Abstract
Mycobacterium tuberculosis (Mtb) infection remains a major public health concern. The STING (stimulator of interferon genes) pathway contributes to the cytosolic surveillance of host cells. Most studies on the role of STING activation in Mtb infection have focused on macrophages. Moreover, a detailed investigation of the role of STING during Mtb infection in vivo is required. Here, we deciphered the involvement of STING in the activation of dendritic cells (DCs) and the host response to Mtb infection in vivo. In DCs, this adaptor molecule was important for Ifn-β expression and IL-12 production as well as for the surface expression of the activation markers CD40 and CD86. We also documented that Mtb DNA induces STING activation in murine fibroblasts. In vivo Mtb aerogenic infection induced the upregulation of the STING and cGAS (cyclic GMP-AMP synthase) genes, and Ifn-β pulmonary expression was dependent on both sensors. However, mice deficient for STING or cGAS presented a similar outcome to wild-type controls, with no major alterations in body weight gain, bacterial burden, or survival. Lung inflammation, proinflammatory cytokine production, and inflammatory cell recruitment were similar in STING- and cGAS-deficient mice compared to wild-type controls. In summary, although the STING pathway seems to be crucial for DC activation during Mtb infection, it is dispensable for host protection in vivo.
Keywords: STING, cGAS, Mycobacterium tuberculosis, Dendritic cells
Introduction
Innate immunity is the first line of defense responsible for sensing and initiating the fight against pathogens. The protein STING (stimulator of interferon genes) is an important member of the cytosolic surveillance system [1]. STING is an endoplasmic reticulum adaptor protein able to sense cyclic dinucleotide (CDN) metabolites produced by pathogens, such as c-di-AMP, c-di-GMP, and 3′3′cGAMP [2]. Additionally, upon DNA sensing by cGAS (cyclic GMP-AMP synthase), 2′3′cGAMP (from now on referred to as cGAMP) is generated, also activating STING [3]. Other cytosolic sensors such as IFI16 and DDX41 have been reported to engage the STING pathway, although their relevance remains elusive [4, 5]. The binding of CDNs on STING induces conformational changes, culminating in the activation of TANK-binding kinase I (TBK1) and interferon regulator factor 3 (IRF3), and migration from the ER [1]. This pathway induces the expression of type I interferons (IFNs) and IFN-stimulated genes [1]. Additionally, STING can activate the STAT6 and NF-κB pathways [6, 7] and contribute to autophagy triggering [8].
The protein STING has been found to influence several infectious diseases and autoimmune disorders [9, 10, 11]. Concerning bacterial infections, STING activation has a wide potential since these pathogens carry both DNA and CDNs; the latter play a prominent role in bacterial metabolism, e.g., virulence regulation and biofilm formation [12]. Surprisingly, the involvement of STING during bacterial infections is not limited to obligatory intracellular or secretion system harboring pathogens. Furthermore, although several bacteria can activate STING signaling, the outcomes are diverse, ranging from necessary for immunity from Streptococcus pneumoniae, Francisella tularensis, or Chlamydia trachomatis, or promoting bacterial survival in Neiseria gonorrhoeae infection (review [13]).
The Mycobacterium genus comprises bacilli of great relevance for human and animal health. Among these bacteria, Mycobacterium tuberculosis (Mtb) stands out as a human-restricted infectious agent that leads to the establishment of tuberculosis, a major cause of morbidity and mortality worldwide [14]. Mtb possesses a type VII secretion system (ESX-1) that induces phagosome permeabilization, which allows mycobacterial DNA and other effectors to gain access to the host cytoplasm [15]. The bacterial DNA can be sensed by cGAS or IFI16, leading to STING engagement [15]. Similarly, mycobacterial c-di-AMP can activate STING; however, the deletion of di-guanylate cyclase (responsible for c-di-GMP production) seems to have no effect on infection [15, 16]. Upon STING activation, IFN-β production and autophagy induction are detected [8, 15, 16, 17, 18, 19]. Most of these findings were obtained by experiments with macrophages, a main cellular reservoir during mycobacterial infection. However, STING activation in response to Mtb may also take place in other innate immune cells, such as dendritic cells (DCs). Together with macrophages, DCs play a prominent role during Mtb, being responsible for orchestrating the establishment of the immune response. Additionally, to date, there has been no detailed investigation on the role of STING during infection in vivo.
In this study, we addressed the involvement of STING in DC activation and during Mtb infection in vivo. We report here that STING is important for DC activation in response to Mtb DNA, upregulating Ifn-β expression, proinflammatory IL-12 and CXCL10 release, or CD86 and CD40 surface expression. Indeed, in vitro, Mtb DNA induced STING intracytoplasmic aggregation. In vivo, Mtb infection led to the upregulation of STING and cGAS gene expression, and both STING and cGAS are critical for pulmonary Ifn-β expression. However, the clinical outcomes in mice lacking STING or cGAS were similar to in wild-type (WT) mice, with no major alterations in survival, body weight, bacterial burden, or lung inflammation 1–3 months after infection. Thus, although the STING pathway seems to be important for DC activation during Mtb infection, it is dispensable for host protective immunity in vivo.
Materials and Methods
Mice
We used C57BL/6 mice (WT) obtained from Janvier Labs (Le Genest-Saint-Isle, France) or from the Universidade Federal de Minas Gerais (UFMG) Animal Facility (Brazil). Mice deficient for STING (STING–/–) [20] or cGAS (cGAS–/–) [21] were provided by Dr. G. Barber (University of Miami). Mice deficient for the IFNAR1 subunit of the type I IFN receptor (IFNAR–/–) [22], the IFN-γ receptor (IFNGR1–/–) [23], TNFα (TNFα–/–) [24], or the adaptor molecule MyD88 (MyD88–/–) [25] were bred in the Transgenose Institute animal facility (TAAM UPS44 CNRS, Orleans, France). All mice were housed in specific pathogen-free animal facilities at CNRS (France) or at the UFMG (Brazil). After infection, male and female mice (8–12 weeks old) were kept in isolators in a biohazard animal unit. The infected mice were monitored daily for clinical status and were weighed twice weekly. All animal experiments complied with the French Government's animal experiment regulations, and were approved by the Ethics Committee for Animal Experimentation of the CNRS Campus Orleans (No. CLE CCO 2015-1071).
DNA Extraction
Mtb H37Rv (3 × 108 bacteria/mL) were heat-killed at 90°C for 30 min and incubated with lysozyme solution (10 mg/mL) at 37°C for at least 12 h. SDS 10% and proteinase K (10 mg/mL) were added to the samples, vortexed and kept at 65°C for 10 min. NaCl 5 M was added and mixed gently. A solution of cetyl-trimethyl-ammonium-bromide (CTAB) 0.3 M/NaCl 0.5 M was added, and the sample was kept at 65°C for 10 min. After this, a solution of chloroform/isoamyl alcohol 24/1 was added, and the mixture centrifuged for 5 min 12,000 rpm at room temperature. The supernatant was collected and mixed with isopropanol for DNA precipitation. The solution was centrifuged for 15 min at 12,000 rpm, the pellet washed with cold ethanol 70% and, when dry, suspended in TE buffer. The samples were quantified and kept at −20°C.
