Abstract
Low social integration is commonly described in acutely suicidal individuals. Neural mechanisms underlying low social integration are poorly understood in depressed and suicidal patients. We sought to characterize the neural response to low social integration in acutely suicidal patients. Adult depressed patients within three days of a suicide attempt (n= 10), depressed patients with suicidal ideation (n= 9), non-suicidal depressed patients (n= 15), and healthy controls (N= 18) were administered the Cyberball Game while undergoing functional magnetic resonance imaging. We used complementary functional connectivity and region of interest data analysis approaches. There were no group differences in functional connectivity within neural network involving the pain matrix, nor in insula neural activity or the insula during either social inclusion. Superior anterior insula activity exhibited an inverted U-shaped curve across the suicide risk spectrum during social inclusion. Superior insula activity during social inclusion correlated with depression severity and psychological pain. Dorsal anterior cingulate cortex activity during social exclusion correlated with physical pain severity. Neural responses in the anterior insula significantly correlated with depression severity and with psychological pain during social inclusion; whereas dACC activity significantly correlated with physical pain during social exclusion. Recent suicidal behavior seems associated with a distinct neural response to social exclusion independently of presence of depression or suicidal thoughts.
Keywords: suicide, depression, social exclusion, pain, fMRI, Cyberball
1. Introduction
Suicide, a growing public health problem in the US [1] and worldwide [33], is a complex and heterogeneous behavior associated with a variety of biological, psychological and social risk factors [47]. A pervasive element in suicide is overwhelming psychological or mental pain [53, 63], usually triggered by psychosocial crises, such as, romantic, familial, social, or work-related conflict [8, 20, 31, 56, 76]. Epidemiological studies have shown incidence of suicide to be negatively correlated with social integration [66, 67]. The relevance of the social milieu, and the reaction of the suicidal individual to it, is further emphasized by the current major theoretical frameworks about suicide. Thwarted belongness, defeat, entrapment, impaired connectedness are considered key elements in the progression to suicidal thoughts and actions [41, 48, 68].
Heightened psychological pain, also referred to as psychache [63], mental pain [51], psychic pain [74], or emotional pain, is considered as a sin equa non for suicidal behavior [63]. Elevated psychological pain is shared by both recent suicide attempters and depressed patients with current suicidal ideation [9, 10, 14, 21, 49]. It has been proposed that a diminished ability to tolerate psychological pain may precede suicidal ideation and behavior [45]. Furthermore, only the level of perceived psychological pain and recent social victimization differentiated recent suicide attempters and those with suicidal ideation [9].
A considerable body of evidence demonstrates an overlap between the subjective response to physical and psychological pain (in response to social loss or rejection) and their shared neural representation in the “pain matrix “corresponding to the dorsal anterior cingulate cortex (dACC) and anterior insula [22, 43], which shows marked overlap with the salience network [62]. However, other fMRI studies distinguish the neural processing representations of physical and psychological pain (26, 27). A large-scale functional neuroimaging study (n=114) showed that a neural pattern classifier for somatic pain did not similarly classify social pain (romantic rejection) in healthy subjects [69]. A caveat is that these studies focused on healthy participants, and it is unknown if physical pain and social exclusion share dissociable or common neural processing correlates in patients with depression or suicidal intent [26].
Accumulating evidence suggests that suicide is not only associated with a propensity to be involved in adversarial social situations but limited ability to manage them. Social exclusion allows for a wide array of experimental approaches, including the Cyberball game, a virtual ball tossing game that simulates social exclusion [72]. To date, only one study has focused on the neural substrates of social exclusion in patients with a lifetime history of suicide attempts [50]. In order to explore the underlying brain mechanisms of social exclusion during acute suicidality we compared functional magnetic resonance imaging (fMRI) responses of recent suicide attempters, suicidal ideators, non-suicidal depressed patients and healthy controls while playing the Cyberball game. A data analysis plan of game behavior used both region of interest (ROI) and functional connectivity approaches to examine group differences in neural responses during social exclusion. We focused on the two brain regions traditionally linked to the ‘pain matrix’: insula and dACC. We tested a hypothesis that acutely suicidal patients will show alterations in the neural processing correlates of psychological pain related to social rejection, and that this brain-behavior relationship exhibits individual differences.
2. Methods
2.1. Subjects
Four groups of adults of both sexes, ages 18–60 years, were recruited between March 2014 and March 2016. The specific characteristics of the four assembled groups included:
Recent Suicide Attempters (SA; N=15): depressed adults with a recent (within the previous three days) suicide attempt rated as being moderate–high in intent and lethality as defined by a score of ≥ 2 in the actual lethality/medical damage subscale of the Columbia Suicide Severity Rating Scale (C-SSRS) [57] (19 individuals were screened and 15 met inclusion/exclusion criteria and agreed to participate);
Suicidal Ideators (SI; N=13): depressed adults with current suicidal ideation and no suicidal behavior in the last six months (22 individuals were screened and 13 met inclusion/exclusion criteria and agreed to participate);
Non-Suicidal Depressed patients (NSD; N=18): depressed adults with no self-reported history of suicidal ideation or suicidal behavior in the last six months (all 18 screened individuals met inclusion/exclusion criteria); and
Healthy Controls (HC; N=21): age- and sex-matched adults without a history of mental illness or drug abuse.
Subjects were recruited consecutively from the psychiatric inpatient units of the University of Arkansas for Medical Sciences (UAMS) (SA and SI groups), the psychiatric outpatient clinics of the UAMS Psychiatric Research Institute (SI and NSD), and the local community (NSD and HC). All subjects in the SA, SI and NSD groups fulfilled Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for Major Depressive Episode and either Major Depressive Disorder, Bipolar Disorder or Depression not otherwise specified.
Study exclusion criteria were: a) inability to speak, read and write English; b) inability to provide informed consent; c) history of dementia, neurovascular or neurodegenerative conditions; d) physical disabilities that prohibit task performance, such as blindness or deafness; e) current or chronic pain of any kind; f) use of analgesic agents, either opioid or non-opioids within the last month; g) current or past history of non-suicidal self-harm, h) undergoing alcohol, benzodiazepine, opioid or barbiturate withdrawal; i) non-removable ferromagnetic objects; j) history of claustrophobia; and k) positive pregnancy test. The University of Arkansas for Medical Sciences Institutional Review Board approved all procedures. The study conformed to the principles of the Declaration of Helsinki. Participants were compensated for their participation in the study.
2.2. Procedures
After providing written informed consent, all participants underwent a study interview to obtain demographic data, psychiatric and medical history, behavioral self-ratings, and measurement of pressure pain threshold, followed by fMRI scanning. Psychiatric diagnosis was established with the Structural Clinical Interview for DSM-IV Diagnoses (SCID) [28]. The C-SSRS and Beck Depression Inventory (BDI-2) [6] were used to quantify suicidal ideation and behavior, as well as depression severity. Known risk factors associated with suicide were characterized with the Beck Anxiety Inventory (BAI) [5], Beck Hopelessness Scale (BHS) [4], Psychache Scale [32] and Childhood Trauma Questionnaire (CTQ) [7]. Question 16 of the BDI-2 was used as proxy for sleep health. Self-reported states of physical and psychological pain were measured with the Physical and Psychological Pain Scale [49]. The Trail Making Test was administered to provide a measure of attention, given that attention impairments can affect the perception of pain [16].
