Case Report
A 72 year old right handed woman with a history of hypertension was brought to a local Emergency Department (ED) by her son approximately 6 hours after she fell at home. The day prior to ED arrival she had recurrent episodes of vertigo with nausea and vomiting associated with mild gait instability. She went to bed the night prior to presentation feeling unwell and reported that when she woke up in the morning she fell onto her right side. Upon further questioning, she reported that for the past two to three weeks she had been having dull, throbbing, intermittent bi-temporal headaches with occasional radiations of pain to her right ear. Over the same time period, chewing had become uncomfortable. She denied any additional neurological, ophthalmological, or constitutional symptoms.
On physical examination she was afebrile and in normal sinus rhythm with an initial blood pressure of 140/62. Her general exam was unremarkable. On neurological examination she was noted to have direction changing nystagmus with horizontal gaze, flattening of her right nasolabial fold, and mild dysarthria with normal facial sensation to light touch and temperature. Additionally, she had decreased sensation to temperature on her left arm and left leg, dysmetria in her right arm and right leg, and truncal ataxia. She was unable to ambulate without assistance due to gait instability.
She was treated for presumed posterior circulation infarct and received aspirin in the ED after an unrevealing computed tomography (CT) scan of the head. A few hours after ED arrival, magnetic resonance imaging (MRI) of the brain showed an acute right lateral medullary infarct (Figure 1A). After the MRI, she complained of new onset oscillopsia as well as worsening dysarthria. Due to clinical worsening, she was transferred to our tertiary referral center.
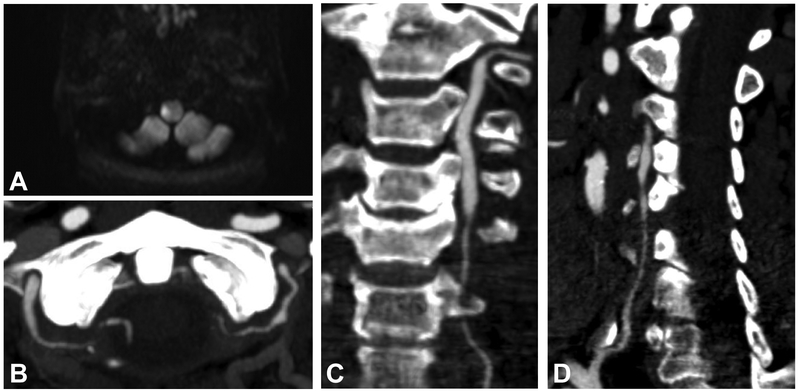
Figure 1. Computed tomography (CT) and magnetic resonance (MR) imaging.
Acute right lateral medullary infarct shown on MR diffusion weighted imaging sequence (A). On CT angiogram, both intradural and extradural segments of the vertebral arteries demonstrate multiple areas of irregular stenoses (B). The first and second parts of the left (C) and right (D) vertebral arteries are also severely stenosed; these vessel irregularities are consistent with the diagnosis of giant cell arteritis.
Upon arrival at our center, her clinical exam stabilized. Urgent CT angiogram demonstrated multifocal stenoses involving both vertebral arteries throughout their course with complete occlusion of the fourth segment of the right vertebral artery (Figure 1B-D). The basilar artery was patent. There was no new evidence of cerebral infarction on repeat MRI of the brain. Initial laboratory testing including complete blood counts and basic metabolic testing were unremarkable aside from thrombocytosis to 450,000/μL. Her erythrocyte sedimentation rate (ESR) was mildly elevated at 37 mm/h as was her C-reactive protein (CRP) at 1.7 mg/dL.
Our patient’s age, presenting symptoms of headache and jaw claudication, as well as her imaging findings were highly suggestive of an inflammatory vasculitic process. Therefore, high dose methylprednisolone was initiated a few hours after her arrival at our center and aspirin was continued. On day three of her hospitalization, a right temporal artery biopsy was performed. Pathological examination of her temporal artery showed lymphohistiocytic inflammation, giant cells, and intimal hyperplasia all consistent with giant cell arteritis (GCA) (Figure 2). By hospital day four, she had complete resolution of her headache symptoms but had persistent right sided dysmetria and mild dysarthria. At the time of hospital discharge, her headaches had completely resolved though her gait remained unstable requiring acute rehabilitation. At six month follow up, our patient reported feeling well overall and was able to ambulate with a cane.
