Abstract
INTRODUCTION:
Vascular factors increase the risk of Alzheimer disease (AD). We investigated the associations between such factors, longitudinal AD cerebrospinal fluid (CSF) biomarkers and cognition.
METHODS:
433 cognitively normal participants were classified into four biomarker groups using their baseline amyloid (A+/−) and tau status (T+/−). 184 participants had undergone serial CSF collection. Frequencies of risk factors and the Framingham Risk Score (FRS) were compared, and we tested the influence of risk factors on change in biomarker concentrations and cognition.
RESULTS:
The absence of obesity, presence of hypertension and a high FRS were associated with an increase in tau levels, particularly in A+T+ individuals. Risk factors were not associated with amyloid. Depression was associated with higher cognitive scores, while high FRS was associated with lower scores and a faster decline.
DISCUSSION:
Our results demonstrate that vascular risk factors may enhance neurodegeneration but not amyloid accumulation in preclinical AD.
Keywords: Alzheimer disease, dementia, cerebrospinal fluid, biomarkers, amyloid-beta, tau, neurodegeneration, vascular risk factors, hypertension, obesity, hypercholesterolemia, depression, cardiovascular disorders, Framingham Risk Score
Introduction
Lifestyle factors and vascular co-morbidities have been associated with an increased risk of cognitive decline and Alzheimer disease (AD) dementia [1-4]. The availability of biomarkers for AD now provide an opportunity to study the associations of these risk factors with AD pathology before the onset of dementia. However, previous cross-sectional studies on the relationship between risk factors and AD pathology in the pre-dementia stages are inconclusive [5-11], and there have been only few studies that examined risk factors, longitudinal biomarker and cognitive changes [12, 13]. Understanding the associations between risk factors and changes in AD biomarkers and cognition in cognitively normal older individuals will be critical for the development of primary and secondary prevention strategies and may improve prognostic accuracy for future patients.
The two primary types of AD biomarkers are those reflecting amyloid deposition and those reflecting neuronal injury. Although results regarding the relationship between AD biomarkers and vascular risk factors have been mixed, an increasing number of cross-sectional biomarker studies report associations with neuronal injury markers [6, 7, 10, 12], but few found a relationship with amyloid [5, 6]. It remains unknown how vascular risk factors are associated with changes in AD pathology over time. To our knowledge only two studies thus far have examined the impact of vascular risk factors in a longitudinal biomarker design, including one that only focused on hypertension [12, 13]. Additionally, studying this in a longitudinal biomarker design focusing both on amyloid and neurodegenerative markers seems especially important because a recent study showed that in cognitively normal individuals the temporal ordering of biomarker changes might be different from the classic hypothesis that in AD amyloid deposition precedes neurodegeneration [14]. Regarding cognitive decline, previous findings suggest that vascular risk factors increase the rate of decline, in particular in individuals who have amyloid pathology and are cognitively normal [8]. Yet, it is less well known which risk factors have the strongest impact on AD pathology and cognitive decline, making it difficult to develop targeted prevention strategies [15, 16].
Therefore, our first aim was to investigate the associations between risk factors, the Framingham Risk Score (FRS; [17]), AD biomarker profiles and changes in AD biomarkers over time, in a cohort of cognitively normal individuals. Second, we examined whether these risk factors influenced the relationship between AD biomarkers and cognitive decline.
Methods
Participants
Participants were selected from longitudinal studies of memory and aging at the Knight Alzheimer’s Disease Research Center (ADRC) at Washington University School of Medicine in St. Louis [18]. Participants were included in the current study (n=433) if they met the following criteria: 1) Clinical Dementia Rating (CDR [19]) of 0 at baseline, indicating normal cognition; 2) CSF collection within one year of the baseline clinical assessment with available data on levels of amyloid-β42 (Aβ42), total tau (t-tau) and phosphorylated tau181 (p-tau); and 3) Completed a clinical assessment and psychometric battery at the baseline visit and at least one follow-up visit. For the current study, the clinical visit closest to the first CSF collection was considered the baseline visit. The study was approved by the Human Research Protection Office at Washington University, and all participants provided written informed consent.
Risk factors
The following risk factors were assessed at baseline: hypertension, hypercholesterolemia, diabetes mellitus, vitamin B12 deficiency, depression, current smoking, alcohol abuse, transient ischemic attack (TIA), stroke, obesity and cardiovascular disorders. Risk factors were based on self- or proxy-reported information during the baseline assessment. Using a structured questionnaire, risk factors were coded as absent, recent/active, or remote/inactive. For the current study, both the recent/active and the remote/inactive categories were compared to the absent category. Definitions of risk factors are listed in Supplemental Table 1 Statistical comparisons were conducted only on risk factors that were relatively common in our study population (frequency >10%) in order to have sufficient statistical power for all statistical analysis, including interaction effects. The FRS was calculated based on plasma total and high-density lipoprotein (HDL) cholesterol, systolic blood pressure, smoking status and medical history of diabetes, as described in detail elsewhere [17].
Clinical assessment and psychometric battery
Clinical assessment with formulation of the CDR was performed annually by trained clinicians who were blinded to the participant’s prior CDR, clinical diagnosis and performance on psychometric tests. The Mini-Mental State Examination (MMSE; [20]) was completed as part of the clinical assessment. A psychometric battery was administered at a separate session. The psychometric tests analyzed for this study were the Free and Cued Selective Reminding Test free immediate recall portion [21]; the Trail making Test parts A & B [22]; the Animal Naming task [23] and the Digit Symbol task from the Wechsler Adult Intelligence Scale-R [24]. A cognitive composite score was created from the available cognitive measures.
CSF collection and analyses
CSF samples (20-30 mL) were collected from all 433 individuals at baseline. Additionally, 184 individuals provided CSF repeatedly during their annual visits: n=107 provided two samples, n=54 provided three samples and n=23 provided four or more samples. All lumbar punctures were performed at 8 AM following overnight fasting. CSF was collected via gravity drip. Following completion of sample collection, the CSF was gently inverted to disrupt potential gradient effects, briefly centrifuged at low speed to pellet any cellular debris, and aliquoted (0.5 mL) into polypropylene tubes prior to freezing at −80°C [25]. Aβ42, t-tau and p-tau were measured with Elecsys immunoassays on the automated cobas e 601 analyzer using a single lot of assays for each analyte [26].
