Abstract
Background
To assess the usefulness of serum C‐terminal hydrolase L1 (UCH‐L1) level as a biomarker for predicting cognitive impairment in patients with acute organophosphorus pesticide poisoning (AOPP).
Methods
Two hundred and seven adult patients with AOPP were included in this study. Serum UCH‐L1 levels were assessed on admission (Day 1 postpoisoning) and on Days 3 and 7 postpoisoning. The associations between serum UCH‐L1 levels, other clinical predictors, and cognitive function evaluated on Day 30 postpoisoning were investigated.
Results
On multivariate analysis, serum UCH‐L1 levels on admission (odds ratio [OR] 1.889, 95% confidence interval [CI] 1.609‐3.082, P = 0.002) and 24‐hour APACHE II score (OR 1.736, 95% CI 1.264‐3.272, P = 0.012) were independent predictors of cognitive impairment on Day 30 postpoisoning. Based on the receiver operating characteristic curve, serum UCH‐L1 levels >5.9 ng/mL on admission predicted cognitive impairment on Day 30 postpoisoning with 86.1% sensitivity and 72.5% specificity (area under the curve, 0.869; 95% CI 0.815‐0.923). On admission [8.51 (6.53‐10.22) ng/mL vs 4.25 (2.57‐6.31) ng/mL, P < 0.001] and Day 3 [9.31 (7.92‐10.98) ng/mL vs 3.32 (2.25‐5.13) ng/mL, P < 0.001] and Day 7 [4.96 (3.28‐7.26) ng/mL vs 2.27 (1.55‐3.24) ng/mL, P < 0.001] postpoisoning, serum UCH‐L1 concentration was significantly higher in patients that developed cognitive impairment compared to those that did not.
Conclusion
This study demonstrates that serum UCH‐L1 level has potential as a novel biomarker for predicting cognitive impairment 30 days after AOPP.
Keywords: acute organophosphorus pesticide poisoning, cognitive impairment, ubiquitin C‐terminal hydrolase L1
1. INTRODUCTION
Acute organophosphorus pesticide poisoning (AOPP) is an important public health problem. AOPP is associated with substantial human morbidity and mortality because of the widespread use of organophosphate compounds.1 The nervous system is particularly sensitive to the effects of AOPP, which manifest as cognitive impairment, including deficits in information processing, sustained attention, memory, problem‐solving, abstraction, flexibility of thinking, and depressed mood.2, 3 Patients experiencing cognitive impairment in AOPP may have long‐lasting or irreversible cognitive and behavioral sequelae and an increased mortality rate. Thus, the identification of patients with AOPP who are at risk of cognitive sequelae could guide treatment decision‐making.
Acute organophosphorus pesticide poisoning causes irreversible inhibition of acetylcholinesterase (AchE) leading to the accumulation of acetylcholine (Ach) at synapses and muscarine, nicotine, and central nervous system symptoms. Clinically, evidence of AOPP can be confirmed by measuring a decrease in blood AchE activity, which can also be used to monitor treatment response as a prognostic indicator. However, cognitive impairment in AOPP is not always associated with decreased blood AchE activity,4 and evidence for a relationship between blood AchE activity and impaired neurobehavioral function is limited.5
Ubiquitin C‐terminal hydrolase L1 (UCH‐L1) is a neuron‐specific enzyme that is highly abundant in the brain.6 UCH‐L1 is a deubiquitinating enzyme that is required for normal cognitive function.7, 8 UCH‐L1 has been implicated in the pathophysiology of Parkinson's disease (PD),9 Alzheimer's disease (AD),10 Huntington's disease,11 and epileptic seizures.12 Increasingly, UCH‐L1 is recognized as a biomarker of brain injury. Circulating UCH‐L1 levels are significantly increased after acute neurological insults such as traumatic brain injury, subarachnoid hemorrhage, ischemic stroke, hypoxic‐ischemic encephalopathy, and cardiac arrest,13, 14, 15, 16, 17, 18 and altered UCH‐L1 expression is an indicator of brain injury severity and a predictor of neurological outcomes.17, 19 To the authors’ knowledge, no studies have investigated the utility of serum UCH‐L1 level as a predictor of cognitive impairment after AOPP. Therefore, this prospective study compared serum UCH‐L1 levels in patients with and without cognitive impairment following AOPP to evaluate the utility of UCH‐L1 for the prediction of cognitive impairment.
