Abstract
Background
Pseudomonas aeruginosa (PS) infection results in severe morbidity and mortality, especially in immune-deficient populations. Aerobic exercise (AE) modulates the immune system, but its effects on the outcomes of pulmonary PS infection in elderly mice are unknown.
Methods
BALB/c mice (24 weeks old) were randomized to sedentary, exercise (EX), PS, and PS + EX groups for the acute experimental setting, and PS and PS + EX groups for the chronic setting. Low-intensity AE was performed for 5 weeks, 60 min/day; 24 h after the final AE session, mice were inoculated with 5 × 104 colony-forming units (CFU) of PS, and 24 h and 14 days after PS inoculation, mice were studied.
Results
AE inhibited PS colonization (p < 0.001) and lung inflammation (total cells, neutrophils, lymphocytes [p < 0.01] in bronchoalveolar lavage [BAL]), with significant differences in BAL levels of IL-1β (p < 0.001), IL-6 (p < 0.01), CXCL1 (p < 0.001), and TNF-α (p < 0.001), as well as parenchymal neutrophils (p < 0.001). AE increased BAL levels of IL-10 and parenchymal (p < 0.001) and epithelial (p < 0.001) IL-10 expression, while epithelial (p < 0.001) and parenchymal (p < 0.001) NF-κB expression was decreased. AE diminished pulmonary lipid peroxidation (p < 0.001) and increased glutathione peroxidase (p < 0.01). Pre-incubation of BEAS-2B with IL-10 inhibited PS-induced epithelial cell expression of TNF-α (p < 0.05), CD40 (p < 0.01), and dichlorodihydrofluorescein diacetate (p < 0.05).
Conclusions
AE inhibits PS-induced lung inflammation and bacterial colonization in elderly mice, involving IL-10/NF-κB, and redox signaling.
Keywords: Exercise immunology, Pseudomonas, Cytokines, Elderly, Physical training
Introduction
Pseudomonas aeruginosa (PS) is the second most common bacterial cause of both hospital-acquired pneumonia and ventilator-associated pneumonia in the US and worldwide [1]. The incidence of PS-induced pneumonia increases with advancing age [2], as does its associated mortality [3]. This combination makes it a particularly problematic disease in the elderly. In addition, pneumonia caused by PS can also lead to the fatal acute respiratory distress syndrome (ARDS). During the first 24 h after infection, PS induces an intense, early proinflammatory innate immune response, largely characterized by neutrophil infiltration and activation followed by mobilization and directed infiltration of neutrophils into the lungs [4]. Immune senescence, which occurs with advancing age, may contribute to the elderly's increased susceptibility to PS infections [5].
Exercise intensity and duration, the general level of physical fitness, as well as age can directly influence the immune system [6]. Upon bacterial challenge, sedentary, elderly people (age >65 years) and elderly mice (age >18 months) tend to respond with an impaired immune response [7, 8]. In contrast, a decreased bacterial infection rate among physically fit, elderly individuals has been linked to a more competent immune response [9]. For example, moderate aerobic exercise (AE) appears to stimulate a Th1-type cytokine response (IL-2 and IL-12), which may enhance the clearance of pathogens [8, 10]. Likewise, elderly mice that performed moderate AE demonstrated increased antigen-specific IL-2 and IFN-γ production in response to LPS challenge [6, 11, 12]. Moreover, cross-sectional studies indicate that compared to untrained elderly, fit elderly people retain immune function and even demonstrate an enhanced immune response to vaccination [6]. Thus, it is proposed in this study that AE can shift a sedentary, elderly, Th2-type dominant immune response towards a more balanced, competent, bacterial-fighting, Th1-type immune response. Taken together, the link between AE and improved immune function in the elderly appears to be related to the boost in the Th1-type immune response, which occurs as a result of physical training.
Given the susceptibility of the elderly to bacterial pneumonia and the ability of moderate AE to modulate the immune system [12, 13, 14, 15, 16], the present study hypothesized that in PS-induced lung infection in elderly mice, low-intensity AE (running at 50% maximum speed for 60 min, 5 days a week for 5 weeks) increases bacterial clearance accompanied by attenuation of the proinflammatory cytokine and oxidative stress responses.
Materials and Methods
Ethical Approval
This study was approved by the local animal ethics committee (protocol 375/13). Experiments were carried out in accordance with the Declaration of Helsinki in its revised version of 1975 and its amendments of 1983, 1989, and 1996. Animals did not present any alterations in health status, which was monitored 1 week before and during physical training sessions. No mouse died due to training or infection.
Animals and Experimental Groups
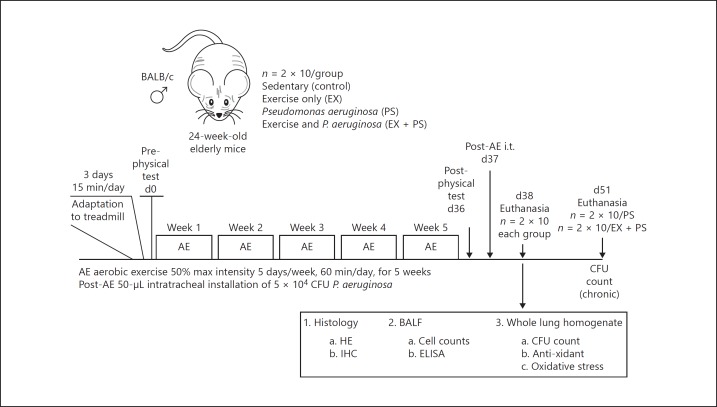
Mice were housed under specific pathogen-free conditions on a 12-h light/dark cycle with free access to food and water. Male elderly BALB/c mice (n = 120; 24 weeks old) were divided into sedentary (control; n = 2 × 10), exercise-only (EX; n = 2 × 10), PS-only (PS; n = 2 × 10) and PS + EX (n = 2 × 10) for the acute experimental setting (evaluation 24 h after PS administration) and PS (n = 2 × 10) and PS + EX (n = 2 × 10) for the chronic experimental setting (evaluation 14 days after PS administration). Adaptation to treadmill training was performed as previously described [17, 18, 19, 20]. Following 3 days of adaptation (15 min/day, 25° incline, 0.2 km/h), animals were submitted to a physical test (beginning at 0.2 km/h, increasing 0.1 km/h every 2.5 min) until animals were exhausted. Exhaustion was defined as failure to run following 10 gentle, mechanical stimuli [17, 18, 19, 20]. Low-intensity AE, defined as 50% maximal speed attained on the treadmill during the first physical test, was performed 4×/week by mice in the EX and PS + EX groups for 5 weeks, 60 min/day. Twenty-four hours before euthanasia, the final physical test was performed [17, 18, 19, 20]. For a complete schematic illustration of the exercise and injury protocol, see Figure 1. For chronic settings, subgroups of PS (n = 2 × 10) and PS + EX subgroups (n = 2 × 10) were maintained for 2 weeks following the administration of PS. Mice in both groups were kept sedentary during this period; 14 days later, mice were euthanized, and CFU were assessed. The experimental protocol is described in Figure 1.
