Abstract
Background and Purpose:
Most pregnancy-related ischemic (IS) and hemorrhagic (HS) strokes occur postpartum. Infections have been identified as a trigger for strokes in young people, and have been associated with strokes during delivery hospitalizations, but a temporal relationship has been difficult to establish. We hypothesized that infections diagnosed during a delivery admission would be associated with an increased risk of readmission for postpartum stroke.
Methods:
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project’s National Readmissions Database from 2010 through 2014. Using weighted survey design Poisson regression analysis, we calculated adjusted risk ratios (aRR) and 95% confidence intervals (CI) for the association between infection during delivery admission and 30-day postpartum readmission for IS or HS.
Results:
Out of 17.2 million delivery admissions during the study period, 2128 were readmitted within 30 days for a stroke of any type. There were 1189 hemorrhagic strokes (ICH and/or SAH) and 720 ischemic strokes, and the remainder unspecified pregnancy-related stroke. Adjusting for age and comorbidities, women with delivery infections were at higher risk of readmission for postpartum stroke of any type (aRR 1.19, 95%CI 1.01–1.41). Women with infections had higher risk of readmission for postpartum IS (adjusted RR 1.75, 95%CI 1.37–2.22), but not for postpartum HS (adjusted RR 0.96, 95%CI 0.75–1.23). The effect of infection on 30-day IS readmission was larger in women without hypertensive disorders of pregnancy (HDP) (aRR 2.04, 95%CI 1.55–2.69 in women without HDP, versus aRR 1.47, 95%CI 0.9–2.38 in women with HDP, p-value for interaction=0.09).
Conclusions:
Infection during delivery hospitalization was associated with increased risk of readmission for IS, but not HS, within 30 days postpartum, particularly in women without HDP. Infection may play a role in triggering postpartum IS even in the absence of other risk factors.
Keywords: Infection, Pregnancy, Stroke, Postpartum
Subject terms: Pregnancy, Women, Risk Factors, Ischemic Stroke, Intracranial Hemorrhage
INTRODUCTION
Mounting attention has focused on alarming rates of maternal morbidity and mortality in the United States (US), which appear to be increasing.1 Maternal stroke accounts for 7.4% of maternal mortality in the US and is a major cause of maternal morbidity.2,3 The majority of pregnancy-associated strokes, both hemorrhagic and ischemic, occur in the postpartum period.4 The risk of postpartum stroke is higher in women with chronic hypertension and hypertensive disorders of pregnancy (HDP), including gestational hypertension, preeclampsia and eclampsia.5 However, up to 80% of postpartum strokes occur in women with no history of chronic hypertension or HDP.6 Even in women with chronic hypertension or HDP, postpartum strokes are difficult to predict, and most occur after women have been discharged home from the hospital following delivery.7
While increasing HDP rates likely contribute to the increased stroke risk postpartum,8 it is imperative that we identify additional potential triggers for postpartum stroke. Infections have been associated with strokes during delivery hospitalizations,9 but administrative data from a single admission have limited ability to discern the temporal relationship between infection and stroke. The Healthcare Cost and Utilization Project’s (HCUP) Nationwide Readmissions Database (NRD) tracks readmissions after hospitalizations in the US. We hypothesized that infections diagnosed during delivery hospitalizations would be associated with an increased risk of readmission for postpartum stroke of any type.
METHODS
We conducted a retrospective cohort study using the NRD from 2010 through 2014 to assess the relationship between infection during a delivery hospitalization and 30-day readmission for a stroke of any type. The NRD is an all-payer database that tracks patients across hospital admissions within a state, using verified patient linkage numbers, and includes public hospitals, community hospitals, and academic medical centers. Data in the NRD are weighted to generate national estimates of readmissions. The requirement for informed consent was waived by Columbia University’s Institutional Review Board due to the de-identified, publically available nature of the data. All data in the NRD are available from HCUP and can be accessed at: https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp.
