Abstract
Objectives:
To investigate the correlation between the characteristics of urethral stricture and incision scars in patients with urethral stricture and median sternotomy incision.
Methods:
We identified 368 patients who had undergone internal urethrotomy between January 2014 and December 2017. A total of 49 male patients with a median sternotomy scar and diagnosed with urethral stricture were retrospectively evaluated. The median sternotomy incision scars were assessed using the Vancouver Scar Scale (VSS) and the patients were divided into 2 groups. Group I consisted of patients with a VSS score of <4 points, and those with ≥4 points constituted group II. The groups were compared in terms of age, smoking habit, body mass index, diabetes mellitus, hypertension, urethral stricture etiology, length and localization, and stricture relapse after intervention.
Results:
The mean total VSS score was 2.0 points in group I and 7.46 points in group II. There was a significant correlation between the VSS total score and the urethral stricture length among the whole study population (correlation coefficient value=0.481; p<0.001). The urethral stricture was longer as the VSS score increased.
Conclusion:
A poorly healed median sternotomy incision scar can predict a poor wound healing in the urethra tissue. Further large scale, multi-center and prospective studies are needed to clarify this relationship.
All urethral strictures develop after an injury to the urethral epithelium, except for congenital strictures.1 Fibrosis occurs as a result of the poor healing of this injured urethral tissue. Iatrogenic causes are the most frequent etiological factors in urethral stricture.2 These include transurethral insertion of Foley catheters and endoscopic interventions. Factors such as advanced age, obesity, diabetes mellitus (DM), smoking, and ischemia negatively affect the urethral health.3,4 However, not every patient having these risk factors develops a urethral stricture. This subject also brings up the question whether the urethrae of some individuals are more prone to stricture or not. It is known that urethral stricture is a wound healing problem.
Urethral strictures and surgical incision scarring are 2 independent examples of poor wound healing. Developments of urethral stricture and incisional skin scar proliferation have common pathophysiological features. Injury is the triggering factor in both the initiation of urethral stricture development and incisional scarring, and then a chronic inflammatory process plays role, in which many cells and molecules, particularly fibroblasts, are involved.5,6 For example, transforming growth factor beta 1 (TGF-β1) has been shown to play a role in the pathophysiology of fibrotic diseases.7,8 Furthermore, the increase of TGF-β1 levels has been demonstrated in the development urethral fibrosis.9
The prevalence of hypertrophic scars is 1.5-4.5% in all populations.10 In addition to individual genetic and systemic factors, some local factors induce pathological scar formation. Special anatomical regions such as the anterior chest wall and posterior ear are more prone to hypertrophic scar formation.11,12 Possessing a higher skin tightness in these regions facilitates scar formation.13 The development of hypertrophic scar after median sternotomy incision is frequently seen in patients undergoing cardiac surgery and the incidence is higher than 50% in the Asian population.14
Determination of the negative predictive factors in patients with urethral stricture may be a guide for planning optimal surgical interventions such as internal urethrotomy or urethroplasty. These factors may warn the surgeon to avoid urethral stricture prior to urethral manipulations. For instance, low-diameter transurethral catheters or instruments may be particularly preferred in these patients to avoid a possible urethral injury. Hypertrophic scars may be an independent factor in predicting the characteristics of urethral stricture. In this study, we evaluated patients with urethral stricture who had undergone median sternotomy incision in the past. We aimed to investigate the correlation between the characteristics of urethral stricture and incision scars in patients with urethral stricture and median sternotomy incision.