Fig. 1.
The effect of Pseudomonas (PS) and aerobic exercise (AE) on the acute respiratory distress syndrome; 120 male BALB/c elderly mice (24 weeks old) were randomized to the following groups: sedentary controls (control), exercise only (EX), Pseudomonas only (PS), and PS + EX for the acute experimental setting, and PS and PS + EX for the chronic experimental setting. n = 2 × 10/group. Following 3 days of adaptation (15 min/day, 25° incline, 0.2 km/h), animals were submitted to a physical test (beginning at 0.2 km/h, increasing 0.1 km/h every 2.5 min) until animals were exhausted. Low intensity AE was performed for 5 weeks, 60 min/day. Twenty-four hours after the final physical test session, an intratracheal inoculation (i.t.) of 5 × 104 colony forming units (CFU) of PS was administered. Inflammatory parameters were measured 24 h after inoculation. Physical tests were performed before and after AE in all groups. Additionally, 2 groups, PS and PS + EX, were evaluated for CFU 2 weeks after PS inoculation.
PS Administration and Culture for Colony Counting
PS (ATCC 9027) was grown and maintained on nutrient agar (Difco 0003) at 4°C and identified by classic biochemical methods. PS (5 × 104 CFU) were diluted in 50 μL of phosphate-buffered saline (PBS) and administered intratracheally. Animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) 24 h and 2 weeks after PS inoculation. Under anesthesia animals were euthanized, the right lungs were surgically removed and subjected to maceration using a tissue lyser (Roche). A 100-µL solution of the mash was inoculated (1: 10 v/v) and distributed onto a Difco nutrient agar medium with a sterile glass loop. The plates were incubated at 37°C in a bacteriological incubator, and readings were taken after 24 and 48 h, respectively, for colony counting. All colonies were stained by the Gram method and confirmed in the selective Rugai medium.
Functional Measurement of Lung Mechanics
Lung mechanics were determined in anesthetized mice using a volumetric ventilator (MV215; Montevideo, Uruguay). Briefly, mice were anesthetized with a ketamine-xylazine mixture (100 mg/kg-10 mg/kg), tracheotomized, and subjected to conventional ventilation with a quasi-sinusoidal flow pattern with a tidal volume of 10 mL/kg of mouse body weight, a frequency of 100 breaths/min, and a positive end expiratory pressure of 2 cm H2O. Flow and pressure signals from the transducers were analogically low-pass filtered (8 poles, 32 Hz; Butterworth) and were sampled at a rate of 100 Hz (PCI-6036; National Instruments) through custom monitoring and recording application (LabView). Lung resistance and elastance were computed from the signals recorded during mechanical ventilation. In the first step, the volume signal (V) was computed by digital integration of the flow signal (V′). Secondly, the tracheal pressure (Ptr) signal was corrected by subtracting the pressure drop (Pcan) caused by the nonlinear resistance of the intubation cannula, which had been previously calibrated and characterized (Pcan = K1V′ + K2|V′|V′, where K1 and K2 are the linear and nonlinear parameters of the Rohrer model). In a subsequent step, effective lung resistance (RL) and elastance (EL) were computed by linear regression fitting of the recorded signals Ptr, V′, and V to the conventional respiratory mechanics model Ptr = Po + EL × V + RL × V′, where Po is a parameter to account for the external positive end-expiratory pressure applied by the ventilator. For each animal, RL and EL were computed from data obtained during 5 breathing cycles [21, 22].
Bronchoalveolar Lavage
Following the measurement of lung mechanics, still under anesthesia and cannulated, the lungs were washed 3× using 0.5 mL of PBS through the tracheal cannula to collect bronchoalveolar lavage (BAL). Total cell counts were obtained in the BAL samples using a hematocytometer (Neubauer chamber). For differential cell counts, cytospins were prepared by centrifugation at 900 rpm for 5 min and stained using Diff-Quik (Medion Diagnostics, Düdigen, Switzerland). The cells were quantified according to the standard morphological criteria. The BAL cellularity data were expressed as cells/mL–1 [17, 18, 19, 20].
ELISA Measurements
The levels of IL-1β, IL-6, CXCL1, TNF-α, and IL-10 were measured in BAL supernatant by using commercially available ELISA kits according to the manufacturer's instructions: IL-1β (#43601; BioLegend), IL-6 (#431301; BioLegend), CXCL1 (#DY453; R&D), TNF-α (#430901; BioLegend), IL-10 (#431411; BioLegend).
Quantitative Histological Analysis
Paraffin sections (5 μm) were placed on slides and stained with hematoxylin and eosin to quantify the number of neutrophils in the lung parenchyma. Fifteen random parenchymal fields of each slide were imaged at a ×400 magnification using an Olympus BX40 microscope and CellSens software. Neutrophils in the lung parenchyma were counted using Image Pro-Plus 4.0 software [17] according to the standard morphological criteria. Results were expressed as the number of neutrophils per square millimeter of parenchymal tissue.
