Abstract
Most studies that focus on understanding how top-down knowledge influences behavior attempt to manipulate either ‘attention’ or ‘expectation’ and often use the terms interchangeably. However, having expectations about statistical regularities in the environment and the act of willfully allocating attention to a subset of relevant sensory inputs are logically distinct processes that could, in principle, rely on similar neural mechanisms and influence information processing at the same stages. In support of this framework, several recent studies attempted to isolate expectation from attention, and advanced the idea that expectation and attention both modulate early sensory processing. Here we argue that there is currently insufficient empirical evidence to support this conclusion, because previous studies have not fully isolated the effects of expectation and attention. Instead, most prior studies manipulated the relevance of different sensory features, and as a result, few existing findings speak directly to the potentially separable influences of expectation and attention on early sensory processing. Indeed, recent studies that attempt to more strictly isolate expectation and attention suggest that expectation has little influence on early sensory responses and primarily influences later ‘decisional’ stages of information processing.
Attention, expectation and perceptual inference
Over the past 40–50 years, a tremendous amount of effort has been spent trying to understand how prior knowledge shapes human information processing from the earliest stages of sensory analysis to decision-making to the execution of motor responses. Prior knowledge is a ‘top-down’ modulatory factor to the extent that priors reflect internal states and neural representations that could influence perception and behavior1. One important ‘top-down’ factor relates to knowledge about the probability that certain stimuli will occur in a specific context: a traffic light is likely to turn red after it turns yellow, a toaster is likely to be on top of a counter instead of under the kitchen sink, and so forth2,3. These top-down priors also code for more complex statistical regularities about stimulus identity and component features: a building is likely to have structures that are composed of straight lines rather than curvatures. Thus, expectations based on fore-knowledge can exert a powerful influence on object identification and scene understanding2,3, and a growing body of research focuses specifically on the impact of expectations on early sensory processing4–6.
Another type of top-down knowledge pertains to the relevance of specific stimuli in the context of current behavioral goals: when looking for your car in the parking lot, knowledge of its color, shape and size can be exploited to improve search efficiency by reducing the set of stimuli that must be interrogated. Critically, expectations about statistical regularities and knowledge about relevant features could have dissociable influences on information processing, as the probability that a stimulus will be encountered in a given context is not necessarily linked to its behavioral relevance4,5. Thus, following Summerfield and de Lange, we define expectation as the mechanism that operates based on the probability of stimulus occurrence, and we define attention as the mechanism that operates based on the behavioral relevance of different stimuli4,5.
The classic Posner cueing paradigm highlights the difficulties associated with dissociating the effects of expectation from the effects of attention. The task manipulates the probability that a target stimulus will appear on the left or the right of fixation, and participants have to press a button when they detect the onset of the peripheral light7. This manipulation alters the probability that the target stimulus will appear in one spatial location, which in turn leads to faster response times and more accurate responses. This seminal result, which has given rise to thousands of subsequent studies using variants of this basic paradigm, was originally interpreted as evidence for more efficient early sensory processing related to the selective deployment of spatial attention. However, later work demonstrated that these results, as well as results from more complex visual search tasks, can often be explained via an increase in the willingness of participants to indicate that they saw a target at the cued location, irrespective of how much sensory evidence was present to support a ‘yes’ response (i.e. the cue led to a change in decisional factors)8–11.
This debate about how to interpret what is perhaps the most widely used paradigm in the field of ‘selective attention’ illustrates two important points. First, this simple variant of a cueing paradigm conflates the theoretically distinct notions of expectation (where a stimulus is likely to appear) and attention to relevant features in the environment (which spatial position is likely to contain the task-relevant information). As a result, any influence of the cue on information processing is difficult to attribute to either factor or to some combination of the two. Second, the behavioral results can be explained either by a change in the sensitivity of early sensory processing or by a change in decisional factors. Importantly, similar issues arise in many other studies within the literature, as experimenters typically manipulate either the probability that a known target stimulus will appear or they manipulate information about which stimulus is most task-relevant. As a result, the field lacks a coherent framework that respects the potentially distinct influence of different types of top-down knowledge on sensory processing. In turn, the lack of a clear framework has important implications for canonical models of information processing such as the notion of perception as inference12,13 [see Box 1], as well as for long-standing debates about the cognitive penetrability of perception14–16.