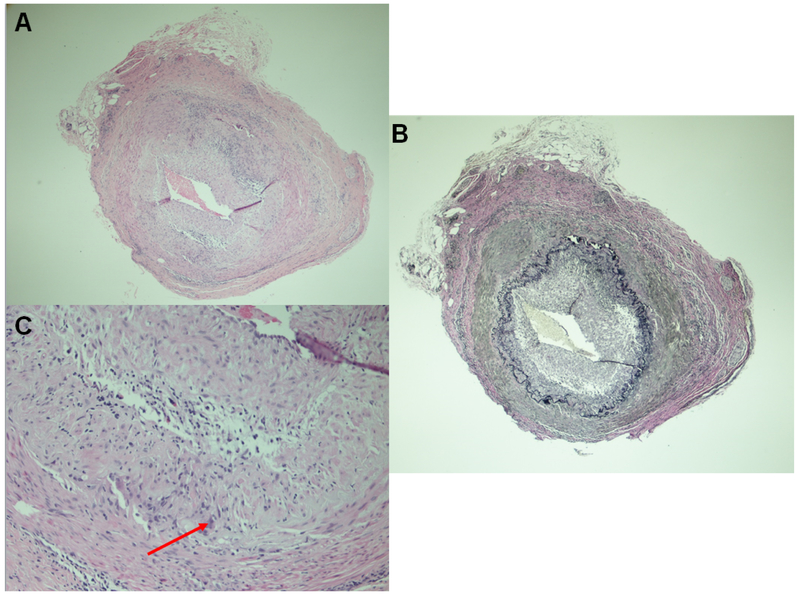
Figure 2. Temporal artery biopsy.
(A) Cross-sections of medium sized artery showing lymphohistiocytic granulomatous inflammation with few giant cells, low power. (B) The internal elastic lamina is focally discontinuous and fragmented (Elastic-Van Gieson stain). (C) Lymphohistiocytic granulomatous inflammation with few giant cells, high power (arrow points to a giant cell).
Case Discussion
Giant cell arteritis (GCA) is a granulomatous autoimmune vasculitis affecting medium to large sized arteries.1 In people over the age of 50, the incidence of GCA has been reported to be 10.9/100,000 cases annually making it the most common vasculitis in older patients.2 The mean age of GCA patients with ischemic complications is 71 years.3 Among patients with GCA, ischemic strokes occur in approximately 2–7% depending on the study, with strokes most frequently occurring in the vertebrobasilar territory.2,4
GCA is a disease that encompasses overlapping phenotypes including cranial symptoms (headache, temporal artery abnormalities, jaw claudication/tongue pain, visual disturbance/loss), extra-cranial symptoms (Raynaud’s phenomena, constitutional symptoms, limb claudication), and polymyalgia rheumatic (bilateral hip and shoulder pain, morning stiffness, peripheral arteritis). The systemic inflammatory symptoms seen in GCA, including fever and weight loss with elevated acute phase reactants (anemia, CRP, ESR, and circulating IL-6 levels), are thought to be related to inflammatory cytokine production by innate immune cells, particularly vascular dendritic cells and monocytes.5 This is in contrast to the vascular component of GCA which leads to cranial symptoms and consists of arterial wall infiltration by T-cells. These lymphocytes release IFNγ and interact with macrophages to form the characteristic multinucleated giant cells for which the disease was named.5 Macrophages and giant cells around the internal elastic lamina secrete growth factors which activate vascular smooth muscle cells. This activation leads to myofibroblast proliferation causing intimal hyperplasia and luminal narrowing.6 While it has been noted that the extradural as opposed to intradural portion of the cranial vasculature are more likely to be affected in GCA due to lower amounts of elastic tissue in the media and adventitia of the latter, 7 reasons for the higher rate of infarcts in the posterior as opposed to anterior circulation remain poorly understood.2, 4, 7
Ischemic strokes are more common in patients with the vascular component of GCA manifesting with cranial symptoms.1, 6 Patients with GCA who suffer an ischemic stroke are less likely to experience fever, rheumatological symptoms, and weight loss but are more likely to report jaw claudication, transient vision loss, and transient diplopia as compared to other GCA patients.8 A weak systemic inflammatory response with low acute phase reactants, including ESR and CRP, on laboratory testing has been strongly associated with a higher risk of severe ischemic complications among GCA patients.4, 8 One possible explanation for this is that GCA patients with ischemic symptoms present earlier in their disease and have not yet developed a strong inflammatory response. Alternatively, it is possible that a strong systemic inflammatory response may directly promote angiogenesis in ischemic tissue and thus prevent ischemic events.6 Older age, male sex, and the presence of traditional cerebrovascular risk factors, including hypertension, have also been associated with increased stroke risk in some studies.2
The clinical evaluation of elderly patients with posterior circulation ischemic strokes should include an evaluation for GCA particularly when there are associated complaints of headache, jaw claudication, and visual loss/disturbances, even in the absence of markers and manifestations of systemic inflammation. In a recent review of 47 patients with stroke due to GCA, headache was reported by nearly three fourths of patients.3 Non-invasive imaging can help distinguish between strokes due to GCA and other etiologies. For instance, affected vessels in GCA may show characteristic beading indicating underlying inflammation leading to constriction and ischemia.3 The American College of Rheumatology currently accepts five criteria for diagnosis of GCA: age of symptom onset ≥ 50, new onset headache (typically with jaw claudication), temporal artery abnormality, ESR ≥ 50 mm/hr, and positive artery biopsy. At the time of their publication, fulfillment of at least three criteria was associated with 93.5% sensitivity and 91.2% specificity.9 While temporal artery biopsy has long been considered the diagnostic gold standard for GCA, temporal or axillary artery ultrasound and high-resolution MRI are fairly sensitive (73% and 94%, respectively); the European League Against Rheumatism (EULAR) 2018 guidelines allow for these studies to aide in the diagnosis of GCA even in the absence of temporal artery biopsy.5 Suggestions for how the American College of Rheumatology criteria could be updated to address recent improvements in neuroimaging have been proposed and updated guidelines from the college are anticipated.1 Since exclusively cranial symptoms can be seen in stroke due to GCA without elevated acute phase reactants, these patients are unlikely to be diagnosed using current GCA diagnostic criteria until positive imaging or temporal artery biopsy is obtained.8
