Abstract
Objective:
Leptin is critical for central nervous system development and maturation. We aimed to evaluate the potential regualatory role of cord leptin in the neuropsychomotor development of children from 18 months to 6 years.
Methods:
We included 424 children from a prospective mother-child cohort, Crete, Greece (Rhea Study), with available cord leptin levels and data on neurodevelopmental outcomes at 18 months (Bayley Scales of Infant & Toddler Development III), 4 (McCarthy Scales of Children’s Abilities) and 6 years (Raven’s Coloured Progressive Matrices, Trail-Making Test). Multivariable linear regression models were used to explore the associations.
Results:
A per 10 ng/ml increase in cord leptin was associated with increased scores in gross motor scale at 18 months (β-coef: 3.8, 95%CI: 0.0, 7.5), with decreased scores in general cognitive (β-coef: −3.0, 95%CI: −5.5, −0.4), perceptual performance (β-coef: −3.4, 95%CI: −6.0, −9.9), working memory (β-coef: −3.1, 95%CI: −5.7, −0.4), executive function (β-coef −3.1, 95%CI: −5.7, −0.5) and functions of posterior cortex (β-coef: −2.7, 95%CI: −5.2, −0.1) scales at 4 years, and with a 3.7 unit decrease in Raven’s score at 6 years (β-coef: −3.7, 95%CI: −6.9, −0.5).
Conclusion:
Increased cord leptin is associated with enhanced gross motor development at 18 months, but decreased cognitive performance in early and middle childhood.
Keywords: perinatal programming, leptin, cognitive development, children
Introduction
Brain development starts during the embryonic period and extends throughout adolescence. The prenatal period is critical for normal brain development, coordinated neuron growth, differentiation and function; several biological, psychological and social events may influence this process (1). A growing body of evidence suggests the presence of a critical time window during fetal programming, during which neurodegenerative diseases have their origin (2). In this regard, the regulation of normal brain development has been linked to hormones that cross the blood brain barrier and bind to receptors within various brain regions (3).
Leptin is a cytokine-like protein mainly produced by the adipose tissue. It is a well-established regulator of appetite and energy expenditure through its action on the hypothalamus (4). Apart from the hypothalamus, leptin and its receptors are widely expressed in brain regions of the central nervous system (CNS) such as the hippocampus, cerebral cortex, basal ganglia, brainstem and cerebellum, regulating numerous functions including cognition and memory processing (5). Cognitive performance is impaired in leptin deficiency models, while leptin replacement improves memory and learning in mice and in some cases, has improved neurocognitive development in patients with congenital leptin deficiency (6). Leptin has been found to be a crucial regulator of CNS development in both rodents and humans (7). Although leptin in the physiological range serves as an enhancer of cognition (8), elevated levels may act as a pathophysiological marker for impaired cognitive function due to leptin resistance.
Serum leptin levels are proportional to adiposity and higher in individuals with obesity (4). In children, adipose tissue-derived inflammatory mediators, such as leptin, create an inflammatory milieu (9), which can affect brain areas critical for cognition, neural plasticity and neurogenesis (10). Indeed, biomarkers of obesity have been associated with cognitive decline among adults (11), but this association has been less examined in children (12). Studies have shown that increased serum leptin levels were associated with reduced cognitive development in infants 6–24 months of age (13). The main objective of the present work was to investigate the potential regulatory role of cord leptin in the neuropsychomotor development of children from 18 months to 6 years, leveraging existing resources from the Mother–Child Cohort (Rhea Study) in Crete, Greece (14).
Methods
Study participants
The present study is part of the “Rhea” study, a prospective pregnancy cohort that recruited pregnant women at around week 12 of gestation from February 2007 to January 2008, at the prefecture of Heraklion, Crete, Greece (www.rhea.gr). Mothers were contacted again at 24 weeks of gestation and at birth; regarding offspring follow-up, time points were 9th month, 18th month, 4 years, and 6 years of age. Children visits were performed at the University Hospital of Heraklion or at the closest health center for families living in rural areas. The study was approved by the Ethics Committee of the University Hospital of Heraklion and all participants provided written informed consent. Further details are provided elsewhere (14).
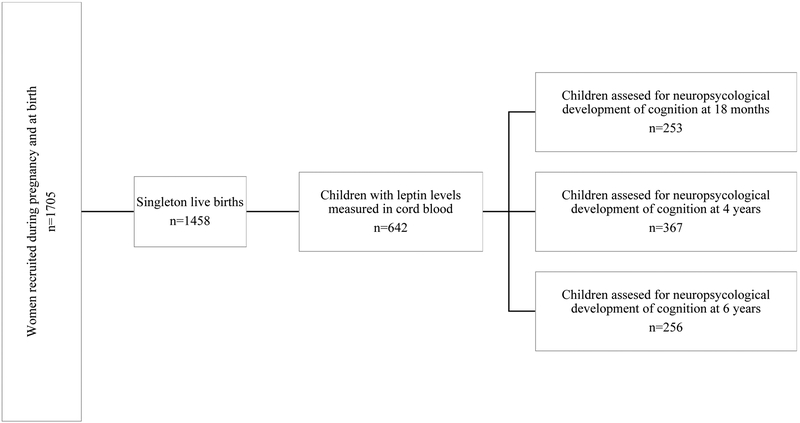
424 offspring had leptin levels measured in cord blood and were assessed at least once from birth to 6 years in terms of neuropsychomotor development (253, 367 and 256 children at 18 months, 4 and 6 years of age, respectively) (Figure 1).
Figure 1:
Flow diagram depicting the study design.
Cord leptin measurements
We collected umbilical cord blood in 10-mL BD gel separator vacutainers. The samples were centrifuged within 2 h after collection at 2500 rpm for 10 minutes. Serum was separated into 0.5 mL aliquots and stored at −80 °C, until assayed. Human leptin was determined in serum with Quantikine Human Leptin [solid phase enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN)], as described elsewhere (15).
Cord leptin was assessed as continuous (increase per 10 ng/ml) and as categorical exposure, according to percentiles of cord leptin levels. Neonates with cord leptin between the 10th and 90th percentile were considered as having a normal leptin status (reference group). All other subjects were divided into two groups: 1) Hyperleptinemia, with cord leptin over the 90th percentile (≥ 14.6 ng/mL); 2) Hypoleptinemia, with cord leptin under the 10th percentile (≤ 2.1 ng/mL). Both groups of hypo- and hyper- leptinemia were compared with the reference group in the analyses (15).
