Abstract
Background and purpose
Stroke patients with type two diabetes mellitus (T2DM) exhibit increased vascular and white matter (WM) damage, and have worse prognosis compared to non-diabetic stroke patients. We investigated the neurorestorative effects of exosomes derived from mouse brain endothelial cells (EC-Exo) as treatment for stroke in T2DM mice, and investigated the role of miR-126 in mediating EC-Exo derived therapeutic benefits in T2DM-stroke mice.
Methods
Adult, male BKS.Cg-m+/+Leprdb/J (T2DM) mice were subjected to photothrombotic stroke model. T2DM mice were intravenously injected at 3 days after stroke with: 1) Phosphate-buffered saline (PBS); 2) Liposome mimic (vehicle control, 3×1010); 3) EC-Exo (3×1010); 4) Knockdown of miR-126 in EC-Exo (miR-126−/−EC-Exo, 3×1010). Behavioral and cognitive tests were performed and mice were sacrificed at 28 days after stroke.
Results
Compared to non-DM stroke mice, T2DM-stroke mice exhibit significantly decreased serum and brain tissue miR-126 expression. Endothelial cells and EC-Exo contain high levels of miR-126 compared to other cell types or exosomes derived from other types of cells, respectively (smooth muscle cells, astrocytes and marrow stromal cells). Compared to PBS or liposome mimic treatment, EC-Exo treatment of T2DM-stroke mice significantly improves neurological and cognitive function, increases axon density, myelin density, vascular density, arterial diameter, as well as induces M2 macrophage polarization in the ischemic boundary zone. MiR-126−/−EC-Exo treatment significantly decreases miR-126 expression in serum and brain, as well as attentuates EC-Exo treatment induced functional improvement, and does not significantly increase axon and myelin density, vascular density, arterial diameter or induce M2 macrophage polarization in T2DM-stroke mice. In-vitro, EC-Exo treatment significantly increases primary cortical neuron axonal outgrowth and increases endothelial capillary tube formation while, miR-126−/−EC-Exo attentuates EC-Exo induced capillary tube formation and axonal outgrowth.
Conclusions
EC-Exo treatment of stroke promotes neurorestorative effects in T2DM mice. MiR-126 may mediate EC-Exo induced neurorestorative effects in T2DM mice.
Keywords: Endothelial cells, exosomes, microRNA-126, neurorestoration, stroke, Type 2 diabetes mellitus, vascular remodeling, white matter remodeling, Basic Science Research, Cell Therapy, Ischemia, Diabetes, Type 2
Introduction
Ischemic stroke patients with diabetes mellitus exhibit distinct clinical pattern and worse neurovascular prognosis compared to non diabetic stroke patients1. Diabetes alters metabolism, affects vasculature and increases inflammation resulting in complicated stroke pathology and aggravated vascular and white matter (WM) damage after stroke2. There is a compelling need to develop therapeutic approaches specifically designed to reduce neurological deficits after stroke in the diabetic population.
Exosomes are small lipid microvesicles between 30–150nm in diameter, that facilitate inter-cellular communication and cell-specific transfer of microRNAs (miRs)3. Exosomes have bi-lipid membranes which protect their biologically active cargo. Exosomes administrated intravenously provide therapeutic benefit at least equivalent to their cellular source, and carry low risk of vascular obstruction, immunogenicity and tumor formation3, 4. Therefore, exosomes can circumvent limitations of cell based therapy and are a promising therapeutic approach to treat diabetic stroke.
MiR-126 is an angiogenic miR and is primarily expressed in endothelial cells (EC)5. Generally, and throughout this manuscript, miR-126 refers to miR-126–3p, while miR-126–5p is referred to as miR-126*. Disruption of miR-126 causes a loss of vascular integrity resulting in a phenotype containing leaky and hemorrhagic vessels6. Type 2 diabetes mellitus (T2DM) patients have lower miR-126 levels in the serum compared to healthy subjects7. In T2DM, reduction of miR-126 contributes to the development of microvascular and macrovascular complications8, 9. In addition to vascular changes, miR-126 also modulates inflammation, and increasing miR-126 after spinal cord injury inhibits leukocyte extravasation into the injured tissue, promotes angiogenesis, and improves functional outcome10. In this study, we investigated the neurorestorative effects of EC-Exo as a treatment of stroke in T2DM mice. We hypothesize that increasing miR-126 by EC-Exo treatment contributes to their neurorestorative effects in T2DM-stroke mice.
Materials and methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and with the approval of Institutional Animal Care and Use Committee of Henry Ford Health System. This manuscript was prepared in accordance with ARRIVE guidelines. The authors declare that all supporting data are available within the article.
Bone marrow stromal cell (MSC), smooth muscle cells (SMC), mouse brain ECs and astrocytes cell culture
Bone marrow was isolated and cultured in MSC growth media (Thermo Scientific HyClone; Classical liquid; MEM Alpha; with L-Glutamine and ribo/deoxyribo-nucleosides; 20% fetal bovine serum and 1% antibiotic/antimycotic) as previously described11. MSC were used within 4 passages. SMC (ATCC, catalog #CRL-1476), mouse brain ECs (ATCC, catalog #CRL-2299), and astrocytes (ATCC catalog #CRL-2541), were cultured in Dulbecco modified Eagle medium (DMEM, Life Technologies) with 10% FBS (fetal bovine serum) and 1% antibiotic/antimycotic (Life Technologies) following the manufacturer`s protocols. To obtain miR-126−/−EC-Exo, knockdown of miR-126 in EC (mouse mmu-miR-126–3p inhibitor, miR-126−/−EC (GE Dharmacon), 5 nmol/ml of a 20 μM stock solution) Lonza electroporation transfection system was used following standard protocol for the Amaxa Nucleofection machine (Lonza)12. MSCs, SMCs, astrocytes, brain ECs and miR-126−/−ECs were cultured up to 3 passages, followed by culture in exosome depleted FBS media (Systembio) for 3 days. The media was collected to isolate exosomes.
