Abstract
Objectives:
To report the genotype-phenotype characteristics, demographic features and clinical outcome of Omani patients with congenital hyperinsulinism (CHI).
Methods:
We retrospectively analyzed the clinical, biochemical, genotypical, phenotypical characteristics and outcomes of children with CHI who were presented to the pediatric endocrine team in the Royal Hospital, Muscat, Oman between January 2007 and December 2016.
Results:
Analysis of 25 patients with CHI genetically revealed homozygous mutation in ABCC8 in 23 (92%) patients and 2 patients (8%) with compound heterozygous mutation in ABCC8. Fifteen (60%) patients underwent subtotal pancreatectomy as medical therapy failed and 2 (8%) patients showed response to medical therapy. Three patients expired during the neonatal period, 2 had cardiomyopathy and sepsis, and one had sepsis and severe metabolic acidosis. Out of the 15 patients who underwent pancreatectomy, 6 developed diabetes mellitus, 6 continued to have hypoglycemia and required medical therapy and one had pancreatic exocrine dysfunction post-pancreatectomy, following up with gastroenterology clinic and was placed on pancreatic enzyme supplements, while 2 patients continued to have hypoglycemia and both had abdominal MRI and 18-F-fluoro-L-DOPA positron emission tomography scan (PET-scan), that showed persistent of the disease and started on medical therapy.
Conclusion:
Mutation in ABCC8 is the most common cause of CHI and reflects the early age of presentation. There is a need for early diagnosis and appropriate therapeutic strategy.
Congenital hyperinsulinism in infancy (CHI) or persistent hyperinsulinemic hypoglycemia of infancy (PHHI) is a heterogeneous disorder that is characterized by inappropriate insulin secretion despite hypoglycemia.1 There is a high risk of brain injury in untreated severe hypoglycemia due to the continued deprivation of the brain tissue from its energy sources, which include glucose (hypoglycemia) and ketones (hyperinsulinism). Hence, early diagnosis and management is of paramount importance to prevent these effects that are usually manifested as seizures and developmental delay.2
Congenital hyperinsulinism in infancy usually has 3 histopathological types: focal, diffuse, and atypical forms. Although these 3 types clinically and biochemically will have same presentation, histological differentiation is crucial in the management.3-5 At present, mutations have been identified in different genes (ABCC8, KCNJ11, GLUD1, GCK, HADH, SLC16A1, UCP2, HNF4A, HNF1A, HK1, PGM1, and PMM2)6-12 that cause dysregulated insulin secretion. Recently there are 2 cases reported with hyperinsulinaemic hypoglycemia (HH) due to other genes (FOXA2, CACNA1D).13,14
The most common causes for CHI are mutations in the genes ABCC8 and KCNJ11 (both autosomal recessive and dominant) that encode the SUR1 and Kir6.2 subunits of the pancreatic b-cell KATP channel.15-19 The incidence rate of this disorder is approximately 1/50,000 live births, and in some countries like Finland, the rate is even higher.20 In Saudi Arabia, the incidence rate is reported to be 1/2500 live births because of the higher rate of consanguinity marriage.21 Typically CHI presents in the first few days after birth in term and preterm infants with symptomatic hypoglycemia, although some patients can present with non-specific symptoms of hypoglycemia such as poor feeding, lethargy, irritability, and seizures. The patients who fail to respond to medical therapy often require pancreatectomy.22
Early diagnosis and treatment is important in babies with CHI because they are at risk for neurological damage that is secondary to severe hypoglycemia and neuroglycopenia. Here, we report the genotype-phenotype characteristics, demographic features, and clinical outcome of Omani patients diagnosed Authors have no conflict of interests, and the work was not supported or funded by any drug company. with CHI at the Royal Hospital, Oman during the 2007-2016 decade.
Methods
We retrospectively analyzed the clinical, biochemical, genotypical, phenotypical characteristics and outcomes in children with CHI who were presented to the pediatric endocrine team in the Royal Hospital, Muscat, Oman between January 2007 and December 2016. Ethical consent approval was obtained from Royal Hospital, Oman. All the patients included in the study had the diagnostic criteria of CHI. These include the following: hypoglycemia (plasma glucose ≤2.6 mmol/L), hyperinsulinism (plasma insulin level ≥2 mIU/L “13.9 pmol/L”) during hypoglycemia, negative urine ketone, normal metabolic, and hormonal profiles with increased glucose requirement at a rate of more than 8 mg/kg/min. Patients with transient hypoglycemia related to intra-uterine growth retardation (IUGR) or other causes who recovered and had no known genetic mutations of CHI we excluded from the study.
