Abstract
Background
This study aimed to explore the correlation of circulating microRNA (miRNA) expression profile with clinical response to tumor necrosis factor (TNF) inhibitor in treating rheumatoid arthritis (RA) patients.
Methods
Baseline PBMC samples from eight responders and eight non‐responders after 24‐week TNF inhibitor (etanercept) treatment were subjected to miRNA microarray. Then, top 10 dysregulated miRNAs were selected and further validated by quantitative polymerase chain reaction (qPCR) in baseline PBMC samples from 92 RA patients treated with 24‐week TNF inhibitor (etanercept). Responders and non‐responders were divided referring to the decline in disease activity score in 28 joints.
Results
In microarray assay, total 59 upregulated and 78 downregulated miRNAs were identified in responders compared to non‐responders, which were mainly enriched in regulating immune‐ and inflammation‐related biological processes and pathways. The top 10 dysregulated miRNAs were as follows: miR‐192‐5p, miR‐146a‐5p, miR‐19b‐3p, miR‐320c, miR‐335‐5p, miR‐149‐3p, miR‐766‐3p, let‐7a‐5p, miR‐24‐3p, and miR‐1226‐5p. In qPCR validation, miR‐146a‐5p was increased, while let‐7a‐5p was decreased in responders compared with non‐responders. Multivariate logistic analysis illuminated that miR‐146a‐5p and CRP independently correlated with higher clinical response, while let‐7a‐5p and biologics history independently associated with lower clinical response. Subsequently, receiver operating characteristic curve showed that combination of these four independent factors presented with a great predictive value for clinical response with area under curve: 0.863, 95% CI 0.781‐0.945.
Conclusion
miRNA expression profile is closely implicated in the treatment efficacy of TNF inhibitor, and combined measurement of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history disclosed a great predictive value for clinical response to TNF inhibitor in RA patients.
Keywords: clinical response, let‐7a‐5p, miR‐146a‐5p, miRNA expression profiles, rheumatoid arthritis, tumor necrosis factor inhibitor
1. INTRODUCTION
Rheumatoid arthritis (RA), one of the common chronic inflammation diseases, affects approximately 0.5% to 1% of adult population worldwide. RA is characterized by joint damage, substantial disability, and other extra‐articular comorbidities, which imposes a huge burden on individuals and society.1, 2 In order to alleviate disease, several effective treatments have been used, including glucocorticoid, conventional disease‐modifying anti‐rheumatic drugs (DMARDs), tumor necrosis factor (TNF) inhibitor, and other biologics.3 Among these, TNF inhibitor could rapidly relieve inflammation and inhibit the progress of radiology to some extent, which are popularly applied in RA patients. However, an estimated 20% to 30% RA patients lack efficacy to TNF inhibitor treatment, or do not tolerate its high cost.4 Therefore, exploring novel biomarkers for predicting the clinical response to TNF inhibitor in RA patients is necessary.
microRNAs (miRNAs), a family of highly conserved small non‐coding RNAs directly binding to the 3′‐untranslated regions (3′‐UTRs), could mediate target genes, protein expression, and signaling pathways to affect cell functions such as proliferation, apoptosis, differentiation, migration, and invasion, thereby contributing to multiple biological processes, such as inflammation formation and immune response.5, 6, 7, 8 Accumulating evidences have proven that several miRNAs have established their value on the progress of various immune diseases, including RA, systemic lupus erythematosus (SLE), and psoriasis, which uncovered the potential of miRNA as marker for RA progression and prognosis.9, 10, 11, 12, 13, 14, 15 However, no studies investigate the effect of comprehensive miRNA expression profile on predicting clinical response to TNF inhibitor in RA patients. Thus, in the present study, the aim was to explore the correlation of circulating miRNA expression profile with clinical response to TNF inhibitor in treating active RA patients.
2. METHODS
2.1. Patients
A total of 92 active RA patients about to receive TNF inhibitor treatment from January 2015 to December 2017 were consecutively enrolled in this study. The inclusion criteria were as follows: (a) diagnosis of RA according to 1987 American College of Rheumatology (ACR) classification of RA; (b) age above 18 years and <75 years; (c) active disease status which was defined as 28‐joint disease activity score (DAS28) ≧3.2 points; and (d) about to initiate TNF inhibitor (etanercept (ETN)) treatment for 24 weeks. The exclusion criteria were as follows: (a) severe deformation of joint; (b) treatment of biologics within 3 months; (c) complicated with or history of hematological malignance disease or solid tumors; (d) history of severe infection, renal dysfunction, or hepatic dysfunction; (e) pregnant or lactating women; and (f) unable to be followed up regularly. This study protocol was approved by the Ethics Committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, and was conducted according to the Helsinki Declaration. All patients provided written informed consents.
2.2. Data collection
Baseline features of RA patients were recorded, including age, gender, disease duration, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP), rheumatoid factor (RF) status, anti‐citrullinated protein antibody (ACPA) status, and biologics history. Besides, DAS28 was evaluated and recorded at baseline as well.
2.3. Sample collection
Baseline peripheral blood sample was obtained from each patient before initiation of TNF inhibitor treatment, and peripheral blood mononuclear cell (PBMC) was subsequently extracted and stored in liquid nitrogen for followed detections.
2.4. Treatment
All patients initiated TNF inhibitor (ETN) treatment for 24 weeks. In brief, patients were given subcutaneous injection of 50 mg ETN per week for 24 weeks. Besides, the concomitant medications of conventional DMARDs were recorded during the study.
2.5. Evaluation of clinical response
DAS28 score was assessed at baseline (W0), 6 weeks (W4), 12 weeks (W12), and 24 weeks (W24). Then, clinical response at W24 was evaluated according to the European League Against Rheumatism (EULAR) response criteria, which was defined as a decrease of 1.2 points in DAS28 score from baseline.16 Referring to achievement of clinical response at W24, patients were divided into responders and non‐responders.