Methods
The medical records of 368 patients who had undergone internal urethrotomy between January 2014 and December 2017 in Sakarya University Medical School, Sakarya, Turkey. The Ethical Board has approved the study (App No: 71522473/050.01.04/67) and the study was conducted in accordance with the Declaration of Helsinki. A total of 49 patients with primary urethral stricture and median sternotomy incision scar, who had undergone internal urethrotomy, were evaluated retrospectively. The patients whose current data were deficient or patients who had undergone median sternotomy incision within the previous year were excluded from the study. Furthermore, those with urethral stricture history prior to thoracic surgery were not included in the study. All patients with symptoms of urethral stricture had undergone urine culture, uroflowmetry and retrograde urethrography (RUG). Direct vision internal urethrotomy was performed using a 21 Fr urethrotome (Karl Storz, Tuttlingen, Germanry). The urethral stricture length was measured intraoperatively during internal urethrotomy. The proximal and distal points of the stricture were determined at the border of the healthy mucosa with the diseased mucosa. The length was measured by the urethrotome. Intraoperative measurement of the stricture length was checked and verified with RUG. Urine culture, uroflowmetry and when necessary, RUG were followed-up on the routine control visit. The median sternotomy incision scars were graded using the Vancouver Scar Scale (VSS), in which pigmentation, flexibility, height, and vascularity items were considered (Table 1). Age, smoking status, DM, hypertension (HT), body mass index (BMI), previous urological interventions, etiology of urethral stricture, stricture length, and the localization and recurrence were evaluated. The median sternotomy scars and urethrography images of the same patients have been displayed in Figure 1 and Figure 2. The patients were divided into 2 groups according to the VSS. Group I consisted of patients with a VSS score of <4 points, and those with ≥4 points constituted group II.
Table 1.
The Vancouver scar scale for the assessment of the skin scars.
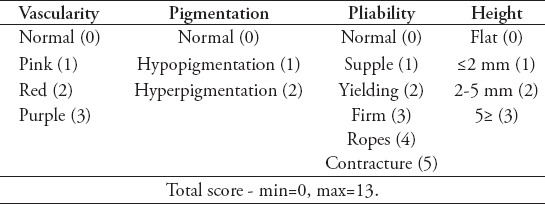
Figure 1.
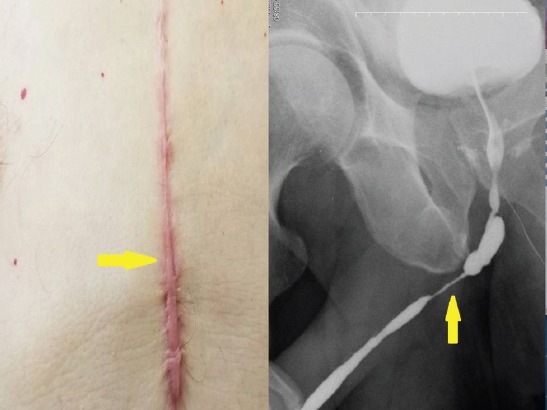
The sternotomy incision scar (Vancouver scar scale=9) and urethrograph of the same patient (yellow arrow).
Figure 2.
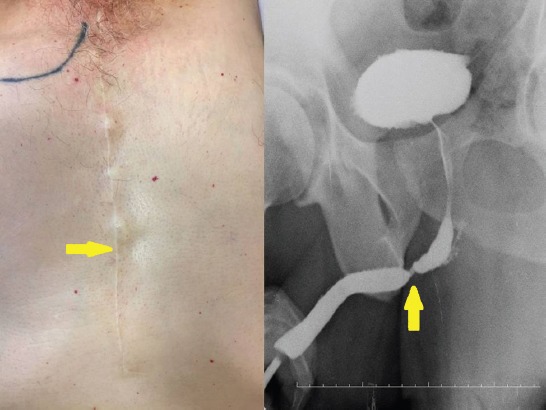
The sternotomy incision scar (Vancouver scar scale=2) and urethrograph of the same patient (yellow arrow).
Statistical analysis
The normal distribution of continuous numerical variables in the groups was tested with the Shapiro-Wilk normality test. The numerical variables were presented as mean±standard deviation (SD) and the categorical variables as number (%). The independent samples t-test was used for the comparison of continuous variables. The Pearson’s Chi-Square test and Fisher exact test were used to compare the categorical variables between the groups. The Spearman’s Rank Correlation Coefficient was calculated to show the direction and power of the relationship between the VSS score and the stricture length. The probability of Type I error was accepted as α<0.05 for all tests. Statistical analysis of the study was conducted with the Statistical Package for the Social Science version 22.0 (IBM Corp., Armonk, NY, USA).
