Abstract
Objectives:
To investigate the use of leukocyte-platelet rich fibrin on suppressing the porphyromonas gingivalis (PG-LPS)-induced secretion of proinflammatory cytokines.
Methods:
This quantitative experimental study was conducted at the School and Hospital of Stomatology, Jilin University, Changchun, China, between September 2017 and January 2019. A modified technique was used to obtain human gingival fibroblast cells (HGFCs). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Cell Counting Kit-8 tests were established to determine the proliferation rate. Human gingival fibroblast cells were treated by PG-LPS at different periods and the isolated mRNA was subjected to reverse transcription polymerase chain reaction and real time quantitative polymerase chain reaction. The release of platelet-derived growth factor and transforming-growth factor-β1 at various time intervals was observed.
Results:
We successfully established a modified technique for the production of HGFCs culture. One µg/mL PG-LPS was the recommended concentration to inhibit fibroblast proliferation. The expression of the pro-inflammatory cytokines messenger ribnucleic acid was notably raised at 3 and 6 hours post-PG-LPS treatment. The cumulative release of growth factors peaked during the first 24 hours and the production continued for 10 days. However, the fibroblast expression of cytokines was significantly suppressed after treatment with leucocyte- and platelet-rich fibrin (L-PRF).
Conclusion:
This study provided a novel way of obtaining HGFCs and greater understanding of the clinical impacts through the assessment of the anti-inflammatory properties of L-PRF in vitro.
Regenerative oral medicine aims to compensate any tissue loss caused by injury or disease with physiologically-identical engineered tissues. Platelet concentrates have drawn wide attention as a potential bioactive material for stimulating bone regeneration as they contain autologous additives that are easy to handle and bioactive constituents in high concentration.1,2 Platelet concentrates are increasingly used in modern research especially in the clinical field of growth factors, tissue engineering, and stem cells.3,4 Lately, an autologous platelet- and leucocyte-enriched fibrin matrix, which is also known as leukocyte-platelet rich fibrin (L-PRF), was presented as a second-generation of platelet concentrate.5 As compared to the first generation of platelet concentrate, namely, platelet rich plasma (PRP), the preparation of L-PRF is simpler and more straightforward.6 The preparation involves a slow and sustained release of growth factors. It can be obtained within 2 minutes by centrifuging freshly collected blood samples, without the need for anticoagulant or thrombin. Furthermore, PRF is able to demonstrate a slow and sustained release of several key growth factors up to 10 days, for example, vascular-endothelial growth factor (VEGF), transforming-growth factor-β1 (TGF-β1), platelet derived growth factor (PDGF), and connective tissue growth factor (CTGF).7 The growth factors do not entirely dissolve when accessed in the culture medium.8 In other words, PRP is able to exhibit nearly all the growth factors during the first few hours, and they completely dissolve only after a few days.9,10
In tissue engineering research, the culture of oral human fibroblasts can be used in many important applications as it works as a good seal for the interface between the teeth and oral mucosa.11 Moreover, oral human fibroblasts play a vital role in immune defense as they can produce a wide variety of cytokines. Furthermore, they also have one of the fastest tissue turnover rates in the body.12 Various techniques have been used in the cultivation of human gingival fibroblast cells (HGFCs), including enzymatic and direct explant techniques.13-15 The enzymatic technique focuses on isolating human keratinocytes using a variety Disclosure. This study was supported by the Department of Implantology, Stomatology Hospital, Jilin University and Jilin Provincial Science and Technological Dpartment for Development, Changchun, China (No. 20160101138JC). of materials (trypsin, dispase, or collagenase), while in the direct explant technique, the epithelial layer of gingival tissue will be removed and then sliced into small pieces (one mm3) before being placed directly into culture flask. This study applied the direct explant technique. As compared to the enzymatic technique, direct explant technique involves fewer steps and shows a higher cultivation rate (82%) and thus this technique has been widely used.16
Based on the theory proposed by Lang et al,17 a series of events would take place during acute localized inflammation. Firstly, cytokines such as IL-1β, TNF-α, and IL-6 are released by the activated macrophages in the acute localized inflammatory site. This, in turn, induces chemokines secretion that would interact with proteoglycans in order to form a gradient in the inflamed side and along the endothelial wall.17 Inflammatory mediators which play a crucial part in programming inflammatory response are also involved in neoplasia events via the secretion of prostaglandins and inflammatory cytokines such as TNF-α, IL-1β, and IL-6. These series of activities create an environment that encourages cell proliferation and survival.18-20
In spite of the extensive use of PRF in a variety of surgical and clinical procedures, very few researches have investigated the microbicidal activity of platelets to date.21-23 In addition, there are many protocols for platelet concentrates preparation and various possibilities to produce different components from the concentrates, making it difficult to define the biological and antibacterial properties of platelet concentrates.24 The aim of our research to prove that L-PRF can suppress the secretion of pro-inflammatory cytokine in vitro. To investigate this hypothesis, HGFCs must first be cultured, then challenged with lipopolysaccharide from Porphyromonas gingivalis (PG-LPS) and treated with L-PRF. This study aims to clarify a modified preparation of HGFCs culture. It also aims to highlight the growth factors that are released at different time points when HGFCs were challenged with PG-LPS. Thereafter, the concentration of inflammatory cytokines (TNF-α, IL-1β, and IL-6) would be demonstrated to support the results of this research. Thirdly, the expression of growth factors would be observed at different time points when the PG-LPS challenged HGFCs are treated with PRF.
