ABSTRACT
The sea anemone Entacmaea medusivora (Actiniaria, Anthozoa) commonly feeds on the golden jellyfish Mastigias papua (Rhizostomeae, Scyphozoa) which harbours an endosymbiotic dinoflagellate of the genus Cladocopium (Symbiodiniaceae). In this study, we monitored the photosynthetic activity of the endosymbiotic microalgae while their host jellyfish were ingested and digested by starved medusivorous anemones. By analyzing the photosynthetic yield of photosystem II, we observed that Cladocopium cells remain photosynthetically competent during the whole digestion process, thus confirming the exceptional resistance of Symbiodiniaceae to digestive enzymes. In the gastric cavity of E. medusivora, Cladocopium cells release oxygen, which could broadly stimulate the gastric microbiotic flora of the sea anemone. Ultimately, E. medusivora is not able to retain Cladocopium cells more than few days and physiologically-unaltered cells are therefore expelled in faecal pellets. The potential contribution of E. medusivora to maintain a reservoir of Cladocopium symbionts and its role in the life cycle of M. papua is discussed.
Keywords: endosymbiotic dinoflagellate Cladocopium, medusivorous sea anemone, golden jellyfish, photosynthesis, Palau meromictic lake
The symbiotic Cladocopium cells (Dinoflagellate, Symbiodiniaceae) are photosynthetically active during the whole digestion process of its host, the golden jellyfish Mastigias papua, by the asymbiotic sea anemone Entacmaea medusivora.
INTRODUCTION
Dinoflagellates from the Symbiodiniaceae family have the capacity to establish an endosymbiotic relationship with many species of cnidarians (e.g. corals, sea anemones and jellyfish) (LaJeunesse et al. 2018). The establishment of symbiosis implies a multi-step process involving the host and the endosymbiont, the very first step being the physical interaction between both partners. The recognition mechanisms are similar to those acting in the recognition of pathogenic organisms and imply the production of pattern recognition receptors (PRR) able to bind to specific microbial compounds (named microbe-associated molecular patterns or MAMPs). In the case of cnidarian–dinoflagellate symbiosis, the PRR are carbohydrate-binding proteins (e.g. lectins) that are either secreted or present at the surface of the host, while the MAMPs are glycans harbored on the cell surface of the symbionts (Davy, Allemand and Weis 2012; Fransolet, Roberty and Plumier 2012). Initial contacts between algae and animal host cells, and the subsequent phagocytosis of algal cells lead to the sorting of the symbionts engulfed in functional structures known as symbiosomes (Fransolet, Roberty and Plumier 2012). On the other hand, many cnidarians such as the actiniarian Nematostella vectensis (Hand and Uhlinger 1992), the jellyfishes Clytia hemisphaerica (hydrozoan) (Houliston, Momose and Manuel 2010) and Aurelia aurita (schyphozoan) (Hernroth and Gröndahl 1983), some strains of the freshwater-living polyp Hydra vulgaris (Ishikawa et al. 2016), and even some scleractinian corals (Daly et al. 2007; Barbeitos, Romano and Lasker 2010) are asymbiotic.
In the Palau archipelago, the meromictic saline lake on the island of Mecherchar (commonly known as Jellyfish Lake) comprises an upper layer (up to 15 m deep) of oxygenated, nutrient-poor and brackish water which the asymbiotic sea anemone Entacmaea medusivora (Fautin and Fitt 1991) and the golden jellyfish Mastigias papua (De Souza and Dawson 2018) inhabit (Hamner, Gilmer and Hamner 1982). In Palau, M. papua usually lives in symbiosis with dinoflagellates of the genus Cladocopium (formerly Clade C) (Krueger et al. 2015; LaJeunesse et al. 2018). About 10% of the jellyfish protein biomass corresponds to endosymbiotic Cladocopium cells which reside within the mesoglea of the coelenterate (Muscatine, Wilkerson and McCloskey 1986). Because of this symbiotic relationship, M. papua is responsible for about 20% of the carbon primary production of the lake (Hamner, Gilmer and Hamner 1982). These jellyfish swim horizontally close to the surface following the sun position to the shadow line (Dawson and Hamner 2003) and avoid reaching the shoreline where the sea anemone E. medusivora, its natural predator, lives (Fautin and Fitt 1991).
E. medusivora is considered as asymbiotic (Fautin and Fitt 1991). However, given that this sea anemone feeds on symbiotic jellyfish, its gastric cavity is exposed to large amounts of Cladocopium cells. The fate of these algae upon digestion is not yet known. However, it has been documented in some symbiotic cnidarian-eating parrotfishes or sea stars that Symbiodiniaceae algae survive digestion and are present in faeces of these animals (Castro-Sanguino and Sánchez 2012; Bachman and Muller-Parker 2007). In this report, we studied the photosynthetic capacity and distribution of Cladocopium cells during digestion of golden jellyfish by the sea anemone E. medusivora.
