Abstract
Background
Abnormal restricted diffusion on magnetic resonance imaging is often associated with ischemic stroke or anoxic injury, but other conditions can present similarly. We present six cases of an unusual but consistent pattern of restricted diffusion in bilateral hippocampi and cerebellar cortices. This pattern of injury is distinct from typical imaging findings in ischemic, anoxic, or toxic injury, suggesting it may represent an under-recognized clinicoradiographic syndrome. Despite initial presentation with stupor or coma in the context of obstructive hydrocephalus, patients may have acceptable outcomes if offered early intervention.
Methods
We identified an ad hoc series of patients at our two institutions between years 2014 and 2017 who presented to the neurocritical care unit with severe, otherwise unexplained cerebellar edema and retrospectively identified several commonalities in history, presentation, and imaging.
Results
Between two institutions, we identified six patients—ages 33–59 years, four male—with similar presentations of decreased level of consciousness in the context of intoxicant exposure, with acute cytotoxic edema of the cerebellar cortex, hippocampi, and aspects of the basal nuclei. All patients presented with severe cerebellar edema which led to obstructive hydrocephalus requiring aggressive medical and/or surgical management. The five patients who survived to discharge demonstrated variable degrees of physical and memory impairment on discharge and at follow-up.
Conclusions
We present findings of a potentially novel syndrome involving a distinct pattern of cerebellar and hippocampal restricted diffusion, with imaging and clinical characteristics distinct from ischemic stroke, hypoxic injury, and known toxidromes and leukoencephalopathies. Given the potential for favorable outcome despite early obstructive hydrocephalus, early identification and treatment of this syndrome are critical.
Keywords: Acute brain injuries, Cerebral edema, Drug overdose, Cerebellar syndromes, Hippocampus proper
Introduction
Cytotoxic edema, identified by restricted diffusion on magnetic resonance imaging (MRI), can be associated with acute ischemic stroke, hypoxic-ischemic encephalopathy (HIE) [1], posterior reversible encephalopathy syndrome (PRES) [2], and various toxidromes [3] including the use of inhaled opiates (i.e., “chasing the dragon”) [4]. When cerebellar and hippocampal edema are seen in HIE from anoxic injury or cardiac arrest, almost invariably other areas of high metabolic demand are also affected, including the cerebral cortex, thalamus, and subcortical white matter [5–7]. Acute obstructive hydrocephalus is not an expected complication of HIE [8]. When patients present unresponsive, with multifocal areas of restricted diffusion accompanied by obstructive hydrocephalus, medical or surgical interventions may not be offered out of the belief that cerebellar edema represents devastating and irreversible cell death, and that patients with these patterns of injury have minimal chance for neurologic improvement despite intervention.
Here, we describe a unique series of cases with a clinicoradiographic syndrome of stupor or coma with imaging findings of bilaterally symmetric abnormally restricted diffusion in the cerebellar cortex, hippocampi, and variable aspects of the basal nuclei. This pattern of acute injury is distinct from previously described conditions and may represent an under-recognized clinical syndrome we term Cerebellar Hippocampal And basal Nuclei Transient Edema with Restricted diffusion (CHANTER) Syndrome. Despite the initial severity of presentation, patients who survive CHANTER syndrome may demonstrate clinical and imaging improvement over time.
Methods
We identified an ad hoc series of patients seen at the University of Cincinnati Medical Center and University of Tennessee Methodist University Hospital between years 2014 and 2017 who presented to the neurocritical care unit with severe, otherwise unexplained cerebellar edema and retrospectively identified several commonalities in history, presentation, and imaging.
Results
Demographics and Clinical Presentation
We identified four patients at the University of Cincinnati Medical Center and two at University of Tennessee Methodist University Hospital with similar imaging findings of severe, bilateral, otherwise unexplained cerebellar cortical edema, identified by restricted diffusion on MRI. In all cases, cerebellar edema was accompanied by restricted diffusion in the bilateral hippocampi and basal nuclei, but sparing the cerebral cortex. Patients ranged in age from 33 to 59 years; two were female and four were male. Five had a prior history of exposure to drugs of abuse and one to prescribed narcotics. Three patients were brought to the emergency department after being found unresponsive in the context of acute opiate exposures; one was brought to the emergency department after using ethanol and stimulants and developing decreased level of consciousness; and one unresponsive after exposures to multiple substances including ethanol, cocaine, and benzodiazepines. One patient received hydromorphone in a post-anesthesia care unit following an elective gastroenterological surgery and became unresponsive and apneic. Patient characteristics, hospital course, and findings are given in Table 1.
