Abstract
Critical changes in adolescence involve brain cognitive maturation of inhibitory control processes that are essential for a myriad of adult functions. Cognitive control advances into adulthood as there is more flexible integration of component processes, including inhibitory control of conflicting information, overwriting inappropriate response tendencies, and amplifying relevant responses for accurate execution. Using a modified Stroop task with fMRI, we investigated the effects of age, sex, and puberty on brain functional correlates of cognitive and motor control in 87 boys and 91 girls across the adolescent age range. Results revealed dissociable brain systems for cognitive and motor control processes, whereby adolescents flexibly adapted neural responses to control demands. Specifically, when response repetitions facilitated planning-based action selection, frontoparietal-insular regions associated with cognitive control operations were less activated, whereas cortical-pallidal-cerebellar motor regions associated with motor skill acquisition, were more activated. Attenuated middle cingulate cortex activation occurred with older adolescent age for both motor control and cognitive control with automaticity from repetition learning. Sexual dimorphism for control operations occurred in extrastriate cortices involved in visuo-attentional selection: While boys enhanced extrastriate selection processes for motor control, girls activated these regions more for cognitive control. These sex differences were attenuated with more advanced pubertal stage. Together, our findings show that brain cognitive and motor control processes are segregated, demand-specific, more efficient in older adolescents, and differ between sexes relative to pubertal development. Our findings advance our understanding of how distributed brain activity and the neurodevelopment of automaticity enhances cognitive and motor control ability in adolescence.
Keywords: functional MRI, executive control, age and gender, puberty, automaticity of behavior
Increasingly sophisticated cognition, faster processing, enhanced learning efficiency, and the ability to exert self-control over behavior are key functions that mature throughout healthy adolescence. During this time, the brain undergoes major changes in structure (e.g., Breukelaar et al. 2016; Pfefferbaum et al. 2016; Zhao et al. 2015) and neural functioning (Marek et al. 2015; Squeglia et al. 2013; for a review see Stevens 2016). As the brain develops from early adolescence into adulthood, the prefrontal cortex exerts greater control over higher-order cognition (Adleman et al. 2002; Andrews-Hanna et al. 2011; Luciana et al. 2005; Schroeter et al. 2004), but also lags behind other brain regions in structural (Raznahan et al., 2014; Pfefferbaum et al., 2018) and functional maturation (Casey et al. 2005, 2008; Kerns et al. 2004; Simmonds et al., 2017). Sex differences in pubertal timing, with development occurring later in boys (Juraska and Willing, 2017), are implicated in later-maturing prefrontal cognitive control regions (Blakemore, 2008; Herting et al., 2015; Raznahen et al., 2014). For example, boys lag behind girls by approximately two years in the peak frontal and parietal gray-matter volume and show steeper developmental slopes in gray matter reduction and white matter increase (Giedd et al., 1999; Gogtay et al., 2004). The different trajectories of regional brain maturation may in part explain sex differences in the development of cognitive abilities and motor response inhibition (Gur et al., 1999; Rubia et al., 2013; Spielberg et al., 2015).
In the progression toward adulthood, the ability to exert higher-order cognitive control is a key process of flexible behaviors needed to adapt to changes in social roles, responsibilities and to meet long-term ‘adult’ goals (Crone and Dahl, 2012), especially in conflict situations where focusing on goals requires the inhibition of inappropriate responses (Chen et al., 2013). Cognitive control and response inhibition processes are measured widely with the Stroop task (Stroop, 1935; Carter et al. 1999; Fan et al. 2003; MacDonald et al. 2000; Salo et al. 2001). The Stroop effect is characterized by slowed responses to naming the ink color of incongruent words, e.g., the word RED printed in blue ink, compared to naming the ink color of congruent words, e.g., the word RED printed in red ink. Cognitive control mechanisms must engage to override the prepotent response to the word’s meaning. Half a century ago, this process was conceptualized as “an aspect of the functioning of the mature organism” and studied in healthy subjects ranging from 7 to 80 years of age (Comalli et al., 1962). These data showed greatest Stroop color-word interference in children (7–13 years old), less interference in adolescents (17–19 years old), least in adults (25–44 years old), and an increase in interference with older adult age (65–80 years). Thus, the ability to deal with conflicting information, to exert control and override automatic response tendencies for goal-directed behavior evolves dramatically during adolescence (Tyborowska et al. 2016) to enhance processing efficiency in adulthood (Kelly et al. 2009; Luna et al. 2015).
Several studies have investigated developmental changes in the functional activation pattern of brain regions to cognitive tasks (e.g., Cohen et al., 2014; Satterthwaite et al., 2013; Supekar and Menon, 2012; for a review see Luna et al., 2015). Studies targeting the role of frontal adolescent brain development for inhibitory control have reported both increased and decreased activation in prefrontal, anterior insular, anterior cingulate and dorsolateral prefrontal regions (Booth et al., 2003; Luna et al., 2001; Rubia et al., 2007; Tamm et al., 2002; Veroude et al., 2013). These conflicting findings may be due to differences in the temporal maturation of component frontal control processes tested with these cognitive tasks; the reconfiguration of limbic-insular-frontal (Christakou et al., 2011; Ladouceur et al., 2018) and frontal-basal gangliathalamic loops (Rubia et al., 2007) varies by age and pubertal stage. While maturation of limbic-insular-frontal circuitry is associated with the ability to exert inhibitory control over impulses (Dambacher et al., 2015; Luna et al., 2001; White et al. 2011), emotion (Tyborowska et al., 2016) and reward (Banich et al. 2007; Chambers et al. 2009; Schramm-Sapyta et al. 2009; van Duijvenvoorde, 2016), the maturation of frontal-basal ganglia-thalamic loops (Rubia et al., 2007) supports increases in the efficiency of planning-based action selection (Randerath et al., 2017) and automaticity of procedures (Beauchamp et al. 2003; Erickson et al. 2010; Leisman et al. 2014), which work together with fronto-cerebellar motor loops to store the most efficient representations of behavior (Ito 1993; Koziol et al. 2012). As repeated procedures become more automatic, they require less cognitive control (Gratton et al., 1992; Mayr et al., 2003; Purmann and Pollmann, 2015; Ullsperger at al., 2005) associated with reduced frontoparietal activity (Egner and Hirsch, 2005; Kerns et al., 2004; Larson et al., 2009). Thus, while the maturation of inhibitory control ability would be related to increased frontal activation, increased efficiency for motor response selection with repeated procedures would be associated with decreased frontoparietal activation and be equally part of the development of inhibitory control ability. In addition, sex-dimorphic activation patterns have been found for fronto-striatal and parietal activation during motor inhibition suggesting underlying sex differences in the functional maturation of these brain regions (Rubia et al., 2013).
Sex differences in brain structural and functional development depend in part on the timing of puberty and have been attributed to hormone effects (Hertling et al., 2014; Nguyen et al., 2016; Piekarski et al., 2017; Savic et al., 2017), although the literature on the critical timing and brain regions affected by hormonal changes is discrepant (Koolschijn et al., 2014). In particular, sexual differentiation in brain structural development of subcortical (Wierenga et al., 2018) and prefrontal-limbic regions with implications for executive functions (Nguyen et al., 2017) have been attributed to changes in testosterone levels. In contrast to research on brain structural development (Giedd et al. 2012), the investigation of sexual dimorphism in brain functional maturation, considered in the context of puberty, is nascent (Ala0rcón et al., 2014; Müller-Oehring et al. 2017; Satterthwaite et al. 2014, 2015; Scheinost et al. 2015).
Here, we aimed to address gaps in the role of sex differences in brain functional development of efficient cognitive and motor control specifically during the heterogeneous developmental phase of puberty. We investigated age-related neurofunctional correlates of component inhibitory control processes by systematically varying cognitive and motor control demands in a task-activated functional MRI study in youth spanning the adolescent age range (12–21 years). As pubertal changes may have sex-specific organizing effects on the brain beyond chronological age (Wierenga et al., 2018), we investigated brain activation to cognitive and motor control in relation to age, sex, and pubertal stage. We propose that successful adolescent development results in enhanced neurofunctional efficacy of control processes that is supported by the fine-tuning of cognitive and motor control functions and the enhancement of repetition-related efficiency of response selection procedures. Accordingly, we tested the hypothesis that, with older age, adolescents would invoke greater frontoparietal-insular activation during cognitive control, greater frontal-basal ganglia-thalamic and cerebellar activation during motor control, and reduced activation in both systems with repetition, which we would interpret as enhanced efficiency of control procedures. As pubertal development differs temporally between boys and girls (Alarcón et al., 2014), we further tested whether sexual dimorphism in brain activations consistent with maturation is related to pubertal development.
MATERIALS AND METHODS
Participants.
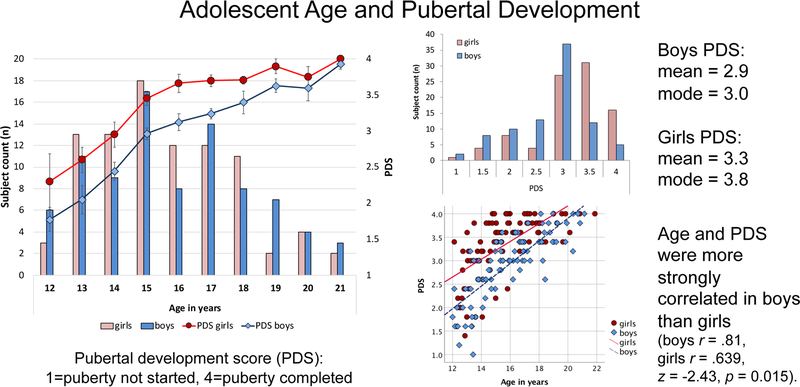
Participants were 87 male and 91 female youth, age 12 to 21 years, recruited by two data collection sites (SRI International and University of California San Diego) of the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA). All adolescents met the entry NCANDA criteria for no/low alcohol and drug use (Brown et al. 2015). Consent (for majors) and assent (for minors) were obtained according to the Declaration of Helsinki. Participants were characterized by age, sex, pubertal stage using the self-assessment Pubertal Development Scale (PDS; Petersen et al. 1988), socioeconomic status (SES) measured by the highest education of either parent (Akshoomoff et al. 2014), and reading and math performance on the Wide Range Achievement Test (WRAT; Wilkinson and Robertson 2006). Boys and girls did not differ in reading or math performance, parental SES, or age, but differed in pubertal status (Table 1). Higher puberty scores derived from the PDS have been associated higher testosterone levels (Huang et al., 2012; Tyborowska et al., 2016). Figure 1 shows that, as expected, puberty in boys was delayed in comparison with girls of the same age. Age and puberty were more strongly related in boys than girls (Figure 1).
