Abstract
Objective:
While most transient ischemic attack and minor stroke (TIAMS) patients in U.S. emergency departments (EDs) are admitted, experience in other countries suggests that timely outpatient evaluation of TIAMS can be safe. We assessed the feasibility and safety of a rapid outpatient stroke clinic for TIAMS: Rapid Access Vascular Evaluation-Neurology (RAVEN).
Methods:
TIAMS patients presenting to the ED with a NIH stroke scale of 5 or less and non-disabling deficit were assessed for potential discharge to RAVEN using a protocol incorporating social and medical criteria. Outpatient evaluation by a vascular neurologist, including vessel imaging, was performed within 24 hours at RAVEN. Participants were evaluated for compliance with clinic attendance and 90-day recurrent TIAMS and hospitalization rates.
Results:
Between December 2016 and June 2018, 162 TIAMS patients were discharged to RAVEN. 154 (95. 1%) appeared as scheduled and 101 (66%) had a final diagnosis of TIAMS. Two patients (1.3%) required hospitalization (one for worsening symptoms, another for intracranial arterial stenosis due to zoster) at RAVEN evaluation. Among the 101 patients with confirmed TIAMS, 18 patients (19.1%) had returned to an ED or been admitted at 90 days. Five were noted to have had recurrent neurological symptoms diagnosed as TIA (4.9%), while one had a recurrent stroke (0.9%). No TIAMS individuals died, and none received thrombolytics or thrombectomy, during the interval period. These 90-day outcomes were similar to historical published data on TIAMS.
Conclusions:
Rapid outpatient management appears a feasible and safe strategy for TIAMS patients evaluated in the ED, with recurrent stroke and TIA rates comparable to historical published data.
Introduction
Background and Importance
Stroke is the third most common cause of death among women, the fifth most common cause of death among men, and the leading cause of serious long-term disability in the United States.1 One of the major precursors of ischemic stroke is a transient ischemic attack (TIA), defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, without acute infarction.2 TIA is a common condition, with an estimated 250,000–300,000 events occurring annually in the United States (U.S.).1 TIA precedes stroke in approximately 15-26% of patients.3 The majority of strokes occur in the first week following TIA, and the first two days after TIA are the highest risk period where rates of stroke vary from 4% to 10%.4 At 90 days, the rate of stroke after TIA is approximately 9-10%.5, 6 This early short-term risk emphasizes the critical importance of timely evaluation of TIA, particularly since risk of stroke is determined largely by underlying etiology.7 While bedside scoring systems, such as the ABCD2 score, have been explored as potential risk stratification tools, they are limited without the use of vascular imaging in identifying symptomatic large arterial stenosis.8, 9 For example, symptomatic extracranial internal carotid artery stenosis is associated with a high short-term risk of recurrence and is not readily captured by current bedside decision tools or scoring systems.10
Rapid diagnostic evaluation of TIA/minor stroke (TIAMS) patients is recommended by the American College of Emergency Physicians and the American Heart Association/American Stroke Association (AHA/ASA) in order to initiate secondary stroke prevention and identify patients who may need rapid carotid revascularization, yet practice variability exists whether such timely follow-up can occur in the inpatient versus outpatient setting.2, 11–13 While most TIAMS patients evaluated in U.S. emergency departments (EDs) are admitted,14 experience in other countries suggests expedited outpatient evaluation can be both safe and cost-effective.15–17 In the U.S., ED and neurology observation units have been developed in select hospitals for the expedited evaluation of TIAMS.18, 19 Outcomes data have been encouraging, with patients treated in observation units having shorter lengths of stay, lower total direct costs, comparable 90-day clinical outcomes, and similar degree of adoption of secondary prevention medication strategies in comparison to patients admitted for TIAMS.18–24 While the evidence in support of these observation units is promising, these units are not widely available, still involve the risk and cost of a hospital stay, and can contribute to hospital crowding. A safe and rapid outpatient discharge strategy may offer benefit in the management of TIAMS patients beyond observation or short inpatient stay.
Based on evidence suggesting clinical equipoise for the disposition of TIAMS patients, we undertook an interdisciplinary endeavor between the Departments of Emergency Medicine and Neurology at Columbia University Irving Medical Center to create a novel outpatient management strategy for the management of TIAMS. This clinic, the Rapid Access Vascular Evaluation-Neurology (RAVEN) is, to our knowledge, among the first outpatient integrated rapid access stroke clinics in the United States and may offer a safe alternative management strategy to inpatient admission of TIAMS.