Genetic analyses
The Knight ADRC Genetics Core performed DNA extraction and apolipoprotein E (APOE) genotyping from non-fasted blood collected at the time of clinical assessment [27]. APOE genotype was dichotomized as APOE ε4 carrier and non-carrier.
Biomarker classification
We classified participants into four groups based on combinations of baseline amyloid and tau status. Amyloid positivity (A+) was defined as baseline CSF Aβ42 <1098 pg/ml. This cut-off was determined based on the CSF Aβ42 value with the highest Youden index, which best distinguished individuals with and without significant brain amyloid burden by positron emission tomography (PET) using the radiotracer 11C-Pittsburgh Compound B (PIB) in a separate but overlapping cohort (total n=200) [26]. Participants were classified as tau positive (T+) based on either abnormal CSF t-tau (>255 pg/ml) or p-tau (>23 pg/ml) at baseline. These cut-offs were determined based on the CSF t-tau and p-tau values with the highest Youden index, which best discriminated the reference group (CDR=0, amyloid PET-negative, n=216) and symptomatic AD (CDR>0, amyloid PET-positive, n=52) in a separate but overlapping cohort (unpublished data). Tau status was based on either abnormal t-tau or p-tau as the concordance between the two markers was very high (96%).
Statistical analyses
Demographics and baseline characteristics were compared among the four biomarker groups using t-tests for continuous variables and Chi-square tests for categorical variables. The frequency of risk factors were compared among groups using logistic regression, adjusted for age, gender, years of education and APOE ε4 status. When the overall difference between the four biomarker groups reached significance on the tested variables (i.e. demographics, baseline characteristics or frequency of risk factors), we used contrast testing to determine which biomarker groups differed from each other.
General linear mixed models (GLMM), with random intercepts and slopes, were used to analyze the influence of risk factor status on concentrations of CSF Aβ42, t-tau and p-tau over time. For these models, the baseline biomarker levels were estimated based on the total sample (n=433), while change in biomarker levels over time (slopes) were estimated based on a subgroup of subjects (n=184). We also assessed the interaction with the four biomarker groups at baseline and the slopes (risk factor*biomarker groups). Prior to comparisons, Aβ42 values were log-transformed to approximate a normal distribution, but untransformed values were used for visualization.
GLMM were also used to examine the influence of risk factors on cognitive performance and decline. For these models, the main effect of baseline risk factors on cognitive performance (baseline) and decline (slope) was assessed in the total group and in all four biomarker groups separately. In all analyses, we only assessed risk factors that had an overall frequency of >10% as this allowed testing of interaction effects between risk factors and biomarker groups cross-sectionally and longitudinally. Risk factors with a lower frequency would yield smaller subgroups that have the risk factor, especially longitudinally and in the A+T+ group, which would provide unreliable or missing results. All models were adjusted for age, years of education, gender and APOE ε4 status. The FRS was dichotomized in low (<12.95) and high (> 12.95) scores using a median split. We corrected for multiple comparisons, using the false discovery rate (FDR) adjustment [28], taking into account the testing of five risk factors and the FRS. Statistical analyses were performed using R Statistical Software (version 3.3.3) and SPSS (version 24), with significance defined as p<0.05.
Results
We included 433 individuals with an average age of 68.3 (SD 8.5) years at baseline. Two hundred and twenty-nine (53%) were female and 149 (34%) carried at least one APOE ε4 allele. The average clinical follow-up time was 5.2 (SD 2.7) years, and the average biomarker follow-up time was 2.1 (SD 2.8) years. At the last clinical follow-up, 29 (7%) individuals had a CDR ≥ 0.5. Baseline sample characteristics and frequency of risk factors by biomarker groups are shown in Table 1. One hundred and eighty-seven participants (43%) were classified as A−T−, 72 (16%) as A−T+, 116 (27%) as A+T− and 58 (13%) as A+T+.
Table 1.
Demographics and risk factors by biomarker groups
| Variables | All | A−T− | A−T+ | A+T− | A+T+ |
Overall p-value |
|---|---|---|---|---|---|---|
| No. (%) | 433 (100) | 187 (43) | 72 (17) | 116 (27) | 58 (13) | - |
| Baseline age, mean (SD), y | 68.3 (8.5) | 66.1 (8.7)a,b | 70.4 (8.7)b,d | 68.0 (8.7)b,c | 73.3 (7.4)b,d | <0.001 |