Table 1.
Patient characteristics and outcomes
| Patient 1: 54F | Patient 2: 59M | Patient 3: 49F | Patient 4: 50M | Patient 5: 35M | Patient 6: 33M | |
|---|---|---|---|---|---|---|
| Acute exposure | Hydromorphone | BZD, cocaine, opiates | Heroin | Cocaine, fentanyl | Amphetamines, etOH | Cocaine, BZD, etOH |
| Clinical presentation | Unresponsive, apneic | Unresponsive, hypoxic, extensor posturing | Found unresponsive, cyanotic | Found unresponsive; last well 2 days prior | Encephalopathy, decreased activity | Found unresponsive |
| Initial GCS | 3 | 5 | 7 | 3 | 11 | 3 |
| Hospital interventions | MV, HTS, EVD, SubOcc | MV, HTS, EVD, SubOcc | MV, HTS, EVD, SubOcc | MV, HTS, EVD; had preexisting SubOcc | HTS | MV, HTS |
| Disposition | Trach/PEG, acute rehabilitation | In-hospital death | PEG, shelter home | SNF | Acute rehabilitation | Home with supervision |
| Follow-up | 2 months: moderate cognitive impairment; mild–moderate weakness, wheelchair-bound | n/a | 1 month: mild dysarthria only | 6 months: cognitive and memory deficits; living at nursing home | 3 months: abulia, memory and cognitive issues, mild hemiparesis; living with family | 2 years: end-stage renal disease; living alone with home health |
BZD benzodiazepines, etOH ethanol, EVD extraventricular drain, F female, GCS Glasgow Coma Scale, HTS hypertonic saline, M male, MV mechanical ventilation, PEG percutaneous endoscopic gastrostomy, SNF skilled nursing facility, SubOcc suboccipital decompression, Trach tracheostomy
Imaging Findings
All patients were identified as having acute cerebellar edema on initial evaluation, with progressive worsening. Obstructive hydrocephalus developed in all patients between 0 and 6 days from presentation. Computed tomography at all patients’ time of presentation to tertiary care center is shown in Fig. 1. MRI was performed in all patients and revealed true diffusion restriction (diffusion-weighted imaging hyperintensity corresponding to apparent diffusion coefficient hypointensity) in a bilaterally symmetric distribution in the gray matter of the cerebellar cortex and hippocampi, and asymmetrically in the basal nuclei, sparing the cerebral cortex (Fig. 2). MRI of patient #5 demonstrated additional areas of restricted diffusion in the posterior cerebral white matter; however, no other patients had subcortical white matter involvement. All patients underwent evaluation for other potential etiologies, including electroencephalography and vessel imaging, which did not reveal alternative explanations for the clinical and imaging findings.
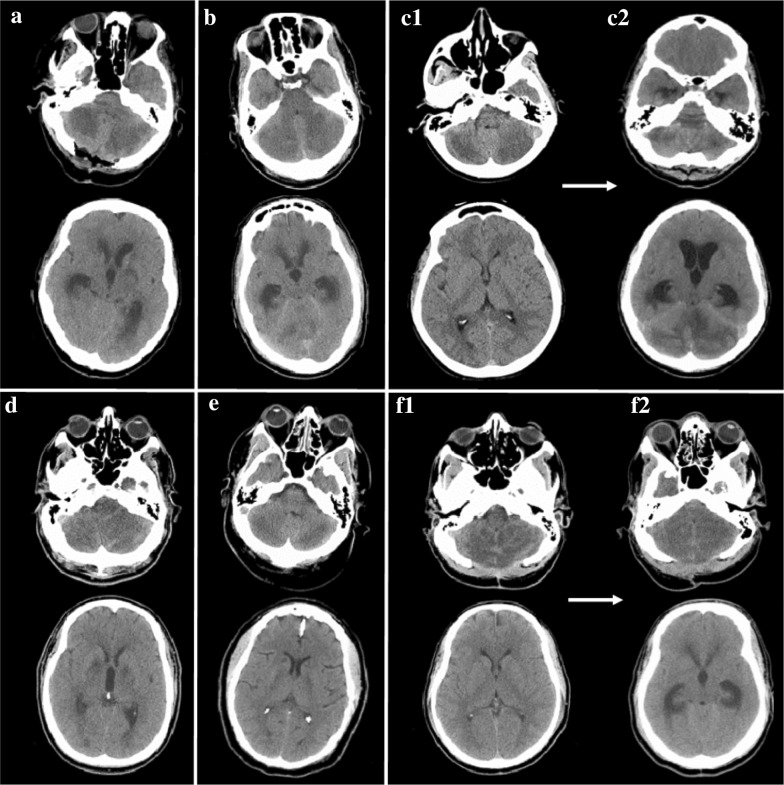
Fig. 1.
