Abstract
Background and Purpose—
Gender differences in the incidence and outcome of stroke have been well documented. The severity of stroke in women is, in general, significantly lower than that in men, which is mediated, at least in part, by the protective effects of β-estradiol. However, the detailed mechanisms underlying the neuroprotection by β-estradiol are still elusive. Recent studies have demonstrated that activation of acid-sensing ion channel 1a (ASIC1a) by tissue acidosis, a common feature of brain ischemia, plays an important role in ischemic brain injury. In the present study, we assessed the effects of β-estradiol on acidosis-mediated and ischemic neuronal injury both in vitro and in vivo and explored the involvement of ASIC1a and underlying mechanism.
Methods—
Cultured neurons and NS20Y cells were subjected to acidosis-mediated injury in vitro. Cell viability and cytotoxicity were measured by MTT and lactate dehydrogenase (LDH) assays, respectively. Transient (60 min) focal ischemia in mice was induced by suture occlusion of the middle cerebral artery (MCAO) in vivo. ASIC currents were recorded using whole-cell patch-clamp technique while intracellular Ca2+ concentration was measured with fluorescence imaging using Fura-2. ASIC1a expression was detected by western blotting and quantitative real-time PCR.
Results—
Treatment of neuronal cells with β-estradiol decreased acidosis-induced cytotoxicity. ASIC currents and acid-induced elevation of intracellular Ca2+ were all attenuated by β-estradiol treatment. In addition, we showed that β-estradiol treatment reduced ASIC1a protein expression which was mediated by increased protein degradation and that estrogen receptor α was involved. Finally, we showed that the level of ASIC1a protein expression in brain tissues and the degree of neuroprotection by ASIC1a blockade were lower in female mice, which could be attenuated by ovariectomy.
Conclusions—
β-estradiol can protect neurons against acidosis-mediated neurotoxicity and ischemic brain injury by suppressing ASIC1a protein expression and channel function.
Keywords: acid-sensing ion channels (ASICs), β-estradiol, cytotoxicity, degradation, ischemic stroke, neuroprotection
Introduction
Stroke is one of the most common causes of morbidity and mortality worldwide1. The incidence of stroke increases with age, but is relatively lower in female than male2, 3. This sex difference in stroke is reduced when comparing postmenopausal women with men4. Although tissue plasminogen activator has long been approved for the treatment of ischemic stroke, there has been limited success5. Searching for new therapeutic interventions for the majority of stroke patients is still a major challenge, even after recent progresses in endovascular thrombectomy6, 7. It has been demonstrated that neuroprotective agents could reduce stroke damage and prolong the thrombolytic treatment window8. Estrogens, a family of cholesterol-derived steroid hormones, have been shown to be protective against a variety of injuries including ischemic stroke9, 10. However, the detailed mechanisms are not clear.
Tissue acidosis is a common phenomenon of ischemia. Brain tissue pH can typically fall below 6.0 during severe ischemia, which aggravates ischemic brain injury11–13. Acid-sensing ion channels (ASICs) are proton-gated cation channels belonging to the degenerin/epithelial sodium channel superfamily14, 15. To date, six subunits encoded by four genes have been identified16. ASIC1a and ASIC2a are the major functional ASIC subunits expressed in central neurons11, 17. Previous findings by our own and others have clearly demonstrated that activation of ASIC1a is involved in acidosis-mediated and ischemic neuronal injury11, 18, 19, suggesting that ASIC1a is a potential therapeutic target for ischemia stroke11, 20, 21.
Numerous studies have demonstrated that female animals suffer much less brain infarction than males in response to ischemia22, 23. Furthermore, direct administration of β-estradiol in animals has been shown to reduce brain injury after stroke22, 24. It has been suggested that the neuroprotective effects of β-estradiol may be caused by its actions on oxidative stress, excitotoxicity, and inflammatory response25, 26. However, the potential neuroprotective benefits and mechanisms of β-estradiol against acidosis-mediated neurotoxicity remain unclear. Here, we demonstrate that β-estradiol can protect neurons against acidotoxicity and ischemic injury by down-regulating ASIC1a protein expression and function.
Material and methods
The data that support the findings of this study are available from the corresponding authors on reasonable request.
Animals
Adult C57BL6 male and female mice (25–30 g, 8 weeks-old), ovariectomized (OVX) female mice, and pregnant Swiss mice were purchased from Charles River. Animals were randomly assigned to different treatment groups and the person who performed animal studies was blinded to the treatments. The experimental procedure for the use of mice in primary neuronal culture and surgery was approved by the Institutional Animal Care and Use Committee of Morehouse School of Medicine.
Cell culture
Mouse cortical neurons were cultured as described previously27, 28. Neurons were cultured with Neurobasal medium supplemented with B-27 and glutamine. Culture medium was changed every three days and neurons were used 12–16 days after plating. NS20Y cells, derived from mouse neuroblastoma, were cultured in Dulbecco’s Modified Eagle’s Medium (Invitrogen), supplemented with 10% fetal bovine serum (Gibco) and 1% antibiotics, as described29.
Electrophysiology
ASIC currents were recorded using patch-clamp techniques as described previously21. Currents were activated 90 seconds apart to achieve a complete recovery from desensitization. A multi-barrel perfusion system (SF-77 Warner Instruments) was used to obtain rapid changes of extracellular solutions. Unless otherwise stated, cells were clamped at a holding potential of −60 mV. Pipette solution contained (in mmol/L): 140 CsF, 1 CaCl2, 2 MgCl2, 11 EGTA, 2 tetraethylammonium chloride, 10 HEPES and 4 MgATP, pH 7.3 adjusted with CsOH, 290 to 300 mOsm. Extracellular fluid/solution (ECF) contained (in mmol/L): 140 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES (pH 7.4 or pH 6.0 adjusted with NaOH/HCl; 320–330 mOsm). PcTX1 (Peptide International) was dissolved in ddH2O at 20 μmol/L before adding to extracellular solutions.