Confocal Microscopy Analysis
Mouse embryonic fibroblasts (MEFs) from WT or STING–/– mice were grown on 12-mm glass coverslips in 24-well plates. Stimulation with Mtb DNA (1 µg/mL) or dsDNA90 (3 µg/mL) in a complex with Lipofectamine (2.5 µl/mL; Invitrogen, Carlsbad, CA, USA) was performed. After 4 h of incubation at 37°C and 5% CO2, the coverslips were washed twice with PBS and fixed in 4% paraformaldehyde, pH 7.4, at 37°C for 30 min. For staining, cells were permeabilized with PBS containing 0.3% Triton X-100 for 30 min, and subsequently blocked for 1 h with 10% BSA in PBS at room temperature. After this, the coverslips were incubated with rabbit polyclonal anti-STING primary antibody (1: 50; as described previously in [26]) at 4°C overnight. The coverslips were then washed and incubated with anti-rabbit secondary antibody conjugated with Alexa Fluor 546 (1: 500; Invitrogen) for 1.5 h at room temperature, and mounted on slides using ProLong Gold with DAPI mounting medium (Invitrogen). The slides were examined in a Nikon C2 confocal microscope using a ×60 oil objective (3× digital zoom) and the images were analyzed in ImageJ software (Bethesda, Maryland).
Bone Marrow-Derived Dendritic Cells
To obtain bone marrow-derived DCs (BMDCs), bone marrow cells were cultured in DMEM with 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 20 ng/mL murine recombinant GM-CSF (Peprotech, Ribeirão Preto, SP, Brazil). Petri dishes containing 1 × 107 cells were incubated at 37°C in 5% CO2. At day 3 of incubation, a further 5 mL of fresh complete medium with GM-CSF was added, and 5 mL of medium was replaced with fresh supplemented medium containing GM-CSF on days 5 and 7. At day 10, nonadherent cells were harvested and seeded in 96-well plates (1 × 105 cells/well) for cytokine and cytometry analysis, or 24-well plates (5 × 105 cells/well) for RNA expression measurement.
BMDC Activation and Cytokine Release Measurements
Stimulation of BMDCs was performed by adding supplemented DMEM with Lipofectamine (2.5 µL/mL; Invitrogen), cGAMP (6 µg/mL; Invivogen, San Diego, CA, USA), Mtb DNA (1 µg/mL) or Mtb (MOI 3: 1). Lipofectamine was used in conjunction with cGAMP and Mtb DNA to deliver the stimuli into the cytoplasm of the cells. Culture supernatants were collected after 24 h of stimulation (37°C, 5% CO2) and assayed for the concentrations of CXCL10 (IP-10) or IL-12/23p40 by ELISA (R&D Systems, Abingdon, UK) according to the manufacturer's instructions.
Flow Cytometry Analysis
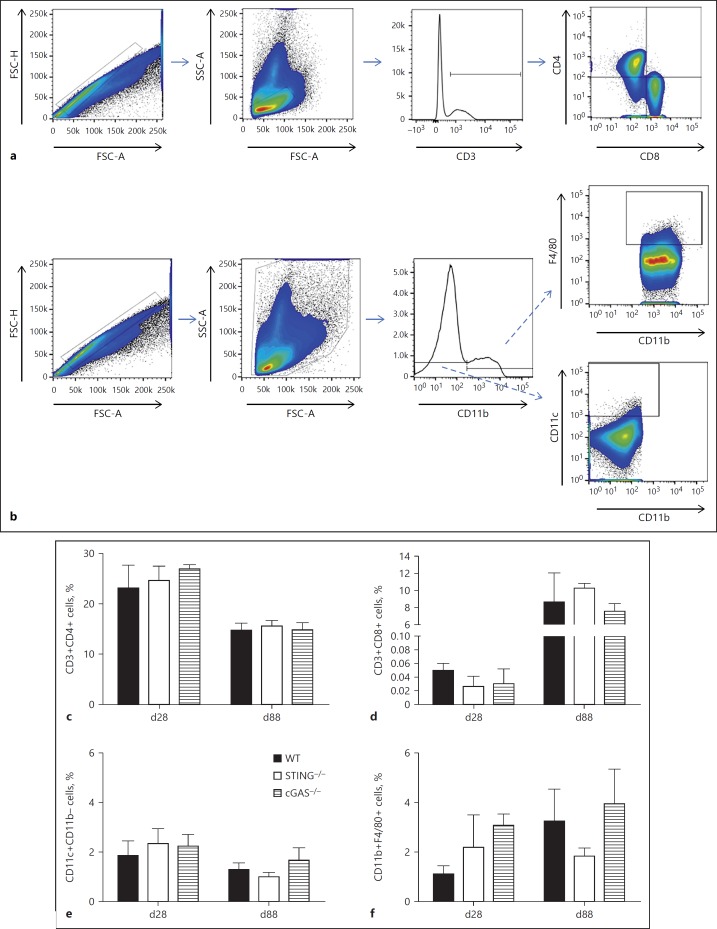
BMDCs were stained for CD11c, CD11b, CD40, and CD86. Briefly, cells were incubated for 20 min with anti-mouse CD16/32 (BD Biosciences) in FACS buffer (PBS, 1% FBS, 1 mM NaN3) and were stained for surface markers for 20 min using FITC-conjugated anti-mouse CD11c (1: 100, clone HL3; BD Biosciences), PE-conjugated anti-mouse CD40 (1: 200, 3/23; BD Biosciences), PE-Cy7-conjugated anti-mouse CD86 (1: 200, clone GL1; BD Biosciences), or APC-Cy7-conjugated anti-mouse CD11b (1: 200, clone M1/70, BD Biosciences). The appropriate isotype controls were used. Next, cells were washed and resuspended in PBS. A FACSVerse (BD Biosciences) was used for collecting approximately 50,000 events and data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). The gating strategy used was: single cells selection by FSC-H and FSC-A followed by CD11c+CD11b+ double-positive cells selection; and finally, MFI of CD40 or CD86 was measured (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000488952).