Delivery hospitalization, strokes, infections, and comorbidities were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic codes. Codes 650 and V27.x were used to identify all delivery hospitalizations in women aged 15–54. These diagnostic codes have been shown to ascertain greater than 95% of delivery hospitalizations.10 Women were considered to have the primary exposure of interest, infection during delivery admission, if they had diagnostic codes for one or more of the following conditions during their delivery hospitalization: sepsis, genitourinary infections, gastrointestinal infections, respiratory infections, sexually transmitted infections, puerperal infections, and other infections (Supplemental Table I, see http://stroke.ahajournals.org), identified using HCUP diagnostic codes.11 Women with a stroke during the delivery hospitalization were excluded from the analysis. The primary outcome, postpartum stroke, was defined as readmission within 30 days of delivery discharge for stroke of any type, including ischemic stroke (IS) (excluding transient ischemic attack), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), or acute cerebrovascular disease in the puerperium (Supplemental Table II, see http://stroke.ahajournals.org). Cerebral venous thrombosis was not included in the outcome, as there is a clearly established strong association between infection and postpartum cerebral venous thrombosis.12 Women who were readmitted for reasons other than stroke were not excluded, and were considered not to have met the primary outcome. Secondary outcomes included readmission for any stroke within 60 days of delivery discharge, and 30-day readmission for hemorrhagic stroke (ICH and/or SAH) and IS separately.
Because the NRD datasets are year-based and cannot be linked, only delivery hospitalizations where discharge occurred from January 1 through November 30 for each year were included in the primary outcome analysis; delivery hospitalizations during December were not included because readmissions for the subsequent 30 days could not be fully ascertained. Similarly, for the secondary outcome of 60-day readmissions, only delivery hospitalizations occurring from January 1 through October 31 for each year were included.
Demographic factors included maternal age, payer, and ZIP-code derived income quartile. Race and ethnicity data are not included in the NRD, and imputation from census data was not possible as the database does not include specific geographic regions or ZIP codes, only income levels. Maternal comorbidities included HDP, defined as gestational hypertension, mild or severe preeclampsia, “superimposed” preeclampsia (preeclampsia with pre-existing chronic hypertension), and eclampsia; chronic hypertension; cesarean delivery during the index hospitalization; and an obstetric comorbidity index that provides weighted scores for comorbidity for individual patients based on the presence of specific diagnosis codes and demographic factors present in administrative data, with higher scores associated with increased risk for severe morbidity.13 Comorbidities included in this index include age and the following conditions: advanced maternal age, pulmonary hypertension, sickle cell disease, chronic renal disease, preexisting hypertension, chronic ischemic heart disease, congenital heart disease, cardiac valvular disease, chronic congestive heart failure, asthma, preexisting diabetes mellitus, systemic lupus erythematosus, HIV, and drug or alcohol use, as well as obstetric comorbidities such as placentia previa, multiple gestation, previous cesarean delivery, gestational hypertension, mild or unspecified preeclampsia, and severe preeclampsia or eclampsia.13 Due to concern for multi-collinearity, in calculating the comorbidity index score, we excluded hypertensive disorders (including chronic hypertension and HDP) and age from the comorbidity index, adjusting for these variables separately. We then categorized women based on their number of obstetric comorbidities contained in the obstetric comorbidity index score: 0 conditions (lowest risk), 1 or 2 conditions, and >2 conditions (highest), without weighting comorbidities. Hospital factors included length of delivery hospitalization; hospital size; and teaching versus non-teaching status.
Statistical analysis
We created weighted survey design Poisson regression models to calculate crude and adjusted risk ratios (aRR) and 95% confidence intervals (95%CI) for the association between infections and readmission for stroke. We considered an alpha of 0.05 significant. To assess if the effect of infection on stroke readmission differed in women with HDP, we tested for interactions and created stratified models for women with and without HDP. To increase our power to detected a clinically important interaction for a rare outcome, we considered a two-sided alpha of 10% significant for an interaction term, recognizing the potential for increased type 1 error.14 We adjusted for demographics, number of maternal comorbidities, and hospital factors in our analysis. Due to concern that categorizing women by a comorbidity index not designed specifically for stroke risk could under- or overestimate comorbidity risk, we performed a secondary analysis of our data which did not use the comorbidity index-based categories, and instead adjusted separately for known maternal stroke risk factors including heart disease, obesity, smoking, migraine, chronic kidney disease, hematological disorders, diabetes, lupus, and history of thromboembolic events.7,12,15,16 All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Primary outcome.
