Abstract
Background
We evaluated the analytical performance of a newly developed electrochemiluminescence immunoassay for everolimus and sirolimus compared to that of liquid chromatography‐tandem mass spectrometry (LC‐MS/MS).
Methods
According to Clinical and Laboratory Standards Institute guidelines, the analytical performance including precision, recovery, linearity, and carryover was evaluated. For correlation evaluation, the results of Elecsys® analysis of everolimus and sirolimus were compared with those of LC‐MS/MS using 120 samples from patients treated with everolimus or sirolimus.
Results
The within‐run and total imprecision values were as follows: 2.3%‐4.5% and 4.5%‐6.4% for the everolimus assay; 3.3%‐4.8% and 4.7%‐8.1% for the sirolimus assay, respectively. The measured concentration was linear over the range of 0.718‐27.585 ng/mL for everolimus analysis and 0.789‐26.880 ng/mL for sirolimus analysis (all R 2 > 0.99). Recovery was 93.5%‐105.5% for the everolimus assay and 99.2%‐109.1% for the sirolimus assay (except lowest levels). Carryover was −1.09% for the everolimus assay and −0.12% for the sirolimus assay. The results of the two chemiluminescence immunoassays showed acceptable correlations with those of LC‐MS/MS (R = 0.9585 and R = 0.9799, respectively). The two immunoassays showed slightly proportional biases compared to LC‐MS/MS.
Conclusion
Elecsys® Everolimus and Sirolimus assays showed acceptable analytical performance in precision, linearity, and correlation compared to LC‐MS/MS These methods can be adopted in the clinical laboratory for rapid therapeutic drug monitoring of patients who require treatment with immunosuppressants.
Keywords: everolimus, immunoassay, liquid chromatography‐tandem mass spectrometry, sirolimus, therapeutic drug monitoring
1. INTRODUCTION
Everolimus and sirolimus are widely used as immunodepressants to prevent rejection in patients who have undergone solid organ transplantation.1, 2, 3 These drugs have narrow therapeutic ranges of 3‐8 ng/mL for everolimus and 4‐12 ng/mL for sirolimus.4 Additionally, immunodepressants show pharmacokinetic variability and can cause severe toxicity including infection, bone marrow suppression, and metabolic effects.4, 5 Therefore, both accurate measurement of their concentrations in the blood and prompt therapeutic drug monitoring are crucial for maximizing their therapeutic effects and minimizing toxicity.6, 7, 8, 9, 10
Because liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) is known to have low interference, LC‐MS/MS is currently used as a gold standard method for determining the concentrations of everolimus and sirolimus.11, 12, 13 However, broad adoption of LC‐MS/MS in clinical laboratories has some limitations including difficulties in automating the test process and high cost of the instrument.14, 15 Therefore, LC‐MS/MS must be combined with other methods such as automated immunoassays, which are cost‐effective, simple, and rapid. The Elecsys® Everolimus and Sirolimus assays manufactured by Roche Diagnostics (Basel, Switzerland) were recently developed for measuring the concentration of everolimus and sirolimus based on automated electrochemiluminescence immunoassays.
In this study, we evaluated the analytical performance of the Elecsys® Everolimus and Sirolimus assays. Particularly, we determined whether Elecsys® Everolimus and Sirolimus assays can be used as an alternative method to LC‐MS/MS for measuring immunodepressant levels. By comparing the results of the Elecsys® Everolimus and Sirolimus assays to those of LC‐MS/MS, we confirmed the accuracy of the automated electrochemiluminescence immunoassays using samples collected from patients who underwent organ transplantation and were treated with everolimus or sirolimus.
2. MATERIALS AND METHODS
The ethics committee of Asan Medical Center approved the use of patient data (2018‐0125).