The evaluation of the ex vivo lung cell population was performed similarly. A single-cell suspension from the lungs of infected mice was obtained by treating the tissue with DNAse I (50 U/mL; Sigma-Aldrich, St. Louis, MO, USA) and Liberase (150 U/mL; Sigma-Aldrich) for 45 min at 37°C. Subsequently, the digested lung tissue was filtered through a 70-µm cell strainer and erythrocytes were lysed with Pharm Lyse (BD Biosciences). The resulting single-cell suspension was stained as described above using FITC-conjugated anti-mouse CD11c (1: 100, clone HL3), PerCP-Cy5.5-conjugated anti-mouse CD11b (1: 100, clone M1/70), and V450-conjugated anti-mouse F4/80 (1: 100, clone BM8); or FITC-conjugated anti-mouse CD3 (1: 100, clone 145–2C11), APC-Cy7-conjugated anti-mouse CD8 (1: 100, clone 53–6.7) and V450-conjugated anti-mouse CD4 (1: 100, clone RMA-5), all from BD Biosciences. A FACSCanto II Cytometer (BD Bioscience) was used for collecting approximately 100,000 events and data were analyzed using FlowJo Software (Tree Star). The gating strategy used was: single cell selection by FSC-H and FSC-A followed by selection of CD11b+ or CD11b– cells (Mix 1) or CD3+ cells (Mix 2) by histogram; finally, CD11b+F4/80+, CD11b–CD11c+, CD3+CD4+, or CD3+CD8+ populations were identified.
Quantitative Real-Time PCR
BMDCs were stimulated in 24-well plates and homogenized in TRIzol reagent (Invitrogen) to isolate total RNA. Reverse transcription of 1 µg of total RNA was performed using IllustraTM ready-to-go RT-PCR beads (GE Healthcare, Little Chalfont, UK), according to the manufacturer's instructions. Quantitative real-time PCR was conducted in a final reaction volume of 10 µL that contained SYBR green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA), cDNA, and primer at a concentration of 10 µM. The PCR was performed in a StepOneTM real-time PCR system (Thermo Fisher Scientific, Waltham, MS, USA). Gene expression data are presented as relative expression to β-actin. Primers sequences were as follows: β-actin forward, 5′-AGT GTG ACG TTG ACA TCC GT-3′; β-actin reverse, 5′-TGC TAG GAG CCA GAG CAG TA-3′; IFN-β forward, 5′-AGC TCC AAG AAA GGA CGA ACA T-3′; IFN-β reverse, 5′-GCC CTG TAG GTG AGG TTG ATC T-3′.
For in vivo experiments, total RNA was isolated from the lungs using TRI-reagent (Sigma-Aldrich), and the RNA integrity and quality determined using the Agilent RNA 6000 nano kit (Agilent Technologies, Santa Clara, CA, USA). Reverse transcription of 1 µg of total RNA was performed using the SuperScript III kit (Invitrogen) according to the manufacturer's instructions. Quantitative real-time PCR was conducted in a final reaction volume of 10 µL following GoTaq qPCR Master Mix protocol (Promega), using commercially available primers for Gapdh, Hprt1, Ifn-β, Tmem173, and Mb21d1 (all from Qiagen, Hilden, Germany) [27, 28, 29]. Gapdh and Hprt1 expression was used for normalization. Raw data were analyzed using the ΔΔCt method and expression relative to noninfected strain-matched mice.
Infection in vivo and Measurement of the Bacterial Burden
Mtb aliquots were thawed, diluted in sterile saline containing 0.05% Tween 20, and clumping was then disrupted by 50 repeated aspirations through 18-, 20-, 26-, and 27-gauge needles (Omnican, Braun, Germany). Pulmonary infection was performed by delivering 103 CFU into the nasal cavities of mice under xylazine-ketamine anesthesia. The inoculum size was verified by determining bacterial load in the lungs on day 1 postinfection. Animals were killed after 28 or 88 days postinfection for the collection of tissues and biological samples. The lungs were weighed and defined aliquots were homogenized in PBS in a Dispomix homogenizer (Axonlab, Baden-Dättwill, Switzerland). Ten-fold serial dilutions of organ homogenates in saline containing 0.05% Tween 20 were plated in duplicates onto Difco Mycobacteria 7H11 (BD Biosciences) agar plates containing 10% OADC and incubated at 37°C. Colonies were counted after 3 weeks of incubation, and the results are shown as log10 CFU/organ. During the experiments, 20% body weight loss was considered as the terminal end point.
Histology
The left lung was collected, fixed in 10% buffered formaldehyde solution for 1 week, dehydrated, diaphanized, and embedded in paraffin. Tissue sections (2–3 µm) were stained with hematoxylin and eosin (H&E). Digital images of the slides were captured with a Leica DM600B microscope using Leica HC plan s 5× or 20× objective lens. The free alveolar space was measured using ImageJ software. Briefly, a binary image was created from the original digital images and the pixels were converted into µm2. The free area was measured and expressed in relation to the total area of each section. Vascular vessels, bronchi, and bronchioles were excluded from the analysis. Cell infiltration, necrosis, and edema were quantified using a semiquantitative score (0–5) of increasing severity of changes by 2 independent observers (noninfected WT mice referenced as 0).
Pulmonary Cytokine Evaluation
Lung homogenates were centrifuged for 3 min at 14,500 rpm, the supernatants sterilized by centrifugation through 0.45- and 0.22-µm filters (3 min at 13,200 rpm; Costar-Corning, Badhoevedorp, The Netherlands), immediately frozen on dry ice, and kept at −80°C. Detection of IFN-γ, CXCL10 (IP-10), IL-12p40, and IL-23p19 was performed by ELISA using the Duoset kit (R&D Systems) according to the manufacturer's instructions.
Statistical Analysis
Statistically significant differences between the results obtained from the WT and genetically deficient mice were evaluated by two-way ANOVA followed by the Bonferroni post hoc test (p < 0.05). Statistical analysis was performed using GraphPad Prism v5.0 (San Diego, CA, USA).