Methods
Human gingival fibroblast cell culture
This experimental study was conducted at the School and Hospital of Stomatology, Jilin University, Changchun, China, between September 2017 and January 2019. Clinically normal gingival fragments were obtained from a healthy patient undergoing implant operations at the Department of Implantology following the bio-ethical guidelines (ID=27) of Jilin University. Three individuals were selected based on the inclusion criteria: 1) healthy men and women over 18 years of age who are eligible for signing consent, 2) patients have understood and consented to the nature and the purpose of the study, and 3) patients with healthy gingival and connective tissues surrounding the tooth that will be extracted. The age and the gender of the subjects providing the gingival samples do not appear to be factors influencing the success rate in the culturing process. Signed informed consent was requested from the patients in accordance to the principles of Helsinki Declaration. The explants were collected under local anesthesia (2x2x1 mm) by scalpel, then directly minced in a sterile 15 mL centrifuge tube containing Dulbecco modified eagle medium (DMEM, HYCLONE, Utah, USA), 10% fetal bovine serum (FBS, GIBCO, Grand island, USA), and 10% penicillin & streptomycin (PS; penicillin 10,000 units/mL, streptomycin 10,000 ug/mL [HYCLONE, Utah, USA]), before being directly transferred to the laboratory. The gingival specimen was then transferred onto a culture plate. Our new technique involved transferring the specimen to the laboratory within 15 minutes after explant, followed by pipette washing of the specimens, firstly with DMEM containing 10% PS, followed by a second wash with phosphate buffered saline containing 10% PS (PBS, HYCLONE, Utah, USA). The samples were then transferred into another centrifuge tube containing dispase (SIGMA, Darmstadt, Germany) for fixation (12 hours at 4°C) to facilitate the removal of the connective tissue from the epithelial layer. Sterile scissors and tweezers were used to scrape the connective tissues to ensure the removal of the remaining epithelial layer debridement. After mincing, the connective tissue was cut into small pieces (0.5 mm3) using sharp scissors and placed into a 25 cm2 culture flask (CORNING CORP, NY, USA). The culture flask was inverted for 2 hours to induce adherence of the small pieces of connective tissue. Following that, the flask was gently turned over, with 4 mL of DMEM supplemented with 10% fetal bovine serum, and incubated at 37°C in 5% CO2 incubator. We have checked the cells microscopically (OLYMPUS cellSens entry system, 40-fold magnification). When the cells reached 80% confluency, the cells were sub-cultured using 0.25% trypsin/EDTA solution (GIBCO, Grand Island, USA).
Cell growth assay
Part of the gingival specimens was used for this. The same number of 0.5 mm3 pieces of connective tissue were cultured in each of the 25 cm3 flasks. Using the above-mentioned method, the flask was gently turned over after 2 hours, with 4 mL of DMEM supplemented with 10% fetal bovine serum and one percent PS. Before harvesting, the tissues pieces were transferred with great care to new flasks for further generation of new fibroblast cells. Fibroblasts were harvested at each time point (days 5, 7, 9, 11, and 13). For harvesting, the cells would be washed 3 times by phosphate buffered saline (PBS), before detaching the cells by using 0.25% trypsin/EDTA solution, and counting them with a Counter Cells Neubauer Hemocytometer (Thomas Scientific, USA).
Assay for cell viability
A modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was established to evaluate cell viability rate. The cellular suspension in the culture medium was added to 24-well plates (1000, 2000, 4000, 6000, 8000, and 10000 cells/mL). After 3 days, 100 µL of MTT solution, 5 mg/mL in PBS (SIGMA, Darmstadt, Germany) was added into each well, followed by incubation under standard conditions for 4 hours. The medium was removed after the incubation, and solubilization was performed by adding acidified alcohol to each well. The solution was then pipetted into a microplate containing 20 µL of 3% sodium dodecyl sulfate (SDS). The change of absorbance at 550 nm was determined using an automated microplate reader (BioTek, Vermont, USA).25
Determination of PG-LPS concentration
Human gingival fibroblast cells were counted manually with a Neubauer Hemocytometer. Firstly, we have used a 96-well plate to culture the fourth and fifth generation of HGFCs at a density of 5x103 cell/well. After 24 hours of incubation, the cells were exposed to different concentrations (0.5, 1, 5 and 100 µg/mL) of PG-LPS (San Diego, NY, USA) for 48 hours. The cell-counting kit-8 (CCK-8) (Kumamoto, Japan) was utilized in this experiment in order to determine the required PG-LPS concentration. After the allocated time, 10 µL of CCK-8 was added into each well and further incubated for an additional 2 hours at 37°C. The change of absorbance at 450 nm was then determined by using an automated microplate reader.
Real time quantitative polymerase chain reaction analysis
The fourth to fifth generation of HGFCs were challenged with PG-LPS (one µg/mL) followed by 3, 6, 12, 24, and 48 hours of incubation at 37°C with 5% CO2. After incubation, the cells were collected for messenger ribonucleic acid (mRNA) extraction in order to proceed to real time quantitative polymerase chain reaction (RT-qPCR). The whole process of total RNA extraction was carried out with TRizol (TAKARA, Dalian, China) based on the manufacturer’s protocol. After this, reverse transcription from RNA to complementary ribonucleic acid (cDNA) was executed with a total of 1000 ng of isolated RNA by using the High Capacity RNA-to-cDNA Master Mix (TAKARA, Nojihigashi, Kusatsu, Japan). The thermal cycling was held at 6 cycles at 37°C for 15minutes, followed by 40 cycles at 85°C for 5 seconds, and at 4°C for ∞. High copy cDNA amplification was performed with SYBR Green Master Mix (TAKARA, Nojihigashi, Kusatsu, Japan) PCR by using an ABI Prism 7700 Sequence Detector (Applied Biosystems, Japan). The primer sequences were selected in this study are listed below:
Human Actin: 5′-AGA GCT ACG AGC TGC CTG AC-3′ and 5′-AGC ACT GTG TTG GCG TAC AG-3′.26
Human IL-1β: 5′- ATG GCA GAA GTA CCT GAG CTC-3′ and 5′-TTC CTT GAG GCC CAA GGC CAC-3′
Human TNF-α: 5′-CCC TCA AGC TGA GGG GCA GCT CCA G-3′ and 5′ GGG CAA TGA TCC CAA AGT AGA CCT G-3′
Human IL-6: 5′-CAA AGA ATC TAG ATG CAA TAA-3′ and 5′-GCC CAT TAA CAA CAA CAA TCT G-3′.27
An endogenous control gene, actin, was included for data normalization in RT-qPCR. The relative expression was calculated based on delta-delta CT method. All of the RT-qPCR tests were repeated twice in this study.
