Abstract
Background
Limited data are available for the diagnostic value, and the diagnostic sensitivity and specificity of pleural fluid periostin (pPOSTN) and serum periostin (sPOSTN) in malignant pleural effusion (MPE) caused by non–small‐cell lung cancer (NSCLC).
Methods
We collected 84 pleural effusion samples, including 44 cases of MPE caused by NSCLC and 40 cases of benign pleural effusions (BPEs) from August 2018 to January 2019. The pPOSTN, sPOSTN, pleural fluid lactate dehydrogenase (pLDH), pleural effusion adenosine deaminase (pADA), pleural effusion total protein (pTP), pleural fluid glucose (pGLU), pleural effusion leukocyte count (pWBC), pleural effusion red cell count (pRBC), pleural effusion carbohydrate antigen 199 (pCA199), pleural fluid carbohydrate antigen 125 (pCA125), pleural effusion ferritin (pFer), serum total protein (sTP), and serum C‐reactive protein (sCRP) were tested, and the obtained data were analyzed by statistical software.
Results
Compared to the BPE group, the pPOSTN level in the MPE group was observably lower, while the levels of sPOSTN, sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN increased. The receiver operating characteristic (ROC) curve showed that the area under the ROC curve (AUC) (=0.844, 0.847, 0.841) of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN (cutoff = 11.86, 0.244, 0.015) was observably higher than other indicators for the diagnosis of MPE caused by NSCLC. Thus, the combined detection of pPOSTN, pCA125/pPOSTN, and pCA125/sCRP suggested that the AUC, sensitivity, and specificity was 0.912%, 95.45%, and 77.50% at the cutoff 0.317 and diagnostic performance was higher than sPOSTN/pADA or pCA199/pADA or pCA199/pPOSTN.
Conclusion
Combined detection of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN can be used as a good indicator for MPE caused by NSCLC.
Keywords: malignant pleural effusion, non–small‐cell lung cancer, periostin
1. INTRODUCTION
Lung cancer is the major cause of cancer‐related death in the world,1 most of which are non–small‐cell lung cancer (NSCLC).2 Malignant pleural effusion (MPE) is one of the common complications to advanced patients with NSCLC.3 The cytopathological detection is used for the diagnosis of MPE caused by NSCLC from benign pleural effusion (BPE), but the missing detection rate is high.4 Thoracoscopy detection can greatly increase the sensitivity of diagnosis; however, it has the risk of injury, the drawback of high cost, and the possibility of missed diagnosis. Currently, the routine clinical detections of pleural effusion, including pleural effusion lactate dehydrogenase (pLDH), pleural effusion adenosine deaminase (pADA), pleural effusion total protein (pTP), pleural effusion glucose (pGLU), pleural effusion white cell count (pWBC), pleural effusion red cell count (pRBC), pleural effusion carbohydrate antigen 199 (pCA199), pleural effusion carbohydrate antigen 125 (pCA125), and pleural effusion ferritin (pFer), can reduce the undetected rate of MPE,5, 6, 7 but have low‐grade specificity and sensitivity for diagnosis of malignant pleural effusion.8
Periostin, a N‐glycoprotein secreting a matrix, which is part of NH2‐terminal signal peptide sequence and internal homologous repeat sequence, is rich in carboxy‐terminal domain and cysteine domain.9 Many studies showed that multifarious tumors in the human body overexpressed the periostin.10, 11, 12, 13 Compared with the expression of normal tissues, the expression of periostin in tumor stroma was more extensive and observably different.14 Accumulating evidences indicated that overexpression of periostin in serum is closely correlated with tumorigenesis and metastasis.15 Especially in NSCLC, the serum periostin (sPOSTN) as the biomarker in patients with malignant tumor is extremely important to the diagnosis and prognosis of the disease.16, 17, 18 However, the level of pleural fluid periostin (pPOSTN) in pleural effusion of patients with non–small‐cell lung cancer has not been studied, and the diagnostic and prognostic value of MPE caused by non–small‐cell lung cancer is still unclear.
In this study, in order to explore the value of pPOSTN and sPOSTN levels in differentiating malignant pleural effusion from benign pleural effusion in patients with non–small‐cell lung cancer, enzyme‐linked immunosorbent assay (ELISA) was used to detect the two indicators. In addition, we investigated the correlations between periostin expression levels and clinicopathological parameters.