There were 17,215,614 admissions for delivery in the US during the years 2010–2014 from January 1 through November 30 of each year. Of these, 2128 were readmitted with a postpartum stroke within 30 days (cumulative incidence 12.36 per 100,000 deliveries). Median time to stroke readmission was 6.7 days (interquartile range, 3.8 to 10.9). Among women with postpartum stroke, 1189 (55.9%) were hemorrhagic, comprising 457 (38.4%) ICH, 646 (54.4%) SAH, and 87 (7.3%) both ICH and SAH; 720 (33.8%) were ischemic; and the remainder had unspecified pregnancy-associated stroke. Women who were readmitted for stroke within 30 days were older, had lower income and a lower proportion of private insurance, and had a higher proportion of cesarean delivery, hypertensive disorders, and obstetric comorbidities, compared to women who were not readmitted or readmitted for reasons other than stroke (Table 1). Stroke readmission rates, including subtypes, are shown in Table 2. Discharge dispositions after 30-day readmission for stroke are shown in Table 3.
TABLE 1.
Characteristics of women delivering in 2010–2014, with and without readmission within 30 days for any stroke
| No stroke readmission (n= 17,215,614) |
Stroke readmission within 30 days (n=2128) | ||
|---|---|---|---|
| n (%) | n (%) | p-value | |
| 1 or more infection during delivery hospitalization | 876,633 (5.1) | 154 (7.3) | 0.01 |
| Median age in years (interquartile range) | 27.4 (22.8–31.8) | 30.0 (25.1–34.2) | <0.0001 |
| Age category | <0.0001 | ||
| 15–19 years | 1,400,688 (8.1) | 72 (3.4) | |
| 20–29 years | 8,829,809 (51.3) | 891 (41.9) | |
| 30–39 years | 6,474,000 (37.6) | 1019 (47.9) | |
| >40 years | 508,988 (3.0) | 146 (6.9) | |
| Payer status | 0.11 | ||
| Medicare | 106,852 (0.6) | 24 (1.1) | |
| Medicaid | 7,381,006 (42.9) | 1018 (47.9) | |
| Private insurance | 8,839,651 (51.4) | 992 (46.6) | |
| Self pay | 273,093 (1.6) | 22 (1.0) | |
| No charge | 10,788 (0.1) | suppressed* | |
| Other | 554,310 (3.2) | 59 (2.8) | |
| Median income by ZIP code | 0.0006 | ||
| Quartile 1 (low) | 4,790,694 (27.8) | 740 (34.8) | |
| Quartile 2 | 4,208,781 (24.5) | 518 (24.3) | |
| Quartile 3 | 4,245,785 (24.7) | 453 (21.3) | |
| Quartile 4 (high) | 3,789,326 (22.0) | 402 (18.9) | |
| Hospital bed size | 0.13 | ||
| Small | 1,933,454 (11.2) | 208 (9.8) | |
| Medium | 4,524,196 (26.3) | 508 (23.9) | |
| Large | 10,755,836 (62.5) | 1412 (66.3) | |
| Hospital teaching status* | 0.007 | ||
| Metropolitan non-teaching | 6,340,768 (36.8) | 753 (35.4) | |
| Metropolitan teaching | 9,008,145 (52.3) | 1218 (57.2) | |
| Non-metropolitan | 1,864,573 (10.8) | 157 (7.4) | |
| Mean delivery length of stay in days | 2.69 | 3.15 | 0.0008 |
| Cesarean delivery | 5686377 (33.0) | 863 (40.6) | <0.0001 |
| Maternal comorbidity index | <0.0001 | ||
| 1 comorbid condition | 4,589,518 (26.7) | 645 (30.3) | |
| 2 or more conditions | 1,281,976 (7.5) | 319 (15.0) | |
| Chronic hypertension without preeclampsia | 290798 (1.7) | 89 (4.2) | <0.0001 |
| Hypertensive disorder of pregnancy (gestational hypertension, preeclampsia or eclampsia)* | 1,376,067 (8.0) | 294 (13.8) | <0.0001 |
Cells with numbers fewer than 10 suppressed according to HCUP guidelines.