Ca2+ imaging
Intracellular Ca2+ was imaged using fluorescent dye Fura-2, as described previously11, 30. Cortical neurons grown on glass coverslips were incubated in ECF with 5 μmol/L Fura-2 AM at room temperature for 30 min, and then washed and incubated in normal ECF for another 30 min. Coverslips with neurons were transferred to a fast perfusion chamber on an inverted microscope (Nikon TE300, Nikon) illuminated using a xenon lamp (75W) and observed with a ×40 oil-immersion objective lens. Digitized images were acquired and analyzed by Axon Imaging Workbench software (AIW, Axon Instruments). Images were acquired at an emission wavelength of 510 nm. Ratio images of 340 nm/380 nm were analyzed by averaging pixel ratio values in circumscribed regions of neurons in the field of view.
Western blotting analysis
Total proteins were isolated as described previously29, 31. Cells were lysed in M-PER™ Mammalian Protein Extraction Reagent with protease inhibitor and phosphatase inhibitor cocktail (Thermo Fisher Scientific). After centrifugation at 13,000 g for 15 min at 4 °C, the lysates were collected and mixed with Laemmli sample buffer (2×), and then boiled for 10 min. Protein concentrations were measured using the Bio-Rad protein assay kit. Proteins were separated by 10 % SDS-polyacrylamide gels and then transferred to PVDF membranes. After blocking, blots were probed with antibodies against ASIC1a (rabbit anti-mouse, 1:1000; Gift from Dr. Xiang-Ming Zha, University of South Alabama) and β-actin (Abcam, 1:1000) followed by horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific, 1:1000). The signals were visualized using an ECL kit (Millipore), and images were acquired using ImageQuant LAS 4000.
Immunofluorescence staining
Neurons cultured on glass coverslips were treated with β-estradiol (1 μmol/L) for 48 h. The cells were fixed with acetone at −20 °C for 15 min, followed by permeabilization in PBS containing 0.3% Triton X-100 for 10 min. After being blocked with 5% bovine serum albumin, the slides were incubated with anti-ASIC1a antibody (1:100) at 4°C overnight, and then incubated with secondary antibodies for 1 h at room temperature.
Quantitative real-time PCR
Total RNAs were isolated by TRIzol reagent (Invitrogen) and cDNA was synthesized using the iScript Select cDNA synthesis kit (Bio-Rad Laboratories) according to manufacturer’s protocols. Quantitative real-time PCR was performed with iQ SYBR® Green supermix (Bio-Rad) in C1000™ Thermal cycler (Bio-Rad). The PCR amplification cycles consisted of denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 5 s, and annealing/extension at 61°C for 10 s, followed by detection of melting curve from 65°C to 95°C. Primers were synthesized by Invitrogen as following: for ASIC1a, forward, 5’-GGCCAACTTCCGTAGCTTCA-3’ and reverse, 5’-ATGCCCTGCTCTGTCGTAGAA-3’; for β-actin, forward, 5’-AGCCATGTACGTAGCCATC-3’ and reverse, 5’-CTCTCAGCTGTGGTGGTGAAC-3’. β-actin was used as an endogenous control, and ΔΔCt values were calculated after β-actin normalization. Relative levels of target mRNAs were calculated as 2−ΔΔCt.
Fluorescein diacetate/propidium iodide (FDA/PI) staining
Staining of the alive/dead cells was performed at 24 h after the beginning of 1–3 h acid treatment using ECF (pH 6.0) as described previously11, 32. Briefly, cells were incubated with normal medium containing FDA (3 mg/ml) and PI (5 mg/ml) for 5 min. Live (FDA positive) and dead (PI-positive) cells were observed and counted with a fluorescent microscope (Nikon Eclipse Ti-S, Nikon) at excitation/emission wavelengths of 585 nm/615 nm for PI and 470 nm/535 nm for FDA.
Lactate dehydrogenase (LDH) assay
Cytotoxicity was measured using LDH assay kit (Roche Diagnostics), as described in our previous studies11. After 48 h exposure to β-estradiol (1 μmol/L), NS20Y cells were washed two times with normal ECF and then incubated in pH 7.4 or pH 6.0 ECF for 3 h and followed by a wash (two times) and incubation in normal culture medium for 21 h. Cultured mouse cortical neurons were treated with the indicated ECF for 1 h and followed by 23 h incubation in normal medium. At the end of the experiments, 50 μl culture medium was transferred from each well into a 96-well plate for measurement of LDH release. For obtaining the maximal releasable LDH, cells were incubated with Triton X-100 (final concentration 0.5 %) for 30 min at room temperature.
MTT assay
MTT assay was performed as described in our previous studies33. NS20Y cells were seeded in 96-well plates overnight and then incubated with fresh medium containing β-estradiol or vehicle for various lengths of time. After the treatments, cell viability was detected by Vybrant® MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific). Absorbance values were measured at 570 nm using a microplate reader (Molecular Devices).
Ischemic stroke models
Transient (60 min) focal ischemia was induced by suture occlusion of the middle cerebral artery (MCAO), as described previously11. Mice were anesthetized using a mixture of 1.5% isoflurane, 70% N2O, and 28.5% O2. Transcranical LASER doppler was used to monitor the change of the cerebral blood flow. Only the mice with a blood flow dropped to below 20% of the normal value were used for data analysis. After 24 hours of ischemia, mice were euthanized and the brains were dissected. Coronal sections at 1 mm intervals were prepared and stained with 2% vital dye 2,3,5-triphenyltetrazolium hydrochloride (TTC). Infarct volume was calculated by summing the infarcted areas (pale) of all sections and multiplying by the thickness of the sections. Intracerebroventricular injection was performed as described previously11.
