Abstract
INTRODUCTION:
We classified individuals based on their baseline performance on cognitive measures, and investigated the association between cognitive classifications and neuropathological findings ~7 years later, as an external validator.
METHODS:
Brain autopsies of 779 decedents were examined. Baseline latent class analysis on 10 neuropsychological measures was previously assigned: Mixed-Domains Impairment (n = 39, 5%), Memory-Specific Impairment (n= 210, 27%), Frontal-Impairment (n = 113, 14.5%), Average Cognition (360, 46.2%) and Superior Cognition (n = 57, 7.3%). Linear regressions and risks ratios were used to examine the relation of latent class assignment at enrollment with neuropathological indices.
RESULTS:
Aβ, Tau, and TDP-43 were associated with Mixed-Domains Impairment and Memory-Specific Impairment classes ~ 7 years prior to death. Moderate arteriolosclerosis was associated with membership in the Frontal Impairment Class.
DISCUSSION:
Our findings support the use of latent class models that incorporate more comprehensive neuropsychological measures to classify cognitive impairment.
Keywords: Alzheimer’s dementia, neuropsychology, neuropathology, latent variable modeling, individual differences, heterogeneity
Background
Alzheimer’s dementia (AD) is a clinically heterogeneous disease, with a long prodromal stage spanning five to ten years [1, 2], some even suggesting this phase may well span between twenty and thirty years [3]. Within the larger population there are specific at-risk subgroups; identifying and defining preclinical cognitive patterns of these subgroups and mapping them onto neuropathology outcomes would improve our ability to capture intra-individual differences across areas of impairment [4–6]. Identifying early-impairment subgroups at enrollment and linking them to specific neuropathology outcomes may help in elucidating differing AD pathways from its preclinical phase to its postmortem end-state.
Neuropathological heterogeneity has been well described in older adults who die with or without cognitive impairment [2, 7–9]. Neurocognitive heterogeneity is also well recognized through the multiple defined forms of mild cognitive impairment (MCI) [10–12]. Previous studies have linked specific neuropsychological measures to postmortem neuropathology; however, these studies have usually studied participants’ neuropsychological performance proximate to death [9, 13–15], assessed average performance with a single test score across participants [9, 16, 17], or were small in sample size [13, 16–18]. To boost the power of clinical trials and improve clinical practice, we need to identify more homogeneous subgroups of individuals at risk of incident dementia. Ideally, the subgroups of individuals free of dementia, identified by neurocognitive measures would predict neuropathological outcomes accurately. Leveraging data from large cohort studies, latent variable modeling may help identify more homogenous subgroups. In prior work using data from Rush Memory and Aging Project (MAP), we examined a variety of approaches linking pathology to change in cognition over multiple years prior to death [19, 20]. We are only aware of one prior study using data from the Religious Orders Study to examine the relation of brain pathologies to latent class measures of cognition at baseline [21].
We previously applied latent class analysis (LCA) to neuropsychological data in the Einstein Aging Study (EAS) cohort [22] and in the MAP [23]. LCA is a statistical model-based probabilistic clustering approach that uses general mixture modeling (GMM) to study individual differences by empirically summarizing large amounts of data [24, 25]. Because it is a probabilistic approach, posterior class-membership probabilities are computed from the estimated model parameters from each individual’s observed scores, resulting in each individual being placed on a scale of prognostic probability [26].
GMM has been widely used in literature investigating mood disorders [27, 28], delinquency [29, 30], and substance abuse, [31], but is still a relatively novel (but growing) approach in AD research. Using the technique, we may be able to group individuals based on their neuropsychological performance and closely approximate their biological underpinnings. Hence, we may be able to identify subgroups of individuals who have evidence of disease but do not have a diagnosis. In both studies, we identified 5 latent classes: 3 with impairment and 2 in the normal range. The classes with cognitive impairment were the “Mixed-Domains Impairment”, “Memory-Specific Impairment”, and “Frontal Impairment” subgroups. The classes with intact cognition were the “Average” and “Superior Cognition” subgroups. These subgroups differed in their cognitive profiles used to define them and, in their ability to predict incident AD and incident all-cause dementia. Despite differences in sample characteristics and in many of the neuropsychological tests, the similar pattern of the class composition between the classes in the EAS and in MAP is the evidence for robustness of these classes. In addition, the ability to predict underlying pathology among subgroups of individuals could have an additional impact on precise classification of the various cognitive profiles in older populations and may have further implications in design of clinical trials. Essentially, potentially important intervention effects can be diluted by enrolling participants who do not need or cannot benefit from these interventions. Identifying homogeneous subgroups of individuals with common cognitive profiles and mapping their phenotypes onto biological markers would help in advancing the assembling of homogeneous cohorts needed for meaningful clinical trials.
The aim of our study was to determine the risk of pathological classification at autopsy based on cognitive class membership at baseline, and how these risks vary by classes. We hypothesized that cognitively impaired classes would display higher risks of pathology at baseline in comparison to cognitively unimpaired classes. Specifically, we hypothesized that amnestic classes i.e. the Mixed-Domains and Memory-Specific Impairment classes would have higher risk for AD-related pathology (high Aβ and Tau, and hippocampal sclerosis), while non-amnestic classes i.e. the Frontal Impairment class would have higher risk of vascular-based pathology (arteriolosclerosis, atherosclerosis and infarctions) in comparison with other classes.
Methods
Sample.
Study participants in the Rush Memory and Aging Project (MAP) are community-dwelling older adults from about 40 retirement communities and senior subsidized housing facilities across northeastern Illinois. Older persons without known dementia consented for annual clinical evaluation, and signed an informed consent and an Anatomical Gift Act for organ donation at the time of death, and a repository consent that permitted data to be repurposed. The study was approved by the Institutional Review Boards of Rush University Medical Center and the Albert Einstein College of Medicine. Annual clinical evaluation includes detailed neuropsychological testing, a medical history, and neurologic examination. More detailed description of the study design can be found in previous reports [2, 32]. Inclusion criteria for the current study were:
-
-
No prevalent dementia at baseline;
-
-
At least one follow-up;
-
-
Deceased at the time of the study;
-
-
Availability of neuropathological data.
Thus, participants were restricted to a subset of MAP participants (N = 779) who were deceased at the time of this study. Although all of the included participants had autopsy data, some of them (n = 138) did not have information on all autopsy measures, but we still included them in the analyses of this paper. In Figure 1 we illustrate a flow chart of the steps we took for participant-inclusion of the current study.
Figure 1.