Outcomes
Neuropsychomotor development assessment at 18 months
At 18 months [mean (SD): 18.2 (0.7) months], children’s mental and psychomotor development was assessed by three trained psychologists (assignment at random, with an excellent inter-rater reliability (16)), using the Bayley Scales of Infant and Toddler Development (Bayley-III) (17). The Bayley-III assesses infant and toddler development across three scales: (i) The Cognitive Scale, (ii) The Language Scale, composed of the Receptive Communication (RC) and the Expressive Communication (EC) subtest, and (iii) The Motor Scale, divided into the Fine Motor (FM) and the Gross Motor (GM) subtest. Raw scores were standardized for examiner and child’s age at test administration using a parametric method for the estimation of age-specific reference intervals and homogenized with a mean (SD) of 100 (15). Scores were treated as continuous variables with higher scores representing better performance.
Neuropsychomotor development assessment at 4 years
At 4 years of age [mean (SD): 4.3 (0.3) years], children’s cognitive and motor development was assessed by two trained psychologists with the McCarthy Scales of Children’s Abilities (MCSA) (18). The MCSA include five conventional scales (verbal, quantitative, memory, perceptual performance, and motor) and a general cognitive scale, which is a composite scale of verbal, perceptual performance and quantitative scales. MCSA raw scores were standardized for child’s age and homogenized with a mean (SD) of 100 (15) (19). Executive function, working memory, memory span and cognitive functions of posterior cortex are four additional scales derived from the MSCA test in accordance with their association with specific neurocognitive function areas (20). Children were assigned to the two psychologists at random and the inter-observer variability was <1%. Scores were treated as continuous variables with higher scores representing better performance. Further details on the methods for the neuropsychomotor developmental assessment at 18 months and 4 years have been reported elsewhere (16, 19).
Neuropsychomotor development assessment at 6 years
At 6 years of age [mean (SD): 6.6 (0.3) years], children’s neuropsychomotor development was evaluated by computerized tests with Raven’s Coloured Progressive Matrices, Trail-Making Test (TMT) A and B and Finger Tapping Test. Raven’s Coloured Progressive Matrices (21) measures clear-thinking ability and is designed for young children ages 5–11 years and older adults. The test consists of 36 items in three sets with 12 items per set. Raven’s raw scores were standardized for child age and then homogenized with a mean (SD) of 100 (15). The TMT is mainly used to assess visual scanning/processing speed (part A) and executive functions (part B). TMT provides information on visual search, scanning, speed of processing, mental flexibility, and executive functions (22). The Finger Tapping Test is designed to evaluate muscle control and motor ability in the upper extremities and is used for the motor speed measurement and for the lateralization index calculation (23). Raw scores were used for TMT and Finger Tapping Test, as suggested by the literature (24, 25); a faster response time and a higher number of taps, respectively, indicate better performance.
Statistical analysis
Continuous, non-normally distributed variables were tested by using Mann–Whitney and Kruskal–Wallis nonparametric statistical tests. Normally distributed variables were tested with t-test and categorical variables with chi-square test (Pearson’s or Cramer’s chi-square with Monte Carlo correction). For our main analysis, we used multivariable linear regression models to assess the association between cord leptin [as continuous exposure (per 10 ng/ml) and as categorical according to percentiles (10th and 90th)] and neurodevelopmental scores and to estimate β-coefficients with 95% CIs. To test differences of the combined neurodevelopmental scales according to cord leptin, we also performed multivariate analysis of covariance (MANCOVA). The independent variable was cord leptin and the outcome variables were: i. the five scales of Bayley-III (cognitive, receptive, expressive, fine and gross motor) as a group, ii. the five MCSA scales (verbal, quantitative, memory, perceptual performance, and motor) as a group and iii. the five neurovelopmental outcomes at 6 years [Raven (Total score), TMT (A and B), and Finger Tapping Test (Sum of dominant and non-dominant hand)]. Generalized additive models (GAMs) were applied to explore the shape of the relationships between cord leptin levels and neurodevelopmental outcomes. Linearity was assumed if the p-gain, defined as the difference in normalized deviance between the GAM model and the linear model for the same exposure and outcome was > 0.05. Since leptin levels in cord blood may be affected by pre-pregnant BMI, weight gain during pregnancy and infant sex (15), we evaluated possible effect modification, by introducing in the model interaction terms between cord leptin and i. pre-pregnant BMI, ii. weight gain during pregnancy and iii. infant sex. Statistical significance was defined by an alpha level of 0.10 for interaction. Stratified analyses by the potential effect modifier were performed when the interaction terms were statistically significant.
We selected the covariates retained in the final models using a combined approach of directed acyclic graphs and change-in estimate procedures (26). The initial DAGs included maternal determinants of cord blood leptin levels in our cohort: maternal origin (Greek/other), parity (primiparous/multiparous), pre-pregnant BMI [underweight/normal (BMI<25 kg/m2)/overweight/obese (BMI>25 kg/m2)], maternal smoking at the first prenatal visit (yes/no), weight gain during pregnancy (within/above/below recommendations, 2009 Institute of Medicine guidelines), type of delivery (caesarian/vaginal), infant sex (male/female), birthweight (g), gestational age (weeks). Other covariates were included based on previous literature: maternal education (low level: ≤6 years of school, medium level: 7 to 12 years of school, high level: university or technical college degree) and breastfeeding duration (months) and maternal age at birth (years). Exact age, quality of assessment (Bayley), examiner and quality of assessment (McCarthy) were included a priori in all models. To evaluate whether the assumed relationships and the minimum adjustment sets provided by the DAGs are supported by our data, we conducted forward and backward 10% change-in-estimate procedures departing from the minimum adjustment sets. The overall DAG of the assumed or known causal relationships between covariates included in the final models is shown in Supplementary Figure 1.
Few covariates had >3% missing values; only information on weight gain during pregnancy was missing in 18.6%. To maximize the sample size, we created missing categories in potential confounders. Results of complete-case analyses with missing observations excluded (data not shown) were similar to results using missing categories.
Sensitivity analyses were conducted after i. excluding preterm and/or low birth weight neonates, to distinguish confounding by these states, and ii. extra adjusting for neonatal ponderal index, to investigate the contribution of neonatal fat mass in our main results.
We did not perform power calculation and sample size estimation a priori; rather, all consecutive mother-child pairs from the Rhea study meeting the inclusion criteria were included. All association testing was conducted assuming a 0.05 significance level and a two-sided alternative hypothesis. DAGs were drawn using the DAGitty version 3.0. Statistical analysis was performed using the statistical package STATA, version 13 (StataCorp, College Station, TX).