2.6. Microarray assay
Sixteen patients including eight responders and 8 non‐responders were selected from total patients. Total RNA was extracted from PBMCs of each patient by TRIzol reagent (Invitrogen), and RNA concentration, purity, and integrity were evaluated and adjusted; then, RNA was subjected to microRNA microarray assay. In brief, the assay started from 500 ng RNA of each sample, which was first polyadenylated and labeled by a biotin‐labeled DNA molecule. Subsequently, the labeled RNA molecule was hybridized and washed in a GeneChip Fluidics Station 450. Finally, a GeneChip Scanner 7G (Affymetrix, USA) was performed to scan that RNA molecule. Owing to the intrinsic background of different chips that might affect the calculation of the expression values, the Robust Multichip Average (RMA) was used for the normalization of the raw data in each chip. As an algorithm applying to create an expression matrix from Affymetrix data, the raw intensity values were background‐corrected, log2‐transformed, and then quantile‐normalized. After that, data were normalized by a linear model, thereby obtaining an expression measure for each probe set on each array.17
2.7. Bioinformatic analysis
Bioinformatic analysis was performed using R software (version 3.3.3) (Lucent Technologies). Principal components analysis (PCA) plot was drawn using Stats package. Dysregulated miRNAs were analyzed using limma package and showed as volcano plot with the statistical significance defined as a P value < 0.05 and the biological significance defined as a difference in fold change (FC) above 1.5 times. (Due to the limited number of miRNA profiles, we set the cutoff value of FC at 1.5 to screen out more candidate miRNAs.) Besides, dysregulated miRNAs were subjected to enrichment analysis based on annotation of (miEAA) database, which consisted of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment, disease enrichment, biological process enrichment, and organ enrichment.18 Fisher's exact test was performed to distinguish overrepresented miRNA‐related items for the enrichment analysis of dysregulated miRNAs and their precursors.
2.8. Quantitative polymerase chain reaction (qPCR) validation
So as to validate the predictive value of several potential miRNAs for clinical response to TNF inhibitor treatment, 10 candidate miRNAs which were the top 10 dysregulated ones between responders and non‐responders in microarray analysis based on the P value were chosen (miR‐192‐5p, miR‐146a‐5p, miR‐19b‐3p, miR‐320c, miR‐335‐5p, miR‐149‐3p, miR‐766‐3p, let‐7a‐5p, miR‐24‐3p, and miR‐1226‐5p), and then, these candidate miRNAs were measured by qPCR assay for validation in total 92 RA patients. In brief, total RNA was extracted from PBMCs of each patient by TRIzol reagent (Invitrogen), and RNA concentration, purity, and integrity were evaluated and adjusted; subsequently, complementary DNA was reverse‐transcribed by QuantiTect Rev. Transcription Kit (Qiagen) and then subjected to qPCR using SYBR Green Kit (TaKaRa). The PCR amplification procedures were as follows: degeneration at 95°C for 5 minutes, followed by 40 cycles at 95°C for 10 seconds, then 60 seconds at 60°C. The expressions of 10 candidate miRNAs were calculated using the 2−ΔΔCt method with U6 as the internal reference. The primer sequences are listed in Table 1.
Table 1.
Primer sequence list
| Gene | Forward primer (5′‐3′) | Reverse primer (5′‐3′) |
|---|---|---|
| miR‐192‐5p | ACACTCCAGCTGGGCTGACCTATGAATTGA | TGTCGTGGAGTCGGCAATTC |
| miR‐146a‐5p | ACACTCCAGCTGGGTGAGAACTGAATTCCA | TGTCGTGGAGTCGGCAATTC |
| miR‐19b‐3p | ACACTCCAGCTGGGTGTGCAAATCCATGCA | TGTCGTGGAGTCGGCAATTC |
| miR‐320c | ACACTCCAGCTGGGAAAAGCTGGGTTGAGA | TGTCGTGGAGTCGGCAATTC |
| miR‐335‐5p | ACACTCCAGCTGGGTCAAGAGCAATAACGA | TGTCGTGGAGTCGGCAATTC |
| miR‐149‐3p | ACACTCCAGCTGGGAGGGAGGGACGGGGGC | TGTCGTGGAGTCGGCAATTC |
| miR‐766‐3p | ACACTCCAGCTGGGACTCCAGCCCCACAGC | TGTCGTGGAGTCGGCAATTC |
| let‐7a‐5p | ACACTCCAGCTGGGTGAGGTAGTAGGTTGT | TGTCGTGGAGTCGGCAATTC |
| miR‐24‐3p | ACACTCCAGCTGGGTGGCTCAGTTCAGCAG | TGTCGTGGAGTCGGCAATTC |
| miR‐1226‐5p | ACACTCCAGCTGGGGTGAGGGCATGCAGGC | TGTCGTGGAGTCGGCAATTC |
| U6 | CGCTTCGGCAGCACATATACTA | ATGGAACGCTTCACGAATTTGC |
2.9. Statistics
Bioinformatic analysis was performed using R software (version 3.3.3) (Lucent Technologies), which was described in the above “Bioinformatic analysis” subsection. Other statistical analysis was carried out using SPSS 22.0 (IBM), and statistical graphs were drawn using GraphPad Prism software 7.01 (GraphPad Int, co, Ltd.). Comparison between two groups was determined by the Wilcoxon rank sum test. Correlation between two parameters was determined by Spearman's test. Predictive factors for clinical response were analyzed by univariate logistic regression model, and factors with a P value below 0.1 were further analyzed by multivariate logistic regression. Predictive value of independent parameters for clinical response was further analyzed by receiver operating characteristic (ROC) curve and evaluated using area under curve (AUC). P < 0.05 was considered as significant in this study.
3. RESULTS
3.1. Study flow
Initially, 125 RA patients about to undergo TNF inhibitor (ETN) treatment were invited to participate in this study, while 13 patients refused the invitation; then, the remaining 112 patients were screened for eligibility, among which 20 patients were excluded (12 refused to provide informed consents, and 8 disobeyed the inclusion criteria or met the exclusive criteria) and the left 92 patients were enrolled in this study. Among the 24‐week duration, nine patients (4 for lacked efficacy, 4 for lost to follow‐up, and 1 for hepatic dysfunction) withdrew; thus, a total of 83 patients completed the study. It is most important that all the 92 patients were included in the final analysis based on the intention‐to‐treatment (ITT) principle with the last observation carried forward (LOCF) method from any of the three post‐baseline measures, and the statistical results based on per protocol (PP) were similar to the data based on ITT; thus, they were not shown repeatedly in this study.
3.2. Characteristics of patients included in microarray
The mean age of 16 RA patients included in the microarray assay was 54.7 ± 9.9 years with 12 (75%) females. The median value of ESR and CRP was 34.6 (14.5‐41.0) mm/hour and 33.2 (21.7‐48.5) mg/L, respectively. The mean value of DAS28 was 5.5 ± 0.8. As to biologics history, there were 3 (19%) patients previously treated with biologics. Other detailed characteristics of these patients are shown in Table 2.