Somatic (pressure) pain threshold was measured using standard procedures [2]. Patients were placed supine, and a point five inches below the patella in the medial facet of the tibia was identified in both legs. Pressure was applied using a hand-held gauge with a 1 cm2 rubber tip (Wagner Instruments, Greenwich, CT, USA) alternately to the left and the right tibia for a total of three times per side. Participants were instructed to verbally report when their sensations change from pressure to pain or discomfort. A mean pressure pain threshold (Lb) was calculated from the six measurements. Measurement of pressure pain threshold was performed immediately before the introduction to lab members as potential confederates in the Cyberball game and the initiation of fMRI scanning.
2.2.1. Analysis of clinical data
Analysis of variance (ANOVA) was used to compare continuous variables. Significant results were followed by Tukey’s test. Chi square tests were used to compare categorical data. All tests were two-tailed. Adjusted p values are reported.
2.2.2. MRI acquisition
Imaging data were acquired using a Philips 3T Achieva X-series MRI scanner (Philips Healthcare, Eindhoven, The Netherlands). Anatomic images were acquired with a MPRAGE sequence (matrix = 256 × 256, 220 sagittal slices, TR/TE/FA = shortest/shortest/8°, final resolution =0.94 × 0.94 × 1 mm3 resolution). Functional images were acquired using a 32-channel head coil with the following EPI sequence parameters: TR/TE/FA = 2000 msec/30 msec/90°, FOV = 240 × 240 mm, matrix = 80 × 80, 37 oblique slices, ascending sequential slice acquisition, slice thickness = 2.5 mm with 0.5 mm gap, final resolution 3.0 × 3.0 × 3.0 mm3. Parameters for the 32-channel coil (at an angle 30 degrees from the AC-PC line) were selected to reduce orbitofrontal signal loss due to sinus artifact.
2.2.3. Social Exclusion Task
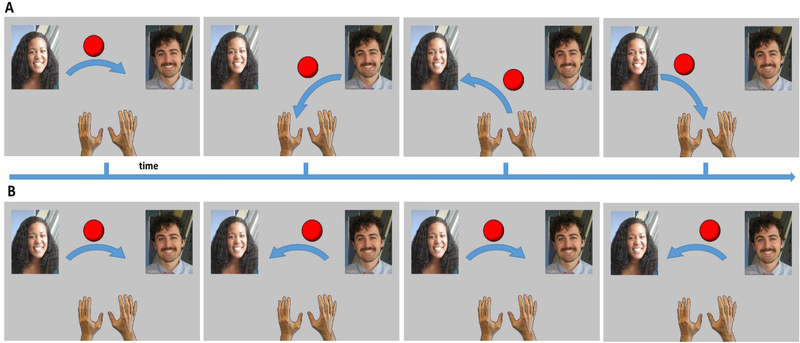
The Cyberball game is a virtual paradigm that simulates the social interactive experience of being excluded by others [25]. Participants were told that they would play a ball-tossing game via the internet with two other subjects in other scanners, in order to examine their coordinated neural response. In actuality, the ball-tossing behavior of these other ‘players’ were controlled by the computer; this aspect of deceit was disclosed at the completion of the MRI acquisitions. Before the scanning session participants were briefly introduced to two laboratory members (confederates) and told they would be playing with them. During scanning, a screen showed photographs of the individuals just met represented the other players, and a cartoon image of their own ‘hand’ that they used to express a ball-toss using a button-box. See Figure 1. Throughout the game, the ball was thrown back and forth among the three players, with the participant choosing the recipient of their own throws, and the throws of the other two ‘players’ determined by the pre-set program. Participants played two rounds of Cyberball: one round in which they were ‘included’ throughout the game, and one round in which they were ‘excluded’ by the other participants. Each round of Cyberball consisted of 60 ball toss trials. During the inclusion round, the confederate players were equally likely to throw the ball to the participant or the other player. During the exclusion round, the two confederate players stopped throwing the ball to the participant after 30 trials and threw the ball only to each other for the remainder of the round. Following the scan session, participants were asked: a) whether they believed the interaction was real, b) to identify their emotional reaction during social exclusion, and c) quantify the intensity of any negative reaction (0–100).
Figure 1.
Outline of social inclusion and social exclusion paradigms presented during the Cyberball game.
2.2.4. fMRI preprocessing
All MRI data preprocessing was conducted in AFNI [17] unless otherwise noted. Anatomic data underwent skull stripping, spatial normalization to the icbm452 brain atlas, and segmentation into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF) with FSL [37]. Functional imaging data underwent despiking; slice correction; deobliquing (to 3×3×3 mm3 voxels); motion correction (using the 10th timepoint); transformation to the spatially normalized anatomic image; regression of 6 motion parameters (lateral movement in x, y, and z; rotational movement in roll, pitch, or yaw) and regression of the mean timecourses of WM and CSF voxels; spatial smoothing with a 6-mm FWHM Gaussian kernel; and scaling to percent signal change. Using Matlab (The Mathworks, Inc.), timepoints with brief spikes in head motion were identified via the frame-wise displacement method [58]; any timepoint for which the sum of these differentials exceeded 0.5 in magnitude was excluded from the timeseries, as these sudden head movements introduce greatest fMRI artifact. Mean activity timecourses were calculated for each ROI by averaging the timeseries of voxels within the ROI. Correlation matrices were generated from these ROIs for each participant and scan, then underwent Fisher’s z-transformation to approximate linearity for subsequent regression.
2.2.5. ROI-level voxel-wise univariate analysis
The fMRI data were analyzed using MATLAB and AFNI in a two-stage, random effects procedure. In the first stage, task-related changes in blood oxygen dependent (BOLD) activity were modeled for each subject using generalized linear modeling (GLM) via AFNI’s 3dDeconvolve command with the standard canonical hemodynamic response function. The GLM included participant head motion as six nuisance parameters. The second stage consisted of one-sample t-tests (if contrasting a task condition versus rest) or two-sample t-tests (if contrasting two conditions) to test if task-related changes in BOLD contrast were consistent for the entire sample. Planned contrasts for Cyberball game results included 1) Inclusion condition; 2) Exclusion condition; and 3) Exclusion - Inclusion. We used a 200 functional ROI atlas derived from parcellation of task-based and resting-state fMRI data [36] to explore the relatedness of suicidality and social exclusion-related neural processing a priori ROIs encompassing dACC, anterior, middle, and posterior insula bilaterally (see supplementary Table 1 for list of ROIs and coordinates). We focused on the dACC and insula as the brain regions with the most supportive data supporting a potential regulatory roles of both physical pain and social exclusion [22, 26, 43, 62].
2.2.6. Regression analysis
For ROIs representing the anterior (L: 113, 33; R: 143, 68) and posterior (L: 165; R: 194) insula, and the dACC (95), timeseries were calculated for each subject and fMRI run by back-projecting specific ROIs spatial β-map to each image timepoint using generalized linear modeling (GLM) via AFNI’s 3dDeconvolve command with the standard canonical hemodynamic response function. The GLM included participant head motion parameters, thus generating weighted timeseries of BOLD activity for each subject and ROI. The GLM identified task-related changes in these weighted timeseries for each subject, and two-tailed one-sample t-tests determined if group-level engagement of ROIs during the contrasted conditions significantly differed from 0. The GLM had 3 contrasts (Inclusion, Exclusion, and Exclusion- Exclusion).
