Abstract
Cellular immune defences in sea urchins are shared amongst the coelomocytes - a heterogeneous population of cells residing in the coelomic fluid (blood equivalent) and tissues. The most iconic coelomocyte morphotype is the red spherule cell (or amebocyte), so named due to the abundance of cytoplasmic vesicles containing the naphthoquinone pigment echinochrome A. Despite their identification over a century ago, and evidence of antiseptic properties, little progress has been made in characterising the immunocompetence of these cells. Upon exposure of red spherule cells from sea urchins, i.e., Paracentrotus lividus and Psammechinus miliaris, to microbial ligands, intact microbes, and damage signals, we observed cellular degranulation and increased detection of cell-free echinochrome in the coelomic fluid ex vivo. Treatment of the cells with ionomycin, a calcium-specific ionophore, confirmed that an increase in intracellular levels of Ca2+ is a trigger of echinochrome release. Incubating Gram-positive/negative bacteria as well as yeast with lysates of red spherule cells led to significant reductions in colony-forming units. Such antimicrobial properties were counteracted by the addition of ferric iron (Fe3+), suggesting that echinochrome acts as a primitive iron chelator in echinoid biological defences.
Keywords: Coelomocytes, Damage response, Degranulation, Invertebrate immunity, Paracentrotus lividus, Psammechinus miliaris
Introduction
Lacking adaptive immune capabilities, invertebrates such as insects and decapod crustaceans are used routinely to study the mechanisms and biological complexities of innate immunity. Unlike those invertebrates, sea urchins are deuterostomes - placing them on the same ancestral branch of life as chordates prior to the divergence of these metazoan lineages. The fully sequenced genome of the purple sea urchin Strongylocentrotus purpuratus has revealed the shared origin of many immune gene families and the genetic synonymity between vertebrates and echinoderms [1, 2]. The canonical view of invertebrate innate immunity describes 3 arms of defence: (1) physical barriers such as the exoskeleton, (2) cellular activities within the equivalents of blood, i.e., coelomic fluid or haemolymph, and (3) humoral factors that include (but are not limited to) antimicrobial peptides, lysozyme, and complement-like proteins [reviewed in [3, 4, 5]]. Cell-derived immunity in sea urchins is provided by the coelomocytes - a heterogeneous population consisting of 4 distinct morphotypes: phagocytes, vibratile cells, and colourless and red spherule cells. The former can be subdivided into discoidal, polygonal, and small phagocytes, which express a myriad of immune effectors belonging to the (Sp)Transformer gene family [6, 7]. The phagocytes are tasked with identifying, ingesting, and destroying invading pathogens, whereas the vibratile cells are said to be involved in hemostasis [8, 9, 10]. The immunological function of colourless spherule cells (CSC) remains unclear, although some evidence supports a cytotoxic role [11].
Despite progress being made in enhancing our understanding of sea urchin immunity over the past 50 years, little is known about the pigmented coelomocytes in adult coelomic fluid, namely, red spherule cells (RSC). RSC owe their distinct colouration to echinochrome A, a 1,4-napthoquinone packaged within cytoplasmic vesicles (or granules) [12]. Initial studies on RSC provided circumstantial evidence in support of immunocompetence [13, 14, 15], further strengthened when Service and Wardlaw [16] deduced the antibacterial activity of echinochrome A from the edible sea urchin Echinus esculentus. Following this, Gerardi et al. [17] fractionated P. lividus coelomocytes and monitored the bactericidal activity of RSC lysates toward several marine Vibrio species (100% inhibition of bacterial growth was achieved within 12 h). The authors confirmed that RSC immune activity was independent of lysozyme, but the mechanism of inhibition of microbial growth remained unknown. More recently, the levels of RSC in the coelomic fluid of P. lividus have been proposed as a good indicator of environmental stress due to their enhanced presence in coelomic fluid in animals living in waters contaminated with heavy metals and/or xenobiotics [18, 19, 20].
Renewed interest in culturing P. lividus and its continued development as an ecotoxicology model presents a greater need to document the immune-capacity and health status indicators of this commercial shellfish. The overall aim of our study was to assess the putative role of RSC in innate immunity. This was addressed by interrogating (1) the physiological responses of RSC in the presence of microbes, their exoplasmic sugar moieties (ligands), and damage-related signals and (2) the nature of the anti-infective properties of liberated contents of cytoplasmic vesicles, i.e., echinochrome A. Our findings demonstrate a capacity of RSC to respond to pathogen- or damage-associated molecular patterns (PAMP or DAMP; e.g., lipopolysaccharides) by undergoing exocytosis through a mechanism most likely involving Ca2+ influx. The extracellular echinochrome A targets bacteria and yeast in vitro, leading to reductions in colony-forming units (CFU). The broad antimicrobial activity of RSC lysates can be offset by the addition of iron - leading us to surmise that echinochrome A's iron-chelating properties impede microbial colonisation of the sea urchin host.
Materials and Methods
Maintenance of Sea Urchins
P. lividus adults (test diameter: 37.4 ± 1.7 mm) were obtained from the FAI Ardtoe Marine Research Facility, Ardtoe, UK. Psammechinus miliaris adults (test diameter: 32.6 ± 2.7 mm) were collected from coastal waters near Oban and Millport, UK. In the laboratory, sea urchins were maintained in closed circulation tanks (30 individuals per 80 L) between 6°C and 10°C containing a mixture of artificial (Instant Ocean) and filtered seawater, and fed dried kelp ad libitum. Particulates were siphoned daily in addition to 25% of the seawater being exchanged weekly.
Coelomocyte Removal and Preparations
All chemicals and reagents (including microbial ligands and membrane phospholipids) of the highest purity available were purchased from Sigma-Aldrich (Dorset, UK) unless stated otherwise.
Coelomic fluid (up to 5 mL) was extracted from sea urchins using a 26-gauge hypodermic needle attached to a sterile syringe containing an equal volume of pre-chilled anticoagulant (20 mM Tris-HCl, 0.5 M NaCl, and 70 mM EDTA, pH 7.5). Each animal was sprayed on the oral (ventral) surface with 70% ethanol prior to needle insertion through the peristomial membrane. Extracted coelomocytes were enumerated using an improved Neubauer haemocytometer or plastic counting chambers (FastRead counting slides; Immune Systems, Torquay, UK). Further cytology work was performed using an Axiovert 135 inverted microscope.