Table 2.
Characteristics of 16 RA patients included in the microarray assay
| Parameters | RA patients in microarray (N = 16) |
|---|---|
| Age (y) | 54.7 ± 9.9 |
| Gender, female (n/%) | 12 (75) |
| Disease duration (mo) | 48.0 (37.3‐60.5) |
| ESR (mm/h) | 34.6 (14.5‐41.0) |
| CRP (mg/L) | 33.2 (21.7‐48.5) |
| DAS28 score | 5.5 ± 0.8 |
| RF positive (n/%) | 14 (88) |
| ACPA positive (n/%) | 13 (81) |
| Biologics history | 3 (19) |
| Concomitant medications | |
| MTX (n/%) | 10 (63) |
| LEF (n/%) | 6 (37) |
Data were presented as mean value ± standard deviation, median (25th‐75th), or count (percentage).
Abbreviations: ACPA, anti‐citrullinated protein antibody; CRP, C‐reactive protein; DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; LEF, leflunomide; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor.
3.3. PCA plot, volcano plot, and heatmap analysis
The PCA plot showed that miRNA expression profile could mostly distinguish responders from non‐responders (Figure 1A). However, R1 sample was close to NR3 sample and could not be clearly differentiated from NR samples, which might be because the patient with R1 sample achieved the response at just a cutoff value. The volcano plot identified 59 upregulated and 78 downregulated miRNAs in responders compared to non‐responders (Figure 1B). Further, heatmap analysis exhibited that these dysregulated miRNAs mostly differentiated responders from non‐responders (Figure 1C). These data implied that miRNA expression profile is implicated in the clinical response to TNF inhibitor treatment in RA patients.
Figure 1.
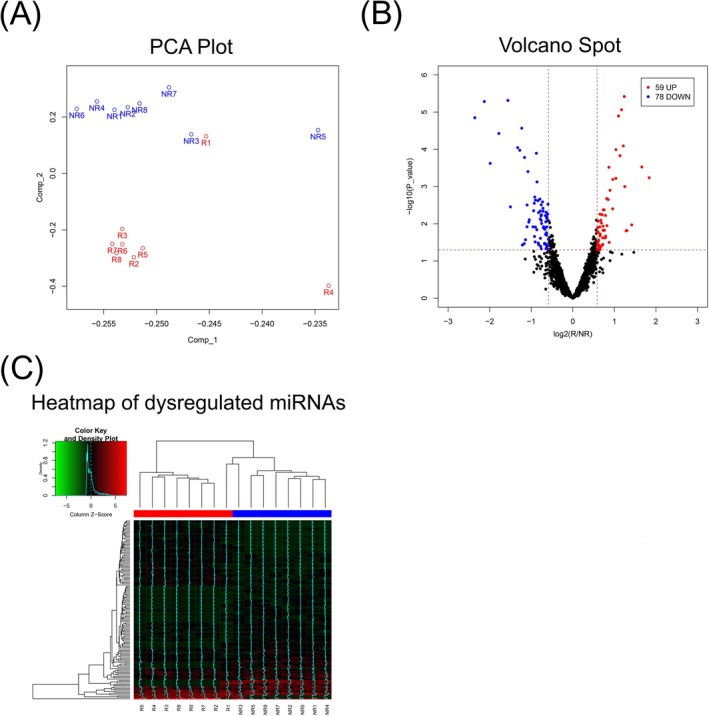
Microarray data analysis. PCA plot of miRNA expression profile between responders (R) and non‐responders (NR) (A). Volcano plot of miRNA expression profile between responders and non‐responders (upregulated miRNAs represent with red plots; downregulated miRNAs represent with blue plots; non‐changed miRNAs represent with black plots) (B). Heatmap of dysregulated miRNAs between responders (R) and non‐responders (NR) (C)
3.4. Enrichment analysis
Enrichment analysis was performed to determine the biological activities and pathways that the dysregulated miRNAs participated in. In brief, KEGG enrichment disclosed that dysregulated miRNAs were mainly implicated in the inflammation‐ and immune‐related pathways such as inflammation‐mediated chemokine and cytokine interaction, androgen receptor signaling, B‐cell activation, and so on (Figure 2A). Disease enrichment illuminated that dysregulated miRNAs were mainly enriched in neoplasms, inflammation, arthritis, and so on (Figure 2B). Biological process enrichment exhibited that dysregulated miRNAs mainly participated in innate immune response, inflammatory response, cell cycle cytokines, and so on (Figure 2C). Besides, organ enrichment showed that they were mainly implicated in fibroblasts, B lymphocytes, and so on (Figure 2D). These data suggested that miRNA expression profile might affect the clinical response to TNF inhibitor via affecting immune‐ and inflammation‐related pathways and biological process.
Figure 2.
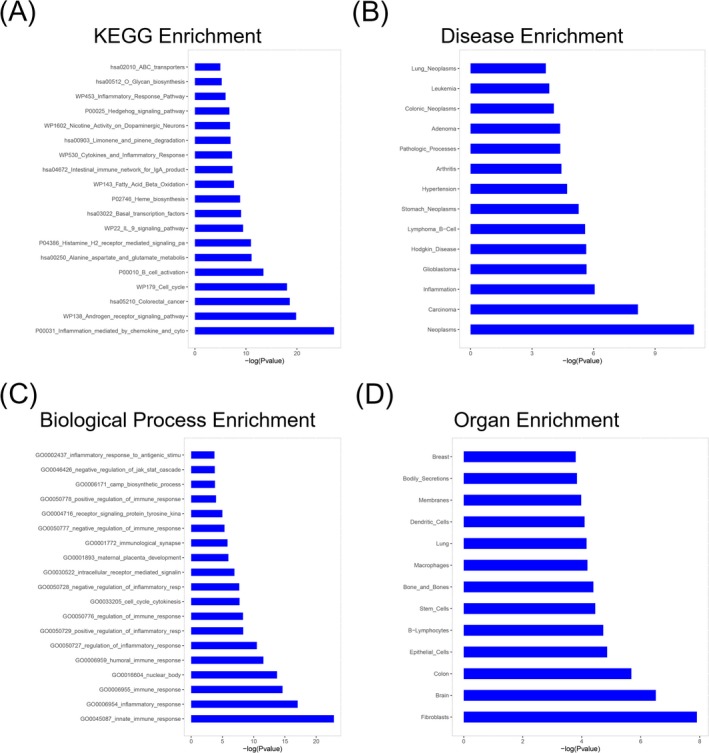
Enrichment analysis. The barplot of enrichment analysis of dysregulated miRNAs on Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (A). The barplot of enrichment analysis of dysregulated miRNAs on disease (B). The barplot of enrichment analysis of dysregulated miRNAs on biological processes (C). The barplot of enrichment analysis of dysregulated miRNAs on organ (D)
3.5. Selection of candidate miRNAs
In order to verify the predictive value of several potential miRNAs for clinical response to TNF inhibitor treatment, 10 candidate miRNAs which were the top 10 dysregulated ones between responders and non‐responders in microarray analysis based on the P value were chosen as follows (Table 3): miR‐192‐5p, miR‐146a‐5p, miR‐19b‐3p, miR‐320c, miR‐335‐5p, miR‐149‐3p, miR‐766‐3p, let‐7a‐5p, miR‐24‐3p, and miR‐1226‐5p.