Platelet rich fibrin preparation and determination of the growth factors release
To prepare the PRF, 3 blood samples were collected from the same 3 patients from which the gingival tissues were explanted for the cell cultures (Ethical approval ID/number: 27). A total of 10mL of blood sample was collected into glass-coated tubes (Vacutainer; BD Bio-sciences, Allschwil, Switzerland) and the blood sample was centrifuged instantly at 3000 rpm (1278 g) for 12 minutes at room temperature (Fixed-angle rotor F-35-30-17, centrifuge 5702; Eppendorf, Darmstadt, Germany). Platelet rich fibrin layer was interspersed in the middle. This clot comprised of white cells and platelets enmeshed within fibrin, a 3-dimensional structure.28 After obtaining the PRF, it was immersed in 15 mL of DMEM without serum or antibiotics to estimate the concentration of TGF-β1 and PDGF (subtype AA) at various time intervals as indicated (1, 2, 3, 24, 48, 72 hours, and 10 days). The growth factors were measured using a commercially available sandwich Enzyme-linked Immunosorbent Assay (ELISA) kit (CLOUD-CLONE CORP. CCC, USA) designed for human use. The ELISAs were carried out based on the manufacturer’s instructions and the absorbances were read at 450 nm by a micro-plate reader. All results were reported as the total weight of molecules (nanograms) per one mL of supernatant volume. The concentration of growth factors at each time point was estimated to decide the optimum point for growth factor release. In addition, the concentration of growth factors at each time point was summed up to compare the cumulative total between the 3 samples.
Estimation of pro-inflammatory cytokines concentration
Fourth and fifth generation of HGFCs were used (density of 6 × 105 cells/mL), and 3 blood samples were taken from the same patient. Leukocyte-platelet rich fibrin was prepared following the standard protocols. In order to determine the maximum cumulative growth factors that were released and could be obtained in the first 24 hours, we collected the medium at that time-point and used it to estimate the effect of cytokines secretion from the cells. At least 24 hours after cell’s sub-culture were enough to let the cells to stick on the surface of the flask before starting proliferation. Three flasks sample were determined as (control group; cells treated with PG-LPS (one µg/mL); and cells treated with PG-LPS (one µg/mL + L-PRF). Flasks-sample were incubated in a standard condition (21% O2, and 5% CO2 at as 37°C). After allocated time, the medium was collected and the released IL-1β, TNF-α, and IL-6 were measured using the ELISA kits (CLOUD-CLONE CORP. CCC, USA) by following the manufacturer provided instructions. The absorbance rate was measured using microplate at 450 nm.
Oral mucositis induction and L-PRF application
We performed this in vivo study on 12 New Zealand white rabbits. A round filter paper with a diameter 4 mm was soaked with 50% acetic acid and applied over the upper labial gingiva for 60 seconds, which created a uniform ulcer. The ulcerated area was measured with a computer program (ImageJ, NIH, USA). Conservative semilunar incision was established to create a pouch under the inflamed site. Leukocyte-platelet rich fibrin was applied over the ulcerated areas in the experimental group (6 rabbits) on the 4th day (day 0) after creation of oral ulcer. Leukocyte-platelet rich fibrin was not applied in the control group (6 rabbits). Measurement started at the fourth day after inflammation induction (Day 0), when the border of the ulcers became clear. Each ulcerated area was expressed as a percentage of the ulcerated area on day 0. All animals were observed macroscopically on days 0, 7, and 14.
Statistical analysis
Quantitative analyses were carried out using GraphPad Prism version 6 (GraphPad Software, San Diego, USA) with one-way and 2-way analyses of variance (ANOVA) as explained in the result figures. In this study, one-way ANOVA was performed to determine the viability rate of HGFC after 48 hours incubation with different concentration (0.5, 1, 5 and 100 µg/mL) of PG-LPS. Furthermore, the data concerning the expression of proinflammatory cytokine by human gingival fibroblast cells (HGFC) please provide the abbreviation in full at different time points after incubation with one µg/mL PG-LPS were also statistically analyzed using one-way analysis of variance (ANOVA). In addition, the cumulative release of 2 differential growth factors at variety time points were performed using 2-way ANOVA.
Results
In this study, the successfully cultured HGFCs were obtained from the donor biopsies collected from the gingival tissue. The first adherent fibroblast cells appeared after 4 days. Under optical microscopy, the primary cells displayed a spindle-shaped fibroblast-like morphology. The tissue samples of smaller pieces (0.5 mm3) grew faster than the larger ones. The explant technique utilized tissues of smaller sizes with sharp edges.29 Approximately 80% confluency was achieved after 7-9 days of incubation and a non-overlapping cell monolayer was observed, indicating that the cells were ready to be sub-cultured. These cells grew and proliferated more rapidly after the first generation and the cell appearance became radial or whorl-shaped (Figure 1).
Figure 1.