2.1. Samples
A total of 120 EDTA whole blood samples were collected from September to November 2017 in Asan Medical Center. For everolimus evaluation, study subjects consisted of 60 patients (51 males and 9 females) with a mean age of 56.1 years. They had received a liver transplantation (n = 51) or heart transplantation (n = 9). For sirolimus evaluation, study subjects consisted of 60 patients (33 males and 27 females) with a mean age of 52.3 years. They had received a kidney transplantation (n = 50), heart transplantation (n = 5), or other organ transplantations (n = 5). After collection, the samples were stored at −20°C until the time of analysis. Drug concentrations were equally distributed based on clinically significant concentrations including the therapeutic range: everolimus: 3‐8 ng/mL; sirolimus: 5‐15 ng/mL.4, 16 Quality control (QC) materials (PreciControl ISD Elecsys, Roche Diagnostics) were used to evaluate precision.
2.2. Electrochemiluminescence immunoassays
To measure everolimus and sirolimus, the samples were pretreated with Elecsys® Everolimus or Elecsys® Sirolimus ISD sample pretreatment, respectively. Calibration was performed using the Elecsys® Everolimus CalSet or Elecsys® Sirolimus CalSet, which consists of two calibrators at concentrations of 0.6 and 23.8 ng/mL for everolimus and 0.5 and 23.6 ng/mL for sirolimus. The immunoassays were performed using the Cobas e602 module (Roche Diagnostics) according to the manufacturers' instructions.
2.3. LC‐MS/MS assays
LC‐MS/MS assays were performed using a Waters ACQUITY UPLC I‐Class/Waters Xevo TQ‐S (Waters Corporation) equipped with an ACQUITY UPLC® HSS C18 SB Column (2.1 × 30 mm, 1.8 µm pore size, Waters Corporation). As an internal standard solution, whole blood samples were mixed with ascomycin (Cerilliant Corporation), d3‐sirolimus (IsoSciences), and d4‐everolimus (Cerilliant). 6PLUS1® Multilevel Calibrator Set Immunosuppressants (Chromsystems Instruments & Chemicals, Gräfelfing, Germany) were used as calibrators, and MassCheck® Immunosuppressants Whole Blood Control (Chromsystems Instruments & Chemicals) was used as a QC material. The samples were eluted and graduated with a mobile phase composed of ammonium acetate, formic acid, and water or methanol. Everolimus and sirolimus were detected in positive‐ion multiple‐reaction‐monitoring mode, and the following precursor/production pairs were detected as 1,220.8 > 1,203.8 m/z for everolimus and 821.5 > 768.5 m/z for sirolimus. The data were quantified using a six‐point standard curve. The limit of detection values were 0.2 and 0.3 ng/mL for everolimus and sirolimus, respectively.17 The limit of quantitation values were 1.2 and 1.1 ng/mL for everolimus and sirolimus, respectively.17
2.4. Precision
For precision evaluation, low, medium, and high concentrations of QC materials were used. Each QC material was measured in duplicate on the same day at more than 2‐hour intervals, and the measurements were performed sequentially over 10 days. The coefficient of variation (CV) for the within‐run assay and total CV were calculated according to the Clinical and Laboratory Standards Institute (CLSI) EP05‐A2.18 The acceptance criteria were based on the recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology expert consensus group.19
2.5. Linearity and recovery
According to CLSI EP06‐A, linearity and recovery were evaluated.20 Patient samples at high and low concentrations were serially diluted to produce five different concentrations. The Elecsys® Everolimus and Sirolimus assays were performed four times on the same day. The means of the concentrations were compared to the expected values.
2.6. Comparison
Each measurement was performed within 4 hour in duplicate according to the CLSI EP9‐A3.21 Using Pearson's correlation analysis, the results determined by Elecsys® Everolimus or Sirolimus assays were compared to those determined by LC‐MS/MS The regression equation between the electrochemiluminescence immunoassays and LC‐MS/MS method was obtained by Deming regression and Passing‐Bablok analysis.22 Additionally, the difference in the two assays was identified using a Bland‐Altman plot.
2.7. Carryover
After patient samples at high concentrations (H1, H2, H3, and H4) were measured, low‐concentration samples (L1, L2, L3, and L4) were sequentially measured. Carryover (%) was calculated according to following equation: .23
2.8. Statistics
Microsoft Excel 2010 (Microsoft), EP Evaluator 8 software package (Data Innovations), and IBM SPSS 23 software (SPSS, Inc) were used for statistical analysis.