Table 1.
Demographic characteristics of the adolescent study group: N=subject count; Mean ± SD (range)
| Boys | Girls | df | p | |
|---|---|---|---|---|
| N | 87 | 91 | 1 | |
| Age | 16±2.34 | 15.67±2.19 | 176 | .332 |
| Site (UCSD/SRI) | 69/18 | 65/26 | .223a | |
| PDS1 | 2.93±0.7 | 3.34±0.65 | 176 | .0001 |
| Parent SES | 92.06±16.05 | 90.36±15.15 | 171 | .476 |
| Parent Years of Education | 16.76±2.98 | 16.8±2.46 | 175 | .92 |
| WRAT2 | ||||
| Reading | 115.86±16.387 | 112.97±15.882 | 176 | .233 |
| Math | 115.63±15.611 | 113.32±16.015 | 176 | .331 |
| Stroop Match-to-Sample task | ||||
| Mean response time (ms) | 568.29±96.66 | 628.66±129.61 | 176 | .001 |
| Median response time (ms) | 544.776±93.82 | 604.56±125.94 | 176 | .001 |
| Stroop conflict: RTINC − RTCON (ms) | 14.88±25.44 | 12.08±24.79 | 176 | .458 |
| Stroop for RR | 20.82±31.26 | 15.62±35.81 | 176 | .305 |
| Stroop for RS | 6.18±40.13 | −0.8±38.21 | 176 | .236 |
| Response switching: RTRS − RTRR (ms) | 3.84±25.96 | 3.35±30.35 | 176 | .909 |
| RR for con (no conflict) | 12.53±39.77 | 16.23±38.14 | 176 | .528 |
| RS for inc (conflict) | −4.86±38.58 | −9.53±43.81 | 176 | .452 |
Pubertal Development Score (PDS): score ranges between 1=puberty not started and 4=puberty completed;
Wide Range Achievement Test (WRAT): Standard scores are reported with an expected mean±SD of 100±15;
Chi-square test
Abbreviations: df: degrees of freedom; p: p-value of statistical significance; SES: socioeconomic status; RS: response switching; RR: response repetition; inc: incongruent; con: congruent; RT: response time; ms: milliseconds.
Note: Despite same average ages of boys and girls, boys had lower pubertal development scores (PDS) than the girls. Age and PDS were more strongly correlated in boys than girls (age/PDS: boys r=0.81, girls r=.639, z=−2.43, p=0.0151). Boys scored lower on TIPI conscientiousness and higher on TIPI emotional stability than girls, the latter being associated with higher PDS scores in the male (r=.236, p=0.028), but not the female (r=−.172, p=0.102) (z=2.72, p=0.0065) group.
Figure 1. Age and pubertal stage distributions for girls and boys.
Despite similar chronological ages (left panel), boys had on average lower pubertal development scores (PDS) than girls (right upper panel) using the self-assessment Pubertal Development Scale (PDS; Petersen et al. 1988) (Table 1). Age and pubertal development were more strongly correlated in boys than girls (right lower panel).
Experimental design.
Task-activated functional MRI using the Stroop Match-to-Sample task.
Participants performed our Stroop Match-to-Sample task, specifically designed for the functional MRI environment (Schulte et al. 2011, 2012). Stimuli were created and presented with PsyScope software. The task required adolescents to match the color of a sample stimulus to the color of a Stroop target stimulus. The color sample was shown prior to the Stroop stimulus and either matched or did not match the font color of the Stroop target, which was either congruent (e.g., the word RED written in red font) or incongruent (e.g., the word RED written in blue font). The incongruent color-word condition is the Stroop condition (cognitive conflict) and the congruent color–word condition is the non-Stroop control condition. Participants were instructed to match the color of the sample to the font color of the Stroop target stimulus by pressing a YES-key for sample-target color matches and a NO-key for nonmatches.
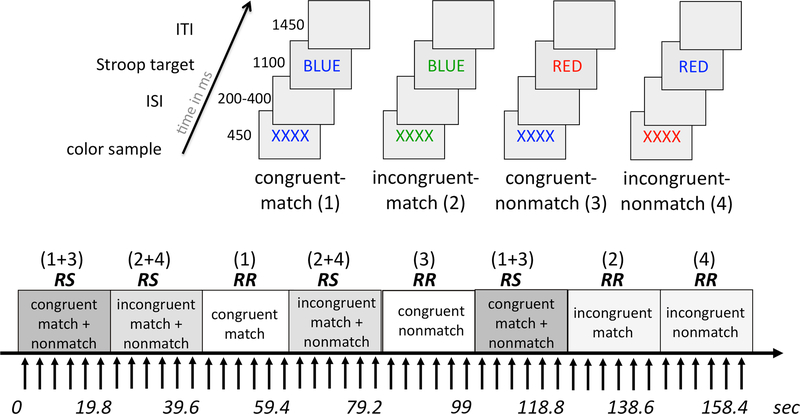
The Stroop Match-to-Sample paradigm has the advantage of using manual key-press responses enabling testing in the MRI environment. Furthermore, by combining Stroop and Match-to-Sample paradigms cognitive and motor response aspects of inhibitory control can be differentiated. In particular, cognitive conflict and motor response were systematically manipulated, i.e., cue and target front colors matched for half of the incongruent and half of the congruent trials, and did not match for the other half of trials (Figure 2, upper panel: YES-response for conditions congruent-match (1) and incongruent-match (3), NO-response for conditions congruent-nonmatch (3) and incongruent-nonmatch (4)). Accuracy and reaction time measures were recorded.
Figure 2. Stroop Match-to-Sample fMRI paradigm.
Subjects were asked to match the color of a sample to the font color of the Stroop target and press a YES-key for color matches and a NO-key for non-matches. Stroop targets were color words printed in a font color that was either congruent (e.g., the word BLUE printed in blue font) or incongruent (the word BLUE printed in green font) to the word’s meaning. The task had four conditions: congruent-match, incongruent-match, congruent-nonmatch, and incongruent nonmatch, which were presented in blocks of trials requiring response switching (RS), i.e., when match and nonmatch trials were mixed in a block, or response repetitions (RR), i.e., when either match or nonmatch trials were presented in a block.
The task contained 144 trials and was performed in an 8-minute run inside the MRI scanner. Each trial started with a string of Xs (i.e., the sample stimulus XXXX printed in red, green, or blue font) presented for 450 ms, followed by a variable inter-stimulus-interval of 200, 300, or 400 ms, and then the Stroop color-word stimulus (i.e., the target stimulus GREEN, RED, BLUE written in red, green, or blue font) was presented for 1100 ms. The inter-trial-interval was 1,450 ms; the total trial length was 3300 ± 100 ms (Figure 2). The task employed a blocked design containing 24 blocks of six trials each. Half of the blocks required response switches (RS), i.e., match and nonmatch trials were mixed in a block, and the other half of blocks required response repetitions (RR) and contained either match trials or nonmatch trials only (Figure 2, lower panel) (see also Schulte et al., 2012). Trials in RS and RR blocks were the same, but presented in a different order.
Stroop effects (cognitive conflict) were examined by comparing incongruent and congruent conditions (Koch and Brown, 1994; Melcher and Gruber, 2006; Pardo et al., 1990). The Stroop effect is characterized by interference from the more automatic word reading when naming the font color of an incongruent word, e.g., the word RED printed in blue ink resulting in slower responses relative to when the word’s meaning and font color are congruent (e.g., BLUE printed in blue font) (Stroop 1935; MacLeod 1991). Accordingly, cognitive control is required to override the prepotent response to the word’s meaning to match the word’s font color to the sample’s color and was measured herein as the difference in response time (RT) or activation pattern between incongruent and congruent trials (e.g., see conditions (2) and (4) versus (1) and (3); Figure 2).
Motor response control was characterized by switching between YES and NO responses (for cue-color matches and nonmatches, respectively) relative to when the same YES or NO response was required repeatedly. The motor control component was measured as the difference in RT or activation pattern between response switching (RS) and response repetition (RR) blocks (Figure 2). The strict-contrast design compares conditions that differ in one specific detail only, i.e., for cognitive control: the incongruent (inc) to the congruent (con) color-word content, and for motor control: the response switching (RS) to the response repetition (RR) blocks. The MR signal change between incongruent vs congruent trials thus reflects the cognitive process that is needed to overcome the semantic conflict elicited by the color-word incongruency (compared to congruency, i.e. the Stroop effect), because all other stimulation and response selection parameters for ‘inc’ and ‘con’ conditions are exactly the same. For the motor control contrast, where blocks of trials requiring response switches (RS) were compared against blocks of trials requiring response repetitions (RR), any activation difference represents this specific modulation of motor control, because all other parameters are identical for the RS and RR conditions. Moreover, the design enabled studying cognitive control in interaction with motor control demands (Stroop-RS) and automaticity with repeated procedures (Stroop-RR), as well as motor control without cognitive control (congruent words only: RSRRcon).
All participants performed a task run outside the scanner to ensure that the task instructions were understood and the task was practiced. The examiner reviewed the test instructions with the subject again in a short practice session (8 trials) before entering the scanner and again via the scanner intercom system before the onset of the task run. Subjects did not receive feedback on their performance. The start of the task was preceded by a countdown of 11 sec (i.e., 5 dummy TRs that were discarded prior to functional image analyses). The total task duration was 8 min and 6.2 sec.
MRI data acquisition.
Imaging was performed on 3.0-T General Electric Discovery MR750 scanners with 8-channel head coils from two sites (134 subjects from UCSD and 44 subjects from SRI). Whole-brain fMRI data were acquired with a T2*-weighted gradient echo spiral pulse sequence (TE=30ms; TR=2200ms; flip angle=79°; FOV=240; matrix=64×64; slice thickness=5mm; skip=0mm; in-plane resolution=3.75mm; 32 slices). Standard T1-weighted structural data were acquired with an inversion recovery-spoiled gradient recalled echo sequence (TE=1.932ms; TR=5.904ms; flip angle=11°; FOV=24cm; matrix=256×256; NEX=1; 146 slices; slice dimensions= 1.2×0.9375× 0.9375mm) for spatial co-registration.
MRI data analysis.
Functional imaging data from a total of 229 adolescents with no/low alcohol use were preprocessed and analyzed using the SPM12 software (Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). For each subject, 215 whole brain functional volumes were acquired. The functional images were realigned, subjected to motion correction, and coregistered to the T1-weighted structural images. All data were inspected for quality and movement artifacts based on SPM12 motion correction parameters and ArtRepair Software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html). The translational movement and rotational absolute motion of all images did not exceed 2 mm and 2 degrees. In addition, no more than 10% of total images within each subject exceeded frame-to-frame movement threshold (0.3 mm/TR) based on ArtRepair Software (see also Power et al. 2010). Data in 44 subjects did not meet these criteria and were excluded from analysis. An additional seven subjects had unusable behavioral fMRI task data. Images from the final sample of 178 subjects were normalized to MNI (Montreal Neurological Institute) space and smoothed with a Gaussian Kernel of 8mm full-width at half-maximum.