Quantitative Immunohistochemistry of IL-10 and NF-κB
After BAL and blood collection (1 mL), lungs were removed and submitted to routine histology. Paraffin sections of lung tissue were processed for standard immunohistochemical (IHC) staining using the streptavidin-biotin method and goat polyclonal anti-mouse IL-10 (sc-1783; diluted 1: 500) and goat polyclonal anti-mouse NF-κB (sc-109-G; diluted 1: 800) (Santa Cruz Biotechnology, CA, USA). An ABC Vectastain kit (Vector Elite PK-6105; Vector Laboratories, CA, USA) was used as secondary antibody. Positive reactions were visualized as brown staining following treatment with 3,3-diaminobenzidine (Sigma Chemical Company, St. Louis, MO, USA). Sections were counterstained with Harris hematoxylin solution (Merck, Darmstadt, Germany). IHC images (5 airways and 15 parenchymal fields) from each slide of each mouse from all experimental groups were taken using an Olympus BX40 microscope at ×400 magnification and CellSens software. The percent epithelial area positive for IL-10 and NF-κB as well as the number of positive cells per square millimeter of parenchymal tissue were presented as previously described [17, 18, 19].
Oxidative Stress Evaluations
Superoxide dismutase activity was assessed spectrophotometrically in lung homogenates by means of inhibition of pyrogallol autooxidation at 420 nm [23]. Enzyme activity was reported as U/mg protein (data not shown). Catalase concentration was measured by monitoring the decrease in H2O2 concentration at 240 nm, and the results are reported as pmol of H2O2/mg protein (data not shown) [24]. Glutathione peroxidase (GPx) activity was determined by monitoring NADPH oxidation spectrophotometrically at 340 nm, and the results are reported as nmol/min/mg protein [25]. Lipid peroxidation was measured by the tert-butyl hydroperoxide-initiated chemiluminescence assay, as previously described [26]. The supernatants were diluted in 140 mmol/L KCl and 20 mmol/L phosphate buffer, pH 7.4, and added to glass tubes, which were placed in scintillation vials; 3 mmol/L tert-butyl hydroperoxide were added and chemiluminescence was determined as the maximum level of emission.
In vitro Epithelial Response Assay and Flow Cytometry
Since AE training modulates immune responses in the airway epithelium, particularly by increased IL-10 release [18], we tested the hypothesis that IL-10 can inhibit PS-induced epithelial activation. Thus, to evaluate the role of epithelial cells in the anti-inflammatory effects of IL-10 mediated by exercise, we have cultivated human epithelial cells (BEAS-2B; 5 × 104/2 mL medium) and pre-incubated the cells with human recombinant IL-10 (10 ng/mL) for 1 h prior to incubation with 1 × 104 CFU/mL of medium. The cells were washed with PBS and resuspended in FACS buffer. The activation of BEAS-2B cells was performed through flow cytometry (Accuri C6; BD Biosciences, USA), and BEAS-2B cell expression of the following markers was determined: TNF-α (Pe; BD Biosciences, USA), CD40 (Pe; BD Biosciences, USA), and dichlorodihydrofluorescein diacetate (DCFH). The Cytofix/CytopermTM kit from BD Biosciences was used for intracellular TNF-α staining.
Statistical Analysis
Statistical analysis and graphs were performed using GraphPad Prism 5.0. Nonparametric data were expressed as box-whisker plots showing ranges, medians, and quartile distributions, while parametric data were expressed as bars and error bars representing means ± SE. Comparisons between groups were carried out by one-way analysis of variance (ANOVA) multiple-comparison test, followed by the Holm-Sidak method for parametric data and by ANOVA on ranks followed by the Dunn test for nonparametric data. Differences were considered significant at p < 0.05.
Results
AE Improves Physical Capacity in PS- and Non-PS-Administered Mice
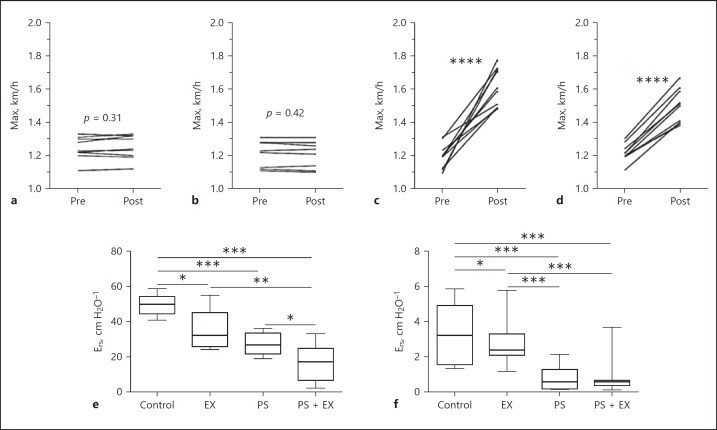
A physical test before and after the AE protocol was performed in all groups. Maximum velocity (km/h) increased significantly in all animals who performed the 5-week AE protocol (p < 0.0001; Fig. 2a–d).
Fig. 2.
Exercise and lung function were tested in sedentary controls (a), Pseudomonas-only (PS; b), exercise-only (EX; c), and PS + EX groups (d). Maximum velocity (Max) was assessed before (Pre) and after (Post) exercise in all mice (n = 2 × 10/group). Animals were submitted to a physical test (beginning at 0.2 km/h, increasing 0.1 km/h every 2.5 min) until animals were exhausted. Exhaustion was defined as failure to run following 10 gentle, mechanical stimuli. **** p < 0.0001. e, f Pulmonary elastance (Ers; e) and resistance (Rrs; f) were measured, and exercise led to a decrease in both in PS and PS + EX groups. * p < 0.05, ** p < 0.01, *** p < 0.001.
AE Fails to Prevent Impaired Lung Mechanics Induced by PS
In the non-PS-administered group, AE slightly reduced both elastance (Ers) and resistance (Rrs) (p < 0.05). PS administration resulted in significant reductions Ers and Rrs (p < 0.001), which was not inhibited by AE, but specifically Ers was even impaired by AE (p < 0.05) (Fig. 2e, f).
