Abstract
Through phase separation some proteins form liquid-like condensates or droplets which can flow, fuse and even deform when pressure is applied. In some cases the condensates ‘mature’ to form gel or solid-like structure. Recent studies suggest that the liquid-like condensates form the structural basis for several membrane-less subcellular organelles such as stress granules and other subcellular structures. Here we review and discuss studies that implicate protein phase separation in the function of spindle apparatus mitotic and centrosomes.
Introduction
Cells need to orchestrate many biochemical reactions and functions spatially and temporally. Although membrane-bound organelles can organize a subset of these activities, other subcellular organelles that are not enclosed in membranes also play important roles. The spindle apparatus assembled after nuclear envelope breakdown and the centrosome represent two large organelles that are not enclosed by membranes. Additional membrane-less organelles include, but are not limited to, stress granules, nuclear speckles, and P granules[1,2]. Recent studies suggest that membrane-less organelles are formed in part through phase separation of proteins and they exhibit liquid-droplet like feature (Box).
Box 1: Protein phase separation and liquid droplet feature of membrane-less organelles.
An even mixture of water and oil can demix or phase separate into oil phase and water phase, a process called phase separation. Phase separation is thermodynamically favored process where strength of molecular interactions between two or more solute molecules is stronger than interactions between molecules and solvent[44]. In the above example, interaction between oil-oil molecules is much favored over the water-oil molecules (poor solvent), this leads to several oil molecules come together (demix) and form oil droplets in water. Purified protein found in membrane-less organelles can also demix (or phase separate) to form liquid droplets, a process often referred to as liquid-liquid phase separation. Proteins engaged in liquid-liquid phase separation are highly concentrated in the liquid droplets, which are often referred to as protein condensates.
Proteins containing intrinsically disordered regions (IDR) with low amino acid complexity (for example, DDX4, FUS and BuGZ) or proteins containing multivalent weak inter-molecular binding modules, such as and NPM1, are shown to undergo phase separation to form liquid droplets in vitro[1,9,45,46]. The weak and multivalent interactions among the protein molecules in the liquid droplets allow the proteins to be concentrated while being mobile and exchange with proteins in solution[47]. The liquid droplets formed by some of proteins (for example, FUS, FIB1 and SPD- 5) can further mature or age into hydro-gel-like or solid state and proteins in these states are less immobile or completely immobile[1,7,9,48].
The transition of proteins from solution phase to liquid droplet phase, hydro-gel like, or solid phase is called phase transition. Most of the membrane-less organelles are considered to have liquid droplet feature because they are observed to undergo fusion and fission.
Protein phase separation by tropoelastin, a precursor of the extracellular matrix elastin, was reported in the 60s[3]. Extensive studies in ensuing decades show that during initial phase separation, tropoelastin self-associate to form reversible liquid droplets. However, prolonged incubation leads to further assembly of elastin filaments and filament bundles that cannot be disassembled[4,5]. In recent years, an increasing number of proteins found in membrane-less organelles are reported to exhibit phase separation behavior in vitro[1,6]. The liquid droplets or protein condensates formed by these phase separation proteins can further recruit and concentrate their binding partners that may not phase separate on their own, suggesting that these condensates can function as membrane-less compartments in cells to segregate different functions in space and time[1]. The phase transition of proteins from liquid droplets into solids has been implicated in causing diseases or forming stable structures such as those found in centrosomes[7-10,37]. Here we focus our discussion of protein phase separation in metazoan mitosis.