Non-contrast computed tomography of the head from patients at the time of arrival or transfer to tertiary care center, at the level of the cerebellum (above) and the lateral ventricles (below). A Patient #1, CT on arrival to tertiary care center, 3 days after initial event; emergent suboccipital decompression was completed on arrival; B Patient #2, at time of presentation, ~ 48 h after last well; C1 Patient #3, on arrival; C2 Patient #3 on hospital day 6, demonstrating evolution of cerebellar edema with obstructive hydrocephalus; D Patient #4, at time of presentation, ~ 24 h from last well; E Patient #5, at time of presentation, ~ 24 h from last well; F1 Patient #4, at time of presentation, ~ 48 h from last well; suboccipital decompression is from a previous event; F2 Patient #4 on hospital day 4, demonstrating evolution of cerebellar edema with obstructive hydrocephalus
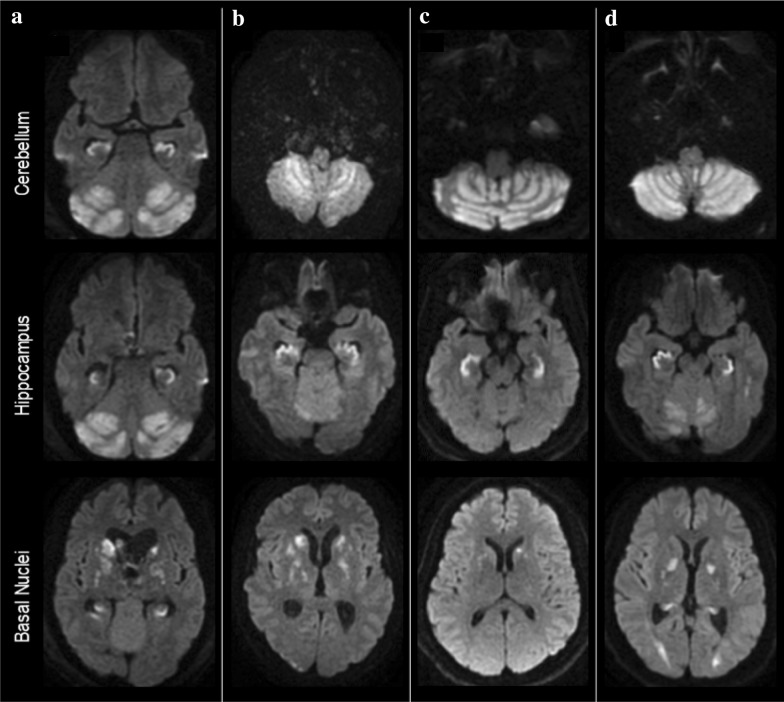
Fig. 2.
Diffusion-weighted magnetic resonance imaging by patient and brain region demonstrating cytotoxic edema in the cerebellar cortices, hippocampi, and basal ganglia of patients #1 (b), #2 (a), #4 (c), and #5 (d). MRI of patients #3 and #6 (not pictured) showed similar patterns
Clinical Course and Outcomes
All patients were admitted to a neurocritical care unit, and all received osmotic therapy with hypertonic saline and/or mannitol. Five of six patients required intubation and mechanical ventilation. Three patients required extraventricular drains for cerebrospinal fluid (CSF) flow diversion and subsequently underwent suboccipital decompressive craniectomy. One patient had suffered an insult 3 years prior, initially diagnosed as a cerebellar ischemic stroke, which was associated with disproportionate cerebellar edema causing obstructive hydrocephalus which required suboccipital decompression at that time. During the hospitalization described in the current series, he required extraventricular drain placement.
One patient died during the hospitalization following brain herniation despite aggressive medical and surgical management. Two patients improved to the point of being discharged to home, two were discharged to acute care rehabilitation, and one to a nursing facility. At follow-up, ranging from 1 month to 2 years after the index event, two patients were functionally independent, two lived at home with assistance, and one required care in a skilled nursing facility, primarily due to profound antegrade amnesia. Repeat MRI was available on patient #4 (9 months after index event) and patient #5 (3 months); both demonstrated resolution of restricted diffusion with marked improvement in T2 hyperintensities, with areas of cerebellar laminar necrosis and hippocampal atrophy (Fig. 3).