Table 3.
Top 10 dysregulated miRNAs between responders and non‐responders in microarray
| miRNA | Log2FC | AveExpr | P Value | Trend |
|---|---|---|---|---|
| miR‐192‐5p | −5.6829178 | 3.28235349 | 5.57E−12 | Down |
| miR‐146a‐5p | 5.3961757 | 3.26313617 | 5.44E−09 | Up |
| miR‐19b‐3p | −3.1327707 | 1.72356641 | 4.95E−08 | Down |
| miR‐320c | −5.0337138 | 3.20315139 | 2.07E−07 | Down |
| miR‐335‐5p | −3.6547919 | 2.40320691 | 4.82E−07 | Down |
| miR‐149‐3p | 5.19999908 | 3.40862467 | 5.37E−07 | Up |
| miR‐766‐3p | 1.23814903 | 0.66925275 | 3.80E−06 | Up |
| let‐7a‐5p | −1.5599407 | 0.92788677 | 4.86E−06 | Down |
| miR‐24‐3p | −2.1284917 | 1.24417515 | 5.18E−06 | Down |
| miR‐1226‐5p | 1.17376876 | 0.58688438 | 8.66E−06 | Up |
Top 10 dysregulated miRNAs were selected according to P value and presented in the table. Significance of the comparison was completed by limma package in R software.
Abbreviations: AveExpr, average of expression level; Log2FC, log2 (fold change); miRNA, microRNA.
3.6. Characteristics of patients included in qPCR validation
The mean age of 92 RA patients included in the qPCR validation was 55.6 ± 8.8 years with 74 (80%) females. The median value of ESR and CRP was 36.4 (20.3‐44.6) mm/hour and 32.8 (19.5‐46.4) mg/L, respectively. The mean value of DAS28 was 5.6 ± 0.9. Besides, 12 (13%) patients had biologics history. Other detailed characteristics of these patients are shown in Table 4.
Table 4.
Characteristics of 92 RA patients included in the qPCR validation
| Parameters | RA patients in qPCR validation (N = 92) |
|---|---|
| Age (y) | 55.6 ± 8.8 |
| Gender, female (n/%) | 74 (80) |
| Disease duration (mo) | 48.0 (34.0‐88.0) |
| ESR (mm/h) | 36.4 (20.3‐44.6) |
| CRP (mg/L) | 32.8 (19.5‐46.4) |
| DAS28 score | 5.6 ± 0.9 |
| RF positive (n/%) | 75 (82) |
| ACPA positive (n/%) | 71 (77) |
| Biologics history (n/%) | 12 (13) |
| Concomitant medications | |
| MTX (n/%) | 51 (55) |
| LEF (n/%) | 41 (45) |
Data were presented as mean value ± standard deviation, median (25th‐75th), or count (percentage).
Abbreviations: ACPA, anti‐citrullinated protein antibody; CRP, C‐reactive protein; DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; LEF, leflunomide; MTX, methotrexate; RA, rheumatoid arthritis; RF, rheumatoid factor.
3.7. Correlation of candidate miRNAs with disease activity
Positive associations of miR‐146a‐5p with ESR (R = 0.231, P = 0.027), miR‐1226‐5p with CRP (R = 0.240, P = 0.021), and let‐7a‐5p with DAS28 (R = 0.261, P = 0.012) were observed, while miR‐335‐5p was negatively correlated with DAS28 (R = −0.226, P = 0.030). No correlation of other candidate miRNAs with disease activity was found (Table 5).
Table 5.
Correlation of 10 candidate miRNAs with disease activity in the qPCR validation
| Parameters | ESR | CRP | DAS28 |
|---|---|---|---|
| miR‐192‐5p | |||
| Correlation coefficient R | 0.146 | −0.042 | 0.115 |
| P value | 0.164 | 0.690 | 0.276 |
| miR‐146a‐5p | |||
| Correlation coefficient R | 0.231 | 0.204 | 0.065 |
| P value | 0.027 | 0.051 | 0.541 |
| miR‐19b‐3p | |||
| Correlation coefficient R | −0.134 | ‐0.095 | −0.099 |
| P value | 0.204 | 0.368 | 0.349 |
| miR‐320c | |||
| Correlation coefficient R | ‐0.068 | −0.161 | 0.119 |
| P value | 0.521 | 0.125 | 0.257 |
| miR‐335‐5p | |||
| Correlation coefficient R | ‐0.140 | −0.008 | −0.226 |
| P value | 0.185 | 0.943 | 0.030 |
| miR‐149‐3p | |||
| Correlation coefficient R | ‐0.040 | 0.041 | 0.068 |
| P value | 0.705 | 0.701 | 0.522 |
| miR‐766‐3p | |||
| Correlation coefficient R | −0.064 | −0.028 | −0.011 |
| P value | 0.546 | 0.790 | 0.920 |
| let‐7a‐5p | |||
| Correlation coefficient R | 0.092 | 0.039 | 0.261 |
| P value | 0.383 | 0.711 | 0.012 |
| miR‐24‐3p | |||
| Correlation coefficient R | 0.051 | −0.089 | −0.115 |
| P value | 0.631 | 0.397 | 0.276 |
| miR‐1226‐5p | |||
| Correlation coefficient R | 0.129 | 0.240 | 0.057 |
| P value | 0.221 | 0.021 | 0.591 |
Correlation was determined by Spearman's test. P < 0.05 was considered significant.