| Female, No. (%) | 229 (53) | 107 (57)b | 48 (67)b,c | 46 (40)a,d | 28 (48)a | 0.001 |
| APOE ε4 carrier, No. (%) | 149 (34) | 40 (21)b,c | 15 (21)b,c | 57 (49)a,d | 37 (64)a,d | <0.001 |
| Education (SD), y | 16.1 (2.7) | 16.2 (2.6) | 15.8 (2.5) | 16.3 (2.9) | 15.6 (3.1) | 0.321 |
| Baseline MMSE score, mean (SD) | 29.1 (1.3) | 29.2 (1.0)c | 29.1 (1.1) | 29.0 (1.5) | 28.8 (1.6)d | <0.001 |
| Baseline CDR-SOB, mean (SD) | 0.02 (0.1) | 0.019 (0.1) | 0.028 (0.1) | 0.013 (0.1) | 0.026 (0.1) | 0.728 |
| Baseline cognitive compound Z-score, mean (SD) | 0.02 (0.7) | 0.20 (0.7)a,b,c | −0.01 (0.6)c,d | 0.00 (0.68)c,d | −0.45 (0.8)a,b,d | <0.001 |
| Individuals with longitudinal biomarkers, No. (%) | 184 (42) | 92 (50) | 25 (35) | 44 (38) | 23 (40) | - |
| Biomarker follow-up (No. of visits) | 1.7 (0.9) | 1.8 (1.0)c,d | 1.5 (0.9)d | 1.5 (0.8)d | 1.6 (0.8) | 0.016 |
| Biomarker follow-up time (years)* | 2.1 (2.8) | 2.6 (3.0) | 1.9 (2.9) | 1.8 (2.5) | 1.7 (2.5) | 0.064 |
| Cognitive follow-up (No. of visits) | 4.8 (2.5) | 4.8 (2.7) | 5.5 (2.3) | 4.4 (2.3) | 4.8 (2.6) | 0.054 |
| Cognitive follow-up time (years) | 5.2 (2.7) | 5.5 (2.9) | 5.5 (2.2) | 4.7 (2.6) | 4.9 (2.7) | 0.071 |
| Baseline Aβ42, mean (SD), pg/ml | 1357.3 (647.5) | 1630.1 (392.7)a,b,c | 2055.4 (728.9)b,c,d | 797.7 (200.5)a,d | 730.4 (186.6)a,d | <0.001 |
| Last follow-up Aβ42, mean (SD), pg/ml* | 1096.6 (526.3) | 1282.7 (478.2)a,b,c | 1391.6 (649.1)b,c,d | 775.0 (312.1)a,c,d | 655.4 (246.2) | <0.001 |
| Baseline T-tau, mean (SD), pg/ml | 225.7 (93.8) | 185.9 (36.3)a,b,c | 325.5 (70.6)b,c,d | 163.2 (44.5)a,c,d | 355.4 (97.0)a,b,d | <0.001 |
| Last follow-up T-tau, mean (SD), pg/ml* | 237.6 (119.5) | 189.8 (54.7)a,c | 340.1 (90.4)b,c,d | 189.5 (82.2)a,c | 421.1 (159.4)a,b,d | <0.001 |
| Baseline P-tau, mean (SD), pg/ml | 20.5 (9.9) | 16.0 (3.2)a,c | 29.2 (7.9)b,c,d | 14.8 (4.3)a,c | 35.7 (11.9)a,b,d | <0.001 |
| Last follow-up P-tau, mean (SD), pg/ml* | 22.1 (13.2) | 16.7 (5.1)a,c | 31.2 (10.0)b,c,d | 17.4 (8.8)a,c | 43.9 (18.9)a,b,d | <0.001 |
| Hypertension, No. (%), (n=433) | 251 (58) | 92 (49) | 46 (64) | 74 (64) | 39 (67) | 0.058 |
| Hypercholesterolemia, No. (%), (n=422) | 273 (65) | 116 (63) | 53 (76) | 70 (61) | 34 (62) | 0.206 |
| Depression, No. (%), (n=421) | 152 (36) | 72 (39) | 28 (41) | 36 (32) | 16 (29) | 0.613 |
| Obesity, No. (%), (n=428) | 113 (26) | 59 (32)c | 12 (17) | 37 (32)c | 5 (9)b,d | 0.048 |
| Cardiovascular disorders, No. (%), (n=423) | 55 (13) | 18 (10)c | 10 (15) | 12 (11) | 15 (26)d | 0.010 |
| Framingham Risk Score, (n=335) | 16.3 (11.4) | 14.0 (9.6) | 17.2 (10.6) | 18.0 (13.3) | 18.7 (12.0) | 0.292 |
Comparisons between groups on frequency of risk factors were adjusted for age, gender, years of education and APOE ε4+. When overall difference between groups was significant, posthoc testing between groups was conducted.
Available in a subgroup: A−T− n=92, A−T+ n=25, A+T− n=44, A+T+ n=23.
p<0.05 compared to A−T+,
p<0.05 compared to A+T−,
p<0.05 compared to A+T+,
p<0.05 compared to A−T−.
Abbreviations: A= amyloid, APOE = Apolipoprotein E, T= tau, TIA=Transient Ischemic Attack.
Frequency of risk factors in baseline biomarker groups
Six of the assessed risk factors had a frequency below 10% and were, therefore, not included in the statistical comparisons: diabetes mellitus (9%), Vitamin B12 deficiency (3%), smoking (7%), alcohol abuse (5%), TIA (2%) and stroke (1%) (Supplementary Table 2). The most common risk factors were hypercholesterolemia (65%), hypertension (58%) and depression (36%) (Table 1). Only 34 (8%) individuals had none of the assessed risk factors, 87 (20%) had a single risk factor and 312 (72%) had more than one risk factor. The frequency of obesity was lower in the A+T+ group compared to the T− groups (A−T−: p=0.033; A+T−: p=0.030). The frequency of cardiovascular disorders was higher in the A+T+ group relative to the biomarker-negative group (p=0.025) (Table 1).
Associations between risk factors and biomarker values at baseline
Table 2 shows the associations between risk factors and baseline biomarker values. Risk factors associated with significant baseline differences or changes in CSF biomarkers are illustrated in Figure 1. We found no associations between risk factors and Aβ42 levels at baseline in the total group, nor in the four biomarker groups (Table 2). In the whole sample, obesity was associated with lower baseline levels of t-tau (p<0.001) and p-tau (p<0.001). When stratifying by biomarker groups, the inverse effect of obesity on p-tau was only significant in the A+T+ group (p=0.032) and showed a trend for t-tau in the A+T+ group (p=0.080) after FDR correction (Table 2).
Table 2.