Exosome isolation and Liposome generation
Mouse brain ECs were cultured as described above. Exosomes were isolated using ExoQuick-TC (Exosome Precipitation Solution kit, System Biosciences) following previously described protocol12, and suspended in PBS. EC-Exo are from non-diabetic condition. Briefly, media was isolated from a 100% confluent T75 tissue culture flask (containing ~5–6 million cells) containing 10 ml of media with exosome free FBS. This media was centrifuged and filtered via a 0.22 μm syringe filter before adding ExoQuickTC (2 ml for 10 ml media). Exosome number was counted using the IZON qNano device (Izon). To decrease the heterogeneity of Exo treatments, we tightly controlled the cell culture conditions, such as passage, density, and culture time. Liposomes are synthetic versions of exosomes mimicking the exosomal lipid layer and were generated using thin-film hydration technique as previously described13.
MiR-126 measurement
Samples were lysed in Qiazol reagents and the total RNA was isolated using miRNeasy Mini kit (Qiagen). Briefly, miRNAs were reverse transcribed with the miRNA Reverse Transcription kit (Thermo Fisher Scientific) and PCR amplification was performed with the TaqMan miRNA assay kit (hsa-miR-126–3p, Thermo Fisher Scientific, catalog #4427975, which is specific for mature miRNA sequences) according to the manufacturer’s protocols, with U6 snRNA as an internal control12. For the qPCR reactions, we used 2 μl of isolated RNA per cDNA reaction, and then used 3 μl of cDNA for each PCR reaction.
Experimental animals and groups
Adult (3–4 months old) male BKS.Cg-m+/+Leprdb/J (db/db-T2DM) and non-diabetic control (db+) mice were purchased from Jackson Laboratory. Fasting blood glucose was tested (AgaMatrix Advanced blood glucose monitoring system). Since a majority of stroke patients can be treated at 3 days after stroke, treatments were initiated at 3 days after stroke. T2DM-stroke mice (n=10/group, sample size after early mortality is indicated for each group in parentheses) were randomized to different groups, and treated intravenously at 3 days after stroke with: 1) PBS (0.2 ml) control (n=7); 2) Liposome mimic as vehicle control: equal volumes of PBS containing an equal number of liposomes consisting of the equivalent lipid/phospholipid content of the exosome (n=7); 3) EC-Exo (3×1010) (n=6); 4) miR-126−/−EC-Exo (3×1010) (n=6). T2DM wild-type naive animals (n=5) were included as reference to test effects/severity of stroke model.
Photothrombotic stroke model
Mice were anesthetized with isoflurane mixed in 70% N2O and 30% O2, fixed on the stereotaxic instrument and rectal temperature was maintained at 37°C with a feedback regulated water heating system. A skin incision was made and a roundabout rubber was placed on the skull surface over the sensorimotor area (1.5 to 3 mm lateral; 0.5 to 1 mm anterior of bregma). A photosensitive dye (Rose Bengal, 40 mg/kg) mixed with saline (4 ml/kg) was injected (i.p). After 20 minutes of cold light exposure, the activated dye induces endothelial damage with platelet activation and thrombosis, resulting in local blood flow interruption14.
EC-Exo labeling
To test whether EC-Exo passes BBB and is internalized by endogenous brain cells, 3×1010 EC-Exo labeled using PKH26 Fluorescent Cell Linker Kit (Sigma, as per manufacturer’s instructions) were injected via tail vein at 3 days after stroke in non-DM mice (n=8). Mice were sacrificed at 24 hours after exosome injection. Using a vibratome, 80 μm thick coronal sections were obtained for immunofluorescence staining (all incubations were for 4 days at 4°C) with NeuN (neuron marker, 1:50, Millipore), Von Willebrand Factor (vWF, EC marker, 1:400, Dako), GFAP (astrocyte marker, 1:5000, Dako), ED1 (macrophage marker, 1:30, BioRad), IBA1 (microglia marker, 1:1000, Wako), APC (oligodendrocyte marker, 1:20, Genway), CD9 (exosome marker, 1:500, Abcam), and CD63 (exosome marker, 1:1000, Abcam) and imaged using a laser-scanning confocal microscope (Zeiss LSM 510 NLO, Carl Zeiss). DAPI counterstain was employed to identify nuclei.
Evaluation of neurological and cognitive function
All functional tests were performed by an investigator who was blinded to the experimental groups. To evaluate somatosensory function, adhesive removal test was performed on days 1, 7, 14, 21 and 28 days after stroke15. To evaluate cognitive outcome and memory, novel odor recognition test was performed on days 25–27 after stroke. The detailed methods for odor test have been previously described16.