Results
In total, 424 children were included in the analysis of cord leptin with at least one neurodevelopmental outcome during childhood (Table 1). Children with cord leptin over the 90th percentile were less likely to be Greek and born with caesarean section, while they were more likely to be females, of higher gestational age and to have higher birthweight and ponderal index, when compared with children with intermediate cord leptin levels. Mothers not included in the analysis due to loss to follow-up were older, Greek and of higher educational level compared to those included in the analysis (Supplementary Table 1). Cord blood levels did not differ between participants and those lost to follow up.
Table 1:
Background characteristics of the study population based on cord leptin status; Rhea birth cohort, Crete Greece (n=424).
| Cord leptin<10th percentile | Cord leptin 10th–90th percentile | Cord leptin>90th percentile | P-value | |
|---|---|---|---|---|
| n=42 | n=345 | n=37 | ||
| Maternal-Paternal socio–demographic and lifestyle characteristics | ||||
| Maternal age at birth (years); Mean (SD) | 30.0 (5.4) | 29.7 (4.9) | 29.4 (4.2) | 0.88 |
| Married; n (%) | 38 (92.7) | 296 (89.7) | 32 (88.9) | 0.82 |
| Greek; n (%) | 39 (92.9) | 328 (95.9) | 31 (86.1) | 0.04 |
| Primiparous; n (%) | 14 (33.3) | 145 (42.3) | 14 (38.9) | 0.52 |
| Maternal education; n (%) | 0.63 | |||
| Low | 6 (14.6) | 63 (18.9) | 9 (25.0) | |
| Medium | 25 (61.0) | 168 (50.3) | 17 (47.2) | |
| High | 10 (24.4) | 103 (30.8) | 10 (27.8) | |
| Smoking at the first prenatal visit; n (%) | 11 (26.8) | 51 (15.6) | 3 (8.3) | 0.08 |
| Pre-pregnant BMI; n (%) | 0.78 | |||
| < 25 kg/m2 | 26 (65.0) | 210 (64.0) | 21 (58.3) | |
| ≥ 25 kg/m2 | 14 (35.0) | 118 (36.0) | 15 (41.7) | |
| Weight gain during pregancy; n (%) | 0.09 | |||
| Below recomendations | 11 (33.3) | 64 (22.8) | 2 (6.5) | |
| According to recommendations | 10 (30.3) | 97 (34.5) | 10 (32.3) | |
| Above recommendations | 12 (36.4) | 120 (42.7) | 19 (61.3) | |
| Gestational diabetes; n (%) | 3 (8.3) | 18 (6.0) | 1 (3.5) | 0.70 |
| Gestationa hypetension; n (%) | 4 (10.8) | 13 (4.4) | 2 (7.1) | 0.23 |
| Obstetrical, neonatal and infant characteristics | ||||
| Caesarian section; n (%) | 25 (59.5) | 168 (49.1) | 9 (24.3) | 0.01 |
| Males; n (%) | 32 (76.2) | 182 (52.8) | 10 (27.0) | 0.00 |
| Birthweight (g); Mean (SD) | 2811.7 (415.3) | 3211.7 (411.7) | 3482.8 (421.7) | 0.00 |
| Ponderal index (kg/m3); Median (IQR) | 23.4 (3.0) | 24.8 (3.0) | 25.7 (3.7) | 0.00 |
| Gestational age (weeks); Median (IQR) | 37 (3) | 38 (1) | 39 (1) | 0.00 |
| Breastfeeding duration (months); Median (IQR) | 2 (3.4) | 3 (5) | 3 (6.5) | 0.18 |
| Adipokine profile at the age of 4 | ||||
| Adiponectin (μg/ml); Median (IQR) | 13.6 (8.9) | 12.9 (8.1) | 11.4 (9.9) | 0.40 |
| Leptin (ng/ml); Median (IQR) | 2.2 (2.2) | 1.9 (1.6) | 2.4 (2.6) | 0.25 |
SD: Standard Deviation, IQR: Interquartile Range; BMI: Body Mass Index
Bold indicates significant differences of ANOVA or Kruskal Wallis test for continuous variables and x2 analysis for categorical variables. Numbers may not correspond to the total due to missing numbers.
Cord leptin levels: under the 10th percentile: <2.1 ng/mL and over the 90th percentile>14.6 ng/mL.
Table 2 presents the multivariate analysis estimating the differences in neurodevelopmental outcomes during childhood: i. per 10 ng/mL increase of cord leptin, and ii. per leptin status according to percentiles (10th and 90th), after adjusting for confounders. An increase per 10 ng/ml in cord leptin was associated with a 3.8 unit increase in gross motor developmental scale (β-coef: 3.8, 95% CI: 0.0, 7.5) at 18 months of life, after adjusting for pre-pregnant BMI, maternal age at birth, maternal education, maternal smoking during the first prenatal visit, weight gain during pregnancy, type of delivery, infant sex, birthweight, gestational age and quality of assessment (Bayley). The MANCOVA did not reveal association with the five scales of Bayley combined (p-value=0.285). At age 4, a per 10 ng/ml increase in cord leptin was associated with three units decrease in general cognitive (β-coef: −3.0, 95%CI: −5.5, −0.4) and in various subscales (perceptual performance scale β-coef: −3.4, 95%CI: −6.0, −0.9, working memory β-coef: −3.1, 95%CI: −5.7, −0.4, executive functions β-coef −3.1, 95%CI: −5.7, −0.5, functions of posterior cortex β-coef −2.7, 95% CI: −5.2, −0.1). The MANCOVA revealed significant association with the five scales of MCSA combined (p-value=0.035). We further examined leptin in percentiles in order to identify the clinical status underlying the observed associations; cord leptin over the 90th percentile was associated with the decreased scores in general cognitive scale (general cognitive scale β-coef −6.6, 95% CI: −12.1, −1.0). The negative effect of cord leptin on cognitive performance persisted at the age of 6 years, wherein an increase per 10 ng/ml in cord leptin was associated with 3.7 units decrease in IQ score (β-coef: −3.7, 95%CI: −6.9, −0.5), after adjusting for pre-pregnant BMI, maternal age at birth, maternal education, maternal smoking during the first prenatal visit, weight gain during pregnancy, type of delivery, infant sex, birthweight, gestational age and age at measurement. Cord leptin levels were not associated with performance in Trail Making Test and in Finger Tapping Test. The MANCOVA revealed significant association with the neurodevelopmental outcomes of 6 years combined (p-value=0.018).