Spindle apparatus, spindle matrix, and centrosomes
Spindle apparatus is a complex bipolar structure consists of antiparallel array of dynamic microtubules (MTs) that capture kinetochores assembled on the duplicated and condensed chromosomes to drive chromosomes segregation equally into daughter cells[11,12]. Although most effort has focused on studying how MTs, motor proteins, and MT-associated proteins assemble into spindle apparatus, some evidence suggests the existence of an independent meshwork, called the spindle matrix, that associates with spindle MTs[13-17]. For example, several Drosophila nuclear proteins permeate the spindle defined by MTs and after MT depolymerization they remain in a spindle-like meshwork[14]. The spindle matrix protein BuGZ is enriched evenly throughout the spindle [16,18], whereas another spindle matrix protein lamin- B is found to associate with spindle MTs in a membranous meshwork[17]. Although the mitotic function of some spindle matrix proteins has been demonstrated[14,17-19], how they exert the function as part of the spindle matrix remains less clear because the biophysical property of the spindle matrix has been difficult to decipher.
The centrosome consists of a pair of MT-based centrioles that function as basal bodies in ciliated or flagellated cells. The centriole pair is surrounded by a pericentriolar material (PCM) that mediates MT nucleation and facilitates MT organization[20,21]. The centrosome duplicates once per cell cycle and as cells enter mitosis, the duplicated centrosomes expand their PCM and MT nucleation activity several fold, which aids spindle assembly. This growth of PCM is in part due to the increased phosphorylation of PCM protein kinases such as Polo-like kinase (PLK-1 or Plk1)[20]. Several PCM proteins, including the Drosophila D-Plp and Centrosomin, human pericentrin, C. elegans SPD-2/Cep192 and SPD-5, contain coiled-coil regions and are required for the growth of mitotic PCM by assembling the PCM scaffold, which in turn recruits other proteins, including γTuRC, TPX2, and XMAP215, involved in MT nucleation[20,22-26], Although studies suggest that the PCM scaffold proteins can self-assemble, the assembly process remains not well understood.
BuGZ phase separation in spindle matrix formation and function
Spindle-like structures can be stimulated by RanGTP and magnetic beads coated with mitotic kinase Aurora A (AurA) in Xenopus egg extracts where AurA induces mitotic PCM-like assemblies on the beads to nucleate MTs and form spindle poles [27-30], MT depolymerization of the AurA-bead spindles results in a meshwork of proteins and membranes ‘the spindle matrix’ that surrounds AurA beads and can be isolated using magnets[27,28], Proteome analyses of this meshwork identified known spindle assembly factors (SAFs), nuclear proteins including lamin-B, membrane-associated proteins and signaling proteins[13,17]. The complexity of spindle matrix proteome could be caused by non-specific protein and membrane precipitation upon MT disassembly, which would suggest that the spindle matrix is an artifact. Alternatively, the proteome may contain proteins that form a physiologically-relevant structure of the spindle matrix. BuGZ was identified in the spindle matrix proteome that functions in mitosis to promote assembly of spindle and its matrix[13,17]. Through binding to both MTs and the spindle assembly checkpoint protein Bub3, BuGZ also stabilizes Bub3 to ensure equal chromosomes segregation and timely metaphase to anaphase transition[18,31,32]. Like other known spindle matrix proteins, BuGZ is present in interphase nucleus where it regulates RNA splicing[33].
The N-terminus of BuGZ contains two zinc fingers (N2ZF), which is followed by an intrinsically disordered region (IDR) (Fig. 1A). The N2ZF binds directly to tubulin, MTs, and AurA[16,18,34]. Purified BuGZ can undergo phase separation to form liquid droplets and efficient phase separation requires the hydrophobic and aromatic residues F and Y in the BuGZ IDR. BuGZ droplets greatly concentrate tubulin and AurA via N2ZF which promotes MT polymerization, MT bundling, and AurA activation, while the binding of N2ZF to MTs stimulates BuGZ phase separation along the MT (Fig. 1B-D)[16,34]. Interestingly, although BuGZ droplets concentrate and activate AurA in vitro, BuGZ depletion in vivo does not reduce the spindle-associated AurA but reduces AurA activation[34]. Thus, BuGZ does not activate AurA in spindles by concentrating AurA. Several activation mechanisms were suggested to explain BuGZ-mediated AurA activation in spindle[34]. Additionally, BuGZ, but not its phase separation mutants, supports assembly of spindle and its spindle matrix[16].