Box 1 – Perception as Bayesian Inference.
In the domain of visual perception, Bayesian models frame inference as the product of the prior probability of a stimulus [denoted p(stimulus), or p(s)] and the probability of a pattern of neural responses (r) given that stimulus [referred to as a likelihood function, denoted p(r|s)]. The prior is a probability distribution over a stimulus space such as orientation or motion direction, and reflects the initial degree of belief in the current state of the world. On the other hand, the likelihood function reflects the probability that a given outcome – for example a pattern of responses over a population of feature-selective sensory neurons – will be observed for each possible stimulus value. The prior and the likelihood function are then combined to form a posterior distribution [denoted p(s|r)]. The peak of the posterior provides an estimate of the most likely stimulus, and the uncertainty associated with the posterior is determined by the precision of the prior and the likelihood functions.
Typically, the prior is thought to encode current expectations held by an observer, and these expectations can be based on a variety of factors such as previous experience in a given context or statistical regularities that are observed in natural scenes71. In contrast, other factors – such as attention induced neural gain27 – can increase the fidelity of a pattern of neural responses and bias the shape of the likelihood function. In this context, better understanding how expectation and attention operate on both early sensory and later decision-related processing will inform questions about how priors and likelihoods are implemented during perception.
The effects of attention and expectation on cortical information processing
Very few studies have independently manipulated expectation and attention to assess the impact of each factor on sensory processing. However, studies that attempt to focus on either expectation or attention have claimed that both factors modulate pre-stimulus neural responses17,18, stimulus-evoked responses19–27, and the efficiency of sensory read-out by putative decision mechanisms in parietal and frontal cortex28–31. For the sake of brevity, we focus here on response modulations in early sensory cortices, both before and after a stimulus has been presented. We first briefly review studies about the effects of selective attention on these responses, and then review recent studies that attempt to experimentally dissociate attention and expectation to assess the separability of their effects on early sensory processing.
The impact of attention to relevant features on early sensory processing
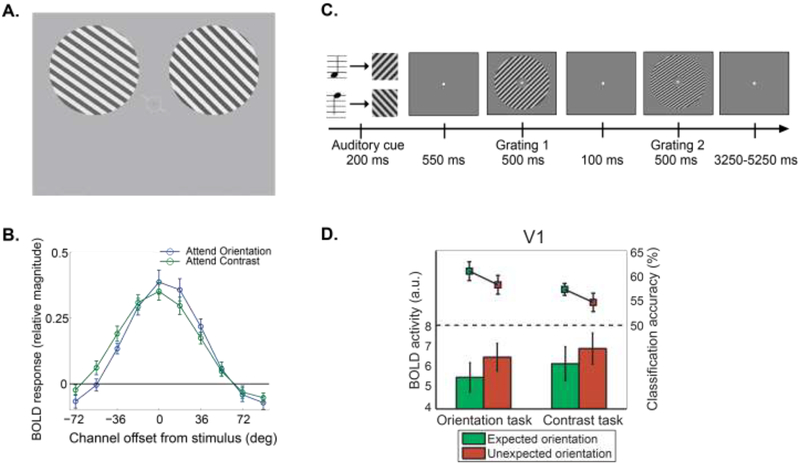
Many single-unit physiology32 and fMRI17 studies demonstrate that attending to relevant locations modulates neural responses in early visual cortex, even before a stimulus is presented33–35. Manipulating the relevance of spatial positions or low-level visual features also modulates the SNR and feature-selectivity of sensory-evoked responses that are associated with attended stimuli24,27,33,36–41. For example, work by Treue and colleagues demonstrated that attention increases the precision of motion-selective population response profiles in MT, and more recent fMRI work shows that these increases in feature-selectivity can occur even in the absence of an overall increase in the BOLD response19,20 (Figure 1A–B). Critically, at least some of these studies cued a behaviorally relevant feature, such as a location or a direction of motion, without inducing any expectation about the probability of the likely target feature19,36,39. Thus, according to the operational definitions of attention and expectation outlined above, both pre- and post-stimulus modulations appear to occur due to manipulations of behavioral relevance, independent of changes in event probabilities.