Individual models were calculated for each subject using a general linear model convolved with a canonical HRF without derivatives, implemented in SPM12. To remove slow signal drifts, a high-pass filter at 158.4 seconds was implemented in the individual models. The six estimated movement parameters were modeled as regressors. Four different conditions of the study design (Figure 2) were specified as congruent color-word trials in response repetition blocks (con RR), incongruent color-word trials in response repetition blocks (inc RR), congruent color-word trials in response switching blocks (con RS), and incongruent color-word trials in response switching blocks (inc RS). The onset of each block of trials was entered for each condition with a block duration of9 scans. Six blocks of trials were modeled for each condition. One image per contrast was computed for each subject for 5 contrasts of interest: Stroop (inc>con) total and for response switching (Stroop-RS: inc RS > con RS) and response repetition (Stroop-RR: inc RR > con RR), and response mode (RS>RR) total and for non-conflicting congruent (RSRR-con: con RS > con RR) block of trials. Testing for site revealed no significant differences in the activation maps between SRI and UCSD for Stroop (inc>con) and response mode (RS>RR) contrasts of interest.
Statistical analysis.
Group functional MR image analyses.
To investigate task-contrast-related regional activation pattern in the whole group, and sex- and age-related effects, a second level group analysis modeled sex (girls, boys) as a factor where the individual images for each contrast of interest were subjected to independent two-sample t-tests and age was modeled as a covariate. An explicit whole-brain mask implemented in SPM12 was applied to the model. Significance threshold was set at p=0.05 cluster-corrected for multiple comparisons using a peak voxel threshold of p=0.001 and a minimum cluster size of 103 contiguous voxels (in addition, see Monte Carlo simulation; afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) (Eklund et al. 2016). Age was modeled as a continuous variable in the second-level two-sample t-test design models, as implemented in SPM12, to identify voxels clusters significantly related to age for each contrast of interest, i.e., cognitive and motor control. For graphing purposes, BOLD signal change for the voxel clusters showing age effects were extracted using the matlab-based SPM-compatible MarsBar toolbox (http://marsbar.sourceforge.net/marsbar.pdf) and graphed using SPSS (SPSS 24).
Brain-behavior relationships.
We extracted individual MR signal change from significant activation clusters using the MarsBar toolbox to test for brain-behavior relationships. Stepwise regression analyses (SPSS 24) tested the explanatory value of activation cluster for behavioral measures separately for cognitive control and motor control for boys and girls separately. Finally, we tested relationships between pubertal development scores (PDS) and activation clusters to cognitive and motor control. Regions showing significant Pearson’s correlations at p<0.05 were Z-transformed to calculate one composite score. The Z-transform results in a group mean Z of 0 and standard deviation of ±1, and allows direct comparisons across regions. Where a negative correlation indicated greater PDS-related differences, scores were multiplied by −1, so that higher Z scores always indicated more difference with higher PDS. A composite score was calculated as the mean of all Z-scores that depicts greater activation difference with higher PDS, for all subjects, and for boys and girls separately.
RESULTS
Behavioral data analysis
Accuracy.
Overall task accuracy was high. On average, less than 2% errors were committed during task performance in the scanner (total number of errors: total=2.3 out of 144, range 0 to 13). Boys and girls did not differ in accuracy (t(176)=−0.67, p=0.51). No significant difference in the number of errors committed was observed between incongruent and congruent Stroop trials (t(177)=0.39, p=0.7) or between response switching and repetition blocks of trials (t(177)=1.3, p=0.19). Task accuracy was not related to age (overall: r=−.11, p=0.13; girls: r=−.13, p=0.23; boys: r=−.11, p=0.31). Also, there was no speed-accuracy trade-off (i.e., which would have been indicated by more errors committed with faster response time) (overall: r=.08, p=0.31; girls: r=.17, p=0.1; boys: r=.00, p=0.99).
Reaction time (RT).
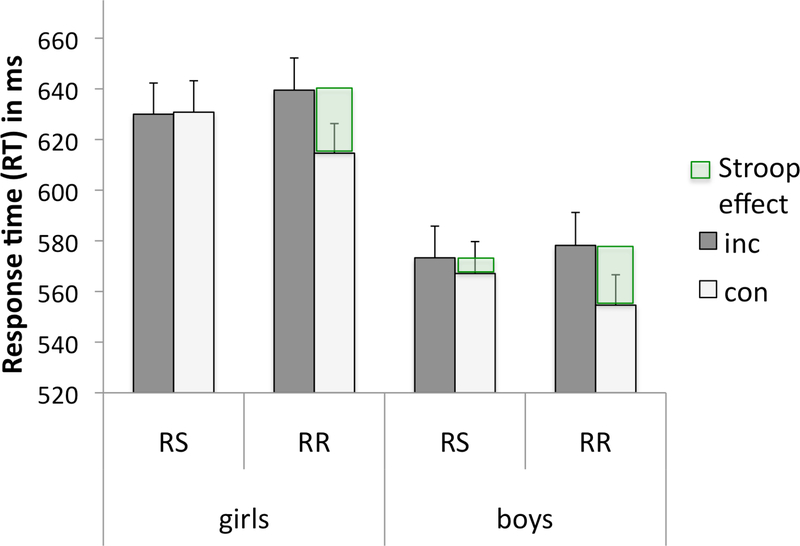
An ANOVA of reaction times (RTs) with Stroop (incongruent, congruent), response block (RS, RR), and sample-word color matching (match, nonmatch) as within-subject factors and sex (girls, boys) as the between-subject factor revealed significant main effects for Stroop (F(1,176)=51.25, p<0.0001, η2p=.23), response mode (F(1,176)=2.89, p=0.016, η2p=.02), match (F(1,176)=146.9, p<0.0001, η2p=.46), and sex (F(1,176)=12.36, p<0.0001, η2p =.07) with boys exhibiting faster RTs than girls. Significant Stroop-by-match (F(1,176)=6.68, p=0.011, η2p=.04) and Stroop-by-response block (F(1,176)=25.5, p<0.0001, η2p=.13) interactions were observed, as was a 3-way interaction among all task conditions (F(1,176)=44.43, p<0.0001, η2p=.20) (Table 2). No interaction between sex and task conditions was found (all p’s>0.05). The Stroop task-derived mean RT in adolescents was 599ms (±118ms). Stroop effects (RTINC minus RTCON) were greater for response repetition (RR: Diff RTINC-CON mean= 18.1±33.6ms) than for response switching blocks of trials (RS: Diff RTINC-CON mean= 2.6±39.2ms) (Figure 3). For congruent trials, mean RTs were slower for RS (RTconRS mean=599.7±121.9ms) than RR blocks (RTconRR mean=585.3±115.7ms) (con: Diff RTRSRR mean=14.4±38.9) (follow-up paired t-test t(177)=4.95, p<0.0001).
Table 2. Cognitive control in adolescents.
A. Stroop-related activation; B. Sex differences (boys vs. girls); C. Age correlation
| Regions | H | BA | k extent | t | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A. Stroop activation (inc>con) | ||||||||
| Anterior insula, frontal inferior operculum, extending to precentral gyrus | R | 48,38,6 | 3534 | 7.95 | 7.33 | 42 | 14 | −2 |
| Anterior insula, extending to SMA, precentral gyrus, anterior and middle cingulate cortex | L/R | 48,6,23,2,32 | 7146 | 7.31 | 6.82 | −34 | 14 | 6 |
| Supramarginal, superior temporal, middle temporal gyri | R | 40,48,42,21,22 | 2553 | 6.91 | 6.49 | 58 | −40 | 24 |
| Fusiform, inferior occipital gyrus/cerebellum, inferior and middle temporal gyri, extending to supramarginal, inferior parietal, superior temporal gyri | L | 37,19,39,40 | 3521 | 6.42 | 6.07 | −40 | −64 | −10 |
| Cerebellum - vermis, areas I-VI | L/R | - | 537 | 5.91 | 5.64 | 0 | −48 | −16 |
| Fusiform, inferior occipital/cerebellum VI | R | 37,19 | 549 | 5.08 | 4.9 | 40 | −62 | −12 |
| dlPFC | L | 46 | 549 | 4.5 | 4.37 | −34 | 48 | 32 |
| Midbrain, thalamus | L/R | - | 691 | 4.39 | 4.27 | −2 | −26 | −16 |
| Hippocampus | R | 20 | 120 | 4.28 | 4.17 | 36 | −6 | −12 |
| Caudate, thalamus | R | - | 163 | 4.2 | 4.1 | 10 | 12 | 8 |
| Inferior parietal lobe | R | 7,40 | 172 | 3.8 | 3.72 | 28 | −48 | 46 |
| Stroop deactivation (con>inc) | ||||||||
| Middle frontal gyrus | L | 8 | 286 | 5.76 | 5.5 | −28 | 20 | 54 |
| Angular and middle occipital gyri | L | 7,19 | 235 | 5.13 | 4.94 | −36 | −74 | 42 |
| Lingual, calcarine gyri | R | 17,18 | 741 | 4.97 | 4.8 | 12 | −88 | −2 |
| Precentral gyrus | R | 6 | 419 | 4.73 | 4.58 | 32 | −20 | 70 |
| Angular and middle occipital gyri | R | 19,7 | 224 | 4.64 | 4.5 | 40 | −76 | 42 |
| Precuneus | L/R | 7 | 291 | 4.5 | 4.37 | 4 | −60 | 44 |
| Superior temporal gyrus, rolandic operculum | R | 22.48 | 430 | 4.5 | 4.37 | 60 | −10 | 6 |
| Superior and middle temporal gyri | L | 21,22,48 | 370 | 4.36 | 4.24 | −56 | −8 | 0 |
| vmPFC | R | 11 | 161 | 4.27 | 4.16 | 8 | 56 | −10 |
| mPFC | L | 10 | 255 | 3.88 | 3.79 | −8 | 66 | 16 |
| vmPFC | L | 11 | 129 | 3.85 | 3.77 | −20 | 48 | −6 |
| Lingual, calcarine gyri | R | 17,18,30 | 155 | 3.75 | 3.67 | 12 | −50 | 2 |
| B. Girls > boys for Stroop (inc>con) | ||||||||
| Lingual, fusiform gyri | L | 17,18 | 122 | 4.08 | 3.98 | −10 | −80 | −8 |
| C. Age for Stroop (inc>con) | ||||||||
| No suprathreshold voxel cluster | ||||||||
Abbreviations: inc: incongruent; con: congruent; H: cerebral hemisphere; BA: Brodmann area; k; number of voxel in a cluster; m: medial; vm: ventromedial, dl: dorsolateral; SMA: supplementary motor area; ACC: anterior cingulate cortex; MCC: middle cingulate cortex; PFC: prefrontal cortex
Figure 3. Stroop Match-to-Sample task: Behavior.
