Abstract
Background.
Male hypogonadism, arising from a range of etiologies including androgen-deprivation therapies (ADT), has been reported as a risk-factor for acquired long-QT syndrome (aLQTS) and Torsades de pointes (TdP). A full description of the clinical features of aLQTS associated with ADT and of underlying mechanisms are lacking.
Methods.
We searched the international pharmacovigilance database VigiBase, for men (n=6,560,565 individual case safety reports) presenting with aLQTS, TdP or sudden death associated with ADT. In cardiomyocytes derived from induced pluripotent stem cells (IPSC-CMs) from men, we studied electrophysiological effects of ADT and dihydrotestosterone.
Results.
Among subjects receiving ADT in VigiBase, we identified 184 cases of aLQTS (n=168) and/or TdP (n=68, 11% fatal), and 99 with sudden death. Of the 10 ADT drugs examined, 7 had a disproportional association (reporting odds-ratio=1.4–4.7,p<0.05) with aLQTS, TdP or sudden death. The minimum and median time to sudden death were 0.25 and 92 days, respectively. The androgen receptor competitive antagonist enzalutamide was associated with more deaths (5,430/31,896[17%],p<0.0001) than other ADT used for prostate cancer (4,208/52,089[8.1%]). In IPSC-CMs, acute and chronic enzalutamide (25μM) significantly prolonged action potential durations (APD90-paced0.5Hz; 429.7±27.1 (control) vs. 982.4±33.2 (acute, p<0.001) and 1062.3±28.9 msec (chronic, p<0.001), and generated afterdepolarizations and/or triggered activity in drug treated cells (11/20 acutely and 8/15 chronically). Enzalutamide acutely and chronically inhibited IKr, and chronically enhanced INa-L. Dihydrotestosterone (30nM) reversed enzalutamide electrophysiological effects on IPSC-CMs.
Conclusions:
QT prolongation and TdP are a risk in men receiving enzalutamide and other ADTs.
NCT:
Keywords: Torsade de Pointes, hypogonadism, acquired Long QT syndrome, testosterone, androgen antagonists
Background
QT interval duration, measured on the electrocardiogram and corrected for heart rate (QTc), represents the duration of ventricular repolarization. Exaggerated QTc prolongation can cause the potentially fatal ventricular tachycardia torsades de pointes (TdP)1 in both the congenital form of the long-QT syndrome (LQTS) as well as an acquired form (aLQTS), often drug-induced. A major mechanism for drug-associated LQTS and TdP is block of the repolarizing potassium-current IKr which in addition to prolonging QTc also generates morphologically distinctive low amplitude bifid T-waves, seen in patients with type 2 congenital LQTS due to reduced IKr.2-6 Recently, Yang et al. showed that IKr-blockers with the greatest propensity to cause TdP can also augment the late sodium current (INa-L) within hours of exposure.7
In healthy individuals, QTc is longer in women than in men from puberty to menopause, and women are at higher risk of aLQTS and TdP. Several lines of evidence support the contention that this sex specificity is attributable to a testosterone effect to shorten QTc.8-12 QTc prolongation and TdP in men have been linked to hypogonadism and correction of testosterone-deficiency was associated with shortening of QTc in interventional studies9-11, 13 and absence of TdP recurrence in a small prospective case-series.14 Modest (10–20 msec) QTc prolongation has been seen with androgen deprivation therapies (ADT) in men with prostatism and with prostate cancer, but cases of ADT-associated TdP and sudden death are limited to case reports.11, 15, 16 We previously reported an association between ADT and aLQTS and TdP in a European database and a US electronic health record.14
Here, we used VigiBase, the World Health Organization’s (WHO) very large global database of individual case safety reports (ICSRs),17 to further validate a role for ADTs in men presenting with aLQTS, TdP, or sudden death. We then studied the effects of the top implicated drug, enzalutamide, in Chinese-hamster-ovary (CHO) cells expressing IKr and cardiomyocytes derived from induced-pluripotent stem-cells (iPSC-CMs).7
Methods
The data that support the findings of this study are available from the corresponding author upon request.
Epidemiological study
VigiBase is the WHO’s global database of >17 million ICSRs. These originate from >130 country members of the WHO Programme for International Drug Monitoring and are reported by diverse sources such as healthcare professionals, patients, and pharmaceutical companies including reports from the US Food and Drug Administration and the European Medicines Agency, with duplicates flagged and dropped.18 We performed a disproportionality case/non-case analysis that considered reports of adverse drug reactions (ADR) in men contained in the deduplicated VigiBase,17 from inception (10/01/1967) to 08/09/2018. This method compares the proportion of specific ADRs reported for the case versus non-case groups. Reactions are based on the medical dictionary for regulatory activities classification of terms for side effects and terms used to define aLQTS, TdP, Sudden death and death are detailed in Supplementary Table 1. For each drug, the number of ADRs under study (i.e, aLQTS, TdP, sudden death) divided by the total number of all ADRs for that drug (in this case, each ADT) is compared to the proportion of the same specific ADR (aLQTS, TdP, Sudden death) over the total number of ADRs for a comparator group. The comparator group used here was the entire database with all other drugs available but restricted to men because ADT are used only in men. Comparisons are performed using a Chi-square test and results are displayed using a reporting odds-ratio (ROR, Supplementary Table 2).19 When the lower end of the ROR 95% confidence interval is >1, signal is deemed significant and the identified association between the specific drug and the reaction is a potential safety signal. This analytical approach has showed, for example, that magnitude of drug-induced IKr blockade correlates with risk for aLQTS/TdP/Sudden death in VigiBase.20 Drugs considered as ADT were the cytochrome-P450–17 inhibitor (abiraterone), gonadotropin-releasing hormone agonists (leuprorelin, goserelin, triptorelin) and antagonists (degarelix), nonsteroidal androgen receptor inhibitors (enzalutamide, bicalutamide, flutamide) indicated for prostate cancer and 5α-reductase inhibitors (finasteride, dutasteride) indicated for androgenic alopecia and prostatism.21 The standard dosage and indications for these drugs have been recently detailed elsewhere.21 The drug used as “positive control” for drug-induced LQTS, TdP and sudden death was sotalol.1, 6 The use of confidential electronically processed patient data was approved by the Vanderbilt University Medical Center institutional review board (IRB#181337).