METHODS
Biological materials
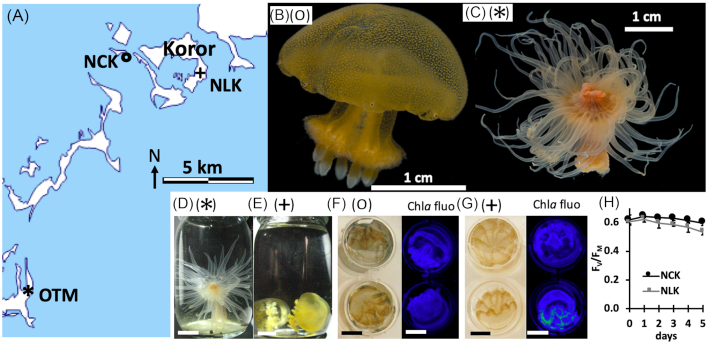
During a sampling campaign conducted in January 2018, six specimens of E. medusivora were collected from Jellyfish Lake (Ongeim'l Tketau, OTM) on the island of Mecherchar in the Republic of Palau. Because the population of golden jellyfish M. papua etpisoni in OTM dropped to zero in 2016 (the causes are most likely similar to causes of a previous event of disappearance in 1998; see Dawson, Martin and Penland 2001 for details on this matter), M. papua remeliiki was sampled in Ngermeuangel Lake (NLK) on 17 January 2018, and the ancestral M. papua from Ngerchaol Cove in the Palau lagoon (NCK) on 19 January 2018 (Fig. 1A-E), under Koror State permit n° 027. Four jellyfish specimens were obtained from each site, 1.5–2 cm diameter and 1.5–2 g fresh weight. Symbiodinaceae associated with NCK and NLK jellyfish were genotyped by T. LaJeunesse at Pennsylvania State University (according to Lajeunesse et al. 2018) which in both populations correspond to Cladocopium sp. (T. Lajeunesse, unpublished data). Sea anemones and jellyfish were maintained at the Palau International Coral Research Center (PICRC) for 10–14 days in diluted seawater (salinity of 25 ppt) for specimens from NLK and OTM or natural seawater (salinity of 34 ppt) for jellyfish from NCK. Sea anemones E. medusivora were maintained individually in 100 mL cylindrical glass jars covered with a metal mesh. They were starved for at least 7 days before being fed with one or two jellyfish (see results section). Jellyfish were maintained in 2 L open plastic containers, allowing them to swim. Light intensity was low, with a maximum of 100 µmol photons m−2 s−1 reached at noon. Water was changed twice a day and water temperature was maintained at 30 ± 1°C (day–night variations). This temperature corresponds to the average temperature of different lakes in Palau (Dawson, Martin and Penland 2001) and to the sea surface temperature of the Western Pacific Ocean (Locarnini et al. 2010) in January. The physiological state of sampled golden jellyfish was monitored in the laboratory every day by transferring the jellyfish into standard 12-well plates and subsequently evaluating the frequency of bell contraction and the maximum quantum yield of photosystem II (FV/FM) by fluorescence imaging (Fig. 1F-H).
Figure 1.
Geographic origin, aspect and size of the golden jellyfish M. papua and the medusivorous anemone E. medusivora from Palau used in this study. (A) Partial map of Palau Archipelago indicating the sampling sites: (*) Ongeim'l Tketau on Mecherchar (OTM, Jellyfish Lake), 7°09′40.2‘N 134°22′35.0″E; (+) Ngermeuangel Lake in Koror (NLK), 7°19′28.5‘N 134°30′31.6″E; (o) Ngerchaol Cove in the Koror lagoon (NCK), 7°20′17.6‘N 134°27′04.6″E. The map was obtained at https://d-maps.com/carte.php?num_car = 3326. (B,F) M. papua from NCK. (C,D) E. medusivora from OTM. (E,G) M. papua remeliiki from NLK. (H) Maximal PSII quantum yield (FV/FM) at day 0 (after sample harvesting) and during 5 days in aquariums. Measurements were performed by chlorophyll fluorescence imaging of jellyfish in standard 12-well plates (mean of four jellyfish with standard errors). Scale bars on (B–G) correspond to 1 cm.
In vivo chlorophyll fluorescence imaging
True-colour pictures along with chlorophyll a fluorescence imaging were used to monitor the distribution of symbiotic microalgae and overall shape during the feeding and digestion events by anemones. Distribution of chlorophyll a fluorescence yield in jellyfish and sea anemones was determined at room temperature with a fluorescence imaging system (SpeedZen, BeamBio/API, France). Chlorophyll a fluorescence was excited by an array of blue LEDs (450–470 nm), filtered with a long-pass red filter and recorded by a CCD camera (UI-3240CP-NIR-GL Rev.2, IDS, Obersulm, Germany). The anemones change their overall shape during digestion and move inside the glass containers, so the geometry of the fluorescence imaging system is different from one image to another. Accordingly, it was not possible to quantitatively compare the overall amount of fluorescence at the different time points.