Mean response time (RT) and standard to incongruent (inc) and congruent (con) Stroop words for response switching (RS) and response repetition (RR) blocks of trials in adolescent girls and boys. The Stroop effect (green) is defined as the difference in RT between incongruent and congruent trials (Diff RTINC-CON). Behavioral Stroop effects were larger for RR than RS in girls and in boys.
RT, age, and puberty.
Age and pubertal development scores (PDS) correlated negatively with overall mean RTs (age: r=−.404, p<0.0001; PDS: r=−.205, p=0.006) and standard deviation (std) (age: r=−.269, p<0.0001; PDS: r=−.251, p=0.001) during Stroop Match-to-Sample task performance; that is, older adolescents responded faster and with less variation than younger ones. No significant sex differences were observed between the correlations between adolescent age and RT (boys r=−.49, p<0.0001, girls r=−.341, p=0.001, z=−1.18, ns), age and std (boys r=−.277, p=0.009, girls r=−.261, p=0.013, z=0.11, ns), as well as between pubertal stage (PDS) and RT (boys r=−.35, p=0.001, girls r=−.275, p=0.008, z=−0.55, ns) and PDS and std (boys r=−.245, p=0.022, girls r=−.344, p=0.001; z=0.71, ns).
Task-activated fMRI data analysis
Group analyses for contrasts of interest were carried testing activation evoked for cognitive control (Stroop conflict, inc>con) (overall, and for RS and RR blocks separately) and motor control (response switches, RS>RR) (overall and for non-conflict congruent trials only).
Cognitive control (Stroop).
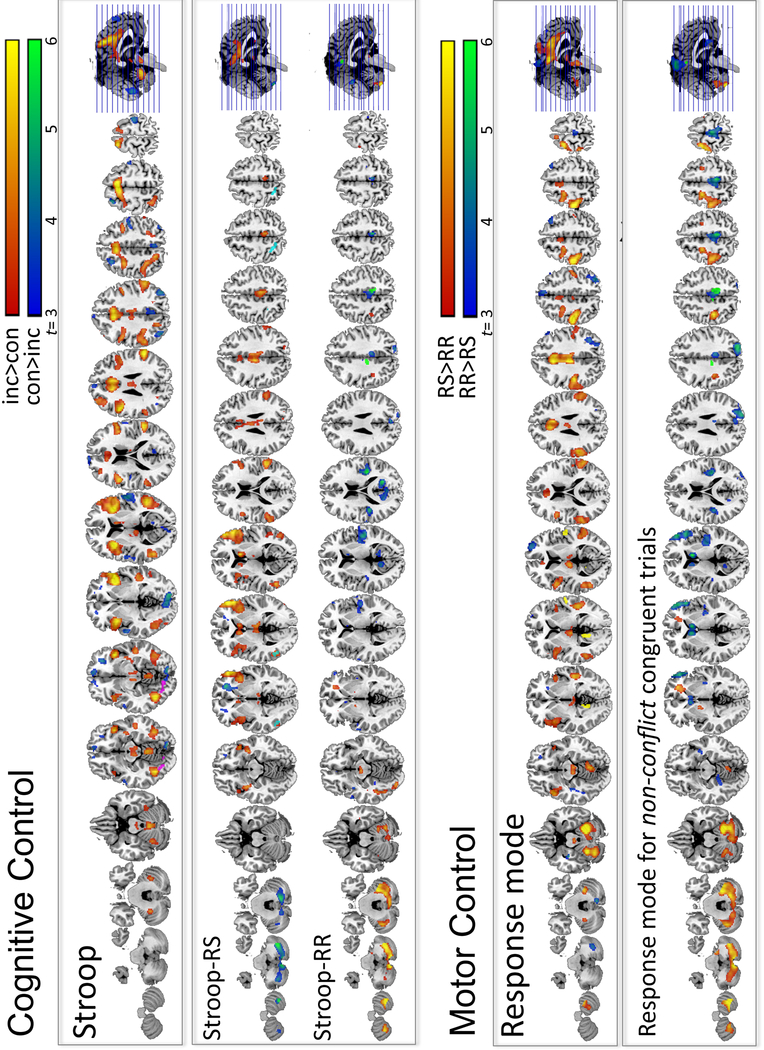
Stroop conflict (inc>con) processing resulted in greater BOLD activation for incongruent (inc) relative to congruent (con) trials in several large fronto-temporo-parietal cortical, thalamo-limbic-striatal subcortical, and cerebellar regions (Table 2). The opposite contrast (con>inc) revealed greater BOLD response to congruent than incongruent trials in medial prefrontal and parietal regions including angular gyrus and precuneus, and in extrastriate cortices including lingual and calcarine gyrus (Figure 4, upper panel).
Figure 4. Cognitive Control.
Stroop-related activation (upper panels): Incongruent in contrast to congruent Stroop conditions (inc>con: in red-to-yellow; all trials) activated a bilateral fronto-parietal network, also including subcortical (thalamus, striatum, limbic) and cerebellar regions; the opposite contrast (con>inc: in blue-to-green) activated medial prefrontal and parietal regions. Stroop contrasts (inc>con) conducted separately for response switching (Stroop-RS) and response repetition blocks of trials (Stroop-RR). Motor Control. Response mode-related activation (lower panels): Response switching (RS), contrasted to response repetition (RR) blocks of trials (RS>RR, all trials) activated a cerebellar-motor cortical circuitry, also including lateral parietal, temporal, insula, and thalamic regions (in red-to-yellow); the opposite contrast (RR>RS) activated prefrontal, lateral and medial frontal areas, also including supplementary motor area, angular, fusiform, and cerebellar regions (in blue-to-green). Response mode contrasts conducted separately for non-conflict congruent trials showed cerebellar–motor cortical network activation (lower panel).
Stroop-RS and Stroop-RR:
Table 3 shows Stroop (inc>con) evoked regional activation separately for response switching (Stroop-RS) and response repetition (Stroop-RR) blocks (Figure 4, middle panel). For Stroop-RS, the BOLD response was greater for incongruent (inc) than congruent (con) trials in frontoparietal-limbic and dorsal striatal regions. By contrast, for Stroop-RR, the BOLD response was greater in cerebellarpallidal-motor cortical regions.
Table 3. Effect of motor control on cognitive control in adolescents.
A. Stroop-RS and Stroop-RR related activation; B. Sex differences (boys vs. girls); C. Age correlation
| Regions | H | BA | k extent | t | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A. Stroop-RS (inc>con) | ||||||||
| Inferior frontal gyri - pars triangularis and opercularis, anterior insula | R | 45,47,48 | 2507 | 6.49 | 6.13 | 54 | 36 | 0 |
| Supramarginal, superior temporal gyri | R | 40,41,42,48 | 1159 | 5.81 | 5.55 | 56 | −40 | 20 |
| Caudate nucleus | L/R | - | 389 | 5.73 | 5.48 | 10 | 12 | 8 |
| Middle cingulate cortex | L/R | 23 | 1710 | 5.39 | 5.18 | −6 | −18 | 38 |
| Middle and superior temporal gyri | L | 21,22,37 | 730 | 5.17 | 4.98 | −52 | −60 | 6 |
| Anterior insula | L | 48 | 980 | 4.78 | 4.63 | −36 | −2 | −14 |
| Midbrain | L/R | - | 219 | 4.67 | 4.53 | 6 | −22 | 2 |
| Rolandic operculum, inferior frontal operculum | L | 6,48 | 127 | 4.46 | 4.33 | −48 | 6 | 14 |
| Superior occipital gyrus, cuneus | R | 18,19 | 111 | 3.65 | 3.58 | 24 | −78 | 34 |
| Stroop−RR (inc>con) | ||||||||
| Cerebellum - areas VI,VIIB/Crus1,2 | L/R | - | 6428 | 7.44 | 6.93 | −6 | −74 | −40 |
| Pallidum and midbrain | L/R | - | 269 | 5.67 | 5.43 | 14 | −4 | −6 |
| Pallidum | L | - | 111 | 4.74 | 4.59 | −14 | −2 | −4 |
| Precentral gyrus | L | 6 | 1574 | 4.71 | 4.56 | −30 | −12 | 64 |
| B. Girls vs. boys for Stroop-RR and Stroop-RS | ||||||||
| No suprathreshold voxel cluster | ||||||||
| C. Age positive correlation for Stroop-RS | ||||||||
| Superior, inferior parietal lobe, precuneus | L | 7,40 | 117 | 3.88 | 3.79 | −24 | −56 | 56 |
| Middle temporal and occipital gyri | L | 37 | 106 | 3.70 | 3.63 | −48 | −64 | 0 |
| Age negative correlation for Stroop-RR | ||||||||
| Middle cingulate cortex | L | 23 | 132 | 4.28 | 4.16 | −8 | −28 | 40 |
| Middle cingulate cortex | R | 23 | 104 | 4.09 | 3.99 | 10 | −34 | 44 |
Abbreviations: RS: response switching blocks; RR: response repetition blocks; inc: incongruent; con: congruent; H: cerebral hemisphere; L: left; R: right; BA: Brodmann area; k; number of voxel in a cluster
Motor control (response mode).
Across all subjects and trials, response switching relative to response repetition (RS>RR) evoked BOLD activity in a cerebellar-motor cortical circuitry that included lateral parietal and temporal regions, bilateral insula, and thalamus (Table 4). The opposite contrast, i.e., RR>RS, showed greater BOLD activity in prefrontal, lateral and medial frontal areas including supplementary motor area, angular and fusiform gyri, and cerebellar regions (Figure 4, lower panel).
Table 4. Motor control in adolescents.