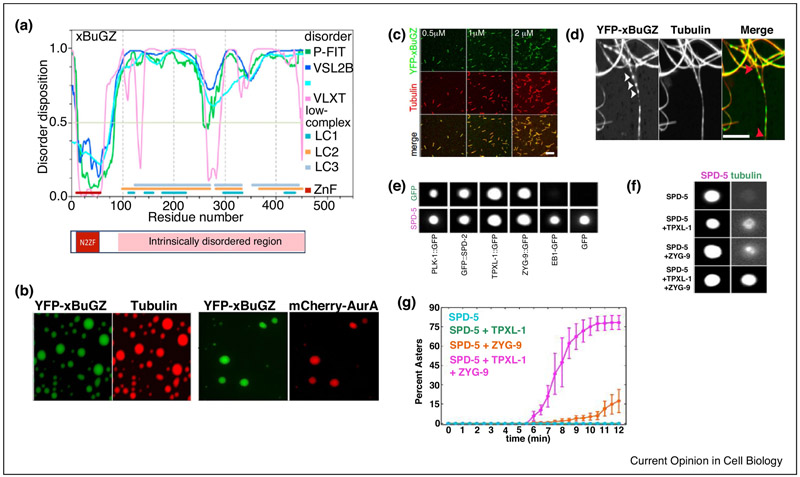
Figure 1.
Protein phase separation can concentrate interacting partners and promote their functions.
A)BuGZ domain structure. The N terminus of xBuGZ contains two zinc fingers, N2ZF, (10-58 aa) and a predicted intrinsically disordered region (IDR, 93-452 aa). The N2ZF is known to bind directly to tubulin, MTs, and Aurora A.
B)Purified xBuGZ undergoes phase separation to form liquid droplets, which can concentrate tubulin and Aurora A.
C)xBuGZ promotes MT polymerization and binds to MTs in vitro.
D)xBuGZ phase separates along MTs and it promotes MT bundling. xBuGZ can be seen in droplet form (arrow heads) along MTs or fulling coating MTs.
E)In the presence of crowding reagent, SPD-5 forms spherical condensates which concentrate several so called PCM client proteins, PLK1, TPXL-1 and ZYG-9.
F)TPXL-1 and ZYG-9 in SPD-5 condensates bind and concentrate tubulin and promote MT aster nucleation as quantified in (G).
Images A, B, C and D are reproduced from[16], and E, F G are from[37].
Several experiments suggest that BuGZ undergoes phase separation in spindle and its matrix, and BuGZ remains dynamic in these structures[16]. The abnormal spindle phenotypes caused by BuGZ phase separation mutants with F and Y replaced by S can be explained by reduced MT assembly and bundling[16]. Purified BuGZ readily diffuses into the preformed pure BuGZ condensates, spindle and spindle matrix, but the phase separation mutant BuGZ failed to do so (Fig. 2A-C). This dynamic behavior of BuGZ allowed further probing of phase separation using BuGZ fragments in the IDR and their corresponding mutants with F and Y replaced by S (Fig. 2A). Each wild type fragment diffused into the preformed pure BuGZ condensates which diminished the droplet size, but the mutant fragments neither diffused into nor affected BuGZ droplets (see Fig. 2D with one fragment as an example)[16]. Incubation of these fragments with preformed spindle and spindle matrix showed that diffusion of wild type fragments into these structures caused disruption, whereas mutant fragments neither diffused into nor disrupted the structures (Fig. 2E&F)[16]. Thus, BuGZ undergoes phase separation in spindle and spindle matrix and the BuGZ fragments can diffuse into BuGZ condensates to disrupt the weak multivalent interactions of BuGZ IDR. The N2ZF of BuGZ is required to promote MT assembly by the spindle matrix (Fig. 2G). These and other findings suggest that BuGZ phase separation during spindle assembly contributes to spindle matrix assembly and function (Fig. 3A).
Figure 2.
Probing BuGZ phase separation and function in spindle and spindle matrix.