Effect of risk factors on baseline biomarker values in total group and by baseline biomarker groups
| Aβ42 | T-tau | P-tau | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference in total group |
p-value interaction groups* risk factor |
Mean difference per biomarker group |
Mean difference in total group |
p-value interaction groups* risk factor |
Mean difference per biomarker group |
Mean difference in total group |
p-value interaction groups* risk factor |
Mean difference per biomarker group |
|
| Hypertension | 7.4 ± 61.6 | 0.603 | A−T−: 18.8 ±61.5 A−T+: 220.1 ± 100.7 A+T−: 75.0 ± 80.4 A+T+: −39.3 ±115.9 |
−6.6 ±9.0 | 0.675 | A−T−: −6.0 ±8.3 A−T+: −11.3 ± 13.6 A+T−: −7.1 ± 10.9 A+T+: −27.2 ± 15.7 |
−0.9 ± 1.0 | 0.759 | A−T−: −0.7 ±0.9 A−T+: −1.9 ±1.5 A+T−: −0.9 ± 1.2 A+T+: −3.0 ±1.7 |
| Hypercholesterolemia | 9.6 ± 194.5 | 0.603 | A−T−: −106.5 ±63.5 A−T+: −164.4 ± 115.9 A+T−: 6.6 ± 79.4 A+T+: 58.3 ± 115.3 |
−3.5 ±9.1 | 0.675 | A−T−: −4.4 ±8.6 A−T+: −22.9 ± 15.6 A+T−: −15.2 ± 10.7 A+T+: 14.1 ± 15.6 |
−0.6 ±0.7 | 0.309 | A−T−: −0.4 ±0.9 A−T+: −2.9 ± 1.7 A+T−: −1.7 ±1.2 A+T+: 2.6 ± 1.7 |
| Depression | 42.0 ±63.1 | 0.603 | A−T−: 13.6 ±63.3 A−T+: 145.5 ±101.9 A+T−: −76.1 ±83.9 A+T+: −32.7 ± 123.8 |
−8.4 ±6.3 | 0.675 | A−T−: 2.5 ±8.2 A−T+: −16.8 ± 13.2 A+T−: −15.5 ± 10.9 A+T+: −4.1 ± 16.1 |
−1.3 ±0.7 | 0.759 | A−T−: .02 ±0.9 A−T+: −2.2 ±1.5 A+T−: −1.6 ±1.2 A+T+: −1.2 ±1.8 |
| Obesity | −180.2 ±275.8 | 0.603 | A−T−: −80.3 ±65.3 A−T+: 169.7 ± 130.6 A+T−: 2.3 ± 82.6 A+T+: −40.4 ± 194.5 |
−28.0 ±8.9*** | 0.675 | A−T−: −4.2 ±8.7 A−T+: −29.4 ± 17.4 A+T−: −18.0 ± 11.0 A+T+: −60.5 ±26.0 |
−3.2 ±0.9*** | 0.309 | A−T−: −0.2 ± 1.0 A−T+: −3.4 ±1.9 A+T−: −1.7 ±1.2 A+T+: −7.6 ±2.9* |
| Cardiovascular disorders |
−77.8 ±91.9 | 0.680 | A−T−: −121.0 ± 105.7 A−T+: −176.1 ± 142.8 A+T−: 26.5 ± 128.3 A+T+: −23.4 ± 125.0 |
−13.4 ± 8.6 | 0.675 | A−T−: −2.7 ± 14.2 A−T+: −17.4 ± 19.2 A+T−: −28.7 ± 17.3 A+T+: −5.0 ± 16.8 |
−1.4 ± 1.0 | 0.759 | A−T−: −0.4 ± 1.6 A−T+: −1.0 ±2.1 A+T−: −3.0 ±1.9 A+T+: −1.1 ±1.9 |
| Framingham Risk Score |
86.0 ±89.1 | 0.680 | A−T−: −98.3 ±80.9 A−T+: −48.0 ± 109.1 A+T−: 51.3 ±93.9 A+T+: −38.4 ± 136.4 |
−9.2 ±8.8 | 0.675 | A−T−: −12.2 ± 11.2 A−T+: −25.3 ± 15.1 A+T−: −8.5 ± 13.0 A+T+: 9.3 ± 18.8 |
−0.7 ±0.9 | 0.759 | A−T−: −1.2 ± 1.2 A−T+: −2.0 ± 1.6 A+T−: −0.8 ± 1.4 A+T+: 1.3 ±2.0 |
Numbers are mean difference ± standard error, between individuals with and without risk factor. Statistical comparisons are conducted on log-transformed Aβ42 values, untransformed values are listed in the table. All analyses are adjusted for age, gender and APOE ε4 status.
p<0.05,
p<0.01,
p<0.001.
Abbreviations: Aβ= amyloid-beta, A= amyloid, T=tau, T-tau = total tau, P-tau = phosphorylated tau181.
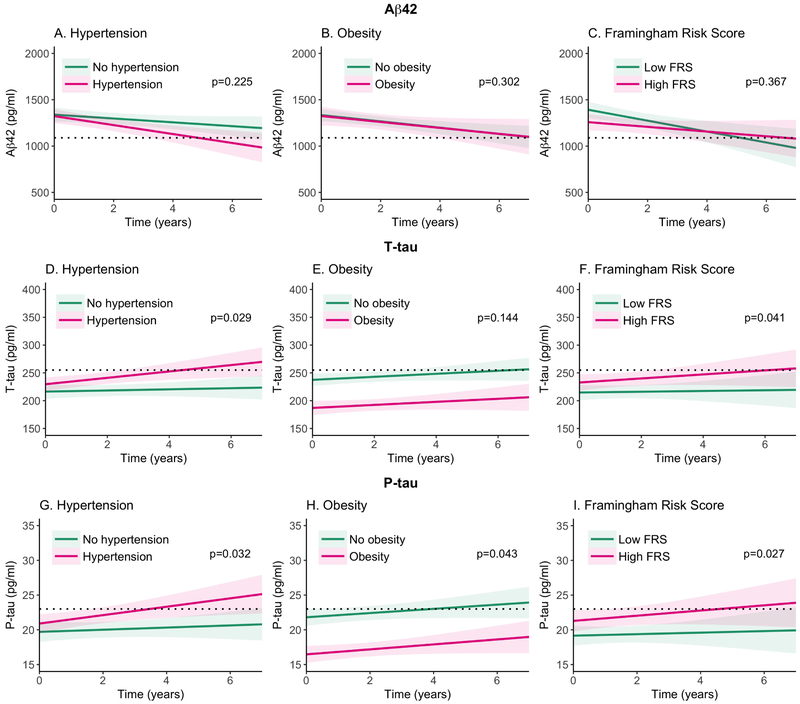
Figure 1. Longitudinal change in Aβ42, t-tau and p-tau values over time by risk factor status.