3. RESULTS
3.1. Precision
The results of precision evaluation are presented in Table 1. The within‐run CV of low, medium, and high levels of QC materials was 4.5%, 2.3%, and 2.6% in the everolimus assay and 4.8%, 4.1%, and 3.3% in the sirolimus assay, respectively. The total run CV of the three QC materials was 6.4%, 4.5%, and 4.9% in the everolimus assay and 8.1%, 5.8%, and 4.7% in the sirolimus assay, respectively. While CVs at low levels were higher than those at other levels, all evaluated levels of QC materials showed acceptable CVs within 8.1%.
Table 1.
Precisions of the Elecsys® Everolimus and Elecsys® Sirolimus assays
| Elecsys® Assay | Level | Mean (ng/mL) | Within‐run imprecision | Total imprecision | ||
|---|---|---|---|---|---|---|
| SD (ng/mL) | CV (%) | SD (ng/mL) | CV (%) | |||
| Everolimus | Low | 2.86 | 0.132 | 4.5 | 0.185 | 6.4 |
| Middle | 9.84 | 0.235 | 2.3 | 0.458 | 4.5 | |
| High | 18.52 | 0.486 | 2.6 | 0.918 | 4.9 | |
| Sirolimus | Low | 3.81 | 0.187 | 4.8 | 0.320 | 8.1 |
| Middle | 10.72 | 0.452 | 4.1 | 0.644 | 5.8 | |
| High | 19.75 | 0.658 | 3.3 | 0.937 | 4.7 | |
Abbreviations: CV, coefficient of variation; SD, Standard deviation.
3.2. Linearity and recovery
The results of linearity evaluation are presented in Figure 1. Linearity was acceptable, showing correlation coefficients (R 2) of 0.9943 for everolimus at 0.718‐27.585 ng/mL and 0.9958 for sirolimus at 0.789‐26.880 ng/mL. Except at the lowest level for both everolimus and sirolimus, the recovery also satisfied the acceptance criteria, which was within ± 10% of the expected value; we observed values of 93.5%‐105.5% in the everolimus assay and 99.2%‐109.1% in the sirolimus assay. At the lowest levels, the differences between the measured and expected values were 0.172 ng/mL for everolimus (mean of the measured value: 0.890 ng/mL; expected value: 0.718 ng/mL) and 0.129 ng/mL for sirolimus (mean of the measured value: 0.916 ng/mL; expected value: 0.789 ng/mL).
Figure 1.
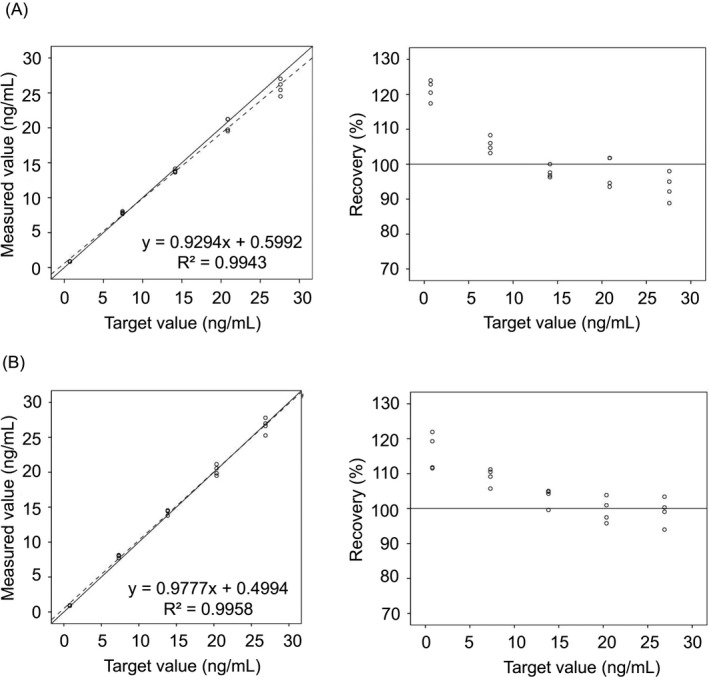
Linearities (left) and %recoveries (right) in Elecsys® Everolimus assays (A) and Elecsys® Sirolimus assays (B). The solid lines are Y = X, and dotted lines represent linear regression
