groups, and no consistent signal for elevations in biomarkers of kidney injury.
The one-year mortality rate for individuals initiating HD in the United States is approximately 22%, and peaks during the first two months post-initiation.1 While some of these deaths may represent expected mortality in medically complex patients, it is possible that perturbations in cardiovascular physiology related to the HD procedure itself may contribute to adverse outcomes. In this regard, much attention has focused on the possible adverse effects of IDH, and its potential effect on end-organ hypoperfusion.8,9 For example, IDH has been independently associated with myocardial hypoperfusion10 and stunning,11 cerebral hypoperfusion,12 decline in residual kidney function,4 and greater mortality.13 Thus, interventions that limit the frequency and magnitude of IDH, especially for incident patients, may represent promising candidates for future clinical application.
Patients receiving maintenance HD typically have higher plasma osmolality than those with normal kidney function,14,15 largely due to the accumulation of urea.16 Rapid dialytic clearance of urea and other molecules may lead to generation of temporary osmotic gradients between the intracellular and extracellular compartments17–20, predisposing to development of disequilibrium and IDH. Indeed, we recently reported that higher pre-dialysis calculated osmolarity is an independent predictor of greater risk of IDH in a cohort of contemporary thrice-weekly HD patients.16 In this respect, despite randomization, the average pre-HD SUN was higher in those assigned to the placebo arm in our trial. As this may have altered our ability to detect differences in SBP decline, we performed sensitivity analyses that adjusted for SUN. There was no evidence for meaningful differences in results according to this approach.
Mannitol has been used for decades in an attempt to ameliorate adverse symptoms associated with hemodialysis. Prior studies reported benefits of mannitol in relation to symptom reduction (primarily cramps) and less IDH, but were limited by small sample size, use of prevalent patients, and lack of placebo controls.21–23 Furthermore, many of these studies were performed decades ago with HD technology and patient characteristics that are not reflective of contemporary practice. As far as we are aware, our study is the largest placebo-controlled trial to date to examine the effect of hypertonic mannitol administration on IDH at the time of HD initiation. Although the observed difference in the primary outcome of SBP decline (15 vs. 19 mmHg) in this pilot trial was not statistically significant between groups, there was a notable signal in favor of the mannitol arm in relation to the secondary outcome of IDH occurrence (25% vs. 43%). In order to have adequate power to detect the observed difference in SBP decline, assuming a SD of 14 mmHg, we would need to enroll approximately 194 participants per group in a future study.
The use of hypertonic solutions in HD patients could, in theory, promote intravascular filling and raises concerns about the development of hypervolemia. Indeed, even prior to their first HD session, the majority of participants in our study had markedly elevated concentrations of hsTnT and NTproBNP. Although higher concentrations of both biomarkers are associated with mortality in patients with CKD25,26 and those on maintenance HD,27,28 reassuringly, we found no difference in the change in concentrations of either NTproBNP or hsTnT between the first and third HD sessions. Further, we found no major differences in patient-reported adverse events between the two groups in our study. In this regard, it should be remembered that mannitol has a molecular weight of 182 Daltons and is highly dialyzable. Our protocol required cessation of mannitol 30 minutes prior to the end of the HD sessions, which may have allowed further clearance of residual circulating mannitol (as evidenced by the similar post-HD POsm measurements between groups). Further studies to test POsm at multiple time points during the HD session may be helpful to determine the optimal dosing strategy for future trials.
Residual kidney function declines with increasing duration of hemodialysis dependence, and its loss is a strong predictor of mortality.29 In keeping with the theory of end-organ hypoperfusion, it is not surprising that IDH has been associated with greater decline in residual kidney function.4 On the other hand, high doses of intravenous mannitol (>200) have been associated with AKI.30,31 In this regard we carefully note the findings from our exploratory analyses of urine and plasma kidney injury biomarkers. Although some biomarkers appeared to increase between HD1 and HD3 in those randomized to mannitol, it must be highlighted that baseline concentrations appeared to be slightly higher in this arm, and that many biomarkers did not increase more in this arm compared with placebo. Furthermore, these exploratory analyses were performed in a subset of participants and were not adjusted for multiple testing. Our protocol specifically mandated that infusions stop 30 minutes before the end of dialysis in order to promote clearance of mannitol during the remaining treatment time. However, this is an important safety issue that requires close attention in any future studies.
