Abstract
BACKGROUND:
The incidence of acute cardiovascular complications is highly time-of-day dependent. However, the mechanisms driving rhythmicity of ischemic vascular events are unknown. While enhanced numbers of leukocytes have been linked to an increased risk of cardiovascular complications, the role that rhythmic leukocyte adhesion plays in different vascular beds has not been studied.
METHODS:
We evaluated leukocyte recruitment in vivo by utilizing real-time multichannel fluorescence intravital microscopy (MFIM) of a TNF-α-induced acute inflammation model in both murine arterial and venous macro- as well as microvasculature. These approaches were complemented with genetic, surgical and pharmacological ablation of sympathetic nerves or adrenergic receptors in order to assess their relevance for rhythmic leukocyte adhesion. In addition, we genetically targeted the key circadian clock gene Bmal1 (also known as Arntl) in a lineage-specific manner to dissect the importance of oscillations in leukocytes and components of the vessel wall in this process.
RESULTS:
In vivo quantitative imaging analyses of acute inflammation revealed a 24h rhythm in leukocyte recruitment to arteries and veins of the mouse macro- and microvasculature. Unexpectedly, while in arteries leukocyte adhesion was highest in the morning, it peaked at night in veins. This phase shift was governed by a rhythmic microenvironment and a vessel type-specific oscillatory pattern in the expression of pro-migratory molecules. Differences in cell adhesion molecules and leukocyte adhesion were ablated when disrupting sympathetic nerves, demonstrating their critical role in this process and the importance of β2-adrenergic receptor signaling. Interestingly, loss of the core clock gene Bmal1 in leukocytes, endothelial cells or arterial mural cells affected the oscillations in a vessel-type-specific manner. Rhythmicity in the intravascular reactivity of adherent leukocytes resulted in increased interactions with platelets in the morning in arteries and in veins at night with a higher predisposition to acute thrombosis at different times as a consequence.
CONCLUSIONS:
Together, our data point to an important and previously unrecognized role of artery-associated sympathetic innervation in governing rhythmicity in vascular inflammation in both arteries and veins and its potential implications in the occurrence of time-of-day-dependent vessel type-specific thrombotic events.
Keywords: leukocyte adhesion, circadian rhythms, sympathetic nervous system, thrombosis
INTRODUCTION
Acute cardiovascular complications in humans occur predominantly at the onset of the behavioral activity phase1. Studies have established a correlation between heightened systemic blood leukocyte counts and an increased risk for cardiovascular complications2. Specifically, the process of leukocyte recruitment to tissues and the associated intravascular interactions between adherent leukocytes and other free-flowing components of the blood exacerbate vaso-occlusive crises3, 4 and lipopolysaccharide (LPS)-induced lethality5. Recent evidence points to a critical role for circadian leukocyte adhesion in the time-of-day-dependent onset of acute vascular inflammation6–10. While steady-state leukocyte recruitment to tissues occurs almost exclusively from venules, inflammation also induces leukocyte adhesion in arteries11. Inflammatory cells, predominantly neutrophils and inflammatory monocytes, roll along and adhere to the vascular endothelium, via interactions mediated by E- and P-selectin, chemokines and the leukocyte integrins leukocyte function associated antigen (LFA)-1 (CD11a/CD18) and macrophage (Mac)-1 antigen (CD11b/CD18) with endothelial intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-112–14.
Circadian rhythms exhibit a period length of approximately 24 h and play a critical role in the adaptation of organisms to the rhythmic light-dark changes of the environment15–17. Light can entrain these rhythms, leading to the synchronization of rhythms in the suprachiasmatic nuclei (SCN) of the hypothalamus, which constitute the master clock of the organism. From there, humoral and neural output mechanisms synchronize clocks in peripheral tissues, thus setting a common phase across the body. The sympathetic nervous system regulates the rhythmic and systemic release of glucocorticoids, adrenaline and noradrenaline from the adrenal glands18, 19. In addition, sympathetic nerves directly innervate tissues, and can modulate physiology locally via the release of noradrenaline from their varicosities20. Local versus systemic application of an inflammatory stimulus can cause different outcomes in the ability of neutrophils to reach inflammatory sites7, 8, 21, indicating the importance of confined environmental signals in inflammatory reactions. Whether sympathetic tone can modulate the inflammatory response in a tissue-specific manner has not been defined. Furthermore, the role of sympathetic nerves in inflammation of distinct vascular beds remains unknown. Here, we show that artery-associated sympathetic innervation is critical for oscillatory inflammatory leukocyte adhesion to both arteries and veins with a functional relevance for the temporal incidence of acute thrombotic events.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
8-10-week old male mice were used for all the experiments. Brain and muscle ARNTL-like (Bmal)1flox/flox, Lyz2cre and Icam1−/− mice were purchased from Jackson Laboratories. Neuron-glial antigen (Ng)2-cre and Ng2-DsRed (Cspg4-DsRed) mice were provided by Konstantin Stark. Lysozyme (Lyz)2-green fluorescent protein (gfp) mice were provided by Markus Sperandio. Per1−/−Per2−/− mice were provided by Urs Albrecht22. Cdh5ERT2-cre mice were a gift from Ralf H. Adams. β2 adrenergic receptor (Adrb2)−/− mice were a gift from Gerard Karsenty to Paul Frenette. Nestin (Nes)-gfp mice were a gift from Grigori Enikolopov to Paul Frenette. C57BL/6 wild-type mice were obtained from Charles River. Animals were housed under a 12 h:12 h light-dark cycle with ad libitum access to food and water. All animal experimental procedures were carried out in accordance with the German Law of Animal Welfare and approved by the Regierung of Oberbayern or the Animal Care and Use Committee of the Albert Einstein College of Medicine or the Swiss Federal Veterinary Office.
Multichannel fluorescence intravital microscopy (MFIM)
For MFIM of the carotid artery and jugular vein, mice were anesthetized by i.p. injection of ketamine (100 mg/kg), xylazine (20 mg/kg) and acepromazine (1%). A ventral incision was made in the neck and the carotid artery and jugular vein were exposed. Suture thread was placed around the sternocleidomastoid muscle and the muscle was pulled to the side. Two surgical knots were placed around the tendon and a cut was made in the middle, in order to separate the tendon and make the carotid visible. An additional suture thread was then tied around the bone and used to pull it to the side (see also23). Afterwards, the exposed area was covered with Tumor Necrosis Factor (TNF)-α (150 ng in 100 μl PBS, containing 0.1% BSA) for 2 h prior to imaging. In the meantime, a catheter was inserted in the femoral artery for administrating a PE-anti-CD11a antibody (clone M17/4, 1 µg, Biolegend) in a very low dose in order to visualize adherent leukocytes. Videos were acquired on a fixed-stage Zeiss Axio Examiner.D1 microscope equipped with a Colibri epifluorescence 405, 488, 563 and 655 nm LED excitation light source (Zeiss).
For MFIM of the cremaster muscle, TNF-α was injected i.p. or into the scrotum 3 h before imaging. Mice were anesthetized with Fentanyl (0,5 mg/kg), Midazolam (5 mg/kg) and Medetomidine (0,5 mg/kg) via i.p. injection. Firstly, a catheter was inserted in the femoral artery for administration of antibodies in different combinations: PE-anti-Ly6G (clone 1A8, 1 μg, BD Biosciences), DyLight649-GPIbβ (3 μg, Emfret Analytics), PE-anti-CD11b (clone M1/70, 1 μg, Biolegend), APC-anti-CD62L (clone MEL-14, 1 μg, Biolegend). Preparation of the cremaster muscle was subsequently performed as explained in a previous publication7. Specifically, the right testis was exposed through an incision of the scrotum. The cremaster muscle surrounding the testis was opened ventrally in a zone with less vasculature, using electrocautery to cut the muscle and at the same time stop any bleeding, and spread over a plexiglas cube of a custom-made microscopy stage. Epididymis and testicle were detached from the cremaster muscle by electrocautery and placed back into the peritoneum. During the procedure as well as after surgical preparation, the muscle was superfused with warm saline (0.9% NaCl). Videos were acquired on a fixed-stage Zeiss Axio Examiner.D1 microscope as described above.