The panels represent the mean scores and 95% confidence intervals based on the general linear mixed model coefficients and standard error of biomarker values during follow-up by baseline risk factor status. The upper panels (A,B,C) show results for Aβ42, the middle panels (D,E,F) for t-tau, and the lower panels for p-tau (G,H,I). The panels on the left (A,D,G) show the effects of hypertension, the middle panels (B,E,H) show the effect of obesity and the panels on the right (C, F, I) show the effect of the Framingham Risk Score. The black, horizontal dotted line indicates the biomarker cut-offs that define positivity for Aβ42 (<1098 pg/ml), t-tau (>255 pg/ml) and p-tau (>23 pg/ml). P-values indicate difference between individuals with and without the risk factor over time.
Associations between risk factors and longitudinal change in biomarker values
Table 3 shows the associations of risk factors on change in Aβ42, t-tau and p-tau values in the total group and in four biomarker groups. We found that none of the factors were associated with a change in Aβ42 levels over time, in the total group nor in the biomarker groups (Table 3). Hypertension was associated with a faster increase in t-tau and p-tau levels over time, but only in A+T+ individuals (t-tau: p=0.002; p-tau p<0.001) (Table 3). A higher FRS was associated with a faster increase in levels of t-tau, but only in A+T+ individuals (p=0.042) (Table 3). Hypercholesterolemia, depression and cardiovascular disorders were not associated with longitudinal change in CSF Aβ42, t-tau or p-tau levels.
Table 3.
Effect of risk factors on change in biomarker values over time
| Aβ42 | T-tau | P-tau | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope in total group |
p-value interaction group by risk factor |
Slope in biomarker groups |
Slope in total group |
p-value interaction group by risk factor |
Slope in biomarker groups |
Slope in total group |
p-value interaction groups by risk factor |
Slope per biomarker group |
|
| Hypertension | −7.2 ± 11.1 | 0.673 | A−T−: −19.4 ± 15.6 A−T+: 5.7 ± 27.1 A+T−: 11.1 ± 24.9 A+T+: −19.1 ± 36.7 |
3.8 ± 1.7 | 0.012 | A−T−: 0.6 ± 2.3 A−T+: −3.6 ± 4.3 A+T−: 1.8 ± 3.6 A+T+: 21.2 ± 5.2** |
0.4 ± 0.2 | 0.030 | A−T−: 0.1 ± 0.2 A−T+: −0.3 ± 0.4 A+T−: 0.1 ± 0.4 A+T+: 2.1 ± 0.5*** |
| Hypercholesterolemia | −2.6 ± 11.5 | 0.539 | A−T−: −17.2 ± 14.6 A−T+: 70.3 ± 28.3 A+T−: −13.5 ± 23.4 A+T+: 10.6 ± 32.9 |
−2.15 ± 1.8 | 0.844 | A−T−: 0.2 ± 2.5 A−T+: −6.6 ± 4.7 A+T−: −3.9 ± 3.6 A+T+: 2.3 ± 5.0 |
−0.2 ± 0.2 | 0.773 | A−T−: −.02 ± 0.2 A−T+: −0.5 ± 0.4 A+T−: −0.5 ± 0.4 A+T+: 0.1 ± 0.4 |
| Depression | −15.2 ± 11.7 | 0.205 | A−T−: −29.9 ± 15.5 A−T+: 50.4 ± 29.7 A+T−: −3.3 ± 24.6 A+T+: 8.7 ± 43.5 |
−0.15 ± 1.8 | 0.844 | A−T−: −0.1 ± 2.5 A−T+: 5.0 ± 4.6 A+T−: −3.7 ± 3.8 A+T+: 10.6 ± 6.2 |
0.0 ± 0.2 | 0.941 | A−T−: 0.1 ± 0.2 A−T+: 0.5 ± 0.5 A+T−: −0.2 ± 0.4 A+T+: 0.8 ± 0.6 |
| Obesity | 16.5 ± 12.8 | 0.442 | A−T−: 9.837 ± 16.7 A−T+: 31.3 ± 45.8 A+T−: 8.5 ± 24.8 A+T+: −8.6 ± 61.2 |
−3.0 ± 2.0 | 0.880 | A−T−: −2.1 ± 2.7 A−T+: −6.5 ± 6.8 A+T−: 0.6 ± 3.7 A+T+: −8.4 ± 8.9 |
−0.4 ± 0.2 | 0.773 | A−T−: −0.2 ± 0.3 A−T+: −0.7 ± 0.7 A+T−: −0.2 ± 0.4 A+T+: −0.8 ± 0.9 |
| Cardiovascular disorders |
−7.9 ± 20.4 | 0.132 | A−T−: −24.5 ± 29.5 A−T+: −79.2 ± 66.1 A+T−: −3.8 ± 38.2 A+T+: 29.2 ± 44.6 |
0.3 ± 3.0 | 0.844 | A−T−: 0.9 ± 4.8 A−T+: −14.4 ± 8.1 A+T−: −7.4 ± 5.5 A+T+: 4.2 ± 6.0 |
0.1 ± 0.3 | 0.941 | A−T−: 0.2 ± 0.5 A−T+: −1.5 ± 0.8 A+T−: −0.7 ± 0.5 A+T+: 0.2 ± 0.6 |
| Framingham Risk Score |
20.4 ± 15.1 | 0.203 | A−T−: 29.3 ± 21.5 A−T+: 45.0 ± 37.8 A+T−: −15.8 ± 34.4 A+T+: 12.0 ± 55.8 |
4.0 ± 2.1 | 0.063 | A−T−: 0.1 ± 2.8 A−T+: 1.1 ± 4.9 A+T−: 6.7 ± 4.2 A+T+: 17.8 ± 6.9* |
0.3 ± 0.2 | 0.090 | A−T−: .05 ± 0.3 A−T+: 0.1 ± 0.5 A+T−: 0.4 ± 0.4 A+T+: 1.8 ± 0.6 |
Slope (difference risk factor – no risk factor) are mean ± standard error, between individuals with and without risk factors. Statistical comparisons are conducted on log-transformed Aβ42 values. All analyses are adjusted for age, gender and APOE ε4 status.
p<0.05,
p<0.01,
p<0.001.
Abbreviations: Aβ= amyloid-beta, T-tau = total tau, P-tau = phosphorylated tau181.