Analysis of mutations in ABCC8 and KCNJ11 genes were carried out for all of our patients and their parents. Blood specimens from these patients and their parents (with the consent forms from the parents) were referred for genetic analysis at the Molecular Genetics, Exeter Clinical Laboratory, Royal Devon & Exeter NHS Foundation Trust, Exeter University Medical School, Exeter, United Kingdom. If the mutation analysis was negative for ABCC8 and KCNJ11 genes in the infant’s blood, other genes were assessed, such as the genes encoding glucokinase (GCK) and hepatic nuclear factor (HNF4A). If a mutation is detected in the patient, the parents were then analyzed for the same mutation to determine the mode of inheritance.
Results
Twenty-five infants (15 males, 10 females; male-to-female ratio of 1.5:1 from unrelated 23 families were diagnosed with CHI over a 10-year period (Jan 2007 and Dec 2016). Four families had 2 affected siblings each, while 11 patients were born to first degree cousin parents. Most babies were born full-term with gestational ages of more than 37 weeks apart from 3 babies (patient-2 at 34 weeks, patient-5 at 36 weeks and patient-21 at 33 weeks). The birth weights for all babies ranged between 2.7 kg and 4.8 kg. Macrosomia babies were detected in 4 babies (patients-1, 9, 17, 19) based on BW -SD over 2 as per World Health Organization chart as 2.5, 3.1, 3.4 and 2.2 with a mean SD of 2.75, and of these, 3 were infants of diabetic mothers. None of the patients were small-for-gestational age (birth weight ≤2.5 kg). The clinical manifestations of all the babies were manifested on day one or 2 of their lives, apart from 2 babies (patient-17 at age of 8 days and patient-13 at age of 4 months).
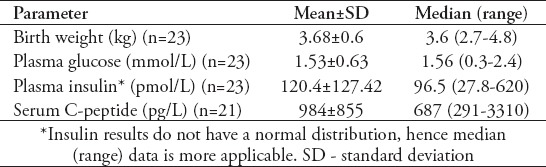
Hypoglycemia (plasma glucose ≤2.6 mmol/l) was detected in all patients. In addition, there were other variable hypoglycemic symptoms that include seizures, apnea, poor feeding, and lethargy. Twenty-four patients had biochemical manifestations of CHI (non-ketotic hypoglycemia, hyperinsulinism at time of hypoglycemia, glucose requirement at rates greater than 8 mg/kg/min, and normal other metabolic and hormonal profiles apart from one baby (patient-6), who had no documentation and neither clinical nor biochemical information. All babies received medical treatment that included oral diazoxide (up to 15 mg/kg/day in 3 divided doses), subcutaneous octreotide injections with doses up to 30 mcg/kg/day in 4 divided doses, and boluses of glucagon intravenously or intramuscularly injection with doses of 30 mcg/kg, apart from patient-6 who had no data available. The data for laboratory results of glycemic parameters (plasma glucose, insulin and C-peptide) are shown in Table 1.
Table 1.
Data for birth weight and laboratory results for patients with congenital hyperinsulinism in infancy.
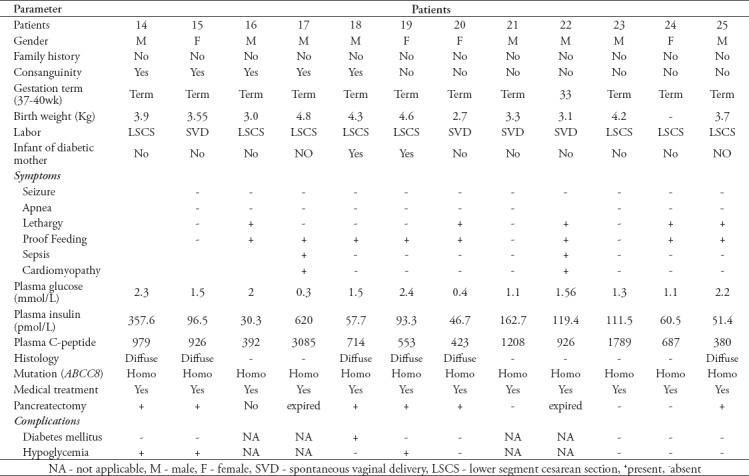
Mutation analyses were carried out at Molecular Genetics, Exeter Clinical Laboratory, Exeter, United Kingdom and revealed that the ABCC8 gene is a consistent gene in our patients with different genotypes (11 patients with homozygous missense mutation, one of them was patient-16 with novel mutation, 5 with homozygous nonsense mutation, 3 with homozygous novel frame shift mutation, 4 with homozygous splicing mutation and 2 with compound heterozygous novel missense mutation). Fifteen patients did not respond to medical therapy and underwent near total pancreatectomy at an age that ranged between one month and 2.6 years.