To determine if algal cells were photosynthetically active, we measured the relative electron transfer rate of photosystem II (rETR-PSII) at different continuous actinic light intensities (up to 1000 µmol photons m−2 s−1) using an LED array emitting at 660 nm, and also delivering 200 ms 6000 µmol photons m−2 s−1 saturating pulses. The maximum quantum yield of photosystem II was measured as FV/FM, where FV = FM−F0, F0 is the initial fluorescence level in dark-adapted sample and FM is the maximum fluorescence level after a saturating pulse of light (Kitajima and Butler 1975). After acclimation to different light intensities, FS and FM′ (stationary fluorescence level in continuous light and maximum fluorescence emission induced by saturating pulse of light, respectively) were measured and the effective quantum yield of photosystem II at steady state (φPSII = (FM′−FS)/FM′, Genty, Briantais and Baker 1989) was calculated. The rETR-PSII was calculated as the product of φPSII and the actinic light intensity (Ralph and Gademann 2005). Because rETR-PSII value is proportional to a ratio of fluorescence measurements, its value is much less affected by the geometry of our experimental setup and the data can be quantitatively compared.
Polarographic measurements of oxygen
Measurements of light-dependent oxygen release of M. papua and E. medusivora (24 h after having being fed with one jellyfish) were carried out by using a Clark-type electrode in a 2.5 mL chamber (Oxygraph+, Hansatech, UK). The translucent acrylic chamber (DW1, Hansatech, UK) was set next to the SpeedZen camera, which was placed in a horizontal position. The same red actinic light intensity used to perform fluorescence measurements was used to monitor oxygen exchange. Measurement of light intensity was performed using a submersible spherical micro quantum sensor (US-SQS, Walz, Germany) connected to a LI-250A light meter (Li-Cor, USA).
Electrochromic shift spectra
The response of photosynthetically competent microalgae to actinic radiation was also assessed by electrochromic shift signal (ECS) detection using a JTS-10 LED pump-probe spectrophotometer (Biologic, France). ECS occurs only in response to an electric potential across the thylakoid membrane (Bailleul et al. 2010). The shape of the ECS spectrum also reflects the membrane pigment composition and is therefore different between various photosynthetic eukaryote lineages (Bailleul et al. 2010). Spectral characteristics of ECS were determined from M. papua and E. medusivora 24 h after being fed with one NLK jellyfish. Absorbance changes were measured in a glass visible-spectrometer cuvette cell (path length of 1 cm), from 480 to 540 nm every 10 nm, using BrightLine (Semrock) single-band bandpass filters (full width at half maximum bandwidth of ∼15 nm). They were determined during 4 ms after a short saturating pulse of red light (9000 µmol photons m−2 s−1, 660 nm), as previously described for cultured Symbiodinium (formerly temperate Clade A) cells (Roberty et al. 2014).
RESULTS
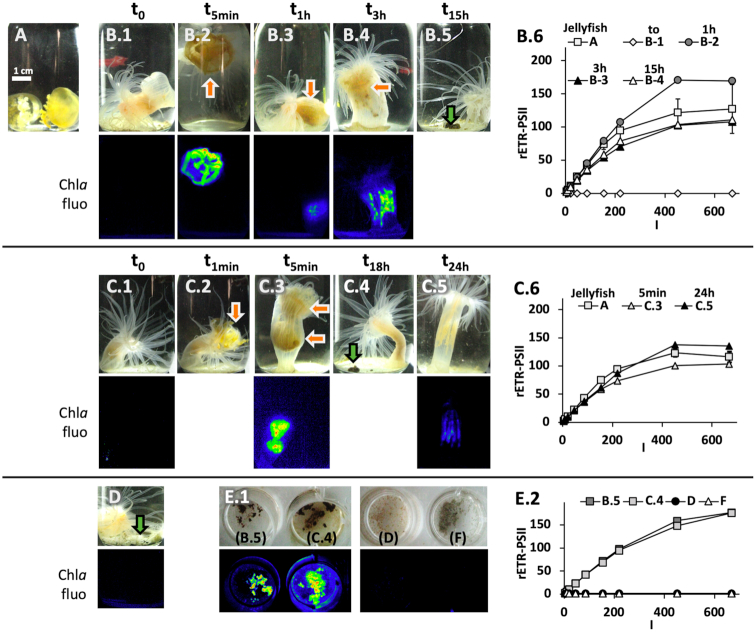
Specimens of the sea anemone E. medusivora collected from Jellyfish Lake (OTM) in the Republic of Palau were on average the same size, about 3 cm high when individuals were fully extended (Fig. 1D). Before being fed, the anemones did not show any chlorophyll a fluorescence signal (Fig. 2B.1, C.1 and D). We first fed an anemone with small M. papua jellyfish collected at NLK. From the moment when a jellyfish was caught by anemone tentacles, it took approximately 5 min for E. medusivora to fully ingest their prey (Fig. 2B.2, C.2 and C.3). The shape of the jellyfish could be distinguished up to 1 h after ingestion inside the sea anemone column (Fig. 2B.3). From 3 h after ingestion, the shape of the jellyfish was no longer distinguishable and chlorophyll a fluorescence was distributed in mesenteries of the anemone (Fig. 2B.4 and C.5), indicating that digestion had occurred. The overall trend was a decrease in the chlorophyll fluorescence (Fig. 2A and 2B) that fully disappeared after 3–4 days (data not shown).