Immunohistochemistry
Mice were sacrificed at 28 days after stroke and transcardially perfused with 0.9% saline and immersion fixed in 4% paraformaldehyde. Seven equally spaced (1 mm) brain coronal sections were processed. Hematoxylin and eosin (H&E) staining was employed for lesion volume measurement. The percentage of the infarction volume compared with the contralateral hemisphere was presented17. Brain coronal tissue sections (6 μm) were prepared and antibody against vWF (1:400; Dako), α-smooth muscle actin (α-SMA, smooth muscle cell marker, mouse monoclonal IgG 1:800, Dako), ED1 (1:30; AbDSerotec), and CD163 (M2 macrophage marker, 1:500, Abcam) were used. Bielschowsky silver (BS) and Luxol fast blue (LFB) staining was used to stain axons and myelin, respectively. Control experiments consisted of similar procedures without addition of primary antibody.
Immunostaining quantification
Five slides from each brain, 4 fields of view within each region- striatum, cortex and corpus callosum of the ischemic boundary zone (IBZ) (or comparable region for non-stroke T2DM-control mice) were digitized under a 20× objective (Olympus BX40) using a 3-CCD color video camera with an MCID image analysis system (Imaging Research). All quantification analysis was performed using MCID image analysis system by an investigator who was blinded to the experimental groups. For BS and LFB immunostaining, data are quantified as density of positive immunolabeling using built-in densitometry function in MCID. The total number of vWF labeled vessels in each field of view was counted; data were averaged to obtain a single value for each animal, and presented as number of vessels per unit area (mm2). Ten α-SMA positively labeled large arteries in the IBZ were selected and lumen diameter (μm) measured along long axis. The number of ED1 and CD163 positive cells was counted in each field of view; data were averaged and presented as number/unit area (mm2).
FITC-dextran perfusion
Additional sets of T2DM-stroke mice treated with PBS or EC-Exo (n=5/group) were injected with fluorescein isothiocyanate (FITC)-dextran (FD2000S, Sigma; 5 mg/mice in 0.2 ml PBS, i.v) at 14 days after stroke 5 minutes before sacrifice18. Brains were immersion fixed in 4% paraformaldehyde for 48 hours in 4°C and 80 μm thick coronal sections obtained using a vibratome. Whole brain sections were imaged under a 10× objective using a laser-scanning confocal microscope (Zeiss LSM 510 NLO, Carl Zeiss), and quantified using a built-in densitometry function in MCID image analysis system.
EC capillary tube formation assay
ECs were subjected to oxygen-glucose deprivation (OGD) in a hypoxia chamber (Forma Anaerobic System, Thermo Scientific) with 37°C incubator for 2 hours. Then, the cells were removed and cultured in high glucose (37.5mmol/l glucose) DMEM media with 10% FBS (Life Technologies) and treated with (30,000 cells/well, n=4 wells/group): 1) Control; 2) +EC-Exo (20 ng/ml); and 3) +miR-126−/−EC-Exo (20 ng/ml). Matrigel (0.1 ml) was added in a 96 well plate. After the matrigel gelled, mouse brain ECs suspended in serum free DMEM were added and incubated for 5 hours. Matrigel wells were digitized under a 10× objective and tracks of ECs were measured and the total length of capillary tube like formation was quantified using MCID image analysis system19.
Primary cortical neurons (PCN) axon outgrowth assay
PCNs were obtained from pregnant (day 18) embryonic Wistar rats (Charles River) and cultured with Neural basal-A medium (GIBCO) containing 2% B27 medium-supplement in vitro2, 15. To separate axons from neuronal soma, a microfluidic chamber (Standard Neuron Device; #SND450, Xona Microfluidics) was used20. PCNs were cultured until they were at 15–20×106 cells/ml, and then 10μl was placed in each microfluidic chamber. Cells were subject to 2 hour OGD and then cultured in high glucose media (37.5 mmol/l glucose) for 3 days and treated with 1) Control; 2) +EC-Exo (20 ng); and 3) +miR-126−/−EC-Exo (20 ng); n=2 chambers/group. pNFH (mature axon marker) positive axons were photographed at 20× magnification to obtain 11 fields of view using a video camera interfaced with MCID image analysis system. The average length of axonal outgrowth of pNFH positive cells was measured using Image J.
Statistical analysis
One-way Analysis of Variance was employed for the evaluation of functional outcome and histology, respectively. “Contrast/estimate” statement was used to test the group difference. If an overall treatment group effect was detected at p<0.05, pair-wise comparisons were made. All data are presented as mean ± standard error (SE).
Results
MiR-126 expression is significantly decreased in T2DM-stroke mice
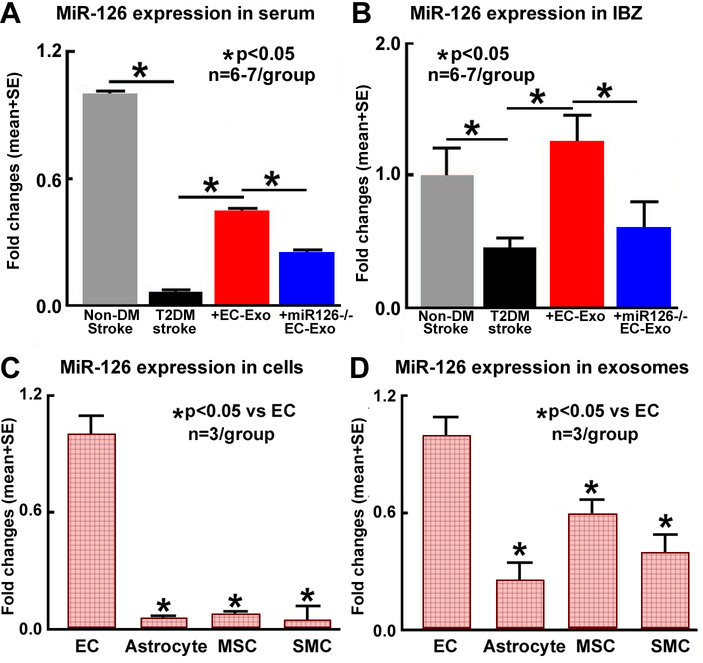
MiR-126 expression was measured in brain and serum samples of non-DM-stroke and T2DM-stroke mice treated with or without EC-Exo. Figure 1A–B show that miR-126 expression is significantly decreased in serum and ischemic brain after stroke in T2DM mice compared to non-DM-stroke mice. We also measured serum exosomal miR-126 expression in non-DM control and non-DM stroke as well as in T2DM control and T2DM-stroke mice. Supplementary Figure I shows that both T2DM as well as stroke significantly decrease miR-126 expression, with greatest reduction observed in T2DM-stroke mice. Treatment of T2DM-stroke with EC-Exo significantly increases miR-126 expression in brain and serum compared to non-treated T2DM-stroke mice.