Results
cGAS/STING Axis Is Important for the Activation of DCs in Response to Mtb DNA
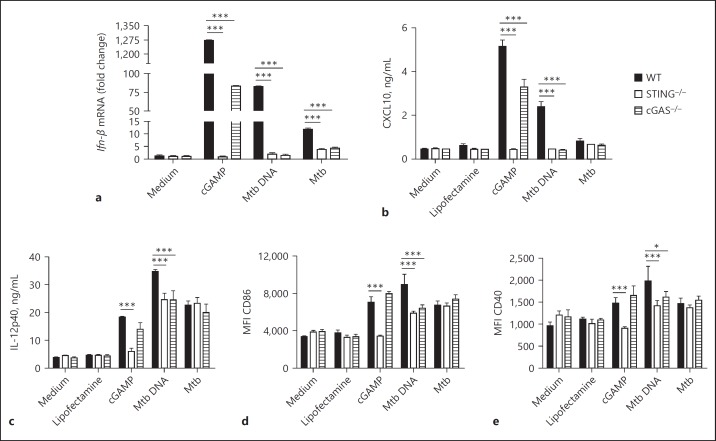
DCs are professional antigen-presenting cells that orchestrate immune responses by producing a variety of cytokines and expressing costimulatory molecules that mediate the interface between innate and adaptive immunity. The relevance of STING activation in macrophages in response to Mtb infection was previously investigated [19] but the role of STING in DC activation was overlooked. To investigate whether the adaptor proteins STING or cGAS interfere with the production of proinflammatory mediators during mycobacterial infection, BMDCs were stimulated for 24 h with Mtb DNA complexed with Lipofectamine, or infected with Mtb H37Rv. STING- or cGAS-deficient DCs showed a diminished expression of Ifn-β in response to either Mtb DNA or infection (Fig. 1a). The levels of CXCL10, a surrogate cytokine for type I IFN, were also reduced in both STING- and cGAS-deficient DCs in response to Mtb DNA. Furthermore, the levels of IL-12, CD86, and CD40 in response to Mtb DNA were reduced in STING- or cGAS-deficient DCs compared to in WT cells (Fig. 1c–e). However, CXCL10 was poorly induced by Mtb infection (Fig. 1b) and there was no difference in the surface expression of IL-12 or the costimulatory molecules CD40 and CD86 in WT, or STING- or cGAS-deficient DCs in response to Mtb infection (Fig. 1c–e). As expected, the response to the CDN cGAMP used as a control was dependent on STING but not on cGAS (Fig. 1).
Fig. 1.
STING is important for dendritic cell activation. Bone marrow-derived DCs from STING–/–, cGAS–/–, or wild-type (WT) mice were infected with M. tuberculosis (Mtb) H37Rv (MOI 3: 1) or stimulated with Mtb DNA (1 µg/mL) during 24 h. a The expression of Ifn-β was analyzed by real-time qPCR using IFN-β and β-actin primers. All data were normalized to β-actin gene expression. b, c Levels of CXCL10 and IL-12p40 were measured in the cell supernatants by ELISA. d, e Mean fluorescence intensity (MFI) of CD86 and CD40 expression was analyzed by flow cytometry. Data are shown as mean values ± SD and are from 1 experiment representative of 3 independent experiments. Statistically significant difference in relation to WT: * p < 0.05; ** p < 0.01; *** p < 0.001 (two-way ANOVA followed by the Bonferroni post hoc test).
Mtb DNA Induces STING Activation in MEFs
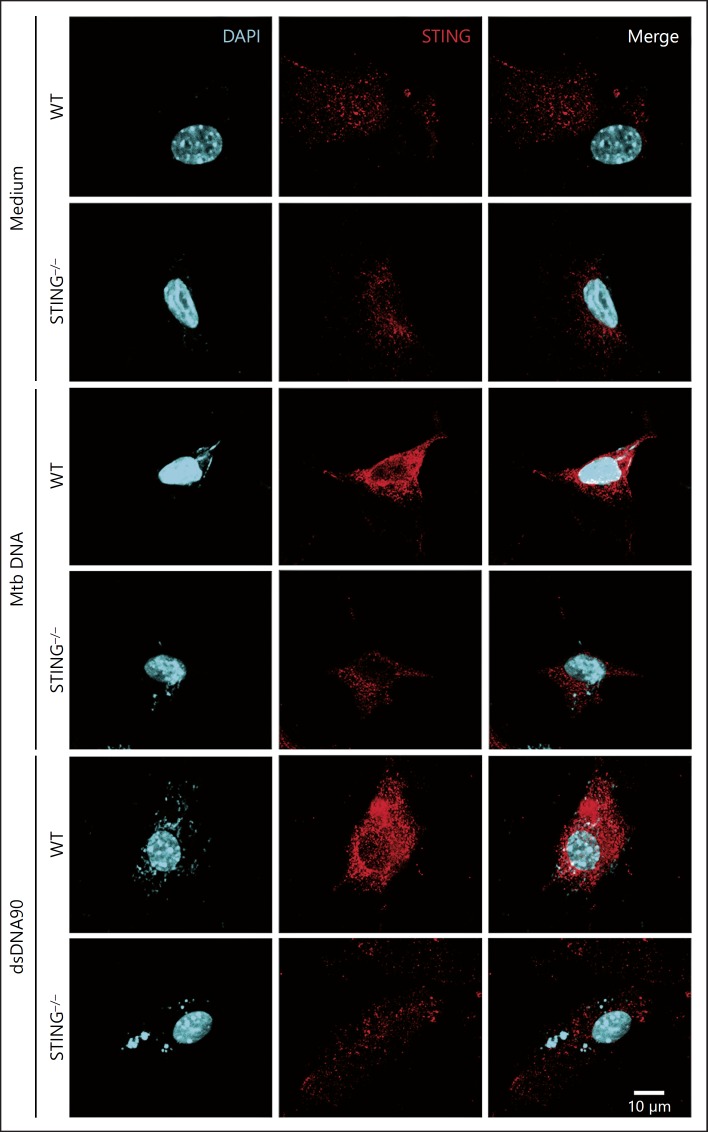
To further provide insight into the intracellular events leading to the activation of STING in response to Mtb, MEFs were transfected with Mtb DNA, and STING aggregation followed with anti-STING antibodies by confocal microscopy. Mtb DNA transfection induced the aggregation of STING in the perinuclear region of WT cells (Fig. 2). In contrast, in the STING–/– MEFs, this speck formation in response to Mtb DNA was not observed. As a control, dsDNA90 transfected into WT MEFs showed an aggregation of STING protein in the perinuclear region which was considerably reduced in the STING-deficient MEFs (Fig. 2). These results confirm that Mtb DNA can activate the STING pathway.
Fig. 2.
Mtb DNA transfection leads to STING aggregation. MEFs from wild-type (WT) or STING–/– mice were transfected with Mtb DNA (1 µg/mL) or dsDNA90 (3 µg/mL) for 4 h, fixed, and subjected to immunofluorescence microscopy analysis using antibodies against STING conjugated with Alexa Fluor 546 (red). A pronounced translocation of STING was observed as aggregated speck formation in the perinuclear region after transfection only in the WT cells. Middle panels, antibody staining for STING; left panels, nucleus staining (DAPI, blue); right panels, merged images of the middle and left panels. Data are representative of 3 independent experiments. Scale bar, 10 µm.