Continuous 40–60% Percoll gradients were used for cell fractionation. Gradients were prepared in sterile Beckman polyallomer tubes using 4 mL Percoll diluted with an equal volume of 2× anticoagulant (40 mM Tris, 1 M NaCl, and 140 mM EDTA, pH 7.5). The mixture was centrifuged at 30,000 g using a fixed angle rotor (23.5°) for 30 min at 4°C. The coelomic fluid extract and anticoagulant mixture were layered onto gradients and centrifuged at 400 g using a swing-out rotor (JS 24.15) for 15 min at 4°C. Polyallomer tubes were pierced using sterile 26-gauge hypodermic needles and fractions were collected (1 mL) into pyrogen-free, conical tubes containing 4 mL anticoagulant buffer. Samples were further centrifuged for 10 min at 500 g (4°C), the supernatant was discarded, and coelomocytes were re-suspended in 500 μL artificial coelomic fluid (ACF) (10 mM CaCl2, 14 mM KCl, 50 mM MgCl2, 398 mM NaCl, 1.7 mM NaHCO3, 25 mM Na2SO4, and 10 mM HEPES, pH 7.4) [21]. The homogeneity of each fraction was assessed by microscopy - only those populations consisting of >95% RSC were used.
Effect of Microbial and Damage-Related Ligands on Coelomocytes in vitro
Approximately 2.5 × 104 ± 3.9 × 103 isolated RSCs (in 500 μL ACF) were seeded into each well of a 24-well (pyrogen-free) culture plate and left for 30 min at room temperature (≤20°C) to settle before centrifugation at 250 g for 5 min at 4°C with no braking. After centrifugation, microbial ligands ranging in concentration from 15–75 μM (mannan from Saccharomyces cerevisiae, laminarin from Laminaria digitata, lipopolysaccharides from Escherichia coli, and lipoteichoic acids [LTA] from Staphylococcus aureus) and inner membrane phospholipids at 25–50 μM (phosphatidylserine [PS] and phosphatidylethanolamine [PE]) were added to each well and incubated at room temperature for 1 h. Controls, absent ligands, were run concurrently. Cellular activity was recorded by calculating the percentage of RSC that released echinochrome (fully de-granulated). For each well, randomly chosen fields of view were selected until 200–300 cells had been assessed. NB: the viability of extracted (unstimulated) coelomocytes at room temperature (<20°C) was monitored in vitro over a 4-h period using trypan blue exclusion (0.2% w/v, [22]). CSC were selected for this task due to the technical challenges encountered when staining the pigmented RSC. Cells staining blue were recorded as dead.
Overnight cultures of Gram-positive bacteria (Bacillus megaterium and B. subtilis), yeast (S. cerevisiae strain AH22), and Gram-negative bacteria (E. coli strain M15) were used to challenge isolated RSC in vitro. S. cerevisiae was cultured at 30°C in YEPD broth (1% [w/v] yeast extract, 2% [w/v] Bacto peptone, and 2% [w/v] D-glucose, pH 7) and all bacteria were grown at 37°C in lysogeny broth (1% [w/v] Bacto tryptone, 0.5% [w/v] yeast extract, and 1% NaCl, pH 7). Optical density readings at 600 nm were recorded for each microbe using a Novaspec 4049 spectrophotometer. An OD600 value of 1.0 is equal to ∼3 × 107 cells/mL for S. cerevisiae and ∼1.2 × 109 cells/mL for E. coli, B. megaterium, and B. subtilis [22]. Microbial cultures (1 mL) were centrifuged at 1,000 g for 5 min (4°C) and re-suspended in 1 mL PBS (pH 7.4) prior to dilution into pre-prepared culture wells containing RSC (2.5 × 104 ± 3.9 × 103). Sea urchin coelomocytes (suspended in ACF) were incubated in the presence of bacteria (2 × 106 cells/mL) or yeast (1 × 106 cells/mL) for 1 h at room temperature (20°C) and responses to each microbe (i.e., degranulation) were quantified as stated above.
The calcium-specific ionophore ionomycin (Tocris, Avonmouth, UK), was used to test whether RSC are reliant on elevated intracellular levels of Ca2+ for degranulation. RSC were maintained ex vivo in ACF as detailed above, treated with 2, 4, or 10 μM ionomycin (stock solution prepared at 2 mM in DMSO), and observed at 0 and 60 min using microscopy. All images were captured using a ×63 1.2 NA objective on a Zeiss Axiovert 135 microscope attached to an Axiocam MRc camera system and analysed using Zen (Zeiss) and/or ImageJ software. Trypan blue exclusion assays were used to determine the viability of RSC at 2 h post-activation with 10 μM ionomycin.
Additionally, an interspecies comparison between mixed coelomocyte populations extracted from P. lividus and P. miliaris was performed in vitro. Coelomocytes were removed from sea urchins and processed as mentioned above but they were not fractionated. Instead, ∼4 × 105 cells were exposed to 25 μM of each microbial ligand (LPS, LTA, and mannan) or 10 μM ionomycin and left for 30 min at room temperature in sterile 15-mL centrifuge tubes (and agitated gently). Post-incubation, 15 μL of the coelomocyte suspension was assessed for the proportion of intact (pigmented) RSC using brightfield microscopy (×40).
Spectrophotometric Detection of Echinochrome A Release
Sea urchins were challenged with 3 μg LPS per mL of coelomic fluid (∼5 μM) via injection into the coelomic cavity through the peristomial membrane using a 26-gauge hypodermic needle. The amount of LPS injected was standardised using a modified formula presented in Smith et al. [23]: weight of the sea urchin (g) × 0.18 = x mL coelomic fluid. The accuracy of this formula to predict dosages was confirmed by removing all coelomic fluid (exsanguination) from a sub-sample of P. lividus (n = 8). Surfaces were sterilised with 70% ethanol before and after treatment. Control injections consisted of ACF only. At 1 and 24 h post-inoculation, 1 mL coelomic fluid was removed. Differential cell counts were performed using 15 μL coelomic fluid, with the remaining sample volume (∼985 μL) being centrifuged at 10,000 g for 5 min to remove the coelomocytes. The acellular coelomic fluid (i.e., supernatant) was placed in a quartz cuvette (1-cm path length) and absorbance values across the range 300–700 nm were recorded using an Ultrospec 2100 pro UV/Vis spectrophotometer. The effect of immune challenge on the acellular coelomic fluid was monitored via absorbance peaks at 346 and 480 nm, which are indicative of echinochrome A [16, 24].
Antimicrobial Properties of RSC-Derived Echinochrome A
Bacteria and yeast were grown as stated above; 1 mL of each culture was centrifuged, re-suspended in PBS (pH 7.4), and subsequently diluted to 1 × 106 microbes per mL. RSC fractions (≥98% homogenous) were centrifuged at 10,000 g (4°C) for 10 min, re-suspended in 1 mL deionized water, and vortexed to lyse the cells. After lysis, cell debris was pelleted using centrifugation (4,000 g for 5 min at 4°C) and the supernatant was retained on ice. Three assays were prepared for each microbe: (1) microbes alone (negative control), (2) microbes treated with 100 µL RSC lysate (1 × 105), or (3) 50 mM EDTA (positive control). Sub-samples of RSC-lysates were spread onto agar to check for potential contamination.