3.3. Correlation with LC‐MS/MS
The results of correlation analysis between the automated electrochemiluminescence immunoassays and LC‐MS/MS are shown in Figure 2. The concentrations determined by the Elecsys® Everolimus and Elecsys® Sirolimus assays showed significant correlation with the results determined by LC‐MS/MS (R = 0.9585 and R = 0.9799, respectively). The differences between electrochemiluminescence immunoassays and LC‐MS/MS were evaluated; the results are shown in Table 2. There were no constant differences in the immunoassays compared to LC‐MS/MS (intercept in everolimus assay: 0.423, 95% CI −0.027‐0.873; intercept in sirolimus assay: 0.188, 95% CI −0.162‐0.538 in Deming regression test). However, a low proportional difference was observed in both immunoassays (slope in everolimus assay: 1.216, 95% CI 1.152‐1.280; slope in sirolimus assay: 1.286, 95% CI 1.239‐1.333).
Figure 2.
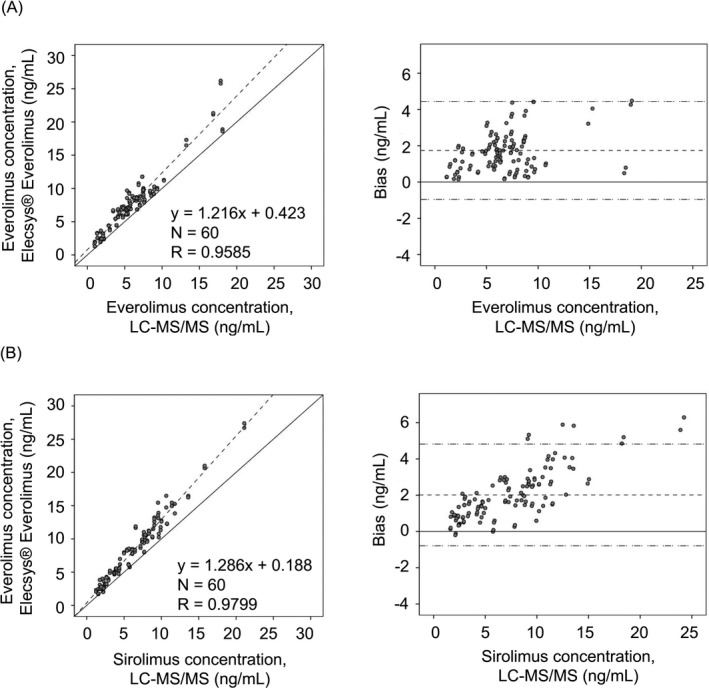
Deming regression (left) and Bland‐Altman plots (right) between electrochemiluminescence immunoassay and liquid chromatography‐tandem mass spectrometry for evaluating everolimus assay (A) and sirolimus assay (B). In the left figures, the solid lines are Y = X and dotted lines represent the Deming regression. In the right figures, the solid lines are Y = X and dotted lines represent the mean value (middle line) and ± 2 SD (upper and lower lines)
Table 2.
Summary of comparison between electrochemiluminescence immunoassay and liquid chromatography‐tandem mass spectrometry
| Elecsys® Everolimus | Elecsys® Sirolimus | |
|---|---|---|
| Deming | ||
| N | 60 | 60 |
|
Slope (95% CI) |
1.216 (1.152‐1.280) |
1.286 (1.239‐1.333) |
|
Intercept (95% CI) |
0.423 (−0.027‐0.873) |
0.188 (−0.162‐0.538) |
| Std Err Est | 1.258 | 1.008 |
| Passing‐Bablok | ||
| N | 60 | 60 |
|
Slope (95% CI) |
1.156 (1.093‐1.219) |
1.254 (1.208‐1.301) |
|
Intercept (95% CI) |
0.788 (0.344‐1.231) |
0.392 (0.045‐0.740) |
| Std Err Est | 1.240 | 1.000 |
| R a | 0.959 | 0.980 |
Abbreviations: CI, confidence interval; N, Number; Std Err Est, standard error of the estimate.