Figure 1. MiR-126 measurement in vivo and in vitro.
(A-B) Compared to non-DM stroke mice, T2DM-stroke mice exhibit significantly decreased serum and brain tissue miR-126 expression. Treatment of T2DM-stroke mice with EC-Exo significantly increases serum and brain tissue miR-126 expression. Loss of miR-126 in EC-Exo attanuates EC-Exo induced increase in seruma nd brain miR-126 expression. (C-D) ECs and EC-Exo contain high levels of miR-126 compared to other cell types or exosomes derived from other types of cells, respectively.
EC-Exo contain high levels of miR-126, can pass the BBB, and are taken up by endogenous brain cells
Exosomes were isolated from cultured mouse brain ECs, astrocytes, MSCs, and SMCs and miR-126 expression was measured. Figure 1C–D show that EC as well as EC-Exo contain high levels of miR-126 compared to MSCs, astrocyte, or SMCs and exosome derived from MSCs, astrocyte, or SMCs, respectively.
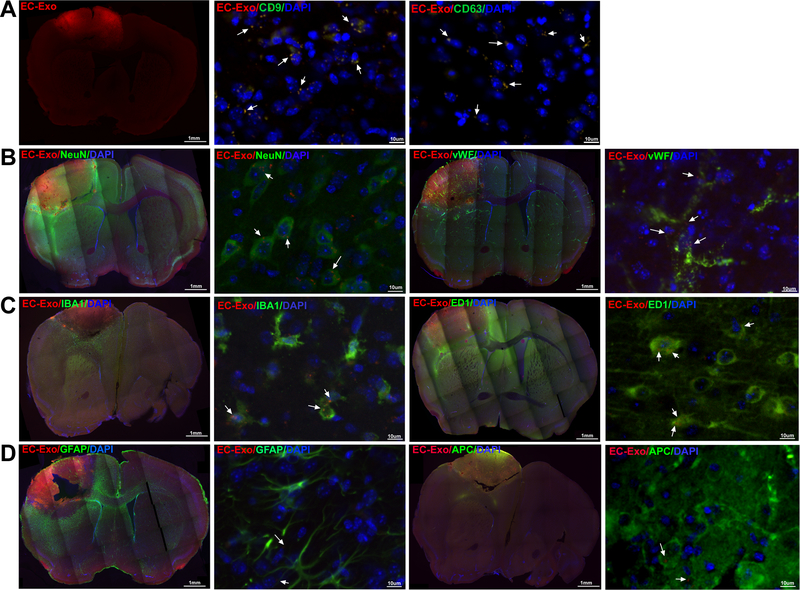
Fluorescently labeled EC-Exo were injected via tail vein at 3 days after stroke and mice were sacrificed 24 hours later. Figure 2 shows that EC-Exo pass the BBB and home to the IBZ. EC-Exo co-localize with CD9 and CD63 which are exosome markers21, thereby verifying the fluorescence as clusters of exosomes. EC-Exo are taken up by endogenous brain cells such as neurons, EC, macrophages, and microglia, while exosomes were not observed in the cell body of astrocytes and oligodendrocytes.
Figure 2. EC-Exo pass the BBB:
Intravenously injected EC-Exo (A) pass the BBB and home to the ischemic brain and co-localize with CD9 and CD63, and (B-D) are internalized by endogenous brain cells such as neurons, ECs, macrophages, microglia but were not detected in the cell body of astrocytes and oligodendrocytes.
EC-Exo treatment significantly improves neurological and cognitive functional outcome in T2DM-stroke mice
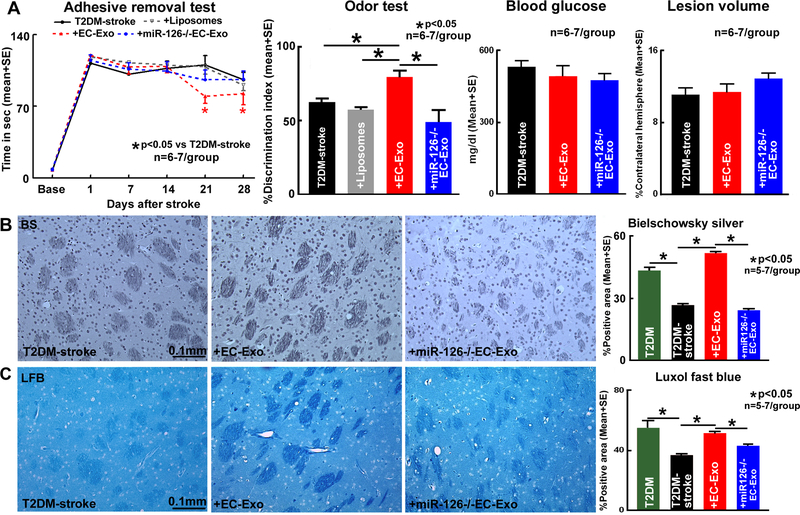
Adhesive removal test and odor test was used to evaluate neurological outcome and cognitive outcome, respectively. EC-Exo treatment in T2DM mice significantly improves neurological function and cognitive outcome compared to PBS or Liposome treated T2DM-stroke mice (Figure 3A). Since, there were no significant differences between PBS and Liposome control groups, PBS treated controls were chosen for further analysis. EC-Exo treatment in T2DM-stroke mice does not alter lesion volume or blood glucose levels compared to T2DM-stroke mice (Figure 3A).