In vitro electrophysiology
FuGENE6-mediated SCN5A and KCNH2 channel expression and cell transfection
Recombinant cDNA for human SCN5A (2μg, encoding the α-subunit of cardiac sodium channel Nav1.5) or for human KCNH2 (2μg, also known as HERG, human Ether-a-go-go-related Gene encoding the α-subunit of cardiac potassium channel Kv11.1) were transiently transfected in CHO-cells, as previously reported.3, 7 In brief, SCN5A and KCNH2 DNA were subcloned into the pRc-CMV vector (Stratagene) and transiently transfected into cultured CHO-cells using FuGENE6 (Roche Applied Bioscience). 0.5μg plasmid encoding the enhanced green fluorescent protein (pEGFP-N3, BD Bioscience Clontech) was cotransfected to identify transfected cells for electrophysiological study. Cells were studied at 48 hours after transfection with or without drug exposures.
Reprogramming and generating iPSC-CMs
iPSC-CM lines were developed using the episomal vector method from 3 men with normal QTc duration.7 Briefly, episomal vectors were transfected into erythroblasts via nucleofection. Cells were then plated onto mouse embryonic fibroblast coated plates. iPSC-like colonies were picked up at ~Day 20 post-transfection. The matrix sandwich method was used to generate iPSC-CM from human iPSCs.7 Single iPSCs were plated onto Matrigel coated 6-well plates, and growth factors (Activin-A, BMP4 and bFGF) were added sequentially to differentiate the iPSCs into cardiomyocytes. iPSC-CMs were then re-plated onto matrigel coated plates and incubated at 37°C for 30–35 days post-induction. Spontaneously-beating iPSC-CMs were used for action potential recordings in current-clamp mode. Single cardiomyocytes were used for ion current recordings in voltage-clamp mode after brief trypsinization. This study was approved by Vanderbilt University Medical Center review committee (IRB#040551) and the subjects gave informed consent.
Action potential (AP) recordings
APs in iPSC-CMs were recorded from spontaneously-beating cells at Day 30–35 post-induction. For these experiments, the bath (extracellular) solution contained (in mmol/L): NaCl 135, KCl 4.0, CaCl2 1.8, and MgCl2 1, HEPES 5, glucose 10, with a pH of 7.4 (adjusted by NaOH). The pipette-filling (intracellular) solution contained (in mmol/L): KCl 130, ATP-K2 5.0, MgCl2 1.0, CaCl2, 1.0, BAPTA 0.1, and HEPES 5.0, with a pH of 7.3 (adjusted by KOH). Microelectrodes with tip resistances of 3–5 mΩ were used. Ten successive traces were averaged for analysis of AP durations at 90% repolarization (APD90). APs were recorded prior to and after acute (15 minutes, min) and/or chronic (5 hours, hrs) exposure to drugs (enzalutamide and dihydrotestosterone) as detailed in Results.
IKr, peak (INa) and late (INa-L) sodium current recordings
Whole-cell voltage clamp experiments were conducted at room temperature (22–23°C). To record sodium currents, two extracellular bath solutions were used. In CHO-cells and iPSC-CMs, the external solution contained (in mmol/L) NaCl 135, KCl 4.0, MgCl2 1.0, CaCl2 1.8, glucose 10, and HEPES 10; the pH was 7.4, adjusted with NaOH. The pipette (intracellular) solution contained (in mmol/L) NaF 10, CsF 110, CsCl 20, EGTA 10, and HEPES 10; the pH was 7.4, adjusted with CsOH. To eliminate L- and T-type inward calcium currents as well as outward potassium currents in iPSC-CMs, 1 μmol/L nisoldipine, 200 μmol/L NiCl2, and 500 μmol/L 4-aminopyride were added into the bath solution, respectively. Glass microelectrodes were heat polished to tip resistances of 0.5–2 MΩ. Cells were held at −120 mV, and sodium current was elicited with a single 200ms pulse from −120 to −30 mV at which maximal peak inward sodium current is usually observed.
To obtain KCNH2-encoded IKr current-voltage relations, activating current was elicited with a 2s voltage-clamp protocol from a holding potential of −80mV to +60mV with 10mV steps and deactivating tail current was measured upon a 2s returning pulse to −40mV. The cycle time between pulses was 15s or slower to accommodate pulse durations. Under these conditions, IKr was stable for >60 min in the absence of a drug intervention.3
Data acquisition was carried out using an Axopatch200B patch-clamp amplifier and pCLAMP-9.2 software (MDS-Inc., Canada). Currents were filtered at 5kHz (−3dB, four-pole Bessel filter) and digitized using an analog-to-digital interface (DigiData1322A, MDS-Inc.). To minimize capacitive transients, capacitance and series resistance were corrected~80%. In some experiments, peak sodium current magnitudes were expressed in units of pA/pF after normalization to cell sizes generated from the cell capacitance calculated by Membrane Test (OUT 0) in pClamp9.2. Clamp protocols used are shown on the figures. INa-L (expressed as a percentage of peak INa) was measured in a 3ms time window (195–198ms after the pulse) before the capacity transient at the end of a 200ms depolarizing pulse. Electrophysiological data were analyzed using pCLAMP-9.2 software and the figures were prepared by using Origin 8.5.1 software (OriginLab Corp., USA) to generate figures. Data were recorded prior to and after acute (15 minutes, min) and/or chronic (24 or 48 hours, hrs) exposure to drugs (enzalutamide and dihydrotestosterone) as detailed in Results.