Figure 2.
Photosynthetic activity of Cladocopium upon digestion of its host golden jellyfish M. papua remeliiki NLK by the anemone E. medusivora. (A) M. papua from NLK (see Fig. 1 for details). A starved E. medusivora was fed with one (B.1–5) or two (C.1–5) NLK jellyfish. Orange arrows point to jellyfish whose shape is still visible inside the anemone. (D) E. medusivora was fed with (non-photosynthetic) mussel flesh collected on the shore of OTM, Jellyfish Lake. (E.1) Faeces from anemones (B.5, C.4 and D; green arrows) and waste matter at the bottom of the glass pot of a starved anemone (F) were transferred in standard 12-well plates. Imaging of the maximal yield of chlorophyll fluorescence (FM, Chl-a fluo) is shown for selected pictures: before ingestion of jellyfish (B.1, C.1); at various moments during digestion of jellyfish (B.2–4, C.3, C.5); after feeding with mussels (D) and for faeces (F). (B.6, C.6, E.2) rETR-PSII as a function of the actinic light intensity (I, μmol photons m−2 s−1) was calculated from chlorophyll fluorescence signals of anemones from B and C series (n = 1) and intact jellyfish (A, n = 3; the series shown in B.6 and C.6 correspond to independant triplicates). Scale bar in (A) corresponds to 1 cm.
To determine if these fluorescence signals corresponded to photosynthetically active microalgae upon digestion, light saturation curves of the rETR-PSII were established. When comparing the light curve obtained with free jellyfish, ingested jellyfish (5 min–1 h) and mesenteries of anemones (from 3 h to 24 h after digestion), no significant differences were found both in the maximum rate of rETR-PSII and in the initial slope of the light curve (Fig. 2B.6 and C.6). These results strongly suggest that an important fraction of Cladocopium cells remain photosynthetically competent during the digestion process of symbiotic jellyfish by E. medusivora.
We also collected faecal pellets expelled by these anemones 12–18 h after feeding with jellyfish (Fig. 2B.5 and C.4) and these excretions were fluorescent (see B.5 and C.4 in Fig. 2E.1). In contrast, faeces from another anemone fed with mussel flesh or waste matter from a starved specimen were not fluorescent (see D and F in Fig. 2E.1). rETR-PSII of the fluorescent faecal matter (Fig. 2E.2) were similar to the one of intact or digested jellyfish. These results indicate that Cladocopium cells can resist digestive enzymes of E. medusivora.
When M. papua (lagoon form) collected in NCK (Fig. 1B and F) was used to feed another starved anemone, the same set of events was observed, meaning that the jellyfish is digested and Cladocopium cells stay photosynthetically active during digestion (Fig. 3A). Because chlorophyll fluorescence is emitted in all directions and may therefore diffuse into the animal tissues, we could not fully exclude that some Cladocopium cells are not located outside the mesenteries. For instance, tentacles were faintly fluorescent in some pictures (see for instance Fig. 3A.1). No chlorophyll a fluorescence was however detected in separated tentacles. All anemones fed only once with jellyfish (NLK or NCK) were also starved thereafter. No remaining fluorescence was detected in these animals after 3 days (data not shown).
Figure 3.
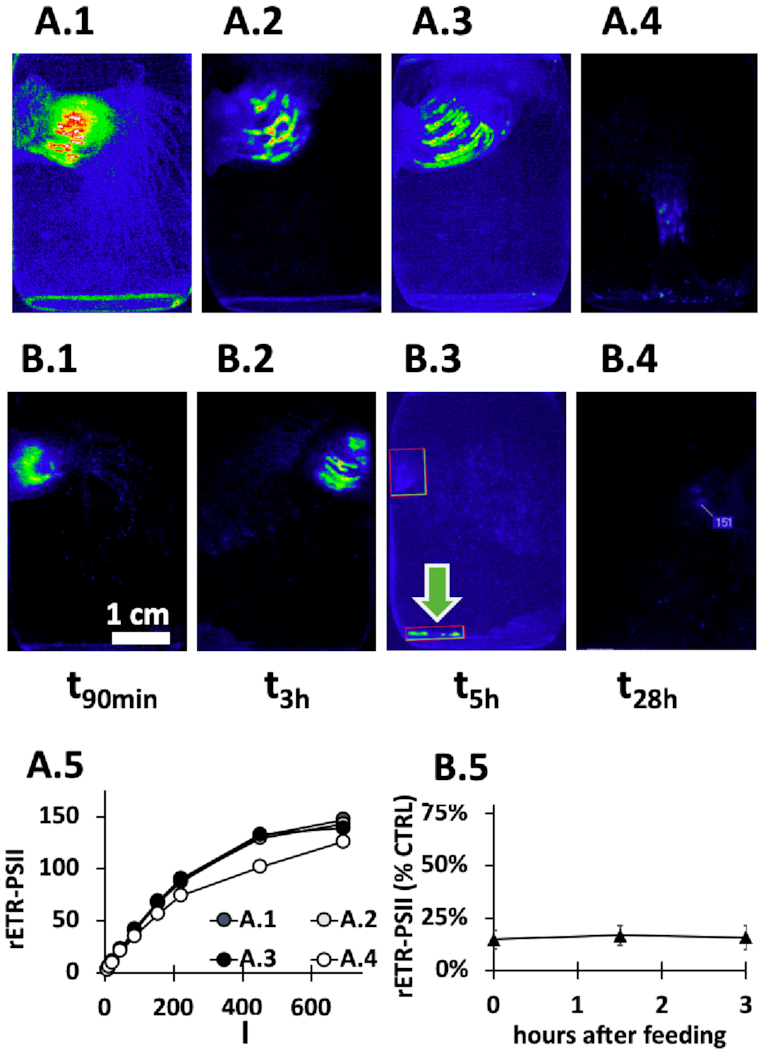
