Abstract
Purpose
Staphylococcus aureus carriage poses an increased risk of S. aureus infection. The aim of this study was to investigate the colonization of S. aureus among healthy individuals and to establish a prospective cohort and biobank for research in the health consequences of colonization.
Population and methods
The Danish Blood Donor S. aureus Carriage Study (DBDSaCS) was established in 2014. So far, a total of 6082 healthy participants have been included with nasal swabs and repeated swabs are performed at subsequent donations. Samples from the first 2217 participants were cultured using a two-step method to evaluate the effect of using enrichment broth. Furthermore, 262 participants were sampled from both the nares and the throat. All participants completed a questionnaire with self-reported health, anthropometric measurements, current smoking status, and physical activity. Plasma samples, nasal swab transport media, and S. aureus isolates were stored.
Results
The prevalence of S. aureus nasal colonization was 41%. The prevalence of colonization was higher in men (46%) than women (34%), lower for smokers, and decreased with increasing age (<25 years: 44% vs >55 years: 35%). In participants swabbed from the nose and throat, the prevalence of S. aureus colonization after enrichment was 55% with significantly higher prevalence in the throat (45%) than in the nose (40%). The use of an enrichment broth increased the proportion of S. aureus colonization.
Conclusion
We describe a large and growing cohort of healthy individuals established to investigate predictors for S. aureus carriage and the health consequences of carriage. Multiple projects using data from DBDSaCS linked with Danish health registers, biomarkers, and genetic markers are ongoing. Results will be published in the coming years.
Keywords: prospective cohort study, Staphylococcus aureus, colonization, epidemiology, blood donor health, risk factors
Introduction
The aim of the Danish Blood Donor Staphylococcus aureus Carriage Study (DBDSaCS) established in 2014 is to investigate the colonization of S. aureus among healthy individuals and to establish a prospective cohort and biobank for research purposes. The DBDSaCS originates from the Danish Blood Donor Study (DBDS, www.dbds.dk).1 The DBDSaCS is unique allowing investigation of the mechanisms involved in S. aureus carriage and the influence of carriage on health and the risk of disease among otherwise healthy individuals.
S. aureus is a bacterium that colonizes the skin and mucous membranes of humans and animals; up to 50% of the healthy adult population are carriers of S. aureus in the nose either intermittently or persistently.2,3 The anterior nares are believed to be the most important colonization site for S. aureus.4–6 However, several studies have highlighted the importance of throat colonization, as throat carriage frequency has been reported to range between 30% and 47%.7–9 Rapid diagnostic tests are often used to determine S. aureus carriage because molecular diagnostic methods, when compared to culture methods, can reduce the “turnaround time” in the laboratory and, in the case of methicillin-resistant S. aureus (MRSA), reduce costs by avoiding any unnecessary isolation days.10 However, culture methods remain highly important as molecular tests have been shown to produce both false-negative and false-positive results.11,12 Culture methods are furthermore less expensive and central for susceptibility testing and surveillance.
Apart from being a commensal in the nasal and throat cavity, S. aureus is also a potent pathogen which may cause a variety of diseases, ranging from simple skin infections to life-threatening soft tissue infections, deep abscesses, arthritis, osteomyelitis, endocarditis, and bacteremia.13 S. aureus is a dominant nosocomial pathogen in both community and health care settings worldwide.14,15 Moreover, S. aureus is the second most frequent pathogen in bloodstream infections in Denmark and accounted for 18% of all bloodstream infections in 2017 (www.danmap.org).16 Invasive S. aureus infections are associated with a high morbidity and mortality, and although colonization itself is harmless, carriers are at increased risk of infection.2,3,17–21 In case of resistance toward various antibiotics, such as in MRSA which is resistant to most of the beta-lactam group of antibiotics complicates treatment of S. aureus infections and constitute an increasing burden on health care resources.15,22 Hence, knowledge of S. aureus carriage and effective measures to prevent S. aureus infections are important.
The mechanisms involved in the transition from carriage to infection are not completely understood but infection is probably a result of both bacterial and host factors.23,24 The causal relation between S. aureus nasal carriage and infection is supported by the fact that decolonization of the nose by nasal application of mupirocin among carriers of S. aureus prior to surgery reduces the risk of subsequent infection.25,26 However, the effect of nasal carriage among healthy individuals on the risk of subsequent infection is not known and therefore there is a need for a population-based study addressing this.
There are only a few population-based cohort studies on S. aureus colonization. The Tromso Staf and Skin Study in Norway,27,28 The Study of Health in Pomerania in Germany,29 and the Generation R Study in The Netherlands30 are all examples of sound population-based studies that have addressed S. aureus colonization and determinants for S. aureus colonization. However, the DBDSaCS is unique because participants are healthy blood donors, a selected group which is generally healthier than the background population, because they must comply with strict criteria to be blood donors. Furthermore, we can assess outcomes in the Danish health registers allowing for complete follow-up with regard to diagnosis codes among both inpatient and outpatient hospital contacts as well as redeemed prescriptions, also after the participants have seized to donate blood. Our participants will be followed for 25 years. Furthermore, genotyping of all participants in the DBDSaCS for more than 650,000 single-nucleotide polymorphisms (SNP) is ongoing. This provides exceptional possibilities in the future investigation of host factors as determinants for S. aureus colonization in addition to addressing bacterial colonization determinants. The DBDSaCS allows us to investigate the mechanisms involved in S. aureus carriage and the influence of carriage on health and the risk of disease.
Here, we describe the setting, organization, and content and present the cohort and results of colonization for quality assurance from the first 6082 participants.
Objectives
The overall aim of the DBDSaCs is to establish a prospective cohort and biobank to investigate the epidemiology, predictors, underlying mechanisms, and associated risk factors of S. aureus nasal colonization/carriage and to examine the effect of S. aureus nasal carriage on health outcomes. Specifically, the objectives of the DBDSaCS can be categorized as follows:
To determine the prevalence of S. aureus nasal carriage among healthy adults and to examine whether carriage is associated with demographic factors
To identify genetic and immunological predictors and risk factors for S. aureus nasal carriage
To investigate whether S. aureus carriage is associated with inflammatory and endocrine markers in serum or plasma
To examine the impact of S. aureus carriage in healthy individuals on the risk of subsequent disease
To examine the nasal microbiome among healthy adults and whether genetic or demographic factors are associated with the composition of microorganisms
Study population and setting
The DBDSaCS is an ongoing multicenter, multiregional, prospective cohort of blood donors. Our study was initiated in January 2014 at Aarhus University Hospital in collaboration between the Department of Clinical Immunology and Department of Clinical Microbiology.