The cGAS/STING Pathway Is Not Essential for Controlling Mtb Infection
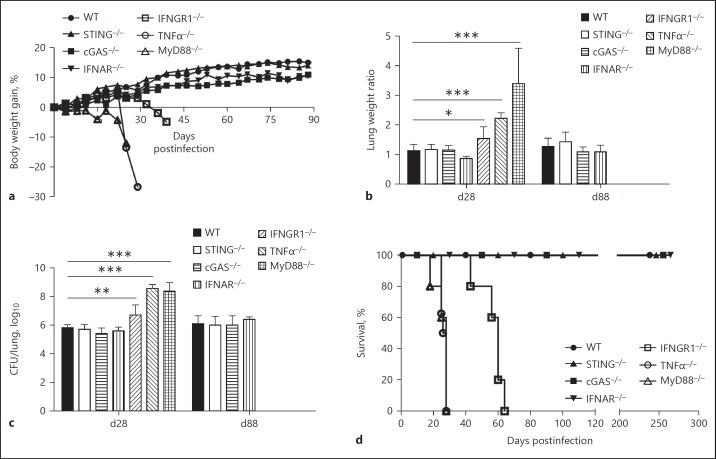
Since STING is critical for the activation of antigen-presenting cells, we decided to address how the STING pathway might impact the host response to Mtb infection in vivo. To this end, mice deficient for the cGAS/STING pathway (i.e., STING–/– and cGAS–/– mice) were compared to mice deficient for type I (IFNAR–/–) or type II (IFNGR1–/–) IFN pathways. Highly susceptible TNFα–/– and MyD88–/– mice [25, 30] were used as controls. All mice were infected intranasally with Mtb (103 CFU) and monitored for up to 12 weeks. The survival experiment was further continued for > 8 months in the resistant strains. The CFU counts in the lungs on day 1 postinfection confirmed that the infection was successfully performed (online suppl. Fig. 2). STING–/– and cGAS–/– mice presented a body weight gain similar to WT mice during Mtb infection (Fig. 3a). The absence of type I IFN signaling did not affect the overall clinical status of the mice since IFNAR–/– mice did not display a body weight loss. Conversely, MyD88–/–, TNFα–/–, and IFNGR1–/– mice showed a dramatic body weight loss at early time points of infection, starting, respectively, on days 18, 22, and 30 postinfection. This deterioration of the overall status of the IFNGR1–/–, TNFα–/–, and MyD88–/– mice correlated with increased lung weight and bacterial load on day 28 postinfection, while the STING–/–, cGAS–/–, and IFNAR–/– mice behaved like WT mice (Fig. 3b, c). Indeed, MyD88–/–, TNFα–/–, and IFNGRI–/– mice succumbed before 30 days (MyD88–/– and TNFα–/–) or 60 days (IFNGRI–/–) (Fig. 3d). In contrast, STING–/– and cGAS–/– mice still presented a lung bacterial burden similar to their WT counterpart after 88 days of infection, and they survived > 250 days like the WT mice (Fig. 3d), indicating that the STING pathway is not essential for controlling Mtb infection in vivo.
Fig. 3.
Absence of the STING pathway does not increase the susceptibility to Mtb infection. WT, STING–/–, cGAS–/–, IFNAR–/–, IFNGR1–/–, TNFα–/–, and MyD88–/– mice were infected by Mtb H37Rv (103 CFU, intranasally). a The relative body weight gain was analyzed twice a week. The relative lung weight (b) and pulmonary bacterial load (c) were determined on days 28 and 88 postinfection (d28 and d88), as indicated. d Additionally, the survival of the infected mice was monitored for > 250 days. The IFNGR1–/–, TNFα–/–, and MyD88–/– mice did not survive for 88 days. Data are shown as mean ± SD of n = 5 mice per group and the results presented are from 1 experiment representative of 3 independent experiments. Statistically significant difference in relation to WT: * p < 0.05; ** p < 0.01; *** p < 0.001 (two-way ANOVA followed by the Bonferroni post hoc test).
cGAS and STING Signaling Do Not Alter Lung Pathology
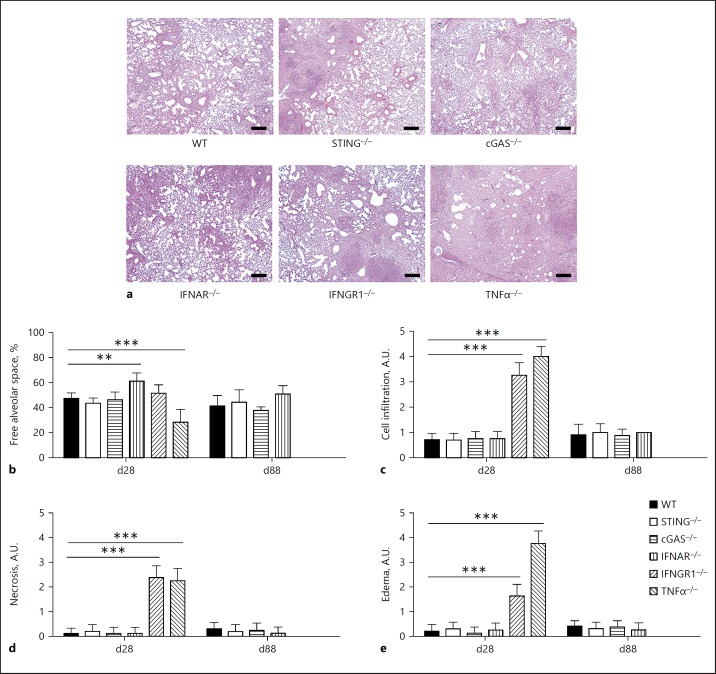
Infection by Mtb causes persistent inflammation in pulmonary parenchyma, culminating in a loss of function in cases of uncontrolled infection and extended granuloma formation. Therefore, lung pathology is a hallmark of tuberculosis and a potential cause of death. Indeed, the lungs of highly susceptible TNFα–/– mice presented large nodules observed macroscopically ([31]; data not shown) and massive inflammation, expressed by a severe reduction in free alveolar space, compared to the WT mice (Fig. 4). Moreover, the lungs from both IFNGR1–/– and TNFα–/– mice had a high histological score for cell infiltration, necrosis, and edema (Fig. 4c–e). The absence of type I IFN signaling led to a slower pace of inflammatory progression, manifested by a higher free alveolar space early in the infection that decreased to a level similar to that observed in the WT mice on day 88 postinfection (Fig. 4b). However, the absence of either STING or cGAS did not alter the establishment of pulmonary inflammation following Mtb infection (Fig. 4). Thus, cGAS/STING pathway is not essential for the development of lung inflammation in response to Mtb infection.
Fig. 4.
Absence of STING does not influence lung pathology during infection by Mtb. Lungs from WT, STING–/–, cGAS–/–, IFNAR–/–, IFNGR1–/–, and TNFα–/– mice infected with Mtb H37Rv (as in Fig. 3) were collected at 28 or 88 days postinfection (d28 or d88), fixed and embedded in paraffin. a Representative pictures of stained slides from lungs after 28 days of infection. H&E. Scale bar, 100 µm. b The free alveolar space was evaluated using ImageJ software and expressed in relation to the total area analyzed. Vascular vessels, bronchi, and bronchioles were excluded from the analysis. Cell infiltration (c), necrosis (d), and edema (e) were also scored from each slide. The results are expressed as arbitrary units (A.U.), ranging from 0 to 5. The IFNGR1–/– and TNFα–/– mice did not survive for 88 days. Data are shown as mean ± SD of n = 5 mice per group and the results presented are from 1 experiment representative of 3 independent experiments performed. Statistically significant difference in relation to WT: * p < 0.05; ** p < 0.01; *** p < 0.001 (two-way ANOVA followed by the Bonferroni post hoc test).