2. MATERIALS AND METHODS
2.1. Materials
Pleural effusion specimens were prospectively collected from 84 patients with lung disease who presented at the First Affiliated Hospital of Wenzhou Medical College, China, from August 2018 to January 2019. Pleural effusion caused by digestive system disease, coronary heart disease, hypoproteinemia, or kidney dysfunction was excluded. These pleural effusion specimens included 44 cases of MPE and 40 cases of BPE. The MPE group consisted of 41 cases of pleural effusion caused by adenocarcinoma and three cases of pleural effusion caused by squamous cell carcinoma, including 30 males and 14 females, aged between 43 and 82 years (64.77 ± 8.72). The BPE group included 30 males and 10 females aged between 20 and 86 years (54.60 ± 19.67). There was no significant difference in age and sex between the two groups in statistical analysis (P > 0.05). These cases were confirmed by bronchoscopic biopsy, bacterial culture of pleural effusion, cell morphology under pleural effusion microscopy, computed tomography, chest X‐ray and tuberculosis polymerase chain reaction detection, and the 7th edition of lung cancer TNM staging system released by International Association for the Study of Lung Cancer (IASLC).19 In this study, all patients agreed and signed a written informed consent which approved by the Institutional Ethics Review Committee of the First Affiliated Hospital of Wenzhou Medical University.
2.2. Methods
The whole blood (3 mL) of the patients was collected and centrifuged at 1509.3 g for 15 minutes, and then, the supernatant serum was separated and frozen at −80°C in a small centrifugal tube. The pleural fluid (8 mL) of the patients was collected and centrifuged at 603.72 g for 15 minutes, and then, the supernatant pleural fluid was separated and frozen at −80°C in a small centrifugal tube. The levels of pCA125, pCA199, and pFer were inspected with the chemiluminescence method in a DXI800 luminescence analyzer (Beckman Coulter). The level of serum C‐reactive protein (sCRP) was inspected with the nephelometry method in a Siemens BN II automatic protein analyzer (Siemens). The levels of pLDH, pADA, pGLU, pTP, and serum total protein (sTP) were inspected in an AU5800 automatic biochemical analyzer (Beckman). The levels of pWBC and pRBC were inspected in a Niu Bao's counting board.
The levels of pPOSTN and the levels of sPOSTN were inspected in an ELISA plates (USCN Life Science Inc) according to the manufacturer's introductions. Detection range of POSTN is 78‐5000 pg/mL. The minimum detectable dose of POSTN is typically less than 31 pg/mL. CV of intra‐assay is lower than 10%, and CV of inter‐assay is lower than 12%.
2.3. Statistical analysis
Kolmogorov‐Smirnov test used to test the normality of the data. The measurement data of non‐normal distribution were expressed as median (minimum, maximum), and Mann‐Whitney U test was used for comparison between the two groups. The diagnostic efficacy was analyzed by drawing the receiver operating characteristic curve (ROC). MedCalc 15.6.1 (MedCalc Software) and SPSS 18.0 (Statistical Package for the Social Sciences Corporation) were used for statistical analysis of all data. The difference was statistically significant when P < 0.05.
3. RESULTS
3.1. Comparison of the parameters in pleural effusion in the MPE group and BPE pleural fluid group
The levels of sPOSTN, pGLU, pRBC, pCA125, pCA199, sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN in the MPE group were obviously higher than those in the BPE group (Mann‐Whitney U = 565.500, 629.500, 597.500, 501.000, 525.000, 265.000, 259.000, and 282.000, P = 0.005, 0.025, 0.011, 0.001, 0.001, 0.000, 0.000, and 0.000); the pPOSTN, pADA, pWBC, and sCRP levels in the MPE group were obviously lower than those in the BPE group (Mann‐Whitney U = 476.000, 309.500, 640.500, and 337.000, P = 0.000, 0.000, 0.032, and 0.000). The levels of pTP, pLDH, pFer, and sTP in the MPE group showed no significant difference when compared with the BPE group (see Figure 1 and Table 1).
Figure 1.