TABLE 2.
Stroke readmission rates among women delivering in 2010–2014
| Number of deliveries | Number of stroke readmissions | Postpartum stroke readmission rate per 100,000 deliveries (95% CI) | |
|---|---|---|---|
| 30 day readmissions | |||
| Any stroke | 17,215,614 | 2128 | 12.4 (11.9, 12.9) |
| Ischemic stroke | 17,215,614 | 720 | 4.2 (3.9, 4.5) |
| Hemorrhagic stroke | 17,215,614 | 1189 | 6.9 (6.5, 7.3) |
| ICH | 457 | ||
| SAH | 646 | ||
| Both ICH and SAH | 87 | ||
| 60 day readmissions | |||
| Any stroke | 15,699,588 | 2192 | 14.0 (13.3, 14.6) |
| Ischemic stroke | 15,699,588 | 825 | 5.3 (4.9, 5.6) |
| Hemorrhagic stroke | 15,699,588 | 1183 | 7.5 (7.1, 8.0) |
CI: confidence interval. ICH: Intracerebral hemorrhage. SAH: Subarachnoid hemorrhage. Ischemic stroke did not include transient ischemic attack (see Supplemental Table 2 for codes used). Stroke type could not be ascertained in all cases.
TABLE 3.
Discharge disposition for US women after re-admission within 30 days of delivery for any stroke, 2010–2014 (n=2128)
| Home | 1617 (76.0%) |
| Other acute care hospital | 45 (2.1%) |
| Skilled nursing or other long term facility | 160 (7.5%) |
| Left against medical advice | 21 (1.0%) |
| Died | 69 (3.3%) |
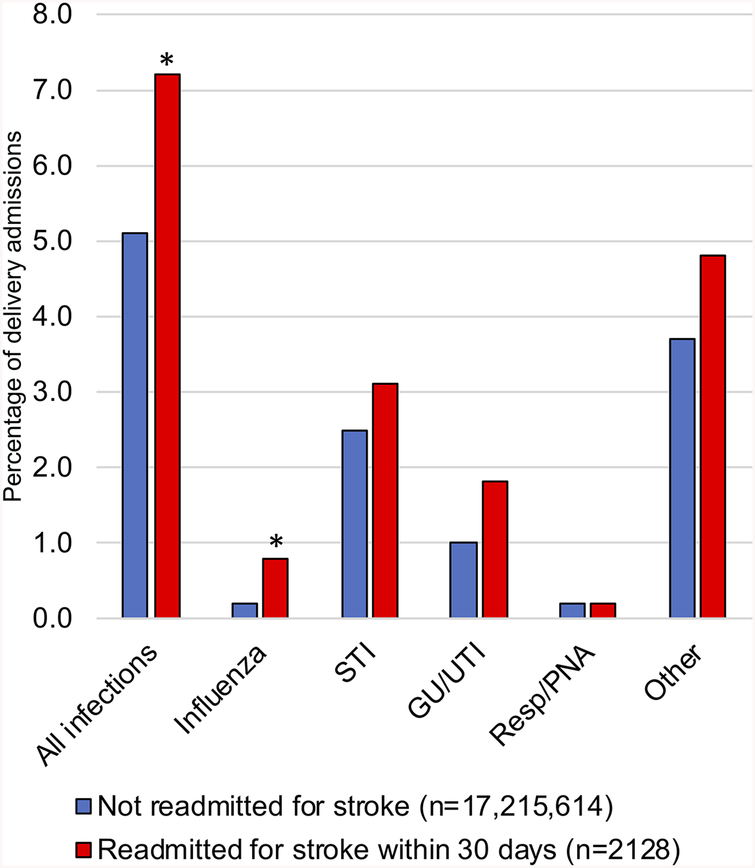
During the initial delivery hospitalization, one or more infections were diagnosed in 876,633 (5.1%) of the 17.2 million women who did not go on to have a postpartum stroke, and 154 (7.3%) of the 2128 women who were readmitted within 30 days with stroke. Adjusting for number of maternal comorbidities and hospital characteristics, women with infections diagnosed during their delivery hospitalizations had higher risk of readmission for postpartum stroke within 30 days (absolute risk difference, 5.5 per 100,000 deliveries, 95% CI 3.1–7.9; adjusted RR 1.19, 95% CI 1.01–1.41). Infections of all types were more common in women who went on to be readmitted within 30 days for stroke (Figure 1). Among infection subgroups, only the difference in influenza infections was statistically significant between the stroke and non-stroke groups. Among women with influenza during the delivery hospitalization, demographics and clinical factors were similar between the stroke and non-stroke groups, with the exception that there was a higher proportion of cesarean deliveries in the stroke group (91.3% in the stroke group, vs. 48.2% in the non-stroke group, p=0.009).