Comparison of photosynthetic activities in DCMU-poisoned and unpoisoned Cladocopium during digestion of M. papua NCK by E. medusivora. (A.1–4) A starved E. medusivora was fed with M. papua jellyfish from NCK (see Fig. 1 for details). Pictures were obtained by in vivo fluorescence imaging of the maximal yield of chlorophyll fluorescence at various moments (from 90 min to 28 h) during digestion of the jellyfish. (A.5) rETR-PSII as a function of the actinic light intensity (I, μmol photons m−2 s−1) was calculated from chlorophyll fluorescence signals of A series (n = 1). (B.1–4) A starved E. medusivora was fed with NCK M. papua jellyfish poisoned by 20 μM DCMU during 5 min of prior ingestion. (B.5) Ratio between rETR-PSII from DCMU-treated (B series) and control experiment (A series) (n = 1). A green arrow indicates fecal pellets. Averaged values and standard deviations were calculated from values at eight different light intensities. Scale bar in (B.1) corresponds to 1 cm.
In parallel, another E. medusivora was fed with a lagoon NCK jellyfish poisoned with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), a potent inhibitor of photosystem II. Although rETR-PSII of the ingested jellyfish was initially <10% of the control (Fig. 3B.5), the same temporal set of events occurred. The overall amount of fluorescence however decreased rapidly in the DCMU-poisoned experiment, being very low 5 h after feeding (compare Fig. 3B.3 and A.3). This suggests that DCMU-poisoned Cladocopium cells lost their fluorescence due to damage to the photosynthetic apparatus and might have been digested or died and been expelled with excrement (green arrow in Fig. 3B.3).
To confirm that Cladocopium cells were still photosynthetically active inside the anemone 1 day after being fed with unpoisoned jellyfish, we examined if an ECS could be measured. A similar ECS spectrum could be determined for both types of golden jellyfish as well as for an anemone fed with a jellyfish from NLK (Fig. 4A). These ECS spectra of Cladocopium are similar to those of cultured Symbiodinium microadriaticum (formerly Clade A) cells, which are characterized by a maximum at 510 nm and a minimum at 550 nm (Roberty et al. 2014). These results confirm that Cladocopium cells were still photosynthetically active in the mesenteries and that membrane pigments responsible for ECS were not impaired.
Figure 4.
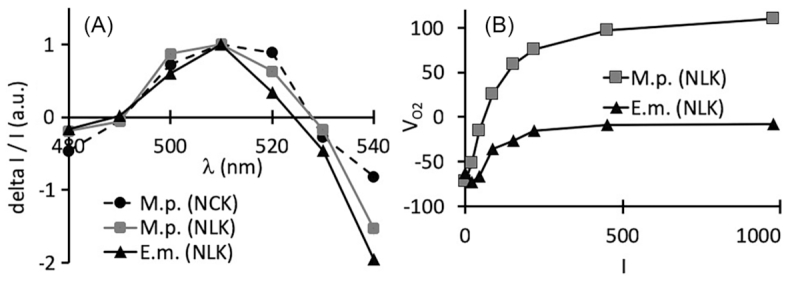
Comparison of photosynthetic features from golden jellyfish M. papua remeliiki and E. medusivora 24 h after feeding with NLK golden jellyfish. (A) ECS spectra upon 4 ms of illumination. (B) Net oxygen exchange rate (VO2; 10−1 nmol O2 s−1 per animal, n = 1) as a function of light intensity (I, μmol photons m−2 s−1, 3 min steps). M.p. (NLK), M.p. (NCK), M. papua from NLK and NCK respectively; E.m. (NLK), E. medusivora fed with NLK golden jellyfish.
Finally, net oxygen exchange rate (VO2) of the anemone analyzed in Fig. 4A was compared with VO2 values of an intact golden jellyfish (Fig. 4B). The dark respiratory rate per animal of both cnidarians was similar. For both organisms, a light-dependent oxygen evolution was observed. Because the experiment was performed only once we cannot comment on the absolute values. Still, this result is another confirmation that Cladocopium cells are able to perform photosynthesis inside the gastric cavity of the anemone.