Statistical analysis
All data are expressed as mean ± SEM. Groups were compared using one-way analysis of variance followed by Dunnett’s test or unpaired Student’s t test as appropriate. Groups in in vivo experiment were compared using two-way ANOVA. p<0.05 was regarded as statistically significant.
Results
β-estradiol attenuates acidosis-induced cytotoxicity in vitro
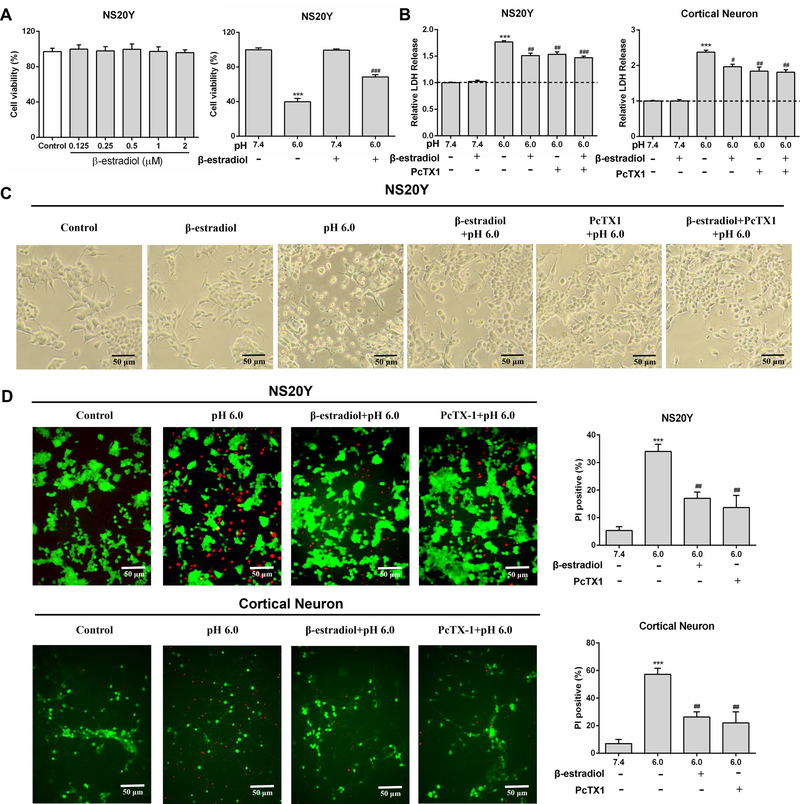
To explore whether β-estradiol has an effect on acidosis-induced neuronal injury, we first examined acid-induced toxicity of cultured mouse cortical neurons and NS20Y cells with and without pretreatment of β-estradiol. MTT assay showed that cell viability was not affected by β-estradiol treatment alone (48 h) at 0.125–2 μmol/L (Figure 1A). Compared to NS20Y cells treated at pH 7.4, 3 h acid incubation (pH 6.0) decreased cell viability. Pretreatment of β-estradiol (1 μmol/L, 48 h) attenuated acidosis-induced decrease in cell viability (Figure 1A). In addition, we analyzed acidosis-induced cell injury by measuring LDH release, as described previously11, 30. As shown in Figure 1B, acid incubation for 3 h dramatically increased LDH release in both types of cells. It has been previously established that activation of ASIC1a plays an important role in acidosis-induced neuronal injury11, 12. As expected, addition of PcTX1, an ASIC1a inhibitor, largely attenuated acidosis-induced cytotoxicity (Figure 1B). Similar to PcTX1, pretreatment of cells with β-estradiol (1 μmol/L, 48 h) also reduced LDH release. Combined treatment of β-estradiol and PcTX1 however did not produce additional protection beyond PcTX1 alone (Figure 1B), suggesting that inhibiting ASIC1a channel is likely involved in β-estradiol-mediated protection. Consistent with LDH assay, acid exposure induced cell body deformation was attenuated by β-estradiol or PcTX1 treatment (Figure 1C). To provide more evidence that β-estradiol is protective against acidosis-mediated cell injury, we also performed fluorescent staining of live and dead cells. As shown in Figure 1D, PI positive cells (dead) were increased in pH 6.0 group but were decreased by β-estradiol pretreatment in both cortical neurons and NS20Y cells. Together, these findings indicated that β-estradiol attenuates acidosis-induced and ASIC1a-mediated cell death.
Figure 1.
β-estradiol attenuates acidosis-induced cytotoxicity. A and B, Viability and cytotoxicity in NS20Y cells and mouse cortical neurons were measured at 24 h after acid treatment by MTT and LDH assays. C, Representative phase-contrast images showing NS20Y cells taken after treatment with the indicated solutions (×200 magnification). D, Analysis of acid-induced injury with fluorescein diacetate (FDA, alive) and propidium iodide (PI, dead) (×200 magnification). ***P<0.001 versus control (pH 7.4). #P<0.05, ##P<0.01, ###P<0.001 versus pH 6.0.