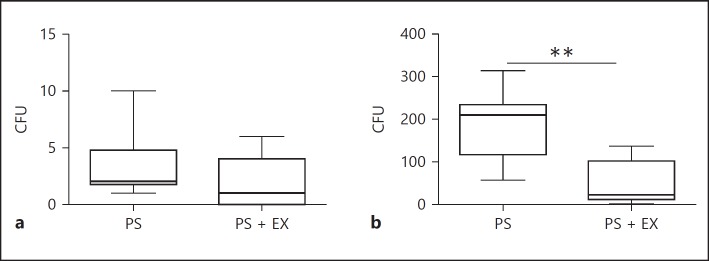
AE Inhibits PS Colonization
A significant PS colonization was not observed in the acute experimental setting (Fig. 3a). AE significantly inhibited PS colonization in the chronic experimental setting (14 days after PS inoculation) (p < 0.001; Fig. 3b).
Fig. 3.
CFU levels in Pseudomonas-only (PS) and PS + exercise (EX) in the lung 24 h (acute; a) and 2 weeks after PS inoculation (chronic; b). n = 2 × 10/group. ** p < 0.01.
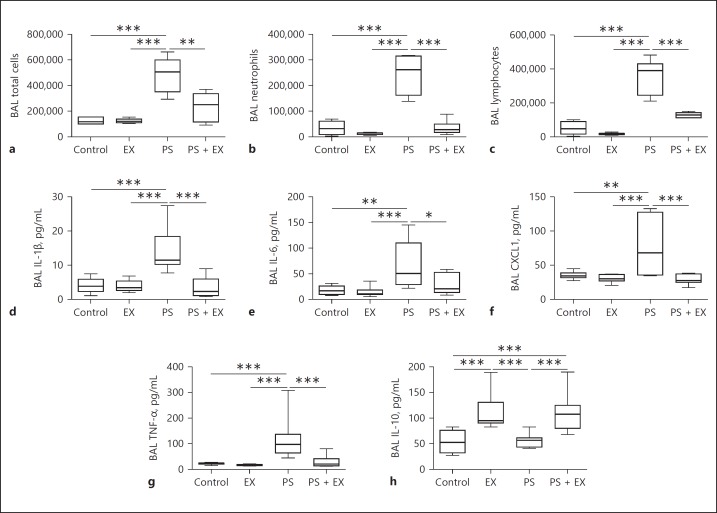
AE Inhibits Pulmonary Inflammation
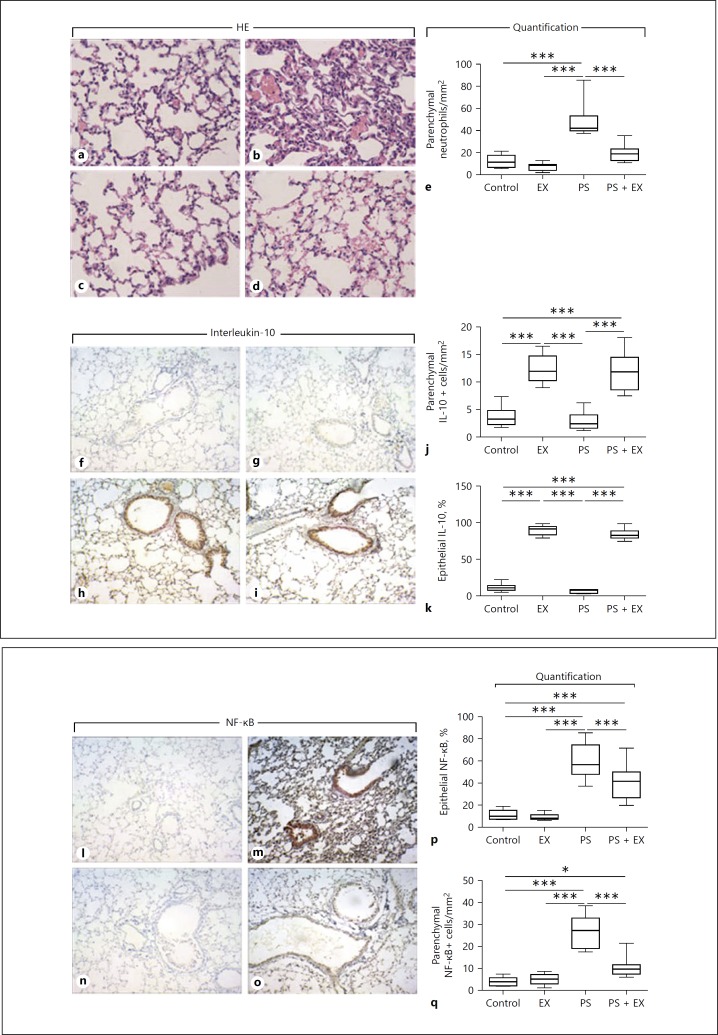
AE significantly inhibited the accumulation of total cells (p < 0.01; Fig. 4a), neutrophils (p < 0.001; Fig. 4b), and lymphocytes (p < 0.001; Fig. 4c) in BAL. Interestingly, not only inflammatory cells decreased, AE also significantly reduced the levels of proinflammatory cytokines IL-1β (p < 0.001; Fig. 4d), IL-6 (p < 0.05; Fig. 4e), CXCL1 (p < 0.001; Fig. 4f), and TNF-α (p < 0.001; Fig. 4g), while increased levels of the anti-inflammatory cytokine IL-10 were observed in EX (p < 0.001; Fig. 4h) and PS + EX (p < 0.001; Fig. 4h) groups. In addition, quantitative histological analysis revealed that PS administration significantly increased neutrophil accumulation in the lung parenchyma compared with control, EX, and PS + EX groups (p < 0.001; Fig. 5a–e), which was reduced by AE (p < 0.001; Fig. 5a–e).
Fig. 4.
Cell count and cytokine levels of bronchial alveolar lavage (BAL). Total and differential cell counts and ELISA were performed on BAL fluid isolated from sedentary controls (controls), exercise-only (EX), Pseudomonas-only (PS), and PS + EX groups. n = 2 × 10/group. Total cells (a), neutrophils (b), lymphocytes (c), and the levels of IL-1β (d), IL-6 (e), CXCL1 (f), TNF-α (g), and IL-10 (h) were assessed. * p < 0.05, ** p < 0.01, *** p < 0.001.
Fig. 5.