2.2.7. Multivariate fMRI data analysis
After preprocessing, group-level independent component analysis (ICA) of functional imaging data sets was conducted using MATLAB and the Group ICA of fMRI Toolbox (GIFT v1.3; http://mialab.mrn.org/software/), an approach for blind-source separation of a complex mixture of noise and signals into spatially and temporally distinct sources (independent components [ICs]) [15]. ICA was run using the Infomax algorithm to solve for 30 components (i.e. 30 networks of activation) using the Cyberball fMRI data. The following processes were used: back-reconstruction using GICA3, subject-specific principal component analysis using expectation maximization and stacked datasets, full storage of covariance matrix to double precision, usage of selective eigenvariate solvers, two-step data reduction with 60 principal components in the first step, and scaling to z-scores. ICA was repeated 20 times using the ICASSO algorithm to identify the most reliable and stable components across all iterations. The ICASSO stability indices (all iQ>0.95) indicated a reliable solution using 30 components.
Based on visual inspection and in comparison with the literature [59], we classified the independent components as follows. Eleven components represented noise (such as ventricle fluctuations or head motion) and were excluded to reduce Type I error. Eight components represented networks not typically associated with decision making and reward processing: four components representing sensorimotor systems, two represented the visual system, and two represented the cerebellum. The eleven remaining ICs represented networks typically associated with cognition and/or pain processing. Three components represented the limbic system, three represented the pain matrix (composed by the insula and anterior cingulate), two components represented the frontoparietal network, and three components represented the default-mode network. See Supplementary Table 1 and Supplementary Figure 1.
To test our study hypotheses, we selected the three components representing the pain matrix and omitted the remaining components from subsequent analyses to reduce Type I error. For these components, timeseries were calculated for each subject and fMRI run by back-projecting the ICA voxelwise spatial β-map to each image timepoint using general linear modeling (GLM) via AFNI’s 3dDeconvolve command with the standard canonical hemodynamic response function. GLM included participant head motion as 6 nuisance parameters, thus generating weighted timeseries of network activity for each subject and component. GLM identified task-related changes in these weighted timeseries for each subject, and two-tailed one-sample t-tests determined if group-level engagement of ICs for the planned contrasts significantly differed from 0. The GLM had 3 contrasts (Inclusion, Exclusion, and Exclusion-Inclusion).
Next, given the colinearity between some of our behavioral variables, we performed a multiple robust regression (using MATLAB’s robustfit command with the Huber weighting function and tuning parameter 1.345) to relate behavioral measures to task-related recruitment of ICs. We modeled age, severity of depression (BDI-2), suicidal ideation severity (C-SSRS), pressure pain threshold, physical and psychological pain (Physical and Psychological Pain Scale [49]), CTQ total score, which were regressed to the dependent variable of task-related brain network activity (i.e. the GLM betas derived from deconvolution of ICA timeseries described above). Robust regression was chosen over standard linear regression for its greater resiliency to the effects of outliers, which are common in neuroimaging data [70]. Each condition contrast underwent false discovery rate (FDR) correction for the number of components (n=3) or ROIs (n=7) being studied at q = 0.05 using the Matlab FDR program [29]. We report corrected p values.
3. Results
We recruited 88 participants aged 18–60 years, 38 men and 50 women. Twenty-one participants were excluded from the Cyberball brain imaging analysis due to loss of data (n=2), incomplete assessments (n=4), head motion artifact (n = 3) or for either not believing they were playing with real humans or not reporting negative emotional responses during the Cyberball game (n = 12; 5 Attempters, 4 Ideators, 4 Non-Suicidal Depressed, and 3 Healthy Controls). Demographics of the 52 subjects included in the imaging data analysis are provided in Table 1.
Table 1.
Clinical characteristics of depressed patients after a recent suicide attempt (Attempters), depressed patients with suicidal ideation (Ideators), non-suicidal depressed patients and healthy subjects.
| Attempter s |
Ideator s |
Non-Suicidal Depressed |
Healthy controls |
Adjuste d p |
|
|---|---|---|---|---|---|
| N | 10 | 9 | 15 | 18 | |
| Age | 34.2±10.9 | 30.1±9.4 | 42.7±14.7 | 33.0±11.3 | 0.08 |
| Gender (m) | 4 (40%) | 3 (33%) | 8 (53%) | 7 (39%) | 0.18 |
| Race | 0.64 | ||||
| White | 8 (80%) | 8 (89%) | 10 (67%) | 14 (78%) | |
| Black | 2 (20%) | 0 (0%) | 4 (27%) | 3 (17%) | |
| Other | 0 (0%) | 1 (11%) | 1 (7%) | 1 (6%) | |
| Marital status | 0.47 | ||||
| Single | 5 (50%) | 5 (56%) | 9 (60%) | 10 (56%) | |
| Long term relationship | 3 (30%) | 3 (33%) | 2 (13%) | 5 (28%) | |