Chemicals used for electrophysiological studies
Enzalutamide and dihydrotestosterone were purchased from SelleckChem and Sigma-Aldrich, respectively. Stock solutions for the tested drugs were prepared according to the vendors’ instructions and then diluted for studies, as needed. The concentrations used for dihydrotestosterone (30nM) and enzalutamide (25μM) were within human physiological and therapeutic range, respectively.22, 23
Descriptive statistical analysis
Results were described in terms of means ± standard deviation (clinical data), ± standard error of the mean (preclinical data) or medians [interquartile-range] for quantitative variables, and in terms of number and proportion for qualitative variables. Comparisons used unpaired t-test or Mann-Whitney tests for quantitative variables, and χ2-test for qualitative variables (Prism-7; GraphPad®). Statistical significance was accepted for p<0.05.
Results
Clinical characteristics of patients with aLQTS, TdP and sudden death associated with ADT
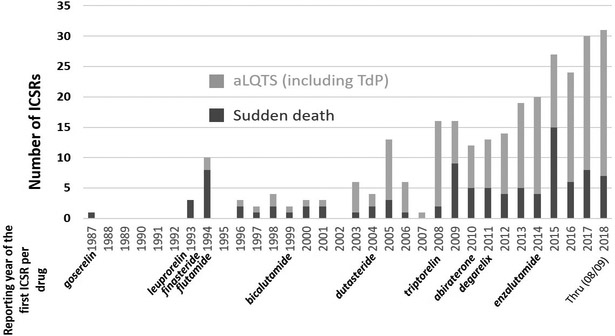
In VigiBase, 283 ICSRs of aLQTS, TdP or sudden death associated with ADT were identified (Figure 1) and their clinical characteristics are detailed in Table 1. These ADT were mainly used to treat prostate cancer, with prostatism and androgenic alopecia as less common indications. Most cases of aLQTS/TdP (115/184,[62%]) and sudden death (87/99,[88%]) associated with ADT were reported without exposure to other drugs known to confer TdP risk. The times to onset between ADT introduction and cardiac events were scattered ranging from few hours with ADT being the only suspected intervention to years after ADT introduction in context of multiple other risk factors or other drugs conferring TdP risk (Table 1).24 Among concurrent reported risk factors for aLQTS/TdP (Table 1), hypocalcemia was present in 16/184 [9%] of ICSRs, usually seen with denosumab (11/16[69%]) which may reflect its use in patients with bone metastases. Hypokalemia was present more often in abiraterone cases (6/21[29%]) vs. other ADT (7/163[4%]), p<0.0001), consistent with the drug’s known action to generate hypermineralocorticism.25
Fig.1.
Number of acquired long QT syndrome (aLQTS) including Torsades de pointes (TdP); and sudden death associated with androgen deprivation therapies (ADT) reported over time within VigiBase. Each ADT is noted below the year corresponding to its first associated individual case safety report (ICSR) related to aLQTS, TdP or sudden death reported in VigiBase.
Table 1.
Details concerning men with aLQTS and/or TdP (n=184) and men with sudden death (n=99) associated with androgen deprivation therapy (ADT) in VigiBase.
| Characteristic | ||
|---|---|---|
| Reported condition | aLQTS and/or TdP | Sudden death |
| Age, years | n=146 available | n=82 available |
| Median, [Interquartile range] | 75[70-82] | 77[68-85] |
| Minimum-maximum | [11-94] | [30-95] |
| Time to onset, days | n=43 available | n=33 available |
| Median,[Interquartile range] | 170[37-473] | 92[21-390] |
| Minimum-maximum | [7-4884] | [0.25-4984] |
| Indication | ||
| Prostate cancer | 70/91(77%) | 35/44(80%) |
| Prostatism | 17/91(19%) | 8/44(18%) |
| Androgenic alopecia | 3/91(3%) | 1/44(2%) |
| Sexual disorders | 1/91(1%) | 0/44(0%) |
| Region of reporting | ||
| Americas | 85/184(46%) | 38/99(38%) |
| Europe | 85/184(46%) | 44/99(45%) |
| Asia, Oceania | 14/184(8%) | 17/99(17%) |
| Concurrent reported drugs at known TdP risk24 | ||
| None | 115/184(62%) | 87/99(88%) |
| 1 other | 55/184(30%) | 11/99(11%) |
| ≥2 others | 14/184(8%) | 1/99(1%) |
| Concurrent reported drugs at conditional, possible or known TdP risk24 | ||
| None | 63/184(34%) | 59/99(60%) |
| 1 other | 43/184(23%) | 18/99(18%) |
| ≥2 others | 78/184(43%) | 22/99(22%) |
| Proton pump inhibitors | 54/184(29%) | 15/99(15%) |
| Diuretics (potassium lowering) | 45/184(24%) | 18/99(18%) |
| Antidepressants | 42/184(23%) | 12/99(12%) |
| Antiarrhythmics | 28/184(15%) | 4/99(4%) |
| Anti-infectious | 25/184(14%) | 4/99(4%) |
| Neuroleptics | 21/184(11%) | 1/99(1%) |
| Opioids | 12/184(7%) | 3/99(3%) |
| Anti-emetics | 12/184(7%) | 3/99(3%) |
| Anti-cancer drugs | 12/184(7%) | 1/99(1%) |
| Anti-histamines | 3/184(2%) | 1/99(1%) |
| Anti-α1-adrenergics | 4/184(2%) | 1/99(1%) |
| Other | 18/184(10%) | 3/99(3%) |
| ADT regimen | ||
| Monotherapy | 155/184(84%) | 82/99(83%) |
| Combination therapy | 29/184(16%) | 17/99(17%) |
| Seriousness | ||
| Serious | 159/159(100%) | 99/99(100%) |
| Fatal | 12/159(8%) | 99/99(100%) |
| Concurrent reported condition favoring aLQTS/TdP | ||
| Hypokalemia | 13/184(7%) | 0/99(0%) |
| Hypocalcemia | 16/184(9%)* | 0/99(0%) |
| Diabetes | 32/184(17%) | 7/99(7%) |
| Uncontrolled hypertension | 13/184(7%) | 0/99(0%) |
| Cardiac ischemia or heart failure | 22/184(12%) | 9/99(9%) |
| Bradycardia or conductive disorders | 26/184(14%) | 0/99(0%) |
| Atrial fibrillation | 33/184(18%) | 1/99(1%) |
| Acute kidney injury | 23/184(13%) | 0/99(0%) |
| Infection (bacteria, fungus or parasite) | 35/184(19%) | 7/99(7%) |
| Acute Stroke or epilepsy | 12/184(7%) | 2/99(2%) |
| Reporting year | ||
| 1987–1997 | 4/184(2%) | 15/99(15%) |
| 1998–2007 | 28/184(15%) | 14/99(14%) |
| 2008–2018 | 152/184(83%) | 70/99(71%) |
*11/16[69%] were taking denosumab.