β-estradiol treatment suppresses ASIC currents and acid-induced [Ca2+]i elevation
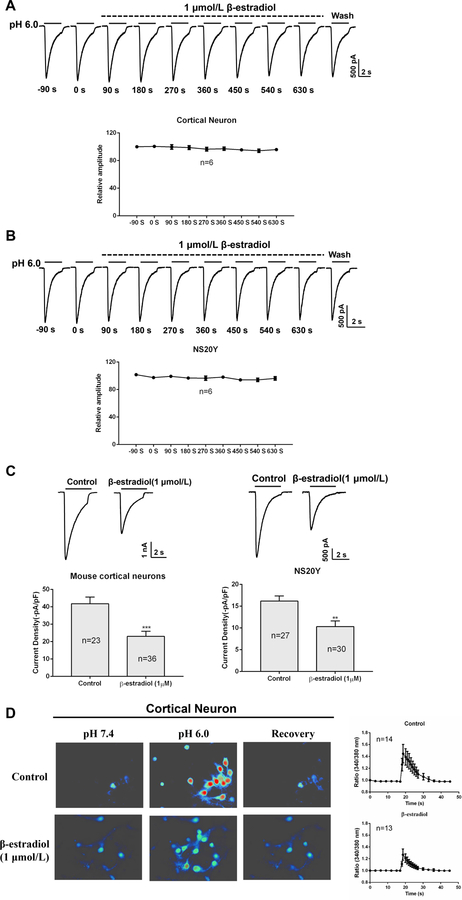
To know whether the protective effect of β-estradiol is mediated by a change in ASIC1a activity, we first determined whether an acute application of β-estradiol has an effect on ASIC currents. ASIC currents were activated by decreasing extracellular pH from 7.4 to 6.011. To examine the effect of β-estradiol on ASIC currents, we added 1 μmol/L β-estradiol to the pH 7.4 and pH 6.0 extracellular solutions for up to 10 min. As shown in Figure 2A and B, 10 min application of β-estradiol did not alter the amplitude of ASIC currents in NS20Y cells and mouse cortical neurons. Next, we explored whether a chronic application of β-estradiol (1 μmol/L, 48 h) has any effect on ASIC currents. As shown in Figure 2C, 48 h pretreatment with β-estradiol significantly inhibited ASIC currents in NS20Y cells and mouse cortical neurons. The amplitude of ASIC currents was suppressed by ~44.93% ± 6.95% (n=23 and 36, p<0.001) and 36.18% ± 7.92 % (n=27 and 30, p<0.01) of control values in cortical neurons and NS20Y cells, respectively. Similar to its effect on ASIC currents, treatment of neurons with 48 h β-estradiol dramatically inhibited acid-induced [Ca2+]i elevation (Figure 2D). Together, these findings suggested that chronic treatment of neurons with β-estradiol can reduce the activity/function of ASIC1a channels.
Figure 2.
β-estradiol suppress ASIC currents and acid-induced [Ca2+]i elevation. A, ASIC currents in cortical neurons were recorded after treatment with β-estradiol (1 μmol/L) for 10 min. B, ASIC currents in NS20Y cells were recorded after β-estradiol (1 μmol/L) treatment for 10 min. C, ASIC currents were recorded after treatment with β-estradiol for 48 h. D, Acid-induced [Ca2+]i was elevated in cortical neurons after β-estradiol treatment (48 h). **P<0.01, ***P<0.001 versus control.
β-estradiol decreases ASIC1a protein expression
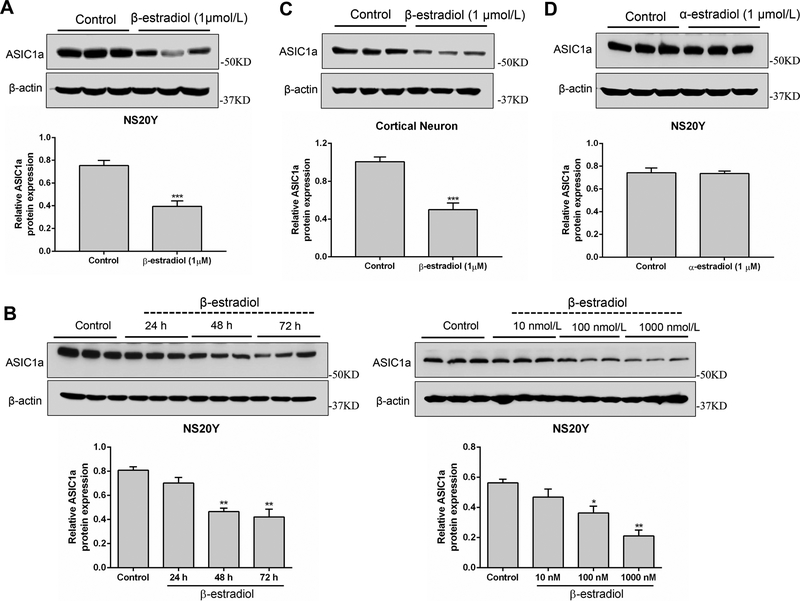
To investigate whether the inhibition of ASIC currents by β-estradiol is mediated by a change in ASIC1a protein expression, NS20Y cells and cortical neurons were treated with β-estradiol (10 nmol/L - 1 μmol/L) or vehicle for 24 – 72 h followed by a measurement of ASIC1a protein expression. As shown in Figure 3A and B, pretreatment with β-estradiol caused a concentration- and time-dependent decrease in ASIC1a protein level in NS20Y cells. Similar to NS20Y cells, treatment of mouse cortical neurons with β-estradiol (1 μmol/L for 48 h) also suppressed the ASIC1a protein expression (Figure 3C). In contrast to β-estradiol, treatment of neurons with 1 μmol/L α-estradiol for 48 h did not produce a clear change in the level of ASIC1a protein (Figure 3D).
Figure 3.
β-estradiol down-regulates ASIC1a protein expression. A, ASIC1a protein expression in NS20Y cells were measured after treatment with β-estradiol (1 μmol/L) for 48 h. B, ASIC1a protein expression in NS20Y cells were measured after 10 nmol/L–1 μmol/L β-estradiol treatment for (24 – 72 h). C, ASIC1a protein expression in cultured mouse cortical neurons were measured after 1 μmol/L β-estradiol treatment for 48 h. D, ASIC1a protein expression in NS20Y cells were measured after 1 μmol/L α-estradiol treatment for 48 h. *P<0.05, **P<0.01, ***P<0.001 versus control.