Figure 1. Infections during US delivery hospitalizations, 2010–2014.
Legend: Percentage of women with infections diagnosed during delivery hospitalizations who were (red) and were not (blue) readmitted for any stroke within 30 days of delivery. GU: genitourinary. UTI: urinary tract infection. Resp: respiratory infection. PNA: pneumonia. STI: sexually transmitted infection. Diagnostic codes used to identify infections are included in eTable 1. *p<0.05
Secondary outcomes
60-day readmission for any stroke.
There were 15,699,588 women admitted for delivery during the years 2010–2014 from January 1 through October 31 of each year, of whom 2192 were readmitted with stroke within 60 days (14 per 100,000 deliveries). Women who had an infection diagnosed during their delivery hospitalization had higher risk of 60-day readmission with stroke (adjusted RR 1.21, 95%CI 1.03–1.42).
Ischemic and hemorrhagic subgroups.
Women who had infections during delivery admissions were at higher risk of readmission for IS in both the 30-day and 60-day time windows (30-day adjusted RR 1.75, 95%CI 1.37–2.22; 60-day adjusted RR 1.71, 95%CI 1.36–2.14). For HS, infection did not confer an increased risk of readmission in either the 30-day or 60-day windows (30-day adjusted RR 0.96, 95%CI 0.75–1.23; 60-day adjusted RR 0.98, 95%CI 0.76–1.26) (Table 4).
TABLE 4.
Relative risk of readmission for stroke within 30 and 60 days among women with infections during delivery hospitalization
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | |
| All stroke, 30 day readmission | 1.46 | 1.24, 1.72 | 1.19 | 1.01, 1.41 |
| All stroke, 60 day readmission | 1.48 | 1.26, 1.74 | 1.21 | 1.03, 1.42 |
| Ischemic stroke, 30 days | 2.27 | 1.79, 2.87 | 1.75 | 1.37, 2.22 |
| Ischemic stroke, 60 days | 2.24 | 1.79, 2.79 | 1.71 | 1.36, 2.14 |
| Hemorrhagic stroke, 30 days | 1.13 | 0.88, 1.44 | 0.96 | 0.75, 1.23 |
| Hemorrhagic stroke, 60 days | 1.12 | 0.88, 1.43 | 0.98 | 0.76, 1.26 |
Interaction of infection with hypertensive disorders of pregnancy.
There was no interaction between infection during delivery hospitalization and HDP on the primary outcome (p-value for interaction = 0.99). However, there was an interaction between infection and HDP for the IS subgroup (p-value for interaction = 0.09). In stratified analyses, the effect of infection on 30-day readmission for IS was larger in women without HDP (adjusted RR 2.04, 95%CI 1.55–2.69, p<0.01 among women without HDP, versus adjusted RR 1.47, 95%CI 0.9–2.38, p=0.12 among those with HDP).
Secondary analysis with separate adjustment for maternal stroke risk factors
Adjusting for maternal stroke risk factors separately, rather than using the obstetric index, we found a similar effect size for the primary outcome of any stroke readmission within 30 days; however, the difference was not statistically significant (adjusted RR 1.15, 95%CI 0.97–1.36). However, for the outcome of readmission within 30 days for IS only, the effect of infection on increased stroke risk remained statistically significant (adjusted RR 1.67, 95%CI 1.31–2.12).