The DBDSaCS has recruited participants among participants in the Danish Blood Donor Study (DBDS) at multiple blood donation facilities in the Central Denmark Region from January 2014 and from September 2014 in the North Denmark Region. DBDSaCS will include participants from the Zealand Region from August 2019. The DBDS is an ongoing nationwide, public-health study and biobank launched 1 March 2010 (www.dbds.dk). So far, more than 125,000 donors from all five administrative regions of Denmark have been included. Blood donors aged from 18 to 67 years (70 years from 1 January 2019) are invited to participate. EDTA plasma samples from each donation and whole blood for DNA purification are stored in a biobank. Participants have consented in merging study data with data from public registers. Blood donation requires compliance with strict criteria. Donation is not allowed if the donor received antibiotics within the preceding 4 weeks. Recent traveling in areas where malaria can be found (most of Africa, South-East Asia, and South America) leads to deferral for 6 months and traveling to areas where flaviviruses are found (most areas outside of Northern Europe) leads to deferral for 4 weeks. Implicitly, all participants were healthy, nonpregnant, and nonlactating adults testing negative for HIV, hepatitis B, and C.1,31–33
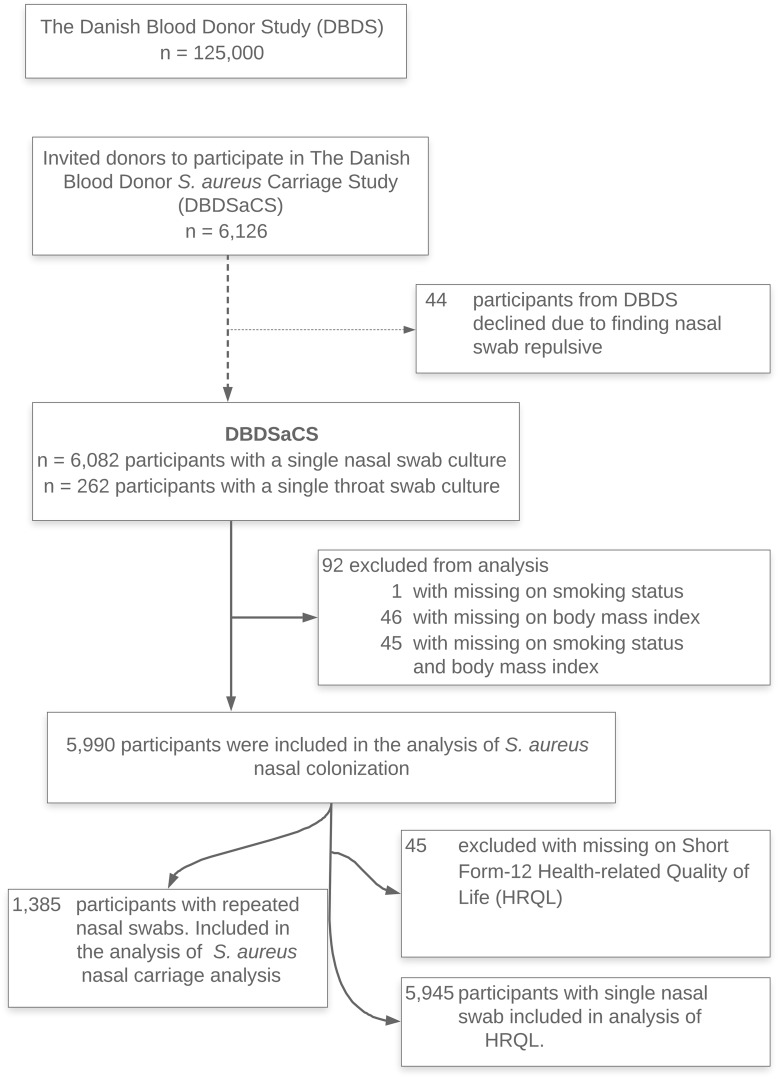
Blood donors return repeatedly for donation; at the time of donation, they are asked to participate in the DBDSaCS and consenting donors complete a questionnaire, a nasal swab is taken by trained health care personnel, and plasma samples are stored. Further, 1385 DBDSaCS participants were at subsequent donations asked to repeat completion of the same questionnaire, and nasal swab and blood samples were taken again. A single throat swab concurrent with a nasal swab was sampled among the first 262 participants included in the study. Only 0.72% declined to participate in the study. Figure 1 depicts a flowchart of the DBDSaCS cohort consisting of the following:
DBDSaCS, Central Denmark Region: 5588 participants (91.9%) recruited in the donation facility at Aarhus University Hospital (4989, 82.0%), primarily, and in the donation facility at Herning-Holstebro Hospital (599, 9.9%) in the western part of Central Denmark Region
DBDSaCS, North Denmark Region: 494 participants (8.1%) recruited from the blood center of Aalborg University Hospital.
Figure 1.
Flowchart of the Danish Blood Donor S. aureus Carriage Study cohort.
See Table 1 for characteristics of participants in DBDSaCS in comparison with blood donors not participating in the study. We defined nonparticipants as Danish blood donors donating whole blood or plasma from January 1st 2014 to April 1st 2019 from the Scandinavian Donations and Transfusions Database (SCANDAT), a database including all electronically recorded Danish and Swedish blood donations since the 1980s and 1970s, respectively. For characteristics of nonparticipants, one donation from each donor was randomly sampled as the index donation.
Table 1.
Characteristics of the DBDSaCS cohort (n=6082) in comparison with the national blood donor cohort (n=234,836)
| DBDSaCSa | Blood donors not participatinga | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| N (%) | 2683 (44.1) | 3399 (55.9) | 126,738 (54.0) | 108,098 (46.0) |
| Age, years | 36.7 (25.7–50.2) | 39.1 (28.5–50.9) | 34.6 (24.6–48.0) | 39.5 (28.0–51.1) |
| Age strata (%) | ||||
| 18–25 | 21.6 | 12.3 | 26.5 | 16.8 |
| 26–35 | 26.0 | 29.3 | 24.2 | 24.0 |
| 36–45 | 17.5 | 21.7 | 18.9 | 20.9 |
| 46–55 | 19.2 | 19.8 | 18.1 | 21.3 |
| 56–67 | 15.7 | 17.0 | 12.4 | 17.1 |
| Hemoglobin, mmol/L | 8.5 (8.2–8.8) | 9.4 (9.1–9.7) | 8.5 (8.1–8.8) | 9.4 (9.1–9.8) |
| Donations during previous: | ||||
| 1 year | 2 (2–3) | 3 (2–4) | 2 (1–3) | 2 (1–3) |
| 3 years | 5 (4–7) | 6 (4–9) | 3 (1–5) | 4 (2–7) |
| 5 years | 7 (5–10) | 9 (6–12) | 4 (2–8) | 7 (3–11) |
| Weight, kg | 70 (63–80)b | 84 (76–93)b | NA | NA |
| Height, cm | 170 (165–174)c | 183 (178–187)c | NA | NA |
| BMI, kg/m2 | 24.3 (22.1–27.5)d | 25.2 (23.3–27.4)d | NA | NA |
| Current smoker (%) | 11.8e | 12.6e | NA | NA |
| Short form-12 | ||||
| Physical Component Score | 56.6 (53.8–57.8)f | 56.6 (54.1–57.9)f | NA | NA |
| Mental Component Score | 55.1 (51.2–57.7)f | 55.8 (52.8–57.7)f | NA | NA |
Notes: Results are reported as medians (interquartile ranges) and numbers (percentages). aThe DBDSaCS cohort includes blood donors from Central Denmark Region and North Denmark Region compared with blood donors from all five regions of Denmark not participating. b=49 missing values; cn=87 missing; dn=91 missing; en=46 missing values; fn=45.
Abbreviations: DBDSaCS, the Danish Blood Donor S. aureus Carriage Study; BMI, body mass index.
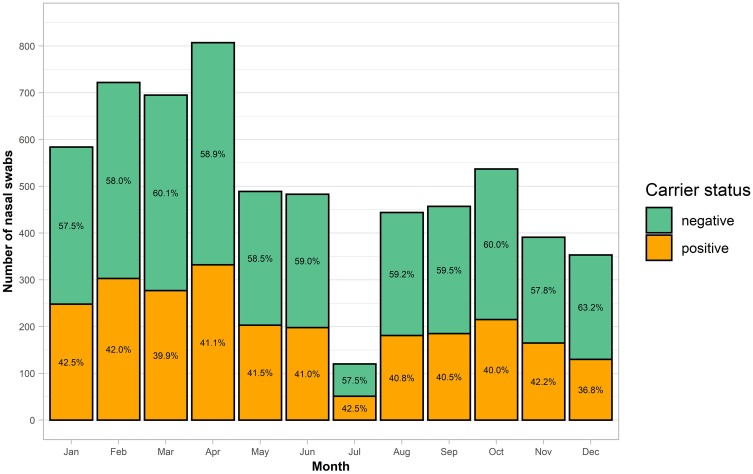
Inclusion of participants is performed year-round except in July due to reduced capacity. The number of included participants per calendar month and their carrier state is presented in Figure 2.
Figure 2.
Bar chart illustrating the number of participants included by calendar month and their carrier status. Orange (or lower pillar) = S. aureus positive; Green (or upper pillar) = S. aureus negative.