Quantification and representative IHC of neutrophils, IL-10, and NF-κB. Representative HE staining of control (a), Pseudomonas-only (PS; b), exercise-only (EX; c), and PS + EX groups (d). The density of neutrophils in the lung parenchyma was quantified (e). Representative IL-10 staining of control (f), PS (g), EX (h), and PS + EX (i) is shown. Parenchymal IL-10+ cells (j) and percent of IL-10+ airway epithelial cells were quantified (k). Representative NF-κB staining of control (l), PS (m), EX (n), and PS + EX (o) is depicted. Epithelial (p) and parenchymal NF-κB+ cells were quantified (q). * p < 0.05, and *** p < 0.001.
AE Increases IL-10 and Reduces NF-κB Expression by Parenchymal Leukocytes and Airway Epithelium
In accordance with ELISA data from BAL supernatant (Fig. 4h), IHC analysis of the anti-inflammatory cytokine IL-10 showed increased expression by parenchymal leukocytes (p < 0.001) and airway epithelium (p < 0.001) in mice subjected to exercise (Fig. 5f–k). In addition, AE significantly inhibited PS-induced NF-κB expression by parenchymal leukocytes (p < 0.001) and airway epithelium (p < 0.001) in mice subjected to exercise (Fig. 5l–q).
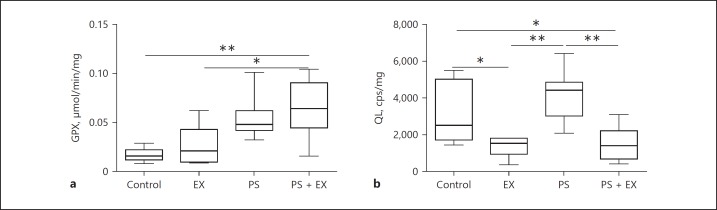
AE Positively Modulates the Oxidant/Antioxidant Imbalance
AE significantly increased GPx levels in PS-administered mice compared to control (p < 0.01; Fig. 6a) and EX groups (p < 0.05; Fig. 6a). AE also inhibited lipid peroxidation measured by the tert-butyl hydroperoxide-initiated chemiluminescence assay, as previously described [23] (Fig. 6b). More specifically, AE reduced lipid peroxidation compared to control (p < 0.05) and PS (p < 0.01) groups, displaying a direct antioxidant effect on PS-induced redox imbalance.
Fig. 6.
Quantification of antioxidant enzyme and lipid peroxidation in sedentary control (control), exercise-only (EX), Pseudomonas-only (PS), and PS + EX groups. n = 2 × 10/group. Measurement for antioxidant activity (GPX) (a) and lipoperoxidation was performed by chemiluminescence reaction initiated by tert-butyl hydroperoxide (T-BOOH) represented as (QL) (b). * p < 0.05, ** p < 0.01.
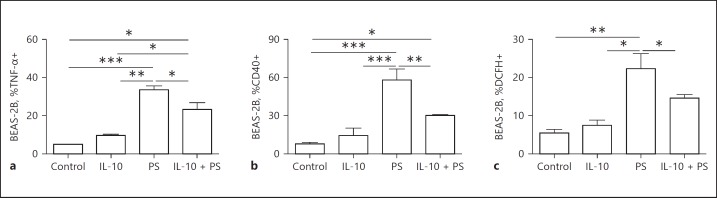
IL-10 Inhibited Airway Epithelial Activation
Since part of the anti-inflammatory effects of AE have been attributed to exercise-induced IL-10 in epithelial cells [18], a translational approach was used using human airway epithelial cells BEAS-2B. The cells were pre-incubated with IL-10 followed by incubation with PS. The results showed that pre-incubation with IL-10 resulted in reduced TNF-α (p < 0.05; Fig. 7a), CD40 (p < 0.01; Fig. 7b), and DCFH (p < 0.05; Fig. 7c) expression induced by PS.
Fig. 7.
IL-10 in airway epithelial cells in face of Pseudomonas (PS) administration. BEAS-2B cells were pre-incubated with IL-10 (10 ng/mL) for 1 h prior PS (1 × 104 CFU/mL) incubation. Flow cytometric analysis shows percentages of TNF-α+ (a), CD40+ (b), and dichlorodihydrofluorescein diacetate (DCHF)+ (c) BEAS-2B cells. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
ARDS is a critical illness characterized by acute lung injury, leading to pulmonary permeability, edema, and respiratory failure [27]. There is no specific therapy, and mortality remains high [28]. The cause of death in patients with ARDS is often due to the underlying causes of ARDS [29]. Sepsis caused by nosocomial lung infections is the most common cause of death among patients who succumb later in their clinical course [30]. A multicenter cohort study comprised of 1,113 ARDS patients who were followed for 15 months found that older patients appear to be at an increased risk for death due to ARDS [31]. Patient mortality ranged from 24% for patients between 15 and 19 years of age up to 60% among patients older than 85 years. While age seems to be a more accurate predictor of ARDS survival, it has also been suggested that obesity may increase the mortality rate of ARDS patients, though evidence is conflicting [32, 33, 34].
Exercise is proven to slow down the lung function decline in chronic obstructive pulmonary disease (COPD) [35], and decrease inflammation in allergic asthma [36, 37, 38]. Early mobilization of critically ill ARDS patients has been shown to attenuate skeletal muscle wasting [39] and likely reduces inflammation [40] as well. Although elderly patients are at higher risk for death due to sepsis during ARDS, whether low-intensity AE (AE) protects against PS-mediated inflammation in the elderly has not been studied to date. Taken together, this study is the first to investigate whether low-intensity AE attenuates the initial inflammatory response to PS in elderly mice.
The low-intensity exercise protocol used in this study resulted in an increase in exercise capacity on the treadmill tested after exercise in the EX group compared to sedentary controls. Though the effect was somewhat attenuated 24 h after PS inoculation (PS + EX), an increase in fitness was still observed despite PS inoculation. In addition, regarding the lung functional response measured through analysis of lung mechanics, 24 h following PS inoculation, PS alone decreased lung elastance and resistance compared to controls. As a result, PS + EX showed even decreased elastance and resistance compared to all groups, indicating that low-intensity exercise could not inhibit lung function impairment due to PS. However, importantly, 2 weeks after inoculation with PS, CFU in the lungs were significantly decreased, suggesting that exercise may enhance pathogen clearance. This very positive effect of AE inhibiting PS colonization could happen due to the initial acute effects of AE, which inhibited PS-induced exacerbation of inflammation, i.e., neutrophil accumulation and hyperactivation, and PS colonization, perhaps preserving the cleaning machinery of the lungs.