Three babies (patient-2 and patient-17) died at the age of one month with sepsis and cardiomyopathy, and patient-22 died with sepsis and severe metabolic acidosis. Three babies refused surgery (patient-3, 4, 16). Patient-3 and 4 were siblings who did not participate in a follow-up, while patient-16 who is now 5 years old and is still undergoing medical treatment. He was on subcutaneous octreotide injection 30 mcg/kg/day then long-acting somatostatin analogue (lanreotide) intramuscular injection (30 mg once monthly) introduced with occasionally use of octreotide but he continued to have hypoglycemia. For the last 12 months he has been treated with sirolimus (a mammalian target of rapamycin inhibitor, mTOR) orally one mg/m2/day, tapered by 0.25-0.5 mg/m2/day with routine monitoring of drug levels and side effects. The patient showed dramatic improvement with less hypoglycemic episodes and was on a nasogastric tube, but got out of it. Patient-23 underwent medical treatments that include octreotide 30 mcg/kg/day subcutaneous injection then a long-acting somatostatin analogue (lanreotide) 30 mg intramuscular injection once monthly introduced and he showed good responses (with occasional hypoglycemia) to these treatments. Those patients remained stable with no side effects of the medications. Two patients (patient-10 and patient-24) resolved from the hypoglycemia and neither required medical therapy nor surgery and are doing well.
The histopathological examinations of all patients that underwent pancreatectomy were diffuse in type. Out of the 15 patients who had pancreatectomy, 6 (patients-1, 3, 7, 11, 12, 18) had secondary diabetes mellitus and were treated by insulin therapy that ranged from multiple daily injections to subcutaneous continuous insulin infusion (insulin pump). Six patients (patients-4, 8, 13, 14, 15, 19) had hypoglycemia and required medical therapy and one patient developed exocrine pancreatic insufficiency and is currently following up in the Gastroenterology Clinic and is placed on pancreatic enzyme supplements. Patient-19 had hypoglycemia post pancreatectomy and was placed on octreotide subcutaneous injection 35mcg/kg/day and lanreotide intramuscular injection 30 mg once monthly, however her hypoglycemia has continued. Sirolimus was given to this patient, but the patient did not respond so this effort was stopped. She was investigated biochemically and showed very high insulin level. Abdomen magnetic resonance imaging (MRI) and 18-F-fluoro-L-DOPA positron emission tomography scan (PET-scan) found to have persistent of the disease. In addition, patient-4 was also found to have persistent of the disease after radiological confirmation with abdominal MRI and still has occasional hypoglycemia on octreotide on/off. Out of those who had surgery, only three patients (patient-9, 20, 25) remained with no complications during follow-up. All babies who were under follow up presented good developmental milestones, apart from patient-16 whose parents refused surgical treatment and (on medical treatment) showed gross motor and speech delayed, started also on sirolimus. At present he caught up in development, but cognitive function of the patient not assessed. Currently, he is walking, running and saying full sentences. The different demographic, clinical and laboratory details of these patients are presented in Table 2 (A and B).
Table 2a.
Demographic, clinical, and laboratory findings of patients with congenital hyperinsulinism in infancy.