Table 2:
Association of cord leptin with child neuropsychomotor development; ‘Rhea’ mother–child cohort, Crete, Greece.
| Cord leptin | ||||||
|---|---|---|---|---|---|---|
| Per 10 ng/ml | Leptin status based on percentiles | |||||
| ≤10th percentile1 (n=42) | Reference (n=345) | ≥90th percentile1 (n=37) | ||||
| n | Adjusted β (95%CI) | Adjusted β (95%CI) | Adjusted β (95%CI) | |||
| 18 months | Bayley Scales of Infant & Toddler Development | |||||
| Cognitive | 248 | −1.0 (−4.5, 2.5) | 1.5 (−5.2, 8.2) | ref | 2.7 (−3.8, 9.1) | |
| Receptive | 248 | −0.4 (−3.8, 3.0) | 3.3 (−3.1, 9.7) | ref | −0.1 (−6.3, 6.2) | |
| Expressive | 248 | 0.6 (−3.1, 4.3) | 1.8 (−5.0, 8.7) | ref | 2.9 (−3.9, 9.6) | |
| Fine Motor | 248 | −0.9 (−4.5, 2.8) | 2.4 (−4.5, 9.3) | ref | −1.8 (−8.6, 4.9) | |
| Gross Motor | 248 | 3.8 (0.0, 7.5) | 0.4 (−6.8, 7.5) | ref | 5.0 (−2.0, 12.0) | |
| 4 years | McCarthy scales of Children’s Abilities | |||||
| General Cognitive scale | 359 | −3.0 (−5.5, −0.4) | 4.4 (−1.0, 9.7) | ref | −6.6 (−12.1, −1.0) | |
| Verbal scale | 359 | −1.6 (−4.2, 0.9) | 3.4 (−2.0, 8.9) | ref | 4.7 (−10.4, 0.9) | |
| Perceptual performance scale | 359 | −3.4 (−6.0, −0.9) | 3.8 (−1.7, 9.3) | ref | −7.7 (−13.4, −2.1) | |
| Quantitative scale | 359 | −2.7 (−5.3, 0.0) | 3.7 (−2.0, 9.4) | ref | −3.9 (−9.8, 2.0) | |
| Memory scale | 359 | −1.5 (−4.2, 1.1) | 1.3 (−4.3, 7.0) | ref | −4.1 (−10.0, 1.8) | |
| Working memory | 359 | −3.1 (−5.7, −0.4) | 2.0 (−2.8, 7.7) | ref | −3.7 (−9.7, 2.3) | |
| Memory span | 359 | −1.7 (−4.4, 1.1) | 2.2 (−3.6, 8.0) | ref | −4.6 (−10.6, 1.4) | |
| Motor scale | 359 | −0.8 (−3.5, 2.0) | 1.3 (−4.6, 7.2) | ref | −1.5 (−7.6, 4.7) | |
| Executive functions | 359 | −3.1 (−5.7, −0.5) | 6.0 (0.5, 11.4) | ref | −5.8 (−11.4, −0.1) | |
| Functions of posterior cortex | 359 | −2.7 (−5.2, −0.1) | 1.9 (−3.5, 7.3) | ref | −6.9 (−12.5, −1.3) | |
| 6 years | Raven | |||||
| Total score | 250 | −3.7 (−6.9, −0.5) | 3.3 (−4.0, 10.6) | ref | −7.6 (−15.3, 0.0) | |
| Trail Making Test | ||||||
| Test A response time (log trans.) | 225 | −0.4 (−8.1, 8.0) | −4.6 (−21.2, 15.5) | ref | −14.2 (−29.2, 3.9) | |
| Test B response time (log trans.) | 225 | −0.5 (−9.4, 9.3) | −8.4 (−27.1, 15.0) | ref | −10.0 (−28.0, 12.5) | |
| Finger Tapping Test | ||||||
| Sum of dominant hand | 232 | −1.8 (−5.7, 2.0) | −1.2 (−9.8, 7.5) | ref | −1.6 (−11.2, 8.1) | |
| Sum of non–dominant hand | 225 | 4.3 (−0.1, 8.6) | −1.1 (−9.8, 7.5) | ref | 8.0 (−1.9, 18.0) | |
Adjusted for pre–pregnant BMI, maternal age at birth, maternal education, maternal smoking during the first prenatal visit, weight gain during pregnancy, type of delivery, infant sex, birthweight, gestational age and for i) 18 months: quality of assessment (Bayley), ii) 4 years: examiner and quality of assessment (McCarthy), and iii) 6 years: age at measurement;
Reference: Cord leptin levels between 10th and 90th percentile; Cord leptin levels <10th percentile: <2.1 ng/mL; Cord leptin levels >90th percentile >14.6 ng/mL;
Values in bold indicate coefficients that are statistically significant at the 95% level.
No evidence for interaction was found between pre-pregnant BMI or infant sex and neurodevelopmental outcomes. The negative effect of cord leptin on perceptual performance scale at 4 years of age was evident only in children of women who gained weight more that recommended (β-coef: −6.6, 95%CI: −10.2, −3.0), and not of women with weight gain below or within recommendations (β-coef: 0.1, 95%CI: −4.4, 4.2, p for interaction=0.041) (Table 3).
Table 3:
Association of cord leptin with child neuropsychomotor neuropsychological development according to pre-pregnant BMI, weight gain during pregnancy and infant sex; ‘Rhea’ mother–child cohort, Crete, Greece.