While a literature search for studies combining exercise, PS, and elderly animals did not turn up any results, a study performed on adult (nonelderly) rats that exercised daily for 4 weeks [41] showed that animals that exercised were protected against LPS-induced sepsis. Lower basal levels of arterial pressure, heart rate, neutrophil count, and creatinine levels were observed in trained mice compared to controls receiving only LPS [40]. Furthermore, trained mice had a higher blood cell count and pathologically less cardiac, hepatic, and pulmonary injuries. In concordance with this study, decreased levels of inflammatory cells, including neutrophils and lymphocytes, were counted in the BAL fluid of trained mice [40, 41, 42]. Likewise, exercise significantly decreased IL-1β, IL-6, CXCL1, and TNF-α [40, 41, 42, 43]. Among these cytokines, IL-1β, IL-6, and TNF-α, the most promising biomarkers for predicting mortality and morbidity [44], were decreased by exercise. Thus, low-intensity exercise had an important anti-inflammatory effect in elderly mice in this model as it significantly reduced inflammatory cytokine production 24 h following inoculation.
Aging results in low-grade chronic inflammation, which can be damaging to cells and compromise the immune response to bacteria and viruses. Exercise may be capable of reducing the chronic inflammation associated with aging [45]. Macrophages, B cells, dendritic cells, NK cells, and subsets of CD4+ and CD8+ lymphocytes express the anti-inflammatory Th2 cytokine IL-10. IL-10 can inhibit costimulatory molecule expression by dendritic cells and regulate both innate and adaptive immune responses. Unlike the BALB/c strain used in this study, the BALB/c mouse strain is notorious for their exceptionally elevated Th2 response to pathogens [46]. BALB/c mice easily clear low-dosage intranasal PS infections due to their aberrantly raised Th2 cytokine (IL-10) response. While currently no immunogerontological studies exist that profile changes in basal IL-10 levels in a single individual over time, one Swedish study showed that basal levels of IL-10 were not different in healthy individuals with a median age of 40 versus 80 years [47]. However, the plasma immunomodulatory cytokine IL-6 as well as the growth factor TGF-β were significantly increased (p < 0.0001) in the older group [47]. These data suggest that, despite unchanged basal IL-10 levels among the elderly, chronic inflammation may render elderly individuals more susceptible to death by sepsis and indicate the importance of investigating mechanisms that lower inflammation. While corticosteroid treatment was found to have no effect on mortality outcome in ARDS, whether prophylactic or chronic corticosteroid use affects mortality in the context of sepsis and ARDS is unknown [48]. In concordance with many of our group's previous studies, in this study, exercise induced IL-10 expression in the BAL as well as parenchymal and epithelial lung cells, and remained elevated for at least 24 h following inoculation [17, 18, 20, 37, 43, 49]. Thus, the anti-inflammatory cytokine IL-10 is not only significantly elevated by exercise alone, levels also persist following a variety of lung injury models, including allergic asthma [17, 18, 20, 37, 49] and lung fibrosis induced by bleomycin [50, 51], COPD [52, 53], and LPS [43, 54]. Conversely, expression of the master inflammatory regulator NF-κB in lung parenchyma was attenuated by exercise. Future studies should incorporate time point experiments to test how long a single bout of exercise sustains IL-10 expression and how sustained expression is influenced by various exercise protocols and intensities.
In patients with ARDS, the antioxidative system is severely compromised. Oxidative stress is thought to be initiated by activated lung macrophages and the products of infiltrated neutrophils that signal to epithelial and endothelial cells, which produce free radicals in response. While a variety of antioxidants has been tested to treat sepsis-induced ARDS in both animal models and patients, whether antioxidants are truly beneficial remains inconclusive [55]. Nonetheless, this study analyzed the ability of low-intensity exercise to modulate the oxidative stress response to PS, which was attenuated by exercise. Similar antioxidant effects in the present study were observed in a model of LPS-induced acute lung injury [43, 54], reinforcing the antioxidant capability of exercise in the context of pulmonary injury.
In addition, AE modulates several aspects of airway epithelial responses, which have been studied in asthma [18] and COPD [56]. In the present study, we demonstrated for the first time that AE induces IL-10 synthesis by pulmonary leukocytes and airway epithelial cells in mice submitted to PS infection, while leukocyte and epithelial NF-kB expression was reduced. Also, we demonstrated that IL-10 incubation was able to inhibit human bronchial epithelial cell (BEAS-2B) hyperactivation, showing a functional role for exercise-derived IL-10 in the face of PS infection. The importance of the airway epithelium as first-line defense against PS is clearly demonstrated and clinically relevant [57, 58]. Here, it was demonstrated that BEAS-2B pre-incubated with IL-10 presented reduced expression of TNF-α, DCFH (a marker of oxidative stress), as well as CD40, indicating that IL-10 may inhibit epithelial damage induced by PS.
Taken together, this is the first study in the literature to provide evidence supporting the beneficial effect of low-intensity exercise on elderly animal's immune responses to acute and chronic pulmonary PS infection: inhibition of inflammation, exacerbation of Th1 immune acute-phase cytokines and oxidative responses, and bacterial colonization, but not impaired lung mechanics.
Disclosure Statement
The authors declare to have no conflicts of interests.
Acknowledgments
This study was supported by the São Paulo Research Foundation (FAPESP; grant Nos. 2012/15165-2, 2012/16498-5, and 2016/08280-0). T.S.D. holds a scientific initiation fellowship from FAPESP (2014/06534-0). F.M.A. holds a FAPESP doctoral fellowship (2012/23305-9). M.C.O.-J. holds a doctoral fellowship with FAPESP (2014/14604-8). B.M. holds a FAPESP postdoctoral fellowship (2014/23196-0). The opinions, hypotheses, conclusions, and recommendations expressed in this paper are the responsibility of the authors and not necessarily reflect the vision of FAPESP.