A. Response mode-related activation; B. Sex differences (boys vs. girls); C. Age correlation
| Regions | H | BA | k extent | t | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A. Response Switching (RS>RR) | ||||||||
| Cerebellum - areas IV-VI/Crus1 | R | - | 1486 | 9.09 | Inf | 22 | −52 | −22 |
| Cerebellum - area VI/Crus1 | L | - | 797 | 7.1 | 6.65 | −26 | −54 | −24 |
| MCC, ACC, SMA | L/R | 23, 24, 6 | 3087 | 7.01 | 6.57 | −6 | −20 | 38 |
| Inferior parietal, postcentral, supramarginal gyri, extending to superior and middle temporal gyri | L | 2,3,48,41,42,21,22 | 4718 | 6.71 | 6.32 | −50 | −26 | 50 |
| Insula, inferior frontal gyri - pars opercularis, orbitalis, triangularis, rolandic operculum | L | 44, 45, 48 | 1397 | 5.54 | 5.31 | −46 | 12 | −10 |
| Cerebellum - area VIII | R | - | 146 | 5.53 | 5.3 | 28 | −48 | −52 |
| Superior and middle temporal gyri | R | 21, 22 | 2146 | 5.43 | 5.21 | 48 | −32 | 0 |
| Thalamus, midbrain | L/R | - | 866 | 5 | 4.83 | −8 | −20 | 8 |
| Anterior insula | R | 48 | 244 | 4.59 | 4.45 | 44 | 10 | −6 |
| Response Repetition (RR>RS) | ||||||||
| Middle and superior frontal cortex | R | 10,11 | 304 | 4.61 | 4.48 | 28 | 58 | 12 |
| Cerebellum - area VIIB/Crus2 | R | - | 348 | 4.61 | 4.47 | 10 | −74 | −34 |
| Fusiform gyrus | L | 20 | 243 | 4.52 | 4.39 | −34 | −18 | −22 |
| Angular, superior occipital gyri | R | 7,39 | 614 | 4.49 | 4.36 | 34 | −70 | 46 |
| SMA, paracentral gyri | L/R | 4,6 | 323 | 4.25 | 4.14 | 4 | −24 | 60 |
| Superior medial frontal gyri | L/R | 8,9,32 | 180 | 4.18 | 4.07 | 8 | 34 | 48 |
| Middle frontal gyrus | R | 44,45 | 209 | 4.15 | 4.04 | 52 | 30 | 34 |
| B. Boys > girls for RS>RR | ||||||||
| Superior temporal gyrus | R | 22,48 | 142 | 3.78 | 3.7 | 58 | −12 | 6 |
| Lingual gyrus | L | 18,27 | 142 | 3.71 | 3.63 | −12 | −48 | 2 |
| C. Age for RS>RR | ||||||||
| No suprathreshold voxel cluster | ||||||||
Abbreviations: RS: response switching blocks; RR: response repetition blocks; inc: incongruent; con: congruent; H: cerebral hemisphere; BA: Brodmann area; k; number of voxel in a cluster; inf: inferior; mid: middle; sup: superior; m: medial; vm: ventromedial, dl: dorsolateral; SMA: supplementary motor area; ACC: Anterior cingulate cortex; MCC: middle cingulate cortex; PFC: prefrontal cortex
Motor control (RS>RR) for congruent non-conflict trials:
Next, we tested activation patterns of adolescent motor control independently from cognitive control. Table 5 shows the response mode-evoked regional activation for non-conflict trials (RS>RR con). In support of our hypothesis, a cerebellar–motor cortical network was engaged for response switches (vs. repetitions; RS>RR con) (Figure 4, lowest panel). For the opposite contrast (RR>RS con), a medial frontoparietal (supplementary motor area, middle cingulate cortex, precuneus) network was engaged that also included orbitofrontal, insular, and occipital cortices.
Table 5. Motor control without cognitive control.
A. Response mode-related activation for congruent trials only; B. Sex differences (boys vs. girls); C. Age correlation
| Regions | H | BA | k extent | t | Z | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| A. Response switching (RS>RR) for congruent (con) trials | ||||||||
| Cerebellum - areas VI, VIIB, VIII/Crus1,2 | R | - | 5608 | 8.31 | 7.62 | 28 | −52 | −28 |
| Pre- and postcentral gyri | L | 2,3,4,6 | 2007 | 6.45 | 6.1 | −30 | −12 | 64 |
| Precentral and superior frontal gyri | R | 6 | 155 | 4.17 | 4.07 | 30 | −8 | 60 |
| Response repetition (RR>RS) for con | ||||||||
| Caudate | L/R | - | 584 | 6.14 | 5.83 | 10 | 12 | 8 |
| SMA, paracentral gyri, MCC, precuneus | L/R | 4,5,23 | 1773 | 5.74 | 5.48 | 6 | −38 | 54 |
| Superior and middle occipital gyri, cuneus | R | 18,19 | 1214 | 5.61 | 5.38 | 24 | −80 | 36 |
| B Inferior frontal gyri - pars triangularis, orbitalis, and opercularis | R | 45,46,47 | 1059 | 5.41 | 5.2 | 50 | 42 | 6 |
| Heschl gyrus, rolandic operculum, post insula | R | 41,48 | 924 | 5.07 | 4.89 | 38 | −26 | 16 |
| Heschl gyrus, rolandic operculum | L | 41,48 | 239 | 4.92 | 4.75 | −38 | −30 | 16 |
| Lingual gyrus | L | 30,37,19 | 322 | 4.21 | 4.11 | −16 | −42 | −8 |
| Calcarine gyrus | L | 17 | 118 | 3.89 | 3.8 | −18 | −60 | 14 |
| B. Girls vs. boys for RS>RR con and RR>RS con | ||||||||
| No suprathreshold voxel cluster | ||||||||
| C. Age negative correlation for RS>RR con | ||||||||
| Middle cingulate cortex | R | 23 | 193 | 4.58 | 4.44 | 10 | −36 | 46 |
| Middle cingulate cortex | L | 23 | 142 | 4.35 | 4.24 | −8 | −28 | 42 |
Abbreviations: RS: response switching blocks; RR: response repetition blocks; con: congruent; H: cerebral hemisphere; L: left; R: right; BA: Brodmann area; k; number of voxel in a cluster; post: posterior; SMA: supplementary motor area; MCC: middle cingulate cortex
Age and brain activation.
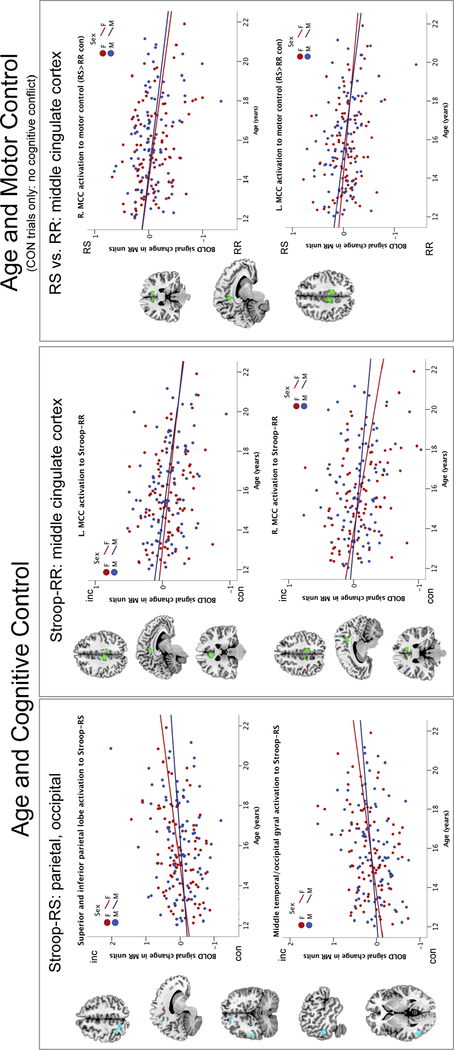
Age-related BOLD signal changes were observed for high cognitive control demands (Stroop-RS) in the left superior parietal lobe, precuneus, middle temporal, and occipital brain regions were more activated in older than younger adolescents (Figure 5, left panel). When cognitive control was less demanding (Stroop-RR), bilateral middle cingulate cortices (MCC) were less activated in older adolescents (Figure 5, middle panel). Also for motor control ability with less demanding conditions (RSRRcon, i.e., no cognitive conflict), bilateral middle cingulate cortical regions were less activated with older adolescent age (Figure 5, right panel).
Figure 5. Age-related Activation.
Correlation graphs depict age effects (Tables 3C, 5C). Cognitive control. With older age, left parietal and occipito-temporal regions were more activated to Stroop-RS (in cyan; left panels) and the middle cingulate cortex (MCC) was less activated to Stroop-RR (in green; middle panels). Motor Control: With older age, the MCC was less activated during RS than RR for non-conflict congruent trials (in green; right panels).
Age-related activation and processing efficiency.
To test if age-related brain signal changes were explained by task performance, we used partial correlation analyses controlling for mean reaction time (RT) and standard variation, i.e., where faster speed (mean RT) and less variation (std) indicate better overall task performance. For all regions, age-activation correlations remained significant (all p’s < 0.0001).
Puberty and brain activation.
Because pubertal development varied widely within the same age groups, we explored if the observed activation pattern for cognitive and motor control were related to PDS. We found moderate positive and negative relationships. In particular, more advanced puberty was related to greater activation for high cognitive control demands, mainly in extrastriate brain regions, and lower activation for less demanding control conditions in extrastriate and MCC regions. We calculated Z-scores for puberty-related regions where negative relations were multiplied by −1. A composite Z-score was derived denoting greater activation differences with more advanced pubertal stage (r=.304; p<0.0001). It included puberty-related signal changes in extrastriate clusters with greater activation during cognitive control in lingual, fusiform gyri (Stroop, Table 2B), middle and superior temporal gyri (Stroop-RS, Table 3A), and middle temporal and occipital gyri (Stroop-RS, Table 3C). Less activation with higher PDS was observed during cognitive control with repeated response selection procedures in the left MCC (Stroop-RR, Table 3C), and during motor control in the left lingual gyrus (RSRR-con, Table 5A), and the right MCC (Table 5C). In sum, puberty-related BOLD signal change to cognitive and motor control was demand-specific and observed in occipital, temporal, and MCC regions.
Sex and brain activation.
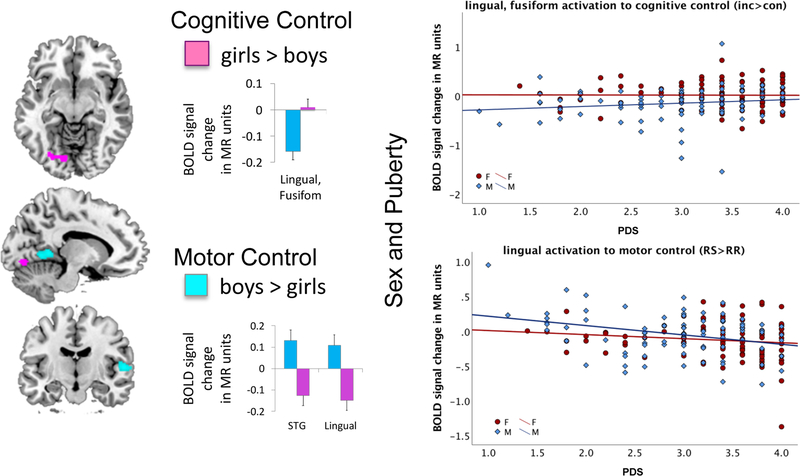
Girls activated extrastriate fusiform and lingual areas more than boys during cognitive control (Stroop conflict, inc>con, Table 2B). Boys activated the right superior temporal and left lingual gyri more than girls during motor control (RSRR, Table 4B). Exploratory analyses (Supplemental Materials) showed that extrastriate activation correlated with PDS scores (cognitive control: r=.151, p=0.044; motor control: r=−.262, p<0.0001; boys: r=−.318, p=0.003; girls: r=−.138, ns) such that sex differences gradually decreased with more advanced puberty (Figure 6).