Goals of This Investigation
The goal of this research report is to describe the feasibility and safety of an outpatient treatment strategy for the management of TIAMS. Specifically, we aimed to report RAVEN feasibility and safety, diagnoses and care at initial evaluation, and outcomes at 90-day follow-up for TIAMS patients treated in RAVEN.
Materials and Methods
Study Design and Setting
This study was a retrospective review of records and outcomes among individuals referred to a rapid outpatient TIAMS clinic in a quaternary academic medical center in an urban setting and a designated Comprehensive Stroke Center. The study protocol was reviewed and approved by the Columbia University Irving Medical Center Institutional Review Board; waiver of informed consent was granted since this was a retrospective review of existing records.
RAVEN protocol development
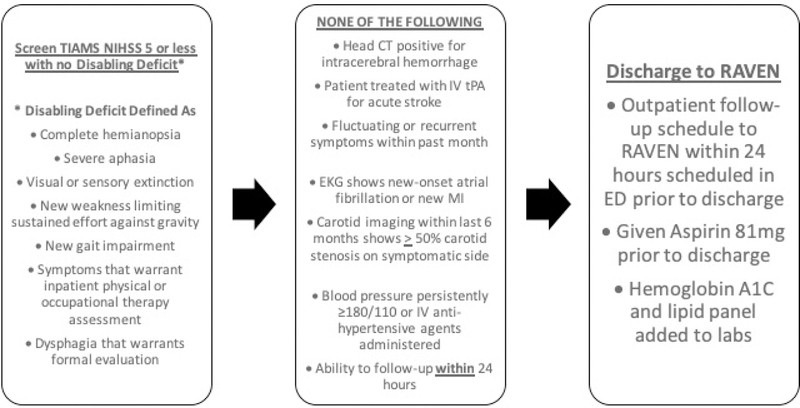
In designing the protocol for RAVEN, we sought to identify TIAMS patients for whom ED discharge to RAVEN follow-up within 24 hours would pose minimal adverse short-term neurologic risk, while considering potential limitations in functional or social factors that would permit rapid outpatient evaluation. The RAVEN protocol and criteria are summarized in Figure 1.
Figure 1.
RAVEN Protocol
Creation of the RAVEN clinic occurred in three phases, spanning approximately 12 months from conception to implementation. First, a team of one emergency physician and four vascular neurologists completed and published a clinical review of extant literature summarizing the use of existing risk stratification methods for TIAMS in the ED setting, evidence for near-term risk of adverse neurologic events in TIAMS, and extant disposition strategies for the management of TIAMS in the emergency setting.13 Based on this clinical review, a preliminary set of criteria and protocol were created. Secondly, building on the initial criteria, in-person meetings were held with the departments of Emergency Medicine and Neurology to obtain feedback and feasibility from the practicing clinicians in both departments to address both acute neurological and medical issues, as well as potential social factors and follow-up concerns. Finally, the draft protocol was reviewed by departmental and senior hospital leadership for approval and administrative support.
Participants: Selection of RAVEN patients
From December 2016 through June 2018, patients seen in the ED with a possible non-disabling TIAMS were screened for potential RAVEN discharge by the consulting neurology resident in the ED. It is the policy at our institution that all patients with a possible TIAMS in the ED undergo brain imaging with CT and have an ED consultation by a neurology resident at the request of the attending ED physician. All such cases are then discussed with a board-certified vascular neurologist prior to ED disposition. As summarized in Figure 1, disabling symptoms were defined following AHA/ASA acute guidelines on the management of acute stroke based on the following: complete hemianopia, severe aphasia, dysphagia, gait disorder, visual or sensory extinction, new weakness limiting sustained effort against gravity, symptoms warranting physical or occupational therapy assessment, or an NIH Stroke Scale (NIHSS) > 5.25 Our criteria for RAVEN eligibility built on this and also included a list of additional criteria for RAVEN discharge, including head CT imaging with no evidence of hemorrhage, no treatment with thrombolysis in the ED, no fluctuating symptoms or recurrent symptoms within the past month, electrocardiogram showing no new onset atrial fibrillation or cardiac ischemia, no evidence of blood pressure persistently elevated over 180/110 mm Hg, no IV hypertensive agents administered in ED, no known large artery stenosis of > 50%, and ability to follow-up within 24 hours. For the initial implementation of RAVEN, due to available hospital and staffing resources, the RAVEN clinic operated on weekdays from Monday to Friday, with no clinic on weekends or holidays. In accordance with AHA/ASA guidelines, patients with non-disabling minor strokes are generally not considered eligible for mechanical thrombectomy.25
If the patient met all criteria for referral to RAVEN, the protocol required that all appointments for follow-up were made during the ED visit by the stroke team on the patient’s behalf, and patients were given specific instructions in their primary language, including a map showing location and the time of the RAVEN appointment. Contact information was collected for each patient, and an email was generated by the stroke team for each patient discharged for RAVEN follow-up and sent to a RAVEN clinic scheduler/administrator as well as the RAVEN clinicians. Patients were given contact information of the clinic for any changes or concerns, and patients were explicitly told to return to the ED for evaluation for any change or worsening of symptoms.