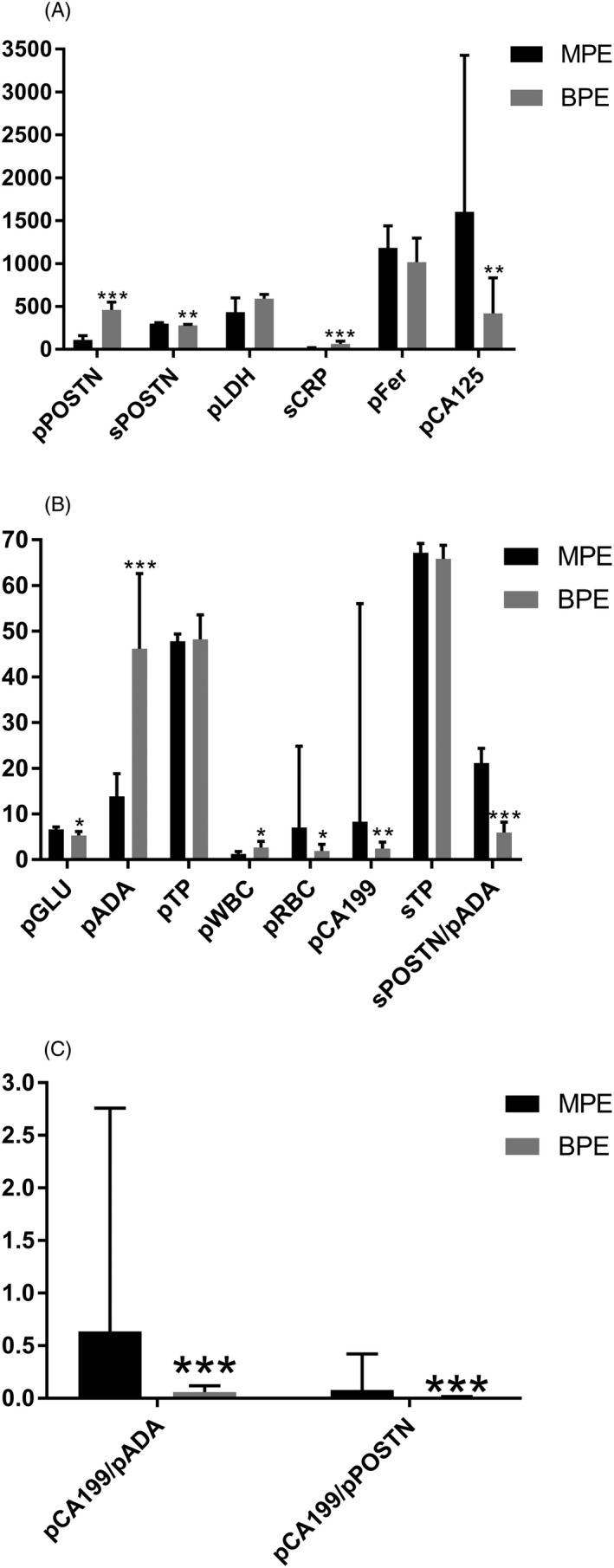
Comparison of the parameters in pleural effusion from the two groups. A, The sPOSTN and pCA125 levels in the MPE group were obviously higher than those in the BPE group. The pPOSTN and sCRP levels in the MPE group were obviously lower than the BPE group. B, The pGLU, pRBC, pCA199, and sPOSTN/pADA levels in the MPE group was higher than those in the BPE group. The pADA and pWBC levels in the MPE group were obviously lower than those in the BPE group. C, The pCA199/pADA and pCA199/pPOSTN levels in the MPE group were obviously higher than those in the BPE group. *P < 0.5, **P < 0.01, and ***P < 0.001
Table 1.