When clinical findings and diagnostic testing are highly suggestive of GCA, treatment should not be delayed. High-dose glucocorticoids are the mainstay initial treatment.1 To minimize morbidity associated with long-term glucocorticoid exposure, steroid-sparing agents can be used. The most common and widely used of these is methotrexate which has shown moderate efficacy.10 Tocilizumab, a biologic that inhibits IL-6, demonstrated efficacy in achieving sustained glucocorticoid-free remission among patients with GCA in a recent trial.5 Secondary stroke prevention with antiplatelet therapy is likely warranted in patients with stroke due to GCA though high-level evidence is lacking.11 Accurate and timely diagnosis of GCA among elderly patients who present with stroke is essential so that the appropriate treatment for secondary stroke prevention can be started. Future work to clarify the relationship between overlapping GCA phenotypes and ischemia is needed as well as a better understanding of how to achieve primary stroke prevention among patients with GCA. An important first step towards these goals is assuring that patients with strokes due to GCA are correctly diagnosed.
Supplementary Material
Take home points.
There should be a high suspicion for GCA in older patients with posterior circulation strokes and multi-focal vertebrobasilar stenoses, even in the absence of laboratory evidence of elevated acute phase reactants such as ESR and CRP
Patients with stroke due to GCA are more likely have cranial symptoms than extra-cranial symptoms or polymyalgia rheumatica
Early diagnosis and prompt initiation of immunosuppressive therapy is essential to reduce mortality and improve outcomes among GCA patients
Acknowledgments
Disclosures
Dr, Kirchoff-Torres receives an honorarium from MedLink Neurology. Dr. Liberman receives research support from NIH grant K23NS107643.
References
- 1.Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: Revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506–515 [DOI] [PubMed] [Google Scholar]
- 2.Samson M, Jacquin A, Audia S, Daubail B, Devilliers H, Petrella T, et al. Stroke associated with giant cell arteritis: A population-based study. J Neurol Neurosurg Psychiatry. 2015;86:216–221 [DOI] [PubMed] [Google Scholar]
- 3.Alsolaimani RS, Bhavsar SV, Khalidi NA, Pagnoux C, Mandzia JL, Tay K, et al. Severe intracranial involvement in giant cell arteritis: 5 cases and literature review. J Rheumatol. 2016;43:648–656 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, Pego-Reigosa R, Lopez-Diaz MJ, Vazquez-Trinanes MC, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore). 2009;88:227–235 [DOI] [PubMed] [Google Scholar]
- 5.Sammel AM, Fraser CL. Update on giant cell arteritis. Curr Opin Ophthalmol. 2018;29:520–527 [DOI] [PubMed] [Google Scholar]
- 6.van der Geest KSM, Sandovici M, van Sleen Y, Sanders JS, Bos NA, Abdulahad WH, et al. Review: What is the current evidence for disease subsets in giant cell arteritis? Arthritis Rheumatol. 2018;70:1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson IM, Russell RW. Arteries of the head and neck in giant cell arteritis. A pathological study to show the pattern of arterial involvement. Arch Neurol. 1972;27:378–391 [DOI] [PubMed] [Google Scholar]
- 8.Cid MC, Font C, Oristrell J, de la Sierra A, Coll-Vinent B, Lopez-Soto A, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 1998;41:26–32 [DOI] [PubMed] [Google Scholar]
- 9.Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The american college of rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128 [DOI] [PubMed] [Google Scholar]
- 10.Watelet B, Samson M, de Boysson H, Bienvenu B. Treatment of giant-cell arteritis, a literature review. Mod Rheumatol. 2017;27:747–754 [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Taboada VM, Lopez-Hoyos M, Narvaez J, Munoz-Cacho P. Effect of antiplatelet/anticoagulant therapy on severe ischemic complications in patients with giant cell arteritis: A cumulative meta-analysis. Autoimmun Rev. 2014;13:788–794 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.