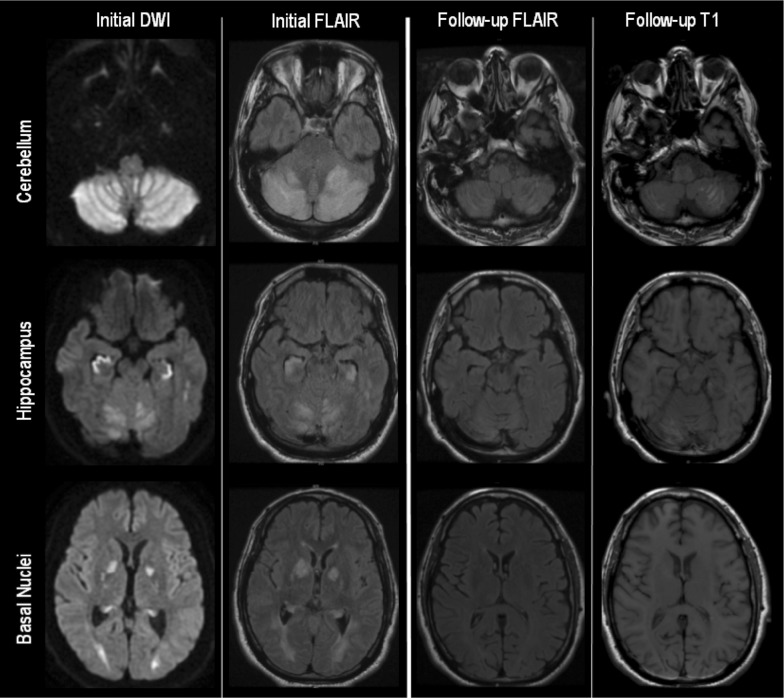
Fig. 3.
Patient #5 MRI brain, selected sequences, initial and three-month follow-up. DWI diffusion-weighted imaging, FLAIR fluid-attenuation inversion recovery, MRI magnetic resonance imaging. Evolution of imaging changes between initial presentation and repeat imaging 3 months later. Abnormal restricted diffusion was not present on follow-up imaging (not shown)
Discussion
All patients described in this series presented after acute exposure to opiates or other drugs of abuse and presented with decreased level of consciousness, with obstructive hydrocephalus secondary to cerebellar edema and with MRI findings of restricted diffusion in the cerebellar cortex, hippocampi, and basal nuclei. With aggressive medical and surgical management, most patients were able to live at home with variable degrees of assistance; this may be considered a favorable outcome given the life-threatening nature of the initial injury.
We propose that this constellation of findings represents a condition we term CHANTER syndrome. CHANTER is distinct from other recognized syndromes including acute ischemic stroke, anoxic brain injury, PRES, and described toxic and metabolic injuries. CHANTER shares some clinical and radiographic features with recently described cases of hippocampal restricted diffusion [9–11] and may represent a more severe spectrum of a similar condition.
It is important to distinguish CHANTER from other known clinical entities. Restricted diffusion in CHANTER syndrome may be mistaken for ischemic stroke, but the CHANTER syndrome pattern is not associated with a vascular distribution and does not correspond to vessel occlusions on angiographic imaging.
Heroin-associated spongiform leukoencephalopathy (HASL) is a toxidrome associated with inhaling heroin vapors in a manner termed “chasing the dragon.” It is characterized primarily by extensive, confluent white matter degeneration in the cerebrum and cerebellum [4, 12], in contrast to the gray matter involvement seen in CHANTER syndrome. It is typically described as a subacute process [12] and may not be associated with restricted diffusion [13]. We believe the acute onset of prominent cerebellar and hippocampal restricted diffusion, in the absence of significant white matter injury, distinguishes CHANTER from HASL.
Similar to HASL, PRES is an entity typically associated with predominantly white matter injury. While PRES can cause posterior fossa edema [2], and occasionally involves the basal nuclei [14], it primarily consists of white matter disease which is essentially absent in CHANTER syndrome.
Specific toxic or metabolic insults could produce gray matter injury, but all have significant imaging and clinical differences than CHANTER syndrome, as given in Table 2 [15–20]. One case report describes a patient exposed to carbon monoxide and benzodiazepines who developed injury in a pattern similar to CHANTER [21].
Table 2.