Abbreviations: CRP, C‐reactive protein; DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; miRNA, microRNA.
3.8. Candidate miRNA expressions between responders and non‐responders
Among totally 92 RA patients, 60 responders and 32 non‐responders to TNF inhibitor were observed. As listed in Figure 3, relative expression of miR‐146a‐5p expression (P = 0.004, Figure 3B) in responders was higher than that in non‐responders, while let‐7a‐5p expression (P = 0.001, Figure 3H) was lower in responders compared with non‐responders. However, relative expressions of miR‐192‐5p (P = 0.272, Figure 3A), miR‐19b‐3p (P = 0.168, Figure 3C), miR‐320c (P = 0.114, Figure 3D), miR‐335‐5p (P = 0.329, Figure 3E), miR‐149‐3p (P = 0.086, Figure 3F), miR‐766‐3p (P = 0.481, Figure 3G), miR‐24‐3p (P = 0.223, Figure 3I), and miR‐1226‐5p (P = 0.073, Figure 3J) between responders and non‐responders had no difference.
Figure 3.
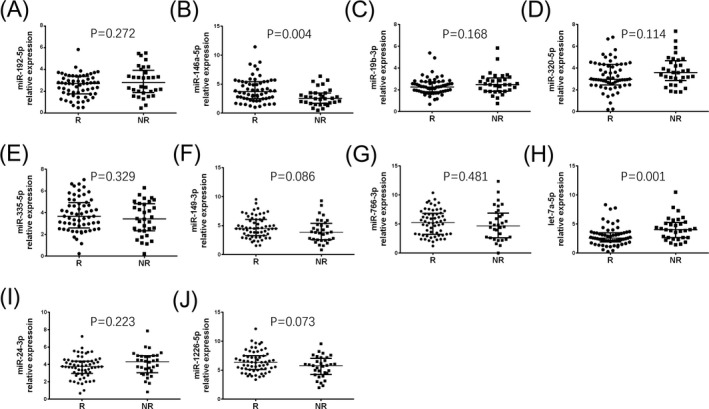
Comparison of 10 candidate miRNAs between responders and non‐responders. miR‐192‐5p (A), miR‐146a‐5p (B), miR‐19b‐3p (C), miR‐320c (D), miR‐335‐5p (E), miR‐149‐3p (F), miR‐766‐3p (G), let‐7a‐5p (H), miR‐24‐3p (I), and miR‐1226‐5p (J) expressions between responders (R) and non‐responders (NR) to TNF inhibitor
3.9. Factors predicting clinical response
Univariate logistic analysis of factors for predicting clinical response to TNF inhibitor in all patients was assessed (Table 6), which observed that miR‐146a‐5p (P = 0.006) and CRP (P = 0.003) were associated with a higher possibility to achieve clinical response, while let‐7a‐5p (P = 0.003) was associated with a lower possibility to achieve clinical response. All factors with a P value ≦0.1 in univariate logistic model were further analyzed by multivariate logistic regression model (Table 7), which suggested that miR‐146a‐5p (P = 0.011) and CRP (P = 0.006) were independent predictive factors for better clinical response, while let‐7a‐5p (P = 0.002) and biologics history (P = 0.037) were independent predictive factors for worse clinical response to TNF inhibitor in RA patients.
Table 6.
Univariate logistic analysis of factors predicting clinical response
| Parameters | Univariate logistic regression model | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| miR‐192‐5p | 0.143 | 0.755 | 0.518 | 1.100 |
| miR‐146a‐5p | 0.006 | 1.508 | 1.127 | 2.018 |
| miR‐19b‐3p | 0.204 | 0.724 | 0.439 | 1.192 |
| miR‐320c | 0.111 | 0.768 | 0.555 | 1.063 |
| miR‐335‐5p | 0.220 | 1.198 | 0.898 | 1.598 |
| miR‐149‐3p | 0.139 | 1.199 | 0.943 | 1.525 |
| miR‐766‐3p | 0.775 | 1.027 | 0.856 | 1.231 |
| let‐7a‐5p | 0.003 | 0.677 | 0.521 | 0.879 |
| miR‐24‐3p | 0.216 | 0.806 | 0.573 | 1.134 |
| miR‐1226‐5p | 0.064 | 1.321 | 1.022 | 1.708 |
| Age | 0.242 | 0.983 | 0.954 | 1.012 |
| Gender, female | 0.328 | 0.619 | 0.237 | 1.617 |
| Disease duration | 0.166 | 0.992 | 0.980 | 1.003 |
| ESR | 0.154 | 1.018 | 0.993 | 1.042 |
| CRP | 0.003 | 1.060 | 1.020 | 1.102 |
| DAS28 score | 0.115 | 1.469 | 0.910 | 2.370 |
| RF positive | 0.189 | 1.894 | 0.730 | 4.914 |
| ACPA positive | 0.154 | 1.971 | 0.776 | 5.011 |
| Biologics history | 0.076 | 0.325 | 0.094 | 1.123 |
| Concomitant medications (MTX vs LEF) | 0.662 | 1.211 | 0.513 | 2.861 |
Univariate logistic regression model was used to analyze the factors at baseline in predicting clinical response in RA patients treated with TNF inhibitors. P value < 0.05 was considered significant.
Abbreviations: ACPA, anti‐citrullinated protein antibody; CI, confidence interval; CRP, C‐reactive protein; DAS28, disease activity score in 28 joints; ESR, erythrocyte sedimentation rate; MTX, methotrexate; LEF, leflunomide; OR, odds ratio; RF, rheumatoid factor.
Table 7.
Multivariate logistic analysis of factors predicting clinical response
| Parameters | Multivariate logistic regression model | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| miR‐146a‐5p | 0.011 | 1.617 | 1.115 | 2.347 |
| let‐7a‐5p | 0.002 | 0.599 | 0.435 | 0.824 |
| miR‐1226‐5p | 0.347 | 1.176 | 0.839 | 1.647 |
| CRP | 0.006 | 1.069 | 1.019 | 1.121 |
| Treatment history of biologics | 0.037 | 0.147 | 0.024 | 0.887 |
Factors with P value no above than 0.1 in univariate analysis were subsequently analyzed by multivariate logistic regression model. P value < 0.05 was considered significant.