Comparison of the parameters in pleural effusion in the two groups
| Parameters | MPE | BPE | Mann‐Whitney U test | P |
|---|---|---|---|---|
| pPOSTN (ng/mL) | 110.1 (11.6‐446.8) | 460.6 (82.6‐636.8) | 476.000 | 0.000 |
| sPOSTN (ng/mL) | 298.3 (204.0‐404.0) | 279.9 (251.7‐294.0) | 565.500 | 0.005 |
| pADA (U/L) | 13.9 (3.1‐61.6) | 46.2 (22.1‐72.5) | 309.500 | 0.000 |
| pTP (g/L) | 47.8 (19.0‐67.4) | 48.2 (41.6‐55.2) | 830.000 | 0.654 |
| pLDH (U/L) | 433 (96‐4346) | 592 (240‐960) | 783.500 | 0.486 |
| pGLU (mmol/L) | 6.64 (0.10‐11.35) | 5.27 (3.52‐6.61) | 629.500 | 0.025 |
| pWBC (103/μL) | 1.28 (0.04‐10.56) | 2.66 (0.87‐4.46) | 640.500 | 0.032 |
| pRBC (103/μL) | 7.00 (0.06‐780.00) | 1.88 (0.83‐6.14) | 597.500 | 0.011 |
| pFer (μg/L) | 1182 (51‐15 000) | 1018 (492‐1457) | 817.500 | 0.576 |
| pCA125 (U/mL) | 1601.9 (2.8‐26 681.9) | 418.9 (119.1‐1373.4) | 501.000 | 0.001 |
| pCA199 (U/mL) | 8.3 (0.8‐20 210.0) | 2.4 (1.6‐4.5) | 525.000 | 0.001 |
| sCRP (mg/L) | 15.4 (3.0‐130.0) | 63.2 (24.9‐125.0) | 337.000 | 0.000 |
| sTP (g/L) | 67.2 (46.9‐90.0) | 65.8 (58.6‐69.7) | 734.000 | 0.191 |
| sPOSTN/pADA | 21.13 (6.56‐109.10) | 5.90 (3.58‐11.09) | 265.000 | 0.000 |
| pCA199/pADA | 0.637 (0.035‐1467.824) | 0.060 (0.030‐0.147) | 259.000 | 0.000 |
| pCA199/pPOSTN | 0.078 (0.005‐272.413) | 0.010 (0.004‐0.033) | 282.000 | 0.000 |
Median (min‐max) in the parameters in the table.
3.2. The AUC, cutoff value, sensitivity, and specificity of parameters for the diagnosis of malignant pleural effusion
The ROC analysis showed that the AUC (=0.844, 0.847, 0.841) of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN (cutoff = 11.86, 0.244, 0.015) observably exceeds pPOSTN (0.735), sPOSTN (0.687), pADA (0.818), pLDH (0.541), pRBC (0.650), pCA125 (0.711), pCA199 (0.699), sCRP (0.803), pGLU (0.632), or pWBC (0.645), and sensitivity and specificity of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN for diagnosis of MPE were 86.36%, 72.73%, and 88.64% and 80.00%, 85.00%, and 65.00%, respectively, showing better diagnostic efficiency (see Figure 2 and Table 2).
Figure 2.
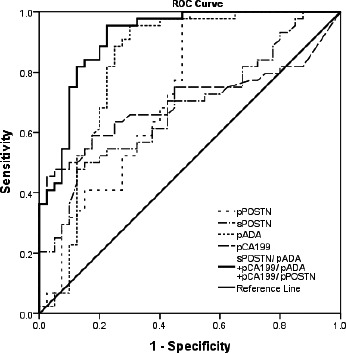
Receiver operating characteristic curve of some parameters for malignant pleural effusion (MPE). At the cutoff = 0.317, the sensitivity and specificity of sPOSTN/pADA+pCA199/pADA+pCA199/pPOSTN were 95.45% and 77.50% for the diagnosis of MPE, and the AUC (0.912) was the highest among all markers tested
Table 2.