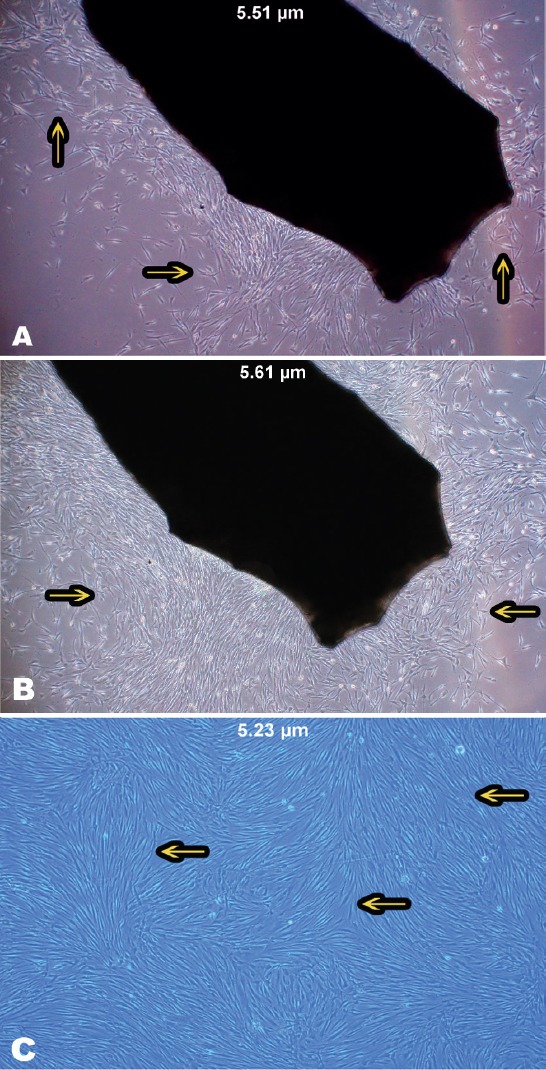
Growth of human gingival fibroblast cells on a gingival biopsy. A) Morphology of spindle-shaped fibroblast cells. B) An 80% confluence of a full monolayer without overlapping cells was observed after 7-9 days. C) Human gingival fibroblast cell appeared radial or whorl-shaped after the first generation. Images of the cells were taken with OLYMPUS cellSens entry system, 40-fold magnification.
The rate of cellular proliferation and growth of the HGFCs were determined from day 5-13. A relative increase in the growth rate was noted during the selected periods, and also the cellular proliferation rate was raised significantly between the 8 gingival biopsy groups during the selected period at each time point (p<0.001) (Figure 2). Currently, colorimetric MTT techniques are used in many studies to assess cellular proliferation.30 In MTT assay, the reaction occurred was restricted only to living cells by cleaving the tetrazolium salt present in active mitochondria. After 3 days, the change of absorbance at 550 nm was determined using an automated microplate reader. The graph of absorbance versus the number of cells is plotted in Figure 2B. The absorbance rate which led to an increase in the number viable cells was significantly increased by the number of cells (p<0.001). Figure 2B shows the means values of triplicate wells from 5 plates. After 48-hours post-treatment with 100 µg/mLlPG-LPS, HGFC viability decreased drastically to 25% (Figure 2C). Incubation with one µg/mL PG-LPS showed a slight decrease in viability rate, indicating the initiation of the inflammatory response. Based on this, one µg/mL PG-LPS was further applied in all the subsequent time course experiments.31
Figure 2.
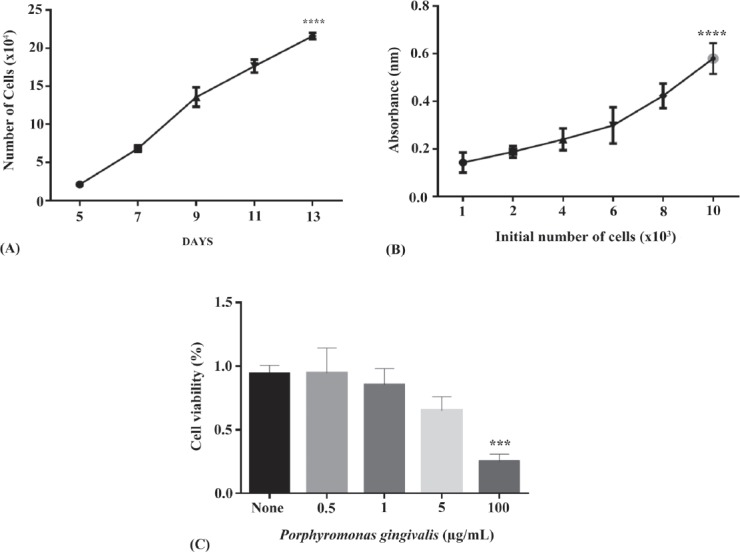
Cell cultures were tested to support the modified methodology. A) Growth rate of human gingival fibroblast cells (HGFCs) was identified from the 5-13th day. B) MTT formazan absorbance played a role as a function of cellular proliferation for a range of cell concentrations. C) Cell viability test on HGFC with porphyromonas gingivalis (PG-LPS) at various concentrations (0.5, 1, 5, and 100 µg/mL) after 48 hours using cell counting kit-8. Based on the one-way analysis (ANOVA) the result showed that 100 µg/mL porphyromonas gingivalis treatment were significantly different compared to control. *** and ****p<0.0001
After incubation with PG-LPS (one µg/mL), the mRNA expression of the proinflammatory cytokines (IL-1β; IL-6; TNF-α) at various time points (3, 6, 12, 24, and 48 hours) was documented and plotted (Figure 3). Cells incubated with one µg/mL PG-PLS for 0 hours, were used as a negative control for this experiment. As illustrated in Figure 3, the mRNA expression for the 3 proinflammatory cytokines exhibited a dramatic increase at earlier culture periods (3 and 6 hours), but not at the later culture periods (24 and 48 hours) as compared to control. These results highlighted the positive connection between PG-LPS and inflammatory responses at different time points.
Figure 3.