| Divorced/widow | 2 (20%) | 1 (11%) | 4 (27%) | 3 (17%) | |
| Right handed | 10 (100%) | 7 (78%) | 12 (80%) | 15 (83%) | 0.87 |
| Education (years) | 12.8±1.3 | 13.7±2.4 | 13.6±1.9 | 15.6±4.6 | 0.08 |
| Functioning level | |||||
| Student or working/unemployed, disabled or retired | 5 (50%) | 7 (78%) | 8 (53%) | 15 (83%) | 0.067 |
| 5 (50%) | 2 (22%) | 7 (47%) | 3 (17%) | ||
| Clinical characteristics | |||||
| Diagnosis | 0.201 1 | ||||
| Major depression | 6 (60%) | 9 (100%) | 11 (73%) | 0 (0%) | |
| Bipolar disorder | 3 (30%) | 0 (0%) | 4 (27%) | 0 (0%) | |
| Depression NOS | 1 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Depression (BDI) | 34.9±16.7d | 39.8±7.2 c,d | 31.2±7.9 b,d | 1.5±1.7a,b,c | <0.001 |
| Anxiety (BAI) | 23.0±17.1d | 28.8±7.5d | 24.0±10.7d | 2.8±3.8 a,b,c | <0.001 |
| Hopelessness (BHS) | 10.3±1.6 d | 10.7±1.7 d | 10.1±2.2 d | 8.6±0.9 a,b,c | 0.005 |
| Individuals on medications | |||||
| Antidepressants | 4 (40%) | 5 (56%) | 7 (47%) | 0 (0%) | 0.001 |
| Mood stabilizers | 3 (30%) | 1 (11%) | 1 (7%) | 0 (0%) | |
| Antipsychotic drugs | 1 (10%) | 3 (33%) | 4 (27%) | 0 (0%) | |
| Benzodiazepines | 0 (0%) | 0 (0%) | 3 (20%) | 0 (0%) | |
| Sleep | 1.9±1.0d | 2.1±.7d | 1.9±.7d | .3±.5 a,b,c | <0.001 |
| Childhood trauma (CTQ) | |||||
| Emotional Abuse | 12.0±6.6 | 14.3±7.0 d | 10.8±5.6 | 7.2±3.5 b | 0.011 |
| Physical Abuse | 9.7±5.9 | 11.9±6.2 d | 9.9±4.0 | 6.3±1.7 b | 0.013 |
| Sexual Abuse | 12.5±7.6 | 9.0±8.4 | 7.9±5.9 | 7.1±5.0 | 0.199 |
| Emotional Neglect | 14.3±7.1 d | 13.2±5.0 d | 13.8±5.0 d | 7.0±2.9 a,b,c | <0.001 |
| Physical Neglect | 9.2±3.8 | 7.0±1.9 | 7.9±3.6 | 6.1±2.1 | 0.06 |
| Total | 57.3±10.6d | 54.3±10.4 d | 47.3±9.5 | 34.7±8.7 a,b | 0.005 |
| Trail Making A (s) | 25.0±7.7 | 24.0±6.6 | 24.5±7.2 | 20.2±5.7 | 0.202 |
| Suicide related measures | |||||
| Presence of suicidal ideation | 4 (40%) | 8 (89%) | 0 (0%) | 0 (0%) | <0.001 1 |
| Severity of suicidal ideation | 1.0±1.6c,d | 1.5±1.0 c,d | 0.0±0 a,b | 0.0±0.0 a,b | <0.001 |
| Lifetime suicide attempts | 10 (100%) | 4 (44%) | 6 (40%) | 0 (0%) | 0.006 1 |
| Number of suicide attempts | 2.8±2.2b,c,d | 0.4±0.5 a | 0.6±0.2 a | 0.0±0.0 a | <0.001 |
| Negative reaction to Social exclusion | 70.0 | 63.6 | 75.0 | 68.4 | 0.326 |
| Pain processing | |||||
| Pressure pain threshold | 13.7±4.5b,c,d | 9.8±4.6a | 10.2±5.1a | 8.8±3.2 a | 0.041 |
| Psychache | 42.4±18.2b, d | 53.9±8.4 a,c,d | 34.9±9.6 b,d | 15.5±3.7 a,b,c | <0.001 |
| Current physical pain | 1.8±2.7 | 0.8±1.2 | 1.8±2.5 | 0.5±1.0 | 0.175 |
| Usual physical pain in last 15 days | 2.9±2.9 d | 1.8±1.7 | 2.9±2.6 d | 0.4±0.9 a,c | 0.004 |
| Maximal physical pain in last 15 days | 4.1±3.9 d | 2.9±3.1 | 3.9±2.4 d | 0.8±1.0 a,c | 0.003 |
| Current psychological pain | 7.2±3.0 c,d | 6.7±2.6 c,d | 3.9±2.8 a,b,d | 0.2±0.9 a,b,c | <0.001 |
| Usual psychological pain in last 15 days | 7.3±3.1 c,d | 7.7±2.6 c,d | 4.9±2.1 a,b,d | 0.7±2.1 a,b,c | <0.001 |
| Maximal psychological pain in last 15 days | 8.2±3.2 c,d | 8.7±2.1 c,d | 6.3±2.6 a,b,d | 1.1±2.1 a,b,c | <0.001 |
| Suicidal Ideas | 5.0±3.8 c,d | 3.8±3.1 c,d | 0.8±1.9 a,b | 0.0±.0 a,b | <0.001 |
Mean ± standard deviation
ANOVA was performed for all demographic variables. GLM with correction for age, gender, race, marital status, education years and functioning level was performed to compare all clinical variables between the four groups, except for * Yates chi square
compared to Attempters group;
compared to Ideators group;
compared to depressed non-suicidal group;
compared to healthy control group;
Chi square between Attempters, Ideators and depressed non-suicidal groups
There were no significant differences in age, gender, race, marital status, level of functioning, handedness, or years of education between the groups. All depressed subjects (groups 1–3) reported more severe depression (F(3,50)=56.2, p<0.001), anxiety (F(3,50)=20.6, p<0.001), hopelessness (F(3,50)=5.3, p<0.001), and sleep abnormalities (F(3,50)=17.4, p<0.001) relative to the healthy control group.
Current (F(3,51)=22.9, p<0.001), usual (F(3,51)=29.5, p<0.001), an maximal self-rated psychological pain during the last 15 days (F(3,51)=42.7, p<0.001), and psychache, another measure of psychological pain (F(3,51)=33.6, p<0.001) were higher in the Attempter and Ideator groups compared with Non-Suicidal Depressed subjects, who in turn reported higher psychological pain than Healthy Controls. Pressure pain threshold was higher in the Attempter group compared with the three other groups (F(3,51)=2.9, p=0.041). Suicide Attempter and Non-Suicidal Depressed groups reported higher physical pain in the last 15 days than Healthy Controls (F(3,51)=5.2, p=0.003). There were no group differences in the subjective negative reaction to social exclusion in the Cyberball Game (F(3,51)=1.2, p=0.36).
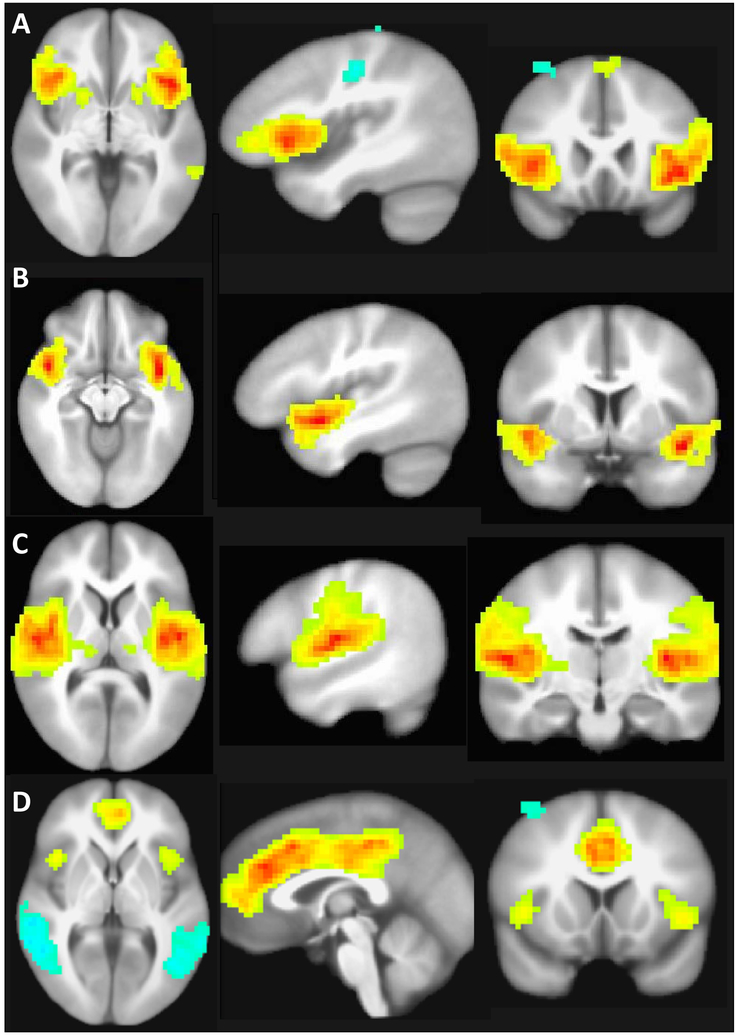
The three insula subregions ICA timecourses did not significantly differ with task during either Inclusion-Rest, Exclusion-Rest or Exclusion-Inclusion conditions. The correlations of insula-related IC neural activity with depression severity, suicidal ideation severity, psychological or physical pain were non-significant. The dACC ICA timecourses did not significantly differ with task during any condition. See Figure 2.