After microbes were incubated at room temperature for 1 h, samples were diluted in PBS so that ∼200 CFU were plated onto pre-prepared 2% agar (in YEPD for yeast and LB for bacteria). Two types of agar recipes were used for each treatment: one containing regular medium and another supplemented with 0.05% (w/v) ferric ammonium citrate (FAC; Fe3+). FAC was selected as this form of iron is more readily available for microbes to utilise (in addition to being a hematinic). The inoculated plates were incubated for 24–48 h (S. cerevisiae at 30°C and E. coli, B. megaterium, and B. subtilis at 37°C). Absorbance readings of RSC lysates from 300–550 nm were recorded in the absence and presence of 200 μM FAC to assess whether iron formed complexes with echinochrome A.
Data Handling
All data were gathered from experiments performed on at least 3 independent occasions (see individual figure legends for sample sizes) and are represented by mean values with 95% CI. Assays concerning ligands, microbes, and damage signals in vitro were run in triplicate (3 technical replicates per biological sample). Analysis of variance (1- or 2-way) with Tukey's multiple comparisons tests were utilised to assess data for significant differences at p ≤ 0.05. Statistical analyses and figure preparation were carried out using GraphPad Prism v7.
Results
Response of RSC to Immune-Stimulants in vitro
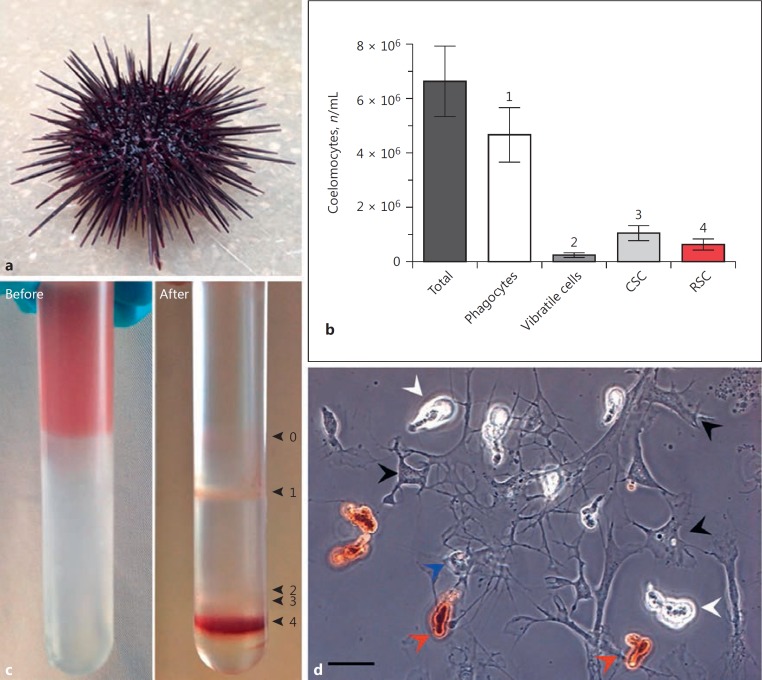
On average, 6.62 × 106 coelomocytes per mL of coelomic fluid were extracted from sea urchin (P. lividus; Fig. 1a) adults, consisting of 70.7% phagocytes (55–84%), 16.1% CSC (7–24%), 9.5% RSC (1.5–25.9%), and 3.7% vibratile cells (<1-10.2%) (Fig. 1b). Fractionation of mixed coelomocyte populations was achieved using 40–60% Percoll gradients. Four cellular bands were observed in addition to a diffuse layer of debris found at the Percoll-coelomic fluid interface (Fig. 1c, d). Bands 1, 2, and 3 consisted mainly of phagocytes and vibratile and colourless spherule cells, respectively. Homogeneity ranged from 82 to >95%. Phagocytes are generally sub-divided into discoidal, polygonal, and small morphotypes, but an enumeration of these sub-types was not within the scope of our experimentation. RSC made-up ≥98% of band 4, with CSC found to be the only contaminant (<2%).
Fig. 1.
Density-dependent fractionation of sea urchin coelomocytes. aP. lividus adult. b Total and differential coelomocytes per millilitre of coelomic fluid (mean ± 95% CI, n = 30). c Continuous Percoll gradient (40–60%) with extracted coelomic fluid, before and after centrifugation. Band 0 consists mainly of cellular debris. Band 1 contains phagocytes; bands 2 and 3 contain vibratile and colourless spherule cells (CSC), respectively; and band 4 is >98% red spherule cells (RSC). d Living coelomocytes removed from P. lividus. The red spherule cells are distinguishable due to the abundance of echinochrome-containing cytosolic vesicles (red arrowheads). Colourless spherule cells, phagocytes, and a vibratile cell are highlighted by white, black, and blue arrowheads, respectively. Scale bar, 20 μm.
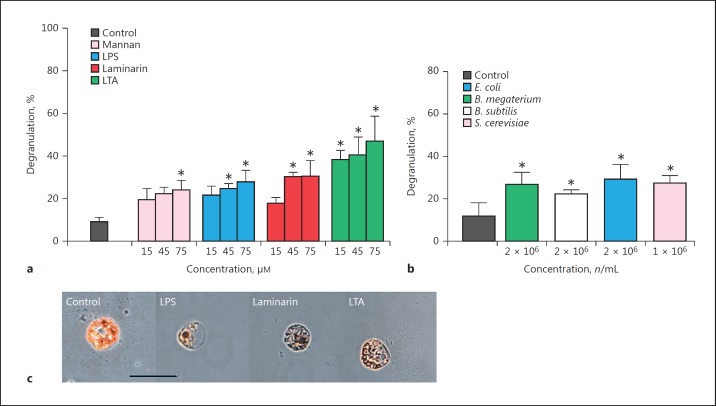
Isolated RSC responded to the presence of various immunostimulants in vitro through the apparent exocytosis of cytoplasmic vesicles containing echinochrome A (Fig. 2, 3, 4). The proportions of cellular degranulation in control samples ranged from 8.8 to 11.5%, whereas treatment of RSC with either microbial ligands (LPS, LTA, mannan, and laminarin), intact microbes (E. coli, B. megaterium, B subtilis, and S. cerevisiae) or inner membrane phospholipids (PS and PE), led to significant increases in echinochrome A release (ligands, F[4, 30] = 65, p < 0.001; microbes, F[4, 10] = 32.27, p < 0.001; and damage, F[2, 12] = 96.59, p < 0.001). LTA from Gram-positive bacteria was the most potent activator of RSC (47%) across the concentration range of 15–75 µM (Fig. 2a). LPS from Gram-negative bacteria and β-glucan (i.e., laminarin) from brown algae were not as effective as LTA at the highest dose tested (75 μM), i.e., 27.8 and 30.4%, respectively, but they were found to be significantly different from the control. On average, the presence of intact microbes led to a significant 2.5-fold increase in the proportion of de-granulated RSC compared to the control (Fig. 2b; E. coli > S. cerevisiae > B. megaterium > B. subtilis). No internalisation of targets (i.e., phagocytosis) was observed in these particular coelomocytes.