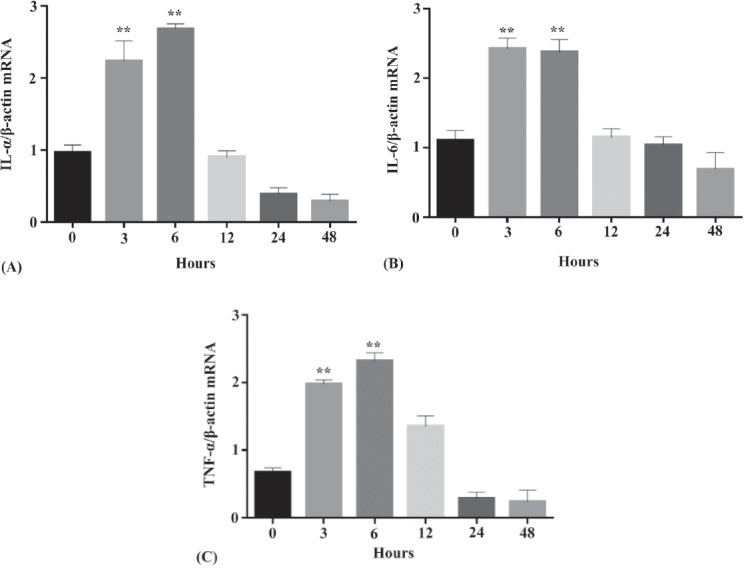
Expression of the 3 recommended proinflammatory cytokines by human gingival fibroblast cells after incubation with one µg/mL PG-LPS at different time points (3, 6, 12, 24, and 48 hours) messenger ribonucleic acid (mRNA) expression level of A) IL-1β/β -actin mRNA, B) IL-6/β -actin, and C) TNF-α/β-actin. Based on the one-way analysis analysis of variance. **p<0.001 demonstrated a statistically significant difference as compared to experimental control. TNF-a - Tumor necrosis factor-alpha, IL-1b - interleukin-1beta, IL-6 - interleukin-6
Leukocyte-platelet rich fibrin was prepared and the released differential growth factors (PDGF-AA and TGF-β1) were quantified at different time points (1-3, 24, 48, 72 hours, and 10 days) using Eliza method. Leukocyte-platelet rich fibrin was considered as one of the most effective platelet concentrates owing to its slow and sustainable release properties.32,33 This procedure was well-tolerated by the donor (ID:27). An obvious correlation was identified between the total subsequent growth factor concentrations of the 3 samples. According to Figure 4, approximately 80 pg/mL of PDGF-AA was released in the first hour and the concentration was reduced to nearly 50% in the second hour. On the contrary, approximately 10 pg/mL of TGF-β1 was released in the first hour, 10 pg/mL of TGF-β1 in second hour, and the concentration of TGF-β1 doubled in the third hour. The cumulative release of growth factors (PDGF-AA and TGF-β1) was measured in term of concentration (pg/mL) over 10 days. Significantly greater values of PDGF-AA were immediately obtained among all the time points between the 2 growth factor groups (p<0.001) (Figure 4). There was a significant decrease in the cumulative yield over time when both growth factors were compared at each time point. It was found that the cumulative release of PDGF-AA was moderately higher in comparison to TGF-β1 (p<0.001) (Figure 4).
Figure 4.

A) The released differential growth factors (PDGF-AA and TGF-β1) were quantified using Enzyme-linked Immunosorbent Assay method at various time points (1-3, 24, 48, 72 hours, and 10 days). B) Cumulative release of differential growth factors (PDGF-AA and TGF-β1) in term of concentration (pg/mL) at different time points. Based on the two-way analysis (ANOVA), **p<0.002 determines a statistically significant higher release than all the other groups, #p<0.002 determines a statistically significant lower release than all the other groups. PDGF-AA - platelet derived growth factor, TGF-b1 - transforming growth factors-beta 1, GF - growth factor
The effect of L-PRF on the PG-LPS-induced secretion of pro-inflammatory cytokines including IL-1β, TNF-α, and IL-6 by HGFCs into the cell culture medium were measured by ELISA after PG-LPS exposure (21% O2, and 5% CO2 at as 37°C, 24 hours). The average concentrations of IL-1β, TNF-α, and IL-6 in the culture medium of HGFCs were significantly increased at 24 hours after challenged with PG-LPS. Another surprising finding was the PG-LPS-induced secretion of proinflammatory cytokine by HGFCs were able to be suppressed with the presence of L-PRF (Figure 5).
Figure 5.
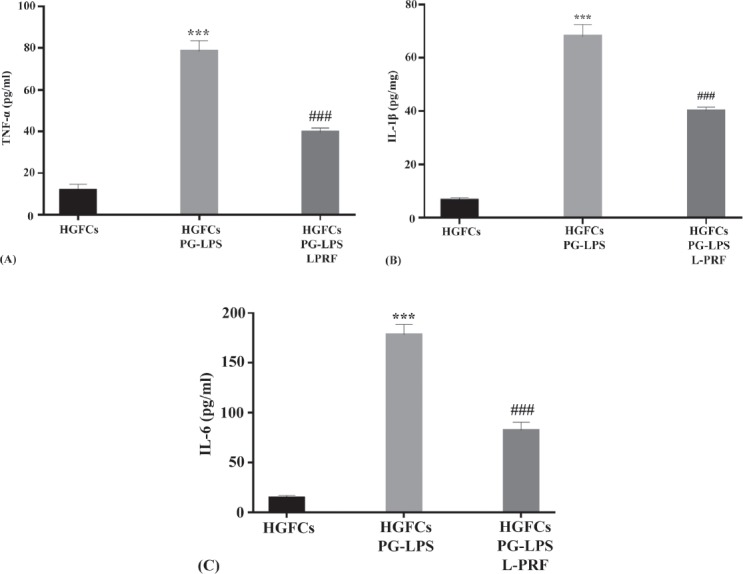
Suppressive effects of L-PRF on the PG-LPS-induced proinflammatory cytokine releasants by HGFCs. The concentration of IL-1β, TNF-α, and IL-6 detected in the culture medium of PG-LPS challenged HGFCs in the presence or absence of L-PRF was quantified with ELISA method after 20 hours. Based on the two-way analysis (ANOVA), ***p<0.001 illustrated a statistically significant higher concentration from HGFCs alone, while ###p<0.001 demonstrated a statistically significant lower concentration as compared to PG-LPS challenged HGFCs. L-PRF - leukocyte-platelet rich fibrin, PG-LPS - lipopolysaccharide from porphyromonas gingivalis, TNF-a - tumor necrosis factor-alpha, IL-1b - interleukin-1beta, IL-6 - interleukin-6, HGFCs - human gingival fibroblast cells
With regard to the preliminary findings from the animal experiments, it was found that the area of ulcer was significantly smaller in the L-PRF-treated group than in the control group on day 7, indicating a more rapid healing process with the application of L-PRF. On day 14, the remaining mucosal ulcer almost disappeared completely in the L-PRF-treated group; while small and tiny ulcers were still detectable in the control group (Figure 6).