Figure 2.
Brain networks associated with activity in the insula and cingulate cortex. A. Neural network including the posterior insula. B. Neural network associated with anterior dorsal insula. C Neural network associated with anterior ventral insula. D. Dorsal anterior cingulate cortex
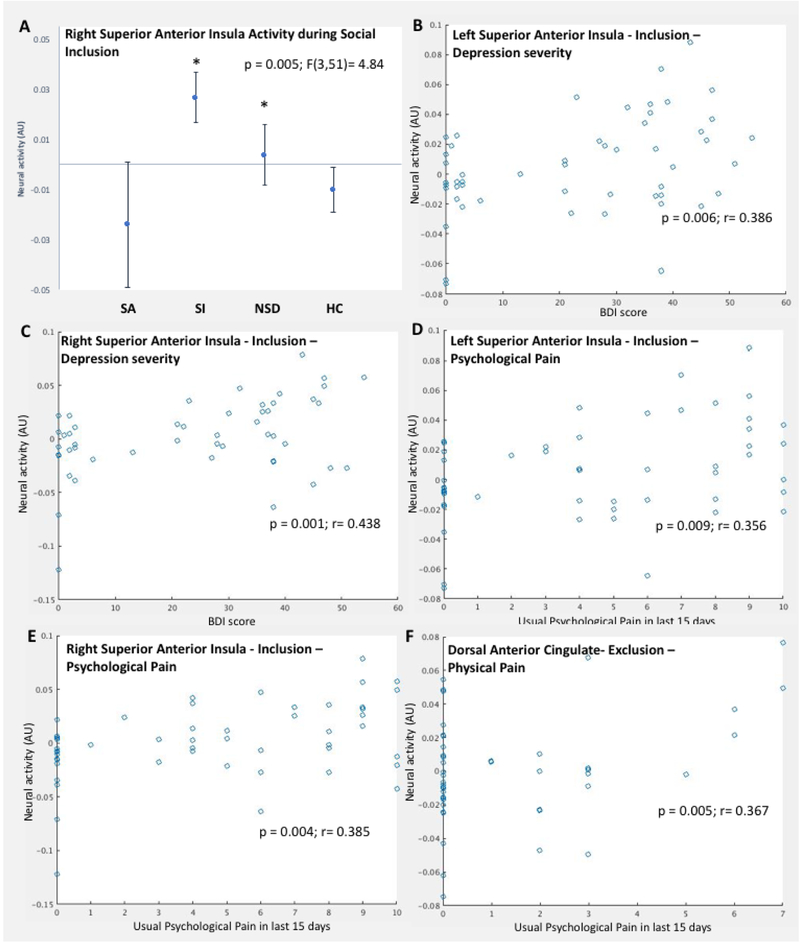
The right superior anterior insula ROI response (ROI #68) significantly differed between the four groups during Inclusion-Rest F(3,51)=4.84, p=0.005) (Figure 3a). Group differences for the middle and posterior insula ROI responses were non-significant or did not survive multiple comparison correction (lest superior anterior insula and inferior anterior insula). The bilateral superior anterior insula response during the Inclusion-Rest condition positively correlated with depression severity (right #68; r = 0.438, p = 0.001; left #33; r = 0.386, p = 0.006) (Figure 3b and 3c), and usual psychological pain in the last 15 days (right #68; r = 0.385, p = 0.004; left #33; r = 0.356, p = 0.009) (Figure 3d and 3e).
Figure 3.
Neural response to social exclusion or inclusion within the pain matrix and its correlation with clinical characteristics. Robust regression analysis outcomes indicated significant association between clinical and pain variables for neural response in: A. Neural responses in the right anterior superior insula ROI (#68) between the four groups during the Social Inclusion condition of the Cyberball game; B) The left superior anterior insula (#33) during Social Inclusion with depression severity (Beck Depression Inventory score); C. The right superior anterior insula (#68) during Social Inclusion with depression severity; D. The left superior anterior insula (#33) during Social Inclusion with usual psychological pain in the last 15 days; E. The right superior anterior insula (#68) during Social Inclusion with usual psychological pain in the last 15 days; and F. The dorsal anterior cingulate cortex (#95) during Social Exclusion with current physical pain. * p<0.01 compared with Suicide attempter group.
Dorsal anterior cingulate cortex (dACC) response (ROI #95) did not differ significantly between the four groups during any of the study conditions. However, dACC (ROI #95) response during social exclusion correlated with current physical pain (r = 0.367, p = 0.005). See Figure 3f. No significant correlations were found with psychache, suicidal ideation severity, pressure pain threshold, CTQ total score, or subjective negative reaction score.
4. Discussion
The present study sought to define the association between suicidal thoughts and actions on the activation and functional connectivity of major nodes in the human pain matrix response to social exclusion. The cross-sectional design further sought to dissociate the ubiquitous co-occurrence of depression from effects related to suicidal actions and thoughts. We report that despite no group differences in functional connectivity of the ‘pain matrix’ or the level of recruitment of the three insula or dACC ROIs related to social exclusion, the neural responses of the anterior insula during inclusion trials in suicide attempters exhibited significant group differences with depressed patients with and without suicidal ideation. Additionally, neural responses in the anterior insula correlated positively with depression severity and psychological pain, whereas, dACC activity correlated positively with current physical pain.
We add to the neurobiology of social intercourse developed mostly in healthy individuals [13, 24, 43, 69, 73], by examining severely depressed suicidal patients. We used social exclusion as a proxy for psychological pain. Even though psychological pain is intimately enmeshed with suicidal ideation and behavior [63], and most suicidal crises are triggered by some form of social conflict – usually rejection- social exclusion and psychological pain are not synonymous constructs. This is one of the initial studies to use the Cyberball game to recreate social interaction in suicidal or even depressed patients in combination with brain imaging [50]. Two previous studies had used variations of mental pain or social exclusion to examine patients with a history of suicide attempts. Reisch and collaborators examined eight moderately depressed women who had attempted suicide by overdose within the previous four weeks showing deactivation of frontal cortical areas (BA 46, 10 and 6) while listening to scripts about the circumstances that triggered their suicide attempts compared with neutral scripts [60]. Olie and collaborators showed decreased neural activity in the left insula and supramarginal gyrus during social exclusion in the Cyberball game in women with history of suicide attempts [50]. In a related approach, studying social reactivity, Jollant et al. presented euthymic individuals with a history of suicide attempts with angry faces and elicited increased responses in the right lateral orbitofrontal cortex (BA 47) and decreased responses in the right superior frontal gyrus (BA 6) compared with euthymic patients with a history of depression but no suicide attempts [39]. Recently, the same group described a negative correlation of N-Acetylaspartate concentration in the right dorsolateral prefrontal cortex with psychological pain, and a psychological pain-mediated positive correlation with suicidal ideation severity [40]. Depressed mood induction with an endotoxin challenge increased dACC and anterior insula responses during social exclusion only in female healthy subjects [23]. It is possible that the variability in brain response to social exclusion induced by the Cyberball game may be related to its condition of a mild-moderate social stressor, as evidenced by minimal hypothalamus-pituitary adrenal (HPA) axis activation [77]. The concurring decrease in insula reactivity in suicide attempters during social exclusion in Cyberball [50] may reflect a distinct transient deficit in the neural representation of social perception independent of depression or suicidal ideation. Perhaps this blunted insular responsivity may be associated with the abnormal emotional reactivity described in suicide attempters [3, 64] or to a diminished value of proprioception in decision making. Noteworthy, the significant correlation of anterior insular response with depression severity and psychological pain during social inclusion suggests that even during the pleasurable social interaction of social inclusion there is a degree of baseline anterior insular activity that correlates with underlying negative states, i.e, depression and psychological pain. These findings are suggestive of a general role of the insula in processing social stimuli [19].