Oral and written informed consent was obtained from all participants. The DBDSaCS project (1-10-72-307-13) and the DBDS (M-20090237) were approved by the Scientific Ethical Committee in the Central Denmark Region. The DBDS GWA study was approved by the Danish National Committee on Health Research Ethics (1700407). The establishment of the biobank was approved by the Danish Data Protection Agency (2007-58-0015). This study was conducted in accordance with the Declaration of Helsinki.
Microbiological methods
S. aureus colonization was determined by a single nasal swab taken at study inclusion when participants donated blood. Until April 2019, 6082 donors have been included. Samples from the first 2217 participants were cultured using the two-step method. Among the 2217 participants with samples from the nose and using the two-step method, 262 participants were also sampled from the throat.
Samples were taken with sterile Copan ESwabs (Copan, Brescia, Italy). The samples from the anterior nares were obtained by a single swab rotated five times in both nostrils while applying a gentle pressure. Throat samples were obtained by rotating a single swab over the surface of both tonsils and the posterior wall of the pharynx while applying a gentle pressure. Samples were stored at +4°C before culturing. Within 24 hrs, all samples were cultured initially using a two-step method later replaced by a one-step method.
In the two-step method, the flocked swab (FLOQSwab, Copan) was first streaked on S. aureus chromID agar plates (SAID) (bioMerieux, Marcy- l’étoile, France) and then submersed into Tryptase Soy Broth (TSB) (SSI Diagnostica, Hillerød, Denmark). The SAID plates and TSB enrichment broths were incubated for 18–24 hrs at 35–37°C. Growth of green colonies on SAID was confirmed as being S. aureus with matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (Bruker Microflex, LT/SH system; Bruker Daltonik GMbH, Bremen, Germany). From samples without S. aureus after primary culture, the TSB enrichment broths were subsequently subcultured on SAID and incubated for another 18–24 hrs at 35–37°C. S. aureus determination was performed as described above.
In the one-step culture method, TSB enrichment broth was inoculated with the flocked swab and incubated for 18–24 hrs at 35–37°C. All samples were subsequently subcultured from the enrichment broth on SAID and incubated for another 18–24 hrs at 35–37°C; then, S. aureus determination was performed as described above. The advantage of the one-step method is a shortening of the cultivation process by 1 day and a reduction of the amount of plates used.
Data collection and variables
Questionnaires
Participants filled out a two-page questionnaire with items including the Short Form-12 (SF-12) health-related quality of life (HRQL) survey, smoking status, anthropometric measurements, and physical activity at work and in leisure time. Questionnaires were double entered. From May 2017, participants completed the questionnaire electronically at enrolment as described previously.34 In addition, between June 2015 and May 2018, a total of 4488 participants in the DBDSaCS completed a comprehensive electronic questionnaire version 2 in the DBDS; among these, donors completed questions regarding allergy, asthma, and place of upbringing (based on standardized questions from the European Community Respiratory Health Survey questionnaire), hidradenitis suppurativa,35 restless leg syndrome,36 attention deficit hyperactive syndrome, and migraine.37
The SF-12 is an abbreviated version of Short Form-36 (SF-36) developed for population surveys for determining general health outcomes, described in detail previously.38,39 Briefly, SF-12 consists of 12 items providing a Physical Component Score and a Mental Component Score, and these scores explain more than 80% of the variance in the original eight SF-36 scores,40 higher scores indicating better HRQL. For missing values in SF-12 items, an imputation algorithm was used as previously described.38,41
Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). We categorized each participant according to BMI using WHO definitions: BMI <18.5 kg/m2 (underweight), 18.5≤ BMI <25 (normal weight as reference group), 25≤ BMI <30 (overweight), and BMI ≥30 (obese).42 Current smokers (yes/no) were defined when answering either “Yes, but less than 1 cigarette/cheroot/pipe per day” or “Yes, more than 1 cigarette/cheroot/cigar/pipe per day” and cumulative tobacco consumption in pack years as defined elsewhere.38 Physical activity at work and in leisure time were categorized as high vs low, see the definition from the previous study in DBDS.38,43
Biochemical material and measures
Biobank
A biobank with plasma samples (frozen at −20°C), Copan ESwab transport media, and S. aureus isolates (frozen at −80°C) was established for the entire cohort. The Copan ESwab transport media were frozen within 24 hrs after they were obtained. Our study and biobank serve as a platform for future research in predictors, underlying mechanisms, and associated risk factors of S. aureus nasal carriage, but potentially also for research purposes on other nasal pathogens. Various biomarkers will be of interest to measure, including immunological and inflammatory markers, markers of iron metabolism, and endocrine markers (Table 2).
Table 2.
Samples and data collected in DBDSaCS
| No. of samples/measurements | Material | |
|---|---|---|
| Baseline plasma samples | 12,164 | 5 mL EDTA plasma from the first inclusion in DBDSaCS |
| Follow-up plasma samples | 4180 | 5 mL EDTA plasma from follow-up in DBDSaCS |
| Baseline nasal swabs | 6082 | Nasal swabs from anterior nares, both nostrils |
| Follow-up nasal swabs, 2nd visit | 1385 | Nasal swabs from anterior nares, both nostrils |
| Follow-up nasal swabs, 3rd visit | 459 | Nasal swabs from anterior nares, both nostrils |
| Follow-up nasal swabs, 4th visit | 165 | Nasal swabs from anterior nares, both nostrils |
| Follow-up nasal swabs, 5th visit | 76 | Nasal swabs from anterior nares, both nostrils |
| Throat swabs | 262 | Throat swabs from oropharynx and both tonsils |
| S. aureus isolates | 3465 | S. aureus isolates after culturing from positive nasal and throat swabs |
| Questionnaire data, DBDSaCS | 8067 | Anthropometric, health-related quality of life, smoking, and physical activity data |
| Questionnaire data, DBDS version 2 | 4488 | Asthma, allergy, upbringing, and screening questions of hidradenitis suppurativa |
| Immunological markers | ||
| Complete blood count | 7886 | Hemoglobin, white blood cells, hemotocrit, platelets, |
| Leukocyte subsets | 6742 | Blood cell counts of basophil, eosinophil, lymphocytes, monocytes, and neutrophil |
| Ferritin | 724 | Marker of iron metabolism |
| Genetics | 3133 | Approx. 650,000 single nucleotide polymorphisms |
| Nasal microbiome | 2161 | Nasal microbiome characterization using 16S rRNA gene-based sequencing |
| Danish registers linkage | 6082 | Entire cohort linked to NPR, DNPR, and socioeconomic data at Statistics Denmark |
Abbreviations: DBDSaCS, the Danish Blood Donor S. aureus Carriage Study; NPR, the Danish National Patient Register; DNPR, the Danish National Prescription Register.
Immunological markers
Erythrocyte and leukocyte subsets were measured with Sysmex® XT-1800i (Sysmex Corp., Kobe, Japan) on a blood sample taken prior to blood donation among the included donors as a part of the daily routine in the Central Denmark Region Blood Center including: hemoglobin, hematocrit, mean erythrocyte volume, mean erythrocyte hemoglobin concentration, white blood cells, platelet counts, basophil, eosinophil, lymphocyte, monocyte, and neutrophil. At this point, ferritin measurement is available for 605 participants and measurements of remaining participants are ongoing.
Genetics
A total of 110,000 participants in the DBDS are genotyped for approximately 650,000 SNPs using the Infinium Global Screening Array (Illumina Inc., San Diego, CA) in collaboration with deCODE Genetics (Reykjavik, Iceland). A further >8 million SNPs have been imputed by use of newly sequenced reference genomes. Standard quality control is applied, thus variants that fail Hardy–Weinberg equilibrium and samples with low genotype yield (less than 98%) will be excluded from the analysis.44 Currently, genetic data on a subgroup of DBDSaCS participants (n=3133) are available. Genetic data are stored and available for analysis at Computerome (Technical University of Denmark, Lyngby).
Nasal microbiome
The nasal bacterial density has been measured in 2161 nasal samples using a broad-coverage quantitative polymerase chain reaction and the nasal microbiota composition has been characterized by 16S rRNA gene-based sequencing. These data are currently being processed.