Adoptive transfer experiments
Single cell suspensions were obtained from spleen and bone marrow from donor mice. Red blood cells were lysed and the remaining cells were resuspended in cell incubation buffer (PBS, 0.2% BSA, 2 mM EDTA). Bone marrow and spleen cells were mixed with a ratio of 50:50 and labeled with 1.5 μM carboxyfluorescein succinimidyl ester (CFSE) (Thermo Fisher Scientific) for 20 min at 37°C. Afterwards, cells were washed 3 times and resuspended in PBS. 20x106 labeled cells were injected intravenously in each recipient mouse. Simultaneously, TNF-α was applied topically on the exposed carotid artery and the jugular vein. After two hours, artery and vein were harvested from recipient mice and digested in order to obtain single cell suspensions for flow cytometry using collagenase IV (Sigma; 1 mg/ml), DNase I (Roche; 0.2 mg/ml) and collagenase D (Roche-Sigma; 3.5 mg/ml) for 30 min at 37°C and smashed through 70 μm cell strainers (ThermoFisher Scientific). Single cell suspensions were stained with antibodies, purchased from Biolegend (dilution 1:200) against PE-anti-CD11a (clone M17/4), PE/DZL594-anti-CD45 (clone 30-F11), PerCP/Cy5.5-anti-Gr-1 (clone RB6-8C5), PE/Cy7-gp38 (clone 8.1.1.), APC-anti-PECAM-1 (clone MEC13.3) and Brilliant Violet 510™-anti-F4/80 (clone BM8). DAPI (Biolegend) was used in order to identify dead cells.
Functional blocking of adhesion molecules and chemokine receptors
Animals were injected i.v. with 200 μg of the following blocking antibodies (BioxCell): anti-CD54 (ICAM-1; clone YN1/1.7.4), anti-CD106 (VCAM-1; clone M/K-2.7) or their isotypes (for ICAM-1 clone LTF-2 and for VCAM-1 clone HRPN) or antagonists against the chemokine receptors CXCR2 and CCR2 (5 mg/kg, Tocris) 1 h before stimulation with TNF-α and 3 h before performing in vivo imaging experiments. In the case of the chemokine antagonist, 1% Tween80 and 5% DMSO in PBS were used as vehicle controls.
Denervation techniques
Mice were denervated in two different ways: chemically, using 6-hydroxydopamine (6-OHDA), and surgically, via removal of the superior cervical ganglion. For the chemical denervation, 6-OHDA was dissolved in ascorbic acid solution at 20 mg/ml (in PBS) and injected twice before the experiment. A first dose of 100 mg/kg was followed by a second one of 250 mg/kg two days later and in vivo imaging was performed three days later. For the surgical denervation, a ventral incision was made in the neck and the superior cervical ganglion, located underneath the carotid bifurcation, was removed unilaterally by careful dissection using forceps. On the contra-lateral side, a sham procedure was carried out without cutting the ganglion. The incision on the neck was closed and the mice were left to recover for 4 weeks in order to re-establish normal blood leukocyte levels indicating the absence of inflammation.
β2-adrenergic receptor antagonist/agonist treatment
For the purpose of investigating β2 adrenergic receptor function, the antagonist butoxamine or the agonist clenbuterol (Sigma-Aldrich) were administered via i.p. injection (5 mg/kg). For acute treatments, injections were performed 2 h prior to imaging. For chronic treatments, injections were performed for 5 consecutive days and imaging was performed 24 h after the last injection.
Thrombosis assay
Mice were anesthetized with Fentanyl (0,5 mg/kg), Midazolam (5 mg/kg) and Medetomidine (0,5 mg/kg) via i.p. injection and a catheter was inserted into the femoral artery for the administration of fluorescein isothiocyanate (FITC)-coupled dextran (2.5% 500 kDa). Once the catheter was placed in the artery, the surgical preparation of the cremaster muscle was performed as explained before. An initial amount of 100 μl FITC-coupled dextran was given through the catheter to the mice and fluorescence was measured until it reached 1800 arbitrary units (AU) inside the vessel. Fluorescence levels were kept constant by obtaining digital images from each vessel and checking the mean fluorescence intensity. FITC phototoxicity was induced using a 488 nm epifluorescence excitation source. This leads to local energy absorption by the dye within the vessel and to endothelial cell damage with the formation of a thrombus as a consequence. Occlusion time was quantified for multiple vessels in each mouse (3 venules and 3 arterioles of the same size, 60 μm and 40 μm diameter, respectively).
Statistics
Statistical analyses were performed using GraphPad Prism 6 software. All data are represented as mean ± SEM. To compare two groups an unpaired Student’s t-test was performed, in case of unequal variances combined with Welch’s correction. For non-parametric analyses a Mann-Whitney test was performed. For three or more groups, one-way ANOVA followed by Tukey’s post hoc test or two-way ANOVA followed by Bonferroni’s post hoc test were used. Statistical significance was assessed as *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
RESULTS
Inflammatory leukocyte adhesion to arteries and veins occurs at different times of the day
We set out for a detailed investigation into time-of-day dependent leukocyte recruitment patterns to arteries and veins in an acute inflammatory scenario. Mice were treated locally with TNF-α and interactions between leukocytes and the vessel wall were visualized in real time in the carotid artery and the jugular vein of the same animals by MFIM and low-dose injections of fluorescent antibodies. Interestingly, leukocyte adhesion in the artery exhibited a striking diurnal rhythmicity (Figure 1A). Moreover, we observed a strong oscillation in leukocyte adhesion in veins but this rhythm exhibited an unexpected phase shift compared to arteries (Figure 1B–C). In the artery, adhesion peaked in the early morning (Zeitgeber time (ZT) 1, i. e. one hour after light onset in a 12 h:12 h light:dark environment) and troughed around midnight (ZT17). In contrast, leukocyte adhesion in the vein reached a trough in the morning and peaked at midnight. Local hemodynamic parameters showed no apparent change between day and night conditions in either vascular bed (data not shown). We confirmed these observations in Lyz2-gfp mice, in which myeloid cells are fluorescently labeled genetically (Figure 1D). We next investigated whether this phenomenon was specific for the macrovasculature and the investigated heterogeneous set of large-caliber vessels situated anatomically apart, or whether the same held true for an organ-embedded microvascular bed, in which arteries and veins share close proximity. Using MFIM of the cremaster muscle microcirculation, we detected a similar time-dependent leukocyte adhesion pattern in smaller arterioles and venules (Figure 1E). In addition, changes in rolling velocities were observed in both vessels (Figure S1A), peaking at night in arterioles and in venules in the morning, a sign of lower levels of tissue activation24 (Figure S1A). This indicated that these rhythms occur in both small- and large-caliber vessels. These data provide for the first time evidence that inflammatory leukocyte recruitment to arteries and veins is diurnally regulated with distinct rhythmicity depending on the type of vascular bed.
Figure 1. Inflammatory leukocyte adhesion to arteries and veins occurs at different times of the day.