The AUC, cutoff value, sensitivity, and specificity of parameters for the diagnosis of malignant pleural effusion
| Parameters | AUC | 95% confidence interval | P | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| pPOSTN (ng/mL) | 0.735 | 0.622‐0.847 | 0.000 | 403.4 | 97.73 | 52.50 |
| sPOSTN (ng/mL) | 0.687 | 0.572‐0.801 | 0.004 | 302.0 | 47.73 | 87.50 |
| pADA (U/L) | 0.818 | 0.715‐0.922 | 0.000 | 26.5 | 95.45 | 70.00 |
| pLDH (U/L) | 0.541 | 0.332‐0.586 | 0.522 | 550 | 62.79 | 52.50 |
| pRBC (103/μL) | 0.650 | 0.529‐0.772 | 0.019 | 3.76 | 63.64 | 70.00 |
| pCA125 (U/mL) | 0.711 | 0.599‐0.823 | 0.001 | 2180.3 | 47.73 | 95.50 |
| pCA199 (U/mL) | 0.699 | 0.581‐0.818 | 0.002 | 13.5 | 45.45 | 97.50 |
| sCRP (mg/L) | 0.803 | 0.705‐0.900 | 0.000 | 36.0 | 81.82 | 74.36 |
| pGLU (mmol/L) | 0.632 | 0.510‐0.754 | 0.040 | 6.62 | 52.27 | 77.50 |
| pWBC (103/μL) | 0.645 | 0.522‐0.767 | 0.024 | 1.79 | 68.18 | 62.50 |
| sPOSTN/pADA | 0.844 | 0.749‐0.940 | 0.000 | 11.86 | 86.36 | 80.00 |
| pCA199/pADA | 0.847 | 0.765‐0.928 | 0.000 | 0.244 | 72.73 | 85.00 |
| pCA199/pPOSTN | 0.841 | 0.758‐0.924 | 0.000 | 0.015 | 88.64 | 65.00 |
| sPOSTN/pADA+pCA199/pADA+pCA199/pPOSTN | 0.912 | 0.850‐0.974 | 0.000 | 0.317 | 95.45 | 77.50 |
3.3. Relationship between pPOSTN concentration and clinicopathological factors in NSCLC patients with MPE
The association between pPOSTN concentration of MPE and clinicopathological factors in NSCLC patients is shown in Table 3. The pPOSTN level in the elder group was higher than that in the younger group (Mann‐Whitney U = 107.000, P = 0.025). No statistically significant associations were found between pPOSTN levels and gender (P = 0.960), smoking status (P = 0.391), histological type (P = 0.592), lymph node metastasis (P = 0.827), and distant metastases (P = 0.652; see Table 3).
Table 3.
Levels of pPOSTN in the MPE group and association with the clinicopathological factors of NSCLC patients
| Clinical variables | Number | pPOSTN (ng/mL) | Mann‐Whitney U test | P |
|---|---|---|---|---|
| Age (y) | ||||
| ≥60 | 32 | 135.0 (62.6,247.5) | 107.000 | 0.025 |
| <60 | 12 | 52.5 (38.5,99.5) | ||
| Gender | ||||
| Male | 30 | 110.1 (48.2,225.5) | 208.000 | 0.960 |
| Female | 14 | 115.0 (45.9,212.2) | ||
| Smoking status | ||||
| Smoker | 23 | 96.4 (47.0,175.6) | 205.000 | 0.391 |
| Non‐smoker | 21 | 131.6 (56.3,218.9) | ||
| Histological type | ||||
| Squamous cell carcinoma | 3 | 159.0 (49.4,/) | 50.000 | 0.592 |
| Adenocarcinoma | 41 | 109.6 (47.7,212.1) | ||
| Lymph node metastasis | ||||
| Positive | 35 | 110.6 (45.8,222.4) | 150.000 | 0.827 |
| Negative | 9 | 109.6 (53.7,132.0) | ||
| Distant metastases | ||||
| Positive | 35 | 130.2 (45.8,255.8) | 142.000 | 0.652 |
| Negative | 9 | 85.2 (53.2,183.9) | ||
Median (P25, P75) in the parameters in the table.
3.4. Relationship between sPOSTN concentration and clinicopathological factors in NSCLC patients with MPE
The association between pPOSTN concentration of MPE and clinicopathological factors in NSCLC patients is shown in Table 4. No statistically significant associations were found between pPOSTN levels and age (P = 0.429), gender (P = 0.465), smoking status (P = 0.124), histological type (P = 0.798), lymph node metastasis (P = 0.142), and distant metastases (P = 0.532; see Table 4).
Table 4.
Levels of sPOSTN in the MPE group and association with the clinicopathological factors of NSCLC patients
| Clinical variables | Number | sPOSTN (ng/mL) | Mann‐Whitney U test | P |
|---|---|---|---|---|
| Age (y) | ||||
| ≥60 | 32 | 307.6 (279.3,328.9) | 162.000 | 0.429 |
| <60 | 12 | 284.9 (258.3,343.2) | ||
| Gender | ||||
| Male | 30 | 302.7 (280.1,331.8) | 181.000 | 0.465 |
| Female | 14 | 293.0 (249.2,330.2) | ||
| Smoking status | ||||
| Smoker | 23 | 310.6.4 (281.4,338.2) | 176.000 | 0.124 |
| Non‐smoker | 21 | 285.2 (252.5,311.3) | ||
| Histological type | ||||
| Squamous cell carcinoma | 3 | 308.2 (209.6,183.9) | 56.000 | 0.798 |
| Adenocarcinoma | 41 | 297.2 (273.4,328.1) | ||
| Lymph node metastasis | ||||
| Positive | 35 | 296.2 (270.0,314.2) | 107.000 | 0.142 |
| Negative | 9 | 323.8 (287.2,361.4) | ||
| Distant metastases | ||||
| Positive | 35 | 299.4 (270.8,338.2) | 136.000 | 0.532 |
| Negative | 9 | 289.2 (260.0,317.2) | ||
Median (P25, P75) in the parameters in the table.