Registers
In Denmark, the unique personal identification number given to each citizen at birth, immigration, and used in public registers enables continuous linkage of data of the entire cohort with the nationwide Danish public registers hosted by Statistics Denmark (www.dst.dk).
Past and future contacts with hospitals in Denmark are available through the Danish National Patient Register (NPR), thus making follow-up of the entire cohort on development of diseases possible. This register was established in 197745,46 and holds records of all hospital contacts in Denmark with information on dates of admission and discharge, and diagnostic information based on the International Classification of Diseases, Eighth Revision (ICD-8) until 1993 and Tenth Revision (ICD-10) from 1994 until present.
Information on redeemed prescriptions from all Danish pharmacies is available from The Danish National Prescription Register, which was established in 1994. This information includes the unique personal identification number of the patient, the type of drug according to the Anatomical Therapeutic Chemical classification system, dosage, pack size, and the date of redeemed prescription.
Data informatics, linkage, and updates
Master data of all participants were recorded in the Danish blood bank system ProSang 9.1 (CSAM Health, Oslo, Norway). This system holds records of following main variables: personal identification number, a unique identification number for each donation contact, contact information, donation history, laboratory test (blood type, hemoglobin, erythrocyte, and leukocyte subsets), and screening results (HIV, hepatitis B and C).
Microbiology test results were recorded in MADS laboratory information system (Dept. of Clinical Microbiology Aarhus University Hospital, Aarhus, Denmark) which hold records of following main variables: personal identification number, a unique sample identification number, requesting department, date and time of receipt and answering of samples, anatomical site, microbiological species, bacteria quantification if relevant, and freeze positions of all nasal swabs and S. aureus isolates.
The DBDSaCS database is stored on a specialized, secure section of the Danish National Supercomputer–Computerome (http://www.computerome.dtu.dk). The DBDSaCS database is updated on a quarterly basis with data exports from ProSang and MADS. At Computerome, the database is linked to data from all Danish blood donors (SCANDAT) and to data from the DBDS currently comprising 125,000 participants. Linkage to national health registers is already in place and updates to Denmark Statistics are performed as needed.
Statistics
Characteristics of the cohort are presented as numbers, percentages, or medians, with interquartile ranges. Groups were compared by the two-sample T-test for normally distributed data, χ2 test, or Fisher’s exact test for categorical data. Correlations between individual characteristics and S. aureus colonization were evaluated by log-binomial regression analysis adjusted for age, smoking status, BMI, and stratified by sex. Results were reported as risk ratios (RR) with 95% CI. Stata/IC 13.1 (StataCorp LP, College Station, Texas, USA) was used for statistical analysis.
First results
In April 2019, the cohort comprised 6082 individuals (Table 3): 44% women and 56% men. The overall prevalence of S. aureus nasal colonization was 41% and colonization was higher in men (46%) than women (34%) (Table 4). The colonization rates of S. aureus for the 2217 participants sampled from the anterior nares, using the two-step method, are shown in Table 5; the overall prevalence of S. aureus nasal colonization was 34% before and 41% after the use of enrichment broth.
Table 3.
Characteristics of the DBDSaCS cohort by carrier status (n=6082)
| Total | S. aureus-positive | S. aureus-negative | |
|---|---|---|---|
| N (%) | 6082 | 2488 (41) | 3594 (59) |
| Age, years | 38.1 (26.9–50.6) | 36.6 (26.5–49.0) | 39.3 (27.5–51.3) |
| 18–25 | 997 (16) | 442 (44) | 555 (56) |
| 26–35 | 1693 (28) | 729 (43) | 964 (57) |
| 36–45 | 1206 (20) | 505 (42) | 701 (58) |
| 46–55 | 1189 (20) | 460 (39) | 729 (61) |
| 56–67 | 997 (16) | 352 (35) | 645 (65) |
| Women | 2683 (44) | 924 (34) | 1759 (66) |
| Men | 3399 (56) | 1564 (46) | 1835 (54) |
| Weight, kga | 79 (70–89) | 80 (71–90) | 78 (69–88) |
| Height, cmb | 177 (170–184) | 178 (172–185) | 176 (170–183) |
| BMI, kg/m2 c | 24.9 (22.7–27.5) | 25.1 (22.9–27.8) | 24.8 (22.6–27.2) |
| BMI, categorized (kg/m2)c | |||
| BMI<18.5, underweight (%) | 26 (0.4) | 12 (0.5) | 14 (0.4) |
| 18.5≤BMI<25, normal weight (%) | 3064 (51.1) | 1191 (48.5) | 1873 (53.0) |
| 25≤BMI<30, overweight (%) | 2167 (36.2) | 899 (36.6) | 1268 (35.9) |
| BMI>30, obese (%) | 734 (12.3) | 353 (14.4) | 381 (10.8) |
| Current smoker (%)d | 739 (12.2) | 255 (10.3) | 484 (13.6) |
| Short form-12e | |||
| Physical Component Score | 56.6 (54.0–57.9) | 56.9 (53.8–57.9) | 56.6 (54.0–57.9) |
| Mental Component Score | 55.7 (52.3–57.7) | 55.7 (52.2–57.7) | 55.7 (52.3–57.7) |
Notes: Results are reported as medians (interquartile ranges), numbers (percentages). an=49 missing values; bn=87 missing; cn=91 missing; dn=46 missing values; en=45.
Abbreviation: BMI, body mass index.
Table 4.
Characteristics of the DBDSaCS cohort by carrier status and stratified by sex (n=6082)
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Total | S. aureus-positive | S. aureus-negative | Total | S. aureus-positive | S. aureus-negative | |
| N (%) | 2683 | 924 (34) | 1759 (66) | 3399 | 1564 (46) | 1835 (54) |
| Age, years | 36.7 (25.7–50.2) | 31.3 (24.9–47.6) | 38.9 (26.3–51.2) | 39.1 (28.5–50.9) | 38.8 (28.0–49.8) | 39.3 (29.0–51.5) |
| 18–25 | 580 (22) | 234 (40) | 346 (60) | 417 (12) | 208 (50) | 209 (50) |
| 26–35 | 697 (26) | 275 (39) | 422 (61) | 996 (29) | 454 (46) | 542 (54) |
| 36–45 | 470 (18) | 146 (31) | 324 (69) | 736 (22) | 359 (49) | 377 (51) |
| 46–55 | 515 (19) | 155 (30) | 360 (70) | 674 (20) | 305 (45) | 369 (55) |
| 56–67 | 421 (16) | 114 (27) | 307 (73) | 576 (17) | 238 (41) | 338 (59) |
| Weight, kga | 70 (63–80) | 70 (64–80) | 70 (63–79) | 84 (76–93) | 85 (77–93) | 83 (76–92) |
| Height, cmb | 170 (165–174) | 170 (165–174) | 170 (165–174) | 183 (178–187) | 183 (178–187) | 182 (178–187) |
| BMI, kg/m2 c | 24.3 (22.1–27.5) | 24.5 (22.1–27.9) | 24.2 (22.0–27.4) | 25.2 (23.3–27.4) | 25.4 (23.4–27.8) | 25.0 (23.1–27.2) |
| BMI, categorized (kg/m2)c | ||||||
| BMI<18.5, underweight (%) | 20 (0.8) | 7 (0.8) | 13 (0.8) | 6 (0.2) | 5 (0.3) | 1 (0.1) |
| 18.5≤BMI<25, normal weight (%) | 1464 (55.6) | 500 (55.2) | 964 (55.9) | 1600 (47.6) | 691 (44.6) | 909 (50.2) |
| 25≤BMI<30, overweight (%) | 775 (29.4) | 252 (27.8) | 523 (30.3) | 1392 (41.4) | 647 (41.8) | 745 (41.2) |
| BMI>30, obese (%) | 373 (14.2) | 147 (16.2) | 226 (13.1) | 361 (10.7) | 206 (13.3) | 155 (8.6) |
| Current smoker (%)d | 312 (11.8) | 88 (9.6) | 224 (12.9) | 427 (12.6) | 167 (10.7) | 260 (14.2) |
| Short form-12e | ||||||
| Physical Component Score | 56.6 (53.8–57.8) | 56.6 (53.8–57.8) | 56.6 (53.7–57.9) | 56.6 (54.1–57.9) | 56.6 (53.9–57.9) | 56.6 (54.2–57.9) |
| Mental Component Score | 55.1 (51.2–57.7) | 55.0 (50.9–57.7) | 55.1 (51.2–57.7) | 55.8 (52.8–57.7) | 55.8 (52.8–57.7) | 55.8 (52.9–57.7) |
Notes: Results are reported as medians (interquartile ranges), numbers (percentages). an=49 missing values; bn=87 missing; cn=91 missing; dn=46 missing values; en=45
Abbreviation: BMI, body mass index.