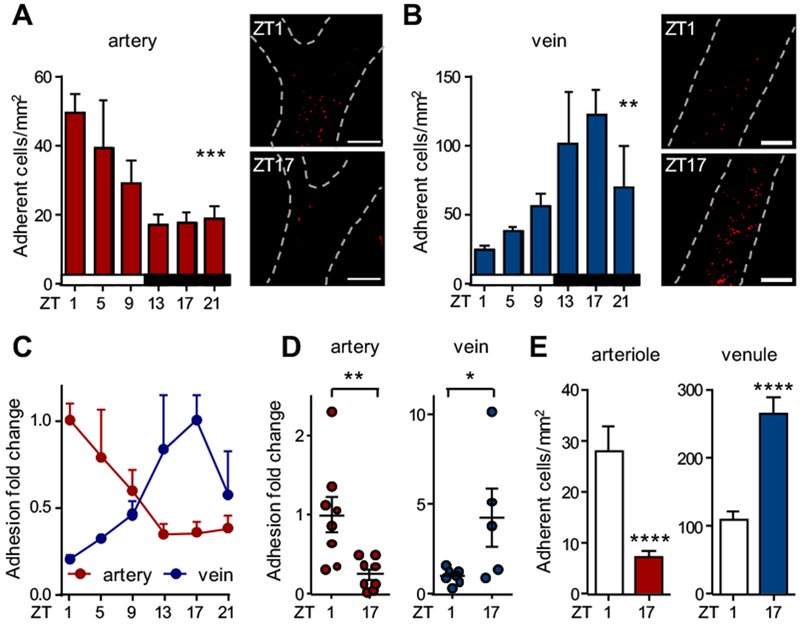
(A-B) In vivo quantification of adherent leukocytes after TNF-α stimulation over 24 h in carotid artery (A) and jugular vein (B); n = 4-13 mice, one-way ANOVA. (C) Normalization of the leukocyte adhesion data from (A-B). Data are normalized to peak levels; n = 4-13 mice. (D) In vivo quantification of adherent cells in Lyz2-gfp mice after TNF-α stimulation in carotid artery and jugular vein. Data are normalized to ZT1 levels; n = 5-8 mice, Student’s t-test (artery) and Mann-Whitney test (vein). (E) In vivo quantification of adherent cells in arterioles and venules of the cremasteric microvasculature after TNF-α stimulation; n = 5 mice, Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Scale bars: 200 μm.
Arterial and venous blood exhibited similar daily changes in leukocyte counts, excluding the possibility that adhesion in arteries might downmodulate venous adhesion levels downstream of the vascular tree, simply by reducing the numbers of available circulating cells (Figure S1B). We next investigated whether the distinct rhythms in both vascular beds were governed by the leukocyte and/or the microenvironment. We first focused on whether the circadian clock in leukocytes could drive rhythmicity, targeting the key circadian gene Bmal1. Interestingly, Lyz2cre:Bmal1flox/flox mice, which exhibit a Bmal1-deficiency specifically in myeloid cells, no longer displayed oscillations in recruitment to arteries or veins (Figure 2A–B). This implicated the circadian clock in myeloid cells to play a critical role in rhythmic leukocyte adhesion, likely via a common mechanism in both vascular beds. However, that loss of Bmal1 in myeloid cells affected rhythmicity in both arteries and veins did not explain the observed phase shift between the vessel types and suggested that the vessels mediated the altered adhesion rhythms between arteries and veins.
Figure 2. Role of rhythmicity in myeloid cells and the microenvironment.
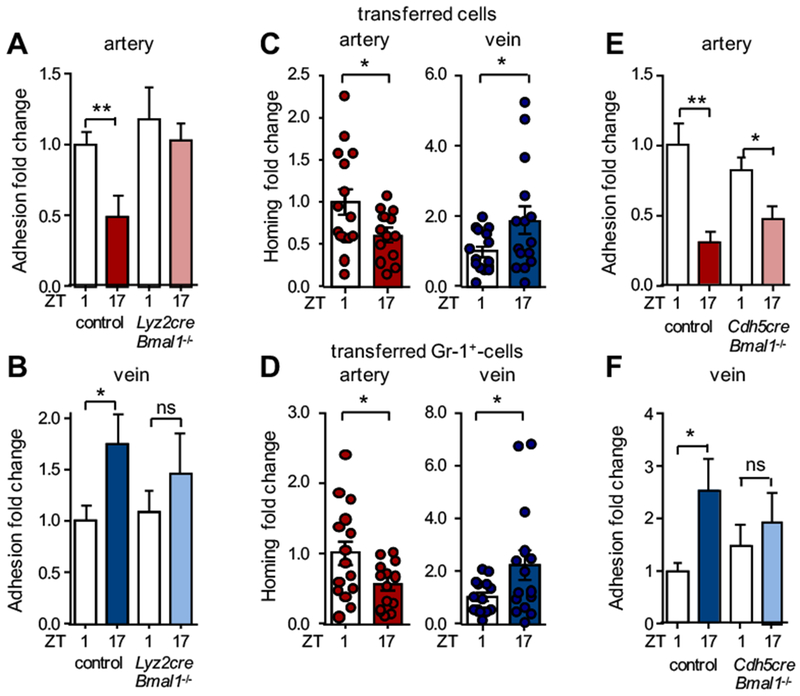
(A-B) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery (A) and jugular vein (B) in control and Lyz2cre:Bmal1−/− mice. Data are normalized to control ZT1 levels; n = 7-10 mice, Student’s t-test. (C) Homing experiments of adoptively transferred leukocytes (C) and Gr1+ cells (D) to carotid artery and jugular vein after TNF-α stimulation. Data are normalized to ZT1 levels; n = 13-15 mice, Student’s t-test with Welch’s correction. (E-F) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery (E) and jugular vein (F) in control and Cdh5creERT2:Bmal1−/− mice. Data are normalized to control ZT1 levels; n = 8-11 mice, Student’s t-test with Welch’s correction. *p < 0.05, **p < 0.01.
We thus focused in subsequent assays on the influence of the microenvironment. We performed adoptive transfer assays using fluorescently-labeled leukocytes harvested from donors during the day, injected them simultaneously into 12 h light: 12 h dark-entrained recipients during their respective day or night phase and performed flow cytometry of the harvested carotid arteries and jugular veins. In this setting, a functional importance of rhythmicity only in the microenvironment was assessed. Transferred leukocytes were recruited with different rhythmicity to arteries and veins (Figure 2C) and neutrophils were the main cell type (Figure 2D and Figure S1C). These results were in line with our in vivo adhesion data and suggested that different oscillations in the microenvironment were responsible for the observed phase shift in leukocyte adhesion. We thus next assessed the role of the circadian clock in endothelial cells – situated at the blood-tissue interface and acting as critical regulators of leukocyte adhesion12–14 – in mediating rhythmicity and a phase shift between the two vascular beds. Interestingly, Cdh5creERT2:Bmal1flox/flox mice, which lack Bmal1 expression selectively in endothelial cells, exhibited no more oscillations in venous leukocyte adhesion but still showed rhythmicity in arterial adhesion (Figure 2E–F). The latter was confirmed in mice deficient in Bmal1 only in arterial endothelial cells (BmxcreERT2:Bmal1flox/flox) (Figure S1D). This implicated a role for the circadian clock in venous – but not arterial – endothelial cells in the process, and provided a potential explanation for the phase shift in adhesion between both tissues. Furthermore, the results demonstrated that both the leukocyte and the microenvironment are involved in the diurnal adhesion rhythms.