Correlation coefficient (R) was calculated by Pearson's correlation analysis.
3.4. Carryover
Carryover analysis showed a value of −1.09% in the everolimus assay and −0.12% in the sirolimus assay.
4. DISCUSSION
In this study, we evaluated the overall analytic performance of the Elecsys® Everolimus and Sirolimus assays. Precision evaluation revealed that the total CVs were acceptable with values of 6.4% and 8.1% in the everolimus and sirolimus assays, respectively. The results showed excellent linearity with an R 2 > 0.99 in the clinically significant range. Carryover was also acceptable within ± 1.1% in both the everolimus and sirolimus assays. Comparison with LC‐MS/MS as a gold standard method showed significant accuracy with R > 0.95 in both the everolimus and sirolimus assays.
However, a low level of positive proportional difference was observed in both immunoassays. Similar results were reported in previous studies. According to Shipkova et al, a positive proportional difference in everolimus immunoassay was observed with a slope of 1.131 (95% CI 1.074‐1.186) in Deming regression analysis.1 According to Fung et al, the intercept for the sirolimus assay results was 2.4 ng/mL (95% CI 1.6‐3.3 ng/mL), indicating a positive constant difference.23 Matrix effect, known to be caused by other components present in samples, can greatly affect the accuracy of immunoassays.24 Additionally, bias may be also caused by inaccurate calibration before measurement, non‐specific binding, or cross‐reactivity between immunoassays.25, 26 For example, the major metabolites of everolimus such as 46‐hydroxy, 24‐hydroxy everolimus, and 25‐hydroxy everolimus can cause approximately 2%‐72% cross‐reactivities in the everolimus immunoassay.27, 28 Moreover, sirolimus and its major metabolites can occur cross‐reactivity in the everolimus immunoassay because of their 46% structural similarity.29
We confirmed that the Elecsys® Everolimus and Sirolimus assays show a generally acceptable correlation with the LC‐MS/MS method. However, some significant differences were observed at a high concentration (>2‐fold the therapeutic range), which has not been frequently observed clinically. Therefore, clinicians may use LC‐MS/MS to confirm accurate drug concentrations if patient samples exceed the therapeutic range. And efforts to maintain qualified test results should be made by the manufacturer.
The Elecsys® Everolimus and Sirolimus assays are conducted using samples from adult patients who have undergone transplant and have been co‐administered with other immunodepressants such as cyclosporine and corticosteroids. In this study, we could not compare patients with various conditions. The correlations of immunoassay compared to LC‐MS/MS may be different according to the type of organ transplantation because of the different metabolism.1, 26, 30, 31 However, because the clinical uses of the everolimus and the sirolimus are different, we cannot collect the same subdivided samples according to the type of organ transplantation. Clinically, the sirolimus has been more frequently used than everolimus in kidney transplantation due to its longer elimination half‐life, which makes clinicians easier to manage in medical practice.32 On the other hand, the everolimus has been mainly used for liver transplantation because the use of sirolimus in the early post‐transplant period has been influenced by the high incidence of hepatic artery thrombosis and decreased graft survival.33 Fortunately, the previous report showed that unlike other immunoassays, the Elecsys® Everolimus assay showed similar results in kidney, liver, and heart transplantations.1
In conclusion, the Elecsys® Everolimus and Sirolimus assays are useful for determining the concentrations of immunodepressants based on automatized electrochemiluminescence immunoassays. We confirmed the good analytical performance of the assays including precision, linearity, and carryover effects. Particularly, high correlations with the gold standard method (LC‐MS/MS) suggest that these assays can be used as alternatives to LC‐MS/MS in clinical laboratories without mass spectrometry instruments. The Elecsys® Everolimus and Sirolimus assays are useful and practical methods for monitoring the concentrations of everolimus and sirolimus in the blood of patients who have undergone organ transplantation.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This study was supported by Roche Diagnostics.