A)Sequence alignment between mouse and Xenopus BuGZ shows conserved F and Y residues (marked in black), Prolines and hydrophobic residues are marked red and green, respectively. Three different xBuGZ protein fragments, A, B and C (underlined in pink, black and orange, respectively) were generated from the IDR region.
B)The wild type YFP-xBuGZ from bulk solution readily diffused into the preformed His-xBuGZ condensates, whereas phase separation mutant xBuGZ, YFP-xBuGZ5S (5F and Y replaced by S) or YFP-xBuGZ13S (all 13F and Y replaced by S), diffused poorly into the droplets.
C)Wild type YFP-xBuGZ was able to diffuse into the isolated spindle matrix (labeled by lamin- B3), whereas the phase separation mutants diffused poorly into the matrix.
D, E) The YFP-xBuGZ fragment xBuGZ-A, but not xBuGZ-A3S (3F and Y residues mutated to S), diffused into preformed His-xBuGZ droplets (D) or isolated spindle matrix (E) and reduced their sizes. Similar results were obtained for the other two fragments GFP-xBuGZ-B, -C, and their corresponding FY to S mutants see [16].
F)The YFP-xBuGZ fragments xBuGZ-A, -B, and -C, but not their corresponding FY to S mutants, disrupted aster and spindle assembly from Aurora A beads in egg extracts. White dashed lines indicate the length of astral MTs and spindle.
G)MT polymerization from the spindle matrix requires N2ZF of BuGZ. Incubation of YFP alone or wild type YFP-xBuGZ with isolated spindle matrix did not disrupt MT assembly from the spindle matrix. Since YFP-xBuGZ13S incorporates poorly into the spindle matrix (see C), it also did not affect MT assembly. However, the YFP-xBuGZΔN (deleted of N2ZF), which can diffuse into the spindle matrix and displace the endogenous xBuGZ see [16], disrupted MT assembly from the spindle matrix. Images are reproduced from[16],
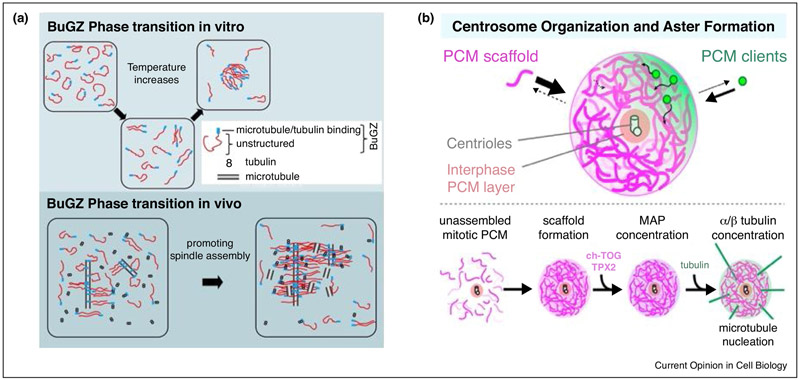
Figure 3.
Protein phase separation and their proposed functions in mitosis.
A) Increase in temperature changes the conformation of BuGZ molecules which allows the otherwise less accessible IDRs of BuGZ molecules to interact with one another to drive phase separation in vitro. Phase separation of BuGZ along MTs in vivo may concentrate tubulin and Aurora A (not shown) to promote MT assembly and bundling. The N2ZF and IDR of BuGZ are indicated.
B) Molecular crowding induces phase separation of SPD-5 to form an amorphous liquid like structure which gradually matures to form a gel or solid-like structure. SPD-5 condensates can recruit several “PCM clients” including tubulin and promote MT aster assembly in vitro. SPD-5 phase separation is proposed to support PCM scaffold formation during mitotic PCM formation and it supports MT nucleation from mitotic centrosomes by concentrating tubulin and MT nucleators.
