Abstract
Background
The differential diagnosis of dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD) is particularly important because DLB patients respond better to cholinesterase inhibitors but sometimes exhibit sensitivity to neuroleptics, which may cause worsening of clinical status. Antemortem voxel-based morphometry (VBM) using structural MRI has previously revealed that patients with DLB have normal hippocampal volume, but atrophy in the dorsal mesopontine area.
Objectives
The aim of this multicenter study was to determine whether VBM of the brain stem in addition to that of medial temporal lobe structures improves the differential diagnosis of AD and DLB.
Methods
We retrospectively chose 624 patients who were clinically diagnosed with either DLB (239 patients) or AD (385 patients) from 10 institutes using different MR scanners with different magnetic field strengths. In all cases, VBM was performed on 3D T1-weighted images. The degree of local atrophy was calculated using Z-score by comparison with a database of normal volumes of interest (VOIs) in medial temporal lobe (MTL) and the dorsal brain stem (DBS). The discrimination of DLB and AD was evaluated using Z-score values in these two VOIs. MRI data from 414 patients were used as the training data set to determine the classification criteria, with the MRI data from the remaining 210 patients used as the test data set.
Results
The DLB and AD patients did not differ with respect to mean age or Mini-Mental State Examination scores. Z-index scores showed that there was significantly more atrophy in MTL of AD patients, compared to DLB patients and in DBS of DLB patients, compared to AD patients. The discrimination accuracies of VBM were 63.3% in the test data set and 73.4% in the training data set.
Conclusion
VBM of DBS in addition to that of MTL improves the differentiation of DLB and AD.
Keywords: dementia with Lewy bodies, Alzheimer’s disease, MRI, voxel-based morphometry
Introduction
Dementia with Lewy bodies (DLB) is one of the main etiologies of neurodegenerative dementia after Alzheimer’s disease (AD).1 Distinguishing DLB from AD is difficult because of their overlapping clinical and pathological features.2–4 Patients with clinically defined DLB may also have AD-type pathological changes as well as the characteristic Lewy bodies. However, the differential diagnosis is particularly important because DLB respond better to cholinesterase inhibitors but are sensitive to neuroleptics, which cause worsening of clinical status.
Volumetric MRI has been extensively used to characterize the patterns of cerebral atrophy in AD, demonstrating involvement of the medial temporal lobe (MTL) and temporoparietal association cortices.5 However, less is known about the patterns of atrophy in DLB. Although voxel-based morphometry (VBM) explores the local volume loss of gray and white matter throughout the whole brain, there has been considerable variation in terms of volume changes among the published studies, probably due to small and heterogeneous samples of participants. Compared with age-matched normal controls, gray matter volume loss has been observed in the cerebral cortex,6–11 such as the frontal, parietal, occipital, temporal, and insula, and in the subcortical structures,12,13 such as the substantia innominata and midbrain. White matter volume loss has been observed in subcortical areas and the brain stem.11,14,15
In patients with pathologically confirmed DLB,16 antemortem VBM studies revealed gray matter volume loss in the MTL that was much less extensive than the changes seen in patients with AD, and also revealed gray matter volume loss in the dorsal mesopontine area in patients with low- to intermediate- to high-likelihood DLB according to the third report of the DLB Consortium. Patients with high-likelihood DLB typically have normal hippocampal volumes but have atrophy in the dorsal mesopontine gray matter nuclei. This atrophy in the dorsal mesopontine area was also observed in our previous VBM study,15 but in the white matter not in the gray matter.
In the fourth report of the DLB Consortium,17 “relative preservation of medial temporal lobe structures on CT/MRI scan” is listed as one of the supportive biomarkers of DLB. This preservation of MTL structures has been consistently observed in most VBM studies of DLB.16,18–21 The aim of the present multicenter study was to determine whether additional VBM of the brain stem to MTL structures improves the differential diagnosis of AD and DLB.