Table 5.
Characteristics of participants sampled from the nose and using the two-step culture method (n=2217)
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | S. aureus-positive | Positive. after 1st culture | Additional positive after enrichment | S. aureus-negative | Total | S. aureus-positive | Positive. after 1st culture | Additional positive after enrichment | S. aureus-negative | |
| Number of participants | 880 | 303 (34) | 236 (27) | 67 (8) | 577 (66) | 1337 | 611 (46) | 520 (39) | 91 (7) | 726 (54) |
| Age<25 | 169 | 74 (44) | 53 (31) | 21 (12) | 95 (56) | 166 | 87 (52) | 76 (46) | 11 (7) | 79 (48) |
| 25≤age<35 | 236 | 94 (40) | 71 (30) | 23 (10) | 142 (60) | 383 | 172 (45) | 141 (37) | 31 (8) | 211 (55) |
| 35≤age<45 | 146 | 52 (36) | 43 (29) | 9 (6) | 94 (64) | 297 | 147 (49) | 127 (43) | 20 (7) | 150 (51) |
| 45≤age<55 | 179 | 44 (25) | 39 (22) | 5 (3) | 135 (75) | 258 | 113 (44) | 98 (38) | 15 (6) | 145 (56) |
| Age≥55 | 150 | 39 (26) | 30 (20) | 9 (6) | 111 (74) | 233 | 92 (39) | 78 (33) | 14 (6) | 141 (61) |
| Age, years | 37.7 (26.1–50.9) | 30.0 (25.1–46.5) | 32.4 (25.3–47.1) | 28.0 (24.3–41.8) | 41.1 (26.8–52.5) | 39.1 (28.7–50.8) | 38.3 (28.0–49.4) | 38.9 (28.4–49.3) | 36.4 (26.7–49.4) | 39.9 (29.4–51.8) |
| Weight, kga | 69 (62–78) | 68 (62–78) | 68 (62–78) | 66 (59–79) | 69 (62–78) | 83 (76–92) | 84 (76–93) | 85 (76–93) | 83 (76–90) | 83 (76–91) |
| Height, cmb | 169 (165–173) | 169 (165–172) | 169 (165–173) | 168 (164–171) | 169 (165–174) | 182 (178–187) | 182 (178–187) | 182 (178–187) | 183 (178–187) | 182 (178–187) |
| BMI, kg/m2 c | 24.0 (21.9–26.7) | 23.7 (21.7–26.5) | 23.7 (21.7–26.6) | 23.7 (21.6–25.5) | 24.2 (22.0–26.8) | 24.9 (23.1–27.2) | 25.1 (23.2–27.8) | 25.1 (23.2–28.1) | 25.1 (23.1–27.1) | 24.8 (23.1–26.9) |
| Current smokerd | 117 (13.3) | 30 (9.9) | 24 (10.1) | 6 (9.0) | 72 (15.1) | 178 (13.3) | 76 (12.5) | 62 (12.0) | 14 (15.4) | 102 (14.1) |
Notes: Numbers with percentages or medians with interquartile ranges. an=2 missing values; bn=33 missing; cn=33 missing; dn=2 missing values.
Abbreviation: BMI, body mass index.
On average, participants colonized with S. aureus in the nostrils were 1.82 years (95% CI: 1.14–2.51) younger than noncolonized participants. In both sexes, the prevalence of S. aureus colonization decreased with increasing age. Thus, the colonization proportion was 13 percentage points lower among women aged 55 years or older when compared to women below 25 years. Similarly, the colonization proportion was 9 percentage points lower among older men (Table 4).
The prevalence of smoking in the cohort was 12.2%: 11.8% for women and 12.6% for men. The prevalence of S. aureus nasal colonization was 34.2% and 41.7% among smokers and nonsmokers, respectively. Obesity (BMI ≥30 kg/m2) was more prevalent among S. aureus colonized participants (14.4%) compared with non-carriers (10.8%) (Tables 3 and 4).
In comparison with the blood donors nationwide not participating in DBDSaCS, the median age and median hemoglobin are similar. The DBDSaCS cohort comprises a larger percentage of men and a smaller age group of 18–25 in return for a larger age group of 26–35. Also, DBDSaCS participants’ number of donations during the previous 5 years are approximately one and a half-fold higher than blood donors not participating (Table 1).
The effect of using a two-step culture method
The effect of using enrichment broth is illustrated in Table 5. In the 2217 individuals sampled from the nose using the two-step method, prevalence of S. aureus nasal colonization prior to and after enrichment was 34% and 41%, respectively. Enrichment broth increased the number of positive samples by 21%. The effect of using enrichment broth was higher for women when compared to men. We found that 8% of women were only positive after enrichment, corresponding to 24% of all S. aureus–positive women. In comparison, we found that 6% of men were positive only after enrichment corresponding to 14% of all S. aureus–positive men (Table 5).
In both sexes, there was no difference between individuals positive for S. aureus after enrichment broth compared to individuals only positive after primary culture with respect to age, BMI, or smoking status.
The effect of including a throat swab
In the group of 262 participants swabbed in the nose and the throat, overall prevalence of S. aureus colonization prior to enrichment was 33% in the nares and 31% in the throat (Table 6). The combined prevalence of S. aureus colonization sampled and cultured from the nares and the throat prior to enrichment was 45%. The combined prevalence of S. aureus colonization after enrichment was 55% with a higher prevalence of colonization in the throat (45%) than in the nose (40%) (Table 6). Enrichment broth increased the number of S. aureus positive samples from both nose and throat swabs. However, the effect of using enrichment broth was more pronounced for throat than for nose swabs with an additional proportion of positive samples after enrichment of 43% and 23%, respectively.
Table 6.
Prevalence of throat and nasal colonization after primary culture and after enrichment (n=262)
| Site of colonization | No. | % | Additional proportion of positive samples after enrichment (%) |
|---|---|---|---|
| S. aureus-positive in nares | 106 | 40 | |
| Before enrichment | 86 | 33 | |
| After enrichment | 20 | 8 | 23 |
| S. aureus-positive in throat | 117 | 45 | |
| Before enrichment | 82 | 31 | |
| After enrichment | 35 | 13 | 43 |
| S. aureus-positive in nares and/or throat | 144 | 55 | |
| Before enrichment | 119 | 45 | |
| After enrichment | 25 | 10 | 21 |
Note: Numbers and percentages of samples positive for S. aureus among 262 participants with concomitant nasal and throat swabs.
Abbreviation: No., number.
Correlation between individual characteristics and colonization
A characterization of the association between S. aureus colonization and age, BMI (categorized cf. WHO definition), and smoking status is presented in Table 7 with crude and adjusted RRs. In the adjusted model, 10-year older age was associated with lower risk of S. aureus colonization among both women (RR=0.87, 95% CI: 0.84–0.91) and men (RR=0.96, CI: 0.93–0.98) and the inverse association of age on nasal colonization was even stronger when comparing the oldest (55–67 years) and the youngest age group (18–25 years) (women: RR =0.66, CI: 0.55–0.79; men: RR=0.80, CI: 0.70–0.92). Similarly, current smoking status was associated with lower risk among both women (RR =0.78, CI: 0.64–0.93) and men (RR=0.83, CI: 0.73–0.94). Conversely, obesity was associated with the risk of S. aureus colonization in both genders (women: RR=1.22, CI: 1.06–1.41; men: RR=1.36, CI: 1.22–1.51).