Alternative interpretation of BuGZ phase separation and spindle matrix in mitosis
Although BuGZ is enriched on spindle and its depletion results in defective spindles in tissue culture cells and egg extracts, BuGZ phase separation behavior has only been inferred in spindles and spindle matrix made in egg extracts. It remains possible that BuGZ does not undergo phase separation in spindles in cells. To show BuGZ phase separation in cells, it is important to probe its behavior as described above. For example, if BuGZ is in a phase separated state, injection or overexpression of excess BuGZ fragments, but not the YF to SS mutants, should displace endogenous BuGZ from spindle and disrupts spindle morphology.
Observation of spindle matrix in cells remains challenging because only several Drosophila spindle matrix proteins that do not have clear homologs in vertebrates are seen to remain in spindle-like assemblages upon MT depolymerization in early embryonic mitosis where the nuclear envelopes do not fully break down[15,35,36]. The known spindle matrix marker proteins, including NuMA, lamin-B, and BuGZ, appear diffusely distributed throughout mitotic mammalian cells when MTs were fully disassembled (unpublished observation). Therefore, it remains possible that spindle matrix only exists in some cells or spindle matrix stability depends on MTs in cells. Additional assays that aim to probe the behaviors of spindle matrix proteins such as BuGZ in spindles in cells are needed to further clarify the existence, biophysical property, and function of the spindle matrix.
SPD-5 phase separation in mitotic PCM assembly and MT nucleation
The C. elegans SPD-5 is required for PCM assembly. SPD-5 belongs to a group of coiled-coil PCM proteins and the multiple coiled-coil regions in these proteins are believed to cause homo-oligomerization and PCM scaffold formation[23,24]. Purified SPD-5 can form micron-scale porous networks upon incubation at elevated temperature for extended time. This network is reversible because it can be dissolved by dilution[24]. Further studies show that the network can recruit several PCM proteins including SPD-2, PLK-1, and ZYG-9 (chTOG and XMAP215 homolog) (Fig. 1E)[37]. These and other findings support the idea that PCM scaffold proteins can self-assemble to recruit additional proteins with known functions in mitotic PCM growth (SPD-2 and PLK-1) and MT nucleation (ZYG-9)[38,39].
Interestingly, instead of forming network, purified SPD-5 undergoes phase separation in the presence of crowding reagents such as PEG to form liquid-like condensates in vitro[37]. The size of crowding reagents appears to be important with >1kDa PEG promoting SPD-5 condensates whereas SPD-5 network formation is promoted by smaller size PEG. The SPD-5 condensates undergo ‘maturation or aging’ and become resistant to dilution. SPD-2 and PLK-1, which are known to recruit other PCM proteins (including SPD-5) and mediate mitotic PCM growth, promote SPD-5 condensates formation in vitro[20]. Similar to SPD-5-mediated recruitment of other PCM proteins at centrosomes in vivo[20-23], SPD-5 condensates recruit PLK-1, SPD-2, ZYG-9, and TPXL-1 (C. elegans TPX2) in vitro. ZYG-9 and TPXL-1 in turn concentrate tubulin and promote MT aster nucleation from the condensates (Fig. 1F & G). These and other studies suggest that molecular crowding initially promotes multivalent inter-SPD-5 interactions at PCM to drive SPD-5 phase separation, which further mature into a stable PCM scaffold (Fig. 3B)[40].