Figure 3. EC-Exo improves neurological and cognitive outcome in T2DM-stroke mice.
(A) Compared to PBS or liposome mimic treatment, EC-Exo treatment of T2DM-stroke mice significantly improves neurological and cognitive outcome, but does not decrease lesion volume or blood glucose. Loss of miR-126 in EC-Exo attenuates EC-Exo induced improvement in stroke outcome. (B-C) Stroke in T2DM mice significantly decreases axon and myelin density in the corpus callosum and striatal WM bundles comapred to non-stroke T2DM control mice. EC-Exo treatment of T2DM-stroke mice significantly increases axon and myelin densityin the corpus callosum and striatal WM bundles in the IBZ. Loss of miR-126 in EC-Exo attenuates EC-Exo induced WM remodeling in T2DM-stroke mice. Representative images are in the striatum.
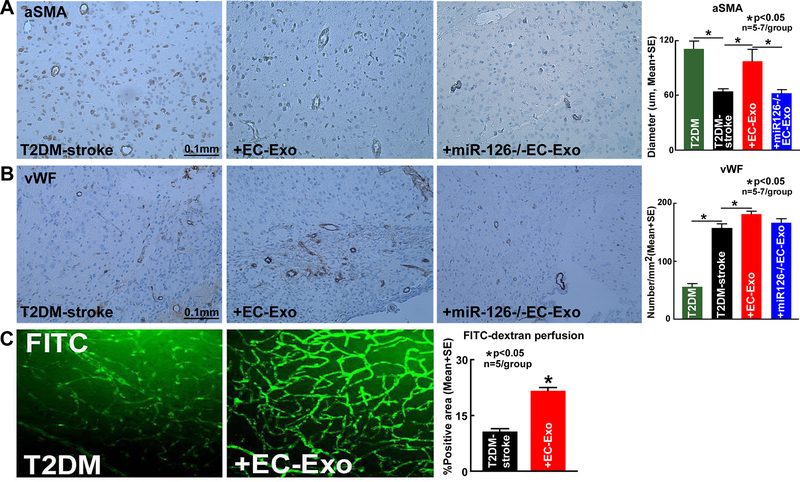
EC-Exo treatment of T2DM-stroke significantly increases axon and myelin density as well as vascular density, arterial diameter and vessel patency in the ischemic brain
Stroke in T2DM mice significantly decreases axon and myelin density in the corpus callosum and striatal WM bundles in the IBZ (Figure 3B–C), reduces average arterial diameter (Figure 4A) and increases vascular density (Figure 4B) in the cortex and striatum region of the IBZ compared to uninjured brain regions of naïve T2DM mice. EC-Exo treatment significantly increases axon density (Figure 3B) and myelin density (Figure 3C) in the corpus callosum and striatal WM bundles in the IBZ compared to PBS treated T2DM-stroke mice. EC-Exo treatment of T2DM-stroke significantly increases artery diameter (Figure 4A) and vessel density (Figure 4B) in the cortex and striatum region of the IBZ compared to T2DM-stroke control mice. Figure 4C shows that EC-Exo treatment significantly increases vessel patency indicated by increased FITC-dextran vascular perfusion in the IBZ (cortex, striatum and corpus callosum) of T2DM-stroke mice compared to T2DM-stroke control mice.
Figure 4. EC-Exo promotes vascular remodeling and increases vessel patency in T2DM-stroke mice.
(A-B) Stroke in T2DM mice significantly decreases arterial diameter and increases vascular density comapred to non-stroke T2DM control mice. EC-Exo treatment of T2DM-stroke mice significantly increases arterial diameter and vascular density in the cortex and striatum of the IBZ. Loss of miR-126 in EC-Exo attenuates EC-Exo induced vascular remodeling in T2DM-stroke mice. (C) EC-Exo treatment significantly increases vessel patency indicated by increased FITC-dextran vascular perfusion in the cortex, striatum and corpus callosum of the IBZ of T2DM-stroke mice at 14 days after stroke.