Table 7.
Predictors of Staphylococcus aureus colonization by log-binomial regression analysis (n=6082)a
| Characteristics | Women | Men | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude model | Adjusted model | Crude model | Adjusted model | |||||||
| Number | RR | 95% CI | RR | 95% CI | Number | RR | 95% CI | RR | 95% CI | |
| Age, 10-year increment | 2683 | 0.88 | 0.85–0.92 | 0.87 | 0.84–0.91 | 3399 | 0.97 | 0.94–0.99 | 0.96 | 0.93–0.98 |
| 18–25 | 580 | 1 | 1 | 396 | 1 | 1 | ||||
| 26–35 | 697 | 0.98 | 0.85–1.12 | 0.97 | 0.85–1.11 | 959 | 0.91 | 0.81–1.03 | 0.90 | 0.81–0.99 |
| 36–45 | 470 | 0.77 | 0.65–0.91 | 0.75 | 0.63–0.89 | 704 | 0.98 | 0.87–1.10 | 0.95 | 0.84–1.08 |
| 46–55 | 515 | 0.75 | 0.63–0.88 | 0.72 | 0.61–0.85 | 653 | 0.91 | 0.80–1.03 | 0.88 | 0.77–1.00 |
| 56-67 | 421 | 0.67 | 0.56–0.81 | 0.66 | 0.55–0.79 | 559 | 0.83 | 0.72–0.95 | 0.80 | 0.70–0.92 |
| BMI (categorized), kg/m2 b | ||||||||||
| Normal weight (18.5≤BMI<25) | 1464 | 1 | 1600 | 1 | ||||||
| Underweight (BMI<18.5) | 20 | 1.02 | 0.56–1.87 | 0.96 | 0.53–1.74 | 6 | 1.93 | 1.34–2.77 | 1.80 | 1.25–2.59 |
| Overweight (25≤BMI<30) | 775 | 0.95 | 0.84–1.08 | 1.00 | 0.88–1.13 | 1392 | 1.08 | 0.99–1.17 | 1.10 | 1.01–1.19 |
| Obese (BMI≥30) | 373 | 1.15 | 1.00–1.33 | 1.22 | 1.06–1.41 | 361 | 1.32 | 1.19–1.47 | 1.36 | 1.22–1.51 |
| Current smokingc | ||||||||||
| Nonsmoker | 2341 | 1 | 1 | 2956 | 1 | 1 | ||||
| Smoker | 312 | 0.80 | 0.66–0.96 | 0.78 | 0.64–0.93 | 427 | 0.83 | 0.73–0.94 | 0.83 | 0.73–0.94 |
Notes: Results presented as risk ratios (RR) with 95% CI. aLog-binomial regression analysis of S. aureus colonization with age (10-year age-groups), body mass index (BMI) categorized cf. WHO definitions, and current smoking as predictors, stratified by sex. Crude model = univariate log-binomial regression without adjustment; adjusted model = based on the crude model with additional adjustment for age, BMI (continuous), and current smoking status. bn=91 missing; cn=46 missing values.
Abbreviation: BMI, body mass index.
In order to determine long-term S. aureus carriage status, multiple nasal swabs are collected randomly from donors returning to donate. A preliminary statement of carriership among participants with 2–5 samples is presented in Table S1.
Discussion
In this cohort of healthy blood donors, we found that the overall prevalence of S. aureus colonization after enrichment was 41% for nasal carriage and 55% for nasal and/or throat carriage; men were more frequently colonized than women. S. aureus colonization was lower among older participants and smokers, whereas obesity was associated with an increased risk of colonization. The use of an enrichment broth increased the proportion of colonization especially among women and for throat carriage of S. aureus.
The prevalence of S. aureus nasal carriage in the general adult population varies according to population, the quality of sampling, and culture techniques.3,47 In sampling, culture, and identification of S. aureus, all our samples were cultured within 24 hrs using enrichment broth and a selective chromogenic media, which lowers the detection limit for S. aureus. Hence, we found that the overall prevalence of S. aureus after enrichment was 41%, slightly higher than the mean S. aureus nasal carriage rate of 37.2% (19.0–55.1), reported in selected cross-sectional studies of S. aureus nasal carriage in a review by Kluytman et al.48
Other explanations for the high prevalence of S. aureus nasal carriage in our cohort could be the male/female ratio (men being more frequently culture-positive than women),49–51 low prevalence of smoking (smokers are less frequent carriers of S. aureus),50,52 and age distribution (elderly are less frequently carriers).2 All these associations were confirmed in this study.
The S. aureus nasal carriage state can be predicted accurately by the use of quantitative and qualitative results from two swabs obtained at 1-week intervals.53 In the current study, the distribution between all positive samples, alternating samples, and all negative samples is in line with previously reported prevalences based on repeated nasal swabs obtained during the study period.2,53,54
We found a significantly higher prevalence of S. aureus carriage among men (46%) than women (34%). Regarding the lower prevalence of colonization among women, a recent study by Liu et al using both culture and PCR methods showed that the difference in S. aureus prevalence between the culture of samples from women and men was most likely due to a lower number of S. aureus in the nares of women.55 This is in line with the findings in the current study where the effect of using enrichment broth was higher for women than men. The reason for this is not known, but could be related to anatomical and gender-dependent differences in the inner wall of a nostril that includes apocrine sweat glands, sebaceous glands, hair follicles, or sex hormones.56
Regarding smoking, we hypothesize that the current decreasing prevalence of smoking in high-income countries could be associated with an increase in the prevalence of S. aureus colonization.
In the current study, S. aureus carriage increased with obesity. It has previously been reported that obesity was found to be a predictor for S. aureus nasal colonization, independent of diabetes, in particular among premenopausal women.27 Furthermore, in a previous study, we have shown that obesity among healthy individuals was associated with an overall increased risk of infection. Obesity was associated with an increased risk of abscesses, infections of the skin, and an increased risk of redeemed prescriptions for dicloxacillin and flucloxacillin.42 We speculate that the higher risk of S. aureus carriage may contribute to the increased risk of S. aureus–related infections in obese participants.
Recently, several studies have highlighted the importance of throat colonization, and throat carriage has been reported to range between 30% and 47%.7–9,47 In this study, we included a throat swab from a subpopulation. When using enrichment broth, the throat was a more common colonization site than the nose and when combined the prevalence of S. aureus in the nose and/or the throat increased to 55%. The use of enrichment broth increased the prevalence, especially in throat samples, possibly because of a lower number of S. aureus or sampling difficulties in the pharyngeal area. Our results confirm that sampling from more than one anatomical site and using enrichment broth more accurately detects colonization with S. aureus.
Strength and weaknesses
Blood donors are a highly selected, healthy population, simply because they comply with criteria regarding health status and lifestyle to be accepted as donors and for their further activity as blood donors. Also, blood donors are permanently excluded from blood donation if diagnosed with certain chronic diseases, including diabetes, hypertension, cancer, or hypercholesterolemia. This can be both an advantage and a limitation. Furthermore, persons weighing less than 50 kg cannot be blood donors. Thus, underweight people are underrepresented in our study. In a socio-demographic description of blood donors, we found that individuals with a low educational level (primary and lower secondary education) and low incomes are underrepresented in the donor populations as well as people of non-ethnic Danish origin and single men.57 Participants in DBDSaCS are similar in characteristics compared with blood donors not participating apart from being more actively bled during the previous 5 years before inclusion. This may reflect that DBDSaCS participants are a selected group of blood donors more willing to donate frequently or healthier in order to donate more; however, these factors do not affect the S. aureus colonization rates. We believe that the higher proportion of men in the DBDSaCS cohort is largely due to women more often being below the hemoglobin deferral guideline, thus affecting their possibility being included in DBDSaCS study.