β-estradiol promotes ASIC1a protein degradation
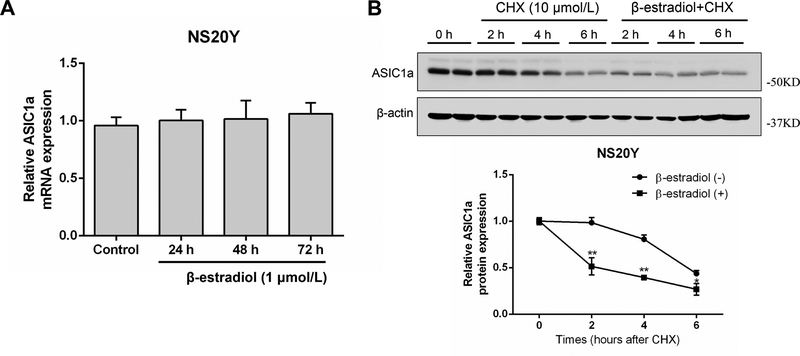
Next, we determined whether the reduction of ASIC1a protein expression by β-estradiol is mediated by a decrease in mRNA expression or an increase in protein degradation. Quantitative real-time PCR analysis showed that β-estradiol treatment did not affect the transcription level of ASIC1a (Figure 4A). To examine the possibility that the decrease in ASIC1a protein expression by β-estradiol is caused by increased protein degradation, NS20Y cells were treated with CHX (10 μmol/L) for 0–6 h to block new protein synthesis. The effect of β-estradiol on ASIC1a protein expression was then analyzed by Western blotting with or without CHX treatment. As shown in Figure 4B, regardless of β-estradiol treatment, the level of ASIC1a protein decreased gradually with time after blocking the protein synthesis. However, it decreased at a much faster rate in β-estradiol-treated cells. After a 4 h treatment, for example, only 54.42% ± 16.01% of the initial level of ASIC1a protein remained in β-estradiol-treated cells, whereas 78.67% ± 13.09% was still present in solvent-treated cells (Figure 4B). These findings suggested that β-estradiol promotes ASIC1a protein degradation.
Figure 4.
β-estradiol promotes ASIC1a protein degradation. A, NS20Y cells were treated with 1 μmol/L β-estradiol for 24–72 h followed by detecting mRNA expression with qRT-PCR. B, NS20Y cells were treated by cycloheximide (CHX) with or without β-estradiol pretreatment for 42–46 h. *P<0.05, **P<0.01 versus CHX.
Estrogen receptor is involved in β-estradiol-induced change of ASIC1a protein expression
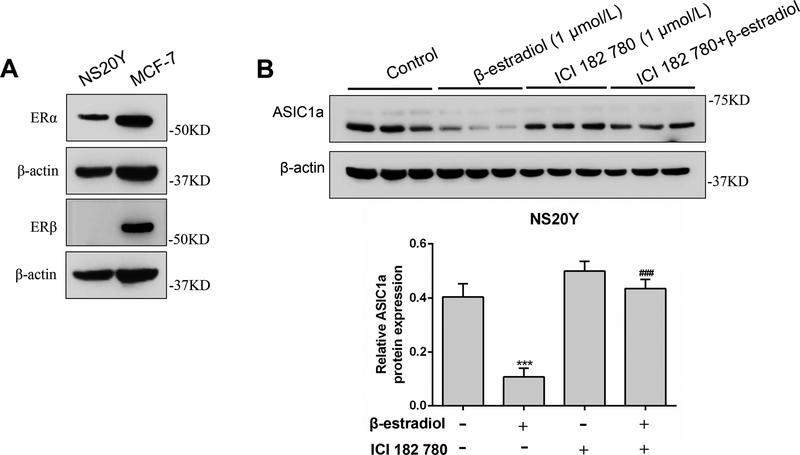
To investigate whether estrogen receptor (ER) is involved in the reduction of ASIC1a protein expression by β-estradiol, we first determined the expression of ERα and ERβ in NS20Y cells. Western blotting results showed that ERα protein, but not ERβ, is expressed in NS20Y cells (Figure 5A). In this experiment, MCF-7, a breast cancer cell line that has high level expression of ERα and ERβ, was used as a positive control. We found that the decrease of ASIC1a protein level by β-estradiol treatment (Figure 5B) was completely blocked by 1 μmol/L ICI 182 780, an antagonist of estrogen receptors. These results suggested that ERα, but not ERβ, is involved in β-estradiol induced decrease of ASIC1a protein expression.
Figure 5.
ER antagonist ICI 182 780 suppresses β-estradiol-induced decrease of ASIC1a protein expression in NS20Y cells. A, Expression of ERα and ERβ protein in NS20Y cells. MCF-7, a breast cancer cell line, was used as a positive control. B, ASIC1a protein expression was analyzed by Western blot after treatment with ICI 182 780 with or without β-estradiol. ***P<0.001 versus control. ###P<0.001 versus β-estradiol.