Fig. 2.
Degranulation of red spherule cells in response to pathogen-associated molecular patterns. Isolated red spherule cells (RSC) were exposed to increasing concentrations of bacterial (LPS, lipopolysaccharide; LTA, lipoteichoic acid), fungal (mannan), and algal (laminarin, a β-glucan) ligands (a) or, intact microbes (Gram-positive/negative bacteria and yeast) (b) for 1 h in vitro. RSC responses to such challenges were recorded as percentage degranulation. All data are presented as means ± 95% CI; n = 3. An asterisk indicates a significant difference (p < 0.05) between the control and treatment groups. c Images depicting RSC in the absence (control) or presence of an immunostimulant. Scale bar, 10 μm.
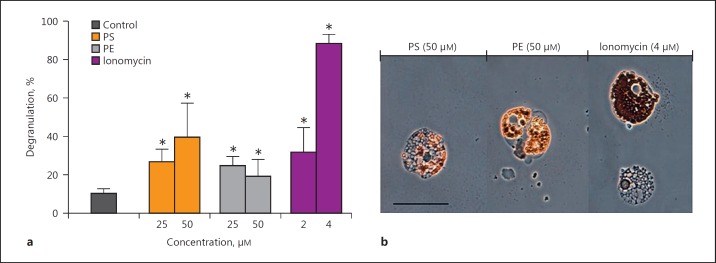
Fig. 3.
Degranulation of red spherule cells in response to damage-associated molecular patterns. a Isolated red spherule cells (RSC) were exposed to inner membrane phospholipids (PS, phosphatidylserine; PE, phosphatidylethanolamine) and the calcium ionophore ionomycin for 1 h in vitro. RSC responses to such challenges were recorded as percentage degranulation. Data are presented as means ± 95% CI; n = 3. An asterisk indicates a significant difference (p < 0.05) between the control and treatment groups. b Images depicting RSC challenged with inner membrane phospholipids (PS, PE) and when intracellular levels of Ca2+ increased due to the presence of an ionophore (ionomycin). Scale bar, 20 μm.
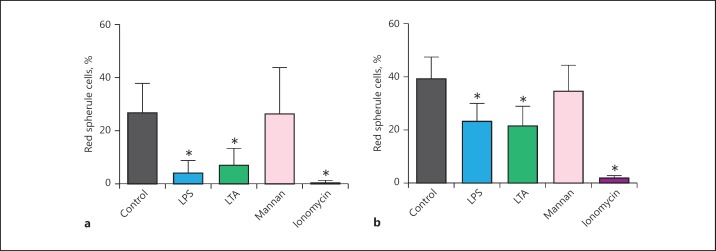
Fig. 4.
Degranulation of red spherule cells in mixed coelomocyte populations from P. lividus (a) and P. miliaris (b). Coelomocytes were extracted into an anticoagulant, pelleted and re-suspended in artificial coelomic fluid, and seeded into wells of a 24-well culture plate without fractionation. Mixed coelomocyte populations were exposed to 25 μM of each microbial ligand (LPS, lipopolysaccharides; LTA, lipoteichoic acids; mannan) and 10 μM ionomycin (positive control). The reduction in red spherule cell numbers due to degranulation was recorded after 1 h. All data are presented as means ± 95% CI; n = 5 (for each species). An asterisk indicates a significant difference (p < 0.05) between the control and treatment groups.
The second most potent inducer of RSC in vitro was the negatively charged phospholipid PS. PS stimulated a 2.5-fold increase in RSC activity compared to the control (10.3%; Fig. 3). A second phospholipid, i.e., PE, was less effective than PS yet it still activated 24.8% of the RSC when applied at the same concentration of 25 µM. Upon doubling the concentration of PS to 50 µM, a reciprocal increase (39.7%) in RSC degranulation was observed; however, this was not the case for PE. Examination of extracted RSC before activation revealed an abundance of refractile, reddish-brown granules (containing echinochrome A) clearly visible within the cytosol (Fig. 1d, 2). Following exposure to PAMP or DAMP, the RSC emptied their cytoplasmic cargo into the surrounding milieu, flattened, and were no longer refractile (Fig. 2, 3b). The extent of the mass exocytosis can be seen in Figure 3b, where vacuole-like compartments occupy the seemingly quiescent RSC.
Role of Calcium in RSC Degranulation
To further interrogate the degranulation process in P. lividus RSC, we employed the Ca2+-specific ionophore ionomycin. Exposure to 2 µM ionomycin led to ∼32% of RSC releasing their granular content in vitro. This proportion increased to ∼90% when the concentration of ionomycin was doubled to 4 µM (Fig. 3), thereby suggesting that an increase in intracellular levels of calcium [Ca2+]i was required for echinochrome release. When coelomocytes were loaded with the Ca2+ chelator BAPTA (20 μM; as the membrane-permeant AM ester) prior to exposure to ionomycin or immune stimulants, there was no release of granular contents (data not shown). A comparison of mixed coelomocyte populations removed from P. lividus and the green sea urchin P. miliaris verified that RSC responded to bacterial cell wall components (LTA and LPS) and ionomycin in a similar manner (Fig. 4). Conversely, RSC in the mixed populations from both species were unresponsive to mannan from S. cerevisiae (p > 0.05). Preliminary experiments to visualise the increase in (Ca2+)i into RSC were performed using the fluorescent indicator Fluo-3 AM (online suppl. Fig. 1; see www.karger.com/doi/10.1159/000484722 for all online suppl. material). Upon addition of 10 µM ionomycin there was a clear increase in fluorescence within vesicular structures and the cytosol, indicative of Ca2+ influx. At such a high concentration of ionomycin, degranulation of echinochrome A was observed in ∼99% of RSC within 60 s. The viability of RSC 2 h after 10 µM ionomycin was >93%. Notably, removal of Ca2+ from the ACF interfered with the activation of RSC despite the presence of immunostimulants (online supp. Fig. 2).