2. MATERIALS AND METHODS
2.1. Study population
Patients with AOPP admitted to the Emergency Department at the First Hospital of Jilin University between January 2016 and June 2018 were eligible for this study. Inclusion criteria were as follows: (a) diagnosis of AOPP based on a history of exposure to an organophosphorus pesticide compound and symptoms of cholinergic and muscarinic toxidromes; (b) aged ≥16 years; (c) presented to the Emergency Department within 24 hours of exposure to the organophosphate compound; and (d) no prior invasive (eg, hemoperfusion) or intravenous therapies. Exclusion criteria were as follows: (a) poisoning caused by other drugs; (b) history of neurological disease or psychiatric disorders; (c) history of severe heart, lung, liver, kidney, or hematological disease; or (d) history of cancer. A control group comprised of age‐ and sex‐matched healthy individuals with no history of exposure to an organophosphorus pesticide compound was recruited from the medical center at the First Hospital of Jilin University between January 2016 and January 2017. The study protocol was approved by the Medical Ethics Committee of the First Hospital of Jilin University (Approval #: 2015‐273). Written informed consent was obtained from study participants or relatives of unconscious patients.
All patients with AOPP were administered atropine, cholinesterase, diuretic, and anti‐inflammatory agents. All patients underwent gastric lavage, catharsis, monitoring and maintenance of life‐sustaining organs, and treatment for acid/base disturbance. Patients with severe poisoning underwent hemoperfusion once a day for at least 3 days.
2.2. Determination of UCH‐L1 in serum
Blood samples for assessment of serum UCH‐L1 were collected within the first 24 hours of admission to hospital (Day 1 postpoisoning) and on Days 3 and 7 postpoisoning. Samples were centrifuged for 10 minutes at 1300 g and stored at −80°C until analysis. Serum UCH‐L1 levels were measured using an enzyme‐linked immunosorbent assay (ELISA) kit (Proteintech Group, Inc, USA), according to the manufacturer's instructions. The lower limit of UCH‐L1 detection was 0.03 ng/mL. Each sample was assayed in duplicate, and the mean of the two measurements was used in the final analyses. Researchers performing the assays were blinded to the patients’ clinical information.
2.3. Data Collection and Definition of Variables
Patients’ demographic data and Acute Physiology and Chronic Health Evaluation (APACHE) II score were recorded within the first 24 hours of admission. Routine clinical laboratory tests, blood lactate levels, serum cholinesterase levels, and blood gases were recorded on admission. Serum cholinesterase activity was calculated as follows: serum cholinesterase level at admission/4500 (reference value). Time from poisoning to treatment (gastric lavage), duration of hospitalization, duration of intensive care unit (ICU) stay, and mechanical ventilation time were also recorded.
The primary endpoint of this study was cognitive function evaluated on Day 30 postpoisoning using the Mini‐Mental State Examination (MMSE) questionnaire. The MMSE evaluates orientation, registration, attention and calculation, recall, and language for a maximum score of 30. A score of ≤23 indicates cognitive impairment.20
2.4. Data analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc) and GraphPad Prism (version 5.01, Inc). Data are reported as counts and percentages for categorical variables and means ± standard deviation (SD) or median and interquartile range for continuous variables. Based on the MMSE, patients were divided into two groups: with or without cognitive impairment on Day 30 postpoisoning. Between‐group comparisons were conducted using the chi‐square or Fisher's exact test for categorical variables and the Student's t test or the Mann‐Whitney U test for continuous variables. Univariate logistic regression analysis was used to select potential predictors of cognitive impairment. Variables with a P value <0.05 in univariate logistic regression were retained for multivariate logistic regression analysis. Those variables that attained a P value of 0.05 were considered significantly associated with cognitive impairment after adjustment for the other investigated covariates. Predictive values, estimations of optimal cutoff points, and area under the curve (AUC) were calculated from receiver operating characteristic (ROC) curves. All hypotheses were two‐tailed, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics of the study patients
Overall, 297 patients were eligible for this study, and 238 patients were included. Of these, 21 patients died, and ten were lost to follow‐up. Data from 207 patients (89 men [43.0%] and 118 women [57.0%]) with a mean age of 54.7 ± 18.3 years (range, 16 to 72 years) were included in the final analysis (Table 1). Among the 207 patients, 192 patients had intentionally and 15 patients had unintentionally consumed an organophosphorus pesticide. Median time from poisoning to treatment was 4.5 hours (IQR 2.2‐6.5 hours). A total of 207 patients were diagnosed with poisoning from an organophosphate compound, including dichlorvos (n = 52), rogor (n = 34), methamidophos (n = 22), omethoate (n = 20), thimet (n = 18), triazophos (n = 16), parathion (n = 16), malathion (n = 14), phoxim (n = 9), and unknown organophosphorus pesticide (n = 6). Median serum cholinesterase activity of the patients on admission to hospital (Day 1 postpoisoning) was 12.2% (range, 4.39%‐25.47%). Serum UCH‐L1 levels on admission were significantly higher in AOPP patients [5.13 (2.92‐7.54) ng/mL; n = 207] compared to healthy controls (0.27 ± 0.13 ng/mL; n = 102) (P < 0.001).