Figure 6. Sex Differences in Activation.
Girls (in pink) activated extrastriate fusiform and lingual areas more than boys (in blue) during cognitive control (Stroop conflict, inc>con, Table 2B). Boys activated the right superior temporal and left lingual gyri more than girls during motor control (RSRR, Table 4B). Relation to Puberty. Pubertal development (PDS) scores correlated with extrastriate activation such that sex differences gradually decreased with more advanced puberty.
Brain-behavior relationships.
Cognitive control (Stroop).
Brain-behavior relationships differed for boys and girls (see also Supplementary Table and Figure). Stepwise regression analysis with activation clusters entered as independent variables (Table 2A) showed that greater behavioral Stroop effects (Diff RTINC-CON) in boys were explained by greater activation (inc>con, Table 2) of bilateral anterior insula-frontal regions (extending into supplementary motor area, precentral gyrus, anterior and middle cingulate cortices) (t=2.691) and lower cerebellar activation (vermis, areas I-IV) (t=−2.033) (F(2,84)=5.706, p=0.005), and in girls by lower precentral gyrus activation (inc<con) (t=−2.069) (F(1,89)=4.281, p=0.041).
Motor control (response mode).
Stepwise regression analysis with activation clusters entered as independent variables (Table 4A) showed that response-mode effects (Diff RTRS-RR) in girls were explained by greater right cerebellar (area IV-VI, Crus1) activation during response switching (RS>RR) (t=2.90) (F(1,89)=8.407, p=0.005), and in boys by lower right cerebellar (area VIIB/Crus2) activation during response repetition (RR>RS) (t=2.488) (F(1,86)=6.189, p=0.015).
Additional analyses testing the moderating effects of sex and puberty on brain-behavior relationships are presented in Supplemental Materials.
DISCUSSION
Our study shows new ways of distinguishing inhibitory control functions between cognitive and motor components and repetition-related enhancement of response selection procedures. During adolescent development, cognitive and motor control mechanisms were dissociable, subserved by different brain systems, and were optimized throughout the adolescent age range. We found evidence for a sexual dimorphism in neural activation pattern that was related to pubertal development, indicating sex-specific organizing effects in the developing brain beyond chronological age.
Adolescent inhibitory control.
Behaviorally, adolescents exhibited interference from cognitive conflict posed by our Stroop task. Cognitive interference was modulated by motor control demands, where Stroop effects were greater for response repetitions than response switches. Similar to previous findings on the Stoop Match-to-Sample task in healthy adults (Schulte et al, 2011), adolescents profited from repetition priming when resolving Stroop color-word conflict. One could expect that repetitive conditions require less cognitive control and, in turn, reduce conflict (see e.g., Gratton et al., 1992; Mayr et al., 2003; Schmidt and Weissman, 2016). However, we did not see this pattern for incongruent conflict trials, but instead for congruent non-conflict trials. Thus, the benefit from response repetition occurred with low need for control, resulting in faster and more efficient processing that was specific to repetitive trials with no conflict (congruent), i.e., the benefit did not occur for repetitive trials with cognitive conflict (incongruent). As a consequence, Stroop effects, i.e., the difference between incongruent and congruent RTs, was greater for repetition than switching blocks. Facilitation of response selection procedures with repetition appears to be independent of conscious awareness and may rely on a type of implicit memory subserved by corticostriatal brain circuits (Mishkin et al., 1984). Because these findings are similar to our previous findings in adult populations (Schulte et al., 2011), these cognitive and motor control functions, together with functions enhancing processing efficiency with repetition, may have already matured to a certain degree even in our youngest adolescents. Nevertheless, we still observed age-related differences, with better processing efficiency in older, more mature adolescents, expressed as faster psychomotor speed and less variance in RTs. Although boys and girls did not differ in cognitive (Stroop effects) and motor control (response mode effects), boys had overall faster responses than girls. It is possible that boys may have employed a different strategy from girls that expedited overall processing speed, unspecific to component control processes. Accordingly, we tested the neurofunctional correlates of task performance and found differentiated regional BOLD activation patterns to cognitive and motor control demands in relation to processing efficiency, that was modulated by age, sex, and pubertal development, described next.
Neural correlates of cognitive control in adolescence.
Cognitive control demands (inc>con) activated a wide reaching, bilateral neural system involving frontotemporo-parietal cortical, thalamo-limbic-striatal subcortical, and cerebellar regions. The opposite contrast showed regions less activated during conflict (inc<con), or alternatively more activated during non-conflict low demand congruent trials, which were the medial prefrontal cortex, angular gyrus, precuneus, as well as extrastriate occipito-temporal cortical regions. Medial prefrontal-parietal cortical regions are typically engaged during rest (Fox et al. 2005; Fox and Raichle 2007; for review, Raichle 2011; for meta-analysis, Fox et al. 2015) and preferentially deactivated during task processing (McCormick and Telzer, 2018; Schulte et al., 2012; Tomasi et al., 2006) likely to enhance efficient and task-specific processing. That activity in non-specific default mode regions was attenuated while control-specific regions were activated suggests refined neurodynamics to the specific cognitive and motor demands in adolescents. The lateral frontoparietal activation was specifically evident for conditions that required both cognitive and motor control and resembled the Stroop activation pattern in adult populations (Bench et al. 1993; Carter et al. 1995; Larrue et al. 1994; Pardo et al. 1990; Peterson et al. 1999; Schulte et al. 2011, 2012).
We found indication for neurofunctional efficacy in adolescents that was supported by the fine-tuning of interacting cognitive and motor control systems and their automaticity with repeated procedures. Similar to behavioral results, brain activation patterns to cognitive control demands were modulated by motor control demands. A cerebellar-pallidal-motor cortical network was activated during Stroop processing for low motor control demands (Stroop-RR). These findings are consistent with the idea that lateral prefrontal and parietal regions, typically recruited for cognitive control operations, would be less activated with the development of automaticity, whereas motor cortical regions and putamen/globus pallidus regions, associated with motor skill acquisition, would be more activated (Poldrack et al. 2005). Specific mechanisms underlying adolescent motor and cognitive associations with respect to skill acquisition and automaticity remain poorly characterized. Our findings explicate this finding and show that control processes were demand-specific and required less brain engagement with low control from automaticity from repetition. In particular, recruitment of cognitive control regions was no longer evident when the same motor response was performed repeatedly (Stroop-RR), whereas frontoparietal-insula regions were engaged in cognitive-motor control during Stroop-RS requiring response switching.
Neural correlates of motor control in adolescence.
Specifically testing the effects of motor control demands shed light on the neurofunctional mechanisms of implicit motor learning with repetition. Pooled over both incongruent and congruent trials, motor control (response switching) activated a cerebellar-thalamic-motor cortical network and bilateral insula, inferior frontal, anterior and middle cingulate, lateral temporal, and occipitoparietal cortical regions. For motor control without cognitive control (RS>RR con), however, only the motor cortico-cerebellar network was activated, validating the successful experimental manipulation of implicit motor control demands within the Stroop Match-to-Sample paradigm.
Moreover, for motor response automaticity with repetition (RR>RS con), greater BOLD responses occurred in a medial frontoparietal (SMA, MCC, precuneus) network that involved the caudate nucleus. This pattern is consistent with cortico-subcortical activation increase with automaticity (Wu and Hallett 2005) and the caudate’s role in perceptual skill learning (Poldrack et al. 2005; for review Poldrack 2002).
While cortico-cerebellar circuitry is engaged during motor control demands (RS>RR), cerebellar regions were not involved during RR (>RS). With learning through practice, the cortico-cerebellar circuitry enables storing of the most efficient representations of behavior in frontal motor association cortices (Ito 1993; Koziol et al. 2012) with reduced participation of the cerebellum when a task is mastered.
Age effects on inhibitory control function.
Sensory association areas, including parietal and occipito-temporal cortices, were more activated with older adolescent age, but only for high executive control demands (Stroop-RS) that required both cognitive and motor control. Age-related parietal activation (Stroop-RS) correlated with mean RT. Because faster mean RT (with no speed-accuracy trade-off) can be considered a measure of processing efficiency, this finding suggests that, for high inhibitory control demands, changes in parietal lobe activation with older age underwrite efficient performance. A functional neuroimaging study using a Stroop task (Adleman et al., 2002) found age-related increases in parietal and parieto-occipital activation mainly during childhood that had matured during adolescence. Our results show that some neurofunctional processes (i.e., an increase in parieto-occipital involvement for control) may not have matured fully during childhood and undergo further refinement during adolescence, e.g., for high control demands, when incongruent stimulus information is additionally in conflict with the planning of movement (Stroop-RS). We had previously investigated the neurofunctional correlates of high control demands in young (19–30 years) and older adults (58–84) using the same task (Schulte et al., 2011). When comparing the finding from those two adult samples (see Figure 2 in Schulte et al., 2011) to that from the adolescent sample in this study, one could infer a linear development for parietal and parieto-occipital activation for high cognitive control. For the bilateral middle cingulate cortex (MCC) engagement, a main motor control region activated for response repetitions (RR>RScon contrast), however, one would rather infer a non-linear age-related neurofunctional development. In the current study, the MCC was less invoked with older adolescent age and, consistent with this, the young adults in the previous study had exhibited low MCC activity, whereas the older adult sample had shown enhanced MCC activation to cognitive control (Schulte et al. 2011). In the current study, the bilateral MCC showed diminishing activation with age for both cognitive and motor control, i.e., the Stroop-RR and RR>RScon contrasts, suggesting older adolescents required less cortical involvement than younger ones for engaging automatic sequences.
Thus, together these findings suggest that adaptive neurofunctional alterations occur during adolescent maturation to enhance efficiency of control processes, a process that likely continues during adult development. It appears that the adolescent fronto-parietal and cerebro-cerebellar cognitive and motor control circuits observed in our study closely resemble that of adults (Schulte et al., 2011; Power et al. 2010); yet, our findings of enhanced partietal and occipito-temporal activation to high control demands in older adolescence and less middle cingulate cortical activation to low demand with automaticity suggests that cortical functioning becomes more efficient with maturation in establishing cortical anticipatory skill learning (Imamizu and Kawato 2009; Saling and Phillips 2007). Our findings are consistent with the interactive component model of control (Luna et al., 2015) that understands adolescence as an adaptive period of continued refinement of an existing set of functions, where improvements in cognitive control ability are related to both the development of component-specific brain systems and their integration.