At the RAVEN clinic visit within 24 hours, patients were evaluated by a board-certified vascular neurologist, and outpatient vascular imaging with carotid duplex and transcranial Doppler were done that same day in keeping with recommendations for evaluation of TIAMS (AHA/ASA and Canadian Best Practices)25, 26. Patients were also referred for an MRI, echocardiogram, and outpatient Holter monitoring at the discretion of the vascular neurologist. Blood pressure, antithrombotic, and statin medications were optimized as clinically indicated. Counseling on lifestyle modifications including smoking cessation and risk factor control was also provided. Notably, referrals to the RAVEN clinic occurred regardless of insurance status, and if uninsured or under-insured, patients were not charged for the visit. Any patients that were deemed to require internal carotid artery intervention or had a change in neurological or medical status were admitted directly to the hospital from RAVEN clinic.
Measurements and Outcomes
Baseline demographics, including age, sex, primary language, and insurance status were collected in all participants. Medical information collected included baseline vital signs (e.g. blood pressure), NIHSS at initial ED presentation, and modified Rankin Scale score for pre-TIAMS disability.27 Rates of participant follow-up to RAVEN clinic were tabulated, together with any adverse events including recurrent stroke, death, or revisit to the hospital during the 24-hour period prior to RAVEN clinic. Frequency of required admission after re-evaluation at RAVEN clinic was tabulated, along with reason for admission. Final diagnosis of the patient was determined by the treating vascular neurologist in the RAVEN clinic after reviewing all clinical variables and imaging. The final diagnosis was categorized as either possible or definite ischemic stroke, TIA, or a TIAMS mimic (migraine, peripheral neuropathy, vestibular, and others).
All patients were contacted by telephone at 90 days as part of our Comprehensive Stroke Center operations to assess if there were any new neurological or cardiovascular events, as well as all-cause rehospitalization (including ED visits). If the patient could not be contacted, the primary care provider and/or family member/next of kin was contacted. In addition, interval chart review was performed. Patients lost to follow-up (e.g. no show) at the 24-hour RAVEN clinic were categorized by reason for no show and also were attempted to be reached again by phone and by their primary care provider (if provided).
Results
Sociodemographic Characteristics of Participants
From December 2016 until June 2018, 253 patients were screened for possible RAVEN discharge, with 162 individuals with TIAMS in the ED enrolled for discharge to RAVEN clinic. Among the 91 individuals who were screened but did not meet RAVEN discharge criteria, 58 were evaluated during a time when RAVEN follow-up within 24 hours was not possible (e.g. weekend or holiday), while 33 were found to have either a disabling deficit or a social reason for being unable to follow up within 24 hours. Baseline demographics for the 162 RAVEN patients are summarized in Table 1.