Disproportionality analysis in VigiBase
The number of total ICSRs reported in men in VigiBase on each of the 10 ADTs analyzed and the subsets of those with aLQTS, TdP, sudden death and death are detailed in Table 2. Overall, we found 184 cases presenting aLQTS (n=168) and/or TdP (n=68, 11% fatal), and another 99 who developed sudden death associated with ADT. Analysis of VigiBase reports in men through 08/09/2018 revealed 6,560,565 ICSRs (n=7,288 aLQTS; n=2,769 TdP; n=4,880 sudden death) for the full database from >130 countries. Seventy-percent of ADT (7/10) had a disproportional association (ROR: 1.4–4.7, p<0.05) with aLQTS, TdP or sudden death (Table 3). Sotalol was used as positive control and had a significant disproportional association with aLQTS, TdP and sudden death (Table 3). For example, there were 9,541 ADRs reported with sotalol, and 152 cases of TdP (1.59%) as compared to 6,551,176 total ADRs on all other drugs in men, including 2,617 cases of TdP (0.04%). This results in a ROR of 40.51 for the association between sotalol and TdP (p<0.0001). Enzalutamide was associated with the highest rate of death (5,430/31,896[17%], p<0.0001; with the numerator being the number of ICSRs with a death outcome and the denominator being the overall number of ICSRs associated with enzalutamide) compared to the other ADTs used for prostate cancer (4,208/52,089[8.1%]; degarelix, abiraterone, flutamide, bicalutamide, goserelin, leuprorelin, triptorelin, Table 2) or prostatism (1,303/48,720[2.7%]; dutasteride, finasteride). Enzalutamide was associated with a total of 32 aLQTS, TDP or sudden death, and enzalutamide was almost always considered a responsible drug by the reporter (n=30/32[93.8%], Table 2).
Table 2.
Number(n) of ICSRs in men in VigiBase by androgen deprivation therapy (ADT), with sotalol (positive control) and in the entire database through 08/09/2018.
| ndeath/ntotal,(%) | aLQTS* | TdP* | Sudden death* |
n/naLQTS±TdP±Sudden-death, (%) with ADT considered suspect by reporter** |
|
|---|---|---|---|---|---|
| Enzalutamide | 5,430/31,896(17%) | 19 | 4 | 13 | 30/32(93.8%) |
| Abiraterone | 1,240/14,261(8.7%) | 19 | 7 | 10 | 29/31(92.5%) |
| Bicalutamide | 724/10,144(7.1%) | 23 | 16 | 11 | 28/41(68.3%) |
| Leuprorelin | 1,871/22,113(8.5%) | 33 | 16 | 18 | 28/55(50.9%) |
| Finasteride | 1,062/33,877(3.1%) | 52 | 20 | 32 | 20/87(23%) |
| Goserelin | 471/5,821(8.1%) | 8 | 2 | 15 | 17/22(77.3%) |
| Degarelix | 82/2,787(2.9%) | 7 | 4 | 3 | 10/11(90.9%) |
| Triptorelin | 52/1,517(3.4%) | 6 | 3 | 2 | 5/8(62.5%) |
| Dutasteride | 248/15,177(1.6%) | 26 | 7 | 11 | 5/38(13.2%) |
| Flutamide | 163/4075(4.0%) | 4 | 2 | 3 | 3/7(42.9%) |
| Sotalol | 210/9541(2.2%) | 134 | 152 | 29 | NA |
| Entire database | 161,130/6,560,565(2.5%) | 7,288 | 2,769 | 4,880 | NA |
These numbers in ADT and sotalol rows correspond to the A values in the contingency table displayed in supplemental Table 2, explaining how ROR (=AD/BC) is calculated. For example, there were 9,541 ADRs reported with sotalol, and 152 cases of TdP (1.59%) as compared to 6,551,176 total ADRs on all other drugs in men, including 2,617 cases of TdP (0.04%). This results in a ROR of 40.51 for the association between sotalol and TdP (see Table 3).
The numerator is the number of ICSRs where the ADT was considered by the reporter to be suspect of directly inducing the aLQTS or TdP or sudden death. The denominator is the number of ICSRs where the ADT was associated to a drug-induced aLQTS or TdP or sudden death, in which the reporter may have considered the ADT as suspect, interacting or concomitant.
Table 3.
Association of androgen deprivation therapies (ADT) with the reporting-Odds-Ratio (ROR) for aLQTS, TdP and sudden death in VigiBase (through 08/09/2018).
| ROR[CI95] | |||
|---|---|---|---|
| aLQTS | TdP | Sudden death | |
| Enzalutamide | (−) | (−) | (−) |
| Abiraterone | (−) | (−) | (−) |
| Bicalutamide | 2.1[1.4-3.1] | 3.8[2.3-6.1] | (−) |
| Leuprorelin | (−) | 1.7 [1.1-2.8] | (−) |
| Finasteride | 1.4[1.1-1.8] | (−) | (−) |
| Goserelin | (−) | (−) | 3.5[2.1-5.8] |
| Degarelix | 2.3[1.1-4.8] | 3.4[1.3-9.1] | (−) |
| Triptorelin | 3.6[1.6-8] | 4.7[1.5-14.6] | (−) |
| Dutasteride | 1.6[1.1-2.3] | (−) | (−) |
| Flutamide | (−) | (−) | (−) |
| Sotalol | 13.03[10.97-15.48] | 40.51[34.36-47.77] | 4.11[2.85-5.93] |
Significantly (p<0.05) increased ROR and their 95% confidence-interval [CI95] when comparing reporting-rate for aLQTS, TdP and sudden death associated with sotalol (positive control) or androgen deprivation therapies versus the full database. Association not significantly increased are displayed by (−).