Table 1.
Baseline characteristics of included patients
| Variable | Cognitive impairment on Day 30 | All (n = 207) | P value | |
|---|---|---|---|---|
| Without (n = 171) | With (n = 36) | |||
| Age (y) | 51.5 ± 20.4 | 56.2 ± 15.8 | 54.7 ± 18.3 | 0.322 |
| Gender (M/F) | 96/75 | 22/14 | 118/89 | 0.584 |
| Time from poisoning to treatment (h) | 4.8 (2.0‐6.9) | 4.1 (1.6‐7.0) | 4.5 (2.2‐6.5) | 0.632 |
| Body temperature (°C) | 36.4 ± 0.7 | 37.0 ± 0.5 | 36.9 ± 0.6 | 0.173 |
| Heart rate (beats/min) | 81.4 ± 16.9 | 88.7 ± 22.6 | 85.3 ± 21.4 | 0.115 |
| Respiratory rate (respirations/min) | 16.4 ± 3.8 | 19.0 ± 4.9 | 18.2 ± 4.0 | 0.344 |
| Mean arterial pressure (mm Hg) | 90.2 ± 18.6 | 84.4 ± 26.2 | 87.3 ± 20.1 | 0.131 |
| APACHE II score | 18.03 ± 6.65 | 29.74 ± 7.08 | 24.32 ± 6.86 | <0.001 |
| Serum cholinesterase level (U/L) | 680.5 (247.0‐1747.5) | 325.0 (130.5‐725.0) | 549.0 (197.5‐1146.0) | 0.116 |
| Serum cholinesterase activity (%) | 15.12 (5.49‐38.83) | 7.22 (2.9‐16.11) | 12.2 (4.39‐25.47) | 0.116 |
| Lactate (mmol/L) | 2.65 (0.44‐4.82) | 4.35 (2.52‐6.23) | 3.42 (1.37‐5.40) | 0.010 |
| pH | 7.32 ± 0.18 | 7.27 ± 0.10 | 7.30 ± 0.15 | 0.142 |
| level (mmol/L) | 18.44 ± 5.47 | 17.53 ± 6.45 | 18.02 ± 5.97 | 0.437 |
| White blood cell (×109/L) | 9.35 (3.73‐14.05) | 14.96 (8.06‐19.32) | 12.15 (6.35‐17.08) | 0.008 |
| C‐reactive protein level (mg/L) | 20.80 ± 9.21 | 24.72 ± 7.84 | 22.57 ± 8.18 | 0.107 |
| Serum UCH‐L1Day1 levels | 4.25 (2.57‐6.31) | 8.51 (6.53‐10.22) | 5.13 (2.92‐7.54) | <0.001 |
| Duration of ICU (h) | 5.15 (3.10‐7.15) | 5.75 (2.95‐8.60) | 5.60 (2.60‐8.05) | 0.709 |
| Mechanical ventilation time (h) | 3.55 (1.05‐6.10) | 4.80 (0.85‐8.35) | 4.05 (0.50‐7.25) | 0.334 |
Numerical variables are presented as mean ± standard deviation (SD) or median (interquartile range) and were analyzed using the unpaired Student's t test or Mann‐Whitney U test. Categorical variables are expressed as counts and were analyzed using the chi‐square test.
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; UCH‐L1, ubiquitin C‐terminal hydrolase L1.