Lee EJ, Kim H‐K, Ahn S, et al. Accuracy evaluation of automated electrochemiluminescence immunoassay for everolimus and sirolimus compared to liquid chromatography‐tandem mass spectrometry. J Clin Lab Anal. 2019;33:e22941 10.1002/jcla.22941
REFERENCES
- 1. Shipkova M, Rapp S, Rigo‐Bonnin R, Wieland E, Peter A. Therapeutic drug monitoring of everolimus: comparability of concentrations determined by 2 immunoassays and a liquid chromatography tandem mass spectrometry method. Ther Drug Monit. 2017;39(2):102‐108. [DOI] [PubMed] [Google Scholar]
- 2. Manito N, Delgado JF, Crespo‐Leiro MG, et al. Clinical recommendations for the use of everolimus in heart transplantation. Transplant Rev (Orlando). 2010;24(3):129‐142. [DOI] [PubMed] [Google Scholar]
- 3. Flechner SM, Goldfarb D, Modlin C, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation. 2002;74(8):1070‐1076. [DOI] [PubMed] [Google Scholar]
- 4. Kovarik JM, Tedesco H, Pascual J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced‐exposure cyclosporine in de novo kidney transplantation. Ther Drug Monit. 2004;26(5):499‐505. [DOI] [PubMed] [Google Scholar]
- 5. Stenton SB, Partovi N, Ensom MH. Sirolimus: the evidence for clinical pharmacokinetic monitoring. Clin Pharmacokinet. 2005;44(8):769‐786. [DOI] [PubMed] [Google Scholar]
- 6. de Wit D, Schneider TC, Moes Djar, et al. Everolimus pharmacokinetics and its exposure‐toxicity relationship in patients with thyroid cancer. Cancer Chemother Pharmacol. 2016;78(1):63‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meier‐Kriesche HU, Kaplan B. Toxicity and efficacy of sirolimus: relationship to whole‐blood concentrations. Clin Ther. 2000;22:B93‐B100. [DOI] [PubMed] [Google Scholar]
- 8. Mohammadpour N, Elyasi S, Vahdati N, Mohammadpour AH, Shamsara J. A review on therapeutic drug monitoring of immunosuppressant drugs. Iran J Basic Med Sci. 2011;14(6):485‐498. [PMC free article] [PubMed] [Google Scholar]
- 9. Dunn C, Croom KF. Everolimus: a review of its use in renal and cardiac transplantation. Drugs. 2006;66(4):547‐570. [DOI] [PubMed] [Google Scholar]
- 10. MacKeigan JP, Krueger DA. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol. 2015;17(12):1550‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan C, Payto D, Gabler J, Wang S. A simple and robust LC‐MS/MS method for measuring sirolimus and everolimus in whole blood. Bioanalysis. 2014;6(12):1597‐1604. [DOI] [PubMed] [Google Scholar]
- 12. Morgan PE, Brown NW, Tredger JM. A direct method for the measurement of everolimus and sirolimus in whole blood by LC‐MS/MS using an isotopic everolimus internal standard. Ther Drug Monit. 2014;36(3):358‐365. [DOI] [PubMed] [Google Scholar]
- 13. Vogeser M, Fleischer C, Meiser B, Groetzner J, Spohrer U, Seidel D. Quantification of sirolimus by liquid chromatography‐tandem mass spectrometry using on‐line solid‐phase extraction. Clin Chem Lab Med. 2002;40(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 14. Busardo FP, Pacifici R, Pichini S. Mass spectrometry vs. immunoassay in clinical and forensic toxicology: qui modus in rebus est? Clin Chem Lab Med. 2017;55(10):e236‐e237. [DOI] [PubMed] [Google Scholar]
- 15. Brandhorst G, Oellerich M, Maine G, Taylor P, Veen G, Wallemacq P. Liquid chromatography‐tandem mass spectrometry or automated immunoassays: what are the future trends in therapeutic drug monitoring? Clin Chem. 2012;58(5):821‐825. [DOI] [PubMed] [Google Scholar]