STING Is Upregulated during Mtb Infection but Does Not Influence Proinflammatory Cytokine Production in the Lungs
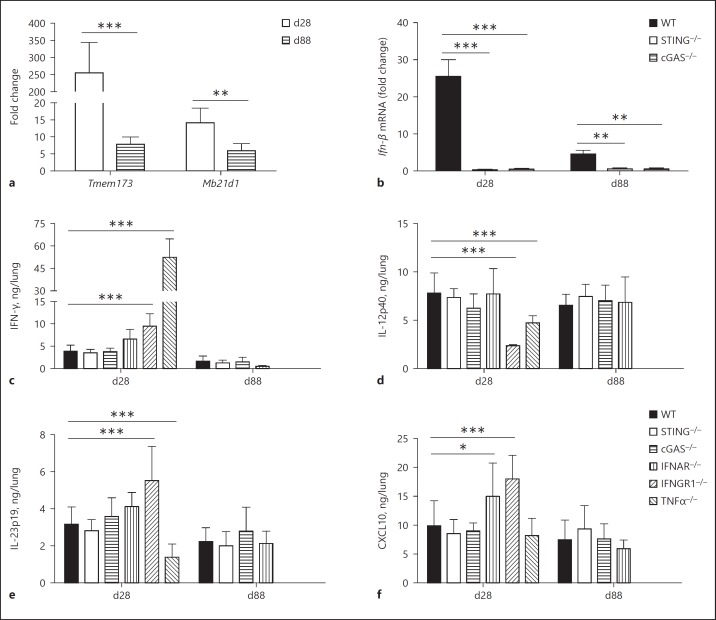
During the course of bacterial infections, several immune events take place in an attempt to restrict and eliminate the pathogen. The production of proinflammatory cytokines in the lungs is important for the establishment of protective immunity against Mtb infection [32]. Expression analysis of the STING or cGAS coding genes in the lungs of infected WT mice revealed that both were upregulated early after infection, especially the STING coding gene (Fig. 5a). However, this was transient, and there was a substantial reduction in the expression levels of both genes at 88 days of infection (Fig. 5a). STING and cGAS gene overexpression was accompanied by a transient overexpression of type I Ifn-β on day 28 postinfection, and the absence of STING or cGAS abolished Ifn-β expression in the lungs of infected mice (Fig. 5b), confirming that they do play a role in the induction of type I IFN responses. Concerning the lung proinflammatory environment, the extremely susceptible TNF-α–/– mice presented a completely altered cytokine pattern compared to the WT mice, with high levels of IFN-γ and reduced IL-12p40 and IL-23p19 production (Fig. 5c–e), as previously reported [31]. Altered cytokine production was also found in mice lacking IFN-γ receptor 1. Indeed, the IFNGR1–/– mice exhibited higher IFN-γ levels, likely due to a compensatory effect, together with increased CXCL10 and IL-23p19 levels, while IL-12p40 was reduced (Fig. 5c–f). On the other hand, the lungs of IFNAR–/– mice exhibited only increased CXCL10 pulmonary levels (Fig. 5f). Notwithstanding, the absence of STING or cGAS did not alter the production of crucial cytokines for the host response to Mtb infection, such as IFN-γ, IL-12p40, IL-23p19, or CXCL10 (Fig. 5). In parallel, the lack of the STING or cGAS pathway did not modify the lung-infiltrating cell populations after Mtb infection. Indeed, we found no difference in terms of CD4+ or CD8+ T cells, CD11c+CD11b– DCs, or CD11b+F4/80+ macrophage proportions throughout the infection (Fig. 6). Additional data supporting the virulence of the Mtb strain used are shown in online supplementary Figure 3. Thus, among the parameters analyzed in this study, STING and cGAS, although highly induced during acute Mtb infection, are essential only for type I Ifn-β expression in mouse lungs.
Fig. 5.
Mtb infection upregulates STING/cGAS expression, but this pathway does not influence proinflammatory cytokine production in the lungs. The lungs of Mtb-infected mice (103 CFU) were collected at 28 or 88 days postinfection (d28 or d88), and homogenized for RNA isolation or supernatant collection. a Expression of the STING coding gene Tmem173 and the cGAS coding gene Mb21d1 was evaluated by real-time qPCR during the infection in the WT mice. Statistically significant difference compared to day 28: * p < 0.05 (two-way ANOVA followed by the Bonferroni post hoc test). b Similarly, Ifn-β expression was determined in WT, STING–/–, and cGAS–/– mice. The fold change of mRNA levels was normalized to the Gapdh level in the uninfected WT mice. c–f Lung homogenates from infected WT, STING–/–, cGAS–/–, IFNAR–/–, IFNGR1–/–, and TNFα–/– mice were analyzed for IFN-γ, IL-12p40, IL23p19, or CXCL10 production by ELISA. The IFNGR1–/– and TNFα–/– mice did not survive for 88 days. Data are shown as mean ± SD of n = 5 mice per group and the results presented are from 1 experiment representative of 3 independent experiments. Statistically significant difference in relation to WT: * p < 0.05; ** p < 0.01; *** p < 0.001 (two-way ANOVA followed by the Bonferroni post hoc test).
Fig. 6.
STING does not influence the immune cell population in the lungs of Mtb-infected mice. The lungs of infected WT, STING–/–, and cGAS–/– mice were collected at 28 or 88 days postinfection (d28 or d88) and processed for flow cytometry analysis. a, b The gating strategy is described in Materials and Methods. c, d Percentages of CD3+CD4+ or CD3+CD8+ cells were determined within the SSC-A/FSC-A lymphocyte selection. e, f Percentages of CD11b–CD11c+ or CD11b+F4/80+ cells were determined within SSC-A/FSC-A total cells. Data are shown as mean ± SD of n = 5 mice per group and the results presented are from 1 experiment representative of 2 independent experiments.
Discussion
The cGAS/STING pathway appeared recently as an important hub of the cytosolic surveillance system [1]. Here, we addressed the influence of the sensor and adaptor proteins on DC activation and in vivo outcome during Mtb infection. We show that, in DCs, STING and cGAS are important for proinflammatory cytokine production and costimulatory molecule expression following Mtb DNA activation. However, in vivo experiments revealed that the cGAS/STING pathway, despite being highly expressed early postinfection, is not essential to control Mtb infection in terms of bacterial burden, lung inflammation, proinflammatory cytokine production, or lung-infiltrating cell populations. Thus, STING activation is dispensable for controlling acute and more chronic Mtb infection even though it affects DC activation.