EC-Exo treatment of T2DM-stroke significantly promotes M2 macrophage polarization in the ischemic brain
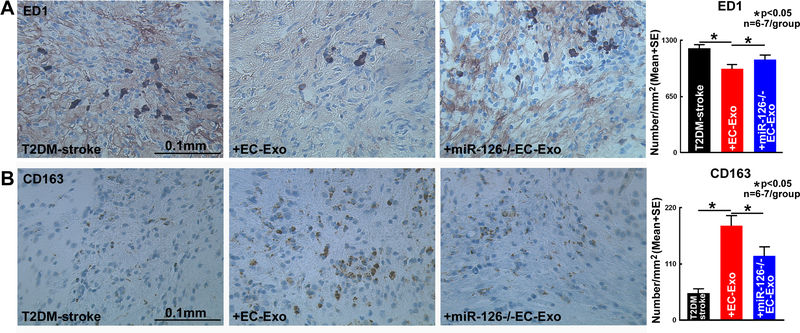
To test whether EC-Exo treatment of stroke induces M2 macrophage polarization, ED1 and CD163 immunostaining were performed. Figure 5A–B indicate that EC-Exo treatment significantly decreases number of M1 macrophages and increases number of M2 macrophages in the IBZ (cortex, striatum and corpus callosum) compared to T2DM-stroke control mice.
Figure 5. EC-Exo promotes M2 macrophage poliraztion in T2DM-stroke mice.
EC-Exo treatment of T2DM-stroke mice significantly decreases ED1 positive M1 macrophages (A) while increasing CD163 positive M2 macrophages (B) in the IBZ (cortex, corpus callosum and striatum) of T2DM-stroke mice. Loss of miR-126 in EC-Exo attenuates EC-Exo induced M2 macrophage polarization.
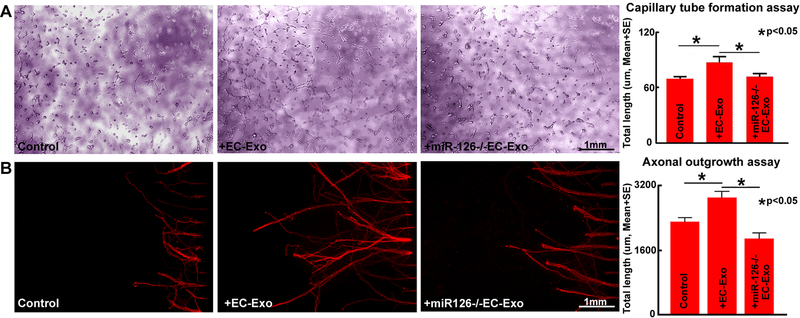
MiR-126 mediates EC-Exo induced capillary tube formation and PCN axonal outgrowth
Figure 6A shows that under conditions of high glucose after OGD, EC-Exo treatment significantly increases capillary tube formation compared to non-treated control while, miR-126−/−EC-Exo significantly decreases tube formation compared to EC-Exo treated group. The in-vitro data indicate that EC-Exo increases angiogenesis and miR-126 may mediate EC-Exo induced angiogenesis. Figure 6B shows that under conditions of high glucose after OGD, EC-Exo significantly increases PCN axonal outgrowth compared to non-treated control and miR-126−/−EC-Exo decreases PCN axonal outgrowth compared to EC-Exo treated group. These data indicate that miR-126 may regulate EC-Exo induced PCN axonal outgrowth.
Figure 6. EC-Exo promotes capillary tube formation and axon outgrowth.
EC-Exo treatment significantly increases endothelial capillary tube formation (A) and PCN axonal outgrowth (B) while, miR-126−/−EC-Exo attenuates EC-Exo induced capillary tube formation and axonal outgrowth.
MiR-126 mediates EC-Exo induced neurorestorative effects after stroke in T2DM mice
To test whether miR-126 mediates EC-Exo induced neurorestorative effects, miR-126−/−EC-Exo treatment was employed in T2DM-stroke mice. Figure 1A–B show that miR-126−/−EC-Exo treated mice exhibit significantly decreased miR-126 expression in ischemic brain and serum compared to EC-Exo treated T2DM-stroke mice. Figure 3 indicates that compared to PBS treated T2DM-stroke mice; miR-126−/−EC-Exo treatment of T2DM-stroke mice does not significantly improve neurological and cognitive function and does not alter lesion volume or blood glucose level. MiR-126−/−EC-Exo treatment does not significantly improve axon and myelin density (Figure 3) or vascular density and arterial diameter (Figure 4) or induce M2 macrophage polarization (Figure 5) compared to T2DM-stroke mice and is significantly worse compared to EC-Exo treated T2DM-stroke mice. Our data indicate that miR-126 plays an important role in EC-Exo induced neurorestorative effects in T2DM-stroke mice.
Discussion
In this study, we found that T2DM-stroke mice exhibit decreased serum and brain miR-126 compared to non-DM stroke mice. EC-Exo contain high levels of miR-126 and EC-Exo treatment of T2DM-stroke mice significantly increases serum and brain miR-126 expression, improves neurological function and cognitive outcome, increases vascular density and arterial diameter, enhances axon and myelin density in the IBZ as well as induces M2 macrophage polarization compared to PBS treated T2DM-stroke mice. Treatment of T2DM-stroke mice with exosomes deficient in miR-126 attentuates EC-Exo treatment derived neurorestorative effects in T2DM mice. Thus, miR-126 may contribute to EC-Exo induced neurorestorative effects in T2DM-stroke mice.