4. DISCUSSION
The causes of pleural effusion can be divided into malignant and benign. There are some false detection and missed detection rates in imaging examination and the pathological examination as the gold standard lacks sensitivity.20, 21 Therefore, it is urgent to find a new marker to identify the type of pleural effusion.
Periostin has a wide range of biological activity and can participate in tumor cell growth, proliferation, and metastasis by multifarious signal transduction pathways.22, 23, 24 Contié et al25 conducted animal experiments on nude mice by quantitative PCR and immunohistochemistry methods and found that peripheral stromal cells of breast cancer of bone metastases transitionally expressed periostin, and in the serum, POSTN levels increased observably. Zhang et al 18 found that the sPOSTN levels in NSCLC patients were significantly higher than in patients with normal subjects and benign lung disease, and the ROC curve showed that the AUC of sPOSTN antidiastole for NSCLC was 0.87. Several studies have shown that the expression level of periostin in serum of various malignant tumors was correlated with tumor stage, metastasis, and survival rate, but research on the expression of POSTN in pleural effusion caused by NSCLC is less.
In this study, the sPOSTN level in the MPE group is observably higher than that in the BPE group, which is consistent with the results of previous report.18 The pPOSTN level in the MPE group is observably lower than the BPE group. It may be due to the high expression of POSTN in the stromal cells neighboring the tumor cells, but not in the tumor cells,16 and MPE often contains tumor cells rather than stromal cells. But the exact mechanism needs furthered exploration and research.
The size of AUC represents the accuracy of diagnosis, and the indicator has high diagnostic value when AUC >0.9. Our results found that at the cutoff 0.317, the sensitivity and specificity of the joint detection of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN were 95.45% and 77.50% for the diagnosis of MPE and the AUC 0.912, giving it a significantly higher diagnostic value of MPE than the other indicators in this study. We report for the first time that joint detection of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN is used to identify MPE caused by NSCLC from BPE.
5. CONCLUSIONS
In a word, the diagnostic efficiency of combined detection of sPOSTN/pADA, pCA199/pADA, and pCA199/pPOSTN for differentiating MPE caused by NSCLC from BPE is very remarkable. Therefore, it is a very important index to differentiate the type of pleural effusion, which can help clinicians to make early diagnosis and precise treatment of pleural effusion. On the other side, the detections of pPOSTN and sPOSTN are convenient, simple, and fast, which are expected to be the new markers for the diagnosis of MPE caused by NSCLC.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent was obtained from all patients. The study was approved by the Ethical Committee of the First Affiliated Hospital of Wenzhou Medical University.
Wang J, Fu J, Shen Q, Zhang F, Wang Y, Wu LL. Identification and diagnostic value of pleural fluid periostin and serum periostin of malignant pleural effusions in patients with non–small‐cell lung cancer. J Clin Lab Anal. 2019;33:e22943 10.1002/jcla.22943
Wang, Fu and Shen contributed equally to this work.
Contributor Information
Yumin Wang, Email: wym0577@163.com.
Ling Ling Wu, Email: wulingling0577@163.com.