Figure 1.
Increased gain modulation of visual responses by attention and expectation. A. Schematic of the experiment design used in19. Participants fixated on the central cue, while attending to either the orientation or contrast of the gratings in alternating blocks of trials. The orientation of one grating always closely matched the oriented cue line presented at fixation, while the orientation of the remaining grating either matched or mismatched the orientation of the first grating by a small CW or CCW offset. Similarly, the contrast of the second grating either matched or mismatched the contrast of the first grating by a small contrast change. On attend-orientation blocks, the participants had to indicate whether the two gratings were rendered at the same orientation (match trials) or at different orientations (mismatch trials). On attend-contrast blocks, the participants had to ignore differences in orientation and to report whether the contrasts of the two gratings matched or did not match. Additionally, on orientation-mismatch trials, the central cue was presented in green or red to indicate either a CW or CCW rotational offset between the two gratings. B. The orientation selectivity of population responses in V169,70, as measured with fMRI, as participants were performing the orientation discrimination task (i.e., attend-orientation) or the contrast discrimination task (i.e., attend-contrast). Data shown here were shifted such that the 0° channel indicates the cued orientation and positive values on the x-axis indicate responses in orientation channels that were offset in the cued direction, whereas negative values indicate responses in orientation channels offset in the uncued direction. Despite similar overall amplitude of responses in attend-orientation and attend-contrast condition, attention shifts the orientation tuning towards the cued offset when participants attend to the orientation of a grating instead of to the contrast of the grating19. In contrast, responses in neural populations away from the attended feature are relatively muted. C. Schematic of the experiment design used in23. Each trial began with an auditory cue, which indicated (with 75% validity) the overall orientation of the subsequent gratings (~45° or ~135°). Following the cue, participants saw two consecutive gratings which differed slightly in terms of orientation, contrast, and spatial frequency. In separate blocks, participants judged whether the second grating rotated CW or CCW with respect to the first (i.e., orientation task); or whether the second grating had higher or lower contrast than the first (i.e., contrast task).
D. Expected orientations evoke less overall activity in V1 relative to unexpected orientations as measured with the BOLD response (bars). However, MVPA orientation classification accuracy of the grating orientation in V1 was higher for expected relative to unexpected orientations (line plots)23 (with permission from the authors).
The impact of expectation on early sensory processing
Initial reports regarding the impact of expectation on sensory-evoked responses demonstrated that large-scale cortical responses measured with fMRI were smaller than responses associated with unexpected stimuli21,22,25. This finding is consistent with generative models that frame perceptual inference as the iterative combination of priors with sensory evidence, because sensory evidence that is consistent with priors can support a rapid perceptual inference without the need for extensive processing. In turn, total cortical activity, as measured using methods such as fMRI, should be lower compared to situations where disparate priors and sensory evidence must be reconciled. In addition to attenuated BOLD responses, studies also suggest that expected stimuli evoke a more precise feature-selective pattern of responses in early visual cortex compared to response patterns associated with unexpected stimuli, similar to the modulations observed with feature-based attention19,42,43. Again, this observation is in line with the idea that consistent priors and sensory evidence should lead to a precise inference, even though overall cortical activity is reduced.