DISCUSSION
Overall, our observations indicate that a significant fraction of Cladocopium cells are not altered and remain photosynthetically active from ingestion by symbiotic jellyfish M. papua to expulsion of excrements by the sea anemone E. medusivora. The sea anemone E. medusivora has been found so far only in Jellyfish Lake located on Mecherchar Island in Palau (Fautin and Fitt 1991) and in Kakaban Lake in Indonesia (Hoeksema, Tuti and Becking 2015). These anemones notably feed on various jellyfish species such as Aurelia aurita (the moon jellyfish), M. papua (golden jellyfish) and Cassiopea sp. (upside-down jellyfish) (Fautin and Fitt 1991; Hoeksema, Tuti and Becking 2015). These two last species live in symbiosis with photosynthetic dinoflagellates of the Symbiodiniaceae family (LaJeunesse et al. 2018). In Palau, the population of M. papua etpisoni disappeared from Jellyfish Lake (OTM) in 2016 following a strong El Niño—southern oscillation event—and began recovering towards the end of 2018 (Coral Reef Research Foundation, pers. comm.). Therefore, E. medusivora sampled in January 2018 had been deprived of photosynthetic jellyfish for several months. Upon digestion of M. papua by starved E. medusivora, the vast majority of Cladocopium chlorophyll fluorescence is distributed in areas that most likely correspond to mesenteries, where digestive enzymes are present. Despite the high amount of algae that remain photosynthetically active from ingestion by their host jellyfish to expulsion with undigested matter, E. medusivora does not establish stable symbiosis with photosynthetic Cladocopium cells. The lack of chlorophyll fluorescence measured in vivo in individuals just after sampling, or several days after feeding with M. papua, confirms that E. medusivora is asymbiotic. This is in good agreement with a previous study that failed to identify dinoflagellate cells using electron microscopy (Fautin and Fitt 1991). But this contrasts with the fact that this sea anemone belongs to a genus containing symbiotic species, such as the bubble-tip anemone Entacmaea quadricolor (Scott and Harrison 2007). Several assumptions can explain why ingested Cladocopium do not establish endosymbiosis: (1) the absence of a recognition mechanism between the two species; (2) the sea anemone recognizes Cladocopium as a prey; (3) Cladocopium is recognized and then phagocytized but the phagosome does not maturate into a symbiosome and fuses with lysosomes. In this respect Fautin and Fitt (1991) suggested that some Symbiodiniaceae are phagocytosed into host digestive cells when introduced with Artemianauplii, but do not remain in the E. medusivora digestive cavity for more than 1 week. A few cases of non-photosynthetic anemones capable of establishing a symbiotic relationship with algae have however been documented, e.g. asymbiotic strains of Hydra vulgaris (Hydrozoa) may establish endosymbiosis with Chlorococcum green algae under laboratory conditions (Ishikawa et al. 2016).
Although E. medusivora is unable to establish a lasting relationship with Cladocopium, it is tempting to suggest that the transient presence of a large amount of photosynthetically active Symbiodiniaceae inside its digestive cavity may have a positive impact on the physiology of the anemone. As the body of E. medusivora is translucent, Cladocopium cells located inside the gastric cavity perform photosynthesis and release O2. Carbon fixed by photosynthesis may also be exported by the microalgae. Indeed, it has been previously reported that isolated Symbiodiniaceae from the sea anemone Exaiptasia released ∼5% of their photosynthates. This proportion even increased to 14–25% in response to extracts of host tissue (host-release factor; Davy and Cook 2001). Therefore, we suggest that the release of photosynthetic products may directly contribute to the metabolism of E. medusivora. This needs to be further investigated by the use of stable isotope tracers of carbon or nitrogen, with labelling of the microalgae before ingestion by anemones (Cleveland, Verde and Lee 2011). The beneficial effects may also be indirect through the stimulation of the bacterial community inhabiting the gastric cavity (Agostini et al. 2012; Cleary et al. 2016).
Our study also demonstrates that expelled Cladocopium from E. medusivora after digestion of M. papua are still photosynthetically competent. Similar results have been previously reported in other corallivorous organisms. For instance, some parrot fishes can carry Symbiodiniaceae algae in their digestive system and expel them alive within their faeces (Castro-Sanguino and Sánchez 2012). The faeces of the sea star Dermasterias imbricata fed with the symbiotic anemone Anthopleura elegantissima also contains almost physiologically-unaltered symbiotic microalgae (Bachman and Muller-Parker 2007). These observations in three different species indicate a general resistance capacity of Symbiodiniaceae to the digestion process of various animals. This is not really surprising since a method aiming to isolate intact and viable Symbiodiniaceae from host tissues requires the use of 1 M NaOH (Zamoum and Furla 2012). Such a resistance to host lysosomal enzymes could be considered as a fundamental prerequisite to the establishment of a symbiotic relationship (e.g. Kodama and Fujishima 2014).