Methods
Patients
This retrospective study used data obtained at 10 institutes from 624 patients who were clinically diagnosed with DLB (239 patients) or AD (385 patients) based on the criteria proposed in the third Consortium on DLB International Workshop1 and the National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria,22 respectively. The demographics of the study population are shown in Table 1. All patients underwent three-dimensional T1-weighted MRI using 1.5-T or 3.0-T MRI scanners. MRI data from 414 patients obtained at institutes in Tokyo (Shinjuku, Sakura, and Yamagata) and from 210 patients obtained at 7 other institutes were used as training and test data sets, respectively.
Table 1.
Subjects Data
| Center | Number Of Patients | DLB | AD | MR Scanner | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Age (Years) | MMSE | Men | Women | Age (Years) | MMSE | |||
| Tokyo (Shinjuku) | 60 | 15 | 18 | 78.5±5.8 | 20.6±6.0 | 8 | 19 | 78.8±5.3 | 21.3±3.8 | Siemens Avanto 1.5T |
| Sakura | 84 | 30 | 29 | 75.9±6.5 | 21.6±3.8 | 6 | 19 | 80.4±3.8 | 19.2±3.4 | Philips Gyroscan 1.5T |
| Yamagata | 270 | 24 | 36 | 81.5±6.4 | 17.7±5.2 | 43 | 167 | 80.5±5.4 | 18.1±4.7 | Siemens Symphony 1.5T |
| Tokyo (Kodaira) | 51 | 11 | 16 | 76.4±6.6 | 18.2±5.7 | 9 | 15 | 75.3±7.8 | 22.0±3.8 | Philips Achieva 3.0T, Siemens Verio 3.0T |
| Obu | 45 | 7 | 9 | 78.2±7.4 | 20.1±7.1 | 12 | 17 | 79.1±6.6 | 17.9±5.4 | Philips Ingenia 1.5T |
| Tsukuba | 7 | 3 | 0 | 76.0±8.9 | 24.0±1.7 | 1 | 3 | 73.0±8.1 | 13.5±6.0 | Siemens Avanto 1.5T |
| Tokyo (Itabashi) | 8 | 2 | 3 | 78.2±4.0 | 20.6±6.3 | 2 | 1 | 83.3±4.5 | 23.0±1.7 | GE Signa HDxt 3.0T, GE Genesis Signa 1.5T |
| Tokyo (Oota) | 51 | 7 | 15 | 77.5±7.3 | 20.0±5.3 | 8 | 21 | 77.8±7.7 | 19.2±5.0 | TOSHIBA Excelart Vantage 1.5T |
| Kawasaki | 27 | 4 | 5 | 75.8±4.6 | 20.8±5.4 | 6 | 12 | 81.8±7.3 | 18.6±5.2 | Philips Gyroscan Intera 1.5T |
| Chiba | 21 | 4 | 1 | 73.0±6.2 | 22.6±1.9 | 4 | 12 | 69.9±9.4 | 18.2±5.7 | Philips Gyroscan Intera 1.5T |
| Total | 624 | 107 | 132 | 78.0±6.7 | 19.8±5.4 | 99 | 286 | 79.3±6.5 | 18.7±4.8 | |
Abbreviations: DLB, dementia with Lewy bodies; AD, Alzheimer’s disease.
All procedures were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval covering the whole study was obtained at ethical committee of National Center of Neurology and Psychiatry (A2015-075). Then, this retrospective study was approved by the ethical committees at other institutes (Supplementary data). The need for informed patient consent was waived because of the retrospective design using secured confidentiality of patient data from daily medical practice.