The paralimbic anterior insula, with reciprocal connectivity with the limbic system [35, 46], is implicated in the affective processing of physical pain [18] and social exclusion [23]. Even though our subjects were selected for absence of current physical pain and for not taking any type of pain medications, we found that physical pain was associated with individual differences in the dACC response during social exclusion. We replicated previous findings of an increased threshold for experimentally-induced physical pain in recent suicide attempters, independent of depression [10, 52, 54, 55]. The correlation of dACC activity during social exclusion with physical pain may represent a possible mechanism for the decreased physical pain sensitivity found in individuals shortly after engaging in suicidal behavior. We did not explore potential underlying molecular mechanisms. Nonetheless, postmortem increased mu opioid receptor mRNA in the mesolimbic system in suicide completers [27, 34] and a promising clinical trial with buprenorphine [75] support the exploration of the opioid system in relation to suicidal ideation and behavior.
The phenomenon of less severe psychopathology (e.g. depression anxiety, suicidal ideation severity) has been observed in other studies with similar populations [11, 12]. Possible interpretations for this finding are that in suicide attempters the zenith of the suicidal process had already occurred within the last three days, whereas suicidal ideators were still in suicidal crisis mode and endorsed suicidal thoughts upon recruitment and assessed in this study. On these lines, a cathartic effect of suicide attempts had been described previously as approximately 50% report resolution of suicidal ideation shortly after a suicide attempt [61].
The strength of study inferences is clearly limited by aspects of its design. We employed a cross-sectional design for which the four groups had relatively small sample sizes. A clear view of the role of insular and dACC activity during inclusion and exclusion conditions may be obscured by the wide interindividual variability in our sample, which may be related to its limited size. We used an ROI approach of the the anterior insula and dACC, brain regions involved in the pain matrix to circumvent the limited sampe size. Social exclusion induced by the Cyberball game has been considered a mild-moderate social stressor, as evidenced by limited HPA axis activation [77]. It is possible that the ongoing intense social contact as part of inpatient milieu treatment may influence subjects’ response during the Cyberball game. Additionally, the experimental social rejection by unknown individuals during Cyberball game may be too removed from real life social rejection or conflict by close friends or family. The available confederates were a white male and a black female. We did not ask for specific differential response to these two confederates, nor quantify sensitivity to social rejection, which may affect the subjective and neural response to social rejection. An inherent limitation to all studies hoping to understand the biology of suicide is that individuals who survive a suicide attempt may differ from those that complete suicide. Three days post-attempt may be too long a delay to capture the state of mind and the neurobiology of an individual during the attempt itself. Our study did not include subjects whose suicide attempts or subsequent treatment prevented them from being able to consent or be MRI scanned. Even though we controlled for use of antidepressants and other psycotropic medications, this strategy is less optimal than studying medication-free individuals (Table 1). The influence of psychotropic medications on social rejection is still formally to be tested. The study did not measure biological markers relevant to pain regulation or stress response, such as enkephalins or cortisol. Finally, Axis II pathology was not systematically measured, however we excluded subjects with nonsuicidal self harm.
The current study has several strengths. To our knowledge this the first brain imaging examination of social inclusion and exclusion in individuals within three days of a suicide attempt. Further, contrasting suicide attempters with suicidal ideation and non-suicidal depressed controls is designed to factor out the ubiquitous influence of depression. We controlled for factors that may influence pain processing such as depression, anxiety, severity of stress, sleep and attention abnormalities, history of self-harm, diagnosis, childhood trauma, substance use, and psychotropic medications. Lastly, in order to avoid confounding factors affecting pain regulation, individuals currently experiencing any type of pain [30], opioid use [65] or non-suicidal self-harm [42] were excluded.
The clinical relevance of social exclusion in suicide is highlighted by the fact that most suicidal crises are triggered by psychosocial conflict. Suicidal individuals show deficits in decision making in social contexts [38] and deficits in verbal communication [71]. Impairment of coping skills in adverse social situations is considered a key element in the progression to suicidal behavior by some of the major suicide theoretical frameworks, i.e. the interpersonal theory of suicide [68], integrated motivational volitional model [48], and the three step theory [41]. Furthermore, improving interpersonal effectiveness is one of the pillars of Dialectical Behavioral Therapy, proven effective in reducing suicide-related events [44].
The decrease in insula reactivity in suicide attempters during social exclusion may reflect a distinct transient deficit in the neural representation of social perception independent of depression or suicidal ideation. The dACC activity correlation with physical pain may represent a possible mechanism for the decreased physical pain sensitivity found in individuals shortly after engaging in suicidal behavior. Overall, our findings illustrate the complexity of physical and psychological pain processing during intricate social contexts in acutely suicidal patients. Addressing the struggles of patients at high risk for suicide in adverse social situations may provide valuable interventions for novel suicide prevention strategies.
Supplementary Material
Electronic supplementary Table 1. Neural activity within independent components identified through Independent Component Analysis and Regions of Interest during the Cyberball game in Suicide Attempters, Suicidal Ideators, non-suicidal depressed and healthy controls.
ICA analyses use data from all voxels. These clusters were arbitrarily thresholded at cluster>20 and p<.005 the aid visualization of the networks.
Supplementary Figure 1. Brain networks identified by independent component analysis (ICA) that were identified during the Cyberball game in Suicide Attempters, Suicidal Ideators, non-suicidal depressed and healthy controls. DMN default-mode network, CEN central executive network, LIM mesolimbic system network, SAL salience network.
Acknowledgments
We thank Ms. Laura Rakes for a critical review of this manuscript.
Role of funding source
This work was partially funded by the Clinician Scientist Program and the Medical Research Endowment Award of the University Arkansas for Medical Sciences and by the National Institute of Health Clinical and Translational Science Award program (UL1TR000039), (National Institute of General Medical Sciences P30 GM110702). No funding source had any role in study design, in the collection, analysis and interpretation of data, in the decision to submit the article for publication.