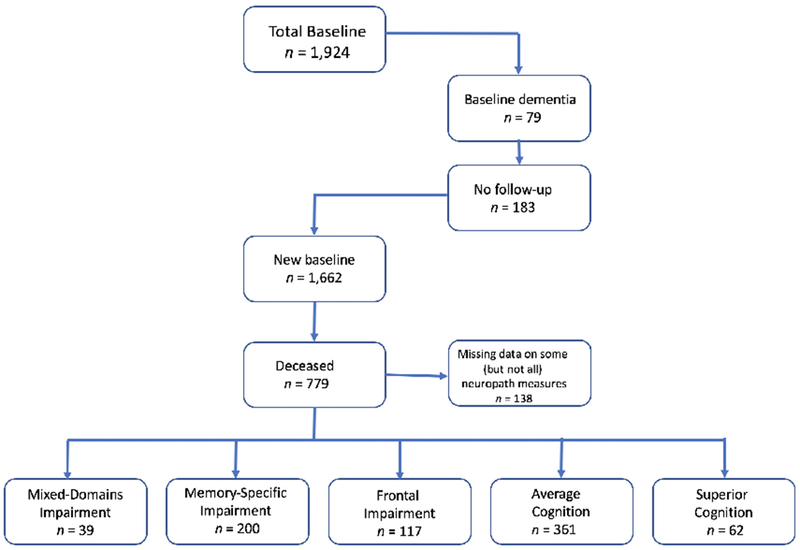
Flowchart for participant inclusion in this study.
Latent Class Analysis.
We previously fit LCA models on baseline performance of 10 neuropsychological indicators (listed below) measured in 1,662 MAP participants [23]. An increasing number of classes were fitted until the best fitting model was identified. Fit indices, including, the Bayesian information criterion (BIC) [33] and entropy [34] were used to compare between k and k+1 models. Lower BIC indicates better model fit, while values for entropy range from 0 to 1, with values closer to 1 indicating higher classification accuracy. A five-class model was deemed optimal based on the BIC and entropy in LCA, with profiles reflecting impaired cognition (mixed-domains impairment, memory-specific impairment, frontal impairment) and intact cognition (superior cognition and average). After identification of the classes, participants were assigned to their most likely class based on posterior probabilities. We then characterized and validated our model using pre-existing characteristics to determine if the classes are distinguishable on core neuropsychological characteristics and external validators that included genetics and vascular risk [23].
Since the subsample who died and went to autopsy may be qualitatively different than those who did not die, and in order to assess the generalizability of 5-classes structure, we repeated the LCA while restricting participants to those participants who died during follow-up. In the current study, we applied LCA using an alternative to the three-step approach. A disadvantage of the three-step approach is that the estimates obtained in the third step (estimating associations) are biased due to classification error introduced when assigning individuals into classes [35–37]. Although several alternatives to the three-step approach exist e.g. maximum likelihood (ML) [38] and the Lanza, Tan, and Bray (LBT) approach [39], we implemented the Block, Croon, and Hagenaars (BCH) approach [40] due to its robustness over ML and LBT in violations of normal distributions within classes and unequal variances across classes (heteroscedasticity). It also obtains unbiased estimates. The BCH approach is also the recommended approach when using stepwise LC modeling. The BCH approach outperforms the LBT method in that it avoids shifts in latent class assignment in the final stage, which the LBT is susceptible to. The BCH method uses a weighted multiple group analysis, similar to ANOVA, where the groups are the classes, hence class-shifting is not possible because the classes are known already. The BCH method performs well when the variance of the auxiliary variable differs across classes (which the LBT is also susceptible to) [37]. Results showed that the five-class structure remained the same (Supplementary Figure 1), and that individuals were highly likely to be placed within the same classes as previously placed when being analyzed with the entire sample (Kappa = .871) (Supplementary Table 2). We therefore proceeded with the present study’s LCA model.
Neuropsychological classes. The classes were formed on baseline performance of ten neuropsychological measures representing domains of:
-
i)
Episodic memory – Logical Memory - Sum [41]and Word list Recall [42]
-
ii)
Semantic memory – Boston Naming Test [43] and Category fluency [44]
- iii)
-
iv)
Perceptual Orientation – Matrices [47] and Line Orientation [48]
-
v)
Perceptual Speed – Symbol Digits Modalities Test [49]and Number Composition
Detailed information on these measures is given in S Table 1. In addition, covariates of baseline age, sex, and education were included in the LCA models.
Table 1.
Baseline demographic information of the 779 Memory and Aging Project deceased participants, stratified by latent class assignment.
| Cognition Impaired |
Cognition Intact |
|||||
|---|---|---|---|---|---|---|
| Characteristics N (%) | All participants 779 | Mixed-Domain N = 39 | Memory-Specific N = 210 | Frontal N = 113 | Average N = 360 | Superior N = 57 |
| Age, years (SD) | 82.6 (5.9) | 84.9 (5.8) | 84.2 (5.4) | 84.2 (6.6) | 81.7 (5.4) | 77.8 (5.1) |
| Time to death from baseline assessment, years (SD) | 7.3 (3.8) | 5.2 (2.8) | 6.4 (3.6) | 6.7 (3.5) | 8.2 (3.8) | 8.6 (4.2) |
| Females (%) | 558 (71.6) | 29 (74.7) | 125 (59.5) | 99 (87.6) | 270 (75.0) | 35 (61.4) |
| White (%) | 750 (96.3) | 35 (89.7) | 203 (96.7) | 103 (92.1) | 352 (97.8) | 57 (100%) |
| Education, years (SD) | 14.4 (2.9) | 11.6 (3.5) | 14.1 (3.1) | 14.1 (2.4) | 14.8 (2.6) | 16.4 (3.3) |
| NART (SD) | 7.9 (2.3) | 5.2 (3.4) | 7.3 (2.4) | 7.5 (2.4) | 8.4 (1.8) | 9.4 (1.2) |
| MMSE (SD) | 27.6 (2.2) | 24.2 (2.4) | 26.2 (2.4) | 27.7 (1.8) | 28.4 (1.4) | 29.1 (1.1) |
Note. NART = National Adult Reading Test. MMSE = Mini Mental State Examination. All percentages are column percentages.
Neuropathological data.
Post-mortem neuropathological evaluations consisted of a uniform structured assessment of global AD pathology [21][50], including β-amyloid load (Aβ) and tangle density (Tau), hippocampal sclerosis [51], TARDNA-binding protein 43 (TDP-43) [52], Lewy bodies, chronic macroinfarcts and microinfarcts [53–55], cerebral amyloid angiopathy [56], atherosclerosis [53], and arteriolosclerosis [57]. Procedures follow pathologic data recommendations by the National Alzheimer’s Disease Coordinating Center (NACC) [58]. In this paper, we stratified global burden of AD pathology into 3 categories: very low < 0.49, low: 0.50 – 1.49, medium: 1.50 – 2.49, and high: ≥2.50 for ease of interpretation. For all other variables, detailed and systematic neuropathologic evaluations of brain specimens were conducted, all blinded to clinical data, as reported elsewhere [59].
Procedure.
We fitted linear regressions to continuous variables (amyloid, and tau) and calculated the relative risk ratios to categorical variables, along with effect sizes, to determine the extent to which class membership of participants at enrollment is associated with risk of neuropathology upon autopsy, thereby using the classes as independent variables and neuropathology as the dependent variable.
Statistical software.
For LCA modeling we used MPlus version 7 [60]. We used SPSS version 25 [61] for descriptive analyses and descriptive figures. Forest plots were done in Excel (Microsoft Corporation, Seattle, Washington). All other analyses were done using R. 3.4.3 (R Core Team, 2018).
Results
Demographic characteristics.