Androgen effects on ventricular repolarization
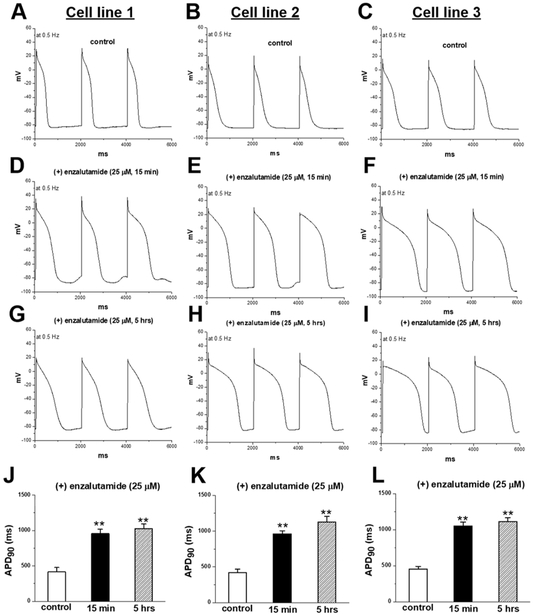
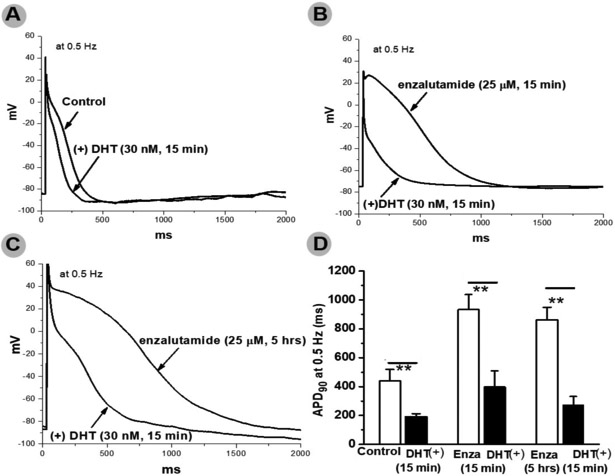
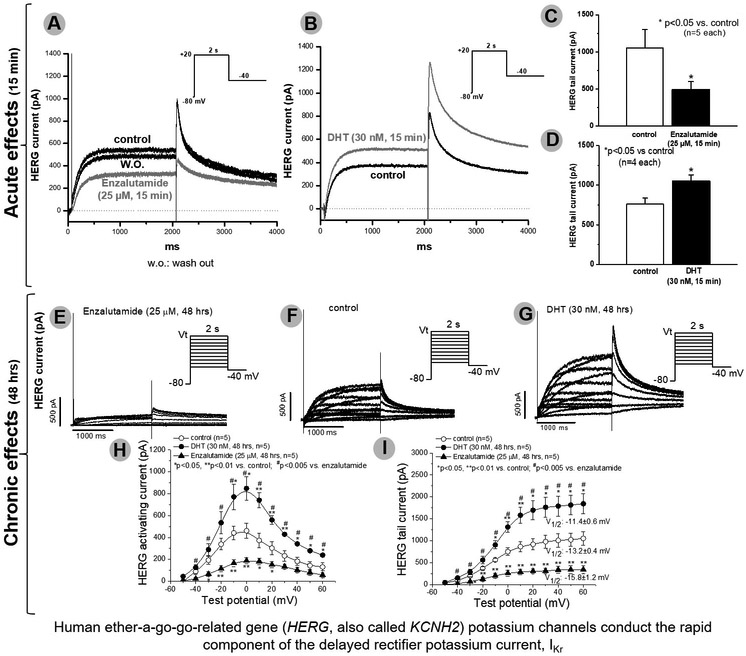
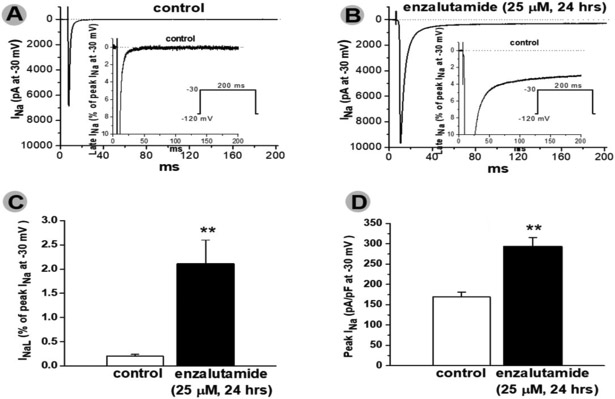
In these experiments, we studied enzalutamide, the ADT most strongly associated with death. In IPSC-CMs from 3 male subjects, acute and chronic exposure to enzalutamide prolonged APD90 recorded during stimulation at 0.5Hz from 429.7±27.1 (control) to 982.4±33.2 (acute, p<0.001) and 1062.3±28.9 msec (chronic, p<0.001), and early-/delayed-afterdepolarizations and/or triggered activity were elicited in enzalutamide treated cells (11/20 acutely and 8/15 chronically vs. 0/15 in non-treated cells, p=0.001, Figure 2). Acute dihydrotestosterone exposure reversed APD90 prolongation observed on acute and chronic enzalutamide exposure at a stimulation of 0.5Hz from 933±105msec (acute enzalutamide) to 397±113msec (dihydrotestosterone acute, p<0.01) and 863±86msec (chronic enzalutamide) to 275±57msec (dihydrotestosterone acute, p<0.01) (Figure 3). Acute dihydrotestosterone exposure in non-enzalutamide-treated cells also shortened APD90 from 439.5±79.2 msec to 189.2± 23.3 msec (p<0.01) (Figure 3). In CHO-cells transfected with KCNH2, acute and chronic exposure to enzalutamide decreased tail current measured after pulses to +20 mV from 1050±253 at baseline to 492±108 (acute, p<0.01) and 307±57 pA (chronic, p<0.01, Figure 4). Conversely, exposure to dihydrotestosterone increased tail current from baseline 760±77 to 1051±79 (acute, p<0.05) and 1698±218 pA (chronic, p<0.01, Figure 4). In IPSC-CMs, chronic exposure to enzalutamide significantly increased both peak INa from 169±12 to 293±23 pA/pF, (p<0.01) and INa-L from 0.2±0.04% to 2.1±0.5% of peak current, (p<0.01, Figure 5). In CHO-cells transfected with SCN5A, similar results were seen (Supplementary Figure 1). Figure 2 and 5 summarizes acute and chronic effects of enzalutamide on APD90 (2.A-D), and peak INa/INa-L (5.A-D) of IPSC-CMs. Figure 3 summarizes acute dihydrotestosterone effects on APD90 of IPSC-CMs from men subjects already exposed acutely or chronically on enzalutamide. Figure 4 summarizes acute and chronic effects of dihydrotestosterone (B,D,G,H,I) and enzalutamide (A,C,E,H,I) on IKr in CHO-cells.