Antimicrobial Activity of RSC Lysates
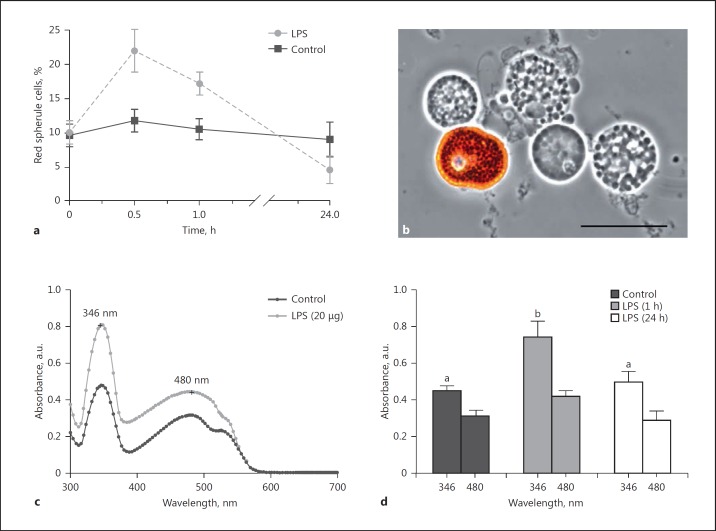
Intra-coelomic injection of LPS (3 µg per mL coelomic fluid) into P. lividus adults led to significant increases in the proportions of circulating RSC within 1 h (p < 0.001) in contrast to coelomocyte numbers from control animals over the same experimental period (p = 0.98) (Fig. 5a, b). RSC increased to 21.9% between 0 and 30 min and then fell to 17.2% at 60 min. Cell numbers correlated inversely with the amount of soluble echinochrome A detected in the coelomic fluid - monitored via absorbance maxima at 346 and 480 nm (Fig. 5c). A hyperchromic effect (2-fold increase) was noted at 346 nm in the coelomic fluid of LPS-stimulated sea urchins within 1 h. LPS caused an initial increase (at 30 min) in the proportion of RSC within the circulating coelomocyte population, which subsequently underwent degranulation (Fig. 5d). The levels of RSC and soluble echinochrome A in challenged sea urchins recovered by 24 h, in line with data from control animals having received an injection of ACF only.
Fig. 5.
Response of red spherule cells to lipopolysaccharides in vivo. a The proportion ofred spherule cells in the free-floating coelomocyte population was determined upon inoculation with 3 μg lipopolysaccharides (LPS) per mL coelomic fluid or artificial coelomic fluid (control). b Living coelomocytes removed from ACF-injected P. lividus. An intact red spherule cell can be seen alongside 4 colourless spherule cells. Scale bar, 20 μm. c Absorbance spectrum of cell-free coelomic fluid from P. lividusat 1 h post-challenge. The observed peaks at 346 and 480 nm are indicative of echinochrome A [see [16], [24]]. The presented spectra are a representation of experiments carried out on 3 independent occasions. d Peak absorbance values for echinochrome were monitored in cell-free coelomic fluid at 1 and 24 h post-injection with LPS. Control values were recorded at 1 h post-injection with ACF. a, d Data are presented as means ± 95% CI; n = 5. Unshared letters indicate significant differences (p < 0.05).
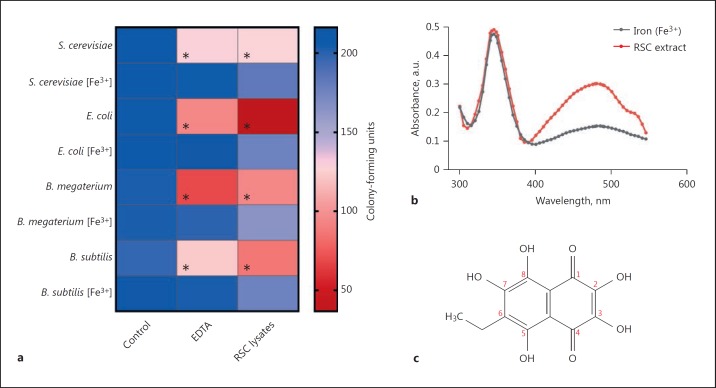
The in vitro antimicrobial activity of RSC lysates was tested against Gram-positive and Gram-negative bacteria as well as yeast. Lysates from 1 × 105 RSC were incubated with 1 × 106 of each microbe for 1 h at room temperature prior to plating ∼200 CFU onto agar with/without FAC. The number of viable microbes (i.e., CFU) decreased significantly to 17.8% for E. coli (p < 0.001), ∼45% for Bacillus sp. (p < 0.001), and 61% for S. cerevisiae (p = 0.003) when compared to untreated (control) microbes (Fig. 6a). CFU recovered to >80% for each treated microbe when grown on agar supplemented with iron (FAC) as opposed to standard agar recipes. Notably, complete recovery of CFU (97.8–104.9%) was achieved when microbes were treated with FAC and RSC lysates simultaneously, prior to plating (online suppl. Fig. 3). Microbes that were exposed to a known antimicrobial iron chelator, i.e., EDTA, displayed similar trends of CFU mortality (Fig. 6a). EDTA-treated microbes recovered to >95% viability when cultured on FAC agar, which was similar to the data for microbes treated with RSC lysates.
Fig. 6.
Antimicrobial activities of red spherule cell lysates from P. lividus. a Gram-positive (B. megaterium and B. subtilis) and Gram-negative (E. coli) bacteria as well as yeast (S. cerevisiae) were incubated in the presence/absence of the iron chelator EDTA or red spherule cell lysates (2.5 × 105) for 1 h at room temperature. Following treatment, microbes were serially diluted in PBS (pH 7.4) so that 200 colony-forming units were plated onto standard agar medium (LB for bacteria and YEPD for yeast) or agar that had been enriched with iron (Fe3+, ferric ammonium citrate). The colour scale on the right indicates the mean number of viable microbes present on agar, i.e., CFU (n = 3). An asterisk indicates a significant difference (p < 0.05) between the control and treatment groups. b Absorbance spectrum of RSC lysate (∼27 μM echinochrome A) in the absence and presence of 200 μM ferric ammonium citrate. The concentration of echinochrome A in 1 × 105 RSC was calculated by following the extraction method developed by Service and Wardlaw [16] and taking into account that there was ∼6.3 × 105 RSC per mL of P. lividus coelomic fluid. c Molecular structure of the 1,4-napthoquinone pigment, echinochrome A.