The biodistribution of extracellular vesicles (EVs) has been reported to be dependent on dose, route of administration and parent cell source22. Following systemic administration, EVs undergo a rapid distribution phase and uptake by organs followed by a longer elimination phase. At 24 hours after systemic injection, EVs were found to preferentially accumulate in the liver, spleen, lungs, and gastrointestinal tract, while low but detectable levels were observed in the kidneys, heart and brain22. Other studies have also reported preferential accumulation of EVs in the spleen and liver followed by other organs23. The elimination phase involves hepatic and renal excretion as well as clearance by macrophages23, 24. It is unlikely that EC-Exo administered at 3 days after stroke persist in the brain days after administration. Rather, EC-Exo likely exert their therapeutic effects by regulating miR expression and downstream signaling pathways. In this study, we show that miR-126 plays a key role in initiating neurorestorative mechanisms in the brain such as vascular and WM remodeling and decreases inflammation creating a conducive environment for brain repair. Thus, the significant improvement in neurological and cognitive function seen at 21–28 days after stroke after treatment with EC-Exo may be attributed to downstream signaling pathways and neurorestorative mechanisms.
In an experimental model of multiple sclerosis, intravenously administered EVs derived from human mesenchymal stem cells (MSC) were detected in the brain 2 hours after injection and found to be taken up by astrocytes as well as neurons25. In this study, we show that EC-Exo can pass the BBB after stroke in T2DM mice and are taken up by endogenous brain cells such as neurons, ECs, macrophages and microglia at 24 hours after intravenous EC-Exo administration. We did not find EC-Exo in the soma of astrocytes or oligodendrocytes. Similarly, following intranasal administration of human MSC derived A1-exosomes after status epilepticus in mice, PKH26-labeled A1 exosomes were found to be internalized by neurons and microglia but were not found in the cell body of astrocytes26. However, following intracerebroventricular delivery of MSC derived exosomes in diabetic mice, PKH26 labeled exosomes were found in damaged neurons, microglia as well as astrocytes27. These differences may be attributed to different cell sources as well as different delivery routes22. In the present study, we demonstrate that intravenously injected EC-Exo pass a disrupted BBB and primarily localize in the ischemic regions. Whether exosomes penetrate an intact and functioning BBB in the db/db-T2DM model remains to be addressed.
Neurorestorative effects after stroke are mediated by many coupled events including vascular remodeling, WM remodeling, neurogenesis and synaptogenesis19, 28. Previously, we have reported that stroke in db/db-T2DM mice aggravates ischemic injury and exacerbates WM damage and inflammation in the brain compared to non-diabetic stroke mice2. Similarly, a recent study has also reported that increased WM injury and exacerbated inflammatory responses after stroke in db/db mice were associated with poor long-term neurological functional outcome29. Additionally, in T2DM rats subjected to stroke, vascular remodeling and increased hemorrhagic transformation have been reported30. Axonal/WM remodeling and remyelination from regenerating axons in the IBZ of cerebral infarcts are crucial to improving brain connectivity and long-term functional outcome31. In this study, we found that EC-Exo treatment of stroke significantly increases vascular density and arterial diameter in the IBZ and significantly increases axon and myelin density in the IBZ, as well as improves neurological function and cognitive outcome compared to control T2DM-stroke mice. The increased vascular and WM remodeling may facilitate neurological functional recovery after stroke in T2DM mice.
Macrophages are known to exert both protective and deleterious effects after stroke which is primarily dependent on its phenotype. In the ischemic brain, the local and infiltrating microglia and macrophages initially assume an anti-inflammatory and protective M2 phenotype aiding in debris removal32. Extending the M2 phase of these macrophages and microglia while attenuating or delaying their transition into a pro-inflammatory M1 phenotype helps create a conductive environment for axonal extension and functional recovery33, decreases the expression of inflammatory factors in the ischemic brain32, and is associated with improved functional recovery after stroke34. Our data show that EC-Exo treatment of stroke in T2DM mice significantly promotes M2 macrophage polarization in the ischemic brain which likely contributes to neurorestorative effects of EC-Exo after stroke. However, we have not investigated whether the effects are direct or indirect.
MiR-126 is one of the most abundant miRs in ECs and plays a crucial role in regulating the function of ECs, angiogenesis and vascular integrity35. High glucose conditions decrease miR-126 content in EC derived microparticles36 and circulating vesicles in plasma5. Disruption of miR-126 leads to loss of vascular integrity resulting in a phenotype containing leaky vessels and hemorrhage6. The drastic loss of circulating miR-126 may contribute to the development of microvascular and macrovascular complications of T2DM8. Exosomal miRs are emerging as novel stroke biomarkers in patients37. MiR-126 expression is significantly decreased in serum and brain after stroke in mice as well as in T2DM patients7, 38, 39. In this study, our data indicate that miR-126 is significantly decreased after stroke in serum, serum exosome as well as ischemic brain tissue of T2DM mice. Consistent with other studies35, 36, we found that miR-126 is highly expressed in ECs and EC-Exo. In this study, we found that EC-Exo increases capillary tube formation and promotes axonal outgrowth indicating that EC-Exo promotes angiogenesis and axonal remodeling. Inhibition of miR-126 in EC-Exo significantly attenuates EC-Exo induced capillary tube formation and axonal outgrowth. Therefore, EC-Exo induced up regulation of miR-126 may regulate vascular changes as well as regulates axonal outgrowth thereby inducing neurorestorative effects after stroke in T2DM mice.
Conclusions
EC-Exo treatment administered at 3 days after stroke in T2DM mice increases vascular density, axon and myelin density as well as M2 macrophage polarization in the ischemic brain of T2DM-stroke mice which may contribute to improvement in neurological function and cognitive outcome compared to control T2DM-stroke mice. EC-Exo contain high levels of miR-126 and EC-Exo treatment in T2DM-stroke mice increases serum and brain miR-126 expression. Inhibition of miR-126 in EC-Exo attentuates EC-Exo treatment induced neurorestorative effects in T2DM-stroke mice. EC-Exo promotes capillary tube formation and PCN axon outgrowth which are also likely mediated by miR-126 pathway. Therefore, miR-126 may mediate the therapeutic neurorestorative effects of EC-Exo treatment of stroke in T2DM mice.