Fig.2.
In cardiomyocytes derived from induced pluripotent stem cells from 3 different male lines (A,B,C), acute (D,E,F) and chronic (G,H,I) exposure to enzalutamide prolonged action potential duration (J,K,L). **p<0.01 vs. control (n=5-7 each)
Fig.3.
Panels A-C are typical action potential traces in three groups of men cardiomyocytes derived from induced pluripotent stem cells in the absence and presence of dihydrotestosterone (DHT). Panel D is the summary of these groups of cells. Acute and chronic enzalutamide (Enza) prolonged action potentials whereas DHT had opposite effect. **p<0.01 (n=4-5 each)
Fig.4.
In Chinese-hamster-ovary cells, acute (A-D) and chronic (E-J) exposure to enzalutamide (A,C,E,H,I) decreased IKr. Conversely, exposure to dihydrotestosterone (DHT) increased IKr (B,D,G,H,I). A p-value <0.005 was deemed significant to account for multiple comparisons (Bonferroni adjustment; H,I). *p<0.05 vs. control, **p<0.01 vs. control, # p<0.005 vs. enzalutamide (n=5 each)
Fig.5.
Chronic exposure to enzalutamide increased ventricular peak (INa) and late (INa-L) sodium current in cardiomyocytes derived from induced pluripotent stem cells (A-D). **p<0.01 vs. control (n=4 each)
Discussion
Taken together, our analysis by multiple translational approaches consistently supports the concept that ADT is a cause of aLQTS and TdP. The in vitro work here provides further support for this concept and specifically for the idea that treatment of hypogonadism by testosterone replacement therapy can shorten QTc duration and treat and/or prevent TdP. 9-11, 13, 14 These results provide a strong justification for a clinical recommendation to systematically investigate the possibility of hypogonadism and ADT intake when men are evaluated for aLQTS or TdP and suggest electrocardiographic monitoring may have a place in the surveillance of men with known hypogonadism or when treated with ADT.
The findings in the experiments do provide insights into mechanisms whereby enzalutamide, a competitive androgen antagonist, and dihydrotestosterone modulate APD90.26 Dihydrotestosterone shortened APD acutely and this appears to be related to IKr enhancement. Conversely, enzalutamide prolonged APD both acutely and chronically. The acute effect likely reflects IKr blockade, while late INa-L enhancement may contribute chronically. This dual time-dependence has also been seen with other potent QT prolonging drugs causing TdP such as dofetilide27 and terfenadine.28 The electrocardiographic effects (data not shown) observed in a case-series of 7 men with aLQTS/TdP associated to androgen deficiency (decreased T-wave maximal amplitude and notching) are also consistent with a predominant role for IKr block.14 Gagliano-Juca et al. recently showed in a 6 month prospective cohort study that ADT shortened QRS and prolonged QTc in men with prostate cancer starting ADT versus a control group of men who previously underwent prostatectomy for cancer and were not receiving ADT.15 The clinical finding of shortened QRS is consistent with the increase in peak INa we observe hereafter chronic exposure to enzalutamide. Other groups have studied effects of androgens in vitro on multiple systems and with heterogeneous and discordant results that have been summarized recently.21 In this study, we used physiological doses of dihydrotestosterone as well as therapeutic concentration of enzalutamide. Enzalutamide is a second generation androgen receptor antagonist active in prostate cancer that has become resistant to first generation androgen receptor antagonists (flutamide, bicalutamide, nilutamide),29, 30 which are weaker blockers of the androgen receptor.31 We selected enzalutamide for in vitro experiments since the pharmacovigilance signal was the largest in terms of absolute numbers of suspected ADR associated with aLQTS, TdP, sudden death and death. Of note, the association of enzalutamide with death was strikingly higher (17% of total ICSRs) than that for other ADT (1.6–8.7%) or entire database (2.5%); this may result in a competition (termed “masking bias”) between sudden death and aLQTS/TdP, accounting for the absence of a positive ROR between enzalutamide and aLQTS, TdP or sudden death.32
ADT are the cornerstone of treatments for prostate cancer or adenoma, and may be also used for androgenic alopecia in younger men. There is no mention in latest European Society of Cardiology and American Heart Association position papers on cancer treatments and cardiovascular toxicity that ADT use might lead to aLQTS and no specific caution is recommended.33, 34 Degarelix and leuprolide are the only ADT considered at possible risk for TdP according to the reference website CredibleMeds.org which presents TdP risk classification of drugs.24 Guidelines will need to be developed to appropriately monitor and manage this risk, particularly knowing that other anticancer and non-cancer related drugs used in combination carry additional TdP risk.1, 35 Interestingly, all classes of ADT, even those with mild ADT effects (5α-reductase inhibitors),36 appeared to be associated with ventricular arrhythmic events. The risk of TdP with 5α-reductase inhibitors is particularly noteworthy since these drugs are indicated in benign conditions, including prostatism and androgenic alopecia.36
Importantly, this study supports the growing concept that IPSC-CMs can contribute to the understanding and possible novel management of clinical conditions such as drug-induced diseases and to move further toward personalized medicine. This concept is just beginning to receive initial support from translational studies such as this work, incorporating a clinical part and an experimental one, using IPSC-CMs. For example, using a similar strategy, a recent study showed that Lumacaftor + Ivacaftor, drugs developed to improve cell surface trafficking of mutant proteins in cystic fibrosis, significantly shortened the QTc in two LQTS type 2 patients with a trafficking defect; these in vivo findings supported in vitro data suggesting improved trafficking of mutant KCNH2 in iPSC-CMs.37
A limitation of the analyses of the pharmacovigilance databases is that the data come from uncontrolled sources. Nevertheless, the preclinical mechanistic studies, the case series,14 the literature,21 and the population analyses of ADT and of the positive control sotalol provide cross validation for the causal – and treatable – relationship we postulate between male hypogonadism, either due to endocrine conditions or ADT and TdP risk. Further mechanistic studies are needed to better decipher mechanisms downstream of androgen receptor pathway leading to IKr and INa/INa-L modulation, but these studies were beyond the objective of this work aiming at raising awareness concerning ADT use and TdP risk, as well as potential therapeutic use of testosterone for aLQTS and TdP.