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; OR, odds ratio.
3.10. Predictive value of 4 independent factors for clinical response
Four independent factors in multivariate logistic analysis were further analyzed by ROC curve (Figure 4), which showed that miR‐146a‐5p (AUC: 0.685, 95% CI 0.574‐0.797), let‐7a‐5p (AUC: 0.714, 95% CI 0.602‐0.826), and CRP (AUC: 0.737, 95% CI 0.625‐0.849) presented with good value in predicting clinical response to TNF inhibitor. Meanwhile, combination of miR‐146a‐5p, let‐7a‐5p, and CRP exhibited a better predictive value for clinical response to TNF inhibitor with AUC: 0.838, 95% CI 0.748‐0.927. It is most important that combination of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history disclosed a great predictive value for clinical response to TNF inhibitor with AUC: 0.863, 95% CI 0.781‐0.945. The sensitivity and specificity were 88.3% and 78.1% at the best cutoff point, respectively.
Figure 4.
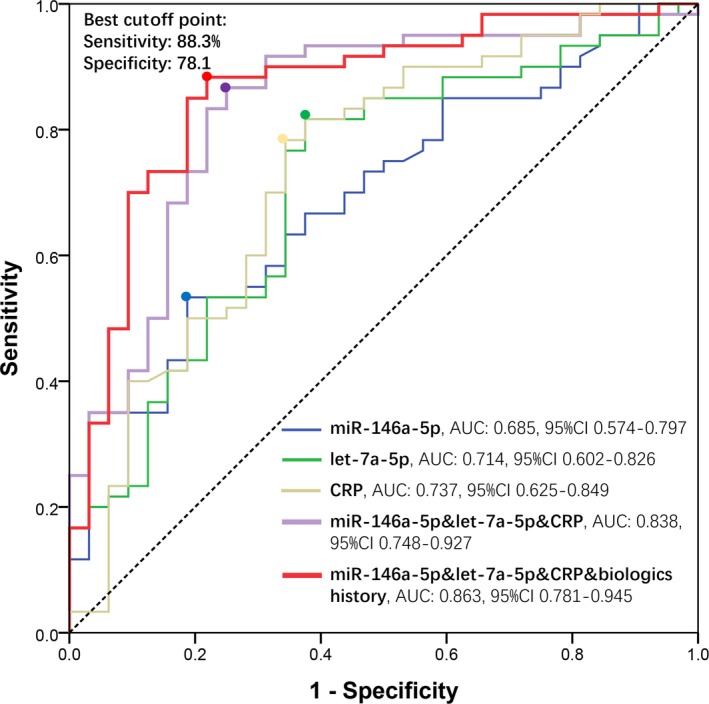
ROC curve analysis for predicting clinical response. Predictive values of miR‐146a‐5p (blue line), let‐7a‐tp (green line), and CRP (yellow line); combination of miR‐146a‐5p, let‐7a‐tp, and CRP (purple line); and combination of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history (red line) for clinical response to TNF inhibitor
4. DISCUSSION
The current study systematically explored the dysregulated miRNA profile in responders and non‐responders to TNF inhibitor by microarray, and subsequently identified the correlation of 10 candidate miRNAs with disease activity as well as clinical response to TNF inhibitor in a larger sample size by qPCR validation in RA patients. The key findings were shown as follows: (a) 59 upregulated and 78 downregulated miRNAs were found in responders than non‐responders to TNF inhibitor by microarray, which were mainly enriched in regulating immune‐ and inflammation‐related biological processes and pathways; and (b) qPCR validation in large samples revealed that miR‐146a‐5p and CRP could independently predict better clinical response, while let‐7a‐5p and biologics history were independent factors for worse clinical response to TNF inhibitor treatment, and the combination of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history exhibited a great predictive value for clinical response to TNF inhibitor.
miRNA is a new class of non‐coding RNAs with length of approximately 23 nucleotides, which potentially construct estimated 1%‐2% of the whole genome and mediate approximately 30% of all protein‐encoding genes. miRNA is proposed to play critical roles in multiple physiological and pathological processes including particular contribution to inflammation formation and autoimmunity response.19, 20, 21 Recent data suggest that circulating miR‐125b expression could be served as a prognostic biomarker in RA patients treated with TNF inhibitor.22 Another interesting study indicates that miR‐146a discloses a potential predictive value for clinical response to Tripterygium wilfordii Hook F (TwHF) treatment in RA patients.23 These previous studies suggest that miRNAs have potential value in predicting treatment efficacy in RA patients. However, no study has explored the predictive value of comprehensive miRNA profile for the clinical response to TNF inhibitor in RA patients. Our study used the microarray to assess dysregulated miRNAs between RA responders and non‐responders to TNF inhibitor, which showed that 59 upregulated and 78 downregulated miRNAs were found in responders than non‐responders to TNF inhibitor by microarray, which were mainly enriched in regulating immune‐ and inflammation‐related biological processes and pathways. These provided novel evidences for the application of miRNA expression pattern in predicting treatment outcome in RA patients underwent TNF inhibitor treatment, which shed a light on optimizing individual treatment strategy and improving prognosis of RA.