The strengths of this study include the use of concealed randomization, an independently prepared placebo, and a double-blinded approach. However, there are limitations to consider. In keeping with a pilot study, the sample size was modest, which reduced the power to detect meaningful differences between groups. This may have been further compromised by the inclusion of participants with AKI. Similarly, all sub-group analyses should be considered as hypothesis generating as the possibility of type 1 errors remain. We did not measure mannitol concentrations (although these could be inferred from plasma osmolality measurements) and did not measure intra-dialytic plasma osmolality, which may have provided additional information on the delivered dose of mannitol in those randomized to the treatment arm. Our primary outcome was related to intra-dialytic SBP changes, and thus we did not follow participants for longer-term outcomes such as hospitalization, residual kidney function decline, or mortality. The timing of intra-dialytic symptoms was not recorded, precluding the distinction of symptomatic and non-symptomatic IDH. Furthermore, accurate timing of administration of anti-hypertensive medications, bioimpedance, and measurements of residual kidney function were not performed.
In summary, in this pilot RCT of 52 patients initiating HD therapy, mannitol did not result in differences in SBP decline, the primary outcome, but did appear to reduce the frequency of IDH episodes (the secondary outcome). While the difference in SBP decline was less than anticipated, mannitol was safe and well tolerated compared with placebo, and there were no major issues encountered in terms of protocol feasibility. Our results provide rationale to design larger multi-center studies using hypertonic mannitol, perhaps with a longer intervention period, to minimize the frequency of IDH in patients initiating HD.
Supplementary Material
Table S1. Dialysis treatment parameters according to randomized treatment assignment.
Table S2. Raw biomarker concentrations according to randomized treatment assignment.
Table S3. Raw pre-HD plasma and urine kidney injury biomarker concentrations according to randomized treatment assignment.
Table S4. Difference in pre-HD biomarkers according to randomized treatment assignment.
Table S5. Summary of adverse events.
Table 2.
Frequency and risk of IDH for Mannitol versus Placebo according to different definitions
| Frequency of IDH events | Risk of IDH (Mannitol vs. Placebo)* | ||||
|---|---|---|---|---|---|
| IDH Definition** | Placebo | Mannitol | P | OR (95%CI) | P |
| decline of ≥20 mmHg | 35/81 (43%) | 19/75 (25%) | 0.02 | 0.38 (0.14–1.00) | 0.05 |
| decline of ≥25 mmHg | 29/81 (36%) | 15/75 (20%) | 0.03 | 0.37 (0.12–1.16) | 0.09 |
| decline of ≥30 mmHg | 18/81 (22%) | 10/75 (13%) | 0.2 | 0.41 (0.12–2.04) | 0.3 |
Unadjusted effect estimates were calculated by fitting repeated measures mixed-effects model that used patient ID as a random effect
Decline measured from pre-dialysis SBP to Intra-dialytic nadirIDH, intradialytic hypotension; OR, odds ratio; CI, confidence interval
Support:
Dr. Mc Causland is supported by the National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511. High sensitivity troponin T reagents were provided by Roche Diagnostics.
Financial Disclosure: Dr. Jarolim reports research support through his institution from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi-Sankyo, Inc.,GlaxoSmithKline, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation; and consulting fees from Roche Diagnostics Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material Descriptive Text for Online Delivery
Data Sharing: There are no plans to share individual patient level data. The study protocol will be available upon request.
Peer Review: Received _______. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form March 4, 2019. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):S1–S434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakim KG, Johnson WJ, Klass DW. Role of blood urea and serum sodium concentrations in the pathogenesis of the dialysis dysequilibrium syndrome. Trans Am Soc Artif Intern Organs. 1968;14:394–401. [PubMed] [Google Scholar]
- 3.Chou JA, Streja E, Nguyen DV, et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant. 2018;33(1):149–159. doi: 10.1093/ndt/gfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansen MAM, Hart AAM, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 5.Mc Causland FR, Prior LM, Heher E, Waikar SS. Preservation of blood pressure stability with hypertonic mannitol during hemodialysis initiation. Am J Nephrol. 2012;36(2):168–174. doi: 10.1159/000341273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitivity cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56(4):642–650. doi: 10.1373/clinchem.2009.134460. [DOI] [PubMed] [Google Scholar]