Mean age at baseline was 82.6 (SD = 5.9), 71.6% were female, and 96.3% were non-Hispanic White. Average follow-up time prior to death was 7.3 years (SD = 3.8). Table 1 shows the demographic characteristics of the sample, and stratified by class membership.
At the last visit before death, 54.3% of the Mixed-Domains Impairment, and 53.7% of the Memory-Specific Impairment classes had an AD diagnosis. The proportions were at 25.7%, 23.6% and 14.8% for the Frontal, Average, and Superior classes. At last visit before death, dementia not due to AD was present in 8.6%, 1.5%, 3.7%, 1.7%, and 1.9% in the Mixed-Domains Impairment, Memory-Specific Impairment, Frontal Impairment, Average, and Superior Cognitive classes at last visit before death respectively. Compared to the Average Class, individuals in the Mixed-Domains Impairment Class, Memory-Specific Impairment Class, and Frontal Impairment Class were at higher risk of death (HR = 2.4, 95%CI=1.7 – 3.3, p < 0.001) (HR = 1.6, 95%CI = 1.3 – 1.8, p<0.001) (HR = 1.4, 95%CI = 1.1 – 1.7, p <0.01). Figure 2 displays the cumulative hazard of death across the classes.
Figure 2.
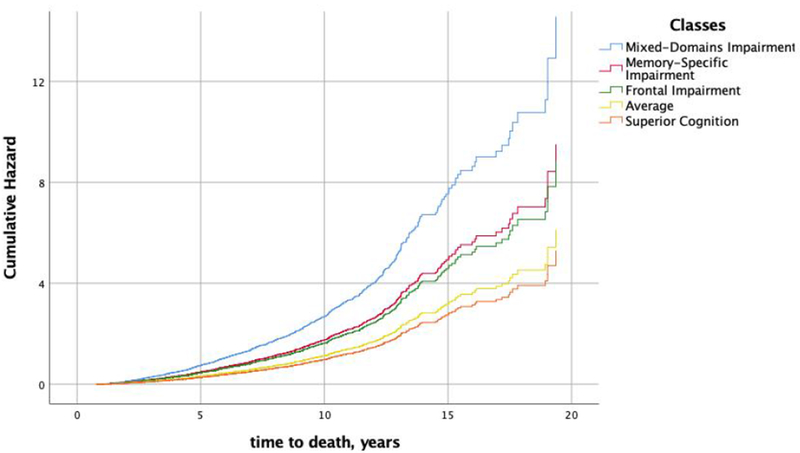
Cumulative hazard of death by latent class membership.
Neuropathological characterization across the classes.
AD pathology.
Table 2 shows that upon autopsy each of the classes had some level of pathology; however, significant differences in global burden of AD pathology as well as in the molecularly specific Aβ load and Tau were present across the classes. The Mixed-Domain Impairment class had the highest global burden of AD pathology, followed by the Memory-Specific class; the Superior Cognition class had the least. The Memory-Specific Class had the highest Aβ load while the Mixed-Domains Impairment had the highest density of Tau, and the Superior Cognition had the least amounts of both Aβ and Tau. The majority of individuals in the Mixed-Domains class had cytoplasmic inclusions (TDP-43) that extended to the limbic region and to the neocortex. Up to 21.7% of individuals in the Mixed-Domains Impairment had hippocampal sclerosis.
Table 2.
Characterization of neuropathology in the deceased participants in Memory and Aging Project, stratified by latent class membership.
| Cognition impaired |
Cognition Intact |
||||
|---|---|---|---|---|---|
| Autopsies | Mixed-Domain N = 39 | Memory-Specific N = 210 | Frontal N = 113 | Average N = 360 | Superior N = 57 |
| Pathology | |||||
| Global burden of AD pathology | 1.1 (0.9) | 0.9 (0.6) | 0.7 (0.5) | 0.7 (0.6) | 0.5 (0.6) |
| Amyloid load, % (SD) | 6.4 (5.7) | 5.8 (4.5) | 4.6 (4.6) | 4.7 (4.4) | 3.4 (3.9) |
| Tangle density, mm2 (SD) | 14.6 (14.1) | 9.3 (8.8) | 6.2 (6.6) | 5.5 (6.0) | 4.5 (6.4) |
| TDP-43 cytoplasmic inclusions, N (%) | |||||
| 0: None | 6 (27.3) | 69 (42.9) | 46 (52.3) | 136 (45.9) | 32 (65.3) |
| 1: Localized to amygdala | 2 (9.1) | 31 (19.3) | 15 (17.0) | 70 (23.6) | 6 (12.2) |
| 2: Extension to hippocampal/entorhinal cortex | 8 (36.4) | 28 (17.4) | 19 (21.6) | 59 (19.9) | 6 (12.2) |
| 3: Extension to neocortex | 6 (27.3) | 33 (20.5) | 8 (9.1) | 31 (10.5) | 5 (10.2) |
| Hippocampal sclerosis, N (%) | |||||
| Present with CA1 region affected | 5 (21.7) | 21 (12.4) | 6 (6.5) | 17 (5.5) | 2 (3.9) |
| Cortical Lewy Body Dementia N (%) | |||||
| Present | 9 (40.9) | 46 (28.0) | 19 (21.3) | 60 (19.8) | 10 (20.4) |
| Vascular Pathology | |||||
| Cerebral amyloid angiopathy (CAA-4GP) N (%) | |||||
| 0-none | 2 (8.7) | 21 (12.4) | 17 (18.7) | 82 (26.6) | 15 (30.0) |
| Mild <1.5 (between 0.25 and 1.4) | 9 (39.1) | 73 (43.2) | 45 (49.5) | 132 (42.9) | 20 40.0) |
| Moderate to severe (1.5 to >2.5) | 12 (15.2) | 75 (44.4) | 29 (31.9) | 94 (30.5) | 15 (30.0) |
| Cerebral atherosclerosis N (%) | |||||
| None | 3 (3.0) | 33 (19.4) | 13 (14.1) | 74 (23.8) | 19 (36.5) |
| Mild | 14 (60.9) | 77 (45.3) | 42 (45.7) | 156 (50.2) | 23 (44.2) |
| Moderate – severe | 6 (26.1) | 60 (35.3) | 37 (40.2) | 81 (26.0) | 10 (19.2) |
| Arteriolosclerosis N (%) | |||||
| None | 6 (26.1) | 38 (22.6) | 20 (21.7) | 100 (32.5) | 19 (37.3) |
| Mild | 10 (43.5) | 70 (41.7) | 30 (32.6) | 118 (38.3) | 16 (31.4) |
| Moderate – severe | 7 (30.4) | 60 (35.7) | 42 (45.7) | 90 (29.2) | 16 (31.4) |
| Gross chronic infarcts N (%) | |||||
| 0: None | 17 (73.9) | 95 (56.9) | 55 (59.8) | 202 (66.0) | 35 (68.8) |
| 1: One or more | 6 (26.1) | 72 (43.1) | 37 (40.2) | 104 (34.0) | 16 (31.4) |
| Chronic mirco infarcts N (%) | |||||
| 0: None | 21 (91.3) | 116 (69.5) | 62 (67.4) | 213 (69.6) | 36 (70.6) |
| 1: One or more | 2 (8.7) | 51 (30.5) | 30 32.6) | 93 (30.4) | 15 (29.4) |
Vascular pathology.