Altered rhythmicity in pro-migratory molecules in arteries and veins
We next focused on the potential molecules mediating leukocyte adhesion. Q-PCR analyses of carotid arteries and jugular veins for promigratory factors showed oscillatory mRNA expression profiles for Icam1 in both arteries and veins, whereas Vcam1, E-selectin (Sele) and P-selectin (Selp) were oscillatory only in venous tissue (Figure 3A). In addition, we observed temporal differences in both tissues for Cxcl1, Cxcl2 and Ccl2, critical chemokines for myeloid cell trafficking25 (Figure 3B). In line with the in vivo recruitment data, expression levels for these molecules peaked in the morning in arteries but at night in the veins, indicating their potential involvement in this process (Figure 3A–B). We quantified protein expression levels of cell adhesion molecules specifically in endothelial cells using frozen tissue sections of carotid arteries and jugular veins. ICAM-1 and VCAM-1 levels peaked at day onset for the artery and at night for the vein (Figure 3C–D). We also observed oscillations in E-selectin in veins but not arteries, whereas P-selectin was not rhythmically expressed (Figure 3C–D and data not shown). We therefore focused on the adhesion-mediating molecules ICAM-1 and VCAM-1 in subsequent functional assays. Mice treated with anti-ICAM-1- or anti-VCAM-1-blocking antibodies as well as Icam1-deficient mice exhibited no more temporal differences in leukocyte adhesion to both arteries and veins (Figure 3E–F). Furthermore, blocking their integrin ligands on leukocytes (LFA-1 and Mac-1) ablated oscillations in a similar manner (Figure 3E). Inhibiting CCR2 function also reduced levels of adhesion and ablated time-of-day differences in both vessels, while blocking CXCR2 only affected rhythmicity in veins, confirming the functional importance of these chemokine receptors in mediating adhesion rhythmicity (Figure 3G). While arterial adhesion relied heavily on ICAM-1, VCAM-1, Mac-1 and CCR2 for rhythmic adhesion, in veins LFA-1 and CXCR2 played additional roles. These results indicated that distinct microenvironmental rhythms in the expression of pro-migratory factors in arteries and veins were the critical determinants for the inverted leukocyte adhesion patterns.
Figure 3. Distinct rhythmicity of pro-migratory molecules in arteries and veins.
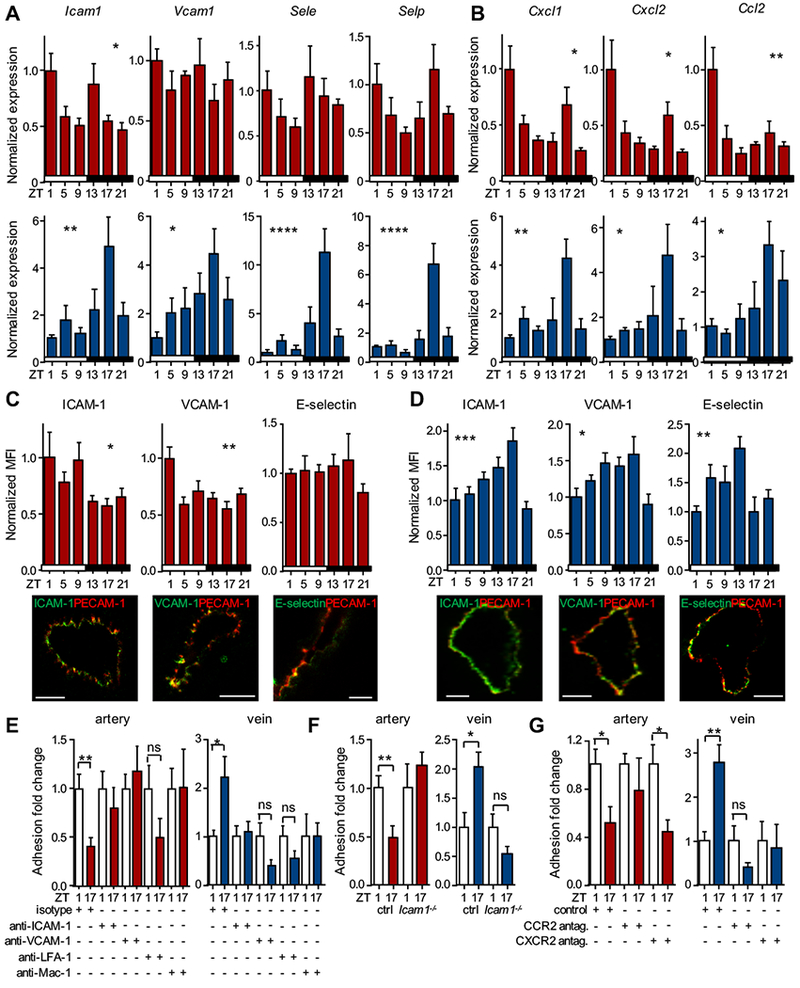
(A-B) Q-PCR analyses of cell adhesion molecules (A) and chemokines (B) in carotid artery (red) and jugular vein (blue) after TNF-α stimulation over the course of the day; n = 3-6 mice, one-way ANOVA. (C-D) Mean fluorescence intensity (MFI) quantifications and representative images of adhesion molecule expression in endothelial cells after TNF-α stimulation in carotid artery (C) and jugular vein (D). Data are normalized to ZT1 levels; n = 3-6 mice, one-way ANOVA. (E) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after antibody blockade. Data are normalized to ZT1 levels; n = 4-6 mice, Student’s t-test. (F) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein in Icam1−/− mice; n = 8-9 mice Student’s t-test. (G) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after antagonist treatment. Data are normalized to ZT1 levels; n = 4-6 mice, Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Scale bars: 50 μm.
Similar circadian clock phases in arteries and veins
We therefore next examined whether overall circadian rhythmicity was altered between arteries and veins using Per2:luc mice, in which luciferase expression is driven by the rhythmically expressed clock gene Per2 26. Harvested arteries and veins exhibited marked circadian oscillations in luciferase expression over multiple days in culture, with arteries showing a much more pronounced rhythm than veins and exhibiting peaks at slightly different phases (Figure 4A). Previous data have indicated different circadian phases in arteries and veins27, but these slight changes in peak times likely do not explain the dramatic phase shifts observed in leukocyte adhesion. In addition, Q-PCR analyses of the core circadian clock genes Bmal1, Clock, Per1, Per2, Cry1, Nr1d1, Nr1d2, Rora and Rorc as well as the output gene D-site of albumin promoter (Dbp) under both steady-state and inflamed conditions revealed peaks and troughs in arteries and veins occurring at very similar times in the light cycle (Figure 4B–D). These data indicated that clock genes are expressed in phase between arteries and veins, making it unlikely that a phase-shift in the overall oscillations of these tissues was responsible for the distinct leukocyte adhesion patterns.
Figure 4. Clock gene expression patterns in arteries and veins.