Supplementary Material
Acknowledgments
The authors wish to thank Qinge Lu and Sutapa Santra for their technical assistance.
Funding
This work was supported by the National Institutes of Health-National Institute of Neurological Disorders and Stroke R01NS099030 (JC), and the National Heart, Lung, and Blood Institute R01HL143432 (JC).
Footnotes
Conflicts of Interest/Disclosures: The authors have no conflicts of interest to disclose. Intellectual Property relating to the topic of this manuscript is subject to patent application (62/586,102) fully owned by Henry Ford Health System.
References
- 1.Nayak AR, Badar SR, Lande N, Kawle AP, Kabra DP, Chandak NH, et al. Prediction of outcome in diabetic acute ischemic stroke patients: A hospital-based pilot study report. Ann Neurosci. 2016;23:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xin H, Li Y, Chopp M. Exosomes/mirnas as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otero-Ortega L, Gomez de Frutos MC, Laso-Garcia F, Rodriguez-Frutos B, Medina-Gutierrez E, Lopez JA, et al. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017:271678×17708917 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microrna profiling reveals loss of endothelial mir-126 and other micrornas in type 2 diabetes. Circ Res. 2010;107:810–817 [DOI] [PubMed] [Google Scholar]
- 6.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microrna mir-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olivieri F, Spazzafumo L, Bonafe M, Recchioni R, Prattichizzo F, Marcheselli F, et al. Mir-21–5p and mir-126a-3p levels in plasma and circulating angiogenic cells: Relationship with type 2 diabetes complications. Oncotarget. 2015;6:35372–35382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regazzi R Diabetes mellitus reveals its micro-signature. Circ Res. 2010;107:686–688 [DOI] [PubMed] [Google Scholar]
- 9.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microrna-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene spred-1. J Mol Cell Cardiol. 2012;53:64–72 [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Zeng L, Huang J, Wang G, Lu H. Mir-126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608:191–202 [DOI] [PubMed] [Google Scholar]
- 11.Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, Lu M, et al. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T, et al. Mir-126 contributes to human umbilical cord blood cell-induced neurorestorative effects after stroke in type-2 diabetic mice. Stem cells. 2016;34:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2d and 3d conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labat-gest V, Tomasi S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Vis Exp. 2013:50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning R, Venkat P, Chopp M, Zacharek A, Yan T, Cui X, et al. D-4f increases microrna-124a and reduces neuroinflammation in diabetic stroke rats. Oncotarget. 2017;8:95481–95494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinetta MJ, Woodlee MT, Feinberg LM, Stroud C, Schallert K, Cormack LK, et al. Alcohol-induced retrograde memory impairment in rats: Prevention by caffeine. Psychopharmacology (Berl). 2008;201:361–371 [DOI] [PubMed] [Google Scholar]
- 17.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293 [DOI] [PubMed] [Google Scholar]
- 18.Yu P, Venkat P, Chopp M, Zacharek A, Shen Y, Ning R, et al. Role of microrna-126 in vascular cognitive impairment in mice. J Cereb Blood Flow Metab.0:0271678X18800593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, et al. The microrna-17–92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiklander OPB, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles. 2015;4:26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS nano. 2014;8:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, et al. Macrophage-dependent clearance of systemically administered b16bl6-derived exosomes from the blood circulation in mice. Journal of extracellular vesicles. 2015;4:26238–26238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laso-García F, Ramos-Cejudo J, Carrillo-Salinas FJ, Otero-Ortega L, Feliú A, Gómez-de Frutos M, et al. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PloS one. 2018;13:e0202590–e0202590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long Q, Upadhya D, Hattiangady B, Kim D-K, An SY, Shuai B, et al. Intranasal msc-derived a1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114:E3536–E3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805–24805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkat P, Chen J, Chopp M. Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab. 2018:271678×18782789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma S, Wang J, Wang Y, Dai X, Xu F, Gao X, et al. Diabetes mellitus impairs white matter repair and long-term functional deficits after cerebral ischemia. Stroke. 2018;49:2453–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi X, Kang Y, Hu Q, Chen C, Yang L, Wang K, et al. A long-term observation of olfactory ensheathing cells transplantation to repair white matter and functional recovery in a focal ischemia model in rat. Brain Res. 2010;1317:257–267 [DOI] [PubMed] [Google Scholar]
- 32.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070 [DOI] [PubMed] [Google Scholar]
- 33.Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, et al. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131–142 [DOI] [PubMed] [Google Scholar]
- 35.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, et al. Mir-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, et al. Endothelial microparticle-mediated transfer of microrna-126 promotes vascular endothelial cell repair via spred1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038 [DOI] [PubMed] [Google Scholar]
- 37.Chen F, Du Y, Esposito E, Liu Y, Guo S, Wang X, et al. Effects of focal cerebral ischemia on exosomal versus serum mir126. Transl Stroke Res. 2015;6:478–484 [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Gao G, Yang C, Zhou K, Shen B, Liang H, et al. The role of circulating microrna-126 (mir-126): A novel biomarker for screening prediabetes and newly diagnosed type 2 diabetes mellitus. Int J Mol Sci. 2014;15:10567–10577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Cui C, Yang X, Xu J, Venkat P, Zacharek A, et al. Mir-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.