Almost half of individuals in the Mixed-domains class had moderate to severe cerebral amyloid angiopathy, while almost half of individuals in the Frontal Impairment class had at least mild cerebral amyloid angiopathy. Up for 45% of individuals in the Frontal Impairment class had moderate to severe arteriolosclerosis.
Class assignment in association to neuropathology.
Membership in the Mixed-Domains and Memory-Specific Classes was associated with more AD pathology (Table 3), in particular participants in the Mixed-Domains Impairment class were more likely to have Aβ and Tau; this association was also present for participants in the Memory-Specific Impairment class (Table 3). Individuals in the Mixed-Domains and Memory-Specific Classes were also at higher risk for hippocampal sclerosis and presence of Lewy Bodies. Figures 3 and 4 summarize the results of associations between the classes and AD neuropathological markers using odds and risk ratios.
Table 3.
Odds and Relative Risk ratios (95%CI), p-value, and effect size, of the association of latent class membership with AD pathology among 779 Memory and Aging participants had autopsy results.
| Mixed-Domain Impairment | ES | Memory-Specific Impairment | ES | Frontal Impairment | ES | Superior Cognition | ES | |
|---|---|---|---|---|---|---|---|---|
| aAmyloid, % | 1.68 (−0.3 – 3.7), 0.095 | 0.37 | 1.11 (0.3 – 2.0), 0.010 | 0.25 | −0.07 (−1.1 – 1.0), 0.903 | 0.02 | −1.3 (−2.6 – 0.5), 0.059 | 0.30 |
| aTau, mm2 | 9.1 (5.9 – 12.3), <0.001 | 1.27 | 3.75 (2.4 – 5.1), <0.001 | 0.53 | 0.63 (−1.1 – 2.4), 0.478 | 0.11 | −1.04 (−3.2 – 1.2), 0.356 | 0.17 |
| bGlobal burden of AD pathology low vs very low | 0.86 (0.49 – 1.51), 0.594 | −0.07 | 1.15 (0.96 – 1.38), 0.129 | 0.08 | 1.04 (0.8 – 1.3), 0.750 | 0.02 | 0.58 (0.37 – 0.92), 0.019 | −0.22 |
| bGlobal burden of AD pathology medium/high vs. very low | 3.22 (1.7 – 6.0), <0.01 | 0.36 | 2.46 (1.55 – 3.90), <0.001 | 0.25 | 1.28 (0.6 – 2.6), 0.485 | 0.06 | 0.90 (0.37 – 2.20), 0.816 | −0.02 |
| bTDP-43 cytoplasmic inclusions localized to amygdala vs. none | 0.74 (0.2 – 2.5), 0.621 | −0.10 | 0.91 (0.6 – 1.3), 0.606 | −0.08 | 0.72 (0.4 – 1.2), 0.186 | 0.18 | 0.47 (0.2–1.0), 0.048 | 0.16 |
| bTDP-43. cytoplasmic inclusions extended to hippocampus and/or entorhinal cortex vs. none | 1.89 (1.1 – 3.1), 0.013 | 0.27 | 0.95 (0.7 – 1.4), 0.807 | −0.15 | 0.97 (0.6 – 1.5), 0.876 | 0.14 | 0.52 (0.2 – 1.1), 0.096 | 0.43 |
| bTDP-43 cytoplasmic inclusions extended to neocortex vs. none | 2.69 (1.4 – 5.2), 0.001 | 0.31 | 1.74 (1.14 – 2.7), 0.010 | 0.16 | 0.80 (0.4 – 1.6), 0.536 | 0.14 | 0.73 (0.3 – 1.7), 0.477 | 0.36 |
| bHippocampal sclerosis | 3.02 (1.2 – 7.4), 0.016 | −0.11 | 2.02 (1.09 – 3.74), 0.026 | 0.09 | 0.91 (0.37 – 2.22), 0.828 | −0.54 | 0.54 (0.13 – 2.28), 0.405 | −0.07 |
| bLewy Bodies vs. none | 2.07 (1.2 – 3.6), 0.011 | 0.32 | 1.42 (1.01 – 1.2), 0.041 | −0.31 | 1.08 (0.7 – 1.7), 0.748 | −0.80 | 1.03 (0.57 – 1.9), 0.921 | 0.01 |
Note. Results show postmortem indices of neurodegenerative and cerebrovascular disease pathologies included in the analyses. “Average class” is the control in all models. ES = Effect Size. TDP-43 = transactive response DNA-binding protein 43.
indicates continuous variables: The odds ratios are given; Hedge’s g is computed for effect size.
indicates categorical variables: The relative risk ratios are given, and effect size is calculated using Cohen’s h for binary variables.
Figure 3.
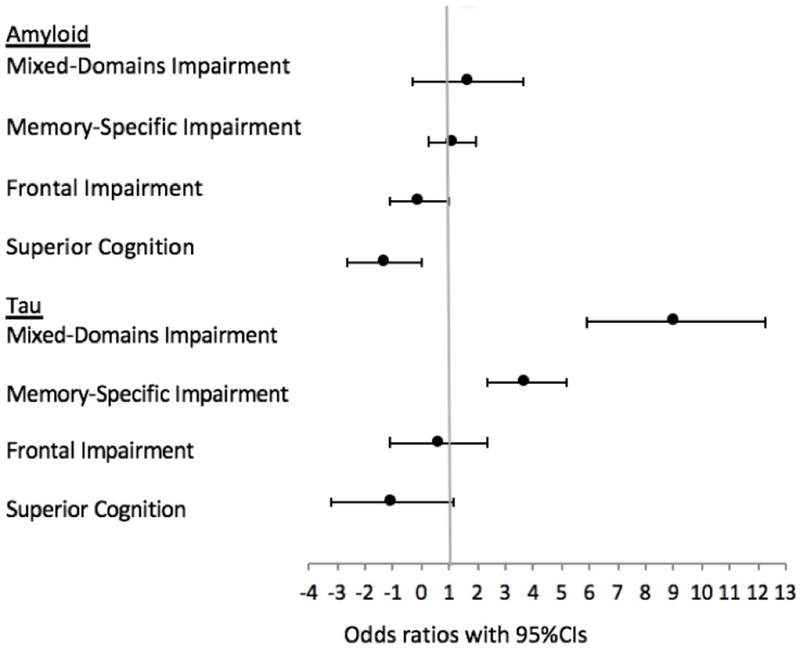
Forest plot summarizing associations between the classes and amyloid and tau using odds ratios, with 95% confidence intervals. Average class is reference in all models.
Figure 4.