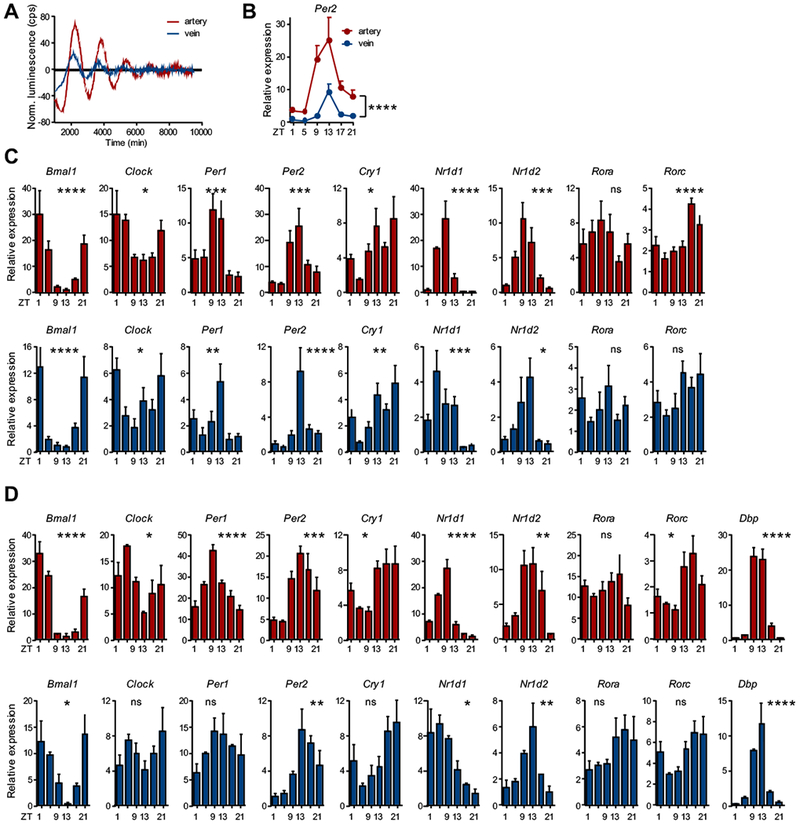
(A) Ex vivo circadian oscillations of Per2 expression levels as quantified in aorta and vena cava harvested from the bioluminescence mPer2:Luc reporter mouse over 6 days under non-inflammatory conditions; n = 3 mice. (B) Comparison of Per2 expression levels between carotid artery (red) and jugular vein (blue) over the course of the day in steady state; n = 6 mice, two-way ANOVA. (C-D) Q-PCR analyses of circadian clock gene expression levels over 24 h in carotid artery (red) and jugular vein (blue) under (C) steady-state and (D) TNF-α-induced inflammatory conditions; n = 6 mice, one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Local sympathetic innervation drives rhythmic inflammatory responses
Since oscillations of core clock genes in arteries and veins occurred at similar times, we speculated that our observations resulted from a difference in the inflammatory response to TNF-α stimulation. However, Tnfrsf1a and Tnfrsf1b expression (as well as Nr3c1, encoding the glucocorticoid receptor) was non-rhythmic in both arteries and veins (Figure S2A–D), making it unlikely that a simple difference in TNF-α-sensing was responsible. We therefore investigated a potential involvement of upstream factors in this process, focusing on sympathetic input as a potential mechanism28. We performed a systemic chemical sympathectomy of mice by administration of 6-hydroxydopamine (6-OHDA), a sympathetic neurotoxin. This treatment ablated the temporal differences in leukocyte recruitment to both arteries and veins (Figure 5A), indicating that the sympathetic nervous system played a critical role in mediating oscillatory leukocyte adhesion to both vascular beds. To distinguish between the effects of direct neural input via local sympathetic nerves in contrast to humoral, circulating factors, we performed microsurgeries to locally ablate the superior cervical ganglion (SCGx), which is responsible for the direct sympathetic innervation of the head and neck area29. Performing these experiments unilaterally while carrying out a sham surgery on the contralateral side, allowed us to investigate nerve-intact and denervated vessels in the same mice. We confirmed that the surgery ablated sympathetic input to the ipsilateral side, while leaving the contralateral side intact (Figure S3A). In addition, no effects on vessel diameters were observed (Figure S3B). SCGx ablated temporal differences in leukocyte recruitment in both arteries and veins, whereas in the contralateral sides rhythms remained intact (Figure 5B). The surgery additionally ablated diurnal oscillations in the expression of pro-migratory factors in arteries and dampened them in veins (Figure 5C–D). This was not due to ablation of circadian rhythms per se in these tissues as denervated vessels retained rhythmic clock gene expression (Figure S3C–D). Thus, sympathetic innervation is not responsible for the rhythmic entrainment of these vascular tissues, consistent with a previous study30. Instead, our data implies a critical role for local sympathetic nerves in governing leukocyte adhesion through the rhythmic expression of adhesion molecules to both arteries and veins after an inflammatory insult.
Figure 5. Local sympathetic nerves drive rhythmic inflammatory responses.
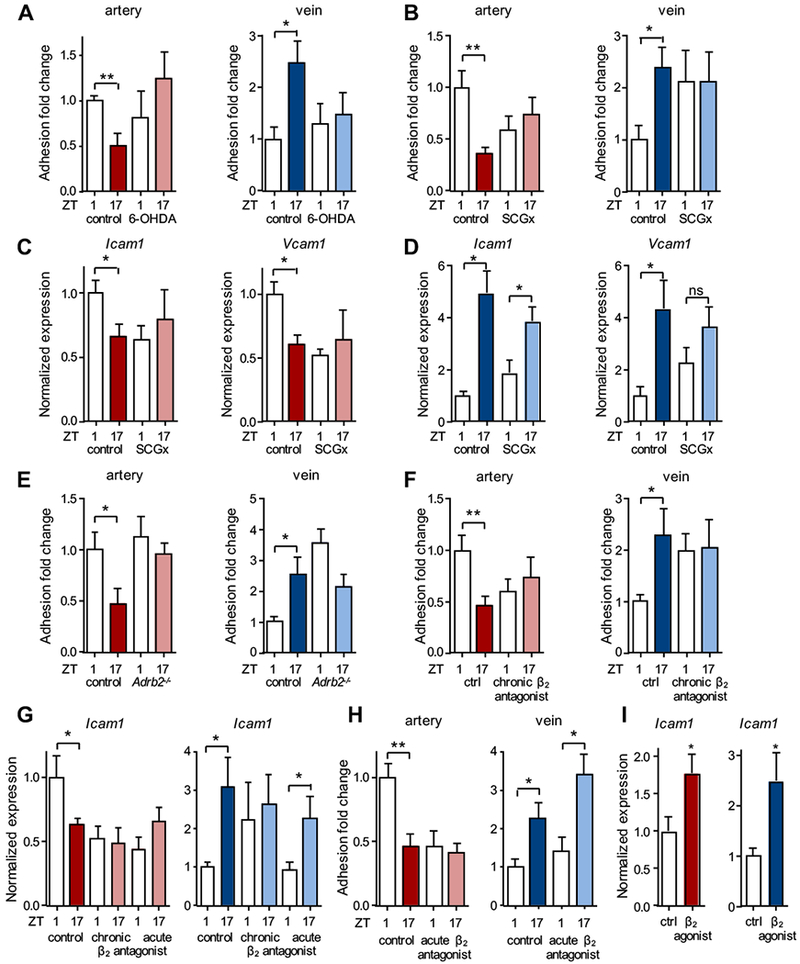
(A) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after 6-OHDA treatment. Data are normalized to ZT1 control levels; n = 5-8 mice, Student’s t-test. (B) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after unilateral surgical denervation of the superior cervical ganglion (SCGx). Data are normalized to ZT1 control levels; n = 5-12 mice, Student’s t-test. (C-D) Q-PCR analyses of cell adhesion molecules after TNF-α stimulation in carotid artery (C) and jugular vein (D) of SCGx mice. Data are normalized to ZT1 control levels; n = 4-7 mice, Student’s t-test with Welch’s correction. (E) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein of Adrb2−/− mice. Data are normalized to ZT1 control levels; n = 6-9 mice, Student’s t-test. (F) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after 5 d (chronic) treatment with a β2 adrenergic receptor antagonist. Data are normalized to ZT1 control levels; n = 4-7 mice, Student’s t-test with Welch’s correction. (G) Q-PCR analyses of cell adhesion molecules after TNF-α stimulation in carotid artery and jugular vein after chronic (5 d) or acute (2 h) treatment with a β2-adrenergic receptor antagonist. Data are normalized to ZT1 control levels; n = 5-6 mice, Student’s t-test with Welch’s correction. (H) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery and jugular vein after 2 h (acute) treatment with a β2-adrenergic receptor antagonist. Data are normalized to ZT1 control levels; n = 6-11 mice, Student’s t-test. (I) Q-PCR analyses of cell adhesion molecules after TNF-α stimulation in carotid artery and jugular vein after acute (2 h) treatment with a β2-adrenergic receptor agonist; n = 5-6 mice, Student’s t-test with Welch’s correction. *p < 0.05, **p < 0.01.
We therefore investigated which adrenoreceptors were involved in this process. Expression analyses revealed the presence of Adrb2 in both arteries and veins as well as expression of downstream G-protein-coupled receptor kinases (Figure S4A–D). Importantly, no significant oscillation in leukocyte adhesion was observed in both types of vessels of Adrb2−/− animals (Figure 5E). To verify that this was not due to a developmental phenotype, we treated wild-type mice pharmacologically for 5 days using a β2 adrenergic receptor antagonist. Consistent with the genetic experiments, animals receiving the antagonist exhibited no oscillation in leukocyte recruitment (Figure 5F) or in the expression of pro-migratory molecules (Figure 5G) without affecting Bmal1 expression (Figure S4E). We next performed the same treatment for a shorter time frame (2 h) in order to tease apart potential differences between arteries and veins that might explain their distinct rhythmic inflammatory responses. Interestingly, with this shorter treatment the functional importance of the β2 adrenergic receptor was confirmed in the artery while it had no effect in veins (Figure 5G–H). In contrast, exogenous, acute (2 h) application of a β2 adrenergic receptor agonist showed an increase in the expression of adhesion molecules in both arteries and veins (Figure 5I). This indicated that both types of vessels could in principle react in a similar manner to β2 adrenergic stimulation if applied exogenously and that, thus, differences in local endogenous sympathetic tone and sensitivity were likely responsible for the distinct effects observed in both vessels.