In one study, Kok et al.23 used fMRI and a task that cued participants on a trial-by-trial basis that an impending target was either going to be a 45° or a 135° oriented grating. The authors analyzed the pattern of responses across voxels in primary visual cortex (V1) using multivariate pattern classification analysis (MVPA) and demonstrated that expectation increased the separability between response patterns associated with each grating, even before stimulus onset (Figure 1C–D). MRI studies have also shown that expectation for a particular object category can bias pre-stimulus activation in face-selective regions of IT cortex44,45. Finally, spontaneous fluctuations in pre-stimulus fMRI signals in sub-regions of visual cortex predict the probability that a particular feature or object will be reported when viewing an ambiguous or weak sensory stimulus42,46. These spontaneous fluctuations may reflect endogenously mediated shifts in expectation, and they highlight the Bayesian notion that small shifts in expectation can have a large impact on perceptual inference when sensory evidence is weak or ambiguous5
Reconciling the effects of attention and expectation on early neural modulations in sensory cortices
Despite the apparent similarity of the early neural modulations attributed to selective attention and to changing expectations, studies that manipulate expectation typically have done so by explicitly providing prior information about the identity of an upcoming stimulus (e.g. a 45° or 135° grating, as in18,23). As a result, participants not only knew what target feature to expect, but they also knew what target feature was relevant to performing the behavioral task on each trial. A similar argument can be made about several other studies18,47–54, and based on the operational definitions of attention and expectation articulated in Summerfield and de Lange, the expectation cue can be expected to induce a shift of attention to the cued (expected) stimulus feature5. Given this consideration, any changes in behavior or associated modulations in early visual cortex were likely influenced to an unknown degree by both expectation and selective attention as opposed to expectation alone.
Recently, several studies have tried to more directly compare the effects of expectation and attention on behavior and on neural responses in visual cortex. One behavioral study used cues to manipulate the probability that a faint stimulus would be presented. These expectation cues increased both hits and false-alarm rates, whereas manipulating stimulus relevance (attention) improved the precision of sensory processing by selectively lowering false-alarm rates54. Using the reverse-correlation method and modelling, this study further suggested that the differential effects of attention and expectation could be accounted for by the fact that attention suppressed internal noise and thus increased precision while expectation biased the baseline activity of sensory processing in favor of the cued stimulus. In addition, a fMRI study found that attention increased the separability of response patterns associated with expected and unexpected stimuli in IT cortex51. However, even in these studies, the cueing scheme is set up so that expectation was manipulated by cueing relevant stimuli over a longer time frame whereas attention was cued on a trial-by-trial basis. So, while this manipulation leads to separate sources of top-down information that operate on different time scales, it is not entirely clear that one type of cue solely modulated expectation and the other attention as both cues provided information about what to expect and what features were more likely to be behaviorally relevant.
One way to isolate the effects of expectation from attention on sensory processing is to design an experiment where stimulus regularities are manipulated without using an explicit cue. For example, Rungratsameetaweemana et al. used a variant of an orientation discrimination task, where targets were either coherently oriented red or blue bars at 0° (horizontal) or 90° (vertical)55. This gave rise to four possible target types: red horizontal, red vertical, blue horizontal, and blue vertical. Each response button was associated with a specific conjunction of color and orientation. The probability that a specific color or orientation was a target feature was independently manipulated on a block-by-block basis such that within each block, targets were presented more frequently in one color (e.g., red; color expectation) or one orientation (e.g., vertical; orientation expectation). Thus, expectations about these sensory features (i.e., color and orientation) were induced through stimulus history without an explicit cue. By not using an explicit probability cue, this study minimized the possibility that participants shifted their attention to the expected stimulus features and thus the results are less likely to be influenced by selective attention. That said, it is possible that an implicitly induced expectation about a target feature could lead participants to allocate more attention towards the feature that is most likely to be presented56. However, even if participants noticed the expectation manipulation, knowledge about the most likely sensory feature would not provide information about the relevant behavioral response because targets were defined by the conjunction of color and orientation.
Using this behavioral paradigm allowed for a manipulation of expectation about two low-level sensory features (color and orientation) while measuring EEG markers that index early sensory processing and the accumulation of sensory evidence during decision-making (the visual negative potential, or VN, and the centroparietal positive potential or CPP, respectively). Importantly, the paradigm also included an independent manipulation of sensory evidence to validate these markers of sensory processing and to provide a point of comparison for any expectation-related modulations. The behavioral results revealed that expectations about likely sensory features improved the speed and accuracy of decision-making in a manner analogous to increasing the amount of available sensory evidence. However, while manipulations of sensory evidence increased the amplitude of the VN and the amplitude and slope of the CPP, expectations about sensory features had no impact on either of these components despite the robust effect of expectations on behavior (Figure 2A–B). Instead, expectation modulated the amplitude of posterior alpha and frontal theta oscillations, signals thought to index overall time-on-task and cognitive conflict. Together, these findings suggest that expectations about low-level sensory features, even when the expectations do not provide information about the behavioral relevance of sensory stimuli, primarily operate at post-perceptual stages of information processing.