Distinctive features of CHANTER syndrome, selected differential diagnoses, and similar cases
| Etiology | Clinical presentation | Injury/imaging pattern | Other notes |
|---|---|---|---|
| Syndromes | |||
| CHANTER | Acute ↓LOC | Bilateral cbel + hippocampi +/− BN | Risk of obstructive HCP |
| Acute ischemic stroke | Focal neurologic deficits | DWI+ in a vascular distribution | +/− Evidence of vascular occlusion |
| HASL (“chasing the dragon”) [4, 12, 13] | Strength or movement abnormalities, ataxia; frequently subacute | Predominantly white matter; unlikely DWI+ | |
| PRES [2, 14] | Variable; +/− headache, vision changes, AMS, seizure | Predominantly white matter | Specific provoking factors |
| Anoxic injury [8, 23–25] | ↓LOC | Cerebral cortex +/− cbel, hippocampi, BN | Not typically associated with obstructive HCP |
| Carbon monoxide (CO) [15, 16, 20, 21] | Headache, AMS | Globus pallidus + BN > cbel + brainstem | Clinical exposure |
| Cyanide (CN) [17] | Headache, agitation, seizures | BN +/− hippocampi; cbel spared | Clinical exposure |
| Mercury (Hg) [18] | Acute: systemic symptoms; chronic: personality changes, erethism | Punctate lesions or degeneration without acute edema or DWI+ | Clinical exposure |
| Similar cases | |||
| Small/Barash et al. [9, 10] | Memory impairment | Hippocampal DWI+ | |
| Bhattacharyya et al. [11] | Various | Hippocampal and other DWI+ areas | |
| Pediatric opiate overdoses [26–31] | ↓LOC | +/− Cerebellar edema | Limited examples with MRI to show potential other areas of injury |
AMS altered mental status, BN basal nuclei, cbel cerebellum, CHANTER Cerebellar Hippocampal And basal Nuclei Transient Edema with Restricted diffusion, DWI+ hyperintensity on diffusion-weighted imaging, HASL heroin-associated spongiform leukoencephalopathy, HCP hydrocephalus, LOC level of consciousness, PRES posterior reversible encephalopathy syndrome, +/− with or without, > more frequently than
Anoxic brain injury following cardiac or respiratory arrest can cause restricted diffusion within gray matter of the hippocampus and cerebellum, specifically within Purkinje cells [22], but this is typically seen co-occurring with cortical gray matter diffusion restriction [23, 24]. Diffuse anoxic injury can be associated with diffuse cerebral edema, but is not typically associated with malignant cerebellar edema and obstructive hydrocephalus [8, 25]. If CHANTER could be considered a variant form of HIE, the disproportionate cerebellar edema with sparing of the cerebral cortex is notable.
CHANTER syndrome may represent a primary metabolic or mitochondrial failure of gray matter with some degree of anoxic injury precipitated by opiates or other drugs of abuse. Single case reports have described cerebellar edema after exposure to opiates, particularly in the pediatric population, either in isolation [26, 27] or with hippocampal [28] or other patterns of injury [29–31]. There are adult case reports of opiate exposure associated with cerebellar edema and hippocampal [32] or basal nuclei damage [33, 34] with some similarities to the cases presented here, although two of these adult cases involved heroin inhalation and were thought to represent HASL-like toxic leukoencephalopathies.
Individuals with active or historical opiate abuse who developed bilateral hippocampal diffusion restriction have been identified, including some with basal nuclei involvement [9]. Recognition of this pattern of injury prompted an alert by the Massachusetts Centers for Disease Control, leading to the identification of ten additional patients with similar findings [10]. The key difference from our case series is the absence of prominent cerebellar edema. A separate case series described sixteen patients with bilateral hippocampal restricted diffusion, including half occurring in the context of recreational drug exposures (most commonly narcotics), and half presumed related to cardiac arrest, seizure, or other etiologies [11]. A majority of patients in this series demonstrated extrahippocampal areas of restricted diffusion, frequently involving the basal nuclei and/or cerebellum. These cases, in conjunction with our own, may suggest the presence of a common pathway and a spectrum of disease severity, with the life-threatening cerebellar edema of CHANTER syndrome representing the most severe end, and isolated hippocampal injury a milder presentation of the same condition. In our series, imaging characteristics and the timing of edema progression are consistent with a pattern of cytotoxic edema, which can explain the development of obstructive hydrocephalus.