Figure 6.
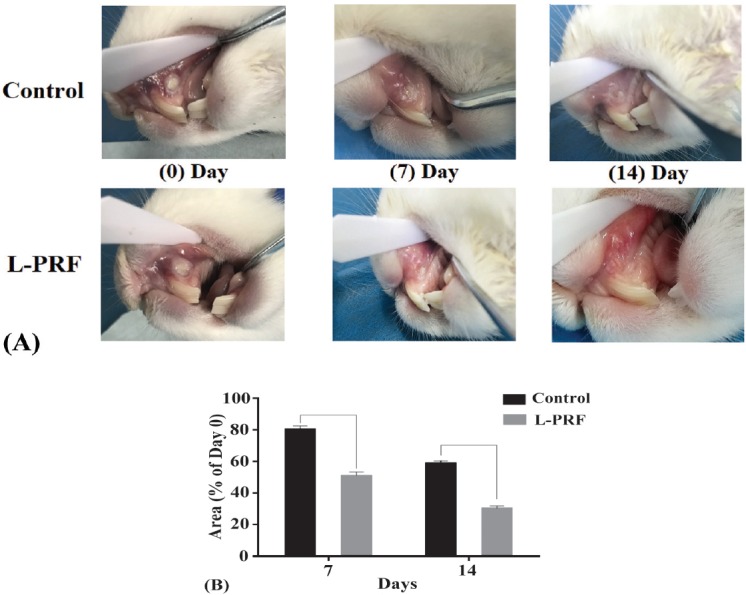
Macroscopic observation showing the comparison of the ulcerated area between the L-PRF-treated group and control group. A) The pictures show the more superior healing progress for the L-PRF group. B) The mucositis induced area was significantly smaller in the L-PRF-treated group than the control group by day 7 and day 14. L-PRF - leukocyte-platelet rich fibrin
Discussion
In recent years, the significant advances in tissue engineering technology and implantology have led to their versatile applications in different areas of dentistry. In this study, we describe a modified method of HGFC primary tissue culture which is simple and rapid to perform. Human gingival fibroblast cells were isolated from a small biopsy sample (2x2x1 mm3) and the efficiency of the in vitro culture of these cells was enhanced. In contrast to our method, some studies described the enzymatic isolation of HGFCs with dispase, or digestion with collagenase.34 Since the fibroblast isolation technique used in this study was based on explanting tissues from the oral gingiva, this procedure has 2 added advantages. Firstly, it resulted in rapid production of HGFCs, as sufficient fibroblasts can be obtained within 10 days. In addition, fibroblasts could be produced continuously and these reusable gingival tissues would be an advantage compared to other procedures.
This study showed that the notable elevation in the mRNA expression of pro-inflammatory cytokines in the early culture periods might have been an absolute immune response towards pathogenic agonist agent (for example PG-LPS) challenge during the acute activation in HGFCs. This scenario was in concordance to the other results reported in the literature.35 In this study, serial dilution of PG-LPS ranging from 0-100 µg/mL was incubated with HGFCs over 48 hours. Porphyromonas gingivalis was selected in this experiment as it was able to trigger cell inflammation which would eventually initiate a series of inflammatory response, including an elevation in the secretion of proinflammatory cytokines.36 As shown in Figure 2, the viability rates of HGFCs when challenged with 1 µg/mL of PG-LPS was almost equivalent to the experimental control. However, the viability rates for HGFCs when challenged with 100 µg/mL of PG-LPS was less than 30%. These results concluded that PG-LPS only had an effect on the cell viability of HGFCs at higher concentration. According to our results, the release of the growth factors peaked at 24 hours, while the release of mRNA pro-inflammatory cytokines peaked at 6 h. Many studies showed that the peak of protein pro-inflammatory cytokines expression was at 24 hours, which was slower that mRNA expression of cytokines. Therefore, we focused on the period between 6-24 hours in our study to examine the pro-inflammatory cytokines expression after the treatment with L-PRF.
Thereafter, the release of growth factors from L-PRF was predicted. PDGF-AA and TGF-β1 were the focus of this study due to the hypothesis that these growth factors exhibit angiogenesis and anti-inflammatory effects.37,38 Platelet-rich concentrates consist of both anabolic and catabolic factors derived from platelets and produced by leukocytes play an important role in promoting tissue healing. Moreover, investigation on growth factor concentration and release kinetics have been extensively discussed in the literature and showed similar results.39 One of the great benefits of PRF over other platelet concentrates lies within the potential of PRF to be handled as a true solid biomaterial.40 The most important adjunct molecule in PRF is fibrin, which provides a therapeutic advantage over traditional PRP. The first 24 hours showed the highest cumulative release of growth factors.