Influence of risk factors on cognitive performance and decline
We assessed the influence of risk factors on cognitive performance and decline in the total group and in the four biomarker groups (Table 4). In the total group there were no associations of risk factors on baseline or longitudinal MMSE scores. In the A+T+ group, a higher FRS was associated with lower baseline MMSE scores (p=0.023), while depression was associated with higher baseline MMSE scores (p=0.045) (Table 4). Longitudinal analyses showed that in the A+T+ group, a high FRS was associated with a faster rate of decline (p=0.031) (Table 4).
Table 4.
Influence of risk factors on MMSE baseline score and change over time in total sample and by baseline biomarker group
| All n=433 |
A−T− n=187 |
A−T+ n=72 |
A+T− n=116 |
A+T+ n=58 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline difference |
Slope difference |
Baseline difference |
Slope difference |
Baseline difference |
Slope difference |
Baseline difference |
Slope difference |
Baseline difference |
Slope difference |
|
| Hypertension | −0.24 ± 0.13 | 0.00 ± 0.03 | −0.03 ± 0.16 | 0.05 ± 0.04 | −0.34 ± 0.25 | −0.04 ± 0.07 | 0.03 ± 0.21 | 0.00 ± 0.06 | −0.57 ± 0.28 | −0.06 ± 0.09 |
| Hypercholesterolemia | 0.23 ± 0.13 | 0.06 ± 0.03 | 0.13 ± 0.16 | −0.00 ± 0.04 | 0.51 ± 0.27 | 0.12 ± 0.08 | 0.34 ± 0.20 | 0.06 ± 0.06 | 0.56 ± 0.27 | 0.15 ± 0.09 |
| Depression | 0.01 ± 0.13 | 0.02 ± 0.03 | −0.05 ± 0.16 | −0.02 ± 0.05 | −0.03 ± 0.24 | −0.04 ± 0.07 | −0.10 ± 0.21 | 0.05 ± 0.07 | 0.71 ± 0.31* | 0.09 ± 0.11 |
| Obesity | −0.27 ± 0.14 | 0.00 ± 0.04 | −0.28 ± 0.16 | −0.01 ± 0.05 | −0.61 ± 0.31 | −0.04 ± 0.09 | −0.31 ± 0.21 | 0.02 ± 0.06 | 0.09 ± 0.48 | −0.11 ± 0.16 |
| Cardiovascular disorders | 0.11 ± 0.17 | 0.03 ± 0.05 | −0.04 ± 0.26 | 0.02 ± 0.10 | −0.18 ± 0.34 | −0.00 ± 0.10 | 0.49 ± 0.31 | 0.11 ± 0.09 | 0.25 ± 0.32 | −0.01 ± 0.13 |
| Framingham Risk Score | −0.21 ± 0.17 | 0.00 ± 0.04 | −0.07 ± 0.21 | 0.04 ± 0.07 | −0.05 ± 0.26 | 0.06 ± 0.09 | −0.34 ± 0.23 | 0.01 ± 0.08 | −1.13 ± 0.31** | −0.24 ± 0.12* |
Baseline differences are mean ± standard error, between individuals with and without risk factors ± for Framingham risk score between low and high score. Slopes are linear mixed model coefficient ± standard error, relative to group without risk factors.
p<0.05,
p<0.01,
p<0.001 compared to group without risk factors, adjusted for age, gender, years of education and APOE ε4 status.
Abbreviations: A= amyloid, T=tau.
Results were fairly similar when CDR sum of boxes was used as the cognitive outcome measure (Supplementary Table 3). When using a cognitive composite score as the outcome measure, baseline results were also generally similar, except that we now found that obesity was associated with lower baseline cognitive composite scores in the A−T− and A+T− groups. Longitudinally, we found no significant associations between risk factors and decline on the cognitive composite score (Supplementary Table 4).
Posthoc, we tested whether results were different when instead of using t-tau or p-tau to define tau status, only t-tau or p-tau were used for the tau classifications. The outcomes of these analyses were similar to the main results.
Discussion
In a large cohort of cognitively normal older individuals we investigated associations of risk factors with AD biomarker profiles, longitudinal CSF biomarker changes, and cognition. Our main findings were: 1) Normal-to-low BMI (i.e. BMI ≦30) was associated with abnormal t-tau and p-tau at baseline; 2) Cardiovascular disorders occurred more frequently in individuals with abnormal amyloid and tau at baseline; 3) Hypertension and a higher FRS were associated with a faster increase in tau levels over time in individuals with abnormal amyloid and tau at baseline; and 4) In the A+T+ group, a higher FRS was associated with lower MMSE scores and a faster rate of decline, while depression was associated with better performance on the MMSE at baseline.
Considering baseline biomarker profiles, we found that a lower frequency of obesity occurred more frequently in preclinical AD (i.e. A+T+) and that the absence of obesity was associated with higher tau values. Although this is partly in line with previous literature suggesting that a decrease in BMI could be indicative of underlying AD pathology in late life [5, 9, 29-31], it also indicates that the absence of obesity is associated with tau and not with amyloid in our study population. This weight loss may also be induced by underlying metabolic or inflammatory changes associated tau accumulation [32, 33]. Nevertheless, validation of this finding in other populations and age groups is necessary. Furthermore, we found that cardiovascular disorders, like carotid artery stenosis and congestive heart failure, occurred more frequently in individuals with preclinical AD compared to individuals with normal AD biomarkers. This is compatible with studies showing that AD pathology and vascular disorders, and concomitant cerebral vascular pathology, often co-exist in late-onset AD [34, 35].
To our knowledge, this study is among the first to investigate the influence of risk factors on longitudinal change in CSF biomarker values in cognitively normal individuals. Moreover, as a single lot of assays for each analyte was used on a fully automated system, potential variability due to analytical procedures is minimized in the current data set [26]. The longitudinal biomarker analyses showed that both hypertension and a higher FRS were associated with a faster increase in tau concentrations over time, and this effect was driven by the individuals who already had amyloid and tau pathology. As blood pressure is a major contributor in the FRS [17], the associations with the FRS can partly be attributed to hypertension. These findings are in line with previous animal and neuropathological studies [36, 37] and partially overlap with a smaller clinical study showing that a change in blood pressure was associated with an increase in p-tau concentrations over time in older individuals with hypertension [12]. Yet, while our results and other previous studies identify hypertension as a contributor to neurodegeneration, results remain inconclusive about the effectiveness of hypertension treatment as an AD prevention strategy. In our study, use of antihypertensive treatment was part of the definition of hypertension, and 77% of individuals diagnosed with hypertension was using antihypertensive treatment at baseline. Despite this high percentage of antihypertensive treatment we still found effects of hypertension on neurodegeneration which could suggest that treatment was initiated too late in life or a more intense treatment is required to slow down the progression of AD [38, 39].