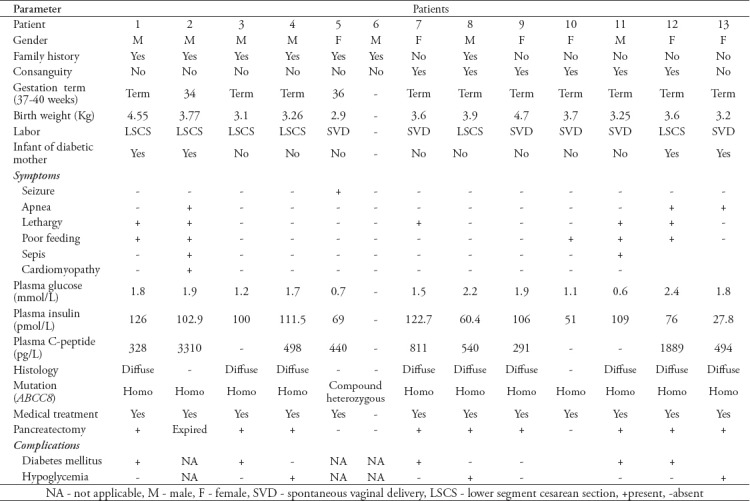
Table 2b.
Demographic, clinical, and laboratory findings of patients with congenital hyperinsulinism in infancy.
Discussion
In this study, we have evaluated the clinical, biochemical presentations, mutational analysis, and outcomes of 25 Omani patients who were confirmed to have CHI, between January 2007 and December 2016. Genetic analysis of the patients in our cohort revealed biallelic mutation in the ABCC8 gene, which represents a higher mutation rate of detection and could reflect the higher rate of consanguinity in the Omani community.23,24 All the patients are homozygous for the ABCC8 mutation, with only 2 siblings being compound heterozygotes that were lost follow-up.
Most patients with biallelic KATP channel mutation were unresponsive to diazoxide treatment despite high doses plus octreotide injection and could not be weaned off from high dextrose concentration apart from 2 patients who were off treatment. Similar findings were reported in different studies. Kapoor et al6 and Sinder et al25 reported in their studies that 63/300 (58%) and 88/417(31%) with biallelic KATP mutations were unresponsive to diazoxide. Both studies have not mentioned the consanguinity rate. In cohort study by Demirbilek et al26 in Turkish patients also found 43% (n=15) had biallelic KATP channel mutations who were unresponsive to diazoxide, our results are in line of these studies.
This study’s response rate is very low with only 2 out of 25 (8%) patients responding to medical therapy, which reflects the severity of the disease in our patient cohort. All those who did not respond well to medical therapy underwent pancreatectomy (n=15) in which showed diffuse type histopathology and none of them had focal type.
In addition to the lower response rate, 3 patients in this study expired with confirmed CHI during the neonatal period and two of them had hypertrophic cardiomyopathy, which has also been reported in other studies.27,28 Six patients developed post-pancreatectomy hypoglycemia, one had frequent hypoglycemia that required octreotide, lanreotide and sirolimus, although her hypoglycemia continued. Investigation showed re-growth of the pancreatic tissue. One patient began lanreotide treatment and responded well. Huang et al28 and Kühnen et al,29 showed that long acting lanreotide lowered the frequency of hypoglycemia and improved blood glucose concentrations with less side effects. All babies under follow-up reached developmental milestones, apart from one patient whose parents refused surgical treatment and was on medical treatment and had gross motor and speech problems. However, his patient caught up and is currently walking, running, and speaking in sentences. We used sirolimus for treating this patient with frequent hypoglycemia and had a good outcome. Sirolimus has been reported to be used in treating severe CHI with good results.30,31 However, extreme caution has to be taken, despite the absence of short-term side effects, the long-term sequels, particularly the risk of malignancy, has to be considered.32
In this cohort, most of our patients clinically during follow up have not detected to have long term neurological sequelae such as cerebral palsy and epilepsy only one patient who had developmental delay but caught up with the time. Demirbilek et al26 reported that 12 out of 35 (34%) patients developed some long-term neurological sequelae.
There are some limitations in this study. First, we do not have the 18-F-fluoro-L-DOPA positron emission tomography scan (PET-scan) in our institute that would enable us to decide if the operation is suitable. Instead, we depend on the clinical follow up of the patients, response to medical therapy and genetic study. Finally, the study was carried out in one tertiary-care hospital, which may not reflect the exact number of cases that are diagnosed at other centers, governmental, or private.