Peri-implants and gingival inflammation are the 2 essential pathological mechanisms involving dental surgeries.41 There have been many published studies focusing on how best to improve tissue repair and tendon injuries in view of the low capacity for tendon regeneration due to the low oxygenation and nutrition in the surrounding area.43 However, very few researches have investigated the platelet concentrates’ microbicidal activity. We focused our experiments on the anti-inflammatory effect of L-PRF. This data set would be able to provide useful information for future studies on tissue regeneration and wound healing in infection or post-operative inflammation. Our study would also add to the evidence available on the critical role PG-LPS on stimulating the secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IL-6. There have been few published studies which used the PG-LPS-induced secretion of pro-inflammatory cytokines as an indicator for determining the anti-inflammatory effects of some extracts.43
From this study, it was found that L-PRF significantly inhibit the PG-LPS-induced secretion of proinflammatory cytokines (TNF-α, IL-1β and IL-6) in HGFCs. Proinflammatory cytokines were secreted into the culture medium after being challenged with PG-LPS. However, L-PRF efficiently attenuated the secretion of cytokines in gingival fibroblasts. Thus, this might be potentially beneficial in the prevention and treatment of gingival inflammations. Furthermore, preliminary results from our ongoing animal experiment showed that L-PRF-treated group had more promising results than control group in reducing inflamed gingival tissues. This finding indicated that the second generation of the platelet concentrates or L-PRF may be of high potential in preventing any inflamed-related deficits.
In conclusion, explant technique used in the culturing of HGFCs was able to provide sufficient number of cells for this experiment within 10 days. Besides, our study showed that 1 µg/mL of GP-LPS was able to elicit the inflammatory condition, which eventually resulted in the release of proinflammatory cytokines by inflamed HGFCs. This study provides the first evidence of potential anti-inflammatory effects of L-PRF. The protective effect was linked to a reduction of the release of cytokines (IL-1β, IL-6, and TNF-α) by inflamed HGFCs. Thus, this helps in the prevention and treatment of possible deficits caused by inflammation. For future research, we intend to clarify the synergistic and additive effects of autologous L-PRF components on anti-inflammation process. The findings will also strengthen the use of HGFCs in the field of tissue engineering.
Acknowledgment
The authors gratefully acknowledge proofreading by UK PhD company (http://www.facebook.com/proofreadingbyphd) for English language editing.
Footnotes
References
- 1.Chen FM, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi: 10.1016/j.progpolymsci.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiran NK, Mukunda K, Tilak Raj TN. Platelet Concentrates:A Promising Innovation in Dentistry. J Den Sci Res. 2011;2:50–61. [Google Scholar]
- 3.Bielecki T, Dohan Ehrenfest DM. Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF):surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr Pharm Biotechnol. 2012;13:1121–1130. doi: 10.2174/138920112800624292. [DOI] [PubMed] [Google Scholar]
- 4.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates:from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF):a second-generation platelet concentrate Part I:technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37-–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Mudalal M, Zhou YM. Biological additives and platelet concentrates for tissue engineering on regenerative dentistry basic Science and concise review. Asian J Pharm. 2017;11:255–263. [Google Scholar]
- 7.Kang YH, Jeon SH, Park JY, Chung JH, Choung YH, Choung HW, et al. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349–359. doi: 10.1089/ten.TEA.2010.0327. [DOI] [PubMed] [Google Scholar]
- 8.Zumstein MA, Bielecki T, Dohan Ehrenfest DM. The future of platelet concentrates in sports medicine:platelet-rich plasma, platelet-rich fibrin, and the impact of scaffolds and cells on the long-term delivery of growth factors. Oper Techn Sport Med. 2011;19:190–197. [Google Scholar]
- 9.Bertrand-Duchesne MP, Grenier D, Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J Periodontal Res. 2010;45:87–93. doi: 10.1111/j.1600-0765.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 10.Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates?An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Curr Pharm Biotechnol. 2012;13:1145–1152. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- 11.Yu X, Ge S, Chen S, Xu Q, Zhang J, Guo H, et al. Human gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs. 2013;198:428–437. doi: 10.1159/000360276. [DOI] [PubMed] [Google Scholar]
- 12.Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–180. doi: 10.1016/j.jdermsci.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Ji Q, Yang J, Xu X, Yu X, Wang Z, et al. Comparison of the characteristics of human gingival fibroblasts isolated by tissue explants and by enzyme digestion methods. Int J Clin Exp Med. 2016;9:19757–19763. [Google Scholar]
- 14.Malekzadeh R, Hollinger JO, Buck D, Adams DF, McAllister BS. Isolation of human osteoblast-like cells and in vitro amplification for tissue engineering. J Periodontol. 1998;69:1256–1262. doi: 10.1902/jop.1998.69.11.1256. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Nguyen AL, Shi S, Hill C, Wilder-Smith P, Krasieva TB, et al. Three-dimensional spheroid culture of human gingiva-derived mesenchymal stem cells enhances mitigation of chemotherapy-induced oral mucositis. Stem Cells Dev. 2012;21:937–947. doi: 10.1089/scd.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saczko J, Dominiak M, Kulbacka J, Chwiłkowska A, Krawczykowska H. A simple and established method of tissue culture of human gingival fibroblasts for gingival augmentation. Folia Histochem Cyto. 2008;46:117–119. doi: 10.2478/v10042-008-0017-4. [DOI] [PubMed] [Google Scholar]