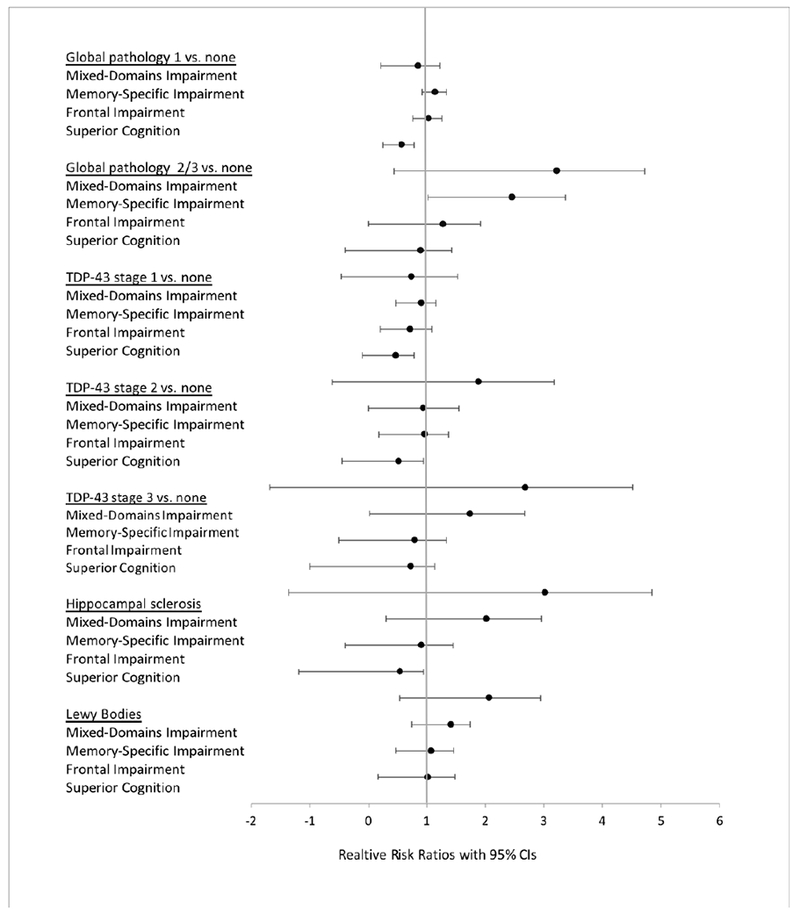
Forest plot summarizing associations between the classes and AD neuropathological markers using risk ratios, with 95% confidence intervals. Average class is reference in all models.
Similar trends were present when investigating vascular pathology (Table 4): Membership in the Mixed-Domains Impairment, Memory-Specific Impairment, and Frontal Impairment class was associated with higher risks for cerebral amyloid angiopathy, arteriolosclerosis, and atherosclerosis. Membership in the The Frontal Impairment class was further associated with high risks for gross and chronic microinfarcts. Figure 4 summarizes the results of associations between the classes and vascular neuropathological markers using effect sizes.
Table 4.
Relative Risks (95% confidence interval), p-value, and effect size of the association of class membership with vascular pathology among 779 Memory and Aging Project participants who had autopsy results.
| Mixed-Domain Impairment | ES | Memory-Specific Impairment | ES | Frontal Impairment | ES | Superior Cognition | ES | |
|---|---|---|---|---|---|---|---|---|
| CAA-4GP: Mild vs. none | 1.33 (1.1 – 1.8), 0.063 | 0.23 | 1.26 (1.1 – 1.5), 0.003 | 0.18 | 1.18 (0.9 – 1.4), 0.086 | 0.12 | 0.93 (0.7 – 1.3), 0.624 | −0.05 |
| CAA-4GP: Moderate/Severe vs. none | 1.60 (1.2 – 2.1), 0.003 | 0.36 | 1.46 (1.2 – 1.7), 0.0001 | 0.26 | 1.18 (0.9 – 1.5), 0.212 | 0.10 | 0.93 (0.6 – 1.4), 0.736 | −0.03 |
| Arteriolosclerosis Mild vs. none | 1.16 (0.8 – 1.7), 0.481 | 0.09 | 1.20 (1.1 – 1.4), 0.056 | 0.11 | 1.11 (0.9 – 1.4), 0.433 | 0.06 | 0.84 (0.6 – 1.2), 0.385 | −0.08 |
| Arteriolosclerosis: | 1.13 (0.7 – 1.9), 0.632 | 0.06 | 1.29 (1.0 – 1.6), 0.021 | 0.14 | 1.43 (1.1 – 1.8), 0.002 | 0.21 | 0.97 (0.7 – 1.4), 0.858 | −0.02 |
| Moderate/Severe vs. none Cerebral atherosclerosis: Mild vs. none | 1.21 (1.1 – 1.5), 0.109 | 0.17 | 1.03 (0.9 – 1.2), 0.683 | 0.02 | 1.13 (0.9 – 1.3), 0.176 | 0.10 | 0.81 (0.6 – 1.1), 0.146 | −0.13 |
| Cerebral atherosclerosis: Moderate/Severe vs. none | 1.28 (0.78 – 2.07), 0.326 | 0.15 | 1.23 (1.1 – 1.5), 0.0525 | 0.12 | 1.42 (1.1 – 1.8), 0.002 | 0.23 | 0.66 (0.4 – 1.1), 0.119 | −0.18 |
| Gross chronic infarcts: 0 vs. ≥1 | 0.77 (0.4 – 1.6), 0.462 | −0.09 | 1.27 (1.0 – 1.6), 0.046 | 0.09 | 1.18 (0.9 – 1.6), 0.262 | 0.06 | 0.92 (0.6 – 1.4), 0.718 | −0.03 |
| Chronic microinfarcts: 0 vs. ≥1 | 0.29 (0.1 – 1.1), 0.066 | −0.28 | 1.005 (0.8 – 1.3), 0.974 | 0.002 | 1.07 (0.8 – 1.5), 0.684 | 0.02 | 0.97 (0.6 – 1.5), 0.888 | −0.01 |
Note. Results list the postmortem indices of neurodegenerative and cerebrovascular disease pathologies included in the analyses. Each cell shows the results of a single regression model, which includes covariates of age at baseline, time from baseline to death, sex, and education (not shown) and the single pathology measure listed in estimates column. “Average class” is the reference class in all models. CAA-4GP = cerebral amyloid angiopathy.
Discussion
In this study, we prospectively assessed the relationship of latent class membership, assigned based on neuropsychological test performance, to neuropathological outcomes based upon autopsy results that were obtained more than seven years later. Results showed that latent classes derived from neuropsychological performance at enrollment are associated with neuropathological outcomes. The results are novel in two ways: First, we illustrated that there are specific subgroups of individuals with certain patterns of cognitive function at enrollment, who are more likely to develop AD pathology. Second, we illustrated that neuropsychological latent classes have the predictive value in distinguishing amongst subgroups with various pathological outcomes, which occurs many years later. The major implications of these finding are that we showed that by using sensitive psychometric measurements to construct subgroups with asymmetric cognitive profiles, i.e. specific impairments in some domains but not in others, can improve our ability to predict neuropathological outcomes, which in turn can have potential in targeting individuals who may be eligible for targeted interventions and clinical-trials.