- 16. Cattaneo D, Merlini S, Pellegrino M, et al. Therapeutic drug monitoring of sirolimus: effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant. 2004;4(8):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 17. National Committee for Clinical Laboratory Standards . Protocols for Determination of Limits of Detection and Limits of Quantitation, Approved Guideline. EP17‐A. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- 18. National Committee for Clinical and Laboratory Standards Institute . Evaluation of Precision Performance of Quantitative Measurement Methods: Approved Guideline. EP05‐A3 (2nd edn). Wayne, PA: Clinical and Laboratory Standards Institute; 2014. [Google Scholar]
- 19. Seger C, Shipkova M, Christians U, et al. Assuring the proper analytical performance of measurement procedures for immunosuppressive drug concentrations in clinical practice: recommendations of the international association of therapeutic drug monitoring and clinical toxicology immunosuppressive drug scientific committee. Ther Drug Monit. 2016;38(2):170‐189. [DOI] [PubMed] [Google Scholar]
- 20. National Committee for Clinical Laboratory Standards . Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach: Approved guideline, EP6‐A. Wayne, PA: National Committee for Clinical Laboratory Standards; 2003. [Google Scholar]
- 21. National Committee for Clinical and Laboratory Standard Institute . Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline. EP09‐A3 (3rd edn). Wayne, PA: Clinical and Laboratory Standards Institute; 2013. [Google Scholar]
- 22. National Committee for Clinical and Laboratory Standards Institute . Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline‐Second Edition (EP5‐A2). Wayne, PA: National Committee for Clinical Laboratory Standards; 2014. [Google Scholar]
- 23. National Committee for Clinical Laboratory Standards . Preliminary evaluation of Quantitative Clinical Laboratory Methods: APPROVED Guideline, EP10‐A2 (2nd edn). Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 24. Fang N, Yu S, Ronis MJ, Badger TM. Matrix effects break the LC behavior rule for analytes in LC‐MS/MS analysis of biological samples. Exp Biol Med (Maywood). 2015;240(4):488‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48(Pt 5):418‐432. [DOI] [PubMed] [Google Scholar]
- 26. Ko DH, Cho EJ, Lee W, Chun S, Min WK. Accuracy evaluation of Roche and Siemens tacrolimus and cyclosporine assays in comparison with liquid chromatography‐tandem mass spectrometry. Scand J Clin Lab Invest. 2018;1–8. [DOI] [PubMed] [Google Scholar]
- 27. Nashan B. Review of the proliferation inhibitor everolimus. Expert Opin Investig Drugs. 2002;11(12):1845‐1857. [DOI] [PubMed] [Google Scholar]
- 28. Thermo Fisher Scientific Inc . QMS Everolimus Immunoassay [package insert]. Fremont, CA: Thermo Fisher Scientific, Inc.; 2015. [Google Scholar]
- 29. Dasgupta A. Therapeutic Drug Monitoring: Newer Drugs and Biomarkers. 1st Ed.
- 30. Manier SK, Keller A, Schaper J, Meyer MR. Untargeted metabolomics by high resolution mass spectrometry coupled to normal and reversed phase liquid chromatography as a tool to study the in vitro biotransformation of new psychoactive substances. Sci Rep. 2019;9(1):2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho EJ, Ko DH, Lee W, Chun S, Lee HK, Min WK. Performance of the dimension TAC assay and comparison of multiple platforms for the measurement of tacrolimus. J Clin Lab Anal. 2018;32(4):e22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moes DJ, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today. 2015;20(10):1243‐1249. [DOI] [PubMed] [Google Scholar]
- 33. Massoud O, Wiesner RH. The use of sirolimus should be restricted in liver transplantation. J Hepatol. 2012;56(1):288‐290. [DOI] [PubMed] [Google Scholar]