TABLE 1:
Participant Demographics
| RAVEN Participant Demographics | n=162 |
|---|---|
| Mean age (SD) | 63.3 (15.6) |
| Age range | 25.1–97.4 |
| Female sex (%) | 96 (59.3) |
| Mean Rankin Score (SD) | 0.6 (SD 1.1) |
| Median Rankin Score (IQR) | 0 (0,1) |
| Mean NIHSS (SD) | 0.98 (1.59) |
| Median NIHSS (IQR) | 0 (0,2) |
| Mean initial systolic Blood Pressure (SD) | 151 (25) |
| Mean initial diastolic Blood Pressure | 86 (15.6) |
| Primary language (%) | |
| English | 91 (56.2) |
| Spanish | 61 (37.7) |
| Bilingual | 3 (1.9) |
| Other | 7 (4.3) |
| Insurance status | |
| Medicare | 61 (37.7) |
| Medicaid | 43 (26.5) |
| Commercial insurance | 46 (28.4) |
| Other | 8 (4.9) |
| Uninsured/self pay | 4 (2.5) |
Patient Characteristics at RAVEN Clinic Follow-up
Patient characteristics at RAVEN follow-up are summarized in Table 2. Of the 162 individuals scheduled and discharged to RAVEN from the ED, 154 (95.1%) followed up in RAVEN clinic the next day. There were no patients who developed a subsequent deficit within 24 hours of the initial visit in the ED who would have been eligible for thrombolytics or thrombectomy. Among the eight individuals who did not show to RAVEN at 24 hours, two returned to RAVEN clinic within 48 hours secondary to scheduling conflicts and confusion about timing of follow-up; two were ultimately not discharged from the ED and were admitted to a medicine service; and another patient, with a known history of metastatic cancer, left against medical advice. Finally, three patients were lost to follow-up with no further information, leaving three (1.8%) of our sample being unexplained “no shows.”
TABLE 2:
RAVEN follow-up Characteristics
| RAVEN patient characteristics | Frequency (Percentage) |
|---|---|
| Followed up in RAVEN within 24 hours (%) | 154 of 162 (95.1) |
| Final diagnosis (%) | |
| TIA | 42 (28) |
| Minor stroke | 59 (38) |
| Unknown | 3(2) |
| Other (e.g. seizure, recrudescence) | 7(4) |
| Peripheral Neuropathy | 23 (15) |
| Migraine | 20 (13) |
In our sample of 162 RAVEN patients, prior to disposition to RAVEN clinic, seven (4.3%) were already maximally medically managed (taking dual antiplatelet therapy and statin), while 56 were already on a single antiplatelet such as aspirin or clopidogrel (38.2%). In the ED, prior to being discharged to RAVEN, 22 (13.5%) had a CTA head/neck and 2 patients (1.2%) had an MRI/MRA before discharge. In terms of neurologic disability, mean NIHSS for patients at time of ED discharge to RAVEN was 0.98 (standard deviation [SD] 1.59), with median NIHSS of 0 (interquartile range [IQR]: 0,2). Mean baseline modified Rankin scale score at time of ED discharge to RAVEN was 0.6 (SD 1.1), with median of 0 (IQR: 0,1). Of the 154 individuals who followed up in RAVEN, 101 (66%) were found to have a final diagnosis of TIA (42) or minor stroke (59). The agreement (kappa) for discharge diagnosis and final diagnosis was .27 for the 154 participants who followed up in RAVEN. The remaining patients were diagnosed with a stroke mimic including 23 (15%) with peripheral neuropathy, 20 (13%) with migraine, and seven (4%) with other conditions such as seizure or recrudescence (transient worsening) of prior stroke symptoms in setting of medical illness or medication; three patients (2%) did not have an established final diagnosis. Five (3.2%) had MRI studies done at RAVEN. One individual was found to have a positive finding on DWI indicative of stroke (right internal capsular) and was subsequently admitted from RAVEN. Out of the patients seen in RAVEN, two (1.3%) required hospitalization (one for the aforementioned capsular stroke, another for diffuse intracranial arterial stenosis due to zoster vasculitis) and were directly admitted to the neurology service from RAVEN.
90-Day Outcomes
The 90-day outcomes are summarized in Table 3. Among the 162 patients discharged to RAVEN clinic, 17 were lost to follow-up at 90 days (10.5%), leaving 145 successfully completed follow-ups. Of these 145 patients, during the 90-day period, 28 (19.3%) presented again to an ED or were admitted to the hospital; six (3.7%) were noted to have recurrent TIA symptoms; one (0.7%) had a recurrent stroke. Two individuals (1.4%) died, one of metastatic cancer and the other of respiratory failure and congestive heart failure. Of the remaining 19 readmissions, nine were for cardiac reasons (syncope, heart failure exacerbation, hypertension, elective valve repair); seven for potential neurologic complaints (lip tingling, dizziness, and vertigo), of whom all were evaluated and discharged from the emergency department without need for inpatient admission; and three for miscellaneous (chemotherapy, urological complaint, alcohol intoxication). None of the readmissions were judged to be preventable based on missed secondary prevention treatment, or related to the index TIAMS. In our sample no patients at follow-up were found to have undergone thrombectomy or received thrombolysis (e.g. tpA).