| Cord leptin (Per 10 ng/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Maternal pre–pregnancy BMI | P for interaction | Weight gain during pregnancy | P for interaction | Infant sex | P for interaction | |||||
| BMI<25kg/m2 | BMI≥25kg/m2 | Less than recommended/According to recommendations | More than recommended | Males | Females | |||||
| Adjusted β (95%CI) | Adjusted β (95%CI) | Adjusted β (95%CI) | Adjusted β (95%CI) | Adjusted β (95%CI) | Adjusted β (95%CI) | |||||
| 18 months | Bayley Scales of Infant & Toddler Development | |||||||||
| Cognitive | −1.0 (−5.3, 3.3) | 2.3 (−6.0, 10.7) | 0.298 | −1.5 (−5.8, 2.9) | 1.5 (−4.8, 7.8) | 0.687 | −0.6 (−6.8, 5.7) | −1.3 (−6.0, 3.5) | 0.812 | |
| Receptive | −0.2 (−4.0, 3.6) | 2.8 (−6.5, 12.1) | 0.878 | 0.5 (−3.5, 4.8) | 2.3 (−4.2, 8.7) | 0.919 | 1.1 (−5.1, 7.3) | −0.3 (−4.8, 4.3) | 0.980 | |
| Expressive | −0.5 (−4.5, 3.6) | 8.7 (−1.2, 18.6) | 0.208 | −0.8 (−5.3, 3.7) | 3.8 (−4.2, 11.7) | 0.359 | 1.0 (−5.6, 7.5) | −1.0 (−6.0, 4.0) | 0.692 | |
| Fine Motor | −2.2 (−8.8, 4.5) | −1.6 (−10.2, 7.0) | 0.839 | −0.7 (−4.1, 5.6) | −1.0 (−6.9, 5.0) | 0.893 | −2.2 (−8.8, 4.5) | 0.2 (−4.6, 5.0) | 0.559 | |
| Gross Motor | 2.4 (−1.8, 6.5) | 11.8 (−3.5, 20.1) | 0.130 | 2.9 (−2.4, 8.2) | 7.2 (0.4, 14.0) | 0.657 | 7.5 (0.8, 14.1) | 1.1 (−4.1, 6.3) | 0.367 | |
| 4 years | McCarthy scales of Children’s Abilities | |||||||||
| General Cognitive scale | −3.2 (−6.8, 0.5) | −1.5 (−5.3, 2.3) | 0.889 | −0.3 (−4.3, 3.8) | −4.9 (−8.7, −1.2) | 0.088 | −1.8 (−6.3, 2.7) | −3.9 (−7.2, −0.6) | 0.706 | |
| Verbal scale | −0.0 (−3.8, 3.8) | −1.9 (−5.5, 1.7) | 0.234 | 0.1 (−3.7, 4.0) | −2.7 (−6.8, 1.4) | 0.187 | −1.2 (−5.8, 3.4) | −1.7 (−5.1, 1.6) | 0.911 | |
| Perceptual performance scale | −5.4 (−9.0, −1.9) | −0.9 (−5.1, 3.3) | 0.066 | 0.1 (−4.4, 4.2) | −6.6 (−10.2, −3.0) | 0.041 | −1.5 (−6.1, 3.2) | −5.1 (−8.4, −1.8) | 0.532 | |
| Quantitative scale | −3.7 (−7.5, 0.1) | −0.6 (−4.6, 3.4) | 0.750 | −0.6 (−5.1, 3.9) | −2.5 (−6.5, 1.5) | 0.549 | −1.5 (−6.2, 3.2) | −3.9 (−7.4, −0.4) | 0.492 | |
| Memory scale | −1.1 (−5.0, 2.8) | −1.0 (−4.9, 3.0) | 0.639 | −0.7 (−3.5, 4.8) | −2.8 (−7.1, 1.5) | 0.302 | −1.1 (−3.6, 5.8) | −3.3 (−6.8, 0.1) | 0.391 | |
| Working memory | −3.0 (−6.8, 0.8) | −2.3 (−6.4, 1.9) | 0.770 | −1.9 (−6.4, 2.7) | −3.4 (−7.5, 0.8) | 0.516 | −3.1 (−7.9, 1.7) | −3.9 (−7.5, −0.3) | 0.851 | |
| Memory span | −1.7 (−5.6, 2.2) | −0.5 (−4.7, 3.8) | 0.663 | −0.2 (−4.1, 4.5) | −2.0 (−6.3, 2.3) | 0.662 | −2.0 (−2.8, 6.8) | −4.2 (−7.7, −0.8) | 0.201 | |
| Motor scale | −2.7 (−6.6, 1.2) | 1.3 (−3.2, 5.8) | 0.145 | 0.3 (−4.2, 4.9) | −1.8 (−5.8, 2.3) | 0.496 | 2.8 (−2.1, 7.7) | −3.6 (−7.3, −0.1) | 0.145 | |
| Executive functions | −0.0 (−3.8, 3.8) | −1.9 (−5.5, 1.7) | 0.537 | −0.0 (−4.2, 4.2) | −4.4 (−8.2, −0.6) | 0.070 | −1.2 (−5.8, 3.4) | −1.7 (−5.1, 1.6) | 0.895 | |
| Functions of posterior cortex | −3.0 (−6.6, 0.7) | −1.3 (−5.0, 2.5) | 0.669 | −0.6 (−4.5, 3.4) | −4.7 (−8.6, −0.9) | 0.217 | −0.3 (−4.7, 4.1) | −4.2 (−7.5, −0.8) | 0.385 | |
| 6 years | Raven | |||||||||
| Total score | −4.6 (−9.0, −0.3) | −3.5 (−8.3, 1.2) | 0.750 | −3.1 (−8.8, 2.6) | −5.7 (−10.2, −1.3) | 0.634 | −5.2 (−11.4, 0.9) | −3.3 (−7.3, 0.6) | 0.293 | |
| Trail Making Test | ||||||||||
| Test A response time (log trans.) | −3.3 (−12.8, 17.2) | −1.4 (−14.1, 13.2) | 0.559 | −12.7 (−23.5, −0.3) | 3.5 (−7.6, 15.9) | 0.089 | −0.0 (−14.5, 16.9) | 3.0 (−6.7, 13.7) | 0.814 | |
| Test B response time (log trans.) | 4.1 (−9.2, 19.4) | −5.3 (−17.1, 8.2) | 0.150 | 0.4 (−14.7, 18.1) | −6.5 (−19.5, 8.6) | 0.720 | −5.1 (−20.8, 13.6) | 3.8 (−8.0, 17.0) | 0.722 | |
| Finger Tapping Test | ||||||||||
| Sum of dominant hand | −3.1 (−8.1, 1.9) | 0.0 (−6.9, 6.9) | 0.337 | 1.3 (−5.4, 7.9) | −5.0 (−11.0, 1.1) | 0.200 | −1.6 (−6.4, 3.2) | 1.8 (−5.0, 8.6) | 0.877 | |
| Sum of non–dominant hand | 0.6 (−6.4, 7.7) | 2.5 (−4.0, 9.0) | 0.655 | 10.2 (−1.4, 21.8) | 1.5 (−3.8, 6.8) | 0.650 | 0.6 (−6.4, 7.7) | 7.0 (1.2, 12.9) | 0.355 | |
Adjusted for pre–pregnant BMI, maternal age at birth, maternal education, maternal smoking during the first prenatal visit, weight gain during pregnancy, type of delivery, infant sex, birth weight, gestational age and for i) 18 months: quality of assessment (Bayley), ii) 4 years: examiner and quality of assessment (McCarthy), and iii) 6 years: age at measurement;
Values in bold indicate coefficients that are statistically significant at the 95% level.