Exclusively sampling from the nose, we miss participants that are carriers at other sites. However, throat swabs require more technical skills and results may thus depend on the skills of different health care workers.8 In DBDSaCS, participants are included while donating blood and, in this setting, a nasal swab was more feasible than a throat swab. Furthermore, exclusively sampling at a single point, we miss the ability to divide participants into persistent carriers, intermittent carriers, and noncarriers. However, this study already included a second swab for 1385 participants, and as this study is an ongoing large-scale study, we aim to include more than 20,000 blood donors. We include participants returning for blood donation and collect a second nasal swab for a large subgroup within the DBDSaCS.
Conclusion and perspectives
In conclusion, we report that S. aureus nasal colonization rates among Danish blood donors are in the upper range compared to previous European population–based studies. Using an enrichment broth increased the detection rate of S. aureus carriers and the effect was more pronounced for throat swabs and among women. Our findings support previously reported prevalences of S. aureus carriage types and determinants of S. aureus colonization, including smoking and aging that are inversely associated with colonization while male sex and obesity are positively associated with colonization. This underscores that the DBDSaCS is a feasible platform for future S. aureus carriage studies. Our study is unique as it includes only healthy blood donors complying with strict criteria to be allowed to donate blood. This minimizes effects from existing diseases and recent antibiotic treatment as impacting on the risk of both S. aureus carriage and subsequent infection. The participants are followed through the Danish national health registers with complete follow-up regarding contacts to hospitals and redeemed prescriptions. We will therefore over time be able to assess the impact of S. aureus carriage on the health of the participants. A number of projects using DBDSaCS data, as described above, have already been initiated in order to fulfill the primary objectives. Specifically, a follow-up study assessing the association between S. aureus nasal and/or throat carriage and the risk of infection with S. aureus or other infections is scheduled in spring 2020. Further, the association of carriage on, eg, diabetes, cardiovascular, and autoimmune diseases, will be addressed in future studies. The findings will be published in the years to come with the first publications being submitted in 2019.
Studying the association between S. aureus and genetic or environmental predictors may provide unique opportunities to understand key elements of the bacteria and the diseases that can follow. If an association exists, this knowledge might facilitate the discovery of new interventions against S. aureus.
Organization
The DBDSaCS is anchored in the DBDS. A growing number of large-scale population-based studies and epidemiological research have been conducted and published using data from the DBDS cohort. DBDS is managed by a steering committee and permanent staff capable of conducting large-scale data collection, analyses, and interpretation in the general population. In DBDSaCS, the principal investigators are Christian Erikstrup and Lise Tornvig Erikstrup who leads the coordination of projects and activities, including scientific, financial, ethics, and communication tasks. Also, Christian Erikstrup is a member of the steering committee in the DBDS making multidisciplinary projects between the DBDSaCS and the DBDS possible. The steering committee in DBDSaCS includes Professor Christian Erikstrup, staff physician Lise Tornvig Erikstrup, PhD fellow Khoa Manh Dinh, and Professor Paal Skytt Andersen with the responsibility of reviewing the scientific progress. Further, the steering committee can grant access to data and biological material based on submitted application accompanied by a research proposal that complies with Danish regulations on ethical approval and data protection.
Acknowledgment
We thank the Danish blood donors for their participation in The Danish Blood Donor S. aureus Carriage Study and The Danish Blood Donor Study as well as the staff at the blood centers and the Department of Clinical Microbiology involved in DBDSaCS making this study possible. We thank our collaborators: associate professor Cindy M. Liu and her research group from the Department of Environmental and Occupational Health, George Washington Milken Institute School of Public Health, and deCODE Genetics, Reykjavík, Iceland, for their contribution to the cohort. The DBDS is funded by The Danish Council for Independent Research – Medical Sciences (grant number: 09-069412); The Danish Administrative Regions (http://www.regioner.dk); The A.P. Møller Foundation for the Advancement of Medical Science; The Danish Bio- and Genome Bank (http://www.regioner.dk/rbgben). The DBDSaCS was supported by The Danish Council for Independent Research – Medical Sciences (grant number: DFF-1333-00147); Bloddonorernes Forskningsfond (FF4-2013/03 and FF4-2018/01); Aase & Ejnar Danielsens Fond (10-001077); National Institute of Allergy and Infectious Diseases (1R01AI125562-01); A.P. Møller Foundation for the Advancement of Medical Science (17-L-0555); Aarhus University (18290985); Health Research Fund of Central Denmark Region (R5-A329); The Højmosegård Grant (2016-1840/124). None of the funders had any influence on study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pedersen OB, Erikstrup C, Kotzé SR, et al. The Danish Blood Donor Study: a large, prospective cohort and biobank for medical research. Vox Sang. 2012;102(3):271. doi: 10.1111/j.1423-0410.2011.01553.x [DOI] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–762. doi: 10.1016/S1473-3099(05)70295-4 [DOI] [PubMed] [Google Scholar]
- 3.Kluytmans J, Van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–520. doi: 10.1128/CMR.10.3.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoefnagels-Schuermans A, Peetermans WE, Jorissen M, et al. Staphylococcus aureus adherence to nasal epithelial cells in a physiological in vitro model. Vitro Cell Dev Biol Anim. 1999;35(8):472–480. doi: 10.1007/s11626-999-0054-0 [DOI] [PubMed] [Google Scholar]
- 5.Williams REO. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27(1):56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole AM, Tahk S, Oren A, et al. Determinants of Staphylococcus aureus nasal carriage. Clin Diagn Lab Immunol. 2001;8(6):1064–1069. doi: 10.1128/CDLI.8.6.1064-1069.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson P, Ripa T. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. J Clin Microbiol. 2006;44(9):3334–3339. doi: 10.1128/JCM.00880-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mertz D, Frei R, Periat N, et al. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med. 2009;169(2):172. doi: 10.1001/archinternmed.2008.536 [DOI] [PubMed] [Google Scholar]
- 9.Mertz D, Frei R, Jaussi B, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007;45(4):475–477. doi: 10.1086/520016 [DOI] [PubMed] [Google Scholar]
- 10.Wassenberg M, Kluytmans J, Erdkamp S, et al. Costs and benefits of rapid screening of methicillin-resistant Staphylococcus aureus carriage in intensive care units: a prospective multicenter study. Crit Care. 2012;16(1):R22. doi: 10.1186/cc11184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanc DS, Basset P, Nahimana-Tessemo I, Jaton K, Greub G, Zanetti G. High proportion of wrongly identified methicillin-resistant Staphylococcus aureus carriers by use of a rapid commercial PCR assay due to presence of Staphylococcal cassette chromosome element lacking the mecA gene. J Clin Microbiol. 2011;49(2):722–724. doi: 10.1128/JCM.01988-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydiner A, Lüsebrink J, Schildgen V, et al. Comparison of two commercial PCR methods for methicillin-resistant Staphylococcus aureus (MRSA) screening in a tertiary care hospital. PLoS One. 2012;7(9):e43935. doi: 10.1371/journal.pone.0043935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandell GL. Principles and Practice of Infectious Disease. 7th ed. Churchill Livingstone; Elsevier. 2009. ISBN 9781437720600. [Google Scholar]
- 14.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective Nationwide Surveillance Study. Clin Infect Dis. 2004;39(3):309–317. doi: 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 15.Benfield T, Espersen F, Frimodt-Møller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13(3):257–263. doi: 10.1111/j.1469-0691.2006.01589.x [DOI] [PubMed] [Google Scholar]
- 16.DANMAP. DANMAP 2017 - Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available from: https://www.danmap.org/-/media/arkiv/projekt-sites/danmap/danmap-reports/danmap-2017/danmap2017.pdf?la=en. Accessed September 11, 2019. ISSN 1600-2032. [Google Scholar]