Inhibition of ASIC1a expression is involved in estrogen-mediated neuroprotection in vivo
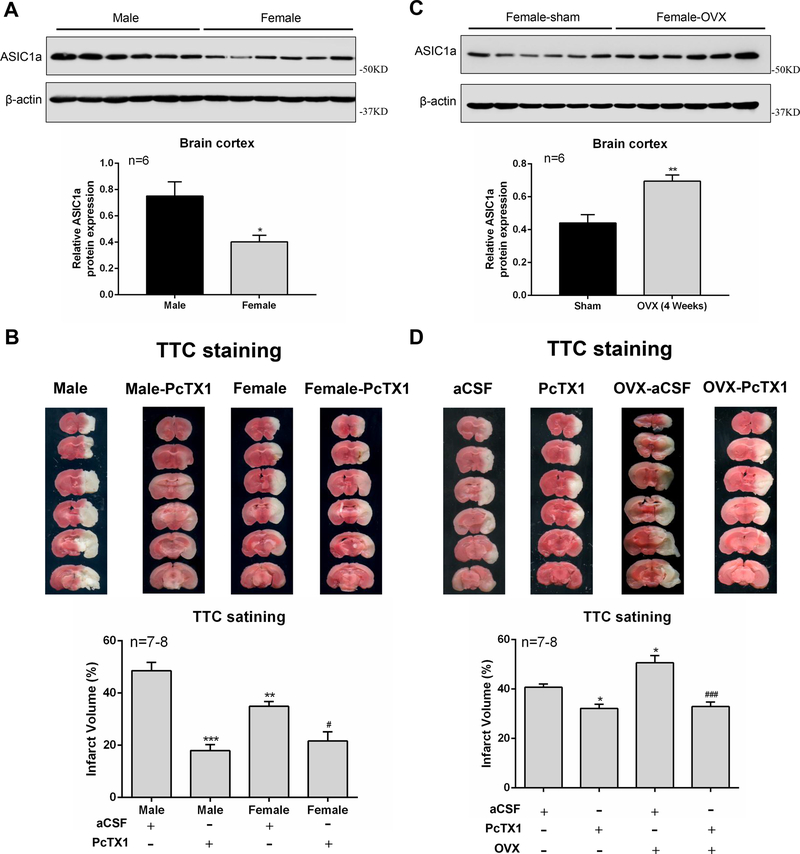
To confirm the relationship between estrogen-mediated neuroprotection and ASIC1a expression in vivo, we first investigated whether there is a difference in the expression of ASIC1a in cerebral cortex of male and female mice. The results showed that the level of ASIC1a protein expression is significantly higher in the cortex of male mice than that of female mice (Figure 6A). As ASIC1a plays an important role in ischemic brain injury, we speculated that there would be a difference in the severity of ischemic stroke and sensitive to ASIC1a blockade between male and female mice. Consistent with previous findings22, 23, infarct volume in male mice produced by 60 MCAO was clearly larger than that in female mice (Figure 6B). A total of 1 μL artificial CSF (aCSF) alone or aCSF-containing PcTX1 (1 μM) were injected intracerebroventricularly 1 h after the start of ischemia. As expected, infarct volume was significantly reduced by PcTX1 in both male and female mice. However, the reduction of infarct volume by PcTX1 in male mice (63.01% ± 4.68% reduction) is clearly larger than that in female mice (38.07% ± 10.11% reduction). This finding is consistent with a reduced ASIC1a protein expression in female mice (Figure 6B).
Figure 6.
Changes in the level of ASIC1a protein expression are involved in estrogen-induced neuroprotection in vivo. A, ASIC1a protein expression in male and female mouse cortex. B, TTC-stained sections showing infarction area in brains from aCSF-injected or PcTX1-injected male and female mice. C, ASIC1a protein expression in Sham-female and OVX-female mouse cortex. D, TTC-stained brain sections showing infarction area in brains from aCSF-injected or PcTX1-injected Sham-female and OVX-female mice. PcTX1 and aCSF were applied 30 min prior to MCAO. *P<0.05, **P<0.01, ***P<0.001 versus male or Sham-female group. #P<0.05, ###P<0.001 versus PcTX1-OVX group.
To provide evidence that estrogen plays a role in reduced ASIC1a expression and stroke-mediated brain injury in female mice, we examined ASIC1a expression in female-sham and female-ovariectomized (OVX) mice. As shown in Figure 6C, 4 weeks after OVX, the level of ASIC1a protein expression in cerebral cortex was increased compared to that of sham female mice. This data further suggests that estrogen plays a role in the reduction of ASIC1a expression in female mice. As expected, infarct volume was significantly increased in OVX female group as compared with sham female group (Figure 6D). In addition, PcTX1 showed more protection against stroke injury in OVX female mice than in sham female mice (Figure 6D, n=7–8). Collectively, our in vitro and in vivo data strongly suggest that β-estradiol mediated inhibition of ASIC1a expression/activity is, at least partially, responsible for its neuroprotective effect in female mice.
Discussion
In the present study, we explored the effect of β-estradiol on acidosis-induced neurotoxicity and ischemic brain injury and determined the underlying mechanism. We first showed that β-estradiol protected NS20Y cells and mouse cortical neurons against acidosis-induced cytotoxicity. We then demonstrated that treatment of neurons with β-estradiol (e.g. 48 h) significantly reduced the ASIC currents and acid-induced [Ca2+]i elevation. Furthermore, we showed that β-estradiol treatment suppressed ASIC1a protein expression, which was mediated by increased protein degradation. Finally, we demonstrated that the level of ASIC1a protein expression is significantly lower in cerebral cortex of female mice which is correlated with a lower degree of stroke damage and protection by ASIC1a inhibitor. These differences can be however attenuated by overiectomy. Together, these results indicated that β-estradiol can protect neurons against acidosis-mediated neurotoxicity and ischemic brain injury by reducing ASIC1a protein expression and channel function.
Stroke is a leading cause of death and long-term disability worldwide. Unfortunately, the effectiveness of current therapies is limited. For decades, neuroprotectants such as NMDA receptor antagonists have shown promise in experimental studies but failed in clinical trials due to limited treatment time window and/or intolerable side effects. Therefore, it is of great clinical significance to actively search for new targets and strategies. Local tissue acidification is a common feature of brain ischemia, which induces neuronal injury and is implicated in a number of neurological disorders including stroke11, 34. ASICs are widely distributed in central and peripheral neurons11, 14. Previous studies by us and others have clearly demonstrated that activation of ASIC1a is largely responsible for acidosis-induced neurotoxicity and ischemic brain injury11, 28. In addition, blockade of ASIC1a activity by PcTX1 can have a prolonged therapeutic time window20. Thus, ASIC1a channel may serve as a novel therapeutic target for ischemic stroke11, 30.