Voxel-Based Morphometry
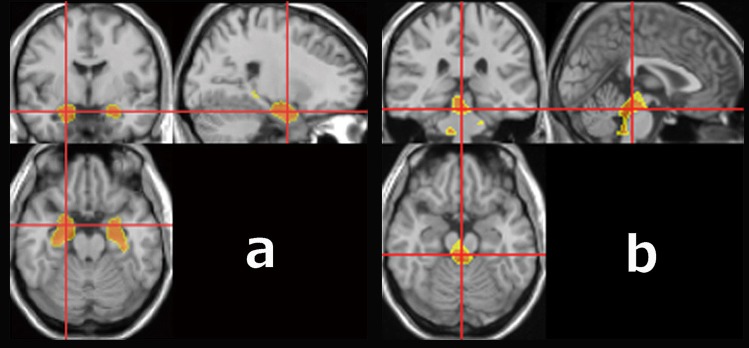
We performed VBM on MRI data using the original stand-alone software program running on Windows, Voxel-based Specific Analysis System for Alzheimer’s Disease (VSRAD®).23 First, MRI data were anatomically standardized with only 12-parameter affine transformation to the statistical parametric mapping template to correct for differences in brain size. Then, MRI data were segmented into gray matter, white matter, and cerebrospinal fluid images using the unified tissue segmentation procedure after image intensity nonuniformity correction. These linearly transformed and segmented images were nonlinearly transformed by diffeomorphic anatomical registration using exponentiated Lie algebra (DARTEL) procedures and then modulated to the customized template for DARTEL followed by smoothing using an 8-mm Gaussian kernel. Each processed segmented image was compared with the mean and standard deviation of gray matter or white matter images segmented from 1.5-T structural MRI of 80 healthy volunteers using voxel-by-voxel Z-score analysis with voxel normalization to global mean intensities (global normalization): Z-score =([control mean]–[individual value])/(control standard deviation). These Z-score maps were displayed by overlay on tomographic sections and surface rendering of the standardized brain. We registered target volumes of interest (VOIs) in the MTL and dorsal brain stem (DBS) as specifically atrophied areas in AD (Figure 1A) and DLB (Figure 1B), respectively, according to our previous studies.15,23
Figure 1.
VOIs in the VSRAD® software program. (A) VOI in medial temporal lobe structures. (B) VOI in the dorsal brain stem.
The following five indices for characterizing atrophy in the target VOIs were used:
MTL_Z: Severity of gray matter atrophy obtained from the averaged positive Z-score in the MTL VOI.
DBS_Z_gray: Severity of gray matter atrophy obtained from the averaged positive Z-score in the DBS VOI.
DBS_Z_white: Severity of white matter atrophy obtained from the averaged positive Z-score in the DBS VOI.
DBS_Z_gray_ratio: Ratio of DBS_Z_gray to MTL_Z.
DBS_Z_white_ratio: Ratio of DBS_Z_white to MTL_Z.
Discrimination Of AD And DLB Using VSRAD
For both single and multiple use of the above five indices for the target VOIs of VSRAD, the training data set was utilized to determine the classification criteria for DLB and AD. Then, the discrimination performance of DLB and AD was evaluated in both the training and test data sets.
For single indices, receiver operating characteristic (ROC) analysis was used to define the diagnostic accuracy for DLB when the cutoff was taken as the point at which [sensitivity+specificity] was maximal. JMP software (ver. 7.0.1; SAS Institute Inc., NC, USA) was used for ROC analysis.
For multiple indices, DLB and AD were classified using a decision tree by machine learning when all five indices were entered as input. A classification and regression tree was adopted as the algorithm of the decision tree. We used the rpart (ver. 4.1–10) software package of R software (ver. 3.3.2; R Foundation for Statistical Computing, Vienna, Austria). Gini indices were used for the optimization function of the decision tree. In order to prevent excessive overfitting in which the tree structure becomes too complicated, branches were pruned by a method based on the prediction error of the 1-standard error rule.24
Association Of VBM Results And Core Clinical Features In DLB
The association of the five indices for characterizing atrophy in the target VOIs with core clinical features – fluctuating cognition, visual hallucination, Parkinsonism, and rapid eye movement (REM) sleep behavior disorder – was investigated in 120 DLB patients of eight institutes other than Sakura and Yamagata.