Alternative mode of PCM scaffold assembly and interpretation
Although SPD-5 condensates can recruit ‘client proteins that nucleate MT asters and exhibit a number of features similar to the PCM scaffold of C. elegans centrosomes, whether SPD- 5 first forms condensates and then PCM scaffold during mitotic PCM growth in vivo remains to be tested. Since SPD-5 can also form a network structure in vitro in the absence of crowding reagents[24,41], it is possible that PCM scaffold formation is driven by SPD-5 network assembly. Interestingly, studies of Drosophila PCM scaffold protein Centrosomin, which performs similar functions as SPD-5 and contains multiple coiled-coil domains like SPD-5, suggest that Centrosomin assembles into network to build PCM scaffold in mitosis[42,43]. Centrosomin has a phospho-regulated multimerization (PReM) domain that contains at least ten different sites for phosphorylation and a Leucin zipper motifs (LZ) that aid dimer formation of PReM domain. Upon phosphorylation, the interaction of LZ and C-terminal Cnn-motif-2 (CM2) within a Centrosomin dimer have been shown to induce self-assembly of Centrosomin to form stable mitotic PCM[25]. Since like Centrosomin purified SPD-5 can form a network, it is possible that PCM scaffold formation is driven by network formation of SPD-5 and Centrosomin. Supporting this idea, Centrosomin sequences involved in network formation in vitro is required for PCM scaffold formation in vivo[25,42]. Alternatively, the two proteins may assemble into PCM via different mechanisms with SPD-5 first forms liquid-like condensates which further mature into solid scaffold, whereas Centrosomin directly assembles into a solid network. Although the coiled-coil regions of SPD-5 is hypothesized to mediate condensate formation, additional mutagenesis work is needed to test whether and how these regions are required to form condensates in vitro and PCM scaffold assembly in vivo. Since both SPD-5 and Centrosomin form stable PCM scaffold, it is important and possible, albeit challenging, to determine SPD-5 and Centrosomin structures at the PCM because the in vivo structure of proteins would provide the best guide to interpret structures formed by purified proteins in vitro.
Concluding remarks
The concept of biomolecular phase separation offers an exciting opportunity to understand assembly of many subcellular structures that have been difficult to decipher. The intense interest in studying the role of phase separation will undoubtedly lead to fast progress. Currently, however, the in vivo biomolecular phase separation function has often been inferred by studying in vitro condensates. Development of new tools that directly probe phase separation in vivo is need to fully understand how biomolecular condensates contribute to the formation of sub-cellular structures and membrane-less organelles.
Acknowledgement
We thank the Cell Press and the Rockefeller University Press for the use of published images. Funded by National Institute of Health of US (GM110151 andGM106023) to Y.Z.
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
••of outstanding interest
- 1.Banani SF, Lee HO, Hyman AA, Rosen MK: Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 2017, 18:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes E, Shorter J: The molecular language of membraneless organelles. J Biol Chem 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo GC, Keeley FW, Weiss AS: Coacervation of tropoelastin. Adv Colloid Interface Sci 2011, 167:94–103. [DOI] [PubMed] [Google Scholar]
- 4.Clarke AW, Arnspang EC, Mithieux SM, Korkmaz E, Braet F, Weiss AS: Tropoelastin massively associates during coacervation to form quantized protein spheres. Biochemistry 2006, 45:9989–9996. [DOI] [PubMed] [Google Scholar]
- 5.Mithieux SM, Weiss AS: Elastin. Adv Protein Chem 2005, 70:437–461. [DOI] [PubMed] [Google Scholar]
- 6.Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. : Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 2018, 28:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin Y, Brangwynne CP: Liquid phase condensation in cell physiology and disease. Science 2017, 357. [DOI] [PubMed] [Google Scholar]
- 8.Murakami T, Qamar S, Lin Julie Q, Schierle Gabriele SK, Rees E, Miyashita A, Costa Ana R, Dodd Roger B, Chan Fiona TS, Michel Claire H, et al. : ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 2015, 88:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. : A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162:1066–1077. [DOI] [PubMed] [Google Scholar]