In a previous study in the same cohort, [21] we reported that cognitive impairment in specific domains of episodic memory and executive function are associated with specific neuropathology (AD pathology and cerebral infarctions) at 1 and 5 years prior to death. Our study is novel and complements our prior work, in that cognition is estimated across different phenotypic subgroups at enrollment (i.e. on average more than 7 years prior to death). Our findings support previous hypotheses on specific associations of AD pathology as illustrated by performance on episodic memory measures [21, 62]; however, results did not support specific effects of infarcts on executive function tests. Our results also support hypotheses that frontal impairment (measures related to perceptual orientation and perceptual speed) is largely related to underlying vascular mechanisms [63], as illustrated by strong associations (effects sizes ranging from 0.65 to 0.95) with mild arteriolosclerosis and mild-severe atherosclerosis.
Our findings provide evidence that neuropsychological measures collected at enrollment provide information on underlying neuropathology upon autopsy. These results support the use of neuropsychological measures as a central role in the assessment, prognosis, and therapeutic strategies of cognitive impairment and dementia [64, 65]. Although biomarker studies have added incremental value to model that predict disease progression, investigators have largely remained unable to provide an accurate prediction of the likelihood of conversion to dementia [66]. This might be in part due to clinical heterogeneity and presence of AD neuropathology in both cognitively healthy as well as cognitively impaired individuals [9, 67].
In this study, we showed that each of the five classes has trends of Aβ and Tau based on neuropathology data. There was a clear exposure-response relationship for Tau across the classes with the Mixed-Domains displaying the highest amounts of Tau, followed by the Memory-Specific, the Frontal Impairment, the Average, and the Superior Cognition. Relatedly, each of the five classes showed trends for AD and dementia diagnosis at the last visit before death, with the Mixed-Domains displaying the highest rates, followed by the Memory-Specific, the Frontal Impairment, the Average, and the Superior Cognition classes. Although recent research has provided strong evidence that abnormal Aβ markers in cognitively normal individuals have prognostic implications and are strong early markers of AD; research has shown that Tau pathology is more strongly associated with clinical and cognitive outcomes than Aβ [65, 68, 69]. Numerous studies have shown that pathology begins many years before onset of clinical diagnosis; however, individuals with higher cognitive reserve are better able to tolerate, and mask, such pathology, to the extent that clinical onset is delayed [70]. It may be the case that specific subgroups of individuals have added reserve, which helps them alleviate the effect of worse underlying neuropathology. In connection with this observation, individuals in the Superior cognition class scored higher on cognitive reserve measures (education and the NART), suggesting class/individual specific indicators of clinical resilience and compensatory mechanisms (fewer individuals in the Superior Cognition class had a dementia/AD diagnosis at the last visit before death) [71]. However, it is also important to note that individuals in the Superior class did not have as much pathology as individuals in the other classes, and it is difficult to make a direct comparison amongst groups with differing levels of pathology. Furthermore, in this study we did not evaluate the onset of diagnosis or the trajectory of cognitive decline, hence we cannot elaborate on the extent of reserve across classes; however, the LC modeling may have helped in targeting specific groups which, are susceptible to the effects of pathology.
Strengths and Limitations.
A major strength of this study is the large number of people enrolled without dementia at baseline and followed to autopsy. The MAP is a large cohort study that has relatively long follow-up with repeated measures of cognition and large incidence of disease. It has a large battery of neuropsychological tests, clinical diagnosis, and well-characterized pathology data. Follow-up and autopsy rates are very high (>80%), which reduces the biases that occur when persons drop out of longitudinal studies or when autopsy is not obtained. This study served as a medium to validate the generated classes using neuropathology data. A particular strength of this study is that participants went through rigorous neuropsychological and neuropathological examination, and that our statistical approach enabled us to test the hypothesis that latent classes displaying various neuropsychological patterns at enrollment associate with patterns of neuropathology upon autopsy.
A limitation inherent in the structure of the latent classes is that the technique groups together individuals depending on their cognitive profile, and thus some classes are more impaired than others. Furthermore, members of some classes (e.g. the Mixed-Domains and the Memory-Specific Class) are at higher risk of dying earlier than members in other classes, which may be due to a host of reasons that include older age and more comorbidity, and because of this, a higher amount of AD pathology might be present at the time of initial assessment.
We did not correct for multiplicity in hypothesis testing. Instead, we follow suggestions made previously [72] and urge readers to focus on effect size rather than statistical significance, and support data using other studies to validate results, which would be in line with the new NIH’s guidelines for rigor and reproducibility. Future studies should consider limiting the number of outcomes in order to limit the number of comparisons between groups, and decrease endpoints.
One limitation of the MAP cohort is that there are relatively few racial and ethnic minorities, which limits generalizability to more diverse populations. Thus, participants may not be representative of the general population of older persons and similar types of studies will need to be done in population-based cohorts with more diversity. It should be mentioned that the LCA might be capturing classes at different time-points, and over time individuals in one class may transition to another class (i.e. the Memory-Specific Impairments class may transition to the Mixed-Domains Impairment class, or individuals from the Average class might transition into one of the Cognition Impaired classes). If so, rather than capturing classes per se, the latent class model is capturing classes at specific time-points. A future study using latent transition analysis on data from several follow-ups can help us in answering these questions.
Conclusions
Our findings support actuarial neuropsychological methods that incorporate more comprehensive neuropsychological measures to classify cognitive impairment with resulting gains in empirical characterization of neuropathological markers. Typically, individual performance on neuropsychological testing is done proximate to death; in this study we showed the predictive ability of neuropsychological measurement in i) distinguishing patterns of cognitive performance amongst subgroups at enrollment, and ii) in subsequently predicting neuropathology outcomes amongst these subgroups upon autopsy.
Applying neuropathological outcomes to cognitive profiles and their clinical outcomes provides a unique opportunity to validate previous results [22]; however, replication across cohorts is essential to continue building upon these models and their implications. Ultimately, a combination of neuropsychological assessment and in vivo imaging may help explain the underlying heterogeneous profiles of preclinical AD [65]. Further studies are urged to extend these findings to other cohort studies to continue to refine the characterization of the latent classes, the psychometric properties of the tests composing the classes, and the biological basis of the classes more fully.
Supplementary Material
Figure 5.