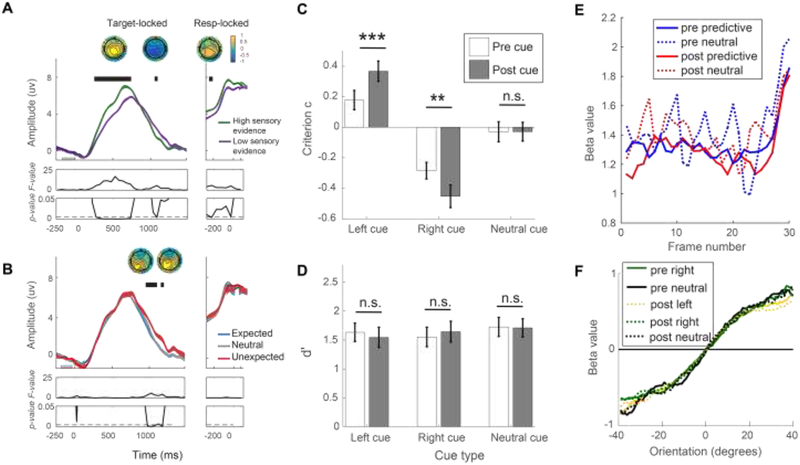
Figure 2.
Recent studies that isolate the effects of expectation from attention on sensory processing. A. The CPP is used as an EEG marker of the accumulation of sensory evidence during decision-making and its pre-peak amplitude is shown to be sensitive to manipulations that increase the amount of sensory evidence in the stimulus display. B. Despite the CPP being sensitive to increases in sensory evidence, expectation does not impact the pre-peak CPP amplitude. Instead, violations of expectation modulate the post-peak CPP amplitude which could be associated with later stages of processing after early sensory processing55. C. Behaviorally, predictive (left and right) cues led to criterion shift towards to the cued direction both when presented before and after the gratings. Critically, direct comparisons of the effects of pre- and post-cues showed that expectation induced via post-cues had a stronger effect on participants’ performance, which must be due to a shift in the decision criterion because the cue was presented after sensory processing of the stimulus was complete57. D. Both pre- and post-cues have comparable influence on stimulus sensitivity (d’)57. E. A reverse correlation analysis was performed to investigate whether pre- or post-cues affected participants’ information usage at any time throughout the 30 frames of stimulus presentation. Higher beta values indicate that participants placed more weight on the information provided by a particular stimulus frame. Temporal information usage for predictive (left and right) and neutral cues did not differ by cue time (pre- or post-cues), showing that expectation induced via pre- and post-cues had similar effects on temporal information usage throughout each trial. Note that noisier plots of neutral-cue condition are due to a smaller number of trials57. F. Feature information usage for predictive (left and right) and neutral cues also did not differ by cue time (pre- or post-cues), suggesting that pre- and post- cues have the same effect on feature-based information usage (reprinted from57 with permission from the authors).
Another recent study by Bang and Rahnev also converges on the idea that expectations do not impact early sensory processing but instead modulate decision criteria57. Participants performed a discrimination task where they judged whether the overall orientation bias in a series of gratings was tilted left (clockwise) or right (counterclockwise) from vertical. The grating stimuli were either preceded or followed by a predictive cue (i.e., pre-stimulus cue or a post-stimulus cue, respectively) indicating with 66.67% validity whether the overall orientation was more likely to be left or right of vertical. An additional condition was also included where neutral (uninformative) cues were presented. A pre-stimulus cue could impact both sensory signals and later decision processes, whereas a post-stimulus cue could only influence decision processes. By comparing the behavioral effects of pre-stimulus cues and post-stimulus cues, the study could assess the impact of expectation on early sensory processing and on decision-related criterion shifts.