References
- 1.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51((suppl 1)):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 2.Venier AG, Gruson D, Lavigne T, et al. Identifying new risk factors for Pseudomonas aeruginosa pneumonia in intensive care units: experience of the French national surveillance, REA-RAISIN. J Hosp Infect. 2011;79:44–48. doi: 10.1016/j.jhin.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Tumbarello M, De Pascale G, Trecarichi EM, et al. Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med. 2013;39:682–692. doi: 10.1007/s00134-013-2828-9. [DOI] [PubMed] [Google Scholar]
- 4.McConnell KW, McDunn JE, Clark AT, et al. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;38:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plackett TP, Boehmer ED, Faunce DE. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 6.Malm C. Exercise immunology: the current state of man and mouse. Sport Med. 2004;34:555–566. doi: 10.2165/00007256-200434090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94:1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. 2001;86:105–111. doi: 10.1007/s004210100521. [DOI] [PubMed] [Google Scholar]
- 9.Nieman DC, Henson DA, Gusewitch G, et al. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen BK, Steensberg A, Fischer C, Keller C, Ostrowski K, Schjerling P. Exercise and cytokines with particular focus on muscle-derived IL-6. Exerc Immunol Rev. 2001;7:18–31. [PubMed] [Google Scholar]
- 11.Gomez CR, Hirano S, Cutro BT, et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 12.Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age-related differences in the neutrophil response to pulmonary Pseudomonas infection. Exp Gerontol. 2014;54:42–46. doi: 10.1016/j.exger.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieman D, Henson D, Gusewitch G, et al. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Akimoto T, Kumai Y, Akama T, et al. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37:76–79. doi: 10.1136/bjsm.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu K, Kimura F, Akimoto T, et al. Effect of moderate exercise training on T-helper cell subpopulations in elderly people. Exerc Immunol Rev. 2008;14:24–37. [PubMed] [Google Scholar]
- 16.Drela N, Kozdron E, Szczypiorski P. Moderate exercise may attenuate some aspects of immunosenescence. BMC Geriatr. 2004;4:8. doi: 10.1186/1471-2318-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira RP, de Andrade VF, Duarte ACS, et al. Aerobic conditioning and allergic pulmonary inflammation in mice. II. Effects on lung vascular and parenchymal inflammation and remodeling. Am J Physiol Lung Cell Mol Physiol. 2008;295:L670–L679. doi: 10.1152/ajplung.00465.2007. [DOI] [PubMed] [Google Scholar]
- 18.Vieira RP, Toledo AC, Ferreira SC, Santos AB, Medeiros MC, Hage M, Mauad T, Martins Mde A, Dolhnikoff M, Carvalho CR. Airway epithelium mediates the anti-inflammatory effects of exercise on asthma. Respir Physiol Neurobiol. 2011;175:383–389. doi: 10.1016/j.resp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Alberca-Custódio RW, Greiffo FR, MacKenzie B, Oliveira-Junior MC, Andrade-Sousa AS, Graudenz GS, Santos AB, Damaceno-Rodrigues NR, Castro-Faria-Neto HC, Arantes-Costa FM, Martins Mde A, Abbasi A, Lin CJ, Idzko M, Ligeiro Oliveira AP, Northoff H, Vieira RP. Aerobic exercise reduces asthma phenotype by modulation of the leukotriene pathway. Front Immunol. 2016;7:237. doi: 10.3389/fimmu.2016.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira RP, Claudino RC, Duarte ACS, et al. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176:871–877. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 21.Nonaka PN, Amorim CF, Paneque Peres AC, et al. Pulmonary mechanic and lung histology injury induced by Crotalus durissus terrificus snake venom. Toxicon. 2008;51:1158–1166. doi: 10.1016/j.toxicon.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Paneque Peres AC, Nonaka PN, de Carvalho Pde T, Toyama MH, Silva CA, Vieira RP, Dolhnikoff M, Zamuner SR, de Oliveira LV. Effects of Tityus serrulatus scorpion venom on lung mechanics and inflammation in mice. Toxicon. 2009;53:779–785. doi: 10.1016/j.toxicon.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Marklund SL, Oreland L, Perdahl E, Winblad B. Superoxide dismutase activity in brains from chronic alcoholics. Drug Alcohol Depend. 1983;12:209–215. doi: 10.1016/0376-8716(83)90062-5. [DOI] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez Flecha B, Llesuy S, Boveris A. Hydroperoxide-initiated chemiluminescence: an assay for oxidative stress in biopsies of heart, liver, and muscle. Free Radic Biol Med. 1991;10:93–100. doi: 10.1016/0891-5849(91)90002-k. [DOI] [PubMed] [Google Scholar]
- 27.Ashbaugh D, Boyd Bigelow D, Petty T, Levine B. Acute respiratory distress in adults. Lancet. 1967;290:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 28.Petty TL, Ashbaugh DG. The adult respiratory distress syndrome: clinical features, factors influencing prognosis and principles of management. Chest. 1971;60:233–239. doi: 10.1378/chest.60.3.233. [DOI] [PubMed] [Google Scholar]
- 29.Papiris SA, Manali ED, Kolilekas L, et al. Clinical review: idiopathic pulmonary fibrosis acute exacerbations - unravelling Ariadne's thread. Crit Care. 2010;14:246. doi: 10.1186/cc9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 32.Dossett LA, Heffernan D, Lightfoot M, et al. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138:568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memtsoudis SG, Bombardieri AM, Ma Y, Walz JM, Chiu YL, Mazumdar M. Mortality of patients with respiratory insufficiency and adult respiratory distress syndrome after surgery: the obesity paradox. J Intensive Care Med. 2012;27:306–311. doi: 10.1177/0885066611411410. [DOI] [PubMed] [Google Scholar]