Regarding the remaining sensitivity analyses, although statistical significance was lost in some cases due to small sample size, results did not differ substantially in terms of magnitude from those derived from the main analyses, when preterm and/or low birth weight neonates were excluded or when models were additionally adjusted for neonatal ponderal index (Table 4).
Table 4:
Association of cord leptin with child neuropsychomotor development, after i. excluding preterm and/or low birth weight neonates, and iii. additional adjusting for ponderal index.
| Cord leptin (Per 10 ng/ml) | ||||||
|---|---|---|---|---|---|---|
| Preterm /low birth weight neonates excluded N=365 | Additional adjustment for ponderal index N=419 | |||||
| n | β (95%CI) | n | β (95%CI) | |||
| 18 months | Bayley Scales of Infant & Toddler Development | |||||
| Cognitive | 213 | −0.7 (−4.5, 3.1) | 238 | −1.1 (−4.6, 2.5) | ||
| Receptive | 213 | −0.6 (−4.2, 3.0) | 238 | −0.3 (−3.7, 3.0) | ||
| Expressive | 213 | 0.6 (−3.4, 4.7) | 238 | 0.8 (−2.9, 4.5) | ||
| Fine Motor | 213 | 0.3 (−3.7, 4.4) | 238 | 0.6 (−4.3, 3.1) | ||
| Gross Motor | 213 | 2.3 (−1.8, 6.4) | 238 | 4.1 (0.3, 7.8) | ||
| 4 years | McCarthy scales of Children’s Abilities | |||||
| General Cognitive scale | 305 | −3.4 (−6.1, −0.6) | 340 | −3.7 (−5.4, 0.1) | ||
| Verbal scale | 305 | −1.9 (−4.6, 0.8) | 340 | −1.3 (−4.1, 1.5) | ||
| Perceptual performance scale | 305 | −4.0 (−6.8, −1.2) | 340 | −3.4 (−6.2, −0.6) | ||
| Quantitative scale | 305 | −2.9 (−5.8, 0.1) | 340 | −2.2 (−5.1, 0.7) | ||
| Memory scale | 305 | −2.1 (−5.0, 0.8) | 340 | −1.3 (−4.2, 1.6) | ||
| Working memory | 305 | −2.9 (−5.9, 0.0) | 340 | −3.1 (−6.0, −0.2) | ||
| Memory span | 305 | −2.2 (−5.2, 0.7) | 340 | −1.3 (−4.2, 1.7) | ||
| Motor scale | 305 | −1.6 (−4.6, 1.4) | 340 | −0.7 (−3.6, 2.3) | ||
| Executive functions | 305 | −3.0 (−5.9, −0.2) | 340 | −2.7 (−5.5, 0.1) | ||
| Functions of posterior cortex | 305 | −3.5 (−6.2, −0.8) | 340 | −2.4 (−5.1, 0.3) | ||
| 6 years | Raven | |||||
| Total score | 214 | −4.1 (−7.5, −0.8) | 246 | −3.9 (−7.5, 0.3) | ||
| Trail Making Test | ||||||
| Test A response time (log trans.) | 193 | 0.2 (−8.1, 9.3) | 221 | −2.6 (−11.0, 6.5) | ||
| Test B response time (log trans.) | 193 | 0.0 (−9.0, 9.9) | 221 | −0.6 (−10.5, 10.3) | ||
| Finger Tapping Test | ||||||
| Sum of dominant hand | 196 | −2.2 (−6.3, 1.9) | 229 | −2.2 (−6.5, 2.1) | ||
| Sum of non–dominant hand | 191 | 3.0 (−1.5, 7.5) | 222 | 3.6 (−1.4, 8.6) | ||
Adjusted for pre–pregnant BMI, maternal age at birth, maternal education, maternal smoking during the first prenatal visit, weight gain during pregnancy, type of delivery, infant sex, birthweight, gestational age and for i) 18 months: quality of assessment (Bayley), ii) 4 years: examiner and quality of assessment (McCarthy), and iii) 6 years: age at measurement.
Values in bold indicate coefficients that are statistically significant at the 95% level.
Discussion
In this prospective, population-based, mother–child cohort, we showed that increased cord leptin levels were associated with enhanced gross motor development at 18 months and with lower cognitive performance in early and middle childhood. The negative effect of cord leptin on perceptual performance at 4 years of age was evident only in children of women who gained weight more that recommended.
The positive association between cord leptin levels and enhanced gross motor corroborates previous knowledge that leptin contributes to brain development (7). Our results are in accordance with animal and human studies. The leptin-deficient (ob/ob) mice have reduced brain volume, weight and DNA content, abnormal development of the cingulate gyrus (7, 27). Interestingly, these mice showed profoundly decreased locomotor activity in comparison to their lean counterparts (28, 29), which could be corrected by exogenous leptin administration (29). A direct effect of leptin on activity independent of adiposity was suggested by Ribeiro et al, who reported increased activity before substantial decreases in body weight following leptin replacement in ob/ob mice (30); the same group also showed decreased activity with abrupt leptin suppression (31). Such an association has not been clearly described in humans, although children with intrauterine growth delay (a state that impairs leptin signaling (32)), exhibit dysfunctions in gross motor skill development (33). The innovation of our findings is that leptin may also have a regulatory role in gross motor development during a critical time window of fetal programming.
Interestingly, while cord leptin served as an enhancer of gross motor during the first 18 months of life, it was negatively associated with subscales of cognition at 4 years (perceptual performance, executive function, working memory and cognitive functions of posterior cortex) and, more importantly, with general cognitive scores at both 4 and 6 years. Executive and memory functions require an optimal function of frontal, pre-frontal, temporal cortex and hippocampus structures (34); leptin, on the other hand, is an important regulator of hippocampal structure and function (35) and neonatal leptin deficiency reduces frontal cortex volume in a mouse model (36). Given the ability of leptin to cross the blood–brain barrier and act upon its receptors (5), it is tempting to speculate that our findings could be attributed to the development of leptin resistance (37, 38, 39) promoted by hyperleptinemia during the critical time of fetal programming (40). Although there is still no solid evidence that leptin resistance develops within brain areas associated with cognition (5), indications towards this hypothesis exist. In animals, high-fat diet triggers neurochemical changes, like leptin resistance, within the hippocampus, which might account for cognitive deficits (41) Studies in humans have shown that increasing age, obesity and metabolic dysfunction impair leptin signaling, at least in the hypothalamus, and are associated with dysfunctional transport of leptin to the brain (5). Another theory could attribute the association between cord leptin and impaired cognitive function during early childhood to epigenetic changes; DNA methylation of the leptin promoter and consequent epigenetic control is influenced by maternal and infant perinatal factors in a tissue-specific manner (42) and cord hyperleptinemia may also represent such a factor. Future studies are needed to investigate the latter hypothesis.