- 7.Gore MO, Seliger SL, Defilippi CR, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol. 2014;63(14):1441–1448. doi: 10.1016/j.jacc.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daugirdas JT. Dialysis hypotension: a hemodynamic analysis. Kidney Int. 1991;39(2):233–246. [DOI] [PubMed] [Google Scholar]
- 9.Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis. 2001;38(4 Suppl 4):S11–S17. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood Purif. 2010;29(2):105–110. [DOI] [PubMed] [Google Scholar]
- 11.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4(12):1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacEwen C, Sutherland S, Daly J, Pugh C, Tarassenko L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J Am Soc Nephrol. 2017;28(8):2511–2520. doi: 10.1681/ASN.2016060704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimizu K, Kurosawa T, Sanjo T. Effect of hyperosmolality on vasopressin secretion in intradialytic hypotension: a mechanistic study. Am J Kidney Dis. 2008;52(2):294–304. doi: 10.1053/j.ajkd.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Cernaro V, Lacquaniti A, Lorenzano G, et al. Apelin, plasmatic osmolality and hypotension in dialyzed patients. Blood Purif. 2012;33(4):317–323. doi: 10.1159/000337104. [DOI] [PubMed] [Google Scholar]
- 16.Mc Causland FR, Waikar SS. Association of Predialysis Calculated Plasma Osmolarity With Intradialytic Blood Pressure Decline. Am J Kidney Dis. 2015;66(3):499–506. doi: 10.1053/j.ajkd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arieff AI, Massry SG, Barrientos A, Kleeman CR. Brain water and electrolyte metabolism in uremia: effects of slow and rapid hemodialysis. Kidney Int. 1973;4(3):177–187. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;1(7226):410–411. [DOI] [PubMed] [Google Scholar]
- 19.Pappius HM, Oh JH, Dossetor JB. The effects of rapid hemodialysis on brain tissues and cerebrospinal fluid of dogs. Can J Physiol Pharmacol. 1967;45(1):129–147. [DOI] [PubMed] [Google Scholar]
- 20.Silver SM, Sterns RH, Halperin ML. Brain swelling after dialysis: old urea or new osmoles? American Journal of Kidney Diseases. 1996;28(1):1–13. [DOI] [PubMed] [Google Scholar]
- 21.Hagstam KE, Lindergard B, Tibbling G. Mannitol infusion in regular haemodialysis treatment for chronic renal insufficiency. Scand J Urol Nephrol. 1969;3(3):257–263. [DOI] [PubMed] [Google Scholar]
- 22.Hothi DK, Harvey E, Goia CM, Geary D. The value of sequential dialysis, mannitol and midodrine in managing children prone to dialysis failure. Pediatr Nephrol. 2009;24(8):1587–1591. doi: 10.1007/s00467-009-1151-8. [DOI] [PubMed] [Google Scholar]
- 23.Henrich WL, Woodard TD, Blachley JD, Gomez-Sanchez C, Pettinger W, Cronin RE. Role of osmolality in blood pressure stability after dialysis and ultrafiltration. Kidney Int. 1980;18(4):480–488. [DOI] [PubMed] [Google Scholar]
- 24.Mc Causland FR, Prior LM, Heher E, Waikar SS. Preservation of blood pressure stability with hypertonic mannitol during hemodialysis initiation. Am J Nephrol. 2012;36(2):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunsolus I, Sandoval Y, Smith SW, et al. Renal Dysfunction Influences the Diagnostic and Prognostic Performance of High-Sensitivity Cardiac Troponin I. J Am Soc Nephrol. 2018;29(2):636–643. doi: 10.1681/ASN.2017030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruch C, Fischer C, Sindermann J, Stypmann J, Breithardt G, Gradaus R. Comparison of the prognostic usefulness of N-terminal pro-brain natriuretic Peptide in patients with heart failure with versus without chronic kidney disease. Am J Cardiol. 2008;102(4):469–474. doi: 10.1016/j.amjcard.2008.03.082. [DOI] [PubMed] [Google Scholar]
- 27.Chen T, Hassan HC, Qian P, Vu M, Makris A. High-Sensitivity Troponin T and C-Reactive Protein Have Different Prognostic Values in Hemo- and Peritoneal Dialysis Populations: A Cohort Study. J Am Heart Assoc. 2018;7(5). doi: 10.1161/JAHA.117.007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paniagua R, Ventura M-D-J, Ávila-Díaz M, et al. NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant. 2010;25(2):551–557. doi: 10.1093/ndt/gfp395. [DOI] [PubMed] [Google Scholar]
- 29.Termorshuizen F, Dekker FW, van Manen JG, et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. JASN. 2004;15(4):1061–1070. [DOI] [PubMed] [Google Scholar]
- 30.Better OS, Rubinstein I, Winaver JM, Knochel JP. Mannitol therapy revisited (1940–1997). Kidney Int. 1997;52(4):886–894 [DOI] [PubMed] [Google Scholar]
- 31.Dickenmann M, Oettl T, Mihatsch MJ. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51(3):491–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dialysis treatment parameters according to randomized treatment assignment.
Table S2. Raw biomarker concentrations according to randomized treatment assignment.
Table S3. Raw pre-HD plasma and urine kidney injury biomarker concentrations according to randomized treatment assignment.
Table S4. Difference in pre-HD biomarkers according to randomized treatment assignment.
Table S5. Summary of adverse events.