Direct comparisons of pre- and post-cues demonstrated similar effects of both cue types on stimulus sensitivity (d’). However, post-cues induced a greater shift in decision criterion (c) compared to pre-cues (Figure 2C–D). To further examine how participants used cue-based-information in both the temporal and feature domains, the authors employed a reverse correlation method in which they compared the impact of predictive and neutral pre- and post-cues. The results demonstrated that pre-cueing and post-cueing exerted a similar influence on the use of temporal information and feature-specific information provided by predictive and neutral cues. Since the post-cues could only influence later decisional processes but not early sensory signals, the comparable effects of pre- and post-cues suggest that expectations primarily impact decision criteria rather than directly modulating the efficiency of sensory processing (Figure 2E–F). Together with the study by Rungratsameetaweemana et al, these results are more in line with classic theoretical frameworks such as signal detection theory (SDT) and suggest that knowledge about statistical regularities of the sensory environment primarily influence later cognitive operations related to response selection and execution58–62.
Conclusions
While we argue here that it is premature to assert that expectations about statistical regularities impact early sensory processing, there is substantial evidence that manipulations of expectation have a profound impact on behavior and on responses in higher-order parietal and frontal regions that are thought to be more directly involved in regulating decision-making and behavioral responses (i.e. saccades, reaching movements63,64). Saccade-selective neurons in frontal cortex show a pre-stimulus response bias as a function of target probability65, stimulus-evoked responses in the superior colliculus are mediated based on the certainty associated with a planned saccade66,67, and disrupting saccade-selective regions in human frontal cortex attenuates the impact of target probability on behavioral performance68. This evidence is consistent with the hypothesis that expectations can mediate priors to influence response selection. These findings are also in line with the idea that expected stimuli might exert a larger impact on sensorimotor decision mechanisms via changes in the ‘read-out’ of sensory-evoked responses rather than affecting the perceptual processing of the sensory signal itself. Moreover, as articulated in Summerfield and de Lange5, observers should exploit information about both statistical regularities and behavioral relevance to guide optimal decision making, as both sources of information should support the efficient processing of information to guide behavior. Future studies are needed to more thoroughly explore when and where expectation impacts information processing, and to orthogonally manipulate expectation and attention within the same paradigm to test for differences in temporal dynamics, modulations in different cortical areas, and influences on behavior.
Acknowledgements
Funding was provided by NIH R01-EY025872 (J.T.S.), the James S. McDonnell Foundation (J.T.S), and through mission funding from the U.S. Army Research Laboratory. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Laboratory or the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests.
References
- 1.Awh E, Belopolsky AV & Theeuwes J Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends Cogn. Sci 16, 437–443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biederman I, Glass AL & Stacy EW Searching for objects in real-world scenes. J. Exp. Psychol 97, 22–27 (1973). [DOI] [PubMed] [Google Scholar]
- 3.Biederman I, Mezzanotte RJ & Rabinowitz JC Scene perception: detecting and judging objects undergoing relational violations. Cogn Psychol 14, 143–177 (1982). [DOI] [PubMed] [Google Scholar]
- 4.Summerfield C & Egner T Expectation (and attention) in visual cognition. Trends Cogn Sci 13, 403–409 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Summerfield C & de Lange FP Expectation in perceptual decision making: neural and computational mechanisms. Nat. Rev. Neurosci 745–756 (2014). doi: 10.1038/nrn3838 [DOI] [PubMed] [Google Scholar]
- 6.Den Ouden HEM, Kok P & de Lange FP How prediction errors shape perception, attention, and motivation. Front. Psychol 3, 1–12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posner MI Orienting of attention. Q J Exp Psychol 32, 3–25 (1980). [DOI] [PubMed] [Google Scholar]
- 8.Palmer J, Ames CT & Lindsey DT Measuring the effect of attention on simple visual search. Journal of Experimental Psychology: Human Perception and Performance 19, 108–130 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Palmer J, Verghese P & Pavel M The psychophysics of visual search. Vision Res 40, 1227–1268 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Eckstein MP, Thomas JP, Palmer J & Shimozaki SS A signal detection model predicts the effects of set size on visual search accuracy for feature, conjunction, triple conjunction, and disjunction displays. Percept Psychophys 62, 425–451 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Eckstein MP et al. Rethinking human visual attention: Spatial cueing effects and optimality of decisions by honeybees, monkeys and humans. Vision Res 85, 5–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helmholtz H Handbook of Physiological Optics (Leopold Voss, 1867). [Google Scholar]