Similarities between CHANTER syndrome and these cases raise the possibility of CHANTER as an opiate-provoked toxidrome [35], perhaps exacerbated in the context of relative hypoxia [36]. This concept is indirectly supported by pathologic data. Postmortem analyses of heroin users have identified hippocampal Purkinje cell loss and evidence of reactive micro- or astroglial reactivity believed to be related to a combination of hypoxic and neurotoxic effects [37]. Fentanyl and related synthetic opiates have been linked to focal brain injury, including in the hippocampus [38], suggesting a potential pathophysiologic association. It is possible that interventions such as naloxone—received by patients described individually in the literature [26, 28–32, 34] as well as our cases—offer relative neuroprotection to the cerebral cortex and white matter [39], areas classically implicated in anoxic injury but relatively spared in CHANTER and similar cases. If indeed an opiate-associated phenomenon, the identification of CHANTER syndrome has important clinical implications in light of the ongoing opiate epidemic [40, 41].
While this case series reflects a consistent pattern of clinical and radiographic findings, it shares limitations inherent to all case series. Individuals came to our attention due to otherwise unexplained cerebellar edema resulting evaluation and management in a neurocritical care unit; it is likely that similar cases may not have been identified by virtue of triage to other intensive care units and/or early death due to herniation. While substance use, in particular with opiates, appears to be a commonality between a majority of our patients, and between our patients and those described in the literature, the documented acute exposure in our cases is heterogeneous. This may indicate a variety of provoking factors for a similar syndrome, or it may reflect an underdiagnosis of exposures, such as synthetic opiates that are not a part of standard toxicology assays [42]. An additional limitation is that we do not have histopathological confirmation of the mechanism and precise location of cellular injury. Nonetheless, the strikingly similar clinicoradiographic presentations in our case series suggest a common pathophysiologic process, and the potential need for urgent intervention highlights the importance of recognizing CHANTER syndrome as a potential novel clinicopathologic entity.
Conclusions
We describe a case series with a clinicoradiographic syndrome of acute, life-threatening cerebellar edema causing obstructive hydrocephalus, possibly provoked by opiates or other drugs of abuse, and associated with symmetric restricted diffusion limited to gray matter of the cerebellum, hippocampus, and basal nuclei. With aggressive medical and/or surgical management of hydrocephalus, some patients are able to achieve reasonable functional outcomes. It is important to differentiate this entity from other disease processes, including ischemic stroke, HASL, or anoxic brain injury. Further work is needed to better understand this syndrome, its risk factors, treatment, and outcomes.
Acknowledgements
The authors wish to thank Dr. M. Taimur Shujaat for his assistance with imaging review and Ms. Lindsey Kuohn for her assistance in figure design.
Author Contributions
ASJ: conception of work; analysis and interpretation of data; drafting of manuscript. KHA: acquisition and analysis of data; revision of manuscript. MSS: analysis and interpretation of data; drafting of manuscript. AP: acquisition and analysis of data; revision of manuscript. AV: analysis and interpretation of data; revision of manuscript. DK: conception of work; acquisition, analysis, and interpretation of data; revision of manuscript; project supervision.
Source of Support
Dr. Jasne’s work was supported by an NIH Training Grant, T32 NS47996-10.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study has been approved by the University of Cincinnati IRB.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ginsberg MD, Hedley-Whyte ET, Richardson EP. Hypoxic-ischemic leukoencephalopathy in man. Arch Neurol. 1976;33(1):5–14. doi: 10.1001/archneur.1976.00500010007002. [DOI] [PubMed] [Google Scholar]
- 2.Grossbach AJ, Abel TJ, Hodis B, Wassef SN, Greenlee JDW. Hypertensive posterior reversible encephalopathy syndrome causing posterior fossa edema and hydrocephalus. J Clin Neurosci. 2014;21(2):207–211. doi: 10.1016/j.jocn.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Sarna JR, Brownell AKW, Furtado S. Cases: reversible cerebellar syndrome caused by metronidazole. CMAJ. 2009;181(9):611–613. doi: 10.1503/cmaj.090591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters EC, Stam FC, Lousberg RJ, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;320(8310):1233–1237. doi: 10.1016/S0140-6736(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez LG, Rovira À, Portela LAP, Leite CC, Lucato LT. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: radiological findings with pathophysiological correlations. Neuroradiology. 2010;52(11):949–976. doi: 10.1007/s00234-010-0728-z. [DOI] [PubMed] [Google Scholar]