miR‐146a is considered as one of the most popularly investigated miRNAs involved in the RA pathogenesis24, 25, 26 with the possible mechanisms as follows: (a) targets IL‐17 leading to the promotion of T‐cell production, thereby inducing inflammation in RA27; (b) represses tumor necrosis factor receptor–associated factor 6 (TRAF6) and IL‐1 receptor–associated kinase 1 (IRAK‐1) to prolong the production of TNF‐α, subsequently contributing to hyperimmune inflammatory responses24; and (c) stimulates Th1 cells to produce IL‐2, IL‐12, and interferon (IFN)‐γ, promoting inflammations in RA. In addition, overexpression of miR‐146a‐5p could target nuclear factor‐kappa (NF‐κB) pathway, inducing the secretion of various pro‐inflammatory cytokines including TNF‐α, IL‐1β, and IL‐17.28 According to several reports, overexpression of miR‐146a is observed in PBMCs and synovial tissue from active RA patients, which also positively correlates with ESR and DAS28.27, 29 In our study, we discovered that miR‐146a‐5p was positively correlated with ESR in 92 RA patients by qPCR validation. The possible explanation was that miR‐146a‐5p affected the disease activity of RA through mediating various inflammation‐ and immune‐related genes and signaling pathways such as TNF‐α, IL‐17, and NF‐κB pathways.24, 27, 28 As to predictive value of miR‐146a for treatment efficacy in RA, an interesting study illuminates that miR‐146a presents with an increased tendency in responders compared with non‐responders to Tripterygium wilfordii Hook F (TwHF) treatment in RA patients.23 However, no study has reported the correlation of miR‐146a with clinical response to TNF inhibitor until now. Our study found that miR‐146a‐5p was an independent predictive factor for higher clinical response to TNF inhibitor treatment in RA patients; these might result from that: (a) miR‐146a‐5p high expression is associated with relatively higher disease activity, which provides a larger gap for DAS28 decrease after TNF inhibitor treatment, thereby achieving a higher possibility of clinical response; (b) miR‐146a‐5p may be correlated with the secretion of TNF‐α, which could directly affect the efficacy of TNF inhibitor treatment; and (c) miR‐146a‐5p might affect TNF inhibitor sensitivity through regulating several critical genes and pathways.6, 24, 28, 30, 31
Let‐7a, one of most common subtypes of let‐7 family, has been also reported to be involved in the development and progression of several diseases, such as lupus, Crohn's disease, and carcinomas,32, 33 with the potential mechanisms as follows: (a) Let‐7a represses mesenchymal stem cells (MSCs) to decrease the apoptosis of T cell, thereby promoting inflammatory responses30; and (b) let‐7a increases cell fraction in G2/M to decrease Cdc34 and stabilize Wee1 kinase directly, thereby stimulating fibroblast formation.34 Partially in line with previous studies, we observed that let‐7a‐5p expression was positively associated with DAS28 in RA patients. The possible explanations might be that let‐7a‐5p promotes inflammatory responses and synovium growth in RA; thus, it correlates with higher disease activity. As to the predictive value of let‐7a for clinical response in RA, no related study has been reported. Our study firstly observed that let‐7a‐5p was an independent factor for worse clinical response to TNF inhibitor treatment in RA patients, which might be explained by that: (a) Let‐7a‐5p regulates ACPA‐induced macrophage activation in RA, which leads to worse treatment outcome35; (b) let‐7a‐5p might affect sensitivity to treatment drugs, resulting in lower clinical response.36
In addition, we also found that CRP independently predicted higher clinical response, while biologics history independently predicted lower clinical response to TNF inhibitor in RA patients. These might result from that: (a) high CRP is correlated with high inflammation which would benefit more from anti‐inflammation treatment, and it has been demonstrated in several studies that high CRP could predict clinical response to DMARDs, TNF inhibitor, and other biologics37, 38; and (b) as to patients with biologics history, they are easy to produce anti‐antibody when they use biologics again, which could induce secondary resistance, thereby decreasing the efficacy of treatment.39
Most interestingly, we further discovered that the combination of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history exhibited a great predictive value for clinical response to TNF inhibitor in RA patients. This provided a novel evidence for optimizing individual treatment strategy and improving prognosis of RA. However, further prospective, multiple‐center study with larger samples exploring the prognostic value of the combination of these four factors in RA patients treated with TNF inhibitor is still needed.
There were several limitations existed in this study: (a) Due to the cost of TNF inhibitor, the sample was relatively small in this study; (b) the follow‐up duration of 24 weeks was relatively short, while the long‐term response was not investigated; and (c) radiographic progress was not investigated, which plays an important role in the progress of RA.
In conclusion, miRNA expression profile is closely implicated in the treatment efficacy of TNF inhibitor, and combined measurement of miR‐146a‐5p, let‐7a‐5p, CRP, and biologics history disclosed a great predictive value for clinical response to TNF inhibitor in RA patients.
Liu Y, Han Y, Qu H, Fang J, Ye M, Yin W. Correlation of microRNA expression profile with clinical response to tumor necrosis factor inhibitor in treating rheumatoid arthritis patients: A prospective cohort study. J Clin Lab Anal. 2019;33:e22953 10.1002/jcla.22953
Liu and Han are equal contributors.
Contributor Information
Mei Ye, Email: 583215728@qq.com.
Wanling Yin, Email: 8347448@qq.com.
REFERENCES
- 1. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360‐1372. [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389(10086):2328‐2337. [DOI] [PubMed] [Google Scholar]
- 3. Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338‐2348. [DOI] [PubMed] [Google Scholar]
- 4. Nam JL, Takase‐Minegishi K, Ramiro S, et al. Efficacy of biological disease‐modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2017;76(6):1113‐1136. [DOI] [PubMed] [Google Scholar]
- 5. Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113(8):6207‐6233. [DOI] [PubMed] [Google Scholar]
- 6. Churov AV, Oleinik EK, Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev. 2015;14(11):1029‐1037. [DOI] [PubMed] [Google Scholar]
- 7. Brennan E, Wang BO, McClelland A, et al. Protective effect of let‐7 miRNA family in regulating inflammation in diabetes‐associated atherosclerosis. Diabetes. 2017;66(8):2266‐2277. [DOI] [PubMed] [Google Scholar]
- 8. Fan Y, Ding S, Sun Y, Zhao B, Pan Y, Wan J. MiR‐377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem. 2018;119(1):327‐337. [DOI] [PubMed] [Google Scholar]
- 9. Huang W, Li Z, Zhao L, Zhao W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer's disease via modulating the expression of miR‐106b. Biomed Pharmacother. 2017;92:46‐57. [DOI] [PubMed] [Google Scholar]