Although cord leptin had a consistent negative effect on general cognitive scores at 4 and 6 years of age, this was not the case for executive function, which was negatively regulated by leptin at age 4, but not at age 6 (trail making test, as a marker of “executive function” of inhibition). Effects of leptin on executive functions may therefore be transient. Neuropsychological assessments must always be interpreted with caution, as some deficits may be persistent due to to irreversible structural brain abnormalities, others could reflect transient effects on cognition, eg. due to leptin levels at the period of examination, while some may represent chronic but potentially reversible effects of metabolic control (ie leptin resistance). Future studies are needed to clarify these different possibilities.
Current data from human studies support a negative association between pre-pregnancy maternal obesity and cognitive function of the offspring during childhood, while lower cognitive performance is also observed in children as a function of weight gain during pregnancy (43). Physiological mechanisms underlying obesity-related neuropsychological disabilities in the offspring are still unknown, but hormone levels (e.g., insulin and leptin) and epigenetic alterations could have a role (44). We have previously shown that both maternal pre-pregnant BMI and weight gain during pregnancy significantly affect the levels of cord leptin (15). Herein, a stratified analysis showed that the negative effect of cord leptin on perceptual performance at 4 years was evident only in women that gained weight more that recommended.
Strengths of the present study include its population-based prospective design, large sample size and number of biological samples, as well as a long follow-up period. The use of internationally recognized psychometric instruments of high reliability, validity and comprehensiveness for neuropsychomotor development assessment is an additional strength of our analysis. Although the administration of different psychometric tools at each follow-up makes the comparisons between assessments suboptimal, these tests were selected, because age-appropriate materials and activities were considered necessary to elicit the performance of the different skills which emerge at each time point and motivate children’s active participation. In order to make these different tests quantitatively comparable, we standardized all scales to a mean (SD) of 100 (15).
Selection bias due to loss to follow-up is always of concern in cohort studies. Although cord leptin levels did not differ between participants and non-participants, children who were lost at follow up were more likely to be females and born to younger, non-Greek mothers with lower educational level. This may limit the generalizability of our results, but we would not expect comparisons of cord blood leptin with neuropsychological outcomes to have been biased. Despite the fact that we tested all scales of neuropsychomotor development together in a single model (using MANCOVA) in order to reduce the multiple comparison problems, a concern of chance findings due to multiple comparisons may be raised. However, an application of Bonferroni correction would be inappropriate in our case, as the outcomes are highly correlated. Although we had previously established gender-specific clinical reference intervals for cord leptin in our cohort (45), we could not apply them due to small sample size; therefore statistical definitions for hypo- and hyperleptinemia were used instead. Finally, we cannot exclude unmeasured residual confounding, in particular with respect to unmeasured perinatal and social factors related to child neuropsychological development. However, we note that results were robust to further adjustment for origin, marital status, ponderal index, or even after excluding preterm /low birth weight neonates, thus residual confounding by further social or perinatal factors would be expected to have minimal effects.
This is the first study, to our knowledge, to examine the impact of cord blood leptin levels on child neuropsychomotor development. Children with high cord leptin may be at increased risk for impaired cognitive development at early and mid-childhood. These results may have important public health and clinical implications as they help to identify targets for prevention and intervention early in life, leading to enhanced child neurodevelopment. In addition, maternal weight during gestation should be carefully controlled though multi-component approaches by healthcare professionals, in order to avoid potential adverse effects on offspring neuropsychomotor development.
Conclusion
In sum, our observations provide support for the programming effect of leptin on the neuropsychomotor development during childhood. Additional longitudinal studies and trials are needed to confirm these data and provide new directions for leptin research.
Supplementary Material
What is already known about this subject?
Leptin regulates brain development in the prenatal and neonatal periods
Leptin serves as an enhancer of cognition, but insensitivity to leptin may also be involved in the development of cognitive deficits.
What does your study add?
Increased cord leptin is associated with enhanced gross motor development at 18 months, but decreased cognitive performance at 4 and 6 years of age.
The negative effect of cord leptin on perceptual performance at 4 years of age was evident only in children of women who gained weight more that recommended.
Acknowledgments
The authors would particularly like to thank all the cohort participants for their generous collaboration.
Funding:
The Rhea project was financially supported by European projects (EU FP6–003-Food-3-NewGeneris - Contract No16320, EU FP6 STREP Hiwate - Contract No36224, EU FP7 ENV.2007.1.2.2.2. Project No 211250 Escape, EU FP7–2008-ENV-1.2.1.4 Envirogenomarkers Contract No226756, EU FP7-HEALTH-2009-single stage CHICOS Contract No241604, EU FP7 ENV.2008.1.2.1.6. Proposal No 226285 ENRIECO, EU-FP7 Proposal No 264357 MeDALL, EU- FP7- HEALTH-2012 Proposal No 308333 HELIX), and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011–2014; “Rhea Plus”: Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015). Dr Chatzi was partially supported by the Southern California Environmental Health Sciences Center (grant # P30ES007048) funded by the National Institute of Environmental Health Sciences. Dr. Mantzoros was supported by NIDDK (K24 81913) award and a discretionary autism research fund.