- 10.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP: Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petry S: Mechanisms of Mitotic Spindle Assembly. Annu Rev Biochem 2016. 85:659–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmke KJ, Heald R, Wilbur JD: Interplay between spindle architecture and function. Int Rev Cell Mol Biol 2013, 306:83–125. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Tsai MY, Wang S, Lu B, Chen R, Yates JR, III, Zhu X, Zheng Y: Requirement for Nudel and dynein for assembly of the lamin B spindle matrix. Nat Cell Biol 2009, 11:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao C, Rath U, Maiato H, Sharp D, Girton J, Johansen KM, Johansen J: A nuclear-derived proteinaceous matrix embeds the microtubule spindle apparatus during mitosis. Mol Biol Cell 2012, 23:3532–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y: A membranous spindle matrix orchestrates cell division. Nat Rev Mol Cell Biol 2010, 11:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Wang S, Huang Y, He X, Cui H, Zhu X, Zheng Y: Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 2015, 163:108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y: A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 2006, 311:1887–1893. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H, He X, Wang S, Jia J, Wan Y, Wang Y, Zeng R, Yates J 3rd, Zhu X, Zheng Y: A microtubule-associated zinc finger protein, BuGZ, regulates mitotic chromosome alignment by ensuring Bub3 stability and kinetochore targeting. Dev Cell 2014, 28:268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman B, Channels W, Qiu M, Iglesias P, Yang G, Zheng Y: Lamin B counteracts the kinesin Eg5 to restrain spindle pole separation during spindle assembly. J Biol Chem 2010, 285:35238–35244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conduit PT, Wainman A, Raff JW: Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol 2015, 16:611–624. [DOI] [PubMed] [Google Scholar]
- 21.Hachet V, Canard C, Gonczy P: Centrosomes promote timely mitotic entry in C. elegans embryos. Dev Cell 2007, 12:531–541. [DOI] [PubMed] [Google Scholar]
- 22.Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O'Connell KF: Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell 2004, 6:511–523. [DOI] [PubMed] [Google Scholar]
- 23.Hamill DR, Severson AF, Carter JC, Bowerman B: Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev Cell 2002, 3:673–684. [DOI] [PubMed] [Google Scholar]
- 24.Woodruff JB, Wueseke O, Viscardi V, Mahamid J, Ochoa SD, Bunkenborg` J, Widlund PO, Pozniakovsky A, Zanin E, Bahmanyar S, et al. : Centrosomes. Regulated assembly of a supramolecular centrosome scaffold in vitro. Science 2015, 348:808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Caballe A, Wainman A, Johnson S, Haensele AFM, Cottee MA, Conduit PT, Lea SM, Raff JW: Structural Basis for Mitotic Centrosome Assembly in Flies. Cell 2017, 169:1078–1089 e1013.(•)Two Centrosomin molecules can interact through their Leucine zipper (LZ) domain present in PReM region and exist as a dimer. This study shows that phosphorylation in PReM bring CM2 domain of Centrosomin to LZ within the dimer which allow the IDRs of Centrosomin to interact and assemble to a scaffold structure. The authors also solved the crystal structure of LZ-CM2 complex.
- 26.Zheng Y, Wong ML, Alberts B, Mitchison T: Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 1995, 378:578–583. [DOI] [PubMed] [Google Scholar]
- 27.Tsai MY, Zheng Y: Aurora A kinase-coated beads function as microtubule-organizing centers and enhance RanGTP-induced spindle assembly. Curr Biol 2005, 15:2156–2163. [DOI] [PubMed] [Google Scholar]
- 28.Tsai MY, Wiese C, Cao K, Martin O, Donovan P, Ruderman J, Prigent C, Zheng Y: A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat Cell Biol 2003, 5:242–248. [DOI] [PubMed] [Google Scholar]
- 29.Wilde A, Zheng Y: Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 1999, 284:1359–1362. [DOI] [PubMed] [Google Scholar]
- 30.Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y: Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science 2001, 291:653–656. [DOI] [PubMed] [Google Scholar]
- 31.Toledo CM, Herman JA, Olsen JB, Ding Y, Corrin P, Girard EJ, Olson JM, Emili A, DeLuca JG, Paddison PJ: BuGZ is required for Bub3 stability, Bub1 kinetochore function, and chromosome alignment. Dev Cell 2014, 28:282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, He X, Feng D, Zhu X, Zheng Y: RanGTP aids anaphase entry through Ubr5- mediated protein turnover. J Cell Biol 2015, 211:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y, Zheng X, Chen H, Guo Y, Jiang H, He X, Zhu X, Zheng Y: Splicing function of mitotic regulators links R-loop-mediated DNA damage to tumor cell killing. J Cell Biol 2015, 209:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y, Li T, Ems-McClung SC, Walczak CE, Prigent C, Zhu X, Zhang X, Zheng Y: Aurora A activation in mitosis promoted by BuGZ. J Cell Biol 2018, 217:107–116.(••)The study shows that BuGZ through its zinc fingers interacts with Aurora A thereby incorporates Aurora A to the BuGZ condensates which in turn activates the Aurora A in vitro. Activation of Aurora A promoted microtuble polymerization.