- 17.Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling:a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20:306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 18.McKay BR, De Lisio M, Johnston AP, O'Reilly CE, Phillips SM, Tarnopolsky MA, et al. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One. 2009;4:e6027. doi: 10.1371/journal.pone.0006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthet J, Damien P, Hamzeh-Cognasse H, Arthaud CA, Eyraud MA, Zéni F, et al. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin Immunol. 2012;145:189–200. doi: 10.1016/j.clim.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Rittig MG, Kaufmann A, Robins A, Shaw B, Sprenger H, Gemsa D, et al. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J Leukoc Biol. 2003;74:1045–1055. doi: 10.1189/jlb.0103015. [DOI] [PubMed] [Google Scholar]
- 21.Filardo G, Kon E, Di Matteo B, Pelotti P, Di Martino A, Marcacci M. Platelet-rich plasma for the treatment of patellar tendinopathy:clinical and imaging findings at medium-term follow-up. Int Orthop. 2013;37:1583–1589. doi: 10.1007/s00264-013-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perdisa F, Filardo G, Di Matteo B, Marcacci M, Kon E. Platelet rich plasma:a valid augmentation for cartilage scaffolds?A systematic review. Histol Histopathol. 2014;29:805–814. doi: 10.14670/HH-29.805. [DOI] [PubMed] [Google Scholar]
- 23.Filardo G, Kon E, Di Matteo B, Di Martino A, Tesei G, Pelotti P, et al. Platelet-rich plasma injections for the treatment of refractory Achilles tendinopathy:results at 4 years. Blood Transfus. 2014;12:533–540. doi: 10.2450/2014.0289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariani E, Canella V, Berlingeri A, Bielli A, Cattini L, Landini MP, et al. Leukocyte presence does not increase microbicidal activity of platelet-rich plasma in vitro. BMC Microbiol. 2015;15:149. doi: 10.1186/s12866-015-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival:application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wu Z, Ni J, Liu Y, Meng J, Yu W, et al. Cathepsin b regulates collagen expression by fibroblasts via prolonging TLR2/NF-κB activation. Oxid Med Cell Longev 2016. 2016 doi: 10.1155/2016/7894247. 7894247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flamand L, Gosselin J, D'Addario M, Hiscott J, Ablashi DV, Gallo RC, et al. Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol. 1991;65:5105–5110. doi: 10.1128/jvi.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bölükbaşı N, Yeniyol S, Tekkesin MS, Altunatmaz K. The use of platelet-rich fibrin in combination with biphasic calcium phosphate in the treatment of bone defects:a histologic and histomorphometric study. Curr Ther Res Clin Exp. 2013;75:15–21. doi: 10.1016/j.curtheres.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klingbeil MF, Herson MR, Cristo EB, dos Santos Pinto D Jr, Yoshito D, Mathor MB. Comparison of two cellular harvesting methods for primary human oral culture of keratinocytes. Cell Tissue Bank. 2009;10:197–204. doi: 10.1007/s10561-009-9122-7. [DOI] [PubMed] [Google Scholar]
- 30.Adan A, Kiraz Y, Baran Y. Cell Proliferation and Cytotoxicity Assays. Curr Pharm Biotechnol. 2016;17:1213–1221. doi: 10.2174/1389201017666160808160513. [DOI] [PubMed] [Google Scholar]
- 31.Cheng R, Liu W, Zhang R, Feng Y, Bhowmick NA, Hu T. Porphyromonas gingivalis-derived lipopolysaccharide combines hypoxia to induce caspase-1 activation in periodontitis. Front Cell Infect Microbiol. 2017;7:474. doi: 10.3389/fcimb.2017.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panda S, Jayakumar ND, Sankari M, Varghese SS, Kumar DS. Platelet rich fibrin and xenograft in treatment of intrabony defect. Contemp Clin Dent. 2014;5:550–554. doi: 10.4103/0976-237X.142830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar RV, Shubhashini N. Platelet rich fibrin:a new paradigm in periodontal regeneration. Cell Tissue Bank. 2013;14:453–463. doi: 10.1007/s10561-012-9349-6. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka K, Iwasaki K, Feghali KE, Komaki M, Ishikawa I, Izumi Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch Oral Biol. 2011;56:380–388. doi: 10.1016/j.archoralbio.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Wu Z, Zhang X, Ni J, Yu W, Zhou Y, et al. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm 2013. 2013 doi: 10.1155/2013/407562. 407562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang W, Wang T, Hu Z, Liu F, Sun Y, Ge S. Metformin inhibits porphyromonas gingivalis lipopolysaccharide-influenced inflammatory response in human gingival fibroblasts via regulating activating transcription factor-3 expression. J Periodontol. 2017;88:e169–e178. doi: 10.1902/jop.2017.170168. [DOI] [PubMed] [Google Scholar]
- 37.Evrard SM, d'Audigier C, Mauge L, Israël-Biet D, Guerin CL, Bieche I, et al. The profibrotic cytokine transforming growth factor-β1 increases endothelial progenitor cell angiogenic properties. J Thromb Haemost. 2012;10:670–679. doi: 10.1111/j.1538-7836.2012.04644.x. [DOI] [PubMed] [Google Scholar]
- 38.Dimmeler S. Platelet-derived growth factor CC--a clinically useful angiogenic factor at last? N Engl J Med. 2005;352:1815–1816. doi: 10.1056/NEJMcibr050670. [DOI] [PubMed] [Google Scholar]
- 39.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 40.Mundala M, Sun Xl, Li X, Fang J, Qi ML, Wang J, et al. Minimally invasive endoscopic maxillary sinus lifting and immediate implant placement:A case report. World J Clin Cases. 2019;7:1234–1241. doi: 10.12998/wjcc.v7.i10.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Majewski M, Ochsner PE, Liu F, Flückiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37:2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 43.Zaccaria V, Curti V, Di Lorenzo A, Baldi A, Maccario C, Sommatis S, et al. Effect of green and brown propolis extracts on the expression levels of microRNAs, mRNAs and proteins, related to oxidative stress and inflammation. Nutrients. 2017;9:1090. doi: 10.3390/nu9101090. [DOI] [PMC free article] [PubMed] [Google Scholar]