TABLE 3:
90-day outcomes for RAVEN patients
| 90 day outcomes assessed for the 162 RAVEN patients | Number/Percentage |
|---|---|
| Readmitted or returned to ED | 28 (19.7) |
| Recurrent TIA | 6 (3.7) |
| Recurrent stroke | 1 (0.7) |
| Death of any cause | 2 (1.4) |
| Lost to follow-up at 90 days | 17 (10.5) |
90-day outcome data for the 154 patients who followed up in RAVEN clinic stratified by their final discharge diagnosis are presented below in Table 4. Patients with a final RAVEN diagnosis of TIAMS (n=101) were compared to those with a final RAVEN diagnosis of a stroke mimic (n=53). Among TIAMS patients, by 90 days, there were no reported deaths. Six individuals in the sample had a new TIA or minor stroke (5.9%).
Table 4:
90 day outcomes stratified by final RAVEN diagnosis
| 90 day outcomes assessed for the 101 RAVEN patients with a final diagnosis of TIAMS | Number/Percentage |
|---|---|
| Readmitted or returned to ED | 18 (19.1%) |
| New TIA or stroke | 6 (5.9%) |
| Death of any cause | 0 |
| Lost to follow-up at 90 days | 9 (8.9%) |
| 90 day outcomes assessed for the 53 RAVEN patients with a final diagnosis of a stroke mimic | Number/Percentage |
| Readmitted or returned to ED | 10 (20.4%) |
| New TIA or stroke | 1 (1.9%) |
| Death of any cause | 2 (3.7%) |
| Lost to follow-up at 90 days | 7 (13.2%) |
Limitations
Our study was a single site cohort study at an academic urban medical center with a comprehensive stroke center designation, with in-house consulting neurology, limiting the generalizability of our findings to sites with different existing neurology infrastructure and different patient populations served. Additionally, while follow-up was conducted both via chart review and 90-day phone calls, we were unable to establish contact with 10.5% of our sample, making it possible that some potential adverse events were not accounted for in our analysis. However, while the overall lost to follow-up was 10.5%, the lost to follow-up for patients with a final diagnosis of TIAMS was 8.9% compared to mimics (13.2%). The lower rate of follow-up among the true TIAMS patients compared to those with TIAMS mimics is reassuring from an acute recurrent stroke outcome perspective. Additionally, the 90-day lost to follow-up rate may in part reflect our study design. As this was a pilot study, using a retrospective chart review design and not a prospective cohort study with planned 90-day follow-up, we did not have any existing infrastructure or mechanisms to optimize follow-up beyond the 24 hour RAVEN clinic assessment. Future prospective trials assessing the near-term and 90-day outcomes in similar TIAMS samples will ideally be better poised to improve near- and long-term follow-up.
Finally, as the primary aim of this study was to assess feasibility and safety of RAVEN, the current study did not have a comparator group.
Discussion
We implemented a novel rapid outpatient management approach, RAVEN clinic, for TIAMS patients evaluated in the ED. While such outpatient strategies have been described in other countries, 16,28, 29 this study represents among the first published efforts of such rapid outpatient strategies for TIAMS in the United States. Our preliminary data suggest that such a protocol is a safe and feasible strategy for selected TIAMS patients. As the goal of this study was focused on assessing the safety and feasibility of such a rapid outpatient neurology strategy, we did not have an a priori comparison group of inpatient strategies or ED observation strategies for this initial study. However, our initial and 90-day safety outcomes are similar to several large published trials in TIA and stroke patients. In a prospective clinical trial of 4,557 TIAMS patients (Platelet Oriented Inhibition in new TIA and minor ischemic stroke: POINT), rates of stroke recurrence (6.3%) and 90 day death of any causes (0.5)% in the control arm were similar or higher in percentage to such outcomes in RAVEN.30 Our 90-day stroke rates were also similar to those seen in the Transient Ischemic Attack Clinic with Round-the-clock Access (SOS-TIA)15 and the Effect of Urgent Treatment of Transient Ischemic Attack and Minor Stroke on Early Recurrent Stroke (EXPRESS) studies, where 90-day rates of stroke among confirmed TIAMS patients ranged from 1.7% to 2.1% 29 and provide further support that urgent outpatient evaluation and vascular imaging may be a feasible approach to the management of TIAMS.15, 16 Administering thrombolytics rapidly if a recurrent disabling deficit is identified is a commonly cited reason for hospitalizing TIA patients, but we did not observe any patients for whom this would have been the case.31