Sympathetic nerves are associated with arteries
How might sympathetic nerves be able to modulate inflammatory leukocyte recruitment to arteries in a different manner than to veins? To explore this, we performed immunofluorescence imaging analyses of the sympathetic innervation status of arteries and veins in the macro- and microvasculature. Interestingly, while large sympathetic nerve bundles accompanied the carotid artery and cremasteric arterioles exhibited heavy sympathetic innervation, the jugular vein and cremasteric venules were devoid of sympathetic fibers (Figure 6A–B). Confirming these results, mRNA expression levels of Th, the rate-limiting enzyme for the generation of catecholamines in sympathetic nerves, was high in arteries but absent from veins (Figure 6C). Sympathetic nerves innervate arteries at the level of alpha smooth muscle actin (αSMA) positive mural cells31 (Figure 6D–F). These cells co-express NG2 (Figure 6F–H), a marker that is absent from veins, which can be used to differentiate between arteries and veins32–34 (Figure 6G–H). We therefore employed Ng2-cre mice to ablate the circadian gene Bmal1 in arterial mural cells. Interestingly, Bmal1-deficiency in NG2+ cells alone resulted in loss of time-of-day differences in Bmal1 expression in the whole arterial tissue, indicating a critical pacemaker function for these cells in arteries (Figure S5A). In addition, it ablated the temporal differences in leukocyte adhesion and Icam1 expression in these tissues (Figure 6I–J) – a phenotype we also observed in clock deficient Per1−/−Per2−/− double knock out mice (Figure S5B) – without affecting systemic blood leukocyte oscillations (Figure S5C). Of importance, although arteries were targeted with this genetic approach, oscillations also ceased in veins (Figure 6I–J and Figure S5A). These data, together with the microsurgical denervation experiments (Figure 5C–D), thus indicate that local sympathetic nerves innervating arteries are critical for the oscillatory inflammation process in both arteries and veins. It furthermore demonstrates the critical importance of circadian oscillations in arterial NG2+ mural cells for a rhythmic inflammatory process in both types of vessel.
Figure 6. Different sympathetic innervation status of arteries and veins.
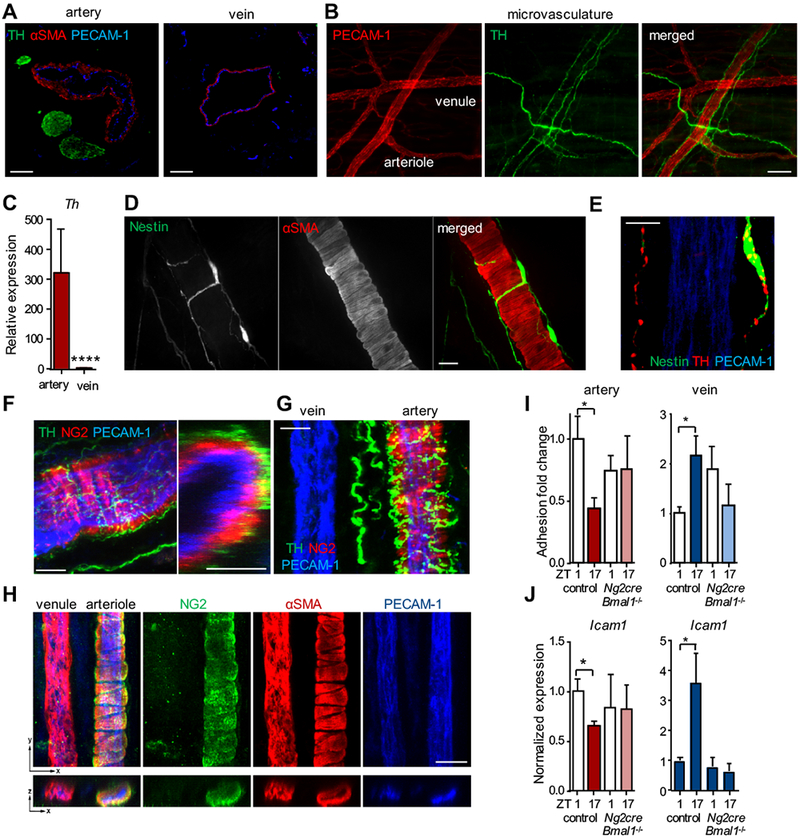
(A) Immunofluorescence images of carotid artery and jugular vein sections stained with antibodies directed against PECAM-1 (endothelial cells), αSMA (smooth muscle cells) and tyrosine hydroxylase (TH, sympathetic nerves) in steady state. (B) Whole-mount immunofluorescence images of sympathetic innervation in the cremaster muscle microcirculation in steady state. (C) Q-PCR analyses of Th expression levels in steady state; n = 11-12 mice, Mann-Whitney test. (D) Whole-mount immunofluorescence images of sympathetic innervation (Nestin) of αSMA+-smooth muscle cells of an arteriole in the cremaster muscle microcirculation in steady state. (E) TH+ sympathetic varicosities within Nestin+ nerve fibers in close proximity to the arterial vessel wall. (F-G) Whole-mount immunofluorescence images of sympathetic innervation (TH) of mural cells (NG2) within the carotid artery and an associated vein (G) in steady state harvested from Ng2-DsRed mice. The right picture in (F) represents an orthogonal view. (H) Whole-mount immunofluorescence images of a cremasteric arteriole and venule stained with antibodies directed against PECAM-1, NG2 and αSMA in steady state. (I) In vivo quantification of adherent leukocytes after TNF-α stimulation in carotid artery (red, left) and jugular vein (blue, right) of Ng2cre:Bmal1−/− mice. Data are normalized to ZT1 control levels; n = 4-10 mice, Student’s t-test with Welch’s correction. (J) Q-PCR analyses of Icam1 expression levels after TNF-α stimulation in carotid artery (red, left) and jugular vein (blue, right) of Ng2cre:Bmal1−/− mice. Data are normalized to ZT1 control levels; n = 3-9 mice, Student’s t-test with Welch’s correction. *p < 0.05, ****p < 0.0001. Scale bars: (A-B) 100 μm, (D) 10 μm, (E) 5 μm, (F) 30 μm, (G) 15 μm, (H) 20 μm.