Results
The mean age of the patients was 72 (40-82) years in group I and 69 (40-78) years in group II. All patients had undergone coronary artery by-pass operation (CABG) due to coronary artery disease (CAD). In all patients, urethral strictures occurred after urological instrumentation. Therefore, they were all considered iatrogenic strictures. Twenty-four (48.97%) of the 49 patients had undergone urethral catheterization due to various indications, and 25 (51.02%) had undergone urological surgery. There was no significant difference between the groups in terms of previous urological interventions. The mean VSS total score of group I was 2.0 points and that of group II was 7.46 points. There was no significant difference between the groups with regard to DM, HT and smoking habit. There was a weak correlation between BMI and the VSS scores (rs=0.309; p=0.042).
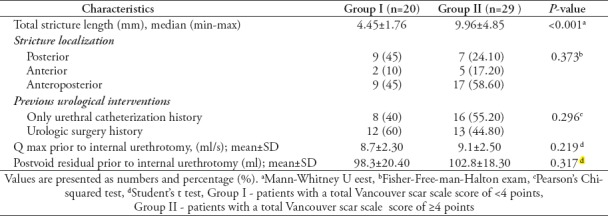
There was a moderate positive correlation between the VSS total score and the stricture length in all patients and this relationship was statistically significant (rs=0.481; p<0.001). As the VSS score increased, the stricture length also increased (Figure 3). The mean stricture length was 4.45±1.76 mm in group I and 9.96±4.85 mm in group II (p<0.001).
Figure 3.
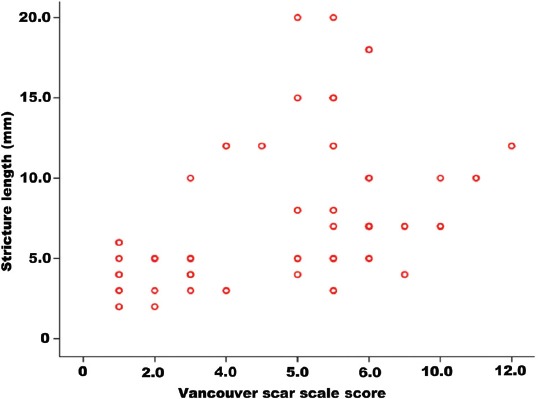
The relationship between total Vancouver scar scale score and stricture length.
When the relationship between the VSS score and stricture recurrence was evaluated, we observed that the recurrence rate in group II was 1.7 times higher than that in group I. There was a weak relationship between the VSS score and stricture recurrence, but this risk was not statistically significant (rs=0.209; p=0.154). The comparative data of the patients have been presented in Table 2 and Table 3.
Table 2.
Comparison of patient's characteristics across group I and group II.
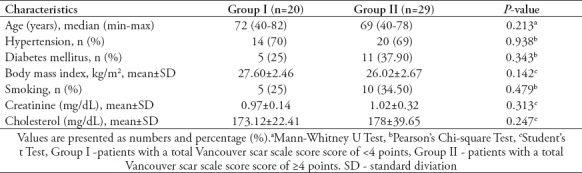
Table 3.
Comparison of the urethral stricture characteristics across group I and group II.
Discussion
Urethral stricture is a consequence of unfavorable wound healing, regardless of the etiology. Proliferative incisional scarring is another example of poor wound healing. It is difficult to show a direct relationship between incision scars and urethral stricture. Both pathologies represent wound healing disorder, but urethral strictures have functional consequences such as weak urine flow, while skin scars cause cosmetic problems. Scar-rating scales objectively evaluate the scar properties. The VSS is often used to examine skin scars. Skin scars are scored according to their physical properties with this scale.15 The 0 point in the scale represents a healthy skin, and 13 points represents the worst scar appearance. A cut-off value for the VSS score has not been identified in the literature for the description of hypertrophic scars. Different values were accepted in previous studies.16,17 Li-Tsang et al,17 accepted a total score of 4 or more for the definition of hypertrophic scars, with at least one point for each item in the scale. With reference to this study, we also divided patients into 2 groups; those with a VSS score of <4 points (group I) and those with a score of ≥4 points (group II).