- 13.Dayan P, Hinton GE, Neal RM & Zemel RS The Helmholtz machine. Neural Comput 7, 889–904 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Firestone C & Scholl BJ ‘Top-Down’ Effects Where None Should Be Found: The El Greco Fallacy in Perception Research. Psychol. Sci 25, 38–46 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Firestone C & Scholl BJ Cognition does not affect perception: Evaluating the evidence for top-down effects. Behav. Brain Sci 39, (2015). [DOI] [PubMed] [Google Scholar]
- 16.Pylyshyn Z Is vision continuous with cognition? The case for cognitive impenetrability of visual perception. Behav. Brain Sci 22, 341–423 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Kastner S, Pinsk MA, De Weerd P, Desimone R & Ungerleider LG Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22, 751–761 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Kok P, Failing M & de Lange F Prior expectations evoke stimulus templates in the primary visual cortex. J Cog Neurosci 26, 1546–54 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Scolari M, Byers A & Serences JT Optimal deployment of attentional gain during fine discriminations. J. Neurosci 32, 7723–7733 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saproo S & Serences JT Attention improves transfer of motion information between V1 and MT. J. Neurosci 34, 3586–3596 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray SO, Kersten D, Olshausen BA, Schrater P & Woods DL Shape perception reduces activity in human primary visual cortex. Proc Natl Acad Sci U S A 99, 15164–15169 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray SO, Schrater P & Kersten D Perceptual grouping and the interactions between visual cortical areas. Neural Netw 17, 695–705 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Kok P, Jehee JFM & de Lange FP Less is more: expectation sharpens representations in the primary visual cortex. Neuron 75, 265–270 (2012). [DOI] [PubMed] [Google Scholar]
- 24.McAdams CJ & Maunsell JH Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron 23, 765–773 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Summerfield C, Trittschuh EH, Monti JM, Mesulam MM & Egner T Neural repetition suppression reflects fulfilled perceptual expectations. Nat. Neurosci 11, 1004–1006 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds JH & Heeger DJ The normalization model of attention. Neuron 61, 168–185 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JH & Chelazzi L Attentional modulation of visual processing. Annu. Rev. Neurosci 27, 611–647 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Pestilli F, Carrasco M, Heeger DJ & Gardner JL Attentional enhancement via selection and pooling of early sensory responses in human visual cortex. Neuron 72, 832–846 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Law C-T & Gold JI Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat. Neurosci 11, 505–513 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Law C-T & Gold JI Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nat. Neurosci 12, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao V, DeAngelis GC & Snyder LH Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J. Neurosci 32, 10063–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luck SJ, Chelazzi L, Hillyard SA & Desimone R Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77, 24–42 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Sylvester CM, Shulman GL, Jack AI & Corbetta M Anticipatory and stimulus-evoked blood oxygenation level-dependent modulations related to spatial attention reflect a common additive signal. J Neurosci 29, 10671–10682 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serences JT, Yantis S, Culberson A & Awh E Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol 92, 3538–3545 (2004). [DOI] [PubMed] [Google Scholar]
- 35.McMains SA, Fehd HM, Emmanouil TA & Kastner S Mechanisms of feature- and space-based attention: response modulation and baseline increases. J Neurophysiol 98, 2110–2121 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Trujillo JC & Treue S Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr. Biol 14, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Mitchell JF, Sundberg KA & Reynolds JH Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55, 131–141 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JF, Sundberg KA & Reynolds JH Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63, 879–888 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treue S & Maunsell JH Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci 19, 7591–7602 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jehee JF, Brady DK & Tong F Attention improves encoding of task-relevant features in the human visual cortex. J Neurosci 31, 8210–8219 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brouwer GJ & Heeger DJ Categorical clustering