A high FRS was not only associated with changes in t-tau values over time but also with an increased rate of decline in MMSE scores and lower scores at baseline in individuals who already had amyloid and tau pathology. This may suggest that tau-related pathology is an important contributor to cognitive impairment [40, 41] and could be a potential mediator in the relationship between amyloid, vascular factors and cognitive decline [42]. Depression was associated with higher MMSE scores at baseline in the A+T+ group. Although this finding seems counterintuitive, it is consistent with results from our previous study in individuals with mild cognitive impairment (MCI; [9]) and others have shown that severity and trajectory of the depressive symptoms could temporarily impact cognitive performance in various stages of AD [43]. In addition, we found that the MMSE and CDR were more sensitive in detecting change in cognition over time compared to a cognitive composite score consisting of five cognitive measures. This observation may reflect the fact that the MMSE and CDR also measure functional status including orientation in time and place, which have been found to be sensitive to detect preclinical AD and cognitive decline [44, 45].
In general we found no associations between Aβ42 levels and vascular risk factors, suggesting separate pathophysiological amyloid and vascular pathways which both enhance neurodegeneration [42, 46]. Moreover, our findings suggest that vascular risk factors enhance neurodegeneration and increase cognitive decline only in individuals that already have abnormal amyloid and tau (A+T+) and not in those with only abnormal tau (A−T+), supporting the classical view of an Ab initiated cascade and not that of a nonlinear relationship between Ab and tau [14]. However, results could be different in younger populations as quadratic effects of Ab seem to be most pronounced in younger individuals [14].
Our study has several limitations that should be mentioned. First, data on risk factors and medication use were based on self or proxy-reported information which could have led to under or over reporting of risk factors. Second, we were unable to assess the influence of all 11 risk factors as the overall frequency was too low. This low overall frequency of risk factors may be due to baseline exclusion of individuals with a medical or psychiatric illness that could interfere with longitudinal follow-up or adversely impact cognition. Third, we only investigated relationships with CSF biomarkers, and imaging markers (i.e. amyloid PET or tau PET) could have led to different results. Fourth, when creating baseline biomarker profiles, we did not apply the newly proposed A/T/N criteria which differentiate between t-tau and p-tau status [47]. As p-tau and t-tau are highly correlated in our sample, the T/N discordant groups would be too small which would limit the statistical power of our analyses. Lastly, as this study included individuals who were willing to participate in biomarker studies, the frequencies of vascular risk and of AD biomarkers found in this sample are not directly comparable to those in the general population. The major strengths of our study include the relatively long clinical follow-up, the diverse spectrum of assessed risk factors and the unique data on longitudinal CSF measurements in a relatively large research cohort of healthy volunteers.
In conclusion, we found that in cognitively normal individuals with preclinical AD (i.e. with abnormal amyloid and tau levels) hypertension and a higher FRS were associated with a faster increase in CSF tau markers. In addition, a normal-to-low BMI later in life may be related to early AD given its association with increased tau levels. These data support the view that hypertension plays a critical role in the progression of AD, however future studies should disentangle how AD prevention strategies could benefit from early treatment and management of hypertension. In addition, our results show that factors, such as BMI and cholesterol, should be monitored from midlife onwards to detect early changes possibly related to AD.
Supplementary Material
HIGHLIGHTS.
Vascular risk factors were not associated with amyloid pathology
The absence of obesity was associated with higher CSF tau levels
Hypertension was associated with a faster increase in CSF tau levels
A high Framingham Risk Score was related to a rise in tau and cognitive decline
Risk factors enhanced neurodegeneration only in individuals with preclinical AD
RESEARCH IN CONTEXT.
Systematic review: Vascular factors have been associated with an increased risk of Alzheimer’s disease (AD). As longitudinal biomarker studies concerning this topic have been scarce, we investigated the associations between risk factors, longitudinal cerebrospinal fluid (CSF) biomarkers and cognition in cognitively normal individuals.
Interpretation: We showed that the absence of obesity, presence of hypertension and a high Framingham Risk score (FRS) were associated with an increase in CSF tau values, whereas risk factors were not associated with amyloid pathology. Depression was associated with better cognition, while high FRS was associated with lower cognitive scores and a faster decline.
Future directions: Our results demonstrate that vascular risk factors, in particular hypertension, may enhance neurodegeneration in preclinical AD. Future studies should disentangle how AD prevention strategies could benefit from treatment of these risk factors as the optimal time window and intensity for treatment is currently uncertain.
Acknowledgements:
We are especially grateful to the research volunteers at the Knight ADRC and their families for their participation.
Funding/Support: This work was funded by National Institute on Aging grants P50 AG05681 (J.C.M.), P01 AG03991 (J.C.M.), and P01 AG026276 (J.C.M.) and Alzheimer Nederland fellowship WE.15-2016.08 (I Bos).