Similarly to Cassiopea (Lampert 2016), a Rhizostomeae jellyfish closely related to the golden jellyfish, the transmission of endosymbiotic dinoflagellates in M. papua occurs horizontally. Indeed, eggs, testicular vesicles and planula larvae of both species are free of endosymbionts, and only scyphistomae (polyps) acquire them during growth (Sugiura 1964; Hofmann, Fitt and Fleck 1996). The colonization of the polyps is even required to initiate the strobilation and the production of symbiont-containing ephyra larvae and adults of M. papua (Sugiura 1964). Consequently, the Symbiodiniaceae community hosted by the jellyfish necessarily originates from an environmental reservoir of free-living symbionts present in sediments and in the water column. This reservoir can be constituted by the release of Symbiodiniaceae (1) from Mastigias polyps, ephyra and adults into the surrounding water (Sugiura 1964), release that could be constant as in symbiotic corals (Hoegh-Guldberg, McCloskey and Muscatine 1987); (2) from the degradation of dead adult jellyfish; (3) from predators feeding on cnidarians (Muller-Parker 1984; Castro-Sanguino and Sánchez 2012; Bachman and Muller-Parker 2007).
Based on the results presented in this report, we propose that, by feeding on M. papua, the medusivorous anemone could significantly contribute to increase the speed of algal release. Because Mastigiasscyphistomae and E. medusivora are living in close vicinity to each other in Jellyfish Lake (Dawson 2005), this proximity could contribute to the maintenance of an environmental reservoir of free-living Cladocopium. Hence, by eating some jellyfish, and participating in microalgal cycling, E. medusivora could play a key role in the life cycle of M. papua.
ACKNOWLEDGEMENTS
All experiments were performed in January 2018 at Palau International Coral Reef Center (PICRC) under permit n° 027 delivered by the Koror State government. We thank Daniel Beal (BeamBio) for technical assistance on Speedzen-II setup during our stay in Palau; Geraldine Rengiil, Randa Johnathan and PICRC staff in Palau for their support during our stay; Lori Colin, Gerda Ucharm and members of Coral Reef Research Foundation in Palau for their help during the sampling campaign of jellyfish and during preparation of this manuscript; Paola Furla for her help during sampling campaign of anemones; Eric Rottinger (CNRS, Kahi Kai Images, Fondation Tara Expéditions) for taking and sharing pictures (Fig. 1B & C), and René Matagne and Lori Colin for their comments on the final revision of this paper. K.-V.D. and P.C. are postdoctoral researcher and senior research associate from Fonds de la Recherche Scientifique—FNRS, respectively.
FUNDING
This work was supported by the European research council [ERC consolidator grant BEAL 682 580 to P.C.] and the Belgian Fonds de la Recherche Scientifique—FNRS [CDR J.0079 to P.C.].
Conflict of interest. None declared.
REFERENCES
- Agostini S, Suzuki Y, Higuchi T et al.. Biological and chemical characteristics of the coral gastric cavity. Coral Reefs. 2012;31:147–56. [Google Scholar]
- Bachman S, Muller-Parker G.. Viable algae released by the sea star Dermasterias imbricata feeding on the symbiotic sea anemone Anthopleura elegantissima. Mar Biol. 2007;150:369–75. [Google Scholar]
- Bailleul B, Cardol P, Breyton C et al.. Electrochromism: A useful probe to study algal photosynthesis. Photosynth Res. 2010;106:179–89. [DOI] [PubMed] [Google Scholar]
- Barbeitos MS, Romano SL, Lasker HR. Repeated loss of coloniality and symbiosis in scleractinian corals. Proc Natl Acad Sci. 2010;107:11877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Sanguino C, Sánchez JA.. Dispersal of Symbiodinium by the stoplight parrotfish Sparisoma viride. Biol Lett. 2012;2:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary DFR, Becking LE, Polónia ARM et al.. Jellyfish-associated bacterial communities and bacterioplankton in Indonesian Marine lakes. FEMS Microbiol Ecol. 2016;92:1–14. [DOI] [PubMed] [Google Scholar]
- Cleveland A, Verde EA, Lee RW. Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar Biol. 2011;158:589–602. [Google Scholar]
- Daly M, Brugler MR, Cartwright P et al.. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. In: Zhang ZQ, Shear WA(eds). Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa2007;1668:127–82. [Google Scholar]
- Davy SK, Allemand D, Weis VM. Cell Biology of Cnidarian-Dinoflagellate Symbiosis. Microbiol Mol Biol Rev. 2012;76:229–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy SK, Cook CB.. The influence of ‘host release factor’ on carbon release by zooxanthellae isolated from fed and starved Aiptasia pallida (Verrill). Comp Biochem Physiol Part A. 2001;129:487–94. [DOI] [PubMed] [Google Scholar]
- Dawson MN, Hamner WM.. Geographic variation and behavioral evolution in marine plankton: The case of Mastigias (Scyphozoa, Rhizostomeae). Mar Biol. 2003;143:1161–74. [Google Scholar]