Conclusion
Cautious prescription and electrocardiogram monitoring should be considered in men on ADT, particularly when at risk of TdP. Androgens might be useful to prevent or treat TdP in men.
Supplementary Material
Clinical perspective.
What is new?
Men receiving androgen deprivation therapy are at increased risk for drug-induced QT-prolongation and Torsade de Pointes.
This study supports the growing concept that cardiomyocytes derived from induced pluripotent stem cells can be useful tool to better understand mechanisms underlying diseases and responses to drugs.
What are the clinical implications?
In men developing acquired long-QT syndrome or Torsade de Pointes, diagnostic work-up should include evaluation of testosterone blood level, androgen deprivation therapy intake, and evaluation for endocrine conditions associated with hypogonadism.
In men treated with androgen deprivation therapy, other risk factors for Torsade de Pointes should be sought and corrected, to avoid accumulation of risks.
In men treated with androgen deprivation therapy, the role of electrocardiographic monitoring to detect QT-prolongation requires further evaluation.
Acknowledgements:
Collaborators: Vanderbilt University Medical Center (Roden lab): M. Blair, H. Lynn, L. Short, C. Ingram, T. Strickland.
Sources of Funding: This study was supported by The Cancer ITMO of the French National Alliance for Life and Health Sciences (AVIESAN): “Plan Cancer 2014–2019“. This study was supported by grant P50 GM115305, R56 HL141466, and by a grant from the Leducq Foundation for Cardiovascular Research.
Footnotes
Disclosures: JJM has served on advisory boards at Bristol Myers, Pfizer, Novartis and Regeneron and has received research funding from Pfizer and Bristol Myers Squibb. The other authors have no conflict of interest to disclose. The supplied data from VigiBase come from a variety of sources. The likelihood of a causal relationship is not the same in all reports. The information does not represent the opinion of the World Health Organization (WHO)..
References.
- 1.Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. [DOI] [PubMed] [Google Scholar]
- 2.Malfatto G, Beria G, Sala S, Bonazzi O and Schwartz PJ. Quantitative analysis of T wave abnormalities and their prognostic implications in the idiopathic long QT syndrome. J Am Coll Cardiol. 1994;23:296–301. [DOI] [PubMed] [Google Scholar]
- 3.Yang T, Snyders D and Roden DM. Drug block of I(kr): model systems and relevance to human arrhythmias. J Cardiovasc Pharmacol. 2001;38:737–744. [DOI] [PubMed] [Google Scholar]
- 4.Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S and Horie M. Exercise stress test amplifies genotype-phenotype correlation in the LQT1 and LQT2 forms of the long-QT syndrome. Circulation. 2003;107:838–844. [DOI] [PubMed] [Google Scholar]
- 5.Graff C, Struijk JJ, Matz J, Kanters JK, Andersen MP, Nielsen J and Toft E. Covariate analysis of QTc and T-wave morphology: new possibilities in the evaluation of drugs that affect cardiac repolarization. Clin Pharmacol Ther. 2010;88:88–94. [DOI] [PubMed] [Google Scholar]
- 6.Salem JE, Germain M, Hulot JS, Voiriot P, Lebourgeois B, Waldura J, Tregouet DA, Charbit B and Funck-Brentano C. GENomE wide analysis of sotalol-induced IKr inhibition during ventricular REPOLarization, “GENEREPOL study”: Lack of common variants with large effect sizes. PLoS One. 2017;12:e0181875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C and Roden DM. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014;130:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makkar RR, Fromm BS, Steinman RT, Meissner MD and Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. [DOI] [PubMed] [Google Scholar]
- 9.Charbit B, Christin-Maitre S, Demolis JL, Soustre E, Young J and Funck-Brentano C. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol. 2009;103:887–890. [DOI] [PubMed] [Google Scholar]
- 10.Abehsira G, Bachelot A, Badilini F, Koehl L, Lebot M, Favet C, Touraine P, Funck-Brentano C and Salem JE. Complex Influence of Gonadotropins and Sex Steroid Hormones on QT Interval Duration. J Clin Endocrinol Metab. 2016;101:2776–2784. [DOI] [PubMed] [Google Scholar]
- 11.Salem JE, Alexandre J, Bachelot A and Funck-Brentano C. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther. 2016;167:38–47. [DOI] [PubMed] [Google Scholar]
- 12.Salem JE, Dureau P, Bachelot A, Germain M, Voiriot P, Lebourgeois B, Tregouet DA, Hulot JS and Funck-Brentano C. Association of Oral Contraceptives With Drug-Induced QT Interval Prolongation in Healthy Nonmenopausal Women. JAMA Cardiol. 2018;3:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliano-Juca T, Icli TB, Pencina KM, Li Z, Tapper J, Huang G, Travison TG, Tsitouras P, Harman SM, Storer TW, Bhasin S and Basaria S. Effects of Testosterone Replacement on Electrocardiographic Parameters in Men: Findings From Two Randomized Trials. J Clin Endocrinol Metab. 2017;102:1478–1485. [DOI] [PubMed] [Google Scholar]