- 35.Ricci C, Terzoni S, Gaeta M, Sorgente A, Destrebecq A, Gigliotti F. Physical training and noninvasive ventilation in COPD patients: a meta-analysis. Respir Care. 2013;59:709–717. doi: 10.4187/respcare.02626. [DOI] [PubMed] [Google Scholar]
- 36.Radom-Aizik S, Zaldivar F, Leu S-Y, Galassetti P, Cooper DM. Effects of 30 min of aerobic exercise on gene expression in human neutrophils. J Appl Physiol. 2008;104:236–243. doi: 10.1152/japplphysiol.00872.2007. [DOI] [PubMed] [Google Scholar]
- 37.Vieira RP, Silva RA, Oliveira-Junior MC, et al. Exercise deactivates leukocytes in asthma. Int J Sports Med. 2014;35:629–635. doi: 10.1055/s-0033-1358477. [DOI] [PubMed] [Google Scholar]
- 38.Vieira RP, Duarte ACS, Claudino RC, et al. Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol Biol. 2007;37:660–667. doi: 10.1165/rcmb.2007-0108OC. [DOI] [PubMed] [Google Scholar]
- 39.Walsh CJ, Batt J, Herridge MS, Dos Santos CC. Muscle wasting and early mobilization in acute respiratory distress syndrome. Clin Chest Med. 2014;35:811–826. doi: 10.1016/j.ccm.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Files DC, Liu C, Pereyra A, et al. Therapeutic exercise attenuates neutrophilic lung injury and skeletal muscle wasting. Sci Transl Med. 2015;7:278ra32–278ra32. doi: 10.1126/scitranslmed.3010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen HI, Hsieh S-Y, Yang F-L, Hsu YH, Lin C-C. Exercise training attenuates septic responses in conscious rats. Med Sci Sports Exerc. 2007;39:435–442. doi: 10.1249/mss.0b013e31802d11c8. [DOI] [PubMed] [Google Scholar]
- 42.Ramos DS, Olivo CR, Quirino Santos Lopes FD, Toledo AC, Martins MA, Lazo Osório RA, Dolhnikoff M, Ribeiro W, Vieira RP. Low-intensity swimming training partially inhibits lipopolysaccharide-induced acute lung injury. Med Sci Sports Exerc. 2010;42:113–119. doi: 10.1249/MSS.0b013e3181ad1c72. [DOI] [PubMed] [Google Scholar]
- 43.Reis Gonçalves CT, Reis Gonçalves CG, de Almeida FM, Lopes FD, dos Santos Durão AC, dos Santos FA, da Silva LF, Marcourakis T, Castro-Faria-Neto HC, Vieira RP, Dolhnikoff M. Protective effects of aerobic exercise on acute lung injury induced by LPS in mice. Crit Care. 2012;16:R199. doi: 10.1186/cc11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 45.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3:130–140. [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- 47.Forsey RJ, Thompson JM, Ernerudh J, et al. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Chen L, Ni H. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci Rep. 2015;5:17654. doi: 10.1038/srep17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacKenzie B, Andrade-Sousa AS, Oliveira-Junior MC, et al. Dendritic cells are involved in the effects of exercise in a model of asthma. Med Sci Sports Exerc. 2016;48:1459–1467. doi: 10.1249/MSS.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 50.Pereira PR, Oliveira-Junior MC, Mackenzie B, Chiovatto JE, Matos Y, Greiffo FR, Rigonato-Oliveira NC, Brugemman TR, Delle H, Idzko M, Albertini R, Ligeiro Oliveira AP, Damaceno-Rodrigues NR, Caldini EG, Fernandez IE, Castro-Faria-Neto HC, Dolhnikoff M, Eickelberg O, Vieira RP. Exercise reduces lung fibrosis involving serotonin/Akt signaling. Med Sci Sports Exerc. 2016;48:1276–1284. doi: 10.1249/MSS.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 51.Andrade-Sousa AS, Rogério Pereira P, MacKenzie B, Oliveira-Junior MC, Assumpção-Neto E, Brandão-Rangel MA, Damaceno-Rodrigues NR, Garcia Caldini E, Velosa AP, Teodoro WR, Ligeiro de Oliveira AP, Dolhnikoff M, Eickelberg O, Vieira RP. Aerobic exercise attenuated bleomycin-induced lung fibrosis in Th2-dominant mice. PLoS One. 2016;11:e0163420. doi: 10.1371/journal.pone.0163420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toledo AC, Magalhaes RM, Hizume DC, et al. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Respir J. 2012;39:254–264. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 53.Toledo-Arruda AC, Vieira RP, Guarnier FA, Suehiro CL, Caleman-Neto A, Olivo CR, Arantes PMM, Almeida FM, Lopes FDTQS, Ramos EMC, Cecchini R, Lin CJ, Martins MA. Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD. J Appl Physiol (1985) 2017;123:674–683. doi: 10.1152/japplphysiol.00819.2016. [DOI] [PubMed] [Google Scholar]
- 54.Rigonato-Oliveira NC, MacKenzie B, Bachi ALL, Oliveira-Junior MC, Santos-Dias A, Andrade-Sousa AS, Delle H, Assumpção-Neto E, Damaceno-Rodrigues NR, Dulley LH, Benetti MA, Malfitano C, de Angelis K, Albertini R, Oliveira APL, Abbasi A, Northoff H, Vieira RP. Aerobic exercise inhibits acute lung injury: from mouse to human evidence. Exerc Immunol Rev. 2018;28:36–44. [PubMed] [Google Scholar]
- 55.Guo R-F, Ward PA. Role of oxidants in lung injury during sepsis. Antioxid Redox Signal. 2007;9:1991–2002. doi: 10.1089/ars.2007.1785. [DOI] [PubMed] [Google Scholar]
- 56.Brandão-Rangel MAR, Bachi ALL, Oliveira-Junior MC, Abbasi A, Silva-Renno A, Britto AA, Oliveira APL, Toledo-Arruda AC, Belvisi MG, Vieira RP. Exercise inhibits the effects of smoke-induced COPD involving modulation of STAT-3. Oxid Med Cell Longev. 2017;2017:6572714. doi: 10.1155/2017/6572714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Curran CS, Bolig T, Torabi-Parizi P. Mechanisms and targeted therapies for Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med. 2018;197:708–727. doi: 10.1164/rccm.201705-1043SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patkee WR, Carr G, Baker EH, Baines DL, Garnett JP. Metformin prevents the effects of Pseudomonas aeruginosa on airway epithelial tight junctions and restricts hyperglycaemia-induced bacterial growth. J Cell Mol Med. 2016;20:758–764. doi: 10.1111/jcmm.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]