Wound healing is quite a complex sequence of pathophysiological events. In this process, the stages of inflammation, proliferation and maturation follow each other.18 Regardless of the etiology, total wound healing is restoration of tissue integrity after injury. This restoration process may be defective in both urethral strictures and incisional scars. Factors such as the anatomy of the incision site, type of surgical operation, wound site inflammation, infection, tension and depth of the wound, and repair techniques affect the wound healing in surgical incision scars. The patient’s age, genetic, and hormonal factors are other important factors in wound healing. Chest wall injuries are more susceptible to healing with scar formation compared to other body regions.19,20 Proliferative scar development has a higher incidence in black people.21 Similarly, the urethral stricture rate has been reported to be higher in the black population.22
One of the most important predictive factors in the recurrence of urethral stricture is the stricture length.23 In our study, we observed that the urethral stricture was significantly longer in patients with marked incision scarring. Other negative predictive factors for urethral stricture development include CAD, smoking, DM and other ischemic conditions. All patients included in the study had CAD. Moreover, most patients had unfavorable factors in wound healing such as smoking, DM, HT and ischemia, which suggests that the urethral tissue of these individuals may have had a tendency towards poor wound healing. In our study, we observed that participants who had marked scarring experienced stricture recurrences more frequently, but this difference was not statistically significant. In our study, we observed that urethral stricture recurrences tended to be more frequent in those with marked incision scars, but there was no statistically significant difference. We think that this was due to the small number of patients enrolled in our study.
All patients had undergone CABG. There are no exclusion criteria in this regard. The patients included in the study did not have a history of heart valve operation or any other thoracic surgery. The development of urethral stricture in some patients after CABG operation has attracted the attention of urologists and many studies have been carried out in the past. Previous studies have investigated the effects of lubricant gels, chemical irritation due to Foley catheter, thickness of the Foley catheter used, trademarks, silicone or latex structure of the catheter, duration of catheterization, urethral ischemia and tissue perfusion changes during surgery on stricture development.24,25 Furthermore, it was demonstrated that providing urinary drainage with a suprapubic catheter during and after the operation reduced the urethral stricture development rates in this group of patients.26
There are few studies in the literature regarding the relationship between wound healing and coronary artery disease. Ziyrek et al,27 showed a close association between proliferative scarring and atherosclerosis in patients who had undergone CABG and reported that atherosclerosis was a wound healing problem. Dustan et al,28 reported that keloid formation may be associated with vascular diseases. Similarly, it has been shown that ischemia negatively affects the urethral tissue healing.29 In our study, there was no significant difference between the groups regarding the factors that could negatively affect wound healing, such as smoking, HT and DM. However, the smoking and DM rates were 2 times higher in patients with proliferative scarring than in others. It was also noted that the urethral stricture was longer in this patient group. Another factor that has a negative effect on wound healing is obesity. A positive correlation between obesity and hypertrophic scar formation has previously been reported.30 In our study, we found a statistically significant but weak correlation between the BMI and the VSS score.
Study limitations
The fact that all patients included in the study had CAD and undergone CABG was a limiting factor for our study. Median sternotomy incisions tend to heal with hypertrophic scar more frequently than other regions. Therefore, it is an area in which it is easy to make scar assessment. However, studies with scars on other body regions may provide further information on this subject. Another limitation was that we could not gather sufficient information regarding previous urologic surgical procedures, infections or traumas in our patients. There were no patients with urethroplasty operation among the patients included in the study. However, studies involving a sufficient number of patients with urethroplasty in this regard will be more helpful. Further studies conducted with a more homogenous patient population are needed to clarify this issue.
In conclusion, the present study has demonstrated that urethral strictures are longer in length in patients who have recovered with proliferative hypertrophic scarring after median sternotomy incision. If urethral stricture develops in the presence of hypertrophic scar in median sternotomy healing, the stricture is longer and tends to recur. In these patients, urethral interventions should be treated more gently. A poorly recovered median sternotomy incision scar may be a negative predictive factor for urethral stricture. Further large scale, multicenter and prospective studies are needed to clarify this relationship.