TIAMS patients are commonly evaluated in the ED; however, wide practice variability exists regarding the ED management and disposition of these patients. The proportion of TIAMS patients admitted in the U.S. is substantial,14, 32 contributing to ballooning healthcare costs of caring for these patients. Prior work has suggested that hospitalizing TIA patients may not be cost effective.33 Emergency Department Observation units and short-term stay inpatient units are other possible sites for TIAMS evaluation, but median length of stays in these units remain around 24 hours and costs may be high.34, 35 The adoption of a rapid outpatient evaluation of TIAMS patients has the potential to reduce unnecessary hospital admissions and reduce overall length of stay for TIAMS patients, with downstream implications for ED and inpatient bed flow management. In addition to these potential positive operational and cost savings, an outpatient strategy may have salutary effects on factors such as patient preferences and decreased nosocomial risk associated with hospital admission. Emerging work from behavioral sciences has shown that hospitalization may be associated with the development of adverse psychological outcomes, including the development of post-traumatic stress disorder.36, 37 Outpatient TIAMS management strategies such as RAVEN clinic may have the additional benefit of reducing risk of psychological distress. Future work building on these initial data from RAVEN clinic may offer additional insight and applications for emergency clinicians with regard to disposition and management of TIAMS.
The role of rapid outpatient approaches should be assessed in a variety of clinical settings to evaluate if such strategies are feasible and safe across multiple practice environments, including the construction of clinical trials and matched comparison groups to evaluate whether such outpatient strategies provide similar neurologic or different health outcomes in TIAMS patients compared to inpatient or observation strategies. Additionally, data garnered from these studies could guide the refinement of existing and future outpatient TIAMS protocols. Outstanding issues, such as whether vessel imaging (e.g. computed tomographic or magnetic resonance angiography) should be done in the ED prior to discharge or at 24-hour follow-up in a rapid outpatient approach could be assessed in future studies. In our study, only a small proportion of patients received vessel imaging in the ED prior to discharge to RAVEN clinic, demonstrating that ours appears a safe approach. In addition, an outpatient TIAMS evaluation strategy is well-poised to adapt to practice changes in acute medical therapy of TIAMS patients. Specifically, two recent large clinical trials have demonstrated the benefit of acute dual antiplatelet therapy on reducing early stroke risk in TIAMS patients.38, 39 This is an intervention that can be easily incorporated into an outpatient TIAMS management strategy as dual antiplatelet therapy can be initiated in the ED and then continued as an outpatient pending RAVEN evaluation and confirmation of TIAMS diagnosis.
The use of such outpatient strategies for TIAMS may also offer potential cost savings for healthcare systems and avoid costly and unnecessary hospital admissions. While the aim of this study was the safety and feasibility of RAVEN rather than cost effectiveness, recent data presented by our group showed that RAVEN patients had decreased direct hospital costs compared to TIAMS patients managed in an inpatient setting.40 Future work evaluating the implications of a RAVEN approach to hospital factors such as total direct cost, revenue, and bed capacity may highlight the broader health economic impact that such an outpatient strategy may have for health systems.
An initial concern about RAVEN implementation was that the wide variability in clinical TIA diagnosis could potentially lead to outpatient evaluation of many non-vascular/stroke patients, leading to an overburden of the rapid access clinic system. However, this did not prove to be true. On average, RAVEN saw approximately 3 patients a week, and we found a 66% concordance rate between ED diagnosis of TIAMS and final discharge diagnosis by vascular neurologists. This rate was similar to prior ED and general practitioner-based studies,35, 41, 42 and notably better than an admitted TIA cohort.43 The subjectivity in the diagnosis of TIA highlights another important role for rapid outpatient TIAMS evaluation: reassessment by another clinician (e.g. in this case a board certified vascular neurologist). There is well-described variability in TIA diagnostic accuracy across a range of practitioners44 and being able to assess a patient multiple times in the acute setting by different providers may help with diagnostic accuracy and prevent unnecessary testing and interventions in patients found to have mimics. The rapid access to vascular neurologists in RAVEN clinic limited the work-up in the 34% of patients who were found to have an alternative non-TIAMS diagnosis. Additional work building on the data from this study may assess the diagnostic accuracy of TIAMS across a range of clinical settings and providers.