To test whether RSC lysates (i.e., echinochrome A) inhibited microbial growth via iron deprivation, we studied the spectral properties of lysates incubated with ferric iron (Fe3+). A hypochromic effect was observed in the absorbance spectrum at 480 nm upon incubation with 200 μM FAC for 15 min (Fig. 6b). Additionally, the shoulder peak at ∼525 nm was no longer distinguishable. These results suggested that iron and echinochrome A formed complexes.
Discussion
RSC are often identified near damaged spines, encapsulated bacteria, and infested epidermal tissues of sea urchins [13, 14, 15, 25, 26], yet until now evidence supporting a role for RSC in immunity has been lacking. Due to the distinct morpho-functional properties of echinoid coelomocytes and the convenience of density separation media (Fig. 1), we were able to examine RSC (>98% homogeneity) in vitro. The introduction of immunostimulants (microbes and PAMP) and membrane phospholipids triggers the exocytosis of echinochrome-containing vesicles in up to 50% of RSC (Fig. 2, 3, 4). Direct injection of lipopolysaccharides into the coelom mobilises RSC to release echinochrome A in vivo (Fig. 5). These data indicate RSC recognise “non-self” motifs leading them to undergo morphological and physiological changes associated with enhanced antimicrobial defence (Fig. 6).
The responses of invertebrate immune cells to PAMP and DAMP are well characterised across diverse taxa; however, sea urchin RSC are an exception [9, 10, 23, 27]. When insect and crustacean hemocytes encounter pathogens they release a battery of immune effectors (through exocytosis) to immobilise/entrap the intruders as part of their inflammatory programmes [28]. RSC alone, and in mixed coelomocyte populations from P. lividus and P. miliaris, degranulate when presented with Gram-negative and Gram-positive bacteria as well as yeast (Fig. 2, 4). The inner-membrane phospholipids PS and PE also stimulate echinochrome A release (Fig. 3). PS location is restricted to the cytoplasmic membrane of healthy coelomocytes. Its relocation onto the cell surface is a hallmark of cell death (apoptosis) in metazoans [29], drawing attention to defective (immune) cells and those tissues compromised by pathogens. Surveys of wounded sea urchins consistently find elevated levels of RSC (>40%) in the coelomic fluid compared to “healthy” conspecifics (∼10%) [13, 25, 26, 30]. These animals play host to noxious bacteria, fungi, and algae - organisms that we have proven RSC react to (Fig. 2, 3). Amorphous red materials and friable layers coincide with hemostasis and spine regeneration, hinting that RSC deposit echinochrome A to prevent the loss of coelomic fluid during infection [13, 25, 26, 30]. Such barriers are found in “hanging-drop” preparations of purple (S. purpuratus) and red (Mesocentrotus franciscanus) sea urchin coelomocytes where RSC form “palisades” on the edges of clots and bacterial aggregates [13]. Our data reinforce these early studies and indicate that RSC are recruited to injury sites (Fig. 3, 5), recognise antigens (Fig. 2, 4), and release echinochrome A. Additionally, we present new evidence for echinochrome A having another, more direct role to play in echinoid immune defence (see the next section).
The effects of ligands/microbes/phospholipids can be mimicked by ionomycin, indicating that elevated intracellular Ca2+ is the trigger for echinochrome A release (Fig. 3, 4). Mechanistic aspects of degranulation events and cell-derived immunity in invertebrates (e.g., Drosophila hemocytes) are largely modulated by calcium [31]. We used the Ca2+ indicator dye Fluo-3 to determine if the release of echinochrome A following application of ionomycin correlates with intracellular Ca2+ in RSC. Shortly after the addition of ionomycin there is enhanced Fluo-3 fluorescence, meaning the Ca2+ concentration has indeed increased (online suppl. Fig. 1). The spatial distribution of Fluo-3 fluorescence reveals that an increase in intra-vesicular Ca2+ accompanies the morphological changes of RSC. This accumulation of Ca2+ in cytoplasmic compartments, as well as in the cytosol, is not unheard of [reviewed in [32]]. Firstly, although ionomycin is a Ca2+-specific ionophore, it is relatively non-selective regarding the membranes into which it can insert. Secondly, Ca2+ indicator dyes such as Fluo-3 AM can accumulate in secretory vesicles, especially those that are acidic [32]. These characteristics of ionomycin likely account for the compartmentalised fluorescent signal visible in activated RSC. Our observations on the requirement for external Ca2+ (online suppl. Fig. 2) and the blocking effect of intracellular BAPTA on RSC responsiveness further support a role for Ca2+ in echinochrome A discharge.
By inoculating P. lividus adults with LPS, we have gained rare insight into RSC activities in vivo (Fig. 5). Within 30 min, the proportion of circulating RSC dou- bles - likely due to cells making their way into circulation from neighbouring tissues where they carry out immunesurveillance. This short period of time is not sufficient for haematopoiesis to occur. By 60 min, there is a noticeable drop in RSC numbers but an increase in absorbance at 346 nm, a signal that more cell-free echinochrome A is in the coelomic fluid (Fig. 5d; online suppl. Fig. 4). Activation of invertebrate defences generally leads to an increase in free-floating immune cell numbers and the liberation of bioactive compounds (e.g., antimicrobial peptides and lysozyme) [33]. Likewise, exposure of sea urchin phagocytes to LPS in vitro induces cellular aggregation (reminiscent of encapsulation) and de novo synthesis of SpTransformer proteins (formerly Sp185/333) [9]. Intriguingly, both phagocytes and RSC from the green sea urchin (S. droebachiensis) increase the gene expression of a defensin-like antimicrobial peptide, i.e., strongylocin 2, when exposed to E. coli in vitro [34]. Whether the mRNA is translated into a functional peptide or degraded in the cytoplasm remains to be determined. Nevertheless, if RSC can produce AMP in addition to echinochrome A, then both could be released to combat sepsis.