3.2. Prediction of cognitive impairment
Among the 207 patients with AOPP, 36 patients (17.4%) had cognitive impairment on Day 30 postpoisoning. Serum UCH‐L1 levels on admission to hospital (Day 1 postpoisoning) were significantly higher in patients with cognitive impairment [8.51 (6.53‐10.22) ng/mL; n = 36] compared to those without [4.25 (2.57‐6.31) ng/mL; n = 171] (P < 0.001) (Figure 1). 24‐h APACHE II score, blood lactate, and white blood cell count were also significantly higher in patients with AOPP and cognitive impairment (Table 1). On univariate analysis, serum UCH‐L1 levels on admission, 24‐h APACHE II score, blood lactate level, and white blood cell count were significantly associated with cognitive impairment on Day 30 postpoisoning (Table 2). On multivariate analysis, serum UCH‐L1 levels on admission (odds ratio [OR] 1.889, 95% confidence interval [CI] 1.609‐3.082, P = 0.002) and 24‐h APACHE II score (OR 1.736, 95% CI 1.264‐3.272, P = 0.012) were independent predictors of cognitive impairment on Day 30 postpoisoning. Based on ROC curves, serum UCH‐L1 levels > 5.9 ng/mL on admission predicted development of cognitive impairment on Day 30 postpoisoning with 86.1% sensitivity and 72.5% specificity (AUC, 0.869; 95% CI 0.815‐0.923), and a 24‐hr APACHE II score > 23 predicted development of cognitive impairment on Day 30 postpoisoning with 74.1% sensitivity and 68.3% specificity (AUC: 0.773, 95% CI: 0.681‐0.876). Serum UCH‐L1 level had better prognostic value than the 24‐h APACHE II score for predicting cognitive impairment on Day 30 postpoisoning vs. no cognitive impairment (Figure 2).
Figure 1.
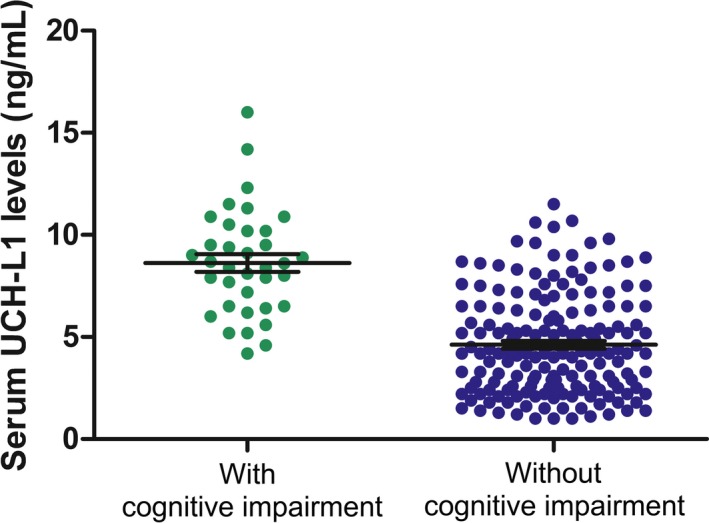
Serum UCH‐L1 levels on hospital admission (Day 1 postpoisoning) in patients with and without cognitive impairment on Day 30 postpoisoning. Serum UCH‐L1 levels on admission were significantly higher in patients with cognitive impairment [8.51 (6.53‐10.22) ng/mL; n = 36] compared to those without [4.25 (2.57‐6.31) ng/mL; n = 171] (P < 0.001). Black horizontal lines indicate the mean, and error bars indicate standard errors. Statistical analysis was performed using the Mann‐Whitney U test
Table 2.
Univariate and multivariate analysis of clinical variables for cognitive impairment at Day 30 postpoisoning
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% confidence interval) | P value | HR (95% confidence interval) | P value | |
| APACHE II score | 2.117 (1.682‐3.502) | <0.001 | 1.736 (1.264‐3.272) | 0.012 |
| Lactate | 2.072 (1.426‐2.741) | 0.024 | 1.883 (1.389‐2.671) | 0.098 |
| White blood cell | 1.962 (1.334‐2.465) | 0.018 | 1.708 (1.268‐2.245) | 0.076 |
| Serum UCH‐L1 level on Day 1 postpoisoning | 2.312 (1.746‐3.804) | <0.001 | 1.889 (1.609‐3.082) | 0.002 |
Figure 2.