- 35.Johansen KM, Johansen J: Cell and molecular biology of the spindle matrix. Int Rev Cytol 2007, 263:155–206. [DOI] [PubMed] [Google Scholar]
- 36.Johansen KM, Forer A, Yao C, Girton J, Johansen J: Do nuclear envelope and intranuclear proteins reorganize during mitosis to form an elastic, hydrogel-like spindle matrix? Chromosome Res 2011, 19:345–365. [DOI] [PubMed] [Google Scholar]
- 37.Woodruff JB, Ferreira Gomes B, Widlund PO, Mahamid J, Honigmann A, Hyman AA: The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Ce// 2017, 169:1066–1077 e1010.(••)This study showed that molecular crowding induces phase separation of SPD-5 proteins in vitro which eventually matures form gel or solid like amorphous structure. The SPD-5 condensates also recruited several mediators for microtubule polymerization and promoted aster formation in vitro.
- 38.Pelletier L, Ozlu N, Hannak E, Cowan C, Habermann B, Ruer M, Muller-Reichert T, Hyman AA: The Caenorhabditis elegans centrosomal protein SPD-2 is required for both pericentriolar material recruitment and centriole duplication. Carr Biol 2004, 14:863–873. [DOI] [PubMed] [Google Scholar]
- 39.Ozlu N, Srayko M, Kinoshita K, Habermann B, O’Toole E T, Muller-Reichert T, Schmalz N, Desai A, Hyman AA: An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Dev Cell 2005, 9:237–248. [DOI] [PubMed] [Google Scholar]
- 40.Gupta GD, Pelletier L: Centrosome Biology: Polymer-Based Centrosome Maturation. Curr Biol 2017, 27:R836–R839. [DOI] [PubMed] [Google Scholar]
- 41.Wueseke O, Zwicker D, Schwager A, Wong YL, Oegema K, Julicher F, Hyman AA, Woodruff JB: Polo-like kinase phosphorylation determines Caenorhabditis elegans centrosome size and density by biasing SPD-5 toward an assembly-competent conformation. Biol Open 2016, 5:1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conduit PT, Feng Z, Richens JH, Baumbach J, Wainman A, Bakshi SD, Dobbelaere J, Johnson S, Lea SM, Raff JW: The centrosome-specific phosphorylation of Cnn by Polo/Plk1 drives Cnn scaffold assembly and centrosome maturation. Dev Cell 2014, 28:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conduit PT, Richens JH, Wainman A, Holder J, Vicente CC, Pratt MB, Dix CI, Novak ZA, Dobbie IM, Schermelleh L, et al. : A molecular mechanism of mitotic centrosome assembly in Drosophila. Elife 2014, 3:e03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyman AA, Weber CA, Julicher F: Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 2014, 30:39–58. [DOI] [PubMed] [Google Scholar]
- 45.Fung HYJ, Birol M, Rhoades E: IDPs in macromolecular complexes: the roles of multivalent interactions in diverse assemblies. Curr Opin Struct Biol 2018, 49:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitrea DM, Cika JA, Stanley CB, Nourse A, Onuchic PL, Banerjee PR, Phillips AH, Park CG, Deniz AA, Kriwacki RW: Self-interaction of NPM1 modulates multiple mechanisms of liquid- liquid phase separation. Nat Commun 2018, 9:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. : Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, et al. : Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]