Acknowledgment
The authors gratefully acknowledge DRFAMEG Healthcare & Consultancy Ltd. for English language editing. They also thank Dr. Fikret Halis and Dr. H. İbrahim Çimen for sharing their patients with them during the course of this research. Lastly, to thank Dr. H. Can Direk for the statistics of the research.
Footnotes
References
- 1.Baskin LS, Constantinescu SC, Howard PS, McAninch JW, Ewalt DH, Duckett JW, et al. Biochemical characterization and quantitation of the collagenous components of urethral stricture tissue. J Urol. 1993;150:642–647. doi: 10.1016/s0022-5347(17)35572-6. [DOI] [PubMed] [Google Scholar]
- 2.Palminteri E, Berdondini E, Verze P, De Nunzio C, Vitarelli A, Carmignani L. Contemporary urethral stricture characteristics in the developed world. Urology. 2013;81:191–196. doi: 10.1016/j.urology.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 3.Harraz AM, El-Assmy A, Mahmoud O, Elbakry AA, Tharwat M, Omar H, et al. Is there a way to predict failure after direct vision internal urethrotomy for single and short bulbar urethral strictures? Arab J Urol. 2015;13:277–281. doi: 10.1016/j.aju.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011;107:6–26. doi: 10.1111/j.1464-410X.2010.09800.x. [DOI] [PubMed] [Google Scholar]
- 5.Bhavsar S, Nimigan A, Hackam DG, O'Gorman DB, Gan BS, Spence JD. Keloid scarring, but not Dupuytren's contracture, is associated with unexplained carotid atherosclerosis. Clin Invest Med. 2009;32:E95–E102. doi: 10.25011/cim.v32i2.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao WT, Yu HS, Arbiser JL, Hong CH, Govindarajan B, Chai CY, et al. Enhanced MCP-1 release by keloid CD14+cells augments fibroblast proliferation:role of MCP-1 and Akt pathway in keloids. Exp Dermatol. 2010;19:e142–e150. doi: 10.1111/j.1600-0625.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 8.Kamath VV, Krishnamurthy S, Satelur KP, Rajkumar K. Transforming growth factor-β1 and TGF-β2 act synergistically in the fibrotic pathway in oral submucous fibrosis:An immunohistochemical observation. Indian J Med Paediatr Oncol. 2015;36:111–116. doi: 10.4103/0971-5851.158842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang K, Chen J, Zhang D, Wang L, Zhao W, Lin DY, et al. microRNA expression profiles of scar and normal tissue from patients with posterior urethral stricture caused by pelvic fracture urethral distraction defects. Int J Mol Med. 2018;41:2733–2743. doi: 10.3892/ijmm.2018.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atiyeh BS. Nonsurgical management of hypertrophic scars:evidence-based therapies, standard practices, and emerging methods. Aesthetic Plast Surg. 2007;31:468–492. doi: 10.1007/s00266-006-0253-y. [DOI] [PubMed] [Google Scholar]
- 11.Jina NH, Marsh C, Than M, Singh H, Cassidy S, Simcock J. Keratin gel improves poor scarring following median sternotomy. ANZ J Surg. 2015;85:378–380. doi: 10.1111/ans.12520. [DOI] [PubMed] [Google Scholar]
- 12.Li YH, Yang J, Liu JQ, Xie ST, Zhang YJ, Zhang W, et al. A Randomized, placebo-controlled, double-blind, prospective clinical trial of botulinum toxin type A in prevention of hypertrophic scar development in median sternotomy wound. Aesthetic Plast Surg. 2018;42:1364–1369. doi: 10.1007/s00266-018-1187-x. [DOI] [PubMed] [Google Scholar]
- 13.Sherris DA, Larrabee WF, Jr, Murakami CS. Management of scar contractures, hypertrophic scars, and keloids. Otolaryngol Clin North Am. 1995;28:1057–1068. [PubMed] [Google Scholar]