- 14.Salem JE, Waintraub X, Courtillot C, Shaffer CM, Gandjbakhch E, Maupain C, Moslehi JJ, Badilini F, Haroche J, Gougis P, Fressart V, Glazer AM, Hidden-Lucet F, Touraine P, Lebrun-Vignes B, Roden DM, Bachelot A and Funck-Brentano C. Hypogonadism as a Reversible Cause of Torsades de Pointes in Men. Circulation. 2018;138:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagliano-Juca T, Travison TG, Kantoff PW, Nguyen PL, Taplin ME, Kibel AS, Huang G, Bearup R, Schram H, Manley R, Beleva YM, Edwards RR and Basaria S. Androgen Deprivation Therapy Is Associated With Prolongation of QTc Interval in Men With Prostate Cancer. J Endocr Soc. 2018;2:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K, Morishita T, Miyanaga D, Hisazaki K, Kaseno K, Miyazaki S, Uzui H, Ohno S, Horie M and Tada H. Medical Castration is a Rare but Possible Trigger of Torsade de Pointes and Ventricular Fibrillation. Int Heart J. 2019;60:193–198. [DOI] [PubMed] [Google Scholar]
- 17.Lindquist M VigiBase, the WHO Global ICSR Database System: Basic Facts. Drug Information Journal. 2008;42:409–419. [Google Scholar]
- 18.Norén GN, Orre R and Bate A. A hit-miss model for duplicate detection in the WHO drug safety database. KDD ‘05 Proceedings of the eleventh ACM SIGKDD international conference on Knowledge discovery in data mining 2005:459. doi: 10.1145/1081870.1081923. [DOI] [Google Scholar]
- 19.Grouthier V, Lebrun-Vignes B, Glazer AM, Touraine P, Funck-Brentano C, Pariente A, Courtillot C, Bachelot A, Roden DM, Moslehi JJ and Salem JE. Increased long QT and torsade de pointes reporting on tamoxifen compared with aromatase inhibitors. Heart. 2018;104:1859–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bruin ML, Pettersson M, Meyboom RH, Hoes AW and Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–597. [DOI] [PubMed] [Google Scholar]
- 21.Barber M, Nguyen LS, Wassermann J, Spano JP, Funck-Brentano C and Salem JE. Cardiac arrhythmia considerations of hormone cancer therapies. Cardiovasc Res. 2019;115:878–894. [DOI] [PubMed] [Google Scholar]
- 22.Lopes RA, Neves KB, Carneiro FS and Tostes RC. Testosterone and vascular function in aging. Front Physiol. 2012;3:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbons JA, Ouatas T, Krauwinkel W, Ohtsu Y, van der Walt JS, Beddo V, de Vries M and Mordenti J. Clinical Pharmacokinetic Studies of Enzalutamide. Clin Pharmacokinet. 2015;54:1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woosley RL, Heise CW, Gallo T, Tate J, Woosley D and Romero KA, www.CredibleMeds.org, QTdrugs List, [Accession Date], AZCERT, Inc; 1822. Innovation Park Dr., Oro Valley, AZ: 85755 [Google Scholar]
- 25.Khan A and Kneale B. Life threatening torsades de pointes due to abiraterone-induced hypokaelemia in a patient with metastatic prostate cancer. N Z Med J. 2016;129:124–127. [PubMed] [Google Scholar]
- 26.Shaw J, Leveridge M, Norling C, Karen J, Molina DM, O’Neill D, Dowling JE, Davey P, Cowan S, Dabrowski M, Main M and Gianni D. Determining direct binders of the Androgen Receptor using a high-throughput Cellular Thermal Shift Assay. Sci Rep. 2018;8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang T, Meoli DF, Moslehi J and Roden DM. Inhibition of the alpha-Subunit of Phosphoinositide 3-Kinase in Heart Increases Late Sodium Current and Is Arrhythmogenic. J Pharmacol Exp Ther. 2018;365:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS and Lin RZ. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med. 2012;4:131ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B and Investigators P. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, Ivashchenko P, Demirhan E, Modelska K, Phung, Krivoshik A and Sternberg CN. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018;378:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schalken J and Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016;117:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juhlin K, Ye X, Star K and Noren GN. Outlier removal to uncover patterns in adverse drug reaction surveillance - a simple unmasking strategy. Pharmacoepidemiol Drug Saf. 2013;22:1119–1129. [DOI] [PubMed] [Google Scholar]
- 33.Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, Milani RV, Sagalowsky AI, Smith MR, Zakai N, American Heart Association Council on Clinical C, Council on E, Prevention tACS and the American Urological A. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM, Authors/Task Force M and Guidelines ESCCfP. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 35.Alexandre J, Moslehi J, Bersell KR, Funck-Brentano C, Roden DM and Salem JE. Anticancer drug-induced cardiac rhythm disorders: Current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018. 189:89–103. [DOI] [PubMed] [Google Scholar]
- 36.Rittmaster RS. Finasteride. N Engl J Med. 1994;330:120–125. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz PJ, Gnecchi M, Dagradi F, Castelletti S, Parati G, Spazzolini C, Sala L and Crotti L. From patient-specific induced pluripotent stem cells to clinical translation in long QT syndrome Type 2. Eur Heart J. 2019. doi: 10.1093/eurheartj/ehz023. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.