At the onset of infection, macrophages and DCs are among the first immune cells to encounter mycobacteria. While macrophages are responsible for the initial restrain of the infection, the primary function of the DCs is to instruct the establishment of an effective adaptive immune response by producing key cytokines and costimulatory molecules [33]. It was previously shown that infection by Mtb overexpressing c-di-AMP increased the production of IFN-β by WT DCs, even rescuing the production to normal levels in cells lacking cGAS [16]. Here, we further show that Mtb induction of Ifn-β mRNA by DCs is partially dependent on STING and cGAS, and that mycobacterial DNA is an important PAMP in this regard. Similarly, the production of CXCL10, a surrogate cytokine for type I IFN expression, was dependent on the STING pathway when induced by cytosol-delivered Mtb DNA. Surprisingly, this was not observed in Mtb-infected cells. This fact indicates that the whole bacterium may induce CXCL10 production independently of the STING pathway. For instance, it has been previously demonstrated that Mtb DNA, which could be released by the pathogen in the extracellular milieu, induces IFN-α release by DCs, involving a TLR9-dependent pathway [34].
The efficient immune instruction mediated by DCs is achieved by the production of proinflammatory cytokines and the expression of costimulatory molecules. IL-12 has an important role in the induction and maintenance of a Th1-biased immune response [33]. The costimulatory molecules CD40 and CD86 are upregulated upon DC maturation, and considered as important activation markers. Moreover, both molecules are involved in the induction of adaptive immune responses [35]. Interestingly, we found that STING is involved in IL-12 production and the expression of CD40 and CD86 after CDN stimulation. Additionally, Mtb DNA delivered into the cytosol was able to induce IL-12 release and CD86 and CD40 overexpression in a STING- and cGAS-dependent manner. However, as indicated above for CXCL10, the infection with the whole bacterium in STING–/– cells induced similar levels of IL-12 or surface activation markers as in WT DCs, suggesting a bypass of the STING signaling and implication of alternative pathways in response to whole Mtb bacilli. Nevertheless, all these results indicate a critical function of the STING pathway in inducing the activation of DCs.
The investigation of the effect of STING and cGAS on macrophages revealed that mycobacterium extracellular DNA or c-di-AMP are important PAMPs for activating this pathway [15, 16]. The consequences of STING/cGAS pathway triggering are type I IFN expression, mainly measured by IFN-β production, the induction of autophagy, and the limited increase of intracellular killing, irrespective of bacterial strain [8, 15, 16, 17, 18, 19]. Moreover, there is intracellular colocalization of Mtb DNA and DNA sensors, mainly cGAS, or some downstream adaptor proteins, such as TBK-1 [15, 18, 19]. Here, we showed, by confocal microscopy, that Mtb DNA induced STING aggregation in the perinuclear region, providing conclusive visual evidence of STING activation during infection.
Most of the studies investigating the influence of the STING pathway on Mtb infection have focused on in vitro experiments [17, 18, 19, 36]. Although it is known that macrophages and DCs serve as intracellular niches for Mtb, STING involvement during the in vivo outcome of tuberculosis has not been thoroughly investigated. Here, we show that the STING and cGAS genes are transiently upregulated early during the infection, presenting a significant reduction of gene expression at a more chronic phase of the disease. Nevertheless, mice deficient for STING or cGAS showed disease progression similar to their WT counterpart. Upon infection with Mtb H37Rv, there was no change in body weight gain, bacterial load, pathology, proinflammatory cytokine production, or cell recruitment in the lungs up to 88 days postinfection, and STING- or cGAS-deficient mice survived for over 8 months with lung inflammation, similar to in the WT mice. Indeed, the absence of STING or cGAS only reduced the expression of Ifn-β, which seems insufficient to induce a critical phenotype influencing the pathogenesis of the disease. Moreover, the survival curve of STING–/– or cGAS–/– mice was similar to that of the WT and IFNAR–/– mice. Indeed, the role of type I IFNs in Mtb infection remains poorly understood. As expected, TNFα–/– and MyD88–/– mice presented early body weight loss and a high bacterial burden, which were correlated with increased inflammation and altered proinflammatory cytokine production in the lungs. IFNGR1–/– mice also presented a high susceptibility to Mtb infection, as expected [37]. The lack of type I IFN signaling in IFNAR–/– mice led to some amelioration of the pathogenesis in comparison to the other mouse strains, as a higher percentage of free alveolar spaces was observed in these animals after 28 days of infection. The other immune parameters evaluated were similar to in WT mice. Thus, although a lack of type I IFN signaling may improve the pathological condition of Mtb infection, the sole absence of STING or cGAS is not sufficient to induce this phenotype.
In vitro studies regarding STING activation during Mtb infection have mostly concentrated on macrophages [8, 15, 16, 17, 18, 19]. However, understanding the role of STING in DCs during bacterial infections is relevant, since DCs are more specialized in DNA sensing than macrophages [38]. The role of STING in DCs was investigated in a model of c-di-AMP-overexpressing Mtb infection, where increased production of IL-1α, IL-6 and TNFα was observed [16].
We extended the notion that the STING pathway influences the activation of DCs, by showing that IL-12, CD86, and CD40 are also upregulated in response to Mtb DNA. STING activation mediated by c-di-GMP was found to induce the upregulation of IL-12 and CD86 by DCs and myeloid-derived suppressor cells, respectively, during cancer vaccination [39]. The correlation between STING and CD40 was demonstrated during Plasmodium yoelii nigeriensis N67 infection [40]. That study suggested that parasite molecules can first activate CD40 expression through TLR signaling pathways, leading to increased STING and type I IFN expression. Our results show that Mtb DNA also induces CD40 expression in a STING-dependent manner, establishing a two-way relationship. STING involvement in different mechanisms for triggering immune responses is of great importance for interventions in several diseases and also for the development of vaccines [9, 10, 20, 39].
Despite the demonstration of the contribution of cGAS/STING on DC activation in vitro, the STING pathway is not essential for in vivo resistance to Mtb infection. Previous reports found that the bacterial burden, gross lung inflammation, and proinflammatory cytokine production in cGAS–/– and STING–/– mice were similar to in their WT counterparts [17, 19]. Reduction on Ifit1 transcripts, an IRF3 target gene, in the lungs of cGAS–/– mice, was also described. Additionally, cGAS–/– mice succumbed earlier than WT mice or STING-deficient mice.