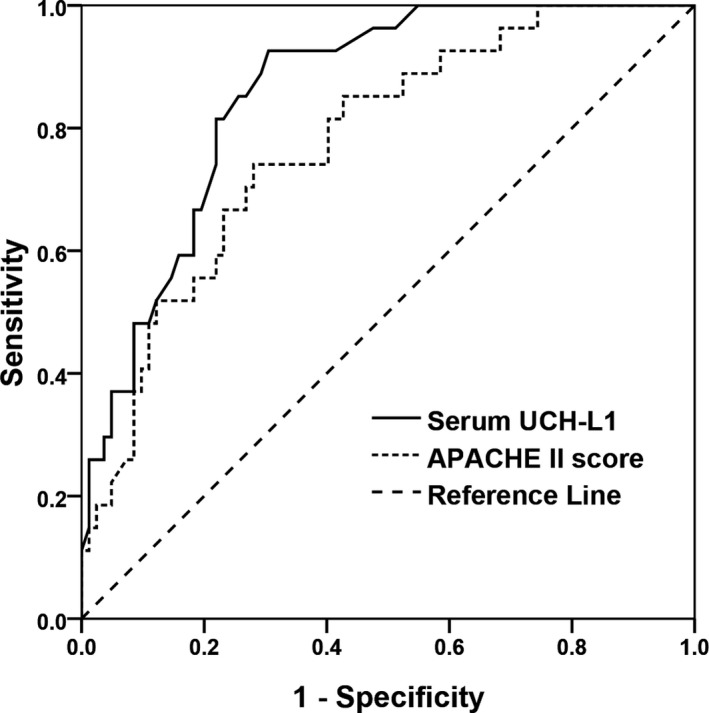
Receiver operating characteristic curve for serum UCH‐L1 levels, 24‐h APACHE II score and the development of cognitive impairment on Day 30 postpoisoning in patients with AOPP
3.3. Time course of serum UCH‐L1 levels
In patients with cognitive impairment on Day 30 postpoisoning, serum UCH‐L1 concentration peaked on Day 3 postpoisoning and slowly decreased over time, while serum UCH‐L1 concentration decreased with time in patients without cognitive impairment (Figure 3). Serum UCH‐L1 concentration did not reach the normal range on Day 7 postpoisoning. On admission to hospital (Day 1 postpoisoning) [8.51 (6.53‐10.22) ng/mL vs 4.25 (2.57‐6.31) ng/mL, P < 0.001) and Day 3 [9.31 (7.92‐10.98) ng/mL vs 3.32 (2.25‐5.13) ng/mL, P < 0.001] and Day 7 [4.96 (3.28‐7.26) ng/mL vs 2.27 (1.55‐3.24) ng/mL, P < 0.001] postpoisoning, serum UCH‐L1 levels were significantly higher in patients with cognitive impairment compared to those without.
Figure 3.
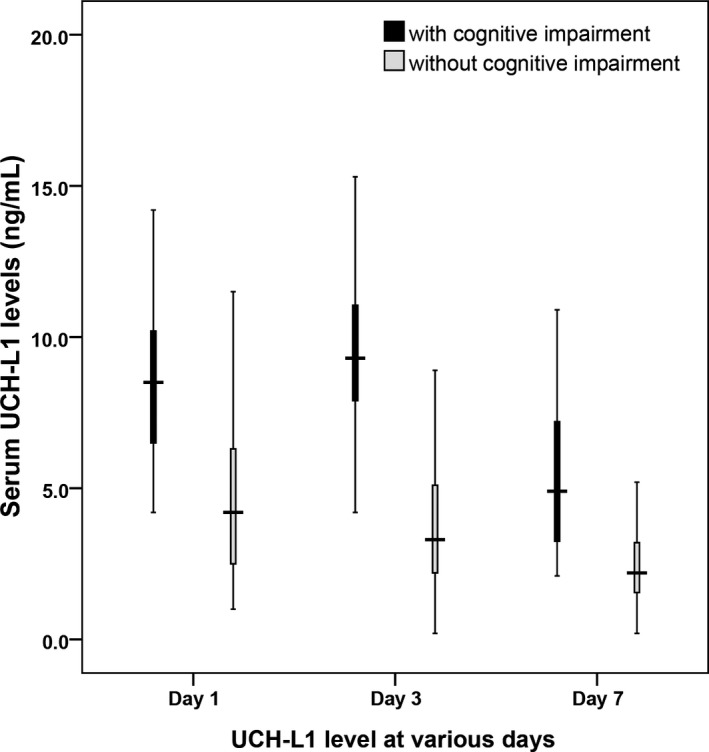
Serum UCH‐L1 concentrations on hospital admission (Day 1 postpoisoning) and Day 3 and Day 7 postpoisoning
4. DISCUSSION
This study investigated the demographic and clinical parameters that are associated with cognitive impairment on Day 30 postpoisoning in patients with AOPP. Findings showed that serum UCH‐L1 levels on admission to hospital (Day 1 postpoisoning) were significantly higher in patients that presented with cognitive impairment on Day 30 postpoisoning compared to those that did not. Multivariate logistic regression analysis identified levels of serum UCH‐L1 on admission and 24‐h APACHE II score as independent predictors of cognitive impairment in patients with AOPP.