Finally, with the emergence of novel platforms for patient care, such as telemedicine, future studies may assess whether such programs may permit remote evaluations and referrals to rapid outpatient stroke clinics by neurology consultants in practice settings where onsite neurology consultation may not be readily available. An integration of these innovative platforms with a rapid outpatient stroke strategy may result in a synergistic comprehensive treatment program for TIAMS, avoiding potential inpatient admissions and allowing greater access to specialized neurologic care in a diverse set of clinical settings.
TIAMS are common conditions evaluated in the ED and are associated with significant morbidity and mortality. Our study has demonstrated that a rapid outpatient follow-up strategy for the management of TIAMS may be a safe and feasible strategy in the acute care setting. Such an outpatient TIAMS management strategy has the potential to improve ED throughput, reduce inpatient hospitalizations, and potentially lead to reductions in secondary hospital-associated adverse outcomes and healthcare-associated costs of such acute illnesses.
Acknowledgments
Grant: This work was supported by a grant by National Institutes of Health (R01 HL141811) and the Empire Clinical Research Investigator Program (New York)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meetings: Presented at National Meeting of American College of Emergency Physicians, San Diego, CA, October 2018 and International Stroke Conference, Honolulu, HI, February 2019.
Conflict of Interest: BC, SR, JW, EM, SS, RM, BK, BN, ME do not have any conflict of interest to declare
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: Heart Disease and Stroke Statistics-2016 update: A report from the American Heart Association. Circulation 2016;133:447. [DOI] [PubMed] [Google Scholar]
- 2.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke; a journal of cerebral circulation 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology 2005;64:817–820. [DOI] [PubMed] [Google Scholar]
- 4.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Archives of internal medicine 2007;167:2417–2422. [DOI] [PubMed] [Google Scholar]
- 5.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology 2004;62:2015–2020. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 7.Amarenco P, Lavallee PC, Labreuche J, et al. One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med 2016;374:1533–1542. [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: meta-analysis and effect per 1,000 patients triaged. Neurology 2015;85:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry JJ, Sharma M, Sivilotti ML, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack 2011;183:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaghi S, Rostanski SK, Boehme AK, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack 2016;73:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo BM, Carpenter CR, Hatten BW, Wright BJ, Brown MD. Clinical Policy: Critical Issues in the Evaluation of Adult Patients With Suspected Transient Ischemic Attack in the Emergency Department. Ann Emerg Med 2016;68:354–370.e329. [DOI] [PubMed] [Google Scholar]
- 12.Johnston SC, Albers GW, Gorelick PB, et al. National Stroke Association recommendations for systems of care for transient ischemic attack. Annals of neurology 2011;69:872–877. [DOI] [PubMed] [Google Scholar]
- 13.Chang BP, Rostanski S, Willey J, Kummer B, Miller E, Elkind M. Can I Send This Patient with Stroke Home? Strategies Managing Transient Ischemic Attack and Minor Stroke in the Emergency Department. J Emerg Med 2018. [DOI] [PMC free article] [PubMed]
- 14.Chaudhry SA, Tariq N, Majidi S, et al. Rates and factors associated with admission in patients presenting to the ED with TIA in the United States-2006 to 2008. The American journal of emergency medicine 2013;31:516–519. [DOI] [PubMed] [Google Scholar]
- 15.Lavallee PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 17.Sanders LM, Cadilhac DA, Srikanth VK, Chong CP, Phan TG. Is nonadmission-based care for TIA patients cost-effective?: A microcosting study. Neurol Clin Pract 2015;5:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stead LG, Bellolio MF, Suravaram S, et al. Evaluation of transient ischemic attack in an emergency department observation unit. Neurocritical care 2009;10:204. [DOI] [PubMed] [Google Scholar]
- 19.Nahab F, Leach G, Kingston C, et al. Impact of an emergency department observation unit transient ischemic attack protocol on length of stay and cost. Journal of Stroke and Cerebrovascular Diseases 2012;21:673–678. [DOI] [PubMed] [Google Scholar]