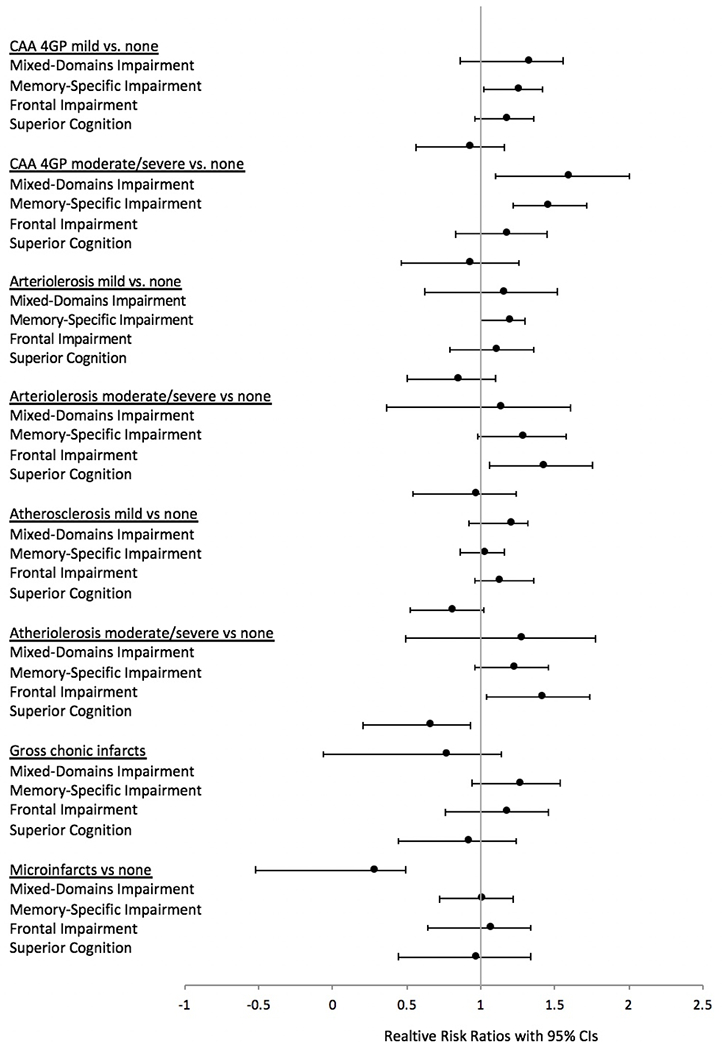
Forest plot summarizing associations between the classes and AD neuropathological markers using risk ratios with 95% confidence intervals. Average class is reference in all models.
Research in Context.
Systematic review: The authors conducted a literature review by using traditional sources (PubMed). Recent studies suggest that actuarial neuropsychological methods that incorporate extensive neuropsychological measures are more accurate in predicting clinical outcomes. Relevant studies are cited.
Interpretation: Our study used latent variable modelling to identify and classify older adults based on their neuropsychological performance. Five classes were identified: Mixed-Domains Impairment, Memory-Specific Impairment, Frontal-Impairment, Average Cognition, and Superior Cognition. Aβ, Tau, and TDP-43 were associated with Mixed-Domains Impairment and Memory-Specific Impairment classes assigned upon enrollment ~ 7 years prior to death.
Future directions: Future studies should explore the combination of neuropsychological assessment and in vivo imaging to continue to construct and refine the characterization of subgroups with asymmetric cognitive profiles, and to understand their biological basis more fully. Results will have potential in targeting individuals who may be eligible for targeted interventions and clinical-trials.
Acknowledgments:
The authors thank the many Illinois residents who have participated in the Rush Memory and Aging Project; Traci Colvin, MPH, for coordination of the clinical data collection; Karen Skish, MS, for coordination of the pathologic data collection; and John Gibbons, MS, and Greg Klein, MS, for data management.
Funding sources: This work was supported by the Memory and Aging Project (R01AG17917, R01AG343749, and R01AG42210) from the National Institute on Aging, the Einstein Aging Study (PO1 AG03949) from the National Institutes on Aging program, by the National Institute On Aging of the National Institutes of Health under Award Number K01AG054700, and by the Sylvia and Leonard Foundation. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: All authors declare that there are no financial, personal, or other potential conflicts of interest.
References
- 1.Ritchie K, et al. , The clinical picture of Alzheimer’s disease in the decade before diagnosis: clinical and biomarker trajectories. J Clin Psychiatry, 2016. 77(3): p. e305–11. [DOI] [PubMed] [Google Scholar]
- 2.Bennett DA, et al. , Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis, 2018. 64(s1): p. S161–s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen WJ, et al. , Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA, 2015. 313(19): p. 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondi MW, et al. , Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s disease : JAD, 2014. 42(1): p. 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmon DP and Bondi MW, Neuropsychological assessment of dementia. Annual review of psychology, 2009. 60: p. 257–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondi MW, et al. , Neuropsychological contributions to the early identification of Alzheimer’s disease. Neuropsychology review, 2008. 18(1): p. 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider JA, et al. , The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol, 2009. 66(2): p. 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelber RP, Launer LJ, and White LR, The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res, 2012. 9(6): p. 664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett DA, et al. , Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology, 2006. 66(12): p. 1837–44. [DOI] [PubMed] [Google Scholar]
- 10.Edmonds E, et al. , Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Vol. 87 2016. 10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J Libon D, et al. , Neuropsychological Syndromes Associated with Alzheimer’s/Vascular Dementia: A Latent Class Analysis. Vol. 42 2014. [DOI] [PubMed] [Google Scholar]
- 12.Delano-Wood L, et al. , ACTUARIAL NEUROPSYCHOLOGICAL CRITERIA FOR MCI DIAGNOSIS IMPROVES ASSOCIATIONS WITH VASCULAR AND IMAGING BIOMARKERS. Vol. 10 2014. P275. [Google Scholar]
- 13.Hulette CM, et al. , Neuropathological and Neuropsychological Changes in “Normal” Aging: Evidence for Preclinical Alzheimer Disease in Cognitively Normal Individuals. Journal of Neuropathology & Experimental Neurology, 1998. 57(12): p. 1168–1174. [DOI] [PubMed] [Google Scholar]
- 14.Wilson RS, et al. , Neurodegenerative basis of age-related cognitive decline. Neurology, 2010. 75(12): p. 1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley KP, Snowdon DA, and Markesbery WR, Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol, 2002. 51(5): p. 567–77. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt FA, et al. , “Preclinical” AD revisited: neuropathology of cognitively normal older dults. Neurology, 2000. 55(3): p. 370–6. [DOI] [PubMed] [Google Scholar]
- 17.Galvin JE, et al. , Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol, 2005. 62(5): p. 758–65. [DOI] [PubMed] [Google Scholar]
- 18.Silver MH, et al. , Distinguishing Between Neurodegenerative Disease and Disease-Free Aging: Correlating Neuropsychological Evaluations and Neuropathological Studies in Centenarians. Psychosomatic Medicine, 2002. 64(3): p. 493–501. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PA, et al. , Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol, 2018. 83(1): p. 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle PA, et al. , Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain, 2017. 140(3): p. 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang FM, et al. , AD pathology and cerebral infarctions are associated with memory and executive functioning one and five years before death. J Clin Exp Neuropsychol, 2013. 35(1): p. 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zammit AR, et al. , Class-Specific Incidence of All-Cause Dementia and Alzheimer’s Disease: A Latent Class Approach. J Alzheimers Dis, 2018. 66(1): p. 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammit AR, et al. , Subtypes Based on Neuropsychological Performance Predict Incident Dementia: Findings from the Rush Memory and Aging Project. J Alzheimers Dis, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLachlan G and Peel D, Finite Mixture Models. 2000: John Wiley & Sons. [Google Scholar]
- 25.Vermunt JK and Magidson J, Latent Class Cluster Analysis, in Applied Latent Class Analysis, Hagenaars J and McCutcheon A, Editors. 2002, Cambridge University Press: New York. [Google Scholar]
- 26.Nagin DS and Odgers CL, Group-Based Trajectory Modeling (Nearly) Two Decades Later. Journal of Quantitative Criminology, 2010. 26(4): p. 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhebergen D, et al. , An examination of generalized anxiety disorder and dysthymic disorder by latent class analysis. Psychological Medicine, 2014. 44(8): p. 1701–1712. [DOI] [PubMed] [Google Scholar]
- 28.Spinhoven P, et al. , Prediction of 6-yr symptom course trajectories of anxiety disorders by diagnostic, clinical and psychological variables. J Anxiety Disord, 2016. 44: p. 92–101. [DOI] [PubMed] [Google Scholar]