Organophosphate compounds induce cholinergic neuronal excitotoxicity, which can cause persistent profound neuropsychiatric and neurological impairments, including memory, cognitive, mental, emotional, motor, and sensory deficits. The underlying mechanisms involve cellular edema, oxidative stress, cytotoxicity, neuroinflammation, and neuronal apoptosis.21 Brain magnetic resonance imaging (MRI) findings in patients after organophosphate poisoning showed localized high signal intensity lesions in the white matter.22 An animal study associated organophosphate poisoning with reduced white matter integrity within the striatum and amygdala that correlated with spatial learning performance.23
UCH‐L1 is a soluble protein localized in the cell body of neurons in the central nervous system that has important roles in the regulation of synaptic plasticity and learning and memory.24 Circulating UCH‐L1 has been identified as a biomarker specific to neuronal injury16, 25 as it is released into the circulation when the integrity of the blood‐brain barrier (BBB) is disrupted.26, 27 In a piglet model, serum UCH‐L1 predicted neuronal apoptosis induced by deep hypothermic circulatory arrest.28 In observational studies, cerebrospinal fluid (CSF) and serum levels of UCH‐L1 had utility as diagnostic and prognostic biomarkers of traumatic brain injury.13, 14, 29 In patients with aneurysmal subarachnoid hemorrhage, elevated CSF levels of UCH‐L1 correlated with neurological outcomes and mortality.15 In patients with white matter lesions, increased serum UCH‐L1 levels were correlated with white matter lesion severity.30 Taken together, these data and findings from the present study suggest that serum UCH‐L1 level has potential as a predictive marker for cognitive impairment after AOPP. This study showed that serum UCH‐L1 levels >5.9 ng/mL could predict the development of cognitive impairment after AOPP with relatively high sensitivity and specificity. These results are consistent with our previous report describing the prognostic value of serum UCH‐L1 levels in acute carbon monoxide poisoning.31 Other studies suggest optimal UCH‐L1 values for predicting outcomes differ between disease types. In patients with traumatic brain injury, the UCH‐L1 cutoff value for the prediction of poor outcomes was 1.03 ng/mL.29 In patients with severe traumatic brain injury, the UCH‐L1 cutoff value for in‐hospital mortality was 1.89 ng/mL.32
APACHE II scoring is a classification system that is used to evaluate the severity and prognosis of disease.33 In the present study, APACHE II score was significantly higher in patients with AOPP that presented with cognitive impairment on Day 30 postpoisoning compared to those that did not. Accordingly, a previous report showed that APACHE II score had the capability to discriminate and estimate early in‐hospital mortality in patients with AOPP.34 However, the APACHE II score is highly complex as it evaluates disease severity based on 12 physiological measurements taken at admission, patient age, and patient medical history. Consequently, the APACHE II score may not be easily applied in the Emergency Department.
Published evidence suggests that blood lactate and leukocyte level can be used as markers of severity and prognosis in patients with AOPP.35, 36 In the present study, blood lactate level and white blood cell count were significantly higher in patients with AOPP that presented with cognitive impairment on Day 30 postpoisoning compared to those that did not. However, blood lactate level and white blood cell count were not selected as independent predictive factors for cognitive impairment on multivariate analysis. An evidence‐based review of the management of AOPP recommends quantification of erythrocyte AchE activity during the initial diagnosis of AOPP. Erythrocyte and synaptic AchE are structurally similar; therefore, erythrocyte AchE activity is thought to reflect synaptic AchE activity.37 Plasma cholinesterase activity has also been accepted as a biomarker of exposure/toxicity in AOPP; however, more recent reports suggest that plasma cholinesterase activity does not accurately reflect the severity of AOPP as it is not involved in cholinergic transmission in the nervous system.4 Accordingly, the present study found no significant differences in serum cholinesterase activity in patients that presented with cognitive impairment on Day 30 postpoisoning compared to those that did not.
The present study was associated with several limitations. First, the sample size was small. Second, the study was conducted in a single hospital and may not be generalizable to other healthcare settings or patient populations. Third, the incidence of cognitive impairment may have been underestimated as the follow‐up period of 30 days was relatively short. Last, assessment of erythrocyte AchE would have provided valuable information; however, erythrocyte AchE cannot be measured at our institution. Further studies with larger sample sizes are warranted to validate our findings.