- 10. Sun HY, Lv AK, Yao H. Relationship of miRNA‐146a to primary Sjogren's syndrome and to systemic lupus erythematosus: a meta‐analysis. Rheumatol Int. 2017;37(8):1311‐1316. [DOI] [PubMed] [Google Scholar]
- 11. Rai G, Rai R, Saeidian AH, Rai M. Microarray to deep sequencing: transcriptome and miRNA profiling to elucidate molecular pathways in systemic lupus erythematosus. Immunol Res. 2016;64(1):14‐24. [DOI] [PubMed] [Google Scholar]
- 12. Jiang H, Ma R, Zou S, Wang Y, Li Z, Li W. Reconstruction and analysis of the lncRNA‐miRNA‐mRNA network based on competitive endogenous RNA reveal functional lncRNAs in rheumatoid arthritis. Mol Biosyst. 2017;13(6):1182‐1192. [DOI] [PubMed] [Google Scholar]
- 13. Sharma AR, Sharma G, Lee SS, Chakraborty C. miRNA‐regulated key components of cytokine signaling pathways and inflammation in rheumatoid arthritis. Med Res Rev. 2016;36(3):425‐439. [DOI] [PubMed] [Google Scholar]
- 14. Hermann H, Runnel T, Aab A, et al. miR‐146b probably assists miRNA‐146a in the suppression of keratinocyte proliferation and inflammatory responses in psoriasis. J Invest Dermatol. 2017;137(9):1945‐1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Gele M, Bracke S, Alves de Medeiros AK, Lambert J. Exploring the feasibility of whole blood to identify systemic miRNA biomarkers for patients with moderate to severe psoriasis. Eur J Dermatol. 2016;26(2):195‐198. [DOI] [PubMed] [Google Scholar]
- 16. van Gestel AM, Prevoo M, van't Hof MA, van Rijswijk MH, van de Putte L, van Riel P. Development and validation of the european league against rheumatism response criteria for rheumatoid arthritis: Comparison with the preliminary american college of rheumatology and the world health organization/international league against rheumatism criteria. Arthritis Rheum. 1996;39(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 17. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249‐264. [DOI] [PubMed] [Google Scholar]
- 18. Backes C, Khaleeq QT, Meese E, Keller A. miEAA: microRNA enrichment analysis and annotation. Nucleic Acids Res. 2016;44(W1):W110‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poole EM, Lee IM, Ridker PM, Buring JE, Hankinson SE, Tworoger SS. A prospective study of circulating C‐reactive protein, interleukin‐6, and tumor necrosis factor alpha receptor 2 levels and risk of ovarian cancer. Am J Epidemiol. 2013;178(8):1256‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu XM, Zhang HJ. miRNAs as new molecular insights into inflammatory bowel disease: crucial regulators in autoimmunity and inflammation. World J Gastroenterol. 2016;22(7):2206‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bordon Y. Autoimmunity: microRNA‐23b keeps TABs on tissue inflammation. Nat Rev Immunol. 2012;12(7):474. [DOI] [PubMed] [Google Scholar]
- 22. Castro‐Villegas C, Perez‐Sanchez C, Escudero A, et al. Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti‐TNFalpha. Arthritis Res Ther. 2015;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen ZZ, Zhang XD, Chen Y, Wu YB. The role of circulating miR‐146a in patients with rheumatoid arthritis treated by Tripterygium wilfordii Hook F. Medicine. 2017;96(20):e6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR‐146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10(4):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu K, Xu P, Yao JF, Zhang YG, Hou WK, Lu SM. Reduced apoptosis correlates with enhanced autophagy in synovial tissues of rheumatoid arthritis. Inflamm Res. 2013;62(2):229‐237. [DOI] [PubMed] [Google Scholar]
- 26. Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160(3):198‐206. [DOI] [PubMed] [Google Scholar]
- 27. Niimoto T, Nakasa T, Ishikawa M, et al. MicroRNA‐146a expresses in interleukin‐17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet Disord. 2010;11:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bogunia‐Kubik K, Wysoczanska B, Piatek D, Iwaszko M, Ciechomska M, Swierkot J. Significance of polymorphism and expression of miR‐146a and NFkB1 genetic variants in patients with rheumatoid arthritis. Arch Immunol Ther Exp (Warsz). 2016;64(Suppl 1):131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng ZT, Li J, Ren J, Lv Z. Expression of miR‐146a and miR‐16 in peripheral blood mononuclear cells of patients with rheumatoid arthritis and their correlation to the disease activity. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(2):320‐323. [PubMed] [Google Scholar]
- 30. Yu Y, Liao LI, Shao B, et al. Knockdown of microRNA Let‐7a improves the functionality of bone marrow‐derived mesenchymal stem cells in immunotherapy. Mol Ther. 2017;25(2):480‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakasa T, Shibuya H, Nagata Y, Niimoto T, Ochi M. The inhibitory effect of microRNA‐146a expression on bone destruction in collagen‐induced arthritis. Arthritis Rheum. 2011;63(6):1582‐1590. [DOI] [PubMed] [Google Scholar]
- 32. Burt RK, Loh Y, Pearce W, et al. Clinical applications of blood‐derived and marrow‐derived stem cells for nonmalignant diseases. JAMA. 2008;299(8):925‐936. [DOI] [PubMed] [Google Scholar]
- 33. Roush S, Slack FJ. The let‐7 family of microRNAs. Trends Cell Biol. 2008;18(10):505‐516. [DOI] [PubMed] [Google Scholar]
- 34. Legesse‐Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazoie S, Coller HA. let‐7 overexpression leads to an increased fraction of cells in G2/M, direct down‐regulation of Cdc34, and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem. 2009;284(11):6605‐6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu W, Yu J, Qiu S, et al. MiR‐let‐7a regulates anti‐citrullinated protein antibody‐induced macrophage activation and correlates with the development of experimental rheumatoid arthritis. Int Immunopharmacol. 2017;51:40‐46. [DOI] [PubMed] [Google Scholar]
- 36. Xiao G, Wang X, Yu Y. CXCR36/Let‐7a axis regulates metastasis and chemoresistance of pancreatic cancer cells through targeting HMGA2. Cell Physiol Biochem. 2017;43(2):840‐851. [DOI] [PubMed] [Google Scholar]
- 37. Alberdi‐Saugstrup M, Nielsen S, Mathiessen P, Nielsen CH, Muller K. Low pretreatment levels of myeloid‐related protein‐8/14 and C‐reactive protein predict poor adherence to treatment with tumor necrosis factor inhibitors in juvenile idiopathic arthritis. Clin Rheumatol. 2017;36(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 38. Plant D, Ibrahim I, Lunt M, et al. Correlation of C‐reactive protein haplotypes with serum C‐reactive protein level and response to anti‐tumor necrosis factor therapy in UK rheumatoid arthritis patients: results from the biologics in rheumatoid arthritis genetics and genomics study syndicate cohort. Arthritis Res Ther. 2012;14(5):R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joensuu JT, Huoponen S, Aaltonen KJ, Konttinen YT, Nordstrom D, Blom M. The cost‐effectiveness of biologics for the treatment of rheumatoid arthritis: a systematic review. PLoS ONE. 2015;10(3):e0119683. [DOI] [PMC free article] [PubMed] [Google Scholar]