Echinochrome A Is a Putative Immune Factor in Sea Urchins
The cytoplasmic granules of RSC are replete with the pigment echinochrome A (6-ethyl-2,3,5,7,8-pentahydroxy-1,4-naphthoquinone; Fig. 6c). Service and Wardlaw [16] first assigned antimicrobial properties to echinochrome A using ethanol/acetone extractions of whole coelomocyte lysates from E. esculentus. The antibacterial activity of echinochrome A toward Pseudomonas strain 111 was concentration (20–200 μM) and time (4–48 h) dependent [16]. Following this, lysates of fractionated RSC (1 × 105) from P. lividus were found to be 100% effective at killing marine bacteria (Vibrio species, Photobacterium sp. strain 56) over a 12-h period at 20°C [17]. More recently, extracts of EchinochromeA: SpinochromeC (75:25) from the tests/spines of several tropical sea urchin species were found to be effective at killing E. coli, B. subtilis, and Shewanella oneidensis [35]. An EC50 value of 61 μM has been calculated for the metal-reducing bacterium S. oneidensis. None of these studies investigated the mechanism behind RSC and echinochrome's anti-infective characteristics. That said, lysozyme (muramidase activity) was ruled out as a contributing factor in RSC lysates [16, 17, 35]. Based on our evidence, we argue that the iron-chelating properties of echinochrome A underpin these earlier observations (Fig. 6). By following the protocol of Gerardi et al. [17], RSC lysates inhibited CFU by 82.2% in E. coli, 53.1% in B. megaterium, 56.3% in B. subtilis, and 38.9% in S. cerevisiae after 1 h of incubation at 20°C (Fig. 6; online suppl. Fig. 3). The microbicidal nature of RSC-lysates can be counteracted by plating the treated microbes onto iron-supplemented agar (0.05% FAC). Re-introducing iron reduces CFU mortality to 13.3–19.1%. Also, treating bacteria and yeast with RSC lysates in the presence of Fe3+ prior to plating has no (measurable) negative impact on microbial growth (online suppl. Fig. 3). In its purified form, echinochrome A is a potent antioxidant and metal chelator capable of scavenging oxidising/nitrosative radicals and forming complexes with ferric/ferrous states of iron in a molar ratio of 1:2 (echinochrome:iron) [36]. The addition of iron (≤80 μM FeSO4) alters the absorbance profile of purified echinochrome A (∼40 μM) from S. intermedius[36]; a result comparable to the effects of FAC (200 μM) on RSC lysates containing (∼27 μM) echinochrome A observed here (Fig. 6b). The free ortho-hydroxyl groups and ketol structure of echinochrome A facilitates the chelation of iron (Fig. 6c). Collectively, these data suggest that echinochrome A from RSC lysates gathers unbound iron from the environment, thereby depriving microbes of this essential metal. Iron reintroduction post-treatment does not restore the CFU viability to 100% (online suppl. Fig. 3). This is only achieved when excess iron (Fe3+) is added to RSC lysates during treatment, implying that echinochrome A may also interfere with microbes directly - analogous to the antimicrobial mechanism of synthetic metal chelators like EDTA [37]. Given its hydrophobicity, it is also possible that echinochrome A directly enters microbes and has some intracellular effects.
We postulate that the ability of echinochrome A to switch between oxidised and reduced forms (via hydroxyl groups; Fig. 6c) benefits the sea urchin host through the disarmament of reactive by-products (e.g., H2O2 and ONOO⁻) caused by immune activities, i.e., phagocytosis-associated respiratory burst [38]. The withholding of metals, particularly iron, is an important component of innate immunity in vertebrates and invertebrates alike, because iron is an essential factor for microbial growth and pathogenicity [39]. We demonstrated that LPS activates P. lividus RSC into releasing the metal-binding pigment echinochrome A in vitro and in vivo (Fig. 2, 3, 4, 5). LPS has also been shown to induce the synthesis of 60 stress and immune-related factors within the coelomic fluid of S. purpuratus, notably transferrin and ferritin [40]. These proteins have well-defined roles in immunity and metabolism as they coordinate the detoxification, transport, and storage of iron.
Concluding Remarks
Based on our observations, and after careful consideration of the available literature, we propose a dual functionality of RSC in vivo. First, RSC detect and respond to microbes by releasing echinochrome A to sequester iron from the environment. Second, pathological trauma mobilises RSC to prevent the systemic spread of microbes and deploy echinochrome A to disarm oxidising and nitrosative radicals produced as a consequence of immune vigour. The iron-chelating properties of echinochrome A would serve as a microbial deterrent and its ability to act as a chemical antioxidant would reduce the likelihood of collateral damage.
Disclosure Statement
The authors declare no conflict of interests, financial or otherwise.
Funding Sources
This research was supported financially by the University of Stirling and Swansea University.
Author Contributions
C.J.C. and T.W. designed the research. All authors performed the experiments. C.J.C. collated and analysed the data. C.J.C. prepared this paper with input from T.W.
Supplementary Material
Supplementary data
Acknowledgements
We are grateful to Prof. Andrew F. Rowley (Swansea University) for constructive comments on multiple versions of this paper, and to the 2 anonymous reviewers for their candour.
References
- 1.Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, Terwilliger DP, et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev Biol. 2006;300:349–365. doi: 10.1016/j.ydbio.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 3.Smith LC, Ghosh J, Buckley KM, Clow LA, Dheilly NM, Haug T, et al. Echinoderm immunity. In: Söderhäll K, Invertebrate Immunity, editor. New York: Springer US; 2010. pp. pp 260–301. [Google Scholar]
- 4.Stokes BA, Yadav S, Shokal U, Smith LC, Eleftherianos I. Bacterial and fungal pattern recognition receptors in homologous innate signalling pathways of insects and mammals. Front Microbiol. 2015;6:19. doi: 10.3389/fmicb.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA. Entomopathogenic fungi: new insights into host-pathogen interactions. Adv Genet. 2016;94:307–364. doi: 10.1016/bs.adgen.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Lun CM, Samuel RL, Gillmor SD, Boyd A, Smith LC. The recombinant sea urchin immune effector protein, rSpTransformer-E1, binds to phosphatidic acid and deforms membranes. Front Immunol. 2017;8:481. doi: 10.3389/fimmu.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith LC, Lun CM. The SpTransformer gene family (formerly Sp185/333) in the purple sea urchin and the functional diversity of the anti-pathogen rSpTransformer-E1 protein. Front Immunol. 2017;8:725. doi: 10.3389/fimmu.2017.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross PS, Clow LA, Smith LC. SpC3, the complement homologue from the purple sea urchin, Strongylocentrotus purpuratus, is expressed in two subpopulations of the phagocytic coelomocytes. Immunogenetics. 2000;51:1034–1044. doi: 10.1007/s002510000234. [DOI] [PubMed] [Google Scholar]