5. CONCLUSION
In conclusion, this study demonstrates that serum UCH‐L1 level measured at the time of hospital admission has potential as a novel biomarker for predicting cognitive impairment 30 days after AOPP.
Pang L, Liu J, Li W, Xia Y, Xing J. Serum ubiquitin C‐terminal hydrolase L1 predicts cognitive impairment in patients with acute organophosphorus pesticide poisoning. J Clin Lab Anal. 2019;33:e22947 10.1002/jcla.22947
Contributor Information
Yan Xia, Email: xiayanzzzz@sina.com.
Jihong Xing, Email: jhxing1@qq.com.
REFERENCES
- 1. Hrabetz H, Thiermann H, Felgenhauer N, et al. Organophosphate poisoning in the developed world ‐ A single centre experience from here to the millennium. Chem Biol Interact. 2013;206:561‐568. [DOI] [PubMed] [Google Scholar]
- 2. Terry AV Jr. Functional consequences of repeated organophosphate exposure: Potential non‐cholinergic mechanisms. Pharmacol Ther. 2012;134:355‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malekirad AA, Faghih M, Mirabdollah M, Kiani M, Fathi A, Abdollahi M. Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arh Hig Rada Toksikol. 2013;64:1‐8. [DOI] [PubMed] [Google Scholar]
- 4. Jokanovic M. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology. 2018;410:125‐131. [DOI] [PubMed] [Google Scholar]
- 5. Pancetti F, Olmos C, Dagnino‐Subiabre A, Rozas C, Morales B. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: Implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev. 2007;10:623‐630. [DOI] [PubMed] [Google Scholar]
- 6. Bishop P, Rocca D, Henley JM. Ubiquitin C‐terminal hydrolase L1 (UCH‐L1): structure, distribution and roles in brain function and dysfunction. Biochem J. 2016;473:2453‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hegde AN, DiAntonio A. Ubiquitin and the synapse. Nat Rev Neurosci. 2002;3:854‐861. [DOI] [PubMed] [Google Scholar]
- 8. Bourtchouladze R, Lidge R, Catapano R, et al. A mouse model of Rubinstein‐Taybi syndrome: Defective long‐term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518‐10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Contu VR, Kotake Y, Toyama T, et al. Endogenous neurotoxic dopamine derivative covalently binds to Parkinson's disease‐associated ubiquitin C‐terminal hydrolase L1 and alters its structure and function. J Neurochem. 2014;130:826‐838. [DOI] [PubMed] [Google Scholar]
- 10. Jara JH, Genç B, Cox GA, et al. Corticospinal Motor Neurons Are Susceptible to Increased ER Stress and Display Profound Degeneration in the Absence of UCHL1 Function. Cereb Cortex. 2015;25:4259‐4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naze P, Vuillaume I, Destee A, Pasquier F, Sablonniere B. Mutation analysis and association studies of the ubiquitin carboxyterminal hydrolase L1 gene in Huntington's disease. Neurosci Lett. 2002;328:1‐4. [DOI] [PubMed] [Google Scholar]
- 12. Li Y, Wang Z, Zhang B, et al. Cerebrospinal fluid ubiquitin C‐terminal hydrolase as a novel marker of neuronal damage after epileptic seizure. Epilepsy Res. 2013;103:205‐210. [DOI] [PubMed] [Google Scholar]
- 13. Diaz‐Arrastia R, Wang K, Papa L, et al. Acute Biomarkers of Traumatic Brain Injury: Relationship between Plasma Levels of Ubiquitin C‐Terminal Hydrolase‐L1 and Glial Fibrillary Acidic Protein. J Neurotrauma. 2014;31:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bazarian JJ, Biberthaler P, Welch RD, et al. Serum GFAP and UCH‐L1 for prediction of absence of intracranial injuries on head CT (ALERT‐TBI): a multicentre observational study. Lancet Neurol. 2018;17:782‐789. [DOI] [PubMed] [Google Scholar]
- 15. Lewis SB, Wolper R, Chi Y‐Y, et al. Identification and preliminary characterization of ubiquitin c terminal hydrolase 1 (UCHL1) as a biomarker of neuronal loss in aneurysmal subarachnoid hemorrhage. J Neurosci Res. 2010;88:1475‐1484. [DOI] [PubMed] [Google Scholar]