In fact, we found that STING and cGAS are upregulated throughout Mtb infection. However, the absence of either proteins did not translate into a determinant survival phenotype. In addition, we showed that body weight gain, lung cell population during infection, and even a more detailed analysis of the pulmonary pathology remained without changes in the STING–/– and cGAS–/– mice when compared to the WT mice. Indeed, only Ifn-β transcripts were greatly reduced in the lungs of infected STING–/– and cGAS–/– mice. The differences observed in the survival curve in our study and Collins et al. [17] (who used an Erdman strain of H37Rv) could be attributed to the different strains and infection doses used, as only highly susceptible mice succumbed in our infection protocol. Different Mtb strains possess distinct virulence and even particular ways of triggering STING activation [36, 41]. Strikingly, we observed that, although the STING pathway presents a substantial contribution to overall Ifn-β expression in vivo, the absence of STING or cGAS was not sufficient to mimic the small amelioration observed in the lung inflammation of IFNAR–/– mice. Thus, despite the relevance of the cGAS/STING pathway observed in vitro, it is actually dispensable for host immunity against Mtb infection in vivo.
Disclosure Statement
The authors declare no conflicts of interest.
Supplementary Material
Supplementary data
Acknowledgements
This study was supported by grants from Brazilian CNPq/ PDE (201452/2015-4), CNPq/PDJ (150044/2017-8), CAPES/PVE (030448/2013-01 and 030490/2013-01), CNPq/INCT-DT (465229/ 2014-0), CNPq (443662/2014-2), CAPES/COFECUB (868/2015), French Region Centre-Val de Loire (No. 2015-00099232), and European funding in Region Centre-Val de Loire (FEDER No. 2016-00110366) and CNRS LIA (No. 1047).
References
- 1.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan M, Harms JS, Marim FM, Armon L, Hall CL, Liu YP, Banai M, Oliveira SC, Splitter GA, Smith JA. The bacterial second messenger cyclic di-GMP regulates Brucella pathogenesis and leads to altered host immune response. Infect Immun. 2016;84:3458–3470. doi: 10.1128/IAI.00531-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvatiyar K, Zhang Z, Teles RM, Ouyang S, Jiang Y, Iyer SS, et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat Immunol. 2012;13:1155–1161. doi: 10.1038/ni.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Sun H, You F, Sun W, Zhou X, Chen L, et al. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol. 2014;88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson RO, Manzanillo PS, Cox JS. Extracellular M.tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lio CW, McDonald B, Takahashi M, Dhanwani R, Sharma N, Huang J, Pham E, Benedict CA, Sharma S. cGAS-STING signaling regulates initial innate control of cytomegalovirus infection. J Virol. 2016;90:7789–7797. doi: 10.1128/JVI.01040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaulding E, Fooksman D, Moore JM, Saidi A, Feintuch CM, Reizis B, et al. STING-licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog. 2016;12:e1005975. doi: 10.1371/journal.ppat.1005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konig N, Fiehn C, Wolf C, Schuster M, Cura Costa E, Tungler V, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. 2017;76:468–472. doi: 10.1136/annrheumdis-2016-209841. [DOI] [PubMed] [Google Scholar]
- 12.Tamayo R, Pratt JT, Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol. 2007;61:131–148. doi: 10.1146/annurev.micro.61.080706.093426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinho FV, Benmerzoug S, Oliveira SC, Ryffel B, Quesniaux VFJ. The emerging roles of STING in bacterial infections. Trends Microbiol. 2017;25:906–918. doi: 10.1016/j.tim.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zumla A, George A, Sharma V, Herbert RH, Baroness Masham of I, Oxley A, Oliver M. The WHO 2014 Global Tuberculosis Re port - further to go. Lancet Glob Health. 2015;3:e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 15.Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe. 2012;11:469–480. doi: 10.1016/j.chom.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins AC, Cai H, Li T, Franco LH, Li XD, Nair VR, et al. Cyclic GMP-amp synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe. 2015;17:820–828. doi: 10.1016/j.chom.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassermann R, Gulen MF, Sala C, Perin SG, Lou Y, Rybniker J, et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 2015;17:799–810. doi: 10.1016/j.chom.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Watson RO, Bell SL, MacDuff DA, Kimmey JM, Diner EJ, Olivas J, et al. the cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 2015;17:811–819. doi: 10.1016/j.chom.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 24.Marino MW, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, et al. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B. Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest. 2004;114:1790–1799. doi: 10.1172/JCI21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas KE, Galligan CL, Newman RD, Fish EN, Vogel SN. Contribution of interferon-beta to the murine macrophage response to the toll-like receptor 4 agonist, lipopolysaccharide. J Biol Chem. 2006;281:31119–31130. doi: 10.1074/jbc.M604958200. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Q, Man SM, Gurung P, Liu Z, Vogel P, Lamkanfi M, Kanneganti TD. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 31.Bourigault ML, Segueni N, Rose S, Court N, Vacher R, Vasseur V, et al. Relative contribution of IL-1α, IL-1β and TNF to the host response to Mycobacterium tuberculosis and attenuated M.bovis BCG. Immun Inflamm Dis. 2013;1:47–62. doi: 10.1002/iid3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domingo-Gonzalez R, Prince O, Cooper A, Khader SA. Cytokines and chemokines in Mycobacterium tuberculosis infection. Microbiol Spectr. 2016:4. doi: 10.1128/microbiolspec.TBTB2-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect. 2008;10:995–1004. doi: 10.1016/j.micinf.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 34.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moliva JI, Turner J, Torrelles JB. Immune responses to Bacillus Calmette-Guerin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol. 2017;8:407. doi: 10.3389/fimmu.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiens KE, Ernst JD. The mechanism for type I interferon induction by Mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog. 2016;12:e1005809. doi: 10.1371/journal.ppat.1005809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown N, Jacobs M, Parida SK, Botha T, Santos A, Fick L, et al. Reduced local growth and spread but preserved pathogenicity of a DeltapurC Mycobacterium tuberculosis auxotrophic mutant in gamma interferon receptor-deficient mice after aerosol infection. Infect Immun. 2005;73:666–670. doi: 10.1128/IAI.73.1.666-670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu MM, Pu Y, Han D, Shi Y, Cao X, Liang H, et al. dendritic cells but not macrophages sense tumor mitochondrial DNA for cross-priming through signal regulatory protein alpha signaling. Immunity. 2017;47:363–373. doi: 10.1016/j.immuni.2017.07.016. e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, et al. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X, Wu J, Lin M, Sun W, He X, Gowda C, et al. Increased CD40 expression enhances early STING-mediated Type I interferon response and host survival in a rodent malaria model. PLoS Pathog. 2016;12:e1005930. doi: 10.1371/journal.ppat.1005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn PL, North RJ. Virulence ranking of some Mycobacterium tuberculosis and Mycobacterium bovis strains according to their ability to multiply in the lungs, induce lung pathology, and cause mortality in mice. Infect Immun. 1995;63:3428–3437. doi: 10.1128/iai.63.9.3428-3437.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data