Acute vaso-occlusion in arteries and veins peaks at different times of the day
Finally, we explored the relevance of oscillatory leukocyte adhesion patterns in the different vascular beds. We assessed the activation status of adherent leukocytes in situ using L-selectin as a marker as it is shed upon aging and activation in the circulation35. Interestingly, cells adherent to arteries exhibited lower surface L-selectin levels in the morning, indicating a higher activation status and/or higher recruitment of aged neutrophils35, whereas the inverse was observed in veins (Figure 7A). Thus, not only were more leukocytes recruited at specific times, the proportion of activated leukocytes was also increased. Adherent L-selectinlo leukocytes were more prone to bind BSA-coated beads that specifically interact with Mac-1 integrin (Figure S5D)3, 35. This resulted in more interactions between leukocytes and platelets in arteries in the morning whereas in veins more interactions occurred at night (Figure 7B). Analogous to leukocytes also numbers of adherent platelets exhibited a striking diurnal rhythmicity with a phase shift between arteries and veins (Figure 7C). In contrast to leukocytes, systemic platelet counts did not oscillate (data not shown), corroborating the importance of a rhythmic microenvironment for diurnal cellular adhesion. We hypothesized that these interactions might be of relevance for acute thrombotic events. We therefore employed a model of phototoxicity-induced thrombus generation, which allowed for the well-controlled induction of an acute and local occlusion of arterioles and venules, side-by-side within the same cremasteric microcirculation. Interestingly, arteries exhibited shorter occlusion intervals at the times of higher cellular adhesion and leukocyte activation in the morning (ZT2 vs ZT8), indicative of a more inflammation-prone environment. In contrast, in veins shorter occlusion intervals occurred at later times (ZT8 vs ZT2) (Figure 7D). These differences were ablated in mice lacking Bmal1 in NG2+ mural cells (Figure 7E) demonstrating that altering circadian oscillations in microenvironmental arterial mural cells had an impact on thrombotic events within the vasculature. We finally assessed whether time-of-day dependency in thrombus formation would still be observable in a general hemostatic model that does not specifically target the venous or arterial vasculature, or whether these rhythms would cancel each other out. Performing bleeding assays after tail tip transection, we observed a clear time-of-day difference, with significantly shorter bleeding time occurring at ZT8 compared to ZT2 (Figure 7F). These data thus indicate that timing of venous thrombosis correlates with overall time to bleeding arrest.
Figure 7. Acute thrombosis in arteries and veins peaks at different times of the day.
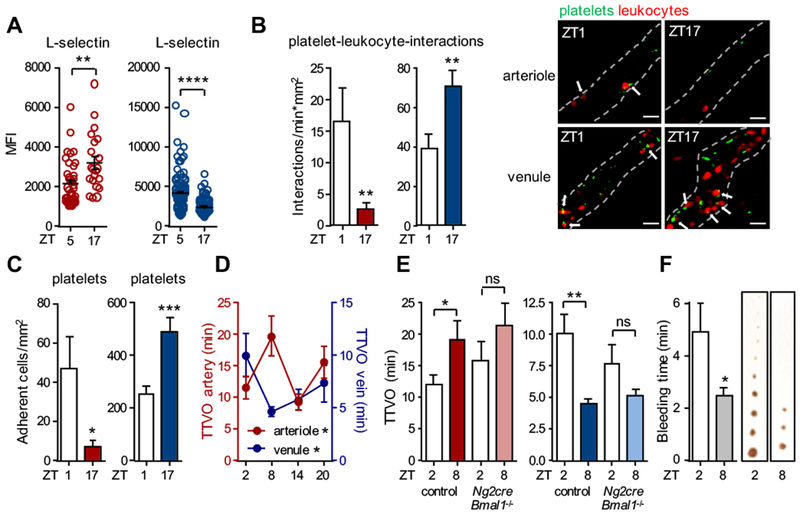
(A) In vivo quantification of L-selectin mean fluorescence intensity (MFI) levels on the surface of adherent leukocytes after TNF-α stimulation in cremasteric arterioles (red) and venules (blue); n = 24 cells, Student’s t-test. (B) In vivo quantifications of heterocellular interactions between platelets (CD41+) and adherent neutrophils (Ly6G+) after TNF-α stimulation in cremasteric arterioles (red) and venules (blue); n = 28-38 vessels, Student’s t-test. (C) In vivo quantification of adherent platelets after TNF-α stimulation in cremasteric arterioles (red) and venules (blue); n = 28-38 vessels, Student’s t-test with Welch’s correction. (D) In vivo quantification of time to vaso-occlusion (TTVO) after light-induced thrombus formation in cremasteric arterioles (red) and venules (blue); n = 6-22 vessels, one-way ANOVA. (E) In vivo quantification of time to vaso-occlusion (TTVO) after light-induced thrombus formation in cremasteric arterioles (red) and venules (blue) of control and Ng2cre:Bmal1−/− mice; n = 12-15 vessels, Student’s t-test. (F) Tail bleeding time after 2mm tail transection, assessed at two different time points with representative images of blood drops on paper every 30s until complete stop of bleeding; n = 18-20 mice, Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Scale bars: 10 μm.
DISCUSSION
We show here a striking time-of-day difference in the level of leukocyte adhesion to arteries with an unexpected phase shift from that observed in veins. Our data implicate rhythms in leukocytes to be important for the general rhythmicity but point to a critical role of vascular cells in governing vessel-type specific timing in acute vascular inflammation. Although circadian rhythmicity of the clock machinery peaks at similar times in arteries and veins, the timing of an acute inflammatory response is distinct, with pro-migratory molecules exhibiting their acrophase at different times. We find local sympathetic innervation and β2-adrenergic receptors to be important in driving vessel-type specific rhythmicity in inflammation in arteries and veins. While the functional interactions between sympathetic nerves and arteries appears to be direct and involve NG2+ mural cells, the effect on veins appears to be indirect but depend on arterial innervation. Together, our data point to an important role of arteries in driving rhythmic inflammatory responses within the vasculature with effects on the time-of-day-dependent onset of induced acute vaso-occlusive events. Whether these rhythms in TNF-α-driven, acute thrombotic events in mice also govern the chronic inflammatory scenarios that culminate in time-of-day cardiovascular events in humans should be the focus of future investigations. Care should be taken, however, when comparing timing in cardiovascular events between humans and mice since at least with respect to the general ability of blood to clot, these rhythms appear to be inversed between mice and humans36, 37.
Interplay between rhythms of the vasculature and of leukocytes
Our data demonstrate that overall rhythmicity in leukocyte adhesion in an inflammatory scenario is dependent on the leukocyte clock. This confirms the role of Bmal1 in myeloid cells as a critical factor in the generation of rhythmic acute immune responses9, 21, 38. Importantly, however, myeloid cell rhythmicity does not explain the phase-shifted phenotype between arteries and veins as it affects equally both vessel types. Instead, our homing and expression data implicate the microenvironment in this process. This appears to be at least partly due to a different level of importance of the endothelial cell clock in both vessels. While in veins endothelial cell-specific Bmal1 deficiency ablated time-of-day-dependent leukocyte adhesion, in arteries it did not. This may be due to the fact that leukocyte adhesion and emigration from blood generally occurs via veins and not arteries. Thus, venous endothelial cells are predestined to recruit leukocytes and facilitate tissue infiltration, as compared to arteries, pointing to a more prominent role of the venous endothelial clock in this process. Indeed, although arteries exhibited much more pronounced amplitudes in their circadian gene expression compared to veins, molecules implicated in leukocyte migration, specifically those whose expression in these tissues are restricted to the endothelium – such as E- and P-selectin – showed greater expression amplitudes in veins. Thus, a different contribution of the endothelial cell clock in arteries and veins contributes to the phase shift in inflammatory adhesion rhythmicity.
Robustness of rhythmicity in arteries and veins
The stronger amplitudes in the expression of clock genes in arteries compared to veins point to a more robust circadian rhythm in the former. These data are in line with previous observations demonstrating that arteries exhibit similar acrophases in their circadian amplitudes, independent of their respective localization within the body while rhythms in veins can vary significantly by anatomical location27. Thus, within the vasculature, robustness in rhythmicity in arteries may be important to preserve blood pressure conformity and stable oxygenation of tissues. Although sympathetic nerves are densely innervating arteries, they appear to not play a role in governing their overall circadian rhythmicity. Instead, local arterial innervation affected rhythms in the inflammatory response. Interestingly, rhythmicity in veins appears to be affected by arteries. This is based on our findings that inflammatory time-of-day effects were lost after local surgical removal of the superior cervical ganglion, which is directly responsible for the sympathetic innervation of local arteries but not veins. In addition, deficiency of Bmal1 in Ng2+ arterial mural cells ablated daily changes not only in arteries but also in veins. Lastly, pharmacological interference of β-adrenergic signaling resulted in similar effects in arteries and veins, however, arteries were more sensitive to these stimuli. Thus, venous rhythmicity in the inflammatory response appears to be partly governed by arteries and their associated nerves. Since many of the functions of the sympathetic nervous system are linked to leukocyte trafficking patterns, which classically have been associated with veins, such as the mobilization of leukocytes and HSCs from the bone marrow39, the recruitment of leukocytes to peripheral tissues6 as well as the retention of lymphocytes within lymph nodes40, these may partly be modulated by arteries and their associated sympathetic nerves. How this arterial-venous signal transduction mechanism is achieved, and how the potential lag in time may contribute to the phase shift in the inflammatory response, is currently unknown and should be the focus of future investigations.