- 16. Ren C, Kobeissy F, Alawieh A, et al. Assessment of serum UCH‐L1 and GFAP in acute stroke patients. Sci Rep. 2016;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douglas‐Escobar MV, Heaton SC, Bennett J, et al. UCH‐L1 and GFAP serum levels in neonates with hypoxic‐ischemic encephalopathy: a single center pilot study. Frontiers in Neurology. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fink EL, Berger RP, Clark R, et al. Exploratory study of serum ubiquitin carboxyl‐terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation. 2016;101:65‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brophy GM, Mondello S, Papa L, et al. Biokinetic analysis of ubiquitin C‐terminal hydrolase‐L1 (UCH‐L1) in severe traumatic brain injury patient biofluids. J Neurotrauma. 2011;28:861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state ‐ practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y. Organophosphate‐induced brain damage: Mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology. 2012;33:391‐400. [DOI] [PubMed] [Google Scholar]
- 22. Lee K‐J, Shin J‐W, Moon J, et al. An illustrative case of mixed pesticide poisoning with remarkable improvement: A case report. J Neurol Sci. 2014;344:232‐233. [DOI] [PubMed] [Google Scholar]
- 23. Mullins RJ, Xu SU, Pereira E, et al. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. Neurotoxicology. 2015;48:9‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo Y‐Y, Lu YI, Zheng Y, et al. Ubiquitin C‐terminal hydrolase L1 (UCH‐L1) promotes hippocampus‐dependent memory via its deubiquitinating effect on TrkB. J Neurosci. 2017;37:5978‐5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mondello S, Shear DA, Bramlett HM, et al. insight into pre‐clinical models of traumatic brain injury using circulating brain damage biomarkers: operation brain trauma therapy. J Neurotrauma. 2016;33:595‐605. [DOI] [PubMed] [Google Scholar]
- 26. Carone D, Librizzi L, Cattalini A, et al. Pravastatin acute neuroprotective effects depend on blood brain barrier integrity in experimental cerebral ischemia. Brain Res. 2015;1615:31‐41. [DOI] [PubMed] [Google Scholar]
- 27. Zhang D, Han S, Wang S, Luo Y, Zhao L, Li J. cPKC gamma‐mediated down‐regulation of UCHL1 alleviates ischaemic neuronal injuries by decreasing autophagy via ERK‐mTOR pathway. J Cell Mol Med. 2017;21:3641‐3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y‐P, Zhu Y‐B, Duan DD, et al. Serum UCH‐L1 as a Novel Biomarker to Predict Neuronal Apoptosis Following Deep Hypothermic Circulatory Arrest. Int J Med Sci. 2015;12:576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takala R, Posti JP, Runtti H, et al. Glial Fibrillary Acidic Protein and Ubiquitin C‐Terminal Hydrolase‐L1 as Outcome Predictors in Traumatic Brain Injury. World Neurosurg. 2016;87:8‐20. [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Sun Y, Li J, et al. Changes of ubiquitin C‐terminal hydrolase‐L1 levels in serum and urine of patients with white matter lesions. J Neurol Sci. 2015;357:215‐221. [DOI] [PubMed] [Google Scholar]
- 31. Pang LI, Wu Y, Dong N, et al. Elevated serum ubiquitin C‐terminal hydrolase‐L1 levels in patients with carbon monoxide poisoning. Clin Biochem. 2014;47:72‐76. [DOI] [PubMed] [Google Scholar]
- 32. Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care. 2011;15:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Akdur O, Durukan P, Ozkan S, et al. Poisoning severity score, Glasgow coma scale, corrected QT interval in acute organophosphate poisoning. Human Exper Toxicol. 2010;29:419‐425. [DOI] [PubMed] [Google Scholar]
- 34. Kim YH, Yeo JH, Kang MJ, et al. Performance Assessment of the SOFA, APACHE II Scoring System, and SAPS II in Intensive Care Unit Organophosphate Poisoned Patients. J Korean Med Sci. 2013;28:1822‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yuan S, Gao Y, Ji W, Song J, Mei X. The evaluation of acute physiology and chronic health evaluation II score, poisoning severity score, sequential organ failure assessment score combine with lactate to assess the prognosis of the patients with acute organophosphate pesticide poisoning. Medicine. 2018;97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar S, Agrawal S, Raisinghani N, Khan S. Leukocyte count: A reliable marker for the severity of organophosphate intoxication? J Lab Physicians. 2018;10:185‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hmoudo H, Ben Salem C, Bouraoui K. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:2169‐2170. [DOI] [PubMed] [Google Scholar]