- 29.Lacourse E, et al. , Developmental trajectories of boys’ delinquent group membership and facilitation of violent behaviors during adolescence. Dev Psychopathol, 2003. 15(1): p. 183–97. [DOI] [PubMed] [Google Scholar]
- 30.Broidy LM, et al. , Developmental trajectories of childhood disruptive behaviors and adolescent delinquency: a six-site, cross-national study. Dev Psychol, 2003. 39(2): p. 222–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linden-Carmichael AN, Dziak JJ, and Lanza ST, Dynamic Features of Problematic Drinking: Alcohol Use Disorder Latent Classes Across Ages 18–64. Alcohol Alcohol, 2019. 54(1): p. 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett DA, et al. , Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res, 2012. 9(6): p. 646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raftery AE, Bayesian model selection in social research. Sociological methodology, 1995: p. 111–163. [Google Scholar]
- 34.Celeux G and Soromenho G, An entropy criterion for assessing the number of clusters in a mixture model. Journal of Classification, 1996. 13(2): p. 195–212. [Google Scholar]
- 35.Bolck A, Croon M, and Hagenaars J, Estimating Latent Structure Models with Categorical Variables: One-Step Versus Three-Step Estimators. Vol. 12 2004. [Google Scholar]
- 36.McIntosh C, Pitfalls in subgroup analysis based on growth mixture models: A commentary on van Leeuwen et al. (2012). Vol. 22 2013. [DOI] [PubMed] [Google Scholar]
- 37.Asparouhov T and Muthén B, Auxiliary Variables in Mixture Modeling: Three-Step Approaches Using Mplus. Structural Equation Modeling: A Multidisciplinary Journal, 2014. 21(3): p. 329–341. [Google Scholar]
- 38.Vermunt J, Latent Class Modeling with Covariates: Two Improved Three-Step Approaches. Vol. 18 2010. [Google Scholar]
- 39.Lanza ST, Tan X, and Bray BC, Latent Class Analysis With Distal Outcomes: A Flexible Model-Based Approach. Structural equation modeling : a multidisciplinary journal, 2013. 20(1): p. 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakk Z and Vermunt J, Robustness of Stepwise Latent Class Modeling With Continuous Distal Outcomes. Vol. 23 2015. [Google Scholar]
- 41.Wechsler D, Wechsler Memory Scale - Revised. 1987, San Antonio: The Psychological Corporation. [Google Scholar]
- 42.Buschke H, Cued recall in amnesia. J Clin Neuropsychol, 1984. 6(4): p. 433–40. [DOI] [PubMed] [Google Scholar]
- 43.Morris JC, et al. , The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 1989. 39(9): p. 1159–65. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan EF, Goodglass H, and Weintraub S, The Boston Naming Test. Second Edition ed. 1983, Philadelphia: Lea & Febiger. [Google Scholar]
- 45.Wechsler D, Adult Intelligence Scale-III. 3rd ed. 1997, San Antonio, TX: Psychological Corporation. [Google Scholar]
- 46.Werheid K, et al. , The Adaptive Digit Ordering Test: Clinical application, reliability, and validity of a verbal working memory test. Archives of Clinical Neuropsychology, 2002. 17(6): p. 547–565. [PubMed] [Google Scholar]
- 47.Raven J, Standard Progressive Matrices [Google Scholar]
- 48.Benton AL, Varney NR, and Hamsher KS, Visuospatial judgment: A clinical test. Archives of Neurology, 1978. 35(6): p. 364–367. [DOI] [PubMed] [Google Scholar]
- 49.Sheridan LK, et al. , Normative Symbol Digit Modalities Test performance in a community-based sample. Archives of Clinical Neuropsychology, 2006. 21(1): p. 23–28. [DOI] [PubMed] [Google Scholar]
- 50.Yang FM, et al. , AD pathology and cerebral infarctions are associated with memory and executive functioning one and five years before death. Journal of clinical and experimental neuropsychology, 2013. 35(1): p. 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nag S, et al. , Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol, 2015. 77(6): p. 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nag S, et al. , TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology, 2017. 88(7): p. 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arvanitakis Z, et al. , The Relationship of Cerebral Vessel Pathology to Brain Microinfarcts. Brain Pathol, 2017. 27(1): p. 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arvanitakis Z, et al. , Microinfarct pathology, dementia, and cognitive systems. Stroke, 2011. 42(3): p. 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennett DA, et al. , Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology, 2003. 60(2): p. 246–52. [DOI] [PubMed] [Google Scholar]
- 56.Boyle PA, et al. , Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology, 2015. 85(22): p. 1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchman AS, et al. , Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke, 2011. 42(11): p. 3183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Honig LS, Kukull W, and Mayeux R, Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology, 2005. 64(3): p. 494–500. [DOI] [PubMed] [Google Scholar]
- 59.Schneider JA, et al. , Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology, 2003. 60(7): p. 1082–8. [DOI] [PubMed] [Google Scholar]
- 60.Muthén LK and Muthén BO, MPlus User’s Guide. 1998–2016, Muthén & Muthén: Los Angeles, CA. [Google Scholar]
- 61.SPSS Inc., IBM SPSS Statistics for Windows. Released 2016, IBM Corp.: Armonk, NY. [Google Scholar]
- 62.Bondi MW, et al. , Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychol Aging, 1999. 14(2): p. 295–303. [DOI] [PubMed] [Google Scholar]
- 63.Jellinger KA, Understanding the pathology of vascular cognitive impairment. Journal of the Neurological Sciences, 2005. 229–230: p. 57–63. [DOI] [PubMed] [Google Scholar]
- 64.Bondi MW, et al. , Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis, 2014. 42(1): p. 275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bondi MW, Edmonds EC, and Salmon DP, Alzheimer’s Disease: Past, Present, and Future. J Int Neuropsychol Soc, 2017. 23(9–10): p. 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sperling RA, Karlawish J, and Johnson KA, Preclinical Alzheimer disease —the challenges ahead. Nature reviews. Neurology, 2013. 9(1): p. 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett DA, et al. , Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology, 2005. 64(5): p. 834–41. [DOI] [PubMed] [Google Scholar]
- 68.Bennett DA, et al. , Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol, 2004. 61(3): p. 378–84. [DOI] [PubMed] [Google Scholar]
- 69.Yau W-YW, et al. , Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. The Lancet Neurology, 2015. 14(8): p. 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stern Y, et al. , Influence of education and occupation on the incidence of Alzheimer’s disease. Jama, 1994. 271(13): p. 1004–10. [PubMed] [Google Scholar]
- 71.Stern Y, Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol, 2012. 11(11): p. 1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ranganathan P, Pramesh CS, and Buyse M, Common pitfalls in statistical analysis: The perils of multiple testing. Perspectives in clinical research, 2016. 7(2): p. 106–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