Previous studies have shown that β-estradiol has an effect on voltage-gated and ligand-gated ion channels such as BK channels, T-type and L-type Ca2+ channels and glutamate receptor channels25, 35, 36. Qu et al have reported that 17β-estradiol could acutely enhance ASIC3-like currents in rat dorsal root ganglia neurons37. However, the role of β-estradiol in modulating the activity/function of ASIC1a in mouse cortical neurons was unknown. Our present results showed that chronic β-estradiol exposure (48 h) could reduce ASIC1a current and ASIC1a-mediated Ca2+ influx by inhibiting ASIC1a protein expression. These findings are not surprising since ASIC1a and ASIC3 channels have distinct responses to modulators and/or pharmacological agents. One example is that amiloride, a commonly used blocker of most ASIC channels, paradoxically potentiates the ASIC3 current38.
Estrogen is a potent neuroprotective agent39. For example, it has been reported that β-estradiol can attenuate glutamate-induced cytotoxicity and hydrogen peroxide-induced apoptosis25, 40. Our present studies provided the first and strong evidence suggesting that estrogen is effective in protecting against acidosis-induced and ASIC1a-mediated neuronal injury. Consistent with a reduction of ASIC1a activity/expression by β-estradiol, relative protection against acidosis-induced damage by PcTX1 is higher in the absence of β-estradiol than that in the presence of β-estradiol. Together, these results suggest that β-estradiol can protect against acidosis-induced neurotoxicity by reducing ASIC1a protein expression and channel function.
The biological effects of β-estradiol are mainly mediated through its interaction with the nuclear estrogen receptors (ERs), ERα and ERβ41. Previous studies have shown that β-estradiol can modulate cell membrane receptors and ion channel function through its interaction with ER42, 43. Our present results showed that ERα, but not ERβ, was expressed in NS20Y cells (Figure 5A). Pretreatment with ICI 182 780, a high affinity estrogen nuclear receptor antagonist, completely blocked β-estradiol-mediated decrease of ASIC1a protein expression.
Epidemiological data demonstrated that premenopausal women have a lower incidence and severity of stroke compared to the same age men, indicating that estrogen has a protective effect44. The removal of estrogen by ovariectomy resulted in an aggravation of the stroke damage that can be reversed by exogenously administered estrogen9, 10, 22. Our present results also showed that the infarct volume in males is bigger than that in females. In addition, we observed a clear difference in the expression level of ASIC1a in cerebral cortex of male and female mice. Consistent with this difference, ASIC1a inhibitor PcTX1 provided more protection against ischemic brain injury in male mice than in female mice. These differences were however attenuated by OVX, further supporting a role for estrogen in regulating the level of ASIC1a expression and ischemic brain injury. In line with our current findings, a recent study has shown that the expression levels of ASIC1 and ASIC2 were increased in bone tissue and bone marrow cells from OVX female mice compared with sham female mice45.
With a very short estrous cycling period (3–5 days), female mice could be in different cycles and therefore estrogen levels could be different, thus increasing the variation of the results. To avoid the fluctuating estrogen level in our experiments, future studies will consider using two additional groups of mice to further investigate the estrogen’s effects on ASIC1a: OVX+vehicle vs. OVX+E2 (pellets).
Conclusions
In summary, the present study suggested that neuroprotection of β-estradiol is, at least partially, mediated by down-regulating ASIC1a expression and function. Our findings highlight a novel mechanism underlying the protective effect of β-estradiol and disclose potential future therapeutic strategies for stroke treatment.
Supplementary Material
Acknowledgments
We thank Dr. Veena N. Rao for providing MCF-7 cell line.
Sources of Funding
This work was partially supported by NIH SC3 GM122593, and S21MD000101.
Footnotes
Disclosures None.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: Ethnic and gender disparities. Neurology. 2003;61:189–194 [DOI] [PubMed] [Google Scholar]
- 3.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. The Lancet. Neurology 2008;7:915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends in endocrinology and metabolism: TEM. 2011;22:467–473 [DOI] [PubMed] [Google Scholar]
- 5.Lo EH, Broderick JP, Moskowitz MA. Tpa and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356 [DOI] [PubMed] [Google Scholar]
- 6.Jadhav AP, Desai SM, Kenmuir CL, Rocha M, Starr MT, Molyneaux BJ, et al. Eligibility for endovascular trial enrollment in the 6- to 24-hour time window: Analysis of a single comprehensive stroke center. Stroke. 2018;49:1015–1017 [DOI] [PubMed] [Google Scholar]
- 7.Kamal N, Majmundar N, Damadora N, El-Ghanem M, Nuoman R, Keller IA, et al. Mechanical thrombectomy - is time still brain? The dawn of a new era. British journal of neurosurgery. 2018;32:245–249 [DOI] [PubMed] [Google Scholar]
- 8.Liu R, Liu Q, He S, Simpkins JW, Yang SH. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. The Journal of pharmacology and experimental therapeutics. 2010;332:1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter RS, Iwuchukwu I, Hinkson CL, Reitz S, Lee W, Kukino A, et al. High-dose estrogen treatment at reperfusion reduces lesion volume and accelerates recovery of sensorimotor function after experimental ischemic stroke. Brain research. 2016;1639:200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, et al. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. Journal of neurosurgery. 1997;87:724–730 [DOI] [PubMed] [Google Scholar]
- 11.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: Blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698 [DOI] [PubMed] [Google Scholar]
- 12.Wang YZ, Wang JJ, Huang Y, Liu F, Zeng WZ, Li Y, et al. Tissue acidosis induces neuronal necroptosis via asic1a channel independent of its ionic conduction. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou RP, Wu XS, Wang ZS, Xie YY, Ge JF, Chen FH. Novel insights into acid-sensing ion channels: Implications for degenerative diseases. Aging and disease. 2016;7:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177 [DOI] [PubMed] [Google Scholar]