- Dawson MN, Martin LE, Penland LK. Jellyfish swarms, tourists, and the Christ-child. Hydrobiologia. 2001;451:131–44. [Google Scholar]
- Dawson MN. Five new subspecies of Mastigias (Scyphozoa: Rhizostomeae:Mastigiidae) from marine lakes, Palau, Micronesia. J Mar Biol Assoc United Kingdom. 2005;85:679–94. [Google Scholar]
- De Souza MR, Dawson MN. Redescription of Mastigias papua (Scyphozoa, Rhizostomeae) with designation of a neotype and recognition of two additional species. Zootaxa. 2018;4457:520–36. [DOI] [PubMed] [Google Scholar]
- Fautin DG, Fitt WK. A jellyfish-eating sea anemone (Cnidaria, Actiniaria) from Palau: Entacmaea medusivora sp. nov. Hydrobiologia. 1991;216–217:453–61. [Google Scholar]
- Fransolet D, Roberty S, Plumier JC. Establishment of endosymbiosis: The case of cnidarians and Symbiodinium. J Exp Mar Bio Ecol. 2012;420–421:1–7. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta - Gen Subj. 1989;990:87–92. [Google Scholar]
- Hamner WM, Gilmer RW, Hamner PP. The physical, chemical, and biological characteristics of a stratified, saline, sulfide lake in Palau. Limnol Oceanogr. 1982;27:896–909. [Google Scholar]
- Hand C, Uhlinger KR.. The culture, sexual and asexual reproduction, and growth of the sea anemone Nematostella vectensis. Biol Bull. 1992;182:169–76. [DOI] [PubMed] [Google Scholar]
- Hernroth L, Gröndahl F.. On the biology of aurelia aurita (L.) 1. Release and growth of aurelia aurita (L.) ephyrae in the gullmar fjord, Western Sweden, 1982–83. Ophelia. 1983;22:189–99. [Google Scholar]
- Hoegh-Guldberg O, McCloskey LR, Muscatine L. Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. Coral Reefs. 1987;5:201–4. [Google Scholar]
- Hoeksema BW, Tuti Y, Becking LE. Mixed medusivory by the sea anemone Entacmaea medusivora (Anthozoa: Actiniaria) in Kakaban Lake, Indonesia. Mar Biodivers. 2015;45:141–2. [Google Scholar]
- Hofmann DK, Fitt WK, Fleck J. Checkpoints in the life-cycle of Cassiopea spp.: control of metagenesis and metamorphosis in a tropical jellyfish. Int J Dev BioI. 1996;40: 331–8. [PubMed] [Google Scholar]
- Houliston E, Momose T, Manuel M. Clytia hemisphaerica: A jellyfish cousin joins the laboratory. Trends Genet. 2010;26:159–67. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Shimizu H, Nozawa M et al.. Two-step evolution of endosymbiosis between hydra and algae. Mol Phylogenet Evol. 2016;103:19–25. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Butler WL.. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. BBA - Bioenerg. 1975;376:105–15. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Fujishima M.. Symbiotic Chlorella variabilis incubated under constant dark conditions for 24 hours loses the ability to avoid digestion by host lysosomal enzymes in digestive vacuoles of host ciliate Paramecium bursaria. FEMS Microbiol Ecol. 2014;90:946–55. [DOI] [PubMed] [Google Scholar]
- Krueger T, Fisher PL, Becker S et al.. Transcriptomic characterization of the enzymatic antioxidants FeSOD, MnSOD, APX and KatG in the dinoflagellate genus Symbiodinium. BMC Evol Biol. 2015;15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse TC, Parkinson JE, Gabrielson PW et al.. Systematic Revision of Symbiodiniaceae Highlights the Antiquity and Diversity of Coral Endosymbionts. Curr Biol. 2018;28:2570–80. e6. [DOI] [PubMed] [Google Scholar]
- Lampert KP. Cassiopea and its Zooxanthelae. In: Goffredo S, Dubinsky Z(eds). The Cnidaria, Past, Present and Future: The world of Medusa and her Sisters. Switzerland: Springer International Publishing, 2016, 415–23. [Google Scholar]
- Locarnini RA, Mishonov AV, Antonov JI et al.. World Ocean Atlas 2009, Volume 1: Temperature. In: Levitus S.(ed). NOAA Atlas NESDIS 68, U.S. Washington, DC: Government Printing Office, 2010, 184. [Google Scholar]
- Muller-Parker GT. Dispersal of Zooxanthellae on Coral Reefs by Predators on Cnidarians. Biol Bull. 1984;167:159–67. [Google Scholar]
- Muscatine L, Wilkerson FP, McCloskey LR. Regulation of population density of symbiotic algae in a tropical marine jellyfish (Mastigias sp.). Mar Ecol Prog Ser. 1986;32:279–90. [Google Scholar]
- Ralph PJ, Gademann R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat Bot. 2005;82:222–37. [Google Scholar]
- Roberty S, Bailleul B, Berne N et al.. PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytol. 2014;204:81–91. [DOI] [PubMed] [Google Scholar]
- Scott A, Harrison PL.. Embryonic and larval development of the host sea anemones Entacmaea quadricolor and Heteractis crispa. Biol Bull. 2007;213:110–21. [DOI] [PubMed] [Google Scholar]
- Sugiura Y. On the life-history of Rhizostomae medusae. II Indispensability of zooxanthellae for strobilation in Mastigias papua. Embryologia (Nagoya). 1964;8:223–33. [Google Scholar]
- Zamoum T, Furla P. Symbiodinium isolation by NaOH treatment. J Exp Biol. 2012;215:3875–80. [DOI] [PubMed] [Google Scholar]