Relevance of arterial and venous leukocyte recruitment
While leukocyte trafficking across arterial endothelial cells plays a minor role for the infiltration of cells into tissues and the general amount of leukocytes in tissues, adhesion of cells within the arterial lumen presents a major obstacle for blood flow. Arterial diameters are generally smaller than veins, due to reduced elasticity compared to veins, which represent the major blood reservoir. Thus, intravascular adhesion within the arterial wall presents a high risk of vascular occlusion with direct effects on blood pressure. Rhythmicity in endothelial cells has previously been linked to be important in the circadian timing of acute thrombotic events37. In addition, diurnal rhythms exist with respect to the coagulation cascade and platelet functions41. We show that differences in the microenvironment and mural cell clock govern thrombosis in a tissue-specific manner. Taken together, our data point to an intricate interplay between tissue- and leukocyte circadian clocks, inflammatory signals and sympathetic innervation in governing vessel type-specific inflammatory responses.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Leukocytes adhere to arteries and veins following a circadian rhythm in mice, with adhesion peaking in arteries in the morning and in veins at night.
What are the clinical implications?
These peaks in leukocyte adhesion at different times in the two vascular beds are associated with increased vascular inflammation and shortened times to local vaso-occlusive events, occurring out of phase between arteries and veins, providing mechanistic insights into the observed time-of-day dependency of cardiovascular complications in patients.
ACKNOWLEDGMENTS
We thank Stéphane Jemelin for technical assistance. The authors are grateful for support from the core facility for animal models (CAM) of the BMC and the animal facility of the WBex.
FUNDING SOURCES
This work was supported by the German Research Foundation (DFG) (Emmy-Noether grant (SCHE 1645/2-1 (C.S.)), SFB914 projects B09 and Z03 (C.S.) and B08 (O.S.), SFB1123 project A6/B5 (O.S.), SFB134 & RTG1957 (H.O.) and DFG project MO2562/1-2 (E.M.)), the European Research Council (ERC) starting grant (635872, CIRCODE (C.S)), the Swiss National Foundation (SNF, 310030_182417/1 (C.S.)), the DZHK (German Centre for Cardiovascular Research) and BMBF (German Ministry of Education and Research), and IMPRS funding (C.S.). H.O. is funded by a Lichtenberg Professorship of the Volkswagen Foundation. The P.S.F. laboratory is supported by the National Institutes of Health (DK056638, HL069438, HL116340).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- SCGx
superior cervical ganglion
- MFIM
Multichannel fluorescence intravital microscopy
- LPS
lipopolysaccharide
- LFA
leukocyte function associated antigen
- Mac-1
macrophage antigen 1
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule 1
- SCN
suprachiasmatic nuclei
- CFSE
carboxyfluorescein succinimidyl ester
- 6-OHDA
6-hydroxydopamine
- Adrb2
β2 adrenergic receptor
- αSMA
alpha smooth muscle actin
- ZT
Zeitgeber time
Footnotes
DISCLOSURES
P.S.F. serves as consultant for Pfizer, has received research funding from Ironwood Pharmaceuticals and is shareholder of Cygnal Therapeutics.
REFERENCES
- 1.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, and Braunwald E and the MILIS Study Group. Circadian variation in the frequency of onset of acute myocardial infarction. New Eng J MEd. 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 2.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arterioscler thromb Vasc Biol. 2005;25:658–670. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY and Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turhan A, Weiss LA, Mohandas N, Coller BS and Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Nat Acad Sci USA. 2002;99:3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA and Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheiermann C, Kunisaki Y and Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M and Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D and Loudon A. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med. 2014;20:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS and Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiermann C, Gibbs J, Ince L and Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423–437. [DOI] [PubMed] [Google Scholar]
- 11.Sumagin R and Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2006;291:H2116–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ley K, Laudanna C, Cybulsky MI and Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. [DOI] [PubMed] [Google Scholar]
- 13.Muller WA. Transendothelial migration: unifying principles from the endothelial perspective. Immunol Rev. 2016;273:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vestweber D How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. [DOI] [PubMed] [Google Scholar]
- 15.Dibner C, Schibler U and Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Ann Rev Physiol. 2010;72:517–549. [DOI] [PubMed] [Google Scholar]
- 16.Golombek DA and Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. [DOI] [PubMed] [Google Scholar]
- 17.Curtis AM, Bellet MM, Sassone-Corsi P and O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. [DOI] [PubMed] [Google Scholar]
- 18.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G and Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell metab. 2005;2:297–307. [DOI] [PubMed] [Google Scholar]
- 19.Ulrich-Lai YM, Arnhold MM and Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr comp physiol. 2006;290:R1128–1135. [DOI] [PubMed] [Google Scholar]
- 20.Vizi ES and Elenkov IJ. Nonsynaptic noradrenaline release in neuro-immune responses. Acta biologica Hungarica. 2002;53:229–244. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW and Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Nat Acad Sci USA. 2012;109:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A and Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. [DOI] [PubMed] [Google Scholar]
- 23.Chevre R, Gonzalez-Granado JM, Megens RT, Sreeramkumar V, Silvestre-Roig C, Molina-Sanchez P, Weber C, Soehnlein O, Hidalgo A and Andres V. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circulation research. 2014;114:770–779. [DOI] [PubMed] [Google Scholar]
- 24.Steeber DA, Campbell MA, Basit A, Ley K and Tedder TF. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Nat Acad Sci USA. 1998;95:7562–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rot A and von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Ann Rev Immunol. 2004;22:891–928. [DOI] [PubMed] [Google Scholar]
- 26.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M and Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Nat Acad Sci USA. 2004;101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson AJ, London B, Block GD and Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens. 2005;27:307–311. [PubMed] [Google Scholar]
- 28.Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, Kalsbeek A, Pevet P and Buijs RM. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PloS one. 2009;4:e5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alito AE, Romeo HE, Baler R, Chuluyan HE, Braun M and Cardinali DP. Autonomic nervous system regulation of murine immune responses as assessed by local surgical sympathetic and parasympathetic denervation. Acta physiol pharmacol latinoam. 1987;37:305–319. [PubMed] [Google Scholar]
- 30.Reilly DF, Curtis AM, Cheng Y, Westgate EJ, Rudic RD, Paschos G, Morris J, Ouyang M, Thomas SA and FitzGerald GA. Peripheral circadian clock rhythmicity is retained in the absence of adrenergic signaling. Arterioscler thromb Vasc Biol. 2008;28:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westfall TC, Macarthur H, Byku M, Yang CL and Murray J. Interactions of neuropeptide y, catecholamines, and angiotensin at the vascular neuroeffector junction. Adv pharmacol. 2013;68:115–139. [DOI] [PubMed] [Google Scholar]
- 32.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A and Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murfee WL, Skalak TC and Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–160. [DOI] [PubMed] [Google Scholar]
- 34.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B and Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M and Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheer FA and Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123:590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA and FitzGerald GA. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 2008;117:2087–2095. [DOI] [PubMed] [Google Scholar]
- 38.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, Xue C, Geiger SS, Hokamp K, Reilly MP, Coogan AN, Vigorito E, FitzGerald GA and O’Neill LA. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Nat Acad Sci USA. 2015;112:7231–7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez-Ferrer S, Lucas D, Battista M and Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki K, Hayano Y, Nakai A, Furuta F and Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. J Exp Med. 2016;213:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paschos GK and FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