- 9.Majeske AJ, Bayne CJ, Smith LC. Aggregation of sea urchin phagocytes is augmented in vitro by lipopolysaccharide. PLoS One. 2013;8:e61419. doi: 10.1371/journal.pone.0061419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero A, Novoa B, Figueras A. Cell mediated immune response of the Mediterranean sea urchin Paracentrotus lividus after PAMPs stimulation. Dev Comp Immunol. 2016;62:29–38. doi: 10.1016/j.dci.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Arizza V, Giaramita FT, Parrinello D, Cammarata M, Parrinello N. Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comp Biochem Physiol A: Mol Integr Physiol. 2007;147:389–394. doi: 10.1016/j.cbpa.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Heatfield BM, Travis DF. Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus. 2. Cell types with spherules. J Morphol. 1975;145:51–71. doi: 10.1002/jmor.1051450104. [DOI] [PubMed] [Google Scholar]
- 13.Johnson PT. The coelomic elements of sea urchins (Strongylocentrotus). 3. In vitro reaction to bacteria. J Invertebr Pathol. 1969;13:42–62. doi: 10.1016/0022-2011(69)90237-7. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PT, Chapman FA. Infection with diatoms and other microorganisms in sea urchin spines (Strongylocentrotus franciscanus) J Invertebr Pathol. 1970;16:268–276. [Google Scholar]
- 15.Coffaro KA, Hinegardner RT. Immune response in the sea urchin Lytechinus pictus. Science. 1977;197:1389–1390. doi: 10.1126/science.331476. [DOI] [PubMed] [Google Scholar]
- 16.Service M, Wardlaw AC. Echinochrome-A as a bactericidal substance in the coelomic fluid of Echinus esculentus (L.) Comp Biochem Physiol B Comp Biochem. 1984;79:161–165. [Google Scholar]
- 17.Gerardi P, Lassegues M, Canicatti C. Cellular distribution of sea urchin antibacterial activity. Biol Cell. 1990;70:153–157. [Google Scholar]
- 18.Matranga V, Pinsino A, Celi M, Bella GD, Natoli A. Impacts of UV-B radiation on short-term cultures of sea urchin coelomocytes. Mar Biol. 2006;149:25–34. [Google Scholar]
- 19.Pinsino A, Della Torre C, Sammarini V, Bonaventura R, Amato E, Matranga V. Sea urchin coelomocytes as a novel cellular biosensor of environmental stress: a field study in the Tremiti Island Marine Protected Area, Southern Adriatic Sea, Italy. Cell Biol Toxicol. 2008;24:541–552. doi: 10.1007/s10565-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 20.Pinsino A, Matranga V. Sea urchin immune cells as sentinels of environmental stress. Dev Comp Immunol. 2015;49:198–205. doi: 10.1016/j.dci.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Brockton V, Henson JH, Raftos DA, Majeske AJ, Kim YO, Smith LC. Localization and diversity of 185/333 proteins from the purple sea urchin - unexpected protein-size range and protein expression in a new coelomocyte type. J Cell Sci. 2008;121:339–348. doi: 10.1242/jcs.012096. [DOI] [PubMed] [Google Scholar]
- 22.Coates CJ, Whalley T, Nairn J. Phagocytic activity of Limulus polyphemus amebocytes in vitro. J Invertebr Pathol. 2012;111:205–210. doi: 10.1016/j.jip.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Smith LC, Britten RJ, Davidson EH. Lipopolysaccharide activates the sea urchin immune system. Dev Comp Immunol. 1995;19:217–224. doi: 10.1016/0145-305x(95)00009-i. [DOI] [PubMed] [Google Scholar]
- 24.Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG. Evaluation of free radical-scavenging activity of sea urchin pigments using HPTLC with post-chromatographic derivatization. Chromatographia. 2013;76:1353–1358. [Google Scholar]
- 25.Pearse JS, Costa DP, Yellin MB, Agegian CR. Localized mass mortality of red sea urchin, Strongylocentrotus franciscanus near Santa Cruz, California. Fish Bull. 1977;75:645–648. [Google Scholar]
- 26.Höbaus E. Coelomocytes in normal and pathologically altered body walls of sea urchins. Echinoderms Present Past. 1980;3:247. [Google Scholar]
- 27.Nair SV, Del Valle H, Gross PS, Terwilliger DP, Smith LC. Macroarray analysis of coelomocyte gene expression in response to LPS in the sea urchin: identification of unexpected immune diversity in an invertebrate. Physiol Genomics. 2005;22:33–47. doi: 10.1152/physiolgenomics.00052.2005. [DOI] [PubMed] [Google Scholar]
- 28.Krautz R, Arefin B, Theopold U. Damage signals in the insect immune response. Frontiers in plant science. 2014;5:342. doi: 10.3389/fpls.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segawa K, Nagata S. An apoptotic “eat me” signal: phosphatidylserine exposure. Trends Cell Biol. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Vevers HG. Pigmentation of the echinoderms. Proceedings 14th Int Congr Zool, Washington. 1963:p 120. [Google Scholar]
- 31.Evans IR, Wood W. Drosophila blood cell chemotaxis. Curr Opin Cell Biol. 2014;30:1–8. doi: 10.1016/j.ceb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan AJ, Davis LC, Galione A. Imaging approaches to measuring lysosomal calcium. Methods Cell Biol. 2015;126:159–195. doi: 10.1016/bs.mcb.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Smith VJ. Immunology of invertebrates: cellular. eLS. 2010 DOI: 10.1002/9780470015902.a0002344.pub2. [Google Scholar]
- 34.Li C, Blencke HM, Haug T, Jørgensen Ø, Stensvåg K. Expression of antimicrobial peptides in coelomocytes and embryos of the green sea urchin (Strongylocentrotus droebachiensis) Dev Comp Immunol. 2014;43:106–113. doi: 10.1016/j.dci.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Brasseur L, Hennebert E, Fievez L, Caulier G, Bureau F, Tafforeau L, et al. The roles of spinochromes in four shallow water tropical sea urchins and their potential as bioactive pharmacological agents. Mar Drugs. 2017;15:179. doi: 10.3390/md15060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebedev AV, Ivanova MV, Levitsky DO. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005;76:863–875. doi: 10.1016/j.lfs.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Kubo I, Lee SH, Ha TJ. Effect of EDTA alone and in combination with polygodial on the growth of Saccharomyces cerevisiae. J Agric Food Chem. 2005;53:1818–1822. doi: 10.1021/jf049363z. [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Matsutani T, Mori K, Nomura T. Phagocytosis and hydrogen peroxide production by phagocytes of the sea urchin Strongylocentrotus nudus. Dev Comp Immunol. 1992;16:287–294. doi: 10.1016/0145-305x(92)90003-u. [DOI] [PubMed] [Google Scholar]
- 39.Ong ST, Ho JZS, Ho B, Ding JL. Iron-withholding strategy in innate immunity. Immunobiology. 2006;211:295–314. doi: 10.1016/j.imbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Dheilly NM, Raftos DA, Haynes PA, Smith LC, Nair SV. Shotgun proteomics of coelomic fluid from the purple sea urchin, Strongylocentrotus purpuratus. Dev Comp Immunol. 2013;40:35–50. doi: 10.1016/j.dci.2013.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data