REFERENCES
- 1. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177‐193. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
- 3. Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non‐small‐cell lung cancer. J Thorac Oncol. 2012;7(10):1485‐1489. [DOI] [PubMed] [Google Scholar]
- 4. Braunschweig R, Yan P, Guilleret I, et al. Detection of malignant effusions: comparison of a telomerase assay and cytologic examination. Diagn Cytopathol. 2001;24(3):174‐180. [DOI] [PubMed] [Google Scholar]
- 5. Gu Y, Zhai K, Shi HZ. Clinical value of tumor markers for determining cause of pleural effusion. Chin Med J. 2016;129(3):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang XF, Wu YH, Wang MS, Wang YS. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev. 2014;15(1):363‐368. [DOI] [PubMed] [Google Scholar]
- 7. Zhang F, Hu L, Wang J, Chen J, Chen J, Wang Y. Clinical value of jointly detection serum lactate dehydrogenase/pleural fluid adenosine deaminase and pleural fluid carcinoembryonic antigen in the identification of malignant pleural effusion. J Clin Lab Anal. 2017;31(5)):e22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang Y, Liu YL, Shi HZ. Diagnostic accuracy of combinations of tumor markers for malignant pleural effusion: an updated meta‐analysis. Respiration. 2017;94(1):62‐69. [DOI] [PubMed] [Google Scholar]
- 9. Li Z, Zhang X, Yang Y, et al. Periostin expression and its prognostic value for colorectal cancer. Int J Mol Sci. 2015;16(6):12108‐12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sung PL, Jan YH, Lin SC, et al. Periostin in tumor microenvironment is associated with poor prognosis and platinum resistance in epithelial ovarian carcinoma. Oncotarget. 2016;7(4):4036‐4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nuzzo PV, Rubagotti A, Zinoli L, Salvi S, Boccardo S, Boccardo F. The prognostic value of stromal and epithelial periostin expression in human breast cancer: correlation with clinical pathological features and mortality outcome. BMC Cancer. 2016;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Du L. Role of pancreatic stellate cells and periostin in pancreatic cancer progression. Tumour Biol. 2015;36(5):3171‐3177. [DOI] [PubMed] [Google Scholar]
- 13. Hu W, Jin P, Liu W. Periostin contributes to cisplatin resistance in human non‐small cell lung cancer A549 cells via activation of Stat3 and Akt and upregulation of survivin. Cell Physiol Biochem. 2016;38(3):1199‐1208. [DOI] [PubMed] [Google Scholar]
- 14. Conway SJ, Izuhara K, Kudo Y, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014;71(7):1279‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66(14):2219‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morra L, Rechsteiner M, Casagrande S, et al. Characterization of periostin isoform pattern in non‐small cell lung cancer. Lung Cancer. 2012;76(2):183‐190. [DOI] [PubMed] [Google Scholar]
- 17. Nuzzo PV, Rubagotti A, Argellati F, et al. Prognostic value of preoperative serum levels of periostin (PN) in early breast cancer (BCa). Int J Mol Sci. 2015;16(8):17181‐17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Yuan D, Yao Y, Sun W, Shi Y, Su X. Predictive and prognostic value of serum periostin in advanced non‐small cell lung cancer patients receiving chemotherapy. Tumour Biol. 2017;39(5):1010428317698367. [DOI] [PubMed] [Google Scholar]
- 19. Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (Seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706‐714. [DOI] [PubMed] [Google Scholar]
- 20. Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy‐which first? Respirology. 2011;16(5):738‐746. [DOI] [PubMed] [Google Scholar]
- 21. Wang XJ, Yang Y, Wang Z, et al. Efficacy and safety of diagnostic thoracoscopy in undiagnosed pleural effusions. Respiration. 2015;90(3):251‐255. [DOI] [PubMed] [Google Scholar]
- 22. Sriram KB, Relan V, Clarke BE, et al. Diagnostic molecular biomarkers for malignant pleural effusions. Future Oncol. 2011;7(6):737‐752. [DOI] [PubMed] [Google Scholar]
- 23. Soltermann A, Ossola R, Kilgus‐Hawelski S, et al. N‐glycoprotein profiling of lung adenocarcinoma pleural effusions by shotgun proteomics. Cancer. 2008;114(2):124‐133. [DOI] [PubMed] [Google Scholar]
- 24. Kim CJ, Isono T, Tambe Y, et al. Role of alternative splicing of periostin in human bladder carcinogenesis. Int J Oncol. 2008;32(1):161‐169. [PubMed] [Google Scholar]
- 25. Contie S, Voorzanger‐Rousselot N, Litvin J, Clezardin P, Garnero P. Increased expression and serum levels of the stromal cell‐secreted protein periostin in breast cancer bone metastases. Int J Cancer. 2011;128(2):352‐360. [DOI] [PubMed] [Google Scholar]