- 14.Sproat JE, Dalcin A, Weitauer N, Roberts RS. Hypertrophic sternal scars:silicone gel sheet versus Kenalog injection treatment. Plast Reconstr Surg. 1992;90:988–992. [PubMed] [Google Scholar]
- 15.Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar:comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21:205–212. doi: 10.1067/mbc.2000.104750. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CM, Sood RF, Honari S, Carrougher GJ, Gibran NS. What score on the Vancouver Scar Scale constitutes a hypertrophic scar?Results from a survey of North American burn-care providers. Burns. 2015;41:1442–1448. doi: 10.1016/j.burns.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li-Tsang CW, Lau JC, Chan CC. Prevalence of hypertrophic scar formation and its characteristics among the Chinese population. Burns. 2005;31:610–616. doi: 10.1016/j.burns.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 19.Truong PT, Abnousi F, Yong CM, Hayashi A, Runkel JA, Phillips T, et al. Standardized assessment of breast cancer surgical scars integrating the Vancouver Scar Scale, short-form McGill pain questionnaire, and patients'perspectives. Plast Reconstr Surg. 2005;116:1291–1299. doi: 10.1097/01.prs.0000181520.87883.94. [DOI] [PubMed] [Google Scholar]
- 20.Jina H, Simcock J. Median sternotomy scar assessment. N Z Med J. 2011;124:57–62. [PubMed] [Google Scholar]
- 21.Child FJ, Fuller LC, Higgins EM, Du Vivier AW. A study of the spectrum of skin disease occurring in a black population in south-east London. Br J Dermatol. 1999;141:512–517. doi: 10.1046/j.1365-2133.1999.03047.x. [DOI] [PubMed] [Google Scholar]
- 22.Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol. 2007;177:1667–1674. doi: 10.1016/j.juro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 23.Kinnaird AS, Levine MA, Ambati D, Zorn JD, Rourke KF. Stricture length and etiology as preoperative independent predictors of recurrence after urethroplasty:A multivariate analysis of 604 urethroplasties. Can Urol Assoc J. 2014;8:E296–E300. doi: 10.5489/cuaj.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruutu M, Alfthan O, Heikkinen L, Järvinen A, Lehtonen T, Merikallio E, et al. “Epidemic” of acute urethral stricture after open-heart surgery. Lancet. 1982;1:218. doi: 10.1016/s0140-6736(82)90775-9. [DOI] [PubMed] [Google Scholar]
- 25.Wilksch J, Vernon-Roberts B, Garrett R, Smith K. The role of catheter surface morphology and extractable cytotoxic material in tissue reactions to urethral catheters. Br J Urol. 1983;55:48–52. doi: 10.1111/j.1464-410x.1983.tb07078.x. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz NP, Riehmann M, Gasser TC. Absence of urethral strictures with suprapubic urinary drainage during extracorporeal circulation. J Urol. 1993;150:337–339. doi: 10.1016/s0022-5347(17)35478-2. [DOI] [PubMed] [Google Scholar]
- 27.Ziyrek M, Şahin S, Acar Z, Şen O. The relationship between proliferative scars and endothelial function in surgically revascularized patients. Balkan Med J. 2015;32:377–381. doi: 10.5152/balkanmedj.2015.15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dustan HP. Does keloid pathogenesis hold the key to understanding black/white differences in hypertension severity? Hypertension. 1995;26:858–862. doi: 10.1161/01.hyp.26.6.858. [DOI] [PubMed] [Google Scholar]
- 29.Nacey JN, Delahunt B. Urinary catheter toxicity. N Z Med J. 1991;104:355–356. [PubMed] [Google Scholar]
- 30.Butzelaar L, Soykan EA, Galindo Garre F, Beelen RH, Ulrich MM, Niessen FB, et al. Going into surgery:Risk factors for hypertrophic scarring. Wound Repair Regen. 2015;23:531–537. doi: 10.1111/wrr.12302. [DOI] [PubMed] [Google Scholar]