- 17.Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2014;364:703–705. doi: 10.1016/S0140-6736(04)16897-9 [DOI] [PubMed] [Google Scholar]
- 18.Kluytmans JAJW, Mouton JW, Ijzerman EPF, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171(1):216–219. doi: 10.1093/infdis/171.1.216 [DOI] [PubMed] [Google Scholar]
- 19.White A. Increased infection rates in heavy nasal carriers of coagulase-positive staphylococci. Antimicrob Agents Chemother. 1963;161:667–670. [PubMed] [Google Scholar]
- 20.Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21(5):319–323. doi: 10.1086/501763 [DOI] [PubMed] [Google Scholar]
- 21.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344(1):11–16. doi: 10.1056/NEJM200101043440102 [DOI] [PubMed] [Google Scholar]
- 22.Lyytikäinen O, Ruotsalainen E, Järvinen A, Valtonen V, Ruutu P. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995–2001. Eur J Clin Microbiol Infect Dis. 2005;24(6):399–404. doi: 10.1007/s10096-005-1345-3 [DOI] [PubMed] [Google Scholar]
- 23.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008;198(3):336–343. doi: 10.1086/589717 [DOI] [PubMed] [Google Scholar]
- 24.Goerke C, Wolz C. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int J Med Microbiol. 2004;294(2):195–202. doi: 10.1016/j.ijmm.2004.06.013 [DOI] [PubMed] [Google Scholar]
- 25.van Rijen MML, Bonten M, Wenzel RP, Kluytmans JAJW. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother. 2008;61(2):254–261. doi: 10.1093/jac/dkm480 [DOI] [PubMed] [Google Scholar]
- 26.van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2008;(4):CD006216. doi: 10.1002/14651858.CD006216.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen K, Danielsen K, Wilsgaard T, et al. Obesity and Staphylococcus aureus nasal colonization among women and men in a general population. Wertheim HFL, ed. PLoS One. 2013;8(5):e63716. doi: 10.1371/journal.pone.0063716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen K, Sangvik M, Simonsen GS, et al. Prevalence and population structure of Staphylococcus aureus nasal carriage in healthcare workers in a general population. The Tromsø Staph and Skin Study. Epidemiol Infect. 2013;141(1):143–152. doi: 10.1017/S0950268812000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtfreter S, Grumann D, Balau V, et al. Molecular epidemiology of Staphylococcus aureus in the general population in Northeast Germany: results of the Study of Health in Pomerania (SHIP-TREND-0). J Clin Microbiol. 2016;54(11):2774–2785. doi: 10.1128/JCM.00312-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebon A, Labout JAM, Verbrugh HA, et al. Dynamics and determinants of Staphylococcus aureus carriage in infancy: the Generation R Study. J Clin Microbiol. 2008;46(10):3517–3521. doi: 10.1128/JCM.00641-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigas AS, Sørensen CJ, Pedersen OB, et al. Predictors of iron levels in 14,737 Danish blood donors: results from the Danish Blood Donor Study. Transfusion (Paris). 2014;54(3 Pt 2):789–796. doi: 10.1111/trf.12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaspersen KA, Dinh KM, Mikkelsen S, et al. Oral iron supplementation is not associated with short-term risk of infections: results from the Danish Blood Donor Study: ORAL IRON SUPPLEMENTATION AND INFECTIONS. Transfusion (Paris). 2019;59:2030–2038. doi: 10.1111/trf.15221 [DOI] [PubMed] [Google Scholar]
- 33.Dinh KM, Pedersen OB, Petersen MS, et al. The impact of CCR5-Δ32 deletion on C-reactive protein levels and cardiovascular disease: results from the Danish Blood Donor Study. Atherosclerosis. 2015;242(1):222–225. doi: 10.1016/j.atherosclerosis.2015.07.031 [DOI] [PubMed] [Google Scholar]
- 34.Burgdorf KS, Felsted N, Mikkelsen S, et al. Digital questionnaire platform in the Danish Blood Donor Study. Comput Methods Programs Biomed. 2016;135:101–104. doi: 10.1016/j.cmpb.2016.07.023 [DOI] [PubMed] [Google Scholar]
- 35.Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2018. doi: 10.1111/bjd.16998 [DOI] [PubMed] [Google Scholar]
- 36.Didriksen M, Rigas AS, Allen RP, et al. Prevalence of restless legs syndrome and associated factors in an otherwise healthy population: results from the Danish Blood Donor Study. Sleep Med. 2017;36:55–61. doi: 10.1016/j.sleep.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Hansen TF, Hoeffding LK, Kogelman L, et al. Comorbidity of migraine with ADHD in adults. BMC Neurol. 2018;18(1). doi: 10.1186/s12883-018-1149-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinh KM, Kaspersen KA, Mikkelsen S, et al. Low-grade inflammation is negatively associated with physical Health-Related Quality of Life in healthy individuals: results from The Danish Blood Donor Study (DBDS). Tauler P, ed. PLoS One. 2019;14(3):e0214468. doi: 10.1371/journal.pone.0214468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health. 1997;19(2):179–186. doi: 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 40.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/S0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 41.Perneger TV, Burnand B. A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol. 2005;58(2):142–149. doi: 10.1016/j.jclinepi.2004.06.005 [DOI] [PubMed] [Google Scholar]
- 42.Kaspersen KA, Pedersen OB, Petersen MS, et al. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology. 2015;26(4):580–589. doi: 10.1097/EDE.0000000000000301 [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Nordestgaard BG, Dahl M. Elevated ACE activity is not associated with asthma, COPD, and COPD co-morbidity. Respir Med. 2009;103(9):1286–1292. doi: 10.1016/j.rmed.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Hansen TF, Banasik K, Erikstrup C, et al. DBDS Genomic Cohort, a prospective and comprehensive resource for integrative and temporal analysis of genetic, environmental and lifestyle factors affecting health of blood donors. BMJ Open 2019;9(6):e028401. doi: 10.1136/bmjopen-2018-028401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landspatientregisteret (LPR) - Sundhedsdatastyrelsen. Available from: http://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/landspatientregisteret.
- 46.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 47.Mernelius S, Löfgren S, Lindgren P-E, Matussek A. The role of broth enrichment in Staphylococcus aureus cultivation and transmission from the throat to newborn infants: results from the Swedish hygiene intervention and transmission of S. aureus study. Eur J Clin Microbiol Infect Dis. 2013;32(12):1593–1598. doi: 10.1007/s10096-013-1917-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21:531–541. doi: 10.1016/j.meegid.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 49.Andersen PS, Pedersen JK, Fode P, et al. Influence of host genetics and environment on nasal carriage of Staphylococcus aureus in Danish Middle-Aged and Elderly Twins. J Infect Dis. 2012;206(8):1178–1184. doi: 10.1093/infdis/jis491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen K, Falch BM, Danielsen K, et al. Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromsø Staph and Skin Study. Eur J Clin Microbiol Infect Dis. 2012;31(4):465–473. doi: 10.1007/s10096-011-1331-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eriksen NH, Espersen F, Rosdahl VT, Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect. 1995;115(1):51–60. doi: 10.1017/s0950268800058118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melles DC, Pauw E, van Den Boogaard L, et al. Host–microbe interplay in persistent Staphylococcus aureus nasal carriage in HIV patients. Microbes Infect. 2008;10(2):151–158. doi: 10.1016/j.micinf.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 53.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, et al. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin Infect Dis. 2004;39(6):806–811. doi: 10.1086/423376 [DOI] [PubMed] [Google Scholar]
- 54.van Belkum A, Verkaik NJ, de Vogel CP, et al. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009;199(12):1820–1826. doi: 10.1086/599119 [DOI] [PubMed] [Google Scholar]
- 55.Liu CM, Price LB, Hungate BA, et al. Staphylococcus aureus and the ecology of the nasal microbiome. Sci Adv. 2015;1(5):e1400216. doi: 10.1126/sciadv.1400216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38(2–3):J282–J291. doi: 10.1016/j.jaut.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 57.Burgdorf KS, Simonsen J, Sundby A, et al. Socio-demographic characteristics of Danish blood donors. PLoS One. 2017;12(2):e0169112. doi: 10.1371/journal.pone.0169112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Landspatientregisteret (LPR) - Sundhedsdatastyrelsen. Available from: http://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygedomme-laegemidler-og-behandlinger/landspatientregisteret.