- 15.Bianchi L, Driscoll M. Protons at the gate: Deg/enac ion channels help us feel and remember. Neuron. 2002;34:337–340 [DOI] [PubMed] [Google Scholar]
- 16.Lingueglia E Acid-sensing ion channels in sensory perception. The Journal of biological chemistry. 2007;282:17325–17329 [DOI] [PubMed] [Google Scholar]
- 17.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (asic2) modulates asic1 h+-activated currents in hippocampal neurons. The Journal of biological chemistry. 2004;279:18296–18305 [DOI] [PubMed] [Google Scholar]
- 18.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, et al. Coupling between nmda receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–646 [DOI] [PubMed] [Google Scholar]
- 20.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of asic1a and the time window for neuroprotection in cerebral ischaemia. Brain : a journal of neurology. 2007;130:151–158 [DOI] [PubMed] [Google Scholar]
- 21.Li MH, Leng TD, Feng XC, Yang T, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel 1a by intracellular ph and its role in ischemic stroke. The Journal of biological chemistry. 2016;291:18370–18383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165; discussion 166 [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40:1842–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Q, Luo Y, Lv F, He Q, Wu H, Chao F, et al. Protective effects of 17beta-estradiol on hippocampal myelinated fibers in ovariectomized middle-aged rats. Neuroscience. 2018;385:143–153 [DOI] [PubMed] [Google Scholar]
- 25.Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting ca2+ influx through l-type voltage-gated ca2+ channels. Brain research. 2009;1276:159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Dykens JA, Perez E, Liu R, Yang S, Covey DF, et al. Neuroprotective effects of 17beta-estradiol and nonfeminizing estrogens against h2o2 toxicity in human neuroblastoma sk-n-sh cells. Molecular pharmacology. 2006;70:395–404 [DOI] [PubMed] [Google Scholar]
- 27.Inoue K, Branigan D, Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. The Journal of biological chemistry. 2010;285:7430–7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng TD, Si HF, Li J, Yang T, Zhu M, Wang B, et al. Amiloride analogs as asic1a inhibitors. CNS neuroscience & therapeutics. 2016;22:468–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Bryant Z, Leng T, Liu M, Inoue K, Vann KT, Xiong ZG. Acid sensing ion channels (asics) in ns20y cells - potential role in neuronal differentiation. Molecular brain. 2016;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, et al. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1247–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue K, Leng T, Yang T, Zeng Z, Ueki T, Xiong ZG. Role of serum- and glucocorticoid-inducible kinases in stroke. Journal of neurochemistry. 2016;138:354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou RP, Leng TD, Yang T, Chen FH, Xiong ZG. Acute ethanol exposure promotes autophagy-lysosome pathway-dependent asic1a protein degradation and protects against acidosis-induced neurotoxicity. Molecular neurobiology. 2019;56:3326–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun H, Leng T, Zeng Z, Gao X, Inoue K, Xiong ZG. Role of trpm7 channels in hyperglycemia-mediated injury of vascular endothelial cells. PloS one. 2013;8:e79540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Advances in neurology. 1996;71:209–233; discussion 234–206 [PubMed] [Google Scholar]
- 35.Song MY, Li CY, Liu XF, Xiao JY, Zhao H. Effect of 17beta-estradiol on t-type calcium channels in the lateral habenula. Journal of neuroendocrinology. 2018:e12629. [DOI] [PubMed] [Google Scholar]
- 36.Evanson KW, Goldsmith JA, Ghosh P, Delp MD. The g protein-coupled estrogen receptor agonist, g-1, attenuates bk channel activation in cerebral arterial smooth muscle cells. Pharmacology research & perspectives. 2018;6:e00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qu ZW, Liu TT, Ren C, Gan X, Qiu CY, Ren P, et al. 17beta-estradiol enhances asic activity in primary sensory neurons to produce sex difference in acidosis-induced nociception. Endocrinology. 2015;156:4660–4671 [DOI] [PubMed] [Google Scholar]
- 38.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through asic3 ion channels at the modest ph changes that occur during myocardial ischemia. Circulation research. 2006;99:501–509 [DOI] [PubMed] [Google Scholar]
- 39.Engler-Chiurazzi EB, Covey DF, Simpkins JW. A novel mechanism of non-feminizing estrogens in neuroprotection. Experimental gerontology. 2017;94:99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen HY, Zhang X, Chen SF, Zhang YX, Liu YH, Ma LL, et al. The protective effect of 17beta-estradiol against hydrogen peroxide-induced apoptosis on mesenchymal stem cell. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2012;66:57–63 [DOI] [PubMed] [Google Scholar]
- 41.Popolo A, Piccinelli AL, Morello S, Sorrentino R, Osmany CR, Rastrelli L, et al. Cytotoxic activity of nemorosone in human mcf-7 breast cancer cells. Canadian journal of physiology and pharmacology. 2011;89:50–57 [DOI] [PubMed] [Google Scholar]
- 42.Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, et al. 17beta-estradiol rapidly attenuates p2×3 receptor-mediated peripheral pain signal transduction via eralpha and gpr30. Endocrinology. 2013;154:2421–2433 [DOI] [PubMed] [Google Scholar]
- 43.Small KM, Nag S, Mokha SS. Activation of membrane estrogen receptors attenuates opioid receptor-like1 receptor-mediated antinociception via an erk-dependent non-genomic mechanism. Neuroscience. 2013;255:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the framingham heart study. Stroke. 2009;40:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanaya K, Iba K, Dohke T, Okazaki S, Yamashita T. Trpv1, asics and p2×2/3 expressed in bone cells simultaneously regulate bone metabolic markers in ovariectomized mice. Journal of musculoskeletal & neuronal interactions. 2016;16:145–151 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.