Footnotes
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.(2014) Centers for disease control and prevention, national center for injury prevention and control. Web-based injury statistics query and reporting system (wisqars). In:Centers for Disease Control and Prevention [Google Scholar]
- 2.Aweid O, Gallie R, Morrissey D, Crisp T, Maffulli N, Malliaras P, Padhiar N (2014) Medial tibial pain pressure threshold algometry in runners. Knee Surg Sports Traumatol Arthrosc 22:1549–1555 [DOI] [PubMed] [Google Scholar]
- 3.Ballard ED, Ionescu DF, Vande Voort JL, Slonena EE, Franco-Chaves JA, Zarate CA Jr., Grillon C (2014) Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. Journal of affective disorders 162:34–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck AT (1988) Beck hopelessness scale. In:The Psychological Corporation [Google Scholar]
- 5.Beck AT, Steer RA (1990) Bai, beck anxiety inventory : Manual. Psychological Corp. : Harcourt Brace Jovanovich, San Antonio [Google Scholar]
- 6.Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of beck depression inventories -ia and -ii in psychiatric outpatients. J Pers Assess 67:588–597 [DOI] [PubMed] [Google Scholar]
- 7.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994) Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136 [DOI] [PubMed] [Google Scholar]
- 8.Buitron V, Hill RM, Pettit JW, Green KL, Hatkevich C, Sharp C (2016) Interpersonal stress and suicidal ideation in adolescence: An indirect association through perceived burdensomeness toward others. Journal of affective disorders 190:143–149 [DOI] [PubMed] [Google Scholar]
- 9.Cáceda R, Durand D, Cortes E, Prendes S, Moskovciak TN, Harvey PD, Nemeroff CB (2014) Impulsive choice and psychological pain in acutely suicidal depressed patients. Psychosomatic Medicine 76:445–451 [DOI] [PubMed] [Google Scholar]
- 10.Cáceda R, Kordsmeier N, Golden LE, Gibbs HM, Delgado PL (2017) Differential processing of physical and psychological pain during acute suicidality. Psychotherapy and Psychosomatics 86 [DOI] [PubMed] [Google Scholar]
- 11.Caceda R, Kordsmeier NC, Golden E, Gibbs HM, Delgado PL (2017) Differential processing of physical and psychological pain during acute suicidality. Psychother Psychosom 86:116–118 [DOI] [PubMed] [Google Scholar]
- 12.Caceda R, Nemeroff CB, Harvey PD (2014) Towards an understanding of decision making in severe mental illness Psychosomatic Medicine 79:36–42 [DOI] [PubMed] [Google Scholar]
- 13.Cacioppo S, Frum C, Asp E, Weiss RM, Lewis JW, Cacioppo JT (2013) A quantitative meta-analysis of functional imaging studies of social rejection. Sci Rep 3:2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calati R, Laglaoui Bakhiyi C, Artero S, Ilgen M, Courtet P (2015) The impact of physical pain on suicidal thoughts and behaviors: Meta-analyses. J Psychiatr Res 71:16–32 [DOI] [PubMed] [Google Scholar]
- 15.Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001) A method for making group inferences from functional mri data using independent component analysis. Hum Brain Mapp 14:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan SC, Chan CC, Kwan AS, Ting KH, Chui TY (2012) Orienting attention modulates pain perception: An erp study. PloS one 7:e40215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox RW (1996) Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 29:162–173 [DOI] [PubMed] [Google Scholar]
- 18.Craig AD (2009) How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70 [DOI] [PubMed] [Google Scholar]
- 19.Dalgleish T, Walsh ND, Mobbs D, Schweizer S, van Harmelen AL, Dunn B, Dunn V, Goodyer I, Stretton J (2017) Social pain and social gain in the adolescent brain: A common neural circuitry underlying both positive and negative social evaluation. Scientific reports 7:42010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duberstein PR, Conwell Y, Caine ED (1993) Interpersonal stressors, substance abuse, and suicide. The Journal of nervous and mental disease 181:80–85 [DOI] [PubMed] [Google Scholar]
- 21.Ducasse D, Holden RR, Boyer L, Artero S, Calati R, Guillaume S, Courtet P, Olie E (2017) Psychological pain in suicidality: A meta-analysis. J Clin Psychiatry [DOI] [PubMed] [Google Scholar]
- 22.Eisenberger NI (2012) The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci 13:421–434 [DOI] [PubMed] [Google Scholar]
- 23.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2009) An fmri study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage 47:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD (2006) An experimental study of shared sensitivity to physical pain and social rejection. Pain 126:132–138 [DOI] [PubMed] [Google Scholar]
- 25.Eisenberger NI, Lieberman MD, Williams KD (2003) Does rejection hurt? An fmri study of social exclusion. Science 302:290–292 [DOI] [PubMed] [Google Scholar]
- 26.Eisenberger NI, Lieberman MD, Williams KD (2003) Does rejection hurt? An fmri study of social exclusion. Science 302:290–292 [DOI] [PubMed] [Google Scholar]
- 27.Escriba PV, Ozaita A, Garcia-Sevilla JA (2004) Increased mrna expression of alpha2aadrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 29:1512–1521 [DOI] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams BW (2002) Structured clinical interview for dsm-iv-tr axis i disorders, research version, patient edition. (scid-i/p). New York State Psychiatric Institute, New York [Google Scholar]
- 29.Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878 [DOI] [PubMed] [Google Scholar]
- 30.Giamberardino MA, Tana C, Costantini R (2014) Pain thresholds in women with chronic pelvic pain. Curr Opin Obstet Gynecol 26:253–259 [DOI] [PubMed] [Google Scholar]
- 31.Haggard-Grann U, Hallqvist J, Langstrom N, Moller J (2006) Short-term effects of psychiatric symptoms and interpersonal stressors on criminal violence--a case-crossover study. Social psychiatry and psychiatric epidemiology 41:532–540 [DOI] [PubMed] [Google Scholar]
- 32.Holden RR, Mehta K, Cunningham EJ, McLeod LD (2001) Development and preliminary validation of a scale of psychche. Can J Behav Sci 33:224–232 [Google Scholar]
- 33.Hoyert DL, Xu J (2012) Deaths: Preliminary data for 2011. Natl Vital Stat Rep 61:1–51 [PubMed] [Google Scholar]
- 34.Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE (1997) Prodynorphin mrna expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry 2:495–500 [DOI] [PubMed] [Google Scholar]
- 35.Jakab A, Molnar PP, Bogner P, Beres M, Berenyi EL (2012) Connectivity-based parcellation reveals interhemispheric differences in the insula. Brain topography 25:264–271 [DOI] [PubMed] [Google Scholar]
- 36.James GA, Hazaroglu O, Bush KA (2016) A human brain atlas derived via n-cut parcellation of resting-state and task-based fmri data. Magnetic resonance imaging 34:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) Fsl. Neuroimage 62:782–790 [DOI] [PubMed] [Google Scholar]
- 38.Jollant F, Guillaume S, Jaussent I, Castelnau D, Malafosse A, Courtet P (2007) Impaired decision-making in suicide attempters may increase the risk of problems in affective relationships. J Affect Disord 99:59–62 [DOI] [PubMed] [Google Scholar]
- 39.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Phillips ML (2008) Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry 165:740–748 [DOI] [PubMed] [Google Scholar]
- 40.Jollant F, Near J, Turecki G, Richard-Devantoy S (2017) Spectroscopy markers of suicidal risk and mental pain in depressed patients. Prog Neuro-Psychoph 73:64–71 [DOI] [PubMed] [Google Scholar]
- 41.Klonsky ED, May AM (2015) The three-step theory (3st): A new theory of suicide rooted in the “ideation-to-action” framework. International Journal of Cognitive Therapy 8:114–129 [Google Scholar]