DISCUSSION
In our retrospective cohort study using the National Readmissions Database, we found that women diagnosed with an infection during their delivery hospitalization had 19% increased risk of 30-day readmission for any stroke. Subgroup analyses revealed a 75% increased risk for IS, but no increased risk for HS. These data support findings from prior studies which have shown an association between infection and maternal stroke,7,9,12 and provide additional evidence that this association may be specific to IS. Interestingly, the association was stronger in women without HDP, potentially indicating a separate mechanism whereby the risk of stroke is increased postpartum.
Our study has important implications for maternal health. While postpartum strokes are rare, occurring in approximately 14.7 per 100,000 deliveries according to a recent systematic review and meta-analysis,17 these events can result in death or permanent disability, with devastating consequences for a young woman and her family.18 HDP are well known to increase the risk of postpartum stroke, but many strokes occur in women without a history of chronic hypertension or HDP.6 It is essential that we identify other risk factors to help identify women at higher risk of postpartum stroke. The association between infection and postpartum stroke may have particular relevance in young patients who lack traditional vascular risk factors.19,20 In identifying these associations, we found an additional high risk group where tailored interventions could be implemented to reduce the risk of postpartum stroke. Considering that there are more than 3.8 million births annually in the US,21 the absolute risk difference we found of 5.5 fewer strokes per 100,000 deliveries could result in an important reduction in maternal morbidity if they could be prevented by a targeted intervention.
The link between infection and IS is well described and not unique to pregnant women, but pregnant women may be more susceptible to infections due to pregnancy.22,23 Pregnancy is an immunomodulated state, with increased susceptibility to some infections and alterations in the inflammatory response triggered by infections such as influenza.24,25 In addition, pregnant women have unique risks of puerperal infections such as chorioamnionitis, endometritis, mastitis, and abdominal or perineal wound infections.26 Infection is a significant contributor to maternal morbidity and remains the third leading cause of maternal death in the US.2 Sexually transmitted infections such as Chlamydia trachomatis are common in young women and may go undetected, particularly in women with low access to prenatal care.27 Interestingly, respiratory infections, which have been associated with IS in other studies, were uncommon in our cohort.20,28,29 However, it is notable that the influenza subgroup was the only infection subgroup to show a statistically significant difference between the stroke and non-stroke groups. This finding is consistent with prior studies in other populations that have shown that influenza is associated with particular risk for ischemic stroke, and raises the possibility that improving influenza vaccination rates among pregnant women might reduce the risk of postpartum stroke.20,30
The mechanisms by which infections could lead to an increased risk of maternal IS warrant investigation. Infections increase the risk of preeclampsia, with proposed mechanisms including increased oxidative stress and pro-inflammatory cytokines, endothelial dysfunction, and a shift from Th2 to Th1 inflammatory profile.31–33 Similar mechanisms may be involved in the increased risk of postpartum stroke. In addition, alterations in the maternal oral, gut and vaginal microbiome have been linked to preterm birth, excessive maternal weight gain, and chorioamnionitis,34,35 possibly due to chronic inflammation, which has also been shown to increase stroke risk.36 Infection-induced platelet activation likely also plays a role, especially in the setting of pregnancy-related hypercoagulability; this is supported by our finding that an effect of infection was seen for IS but not HS.
Our study has limitations. Despite our use of validated diagnostic codes, administrative data are limited and strokes may have been over- or under-diagnosed. In addition, strokes may have been misclassified as hemorrhagic, for example if a woman had hemorrhagic transformation of an ischemic stroke, or hemorrhage due to a venous sinus thrombosis. Similarly, infections may have been over- or under-diagnosed, leading to misclassification. Since the NRD does not contain information about medication use, it is impossible to know whether infections were treated, and whether the increased risk of stroke was due to infection itself, or the treatment (or lack of treatment) for the infection. The use of a higher threshold for significance (alpha of 10%) for interaction may have led to increased Type 1 error, although our stratified analyses suggested effect modification between HDP and infection. The use of an obstetric comorbidity index not designed specifically to estimate stroke risk may have led to under- or overestimation of comorbidity risk, although our secondary analysis adjusting separately for stroke risk factors showed similar effect sizes. As a retrospective, observational study, our results should be interpreted with caution.
The lack of race or ethnicity data in the NRD is an important limitation of our study. Research has consistently shown racial disparities in maternal morbidity and mortality outcomes.37–39 Future studies in other large databases should address whether the effect of infection on postpartum ischemic stroke differs by race or ethnicity.