Footnotes
Role of Funder/Support: The funders had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data: preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest Disclosures: S.E.S. has a family member with stock in Eli Lilly, which is developing drugs for Alzheimer disease. J.J.H. is on the advisory board and consults for both Biogen and Lundbeck A/S. J.C.M. has or is currently participating in clinical trials of anti-dementia drugs sponsored by Janssen Immunotherapy, Eli Lilly and Company, and Pfizer. He has served as a consultant for or has received speaking honoraria from Eisai, Esteve, Janssen Alzheimer Immunotherapy Program/Elan, GlaxoSmithKline, Novartis, and Pfizer. He receives research support from Eli Lilly/Avid Radiopharmaceuticals. A.M.F. has received research funding from Biogen, Fujirebio and Roche Diagnostics. She is a member of the scientific advisory boards for Roche, Genentech and AbbVie and also consults for Araclon/Griffols and DiamiR. All other authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer's disease. BMC Med. 2014;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012;9:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–8. [DOI] [PubMed] [Google Scholar]
- [4].Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. [DOI] [PubMed] [Google Scholar]
- [5].Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, et al. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. Jama. 2017;317:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff-Radford J, et al. Evaluation of Amyloid Protective Factors and Alzheimer Disease Neurodegeneration Protective Factors in Elderly Individuals. JAMA Neurol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Lowe VJ, Graff-Radford J, et al. Age, Vascular Health, and Alzheimer's disease Biomarkers in an Elderly Sample. Annals of neurology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, et al. Interactive Associations of Vascular Risk and beta-Amyloid Burden With Cognitive Decline in Clinically Normal Elderly Individuals: Findings From the Harvard Aging Brain Study. JAMA Neurol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bos I, Vos SJ, Frölich L, Kornhuber J, Wiltfang J, Maier W, et al. The frequency and influence of dementia risk factors in prodromal Alzheimer’s disease. Neurobiology of aging. 2017. [DOI] [PubMed] [Google Scholar]
- [10].Enache D, Solomon A, Cavallin L, Kareholt I, Kramberger MG, Aarsland D, et al. CAIDE Dementia Risk Score and biomarkers of neurodegeneration in memory clinic patients without dementia. Neurobiology of aging. 2016;42:124–31. [DOI] [PubMed] [Google Scholar]
- [11].Kemppainen N, Johansson J, Teuho J, Parkkola R, Joutsa J, Ngandu T, et al. Brain amyloid load and its associations with cognition and vascular risk factors in FINGER Study. Neurology. 2018;90:e206–e13. [DOI] [PubMed] [Google Scholar]
- [12].Glodzik L, Rusinek H, Pirraglia E, McHugh P, Tsui W, Williams S, et al. Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiology of aging. 2014;35:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lo RY, Jagust WJ, Alzheimer's Disease Neuroimaging I. Vascular burden and Alzheimer disease pathologic progression. Neurology. 2012;79:1349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim HJ, et al. The nonlinear relationship between cerebrospinal fluid Abeta42 and tau in preclinical Alzheimer's disease. PloS one. 2018;13:e0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Middelaar T, Hoevenaar-Blom MP, van Gool WA, Moll van Charante EP, van Dalen JW, Deckers K, et al. Modifiable dementia risk score to study heterogeneity in treatment effect of a dementia prevention trial: a post hoc analysis in the preDIVA trial using the LIBRA index. Alzheimer's research & therapy. 2018;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. [DOI] [PubMed] [Google Scholar]
- [17].D'Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. [DOI] [PubMed] [Google Scholar]
- [18].Coats M, Morris JC. Antecedent biomarkers of Alzheimer’s disease: the adult children study. Journal of geriatric psychiatry and neurology. 2005;18:242–4. [DOI] [PubMed] [Google Scholar]
- [19].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [20].Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [21].Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–3. [DOI] [PubMed] [Google Scholar]
- [22].Reitan R Trail-Making Test. Arizona: Reitan Neuropsychology Laboratory 1979.
- [23].Goodglass H, Kaplan E. The assessment of aphasia and related disorders. . Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- [24].Wechsler D Manual: Wechsler Adult Intelligence Scale - Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- [25].Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of neurology. 2006;59:512–9. [DOI] [PubMed] [Google Scholar]
- [26].Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pastor P, Roe CM, Villegas A, Bedoya G, Chakraverty S, Garcia G, et al. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Annals of neurology. 2003;54:163–9. [DOI] [PubMed] [Google Scholar]
- [28].Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society Series B (Methodological). 1995:289–300. [Google Scholar]
- [29].Muller S, Preische O, Sohrabi HR, Graber S, Jucker M, Dietzsch J, et al. Decreased body mass index in the preclinical stage of autosomal dominant Alzheimer's disease. Scientific reports. 2017;7:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Besser LM, Alosco ML, Ramirez Gomez L, Zhou XH, McKee AC, Stern RA, et al. Late-Life Vascular Risk Factors and Alzheimer Disease Neuropathology in Individuals with Normal Cognition. Journal of neuropathology and experimental neurology. 2016;75:955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vidoni ED, Townley RA, Honea RA, Burns JM. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77:1913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mahieux F, Couderc R, Fenelon G, Maachi M. [Relationships between weight loss and circulating cytokines in patients with Alzheimer's disease]. Psychologie & neuropsychiatrie du vieillissement. 2006;4:281–6. [PubMed] [Google Scholar]
- [34].Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease--lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. Journal of Alzheimer's disease : JAD. 2009;18:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiology of aging. 2000;21:57–62. [DOI] [PubMed] [Google Scholar]
- [37].Kruyer A, Soplop N, Strickland S, Norris EH. Chronic Hypertension Leads to Neurodegeneration in the TgSwDI Mouse Model of Alzheimer's Disease. Hypertension. 2015;66:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bosch J, O'Donnell M, Swaminathan B, Lonn EM, Sharma M, Dagenais G, et al. Effects of blood pressure and lipid lowering on cognition: Results from the HOPE-3 study. Neurology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. Journal of neuropathology and experimental neurology. 2012;71:362–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bejanin A, Schonhaut DR, La Joie R, Kramer JH, Baker SL, Sosa N, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain : a journal of neurology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bos I, Verhey FR, Ramakers I, Jacobs HIL, Soininen H, Freund-Levi Y, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimer's research & therapy. 2017;9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3:628–35. [DOI] [PubMed] [Google Scholar]
- [44].Allison SL, Fagan AM, Morris JC, Head D. Spatial Navigation in Preclinical Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2016;52:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Guerrero-Berroa E, Luo X, Schmeidler J, Rapp MA, Dahlman K, Grossman HT, et al. The MMSE orientation for time domain is a strong predictor of subsequent cognitive decline in the elderly. International journal of geriatric psychiatry. 2009;24:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vemuri P, Knopman DS. The role of cerebrovascular disease when there is concomitant Alzheimer disease. Biochimica et biophysica acta. 2016;1862:952–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.