In conclusion, our study reveals the importance of detecting and investigating hypoglycemia in newborns in early neonatal life to prevent the sequels of severe hypoglycemia, particularly neurological damage. The diagnosis of CHI is important to ensure the provision of proper management and counseling regarding the risk of recurrence. The neonatologists and pediatricians must have a high index of suspicion for CHI whenever dealing with persistent hypoglycemia and inappropriate hyperinsulinism.
Footnotes
References
- 1.Palladino AA, Bennett MJ, Stanley CA. Hyperinsulinism in infancy and childhood:when an insulin level is not always enough. Clin Chem. 2008;54:256–63. doi: 10.1373/clinchem.2007.098988. [DOI] [PubMed] [Google Scholar]
- 2.Steinkrauss L, Lipman TH, Hendell CD, Gerdes M, Thornton PS, Stanley CA. Effects of hypoglycemia on developmental outcome in children with congenital hyperinsulinism. J Pediatr Nurs. 2005;20:109–118. doi: 10.1016/j.pedn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Kapoor RR, Flanagan SE, James C, Shield J, Ellard S, Hussain K. Hyperinsulinaemic hypoglycaemia. Arch Dis Child. 2009;94:450–457. doi: 10.1136/adc.2008.148171. [DOI] [PubMed] [Google Scholar]
- 4.Hussain K, Aynsley-Green A. Management of hyperinsulinism in infancy and childhood. Ann Med. 2000;32:544–551. doi: 10.3109/07853890008998834. [DOI] [PubMed] [Google Scholar]
- 5.James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet. 2009;46:289–299. doi: 10.1136/jmg.2008.064337. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor RR, Flanagan SE, Arya VB, Shield JP, Ellard S, Hussain K. Clinical and molecular characterization of 300 patients with congenital hyperinsulinism. Eur J Endocrinol. 2013;168:557–564. doi: 10.1530/EJE-12-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor RR, Heslegrave A, Hussain K. Congenital hyperinsulinism due to mutations in HNF4A and HADH. Rev Endocr Metab Disord. 2010;11:185–191. doi: 10.1007/s11154-010-9148-y. [DOI] [PubMed] [Google Scholar]
- 8.Flanagan SE, Kapoor RR, Hussain K. Genetics of congenital hyperinsulinemic hypoglycemia. Semin Pediatr Surg. 2011;20:13–17. doi: 10.1053/j.sempedsurg.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann AM LC, Freeze HH, Ficicioglu C, Kaestner KH, Stanley CA Hypoglycemia due to lower threshold of glucose-stimulated insulin secretion in phosphoglucomutase 1 deficiency. Platform Presentation at: Annual meeting of the Pediatric Academic Societies. San Diego (CA): University of Pennsylvania; 2015. [Google Scholar]
- 10.Cabezas OR, Flanagan SE, Stanescu H, García-Martínez E, Caswell R, Lango-Allen H, et al. Polycystic kidney disease with hyperinsulinemic hypoglycemia caused by a promoter mutation in phosphomannomutase 2. J Am Soc Nephrol. 2017;28:2529–2539. doi: 10.1681/ASN.2016121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinney SE, Ganapathy K, Bradfield J, Stokes D, Sasson A, Mackiewicz K. Dominant form of congenital hyperinsulinism maps to HK1 region on 10q. Horm Res Paediatr. 2013;80:18–27. doi: 10.1159/000351943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tegtmeyer LC, Rust S, van Scherpenzeel M, Ng BG, Losfeld ME, Timal S, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med. 2014;370:533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri D, Vignola ML, Gualtieri A, Scagliotti V, McNamara P, Peak M, et al. Novel FOXA2 mutation causes Hyperinsulinism, Hypopituitarism with Craniofacial and Endoderm- derived organ abnormalities. Hum Mol Genet. 2017;26:4315–4326. doi: 10.1093/hmg/ddx318. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan SE, Vairo F, Johnson MB, Caswell R, Laver TW, Lango Allen H, et al. A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr Diabetes. 2017;18:320–323. doi: 10.1111/pedi.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senniappan S, Shanti B, James C, Hussain K. Hyperinsulinaemic hypoglycaemia:genetic mechanisms, diagnosis and management. J Inherit Metab Dis. 2012;35:589–601. doi: 10.1007/s10545-011-9441-2. [DOI] [PubMed] [Google Scholar]