Footnotes
Disclosure: The authors declare no conflict of interest
References
- 1.Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108 Suppl 3: 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faa G, Marcialis MA, Ravarino A, Piras M, Pintus MC, Fanos V. Fetal programming of the human brain: is there a link with insurgence of neurodegenerative disorders in adulthood? Curr Med Chem 2014;21: 3854–3876. [DOI] [PubMed] [Google Scholar]
- 3.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr 2009;48 Suppl 1: S31–38. [DOI] [PubMed] [Google Scholar]
- 4.Polyzos SA, Mantzoros CS. Leptin in health and disease: facts and expectations at its twentieth anniversary. Metabolism 2015;64: 5–12. [DOI] [PubMed] [Google Scholar]
- 5.Morrison CD. Leptin signaling in brain: A link between nutrition and cognition? Biochim Biophys Acta 2009;1792: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paz-Filho GJ. The Effects of Leptin Replacement on Neural Plasticity. Neural plasticity 2016;2016: 8528934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udagawa J, Hatta T, Hashimoto R, Otani H. Roles of leptin in prenatal and perinatal brain development. Congenit Anom (Kyoto) 2007;47: 77–83. [DOI] [PubMed] [Google Scholar]
- 8.Harvey J, Shanley LJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans 2005;33: 1029–1032. [DOI] [PubMed] [Google Scholar]
- 9.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev 2010;11: 118–126. [DOI] [PubMed] [Google Scholar]
- 10.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011;25: 181–213. [DOI] [PubMed] [Google Scholar]
- 11.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol 2014;13: 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr Res 2015;77: 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camargos AC, Mendonca VA, Oliveira KS, de Andrade CA, Leite HR, da Fonseca SF, et al. Association between obesity-related biomarkers and cognitive and motor development in infants. Behav Brain Res 2017;325: 12–16. [DOI] [PubMed] [Google Scholar]
- 14.Chatzi L, Leventakou V, Vafeiadi M, Koutra K, Roumeliotaki T, Chalkiadaki G, et al. Cohort Profile: The Mother-Child Cohort in Crete, Greece (Rhea Study). Int J Epidemiol 2017;46: 1392–1393k. [DOI] [PubMed] [Google Scholar]
- 15.Karakosta P, Georgiou V, Fthenou E, Papadopoulou E, Roumeliotaki T, Margioris A, et al. Maternal weight status, cord blood leptin and fetal growth: a prospective mother-child cohort study (Rhea study). Paediatric and Perinatal Epidemiology 2013;27: 461–471. [DOI] [PubMed] [Google Scholar]
- 16.Koutra K, Chatzi L, Roumeliotaki T, Vassilaki M, Giannakopoulou E, Batsos C, et al. Socio-demographic determinants of infant neurodevelopment at 18 months of age: Mother-Child Cohort (Rhea Study) in Crete, Greece. Infant Behav Dev 2012;35: 48–59. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley scales of infant and toddler development (3rd ed.). TX: PsychCorp, Harcourt Assessment Inc.: San Antonio, 2006. [Google Scholar]
- 18.McCarthy D. Manual for the McCarthy Scales of Children’s Abilities. Psychological Corp: New York., 1972. [Google Scholar]
- 19.Kampouri M, Kyriklaki A, Roumeliotaki T, Koutra K, Anousaki D, Sarri K, et al. Patterns of Early-Life Social and Environmental Exposures and Child Cognitive Development, Rhea Birth Cohort, Crete, Greece. Child Dev 2017. [DOI] [PubMed] [Google Scholar]
- 20.Julvez J, Ribas-Fito N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol 2007;36: 825–832. [DOI] [PubMed] [Google Scholar]
- 21.Raven JCRJ. Raven coloured progressive matrices manual Harcourt assessment: San Antonio Texas, USA. [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment: New York, 2004. [Google Scholar]
- 23.Lezak M Neuropsychological Assessment. 3. . Oxford University Press: Oxford, 1995. [Google Scholar]
- 24.Hernandez-Bonilla D, Schilmann A, Montes S, Rodriguez-Agudelo Y, Rodriguez-Dozal S, Solis-Vivanco R, et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology 2011;32: 615–621. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol 2001;30: 256–263. [DOI] [PubMed] [Google Scholar]
- 26.Evans D, Chaix B, Lobbedez T, Verger C, Flahault A. Combining directed acyclic graphs and the change-in-estimate procedure as a novel approach to adjustment-variable selection in epidemiology. BMC Med Res Methodol 2012;12: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun 1999;256: 600–602. [DOI] [PubMed] [Google Scholar]
- 28.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol 2006;290: R894–903. [DOI] [PubMed] [Google Scholar]
- 29.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269: 540–543. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, Mark AL. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One 2011;6: e23364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montez JM, Soukas A, Asilmaz E, Fayzikhodjaeva G, Fantuzzi G, Friedman JM. Acute leptin deficiency, leptin resistance, and the physiologic response to leptin withdrawal. Proc Natl Acad Sci U S A 2005;102: 2537–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coupe B, Grit I, Hulin P, Randuineau G, Parnet P. Postnatal growth after intrauterine growth restriction alters central leptin signal and energy homeostasis. PLoS One 2012;7: e30616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen MB, Pedersen SA, Greisen G, Pedersen JF, Molsted-Pedersen L. Early growth delay in diabetic pregnancy: relation to psychomotor development at age 4. Br Med J (Clin Res Ed) 1988;296: 598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb B, Wishaw I, Atkinson R, Lindzey G, Thompson R. Fundamentals of Human Neuropsychology. NYPa: W.H. Freeman and Company: New York, 1996. [Google Scholar]
- 35.McGuire MJ, Ishii M. Leptin Dysfunction and Alzheimer’s Disease: Evidence from Cellular, Animal, and Human Studies. Cell Mol Neurobiol 2016;36: 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dexter BC, Rahmouni K, Cushman T, Hermann GM, Ni C, Nopoulos PC, et al. Neonatal leptin deficiency reduces frontal cortex volumes and programs adult hyperactivity in mice. Behav Brain Res 2014;263: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irving AJ, Harvey J. Leptin regulation of hippocampal synaptic function in health and disease. Philos Trans R Soc Lond B Biol Sci 2014;369: 20130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides 2006;27: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 39.Valladolid-Acebes I, Fole A, Martin M, Morales L, Cano MV, Ruiz-Gayo M, et al. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol Learn Mem 2013;106: 18–25. [DOI] [PubMed] [Google Scholar]
- 40.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One 2010;5: e11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valladolid-Acebes I, Merino B, Principato A, Fole A, Barbas C, Lorenzo MP, et al. High-fat diets induce changes in hippocampal glutamate metabolism and neurotransmission. Am J Physiol Endocrinol Metab 2012;302: E396–402. [DOI] [PubMed] [Google Scholar]
- 42.Lesseur C, Armstrong DA, Paquette AG, Koestler DC, Padbury JF, Marsit CJ. Tissue-specific Leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Mol Cell Endocrinol 2013;381: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contu L, Hawkes CA. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casas M, Forns J, Martinez D, Guxens M, Fernandez-Somoano A, Ibarluzea J, et al. Maternal pre-pregnancy obesity and neuropsychological development in pre-school children: a prospective cohort study. Pediatr Res 2017;82: 596–606. [DOI] [PubMed] [Google Scholar]
- 45.Karakosta P, Georgiou V, Fthenou E, Margioris A, Castanas E, Kogevinas M, et al. Gender-specific reference intervals for cord blood leptin in Crete, Greece. European Journal of Pediatrics 2012;171: 1563–1566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.