of the neural representation of color. J Neurosci 33, 15454–15465 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hesselmann G, Kell CA, Eger E & Kleinschmidt A Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc Natl Acad Sci U S A 105, 10984–10989 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Trujillo JC & Treue S Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol 14, 744–751 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Esterman M & Yantis S Perceptual expectation evokes category-selective cortical activity. Cereb Cortex 20, 1245–1253 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puri AM, Wojciulik E & Ranganath C Category expectation modulates baseline and stimulus-evoked activity in human inferotemporal cortex. Brain Res 1301, 89–99 (2009). [DOI] [PubMed] [Google Scholar]
- 46.Hesselmann G, Kell CA & Kleinschmidt A Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. J Neurosci 28, 14481–14485 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kok P, Mostert P & de Lange FP Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci 114, 201705652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kok P, van Lieshout LLF & de Lange FP Local expectation violations result in global activity gain in primary visual cortex. Sci. Rep 6, 37706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St. John-saaltink E, Utzerath C, Kok P & Lau HC Expectation suppression in early visual cortex depends on task set. PLoS One 10, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lange FP De, Rahnev, D. a, Donner, T. H. & Lau, H. Prestimulus oscillatory activity over motor cortex reflects perceptual expectations. J. Neurosci 33, 1400–1410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang J, Summerfield C & Egner T Attention sharpens the distinction between expected and unexpected percepts in the visual brain. J. Neurosci 33, 18438–47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Summerfield C & Egner T Feature-based attention and feature-based expectation. Trends Cogn. Sci 20, 401–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheadle S, Egner T, Wyart V, Wu C & Summerfield C Feature expectation heightens visual sensitivity during fine orientation discrimination. J. Vis 15, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyart V, Nobre AC & Summerfield C Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proc Natl Acad Sci U S A 109, 3593–3598 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rungratsameetaweemana N, Itthipuripat S, Salazar A & Serences JT Expectations do not alter early sensory processing during perceptual decision making. J. Neurosci 38, 5632–5648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geng JJ & Behrmann M Spatial probability as attentional cue. Percept. Psychophys 67, 1252–1268 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Bang JW & Rahnev D Stimulus expectation alters decision criterion but not sensory signal in perceptual decision making. Sci. Rep 7, 17072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wald A & Wolfowitz J Bayes solutions of sequential decision problems. Proc. Natl. Acad. Sci 35, 99–102 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DM Green JS, Green DM & Swets J. a. Signal detection theory and psychophysics. Society 1, 521 (1966). [Google Scholar]
- 60.Wolfe JM Visual Search. Attention, Perception, Psychophys 20, 13–73 (1998). [Google Scholar]
- 61.Berti S & Schroger E Distraction effects in vision: behavioral and event-related potential indices. Neuroreport 15, 665–669 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Macmillan N & Creelman D Detection Theory: A User’s Guide (2005).
- 63.Andersen RA & Buneo CA Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25, 189–220 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Gold JI & Shadlen MN The neural basis of decision making. Annu Rev Neurosci 30, 535–574 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Hanks TD, Mazurek ME, Kiani R, Hopp E & Shadlen MN Elapsed decision time affects the weighting of prior probability in a perceptual decision task. J. Neurosci 31, 6339–6352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basso MA & Wurtz RH Modulation of neuronal activity by target uncertainty. Nature 389, 66–69 (1997). [DOI] [PubMed] [Google Scholar]
- 67.Basso MA & Wurtz RH Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18, 7519–7534 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu CL et al. The location probability effects of saccade reaction times are modulated in the frontal eye fields but not in the supplementary eye field. Cereb Cortex 21, 1416–1425 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Brouwer GJ & Heeger DJ Decoding and reconstructing color from responses in human visual cortex. J Neurosci 29, 13992–14003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sprague TC, Saproo S & Serences JT Visual attention mitigates information loss in small- and large-scale neural codes. Trends Cogn Sci 19, 215–226 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geisler WS Visual perception and the statistical properties of natural scenes. Annu Rev Psychol 59, 167–192 (2008). [DOI] [PubMed] [Google Scholar]