- 16.Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 17.Kane C, Shepherd RM, Squires PE, Johnson PR, James RF, Milla PJ, et al. Loss of functional KATP channels in pancreatic beta-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 18.Glaser B. Lessons in human biology from a monogenic pancreatic beta cell disease. J Clin Invest. 2011;121:3821–3825. doi: 10.1172/JCI60002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunne MJ, Kane C, Shepherd RM, Sanchez JA, James RF, Johnson PR, et al. Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med. 1997;336:703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- 20.Otonkoski T, Ammala C, Huopio H, Cote GJ, Chpman J, Cosqrove K, et al. A point mutation inactivating the sulphonylurea receptor causes the severe form of persistent hyperinsulinaemic hypoglycemia of infancy in Finland. Diabetes. 1999;48:408–415. doi: 10.2337/diabetes.48.2.408. [DOI] [PubMed] [Google Scholar]
- 21.Al-Nasser S, Sakati N, Al-Ashwal A, Bin Abbas B. Persistent hyperinsulinaemic hypoglycemia of infancy in 43 children:long term clinical and surgical follow-up. Asian J Surg. 2006;29:207–211. doi: 10.1016/S1015-9584(09)60089-0. [DOI] [PubMed] [Google Scholar]
- 22.Pierro A, Nah SA. Surgical management of congenital hyperinsulinism of infancy. Semin Pediatr Surg. 2011;20:50–53. doi: 10.1053/j.sempedsurg.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Al-Thihli K, Al-Murshedi F, Al-Hashmi N, Al-Mamari W, Mazharul M, Al-Yahyaee S. Consanguinity, endogamy and Inborn Errors of Metabolism in Oman:a Cross sectional Study. Hum Hered. 2014;77:183–188. doi: 10.1159/000362686. [DOI] [PubMed] [Google Scholar]
- 24.Rajab A, Abdel Aty MA, Jaju S, Morsi M, Al-Khaurasi H, Al-Shekaili W National reproductive Health SURVEY 2008. Muscat (Oman): Ministry of Health; 2008. [Google Scholar]
- 25.Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, et al. Genotype and phenotype correlations in 417 children with congenital hyper-insulinism. J Clin Endocrinol Metab. 2013;98:E355–E363. doi: 10.1210/jc.2012-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demirbilek H, Arya VB, Ozbek MN, Akinci A, Dogan M, Demirel F, et al. Clinical characteristics and phenotype–genotype analysis in Turkish patients with congenital hyperinsulinism;predominance of recessive KATP channel mutations. Eur J Endocrinol. 2014;170:885–892. doi: 10.1530/EJE-14-0045. [DOI] [PubMed] [Google Scholar]
- 27.Akcay T, Taskin N, Ulucan K, Kirac D. Congenital hyperinsulinism and cardiomyopathy. Fetal Pediatr Pathol. 2012;31:190. doi: 10.3109/15513815.2012.656831. [DOI] [PubMed] [Google Scholar]
- 28.Huang T, Kelly A, Becker SA, Cohen MS, Stanley CA. Hypertrophic cardiomyopathy in neonates with congenital hyperinsulinism. Arch Dis Child Fetal Neonatal Ed. 2013;98:F351–F354. doi: 10.1136/archdischild-2012-302546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kühnen P, Marquard J, Ernert A, Meissner T, Raile K, Wannenmacher G, et al. Long-term lanreotide treatment in six patients with congenital hyperinsulinism. Horm Res Paediatr. 2012;78:106–112. doi: 10.1159/000341525. [DOI] [PubMed] [Google Scholar]
- 30.Shah P, Arya VB, Flanagan SE, Morgan K, Ellard S, Senniappan S, et al. Sirolimus therapy in a patient with severe hyperinsulinaemic hypoglycaemia due to a compound heterozygous ABCC8 gene mutation. J Pediatr Endocrinol Metab. 2015;28:695–699. doi: 10.1515/jpem-2014-0371. [DOI] [PubMed] [Google Scholar]
- 31.Senniappan S, Alexandrescu S, Tatevian N, Shah P, Arya V, Flanagan S, et al. Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N Engl J Med. 2014;370:1131–1137. doi: 10.1056/NEJMoa1310967. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee I, De Leon D, Dunne MJ. Extreme caution on the use of sirolimus for the congenital hyperinsulinism in infancy patient. Orphanet J Rare Dis. 2017;12:70. doi: 10.1186/s13023-017-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