Strengths of our study include its large size and use of a nationwide weighted database, allowing study of a rare disease, and making the results highly generalizable to geographically and ethnically diverse populations within the US. In addition, among administrative datasets, the NRD offers the unique advantage of confirming that the infection temporally preceded the stroke, since we excluded all delivery admissions that included a diagnostic code for stroke, although it is possible that some strokes may have been missed initially and only diagnosed upon readmission. We used only well-validated diagnostic codes to identify our outcomes, excluding codes for transient ischemic attack and other less reliable codes.40,41
SUMMARY/CONCLUSIONS:
Infection during delivery hospitalization was associated with increased risk of readmission for ischemic, but not hemorrhagic stroke within 30 days postpartum. Infection may play a role in triggering postpartum ischemic stroke, particularly in women without other risk factors such as gestational hypertension or preeclampsia. Women with infections during delivery admissions should be counseled on signs and symptoms of stroke, and may warrant closer postpartum follow-up.
Supplementary Material
Funding:
Dr. Miller received support from the National Center for Advancing Translational Sciences, National Institutes of Health (KL2TR001874), and from the Louis V. Gerstner, Jr. Foundation. Dr. Friedman is supported by a career development award (K08HD082287) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Dr. Elkind receives support from the Giordano Family Foundation.
Footnotes
Disclosures: Dr. Miller receives personal compensation for medicolegal consulting related to maternal stroke. Dr. Elkind receives personal compensation from Merck/Organon for expert witness testimony related to hormonal contraception and stroke, and from UpTodate for chapters related to stroke.
REFERENCES
- 1.Mann S, Hollier LM, McKay K, Brown H. What We Can Do about Maternal Mortality - And How to Do It Quickly. N. Engl. J. Med 2018;379:1689–1691. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Pregnancy Mortality Surveillance System [Internet]. cdc.gov [cited 2019 Feb 14];Available from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html
- 3.Callaghan WM, MacKay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. The American Journal of Obstetrics & Gynecology. 2008;199:133.e1–133.e8. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Chan WS, Ray JG, Kramer MS, Joseph KS, for the Canadian Perinatal Surveillance System (Public Health Agency of Canada). Stroke and Cerebrovascular Disease in Pregnancy. Stroke. 2019;50:13–20. [Google Scholar]
- 5.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstetrics & Gynecology. 2015;125:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, Mack WJ, DʼAlton ME, Friedman AM. Timing and Risk Factors of Postpartum Stroke. Obstetrics & Gynecology. 2018;131:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert LR, Marshall RS, Elkind MSV, Willey JZ. Risk Factors for Pregnancy-Associated Stroke in Women With Preeclampsia. Stroke. 2017;48:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42:2564–2570. [DOI] [PubMed] [Google Scholar]
- 9.Miller EC, Gallo M, Kulick ER, Friedman AM, Elkind MSV, Boehme AK. Infections and Risk of Peripartum Stroke During Delivery Admissions. Stroke. 2018;49:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, Marchbanks PA. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern Child Health J. 2008;12:469–477. [DOI] [PubMed] [Google Scholar]
- 11.Agency for Healthcare Research and Quality, editor. Introduction to the HCUP State Inpatient Databases. HCUP 2017. [cited 2017 Dec 5];Available from: https://www.hcup-us.ahrq.gov/sidoverview.jsp
- 12.Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31:1274–1282. [DOI] [PubMed] [Google Scholar]
- 13.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, Callaghan WM, Gagne JJ. Development of a comorbidity index for use in obstetric patients. Obstetrics & Gynecology. 2013;122:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiol Perspect Innov. 2007;4:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N. Engl. J. Med 1996;335:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516. [DOI] [PubMed] [Google Scholar]