- 42.Koenig J, Thayer JF, Kaess M (2016) A meta-analysis on pain sensitivity in self-injury. Psychological medicine FirstView:1–16 [DOI] [PubMed] [Google Scholar]
- 43.Kross E, Berman MG, Mischel W, Smith EE, Wager TD (2011) Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A 108:6270–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linehan MM (1993) Cognitive-behavioral treatment of borderline personality disorder. Guilford Press, New York [Google Scholar]
- 45.Meerwijk EL, Ford JM, Weiss SJ (2013) Suicidal crises because of diminishing tolerance to psychological pain. Brain Imaging and Behavior 7:245–247 [DOI] [PubMed] [Google Scholar]
- 46.Mufson EJ, Mesulam MM, Pandya DN (1981) Insular interconnections with the amygdala in the rhesus monkey. Neuroscience 6:1231–1248 [DOI] [PubMed] [Google Scholar]
- 47.Niculescu AB, Levey DF, Phalen PL, Le-Niculescu H, Dainton HD, Jain N, Belanger E, James A, George S, Weber H, Graham DL, Schweitzer R, Ladd TB, Learman R, Niculescu EM, Vanipenta NP, Khan FN, Mullen J, Shankar G, Cook S, Humbert C, Ballew A, Yard M, Gelbart T, Shekhar A, Schork NJ, Kurian SM, Sandusky GE, Salomon DR (2015) Understanding and predicting suicidality using a combined genomic and clinical risk assessment approach. Mol Psychiatry 20:1266–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor RC, Kirtley OJ (2018) The integrated motivational-volitional model of suicidal behaviour. Philos Trans R Soc Lond B Biol Sci 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olie E, Guillaume S, Jaussent I, Courtet P, Jollant F (2010) Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. J Affect Disord 120:226–230 [DOI] [PubMed] [Google Scholar]
- 50.Olie E, Jollant F, Deverdun J, de Champfleur NM, Cyprien F, Le Bars E, Mura T, Bonafe A, Courtet P (2017) The experience of social exclusion in women with a history of suicidal acts: A neuroimaging study. Sci Rep 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orbach I (2003) Mental pain and suicide. Isr J Psychiatry Relat Sci 40:191–201 [PubMed] [Google Scholar]
- 52.Orbach I, Mikulincer M, King R, Cohen D, Stein D (1997) Thresholds and tolerance of physical pain in suicidal and nonsuicidal adolescents. J Consult Clin Psychol 65:646–652 [DOI] [PubMed] [Google Scholar]
- 53.Orbach I, Mikulincer M, Sirota P, Gilboa-Schechtman E (2003) Mental pain: A multidimensional operationalization and definition. Suicide Life Threat Behav 33:219–230 [DOI] [PubMed] [Google Scholar]
- 54.Orbach I, Palgi Y, Stein D, Har-Even D, Lotem-Peleg M, Asherov J, Elizur A (1996) Tolerance for physical pain in suicidal subjects. Death Stud 20:327–341 [DOI] [PubMed] [Google Scholar]
- 55.Orbach I, Stein D, Palgi Y, Asherov J, Har-Even D, Elizur A (1996) Perception of physical pain in accident and suicide attempt patients: Self-preservation vs self-destruction. J Psychiatr Res 30:307–320 [DOI] [PubMed] [Google Scholar]
- 56.Pompili M, Innamorati M, Szanto K, Di Vittorio C, Conwell Y, Lester D, Tatarelli R, Girardi P, Amore M (2011) Life events as precipitants of suicide attempts among first-time suicide attempters, repeaters, and non-attempters. Psychiatry research 186:300–305 [DOI] [PubMed] [Google Scholar]
- 57.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ (2011) The columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Power JD, Schlaggar BL, Petersen SE (2015) Recent progress and outstanding issues in motion correction in resting state fmri. Neuroimage 105:536–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, Smith SM, Fox PT, Laird AR (2013) Ica model order selection of task co-activation networks. Front Neurosci 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reisch T, Seifritz E, Esposito F, Wiest R, Valach L, Michel K (2010) An fmri study on mental pain and suicidal behavior. J Affect Disord [DOI] [PubMed] [Google Scholar]
- 61.Rosen DH (1976) Suicide survivors: Psychotherapeutic implications of egocide. Suicide Life Threat Behav 6:209–215 [PubMed] [Google Scholar]
- 62.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shneidman ES (1993) Suicide as psychache. J Nerv Ment Dis 181:145–147 [DOI] [PubMed] [Google Scholar]
- 64.Smith PN, Cukrowicz KC, Poindexter EK, Hobson V, Cohen LM (2010) The acquired capability for suicide: A comparison of suicide attempters, suicide ideators, and non-suicidal controls. Depress Anxiety 27:871–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strigo IA, Matthews SC, Simmons AN (2013) Decreased frontal regulation during pain anticipation in unmedicated subjects with major depressive disorder. Transl Psychiatry 3:e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsai AC, Lucas M, Kawachi I (2015) Association between social integration and suicide among women in the united states. Jama Psychiatry 72:987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai AC, Lucas M, Sania A, Kim D, Kawachi I (2014) Social integration and suicide mortality among men: 24-year cohort study of us health professionals. Ann Intern Med 161:85-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr., (2010) The interpersonal theory of suicide. Psychol Rev 117:575–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E (2013) An fmri-based neurologic signature of physical pain. N Engl J Med 368:1388–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wager TD, Keller MC, Lacey SC, Jonides J (2005) Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage 26:99–113 [DOI] [PubMed] [Google Scholar]
- 71.Wasserman D, Tran Thi Thanh H, Pham Thi Minh D, Goldstein M, Nordenskiold A, Wasserman C (2008) Suicidal process, suicidal communication and psychosocial situation of young suicide attempters in a rural vietnamese community. World Psychiatry 7:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams KD, Cheung CK, Choi W (2000) Cyberostracism: Effects of being ignored over the internet. J Pers Soc Psychol 79:748–762 [DOI] [PubMed] [Google Scholar]
- 73.Woo CW, Koban L, Kross E, Lindquist MA, Banich MT, Ruzic L, Andrews-Hanna JR, Wager TD (2014) Separate neural representations for physical pain and social rejection. Nat Commun 5:5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yager J (2015) Addressing patients’ psychic pain. Am J Psychiatry 172:939–943 [DOI] [PubMed] [Google Scholar]
- 75.Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, Lotan A, Rigbi A, Panksepp J (2015) Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: A randomized controlled trial. Am J Psychiatry:appiajp201515040535 [DOI] [PubMed] [Google Scholar]
- 76.Zaroff CM, Wong HL, Ku L, Van Schalkwyk G (2014) Interpersonal stress, not depression or hopelessness, predicts suicidality in university students in macao. Australasian psychiatry : bulletin of Royal Australian and New Zealand College of Psychiatrists 22:127–131 [DOI] [PubMed] [Google Scholar]
- 77.Zoller C, Maroof P, Weik U, Deinzer R (2010) No effect of social exclusion on salivary cortisol secretion in women in a randomized controlled study. Psychoneuroendocrinology 35:1294–1298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary Table 1. Neural activity within independent components identified through Independent Component Analysis and Regions of Interest during the Cyberball game in Suicide Attempters, Suicidal Ideators, non-suicidal depressed and healthy controls.
ICA analyses use data from all voxels. These clusters were arbitrarily thresholded at cluster>20 and p<.005 the aid visualization of the networks.
Supplementary Figure 1. Brain networks identified by independent component analysis (ICA) that were identified during the Cyberball game in Suicide Attempters, Suicidal Ideators, non-suicidal depressed and healthy controls. DMN default-mode network, CEN central executive network, LIM mesolimbic system network, SAL salience network.