Sex and puberty effects on inhibitory control function.
The most prominent Stroop activation peak in adolescents was in bilateral anterior insular cortices, which correlated with behavioral Stroop effects in boys, who were, on average, not as advanced as girls in their pubertal development despite similar chronological ages. The insula is well situated for orchestrating synchronization between frontal cortical and subcortical limbic, striatal, and thalamic regions (Vogel et al. 2010). As such, the anterior insula has been previously linked to cognitive-motor control (Barber et al. 2013; Sali et al. 2016; Tremel et al. 2016), incentive salience (e.g., Filbey et al. 2009), emotion (e.g., Loeber et al. 2009), and interoceptive regulation (Li et al. 2016). The insula is also a key player in the salience network that filters relevant, interoceptive, autonomic, and emotional information (Seeley et al. 2007; Taylor et al., 2009). As the development of cognitive control is influenced by emotion and changing reward contingencies (Geier et al., 2010) such that motivational systems enhance behaviors (van Duijvenvoorde, 2016), the finding of insula principal engagement for cognitive control in adolescents may denote its role during maturation, particularly in boys, who had a lower pubertal status than girls. It can be speculated that during maturation the insula’s role is to coordinate limbic responses to incoming stimuli, by assigning salience to stimuli in the selection processes in extrastriate regions (see e.g., Rainer et al. 2004) and allocating cognitive-motor control responses in frontoparietal circuits (e.g., Marusak et al. 2015). Although boys and girls in our study exhibited overall similar Stroop-related activation patterns, sex differences were observed for visual extrastriate regions during inhibitory control. Specifically, boys (relative to girls) activated extrastriate cortices less for cognitive control and more for motor control. Although extrastriate regions are not known to be directly involved in cognitive control, increased activity in occipital and temporal regions have been frequently observed in association with motor planning (e.g., Randerath et al., 2017) that has modulatory effects in brain areas concerned with early processes of visual encoding (Danielmeier et al., 2004). An interpretation of these effects includes a possible involvement of extrastriate regions in action planning that affects how Stroop words are encoded with different developmental trajectories in boys than girls for cognitive and motor control. Satterthwaite et al. (2014) found that cerebral perfusion in fronto-parietal, insular and occipito-temporal regions started to diverge between boys and girls in midpuberty, regions that were also activated for cognitive control in our study. In an event-related potential study using Stroop stimuli in young adults (18–23 years old), Shen (2005) reported sex differences in the allocation of attentional resources for perceptual discrimination with a longer P300 latency and smaller amplitude in young men than women. Our fMRI findings add to these results and suggest the use of different control strategies over the course of pubertal maturation in adolescent boys and girls. In our study, the observed sexual dimorphism in extrastriate activation attenuated with increased pubertal status. Also, the insula was less engaged with more advanced puberty in boys (see Supplemental Material). Taken together, sex disparities in inhibitory control functions manifest depending on the timing of pubertal development and brain regions affected.
Although others have linked higher puberty scores, derived from the same PDS scale as in our study, to higher testosterone levels (Huang et al., 2012; Tyborowska et al., 2016), a mechanistic interpretation is limited by the lack of data on circulating hormone levels for our adolescent sample. However, recent MRI studies suggest a role of testosterone and estradiol in mediating sexual dimorphism in pubertal brain development (Cservenka et al., 2015; Nguyen et al., 2013, 2017; Op de Macks et al., 2016; Selmeczy et al., 2018; Tyborowska et al., 2017; Wiereanga et al., 2018). For example, Cservenka et al. (2015) reported testosterone-related changes in frontal-pallidal activation in boys and estradiol-related changes in occipital brain activation in girls during an emotional conflict task. These limitations notwithstanding, using PDS, our results establish that pubertal delay in boys (relative to girls of the same age) was associated with sexual dimorphism in brain activation patterns during inhibitory control. In addition to chronological age as predictive of brain development, pubertal stage may be a useful predictor of sex-specific brain maturation.
Together, our results provide unique insight into the fine-tuning of neurofunctional dynamics underwriting cognitive and motor control during adolescence. In addition to frontoparietal regions, the insula was robustly engaged during executive control potentially marking an adolescent maturation process in that it showed less activation with higher pubertal stage, in particular in adolescent boys. The insula may orchestrate the synchronization between higher-order frontoparietal circuits for cognitive-motor control with bottom-up extrastriate regions to aid efficient stimulus attribute selection. The neural cognitive control circuitry was modulated by motor control demands and specifically by the development of automaticity from repetition, which engaged a different cerebellar-pallidal-motor cortical network associated with motor skill acquisition. Here, attenuated MCC activation with older age suggests development of neural efficiency in establishing cortical anticipatory skill learning. Whether observed associations among age, sex, puberty, and cognitive and motor control circuitries mark developmental trajectories await longitudinal study.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Health Office of the Director, the National Institute of Child Health and Human Development, and the Office of the Director, National Institutes of Health [NCANDA grant numbers: AA021696 (IMC+FCB), AA021695 (SAB+SFT), AA021692 (SFT), AA021697 (AP+KMP)]. Additional funding was provided by NIAAA grant number AA010723 (EVS).
Footnotes
DISCLOSURES
None of the authors have conflicts of interest with the reported data or their interpretation.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL (2002) A developmental fMRI study of the Stroop color-word task. Neuroimage. 16(1): .61–75. [DOI] [PubMed] [Google Scholar]
- Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, Amaral DG, Casey BJ, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Libiger O, Mostofsky S, Murray SS, Sowell ER, Schork N, Dale AM, Jernigan TL (2014) The NIH toolbox cognition battery: Results from a large normative developmental sample (ping). Neuropsychology. 28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G, Cservenka A, Fair DA, Nagel BJ (2014). Sex differences in the neural substrates of spatial working memory during adolescence are not mediated by endogenous testosterone. Brain Res. 1593:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT (2011) Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS One. 6(6):e21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Crowley TJ, Thompson LL, Jacobson BL, Liu X, Raymond KM, Claus ED (2007) Brain activation during the Stroop task in adolescents with severe substance and conduct problems: A pilot study. Drug Alcohol Depend. 90(2–3):175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AD, Caffo BS, Pekar JJ, Mostofsky SH (2013) Effects of working memory demand on neural mechanisms of motor response selection and control. J Cogn Neurosci 25(8), 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JA, Doyon J (2003) Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage. 20(3):1649–1660. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RS, Dolan RJ (1993) Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 31(9):907–922. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nat Rev Neurosci. 9(4): 267–277. Review. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM (2003). Neural development of selective attention and response inhibition. Neuroimage. 20(2):737–751. [DOI] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FC, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF (2015) The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 76(6):895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breukelaar IA, Antees C, Grieve SM, Foster SL, Gomes L, Williams LM, Korgaonkar MS (2016) Cognitive control network anatomy correlates with neurocognitive behavior: A longitudinal study. Hum Brain Mapp. 38(2):631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD (1995) Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 2(4):264–272. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD (1999) The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 10(1):49–57. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S (2005) Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 9(3):104–110. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA (2008) The adolescent brain. Ann N Y Acad Sci. 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA (2009) Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 33:631–646. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lei X, Ding C, Li H, Chen A (2013). The neural mechanisms of semantic and response conflicts: an fMRI study of practice-related effects in the Stroop task. Neuroimage. 66:577–84. [DOI] [PubMed] [Google Scholar]
- Christakou A (2011). Maturation of limbic corticostriatal activa- tion and connectivity associated with developmental changes in temporal discounting. Neuroimage. 54:1344–1354. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Gallen CL, Jacobs EG, Lee TG, D’Esposito M (2014). Quantifying the reconfiguration of intrinsic networks during working memory. PLOS ONE. 9(9):e106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalli PE, Wapner S, Werner H (1962) Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 100:47–53. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE (2012) Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci 13(9), 636–650. Review. [DOI] [PubMed] [Google Scholar]
- Cservenka A, Stroup ML, Etkin A, Nagel BJ (2015). The effects of age, sex, and hormones on emotional conflict-related brain response during adolescence. Brain Cogn. 99:135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T (2015). Out of control: evidence for anterior insula involvement in motor impulsivity and reactive aggression. Soc Cogn Affect Neurosci. 10(4):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Zysset S, Müsseler J, von Cramon DY (2004). Where action impairs visual encoding: an event-related fMRI study. Brain Res Cogn Brain Res. 21(1):39–48. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J (2005). The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 24(2):539–547. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Boot WR, Basak C, Neider MB, Prakash RS, Voss MW, Graybiel AM, Simons DJ, Fabiani M, Gratton G, Kramer AF (2010) Striatal volume predicts level of video game skill acquisition. Cereb Cortex. 20(11):2522–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003) Cognitive and brain consequences of conflict. Neuroimage. 18(1):42–57. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE (2009). Marijuana craving in the brain. Proc Natl Acad Sci U S A. 106(31):13016–13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci, 8, 700–711. [DOI] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K (2015) The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–621. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence (2010). Cereb Cortex. 20(7):1613–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci, 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK (2012). Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 3(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101(21), 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E (1992). Optimizing the use of information: strategic control of activation of responses. Exp Psychol Gen. 121(4):480–506. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsu M, Yan M, Bilker W, Hughett P, Gur RE (1999) Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 19, 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER (2015). A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 10(3), e0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Hillman J, Biro FM, Ding L, Dorn LD, Susman EJ (2012). Correspondence between gonadal steroid hormone concentrations and secondary sexual characteristics assessed by clinicians, adolescents, and parents. J Res Adolesc. 22:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Kawato M (2009) Brain mechanisms for predictive control by switching internal models: implications for higher-order cognitive functions. Psychol Res 73(4), 527–544. [DOI] [PubMed] [Google Scholar]
- Ito M (1993) Synaptic plasticity in the cerebellar cortex and its role in motor learning. Can J Neurol Sci. 20 Supp 3:S70–74. Review. [PubMed] [Google Scholar]
- Juraska JM, Willing J (2017) Pubertal onset as a critical transition for neural development and cognition. Brain Res 1654(Pt B), 87–94. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science. 303(5660):1023–6. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, Stenger VA, Carter CS (2004) Prefrontal cortex guides context-appropriate responding during language production. Neuron. 43(2):283–291. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP (2009) Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 19(3):640–657. [DOI] [PubMed] [Google Scholar]