- 17.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert LR, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. International Journal of Stroke. 2017;12:687–697. [DOI] [PubMed] [Google Scholar]
- 18.Foo L, Bewley S, Rudd A. Maternal death from stroke: a thirty year national retrospective review. Eur. J. Obstet. Gynecol. Reprod. Biol 2013;171:266–270. [DOI] [PubMed] [Google Scholar]
- 19.Elkind MSV, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, deVeber GA, Fullerton HJ. Herpesvirus Infections and Childhood Arterial Ischemic Stroke: Results of the VIPS Study. Circulation. 2016;133:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MSV. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: Final Data for 2017. National Vital Statistics Reports. 2018;67:1–50. [PubMed] [Google Scholar]
- 22.Cowan LT, Alonso A, Pankow JS, Folsom AR, Rosamond WD, Gottesman RF, Lakshminarayan K. Hospitalized Infection as a Trigger for Acute Ischemic Stroke: The Atherosclerosis Risk in Communities Study. Stroke. 2016;47:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller EC, Elkind MSV. Infection and Stroke: an Update on Recent Progress. Curr Neurol Neurosci Rep. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol. 2013;2013:752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kourtis AP, Read JS, Jamieson DJ. Pregnancy and Infection. N. Engl. J. Med 2014;370:2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karsnitz DB. Puerperal infections of the genital tract: a clinical review. J Midwifery Womens Health. 2013;58:632–642. [DOI] [PubMed] [Google Scholar]
- 27.Howie SEM, Horner PJ, Horne AW. Chlamydia trachomatis infection during pregnancy: known unknowns. Discov Med. 2011;12:57–64. [PubMed] [Google Scholar]
- 28.Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fullerton HJ, Hills NK, Elkind MSV, Dowling MM, Wintermark M, Glaser CA, Tan M, Rivkin MJ, Titomanlio L, Barkovich AJ, deVeber GA, VIPS Investigators. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS study. Neurology. 2015;85:1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin H-C, Chiu H-F, Ho S-C, Yang C-Y. Association of influenza vaccination and reduced risk of stroke hospitalization among the elderly: a population-based case-control study. Int J Environ Res Public Health. 2014;11:3639–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: systematic review and metaanalysis. The American Journal of Obstetrics & Gynecology. 2008;198:7–22. [DOI] [PubMed] [Google Scholar]
- 32.Easter SR, Cantonwine DE, Zera CA, Lim K-H, Parry SI, McElrath TF. Urinary tract infection during pregnancy, angiogenic factor profiles, and risk of preeclampsia. American Journal of Obstetrics and Gynecology. 2016;214:387.e1–7. [DOI] [PubMed] [Google Scholar]
- 33.Nourollahpour Shiadeh M, Behboodi Moghadam Z, Adam I, Saber V, Bagheri M, Rostami A. Human infectious diseases and risk of preeclampsia: an updated review of the literature. Infection. 2017; 45(5):589–600 [DOI] [PubMed] [Google Scholar]
- 34.Dunlop AL, Mulle JG, Ferranti EP, Edwards S, Dunn AB, Corwin EJ. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv Neonatal Care. 2015;15:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinturache AE, Gyamfi-Bannerman C, Hwang J, Mysorekar IU, Jacobsson B. Maternal microbiome - A pathway to preterm birth. Semin Fetal Neonatal Med. 2016;21:94–99. [DOI] [PubMed] [Google Scholar]
- 36.Elkind MSV, Ramakrishnan P, Moon YP, Boden-Albala B, Liu KM, Spitalnik SL, Rundek T, Sacco RL, Paik MC. Infectious Burden and Risk of Stroke: The Northern Manhattan Study. Arch. Neurol 2010;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen JJ, Tymkow C, MacMullen N. Disparities in maternal outcomes among four ethnic populations. Ethn Dis. 2005;15:492–497. [PubMed] [Google Scholar]
- 38.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. The American Journal of Obstetrics & Gynecology. 2014;210:435.e1–8. [DOI] [PubMed] [Google Scholar]
- 39.Gyamfi-Bannerman C, Pandita A, Miller EC, Boehme AK, Wright JD, Siddiq Z, D’Alton ME, Friedman AM. Preeclampsia outcomes at delivery and race. J Matern Fetal Neonatal Med. 2019;131:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 41.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert LR, Elkind MSV, Willey JZ. Risk of Pregnancy-Associated Stroke Across Age Groups in New York State. JAMA Neurol. 2016;73:1461–1467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.