- 6.Howard RS, Holmes PA, Koutroumanidis MA. Hypoxic-ischaemic brain injury. Pract Neurol. 2011;11(1):4–18. doi: 10.1136/jnnp.2010.235218. [DOI] [PubMed] [Google Scholar]
- 7.Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. Am J Neuroradiol. 1999;20(6):999–1007. [PMC free article] [PubMed] [Google Scholar]
- 8.Inamasu J, Miyatake S, Suzuki M, et al. Early CT signs in out-of-hospital cardiac arrest survivors: temporal profile and prognostic significance. Resuscitation. 2010;81(5):534–538. doi: 10.1016/j.resuscitation.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Small JE, Butler PM, Zabar Y, Barash JA. Complete, bilateral hippocampal ischemia: a case series. Neurocase. 2016;22(5):411–415. doi: 10.1080/13554794.2016.1213299. [DOI] [PubMed] [Google Scholar]
- 10.Barash JA. Cluster of an unusual amnestic syndrome—Massachusetts, 2012–2016. MMWR Morb Mortal Wkly Rep [Internet] 2017 [cited 2017 Aug 18];66. https://www.cdc.gov/mmwr/volumes/66/wr/mm6603a2.htm. [DOI] [PMC free article] [PubMed]
- 11.Bhattacharyya S, Gholipour T, Colorado RA, Klein JP. Bilateral hippocampal restricted diffusion: same picture many causes. J Neuroimaging. 2017;27(3):300–305. doi: 10.1111/jon.12420. [DOI] [PubMed] [Google Scholar]
- 12.Keogh CF, Andrews GT, Spacey SD, Forkheim KE, Graeb DA. Neuroimaging features of heroin inhalation toxicity: “chasing the dragon”. Am J Roentgenol. 2003;180(3):847–850. doi: 10.2214/ajr.180.3.1800847. [DOI] [PubMed] [Google Scholar]
- 13.Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63(2):146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol. 2007;28(7):1320–1327. doi: 10.3174/ajnr.A0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephen RA, Donal SW, Siobhain OB, Michael CR, Daniel CJ. Carbon monoxide poisoning: novel magnetic resonance imaging pattern in the acute setting. Int J Emerg Med. 2012;5(1):30. doi: 10.1186/1865-1380-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell P, Buxton PJ, Pitkin A, Jarvis LJ. The magnetic resonance imaging appearances of the brain in acute carbon monoxide poisoning. Clin Radiol. 2000;55(4):273–280. doi: 10.1053/crad.1999.0369. [DOI] [PubMed] [Google Scholar]
- 17.Mohan A., Lee T., Sachdev P. Surviving acute cyanide poisoning: a longitudinal neuropsychological investigation with interval MRI. Case Reports. 2014;2014(mar19 1):bcr2013203025–bcr2013203025. doi: 10.1136/bcr-2013-203025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korogi Y, Takahashi M, Okajima T, Eto K. Invited. MR findings of Minamata disease—organic mercury poisoning. J Magn Reson Imaging. 1998;8(2):308–316. doi: 10.1002/jmri.1880080210. [DOI] [PubMed] [Google Scholar]
- 19.Sarafian T, Hagler J, Vartavarian L, Verity MA. Rapid cell death induced by methyl mercury in suspension of cerebellar granule neurons. J Neuropathol Exp Neurol. 1989;48(1):1–10. doi: 10.1097/00005072-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lo C-P, Chen S-Y, Lee K-W, et al. Brain injury after acute carbon monoxide poisoning: early and late complications. Am J Roentgenol. 2007;189(4):W205–W211. doi: 10.2214/AJR.07.2425. [DOI] [PubMed] [Google Scholar]
- 21.Guerra-Schulz E, Pinel A, Montero P, De Miguel C. Magnetic resonance imaging findings after acute carbon monoxide poisoning. Neurol Engl Ed. 2015;30:526–527. doi: 10.1016/j.nrl.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Ellison D, Love S, Chimelli LMC, et al. Neuropathology E-book: a reference text of CNS pathology. Amsterdam: Elsevier; 2012. [Google Scholar]
- 23.White ML, Zhang Y, Helvey JT, Omojola MF. Anatomical patterns and correlated MRI findings of non-perinatal hypoxic–ischaemic encephalopathy. Br J Radiol. 2013;86(1021):20120464. doi: 10.1259/bjr.20120464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlynash M, Campbell DM, Leproust EM, et al. Temporal and spatial profile of brain diffusion-weighted MRI after cardiac arrest. Stroke. 2010;41(8):1665–1672. doi: 10.1161/STROKEAHA.110.582452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayman EG, Patel AP, Kimberly WT, Sheth KN, Simard JM. Cerebral edema after cardiopulmonary resuscitation: a therapeutic target following cardiac arrest? Neurocrit Care. 2017;28:276–287. doi: 10.1007/s12028-017-0474-8. [DOI] [PubMed] [Google Scholar]
- 26.Hosseini F, Nikkhah A. Acute cerebellitis following opium intoxication: a case report and literature review. J Pediatr Rev [Internet] 2017 [cited 2017 Jul 10];5(1). http://jpediatricsreview.com/en/articles/8803.html.