- Koch C, Brown JM (1994). Examining the time course of prime effects on Stroop processing. Percept Mot Skills. 79(1 Pt 2):675–687. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, Peper JS, Crone EA (2014). The influence of sex steroids on structural brain maturation in adolescence. PLoS One. 9:e83929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding DE, Chidekel D (2012) From movement to thought: executive function, embodied cognition, and the cerebellum. Cerebellum. 11(2):505–525. Review. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Schlund MW, Segreti AM (2018). Positive reinforcement modulates fronto-limbic systems subserving emotional interference in adolescents. Behav Brain Res. 338:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrue V, Celsis P, Bès A, Marc-Vergnes JP (1994) The functional anatomy of attention in humans: cerebral blood flow changes induced by reading, naming, and the Stroop effect. J Cereb Blood Flow Metab. 14(6):958–962. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM (2009). Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 47(3):663–670. [DOI] [PubMed] [Google Scholar]
- Leisman G, Braun-Benjamin O, Melillo R. 2014. Cognitive-motor interactions of the basal ganglia in development. Front Syst Neurosci. 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Zucker NL, Kragel PA, Covington VE, LaBar KS (2016) Adolescent development of insula-dependent interoceptive regulation. Dev Sci [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Burke J, Pardini DA (2009) Perspectives on oppositional defiant disorder, conduct disorder, and psychopathic features. J Child Psychol Psychiatry 50:133–142. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS (2005) The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 76(3):697–712. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA (2001). Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 13(5):786–793. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R (2015) An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 38:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288(5472), 1835–1838. [DOI] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B (2015) The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biol. 13(12):e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Etkin A, Thomason ME (2015) Disrupted insula-based neural circuit organization and conflict interference in trauma-exposed youth. Neuroimage Clin. 8:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P (2003). Conflict adaptation effects in the absence of executive control. Nat Neurosci. 6(5):450–452. [DOI] [PubMed] [Google Scholar]
- McCormick EM, Telzer EH (2018). Contributions of default mode network stability and deactivation to adolescent task engagement. Sci Rep. 8(1):18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod CM (1991). Half a century of research on the Stroop effect: an integrative review. Psychol. Bull 109:163–203. [DOI] [PubMed] [Google Scholar]
- Melcher T, Gruber O (2006). Oddball and incongruity effects during Stroop task performance: a comparative fMRI study on selective attention. Brain Res. 1121(1):136–149. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J (1984). Memories and habits: two neural systems In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of learning and memory. New York: Guilford: pp. 65–77. [Google Scholar]
- Müller-Oehring EM, Kwon D, Nagel BJ, Sullivan EV, Chu W, Rohlfing T, Prouty D, Nichols BN, Poline JB, Tapert SF, Brown SA, Cummins K, Brumback T, Colrain IM, Baker FC, De Bellis MD, Voyvodic JT, Clark DB, Pfefferbaum A, Pohl KM (2017) Influences of Age, Sex, and Moderate Alcohol Drinking on the Intrinsic Functional Architecture of Adolescent Brains. Cereb Cortex. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S; Brain Development Cooperative Group (2013). Testosterone-related cortical maturation across childhood and adolescence. Cereb Cortex. 23(6):1424–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Gower P, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins L, Ducharme S, McCracken JT (2016). The developmental relationship between DHEA and visual attention is mediated by structural plasticity of cortico-amygdalar networks. Psychoneuroendocrinology. 70:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Lew J, Albaugh MD, Botteron KN, Hudziak JJ, Fonov VS, Collins DL, Ducharme S, McCracken JT (2017). Sex-specific associations of testosterone with prefrontal-hippocampal development and executive function. Psychoneuroendocrinology. 76:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Wilbrecht L, Kriegsfeld LJ, Kayser AS, Dahl RE (2016). Risky decision-making in adolescent girls: The role of pubertal hormones and reward circuitry. Psychoneuroendocrinology. 74:77–91. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990) The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 87(1):256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A (1988) A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 17:117–133. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC (1999) An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 45(10):1237–1258. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Meloy MJ, Jernigan TL, Dale A, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Luna B, Chung T, Nagel BJ, Sullivan EV (2016) Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 26(10):4101–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, Brown SA, Colrain IM, Baker FC, Prouty D, De Bellis MD, Clark DB, Nagel BJ, Chu W, Park SH, Pohl KM, Sullivan EV (2018) Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry, 175(4):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarski DJ, Boivin JR, Wilbrecht L (2017). Ovarian Hormones Organize the Maturation of Inhibitory Neurotransmission in the Frontal Cortex at Puberty Onset in Female Mice. Curr Biol. 27(12):1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2002) Neural systems for perceptual skill learning. Behav Cogn Neurosci Rev. 1(1):76–83. Review. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ (2005) The neural correlates of motor skill automaticity. J Neurosci. 25(22):5356–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE (2010) The development of human functional brain networks. Neuron, 67(5):735–748. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purmann S, Pollmann S (2015) Adaptation to recent conflict in the classical color-word Stroop-task mainly involves facilitation of processing of task-relevant information. Front Hum Neurosci. 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2011). The restless brain. Brain Connect. 1(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Lee H, Logothetis NK (2004) The effect of learning on the function of monkey extrastriate visual cortex. PLoS Biol. 2(2):E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath J, Valyear KF, Philip BA, Frey SH (2017). Contributions of the parietal cortex to increased efficiency of planning-based action selection. Neuropsychologia. 105:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, Pipitone J, Chakravarty MM, Giedd JN (2014). Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc Natl Acad Sci U S A. 111(4):1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 28(11):1163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Lim L, Ecker C, Halari R, Giampietro V, Simmons A, Brammer M, Smith A (2013). Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage, 83:690–703. [DOI] [PubMed] [Google Scholar]
- Sali AW, Courtney SM, Yantis S (2016). Spontaneous Fluctuations in the Flexible Control of Covert Attention. J Neurosci. 36(2):445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saling LL, Phillips JG (2007). Automatic behaviour: efficient not mindless. Brain Res Bull. 73(1–3):1–20. [DOI] [PubMed] [Google Scholar]
- Salo R, Henik A, Robertson LC (2001). Interpreting Stroop interference: an analysis of differences between task versions. Neuropsychology. 15(4):462–471. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, Hopson R, Jackson C, Prabhakaran K, Bilker WB, Calkins ME, Loughead J, Smith A, Roalf DR, Hakonarson H, Verma R, Davatzikos C, Gur RC, Gur RE (2013). Functional maturation of the executive system during adolescence. J Neurosci. 33(41):16249–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, Elliott MA, Bilker WB, Calkins ME, Prabhakaran K, Davatzikos C, Hakonarson H, Gur RE, Gur RC (2014). Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry. 53(3):341–350.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. (2015). Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cereb Cortex. 25(9):2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Frisen L, Manzouri A, Nordenstrom A, Lindén Hirschberg A (2017). Role of testosterone and Y chromosome genes for the masculinization of the human brain. Hum Brain Mapp. 38(4):1801–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Finn ES, Tokoglu F, Shen X, Papademetris X, Hampson M, Constable RT (2015). Sex differences in normal age trajectories of functional brain networks. Hum Brain Mapp. 36(4):1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JR, Weissman DH (2016). Congruency sequence effects and previous response times: conflict adaptation or temporal learning? Psychol Res. 80(4):590–607. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM (2009) Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl). 206(1):1–21. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Zysset S, Wahl M, von Cramon DY (2004) Prefrontal activation due to Stroop interference increases during development--an event-related fNIRS study. Neuroimage. 23(4):1317–1325. [DOI] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Chanraud S, Rosenbloom MJ, Pfefferbaum A, Sullivan EV (2011). Age-related reorganization of functional networks for successful conflict resolution: a combined functional and structural MRI study. Neurobiol Aging. 32(11):2075–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Sullivan EV, Pfefferbaum A (2012) Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biol Psychiatry. 71(3):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmeczy D, Fandakova Y, Grimm KJ, Bunge SA, Ghetti S (2018). Longitudinal trajectories of hippocampal and prefrontal contributions to episodic retrieval: Effects of age and puberty. Dev Cogn Neurosci. S1878–9293(18)30132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X (2005) Sex differences in perceptual processing: performance on the color-Kanji stroop task of visual stimuli. Int J Neurosci. 115:1631–1641. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Luna B (2017) Protracted development of executive and mnemonic brain systems underlying working memory in adolescence: A longitudinal fMRI study. Neuroimage 157:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Galarce EM, Ladouceur CD, McMakin DL, Olino TM, Forbes EE, Silk JS, Ryan ND, Dahl RE (2015) Adolescent development of inhibition as a function of SES and gender: Converging evidence from behavior and fMRI. Hum Brain Mapp, 36(8), 3194–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, McKenna BS, Jacobus J, Castro N, Sorg SF, Tapert SF (2013) BOLD response to working memory not related to cortical thickness during early adolescence. Brain Res. 1537:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC (2016) The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci Biobehav Rev. 70:13–32. [DOI] [PubMed] [Google Scholar]
- Stroop JR (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol 12:643–662. [Google Scholar]
- Supekar K, Menon V (2012). Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol 8(2), e1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL (2002). Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 41(10):1231–1238. [DOI] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 30(9):2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L (2006). Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 27(8):694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremel JJ, Laurent PA, Wolk DA, Wheeler ME, Fiez JA (2016) Neural signatures of experience-based improvements in deterministic decision-making. Behav Brain Res. 315:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyborowska A, Volman I, Smeekens S, Toni I, Roelofs K (2016) Testosterone during Puberty Shifts Emotional Control from Pulvinar to Anterior Prefrontal Cortex. J Neurosci. 36(23):6156–6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Bylsma LM, Botvinick MM (2005). The conflict adaptation effect: it’s not just priming. Cogn Affect Behav Neurosci. 5(4):467–472. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde AC (2016) What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci. Biobehav. Rev 70, 135–147. [DOI] [PubMed] [Google Scholar]
- Veroude K, Jolles J, Croiset G, Krabbendam L (2013). Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev Cogn Neurosci. 5:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL (2010) Development of the Brain’s Functional Network Architecture. Neuropsychol Rev. 20(4):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, Loeber R (2011) Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcohol Clin Exp Res 35(2), 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Bos MGN, Schreuders E, Vd Kamp F, Peper JS, Tamnes CK, Crone EA (2018). Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology. 91:105–114. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ (2006) Wide range achievement test (WRAT4) Psychological Assessment Resources, Lutz. [Google Scholar]
- Wu T, Hallett M (2005) The influence of normal human ageing on automatic movements. J Physiol. 562(Pt 2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Cao M, Niu H, Zuo XN, Evans A, He Y, Dong Q, Shu N (2015) Age-related changes in the topological organization of the white matter structural connectome across the human lifespan. Hum Brain Mapp. 36(10):3777–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.