- 20.Roberts RR, Zalenski RJ, Mensah EK, et al. Costs of an Emergency Department—Based Accelerated Diagnostic Protocol vs Hospitalization in Patients With Chest Pain: A Randomized Controlled Trial. Jama 1997;278:1670–1676. [PubMed] [Google Scholar]
- 21.Gomez MA, Anderson JL, Karagounis LA, Muhlestein JB, Mooders FB, Group RS. An emergency department-based protocol for rapidly ruling out myocardial ischemia reduces hospital time and expense: results of a randomized study (ROMIO). Journal of the American College of Cardiology 1996;28:25–33. [DOI] [PubMed] [Google Scholar]
- 22.Rydman RJ, Zalenski RJ, Roberts RR, et al. Patient satisfaction with an emergency department chest pain observation unit. Annals of emergency medicine 1997;29:109–115. [DOI] [PubMed] [Google Scholar]
- 23.Ross MA, Compton S, Medado P, Fitzgerald M, Kilanowski P, O’Neil BJ. An emergency department diagnostic protocol for patients with transient ischemic attack: a randomized controlled trial. Annals of emergency medicine 2007;50:109–119. [DOI] [PubMed] [Google Scholar]
- 24.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. The Lancet Neurology 2007;6:1063–1072. [DOI] [PubMed] [Google Scholar]
- 25.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 26.Wein T, Lindsay MP, Côté R, et al. Canadian stroke best practice recommendations: Secondary prevention of stroke, practice guidelines, update 2017 2018;13:420–443. [DOI] [PubMed] [Google Scholar]
- 27.Fish J Rankin Scale. Encyclopedia of Clinical Neuropsychology: Springer; 2011:2110–2112. [Google Scholar]
- 28.Lavallée P, Amarenco P. TIA clinic: a major advance in management of transient ischemic attacks. TIA as Acute Cerebrovascular Syndrome Vol 33: Karger Publishers; 2013:30–40. [DOI] [PubMed] [Google Scholar]
- 29.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. The Lancet 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 30.Johnston SC, Easton JD, Farrant M, et al. Platelet‐oriented inhibition in new TIA and minor ischemic stroke (POINT) trial: rationale and design 2013;8:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen-Huynh MN, Johnston SC. Is hospitalization after TIA cost-effective on the basis of treatment with tPA? Neurology 2005;65:1799–1801. [DOI] [PubMed] [Google Scholar]
- 32.Durrani-Tariq S, Eskin B, Allegra JR. Admission rates of ED patients with transient ischemic attack have increased since 2000. The American journal of emergency medicine 2013;31:1349–1351. [DOI] [PubMed] [Google Scholar]
- 33.Joshi JK, Ouyang B, Prabhakaran S. Should TIA patients be hospitalized or referred to a same-day clinic?: a decision analysis. Neurology 2011;77:2082–2088. [DOI] [PubMed] [Google Scholar]
- 34.Ross MA, Compton S, Medado P, Fitzgerald M, Kilanowski P, O’Neil BJ. An emergency department diagnostic protocol for patients with transient ischemic attack: a randomized controlled trial. Ann Emerg Med 2007;50:109–119. [DOI] [PubMed] [Google Scholar]
- 35.Nahab F, Leach G, Kingston C, et al. Impact of an emergency department observation unit transient ischemic attack protocol on length of stay and cost. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association 2012;21:673–678. [DOI] [PubMed] [Google Scholar]
- 36.Edmondson D, Green P, Ye S, Halazun HJ, Davidson KW. Psychological stress and 30-day all-cause hospital readmission in acute coronary syndrome patients: an observational cohort study. PloS one 2014;9:e91477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White M, Edmonson D, Chang BPJTAjoem. Patient perceptions of stress during evaluation for acute coronary syndrome in the emergency department 2017;35:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med 2018;379:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro SD, Luna J, Mehendale R, et al. Abstract TP277: A Hospital’s Perspective: Economic Evaluation of Hospitalization vs Rapid Outpatient Evaluation for TIA and Minor Strokes. Stroke 2019;50:ATP277–ATP277. [Google Scholar]
- 41.Schrock JW, Glasenapp M, Victor A, Losey T, Cydulka RK. Variables associated with discordance between emergency physician and neurologist diagnoses of transient ischemic attacks in the emergency department. Ann Emerg Med 2012;59:19–26. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs RG, Newson R, Lawrenson R, Greenhalgh RM, Davies AH. Diagnosis and initial management of stroke and transient ischemic attack across UK health regions from 1992 to 1996: experience of a national primary care database. Stroke 2001;32:1085–1090. [DOI] [PubMed] [Google Scholar]
- 43.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis 2008;26:630–635. [DOI] [PubMed] [Google Scholar]
- 44.Ferro JM, Falcao I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke 1996;27:2225–2229. [DOI] [PubMed] [Google Scholar]