- 27.Bazmamoun H, Fayyazi A, Khajeh A, Sabzehei MK, Khezrian F. A study of methadone-poisoned children referred to Hamadan’s Besat Hospital/Iran. Iran J Child Neurol. 2014;8(2):34–37. [PMC free article] [PubMed] [Google Scholar]
- 28.Anselmo M, Rainho AC, do Carmo Vale M, et al. Methadone intoxication in a child: toxic encephalopathy? J Child Neurol. 2006;21(7):618–620. doi: 10.1177/08830738060210071101. [DOI] [PubMed] [Google Scholar]
- 29.Nanan R, von Stockhausen HB, Petersen B, Solymosi L, Warmuth-Metz M. Unusual pattern of leukoencephalopathy after morphine sulphate intoxication. Neuroradiology. 2000;42(11):845–848. doi: 10.1007/s002340000442. [DOI] [PubMed] [Google Scholar]
- 30.Reisner A, Hayes LL, Holland CM, et al. Opioid overdose in a child: case report and discussion with emphasis on neurosurgical implications. J Neurosurg Pediatr. 2015;16(6):752–757. doi: 10.3171/2015.4.PEDS14667. [DOI] [PubMed] [Google Scholar]
- 31.Zanin A, Masiero S, Severino MS, Calderone M, Da Dalt L, Laverda AM. A delayed methadone encephalopathy: clinical and neuroradiological findings. J Child Neurol. 2010;25(6):748–751. doi: 10.1177/0883073809343318. [DOI] [PubMed] [Google Scholar]
- 32.Gupta PK, Krishnan PR, Sudhakar PJ. Hippocampal involvement due to heroin inhalation—“chasing the dragon”. Clin Neurol Neurosurg. 2009;111(3):278–281. doi: 10.1016/j.clineuro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Bui DH, Pace J, Manjila S, et al. Heroin inhalation complicated by refractory hydrocephalus: a novel presentation. Neurology. 2015;84(20):2093–2095. doi: 10.1212/WNL.0000000000001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odia YM, Jinka M, Ziai WC. Severe leukoencephalopathy following acute oxycodone intoxication. Neurocrit Care. 2010;13(1):93–97. doi: 10.1007/s12028-010-9373-y. [DOI] [PubMed] [Google Scholar]
- 35.Barash JA, Lev MH. Opioid-associated acute hippocampal injury with cardiac arrest. Radiology. 2018;289(2):315. doi: 10.1148/radiol.2018181379. [DOI] [PubMed] [Google Scholar]
- 36.Haut MW, Hogg JP, Marshalek PJ, Suter BC, Miller LE. Amnesia associated with bilateral hippocampal and bilateral basal ganglia lesions in anoxia with stimulant use. Front Neurol [Internet] 2017 [cited 2017 Oct 17];8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5296355/. [DOI] [PMC free article] [PubMed]
- 37.Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol (Berl) 1996;91(6):642–646. doi: 10.1007/s004010050478. [DOI] [PubMed] [Google Scholar]
- 38.Kofke WA, Blissitt PA, Rao H, Wang J, Addya K, Detre J. Remifentanil-induced cerebral blood flow effects in normal humans: dose and ApoE genotype. Anesth Analg. 2007;105(1):167–175. doi: 10.1213/01.ane.0000266490.64814.ff. [DOI] [PubMed] [Google Scholar]
- 39.Sinz EH, Kofke WA, Garman RH. Phenytoin, midazolam, and naloxone protect against fentanyl-induced brain damage in rats. Anesth Analg. 2000;91(6):1443. doi: 10.1097/00000539-200012000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Hsu DJ, McCarthy EP, Stevens JP, Mukamal KJ. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001–12. Addict Abingdon Engl. 2017;112(9):1558–1564. doi: 10.1111/add.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedegaard H, Bastian B, Trinidad J, Spencer M, Warner M. National Vital Statistics Reports, vol. 67, no. 9, 12 Dec 2018. p. 14. [PubMed]
- 42.Barash JA, Ganetsky M, Boyle KL, et al. Acute amnestic syndrome associated with fentanyl overdose. N Engl J Med. 2018;378(12):1157–1158. doi: 10.1056/NEJMc1716355. [DOI] [PubMed] [Google Scholar]