Results
There were no significant differences in age and Mini-Mental State Examination (MMSE) scores between the DLB patients and AD patients (Table 1). A comparison of values for the single indices between DLB and AD is shown in Table 2. MTL_Z showed significantly lower values in DLB than in AD. DBS_Z_gray_ratio and DBS_Z_white_ratio showed significantly higher values in DLB than in AD, while DBS_Z_gray and DBS_Z_white showed only slightly higher values in DLB than in AD.
Table 2.
Comparison Of Single Index Values For Characterizing Atrophy In The Target VOIs Between DLB And AD
| Index | DLB (n=239) | AD (n=385) |
|---|---|---|
| MTL_Z | 1.73±0.88 | 2.44±1.05** |
| DBS_Z_gray | 0.57±0.23* | 0.53±0.25 |
| DBS_Z_white | 0.67±0.57 | 0.65±0.72 |
| DBS_Z_gray_ratio | 0.44±0.32** | 0.28±0.20 |
| DBS_Z_white_ratio | 0.47±0.48** | 0.30±0.34 |
Notes: *P<0.05, **P<0.001.
Abbreviations: VOI, volume of interest; DLB, dementia with Lewy bodies; AD, Alzheimer’s disease; MTL, medial temporal lobe; DBS, dorsal brain stem.
Table 3 shows the discrimination performance when a single index is used with cutoff values for DLB in ROC analysis. In the training data set, MTL_Z, DBS_Z_gray_ratio, and DBS_Z_white_ratio showed greater than 60% accuracy, whereas DBS_Z_gray and DBS_Z_white showed less than 60% accuracy. In the test data set, all indices showed less than 60% accuracy.
Table 3.
Discrimination Performance Of DLB And AD Using Single Indices
| Index | DLB Cutoff | Training Data (n=414) | Test Data (n=210) | |||||
|---|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | ||
| MTL_Z | <2.19 | 0.72 | 75.7% | 59.2% | 65.2% | 75.9% | 43.9% | 57.1% |
| DBS_Z_gray | ≥0.34 | 0.54 | 87.5% | 22.5% | 46.4% | 86.2% | 17.9% | 46.2% |
| DBS_Z_white | ≥0.32 | 0.56 | 69.1% | 46.2% | 54.6% | 72.4% | 30.9% | 48.1% |
| DBS_Z_gray_ratio | ≥0.27 | 0.68 | 68.4% | 60.7% | 63.5% | 70.1% | 50.4% | 58.6% |
| DBS_Z_white_ratio | ≥0.33 | 0.66 | 50.7% | 75.6% | 66.4% | 59.8% | 55.3% | 57.1% |
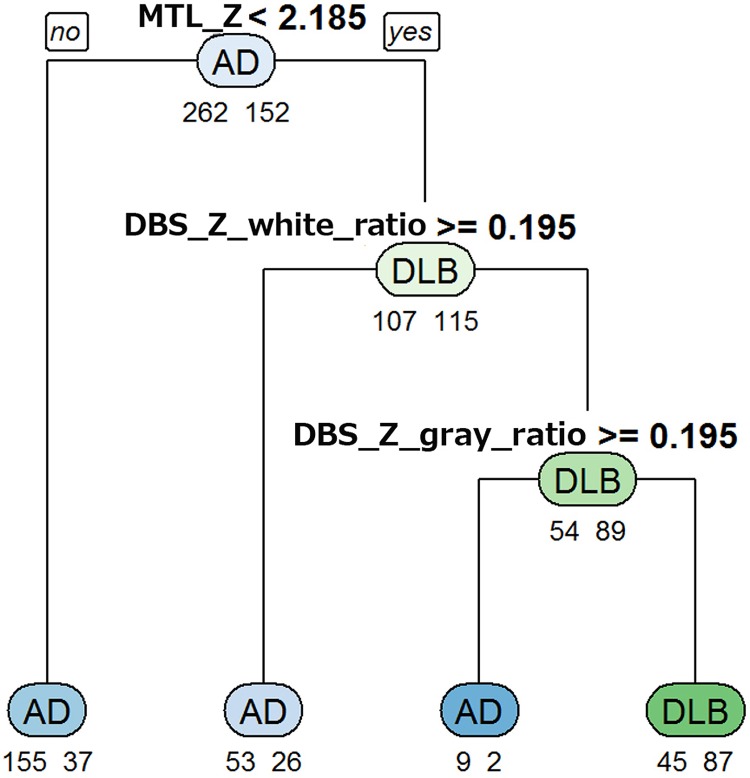
Figure 2 shows the decision tree generated from the training data set with multiple indices. Branching is repeated at each node from the top to the bottom, and the four terminal nodes at final arrival indicate the classification result as a decision tree. In this decision tree, the condition classified as DLB corresponds to the terminal node on the right end, and “MTL_Z < 2.185 and DBS_Z_gray_ratio ≥ 0.195 and DBS_Z_white_ratio ≥ 0.195” is the cutoff. The discrimination performance of the training and test data sets with this decision tree is shown in Table 4. The differentiation accuracy of DLB and AD was greater than 70% in the training data set and greater than 60% in the test data set. When values, “MTL_Z < 2.0 and DBS_Z_gray_ratio ≥ 0.2 and DBS_Z_white_ratio ≥ 0.2”, that are easy to set with VSRAD are used, almost the same accuracies were obtained in both data set. Thus, the multiple indices provided better discrimination accuracy than the single indices.
Figure 2.
Decision tree for differentiation of DLB and AD using VBM results in the training data set. Below each node, the numbers of AD (left) and DLB (right) patients are shown. The class of AD or DLB as the classification result is shown at the center of each node. In this decision tree, the condition classified as DLB corresponds to the terminal node on the right end, and “MTL_Z <2.185 and DBS_Z_white_ratio ≥0.195 and DBS_Z_gray_ratio” is the cutoff.
Table 4.
Discrimination Performance Of DLB And AD Using Multiple Indices
| DLB Cutoff | Training Data (n=414) | Test Data (n=210) | ||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | |
| MTL_Z < 2.185 & DBS_Z_gray_ratio ≥ 0.195 & DBS_Z_white_ratio ≥ 0.195 | 57.2% | 82.8% | 73.4% | 56.3% | 68.3% | 63.3% |
| MTL_Z < 2.0 & DBS_Z_gray_ratio ≥ 0.2 & DBS_Z_white_ratio ≥ 0.2 | 52.0% | 84.4% | 72.5% | 50.6% | 75.6% | 65.2% |
Table 5 shows the association of the values of the five indices in the target VOIs with core clinical features. Only DBS_Z_gray_ratio and DBS_Z_white_ratio showed significantly lower values in DLB patients with visual hallucination than in those without visual hallucination.
Table 5.
Association Of Single Index Values With Core Clinical Features In DLB
| Index | Core Clinical Features | |||||||
|---|---|---|---|---|---|---|---|---|
| Fluctuating Cognition | Visual Hallucination | Parkinsonism | REM Sleep Behaviour Disorder | |||||
| Positive (n=56) | Negative (n=64) | Positive (n=96) | Negative (n=24) | Positive (n=72) | Negative (n=48) | Positive (n=55) | Negative (n=65) | |
| MTL_Z | 1.75±0.94 | 1.63±0.77 | 1.71±0.78 | 1.58±1.12 | 1.74±0.86 | 1.59±0.85 | 1.63±0.96 | 1.73±0.77 |
| DBS_Z_gray | 0.59±0.24 | 0.59±0.25 | 0.57±0.23 | 0.65±0.30 | 0.62±0.25 | 0.54±0.22 | 0.57±0.22 | 0.60±0.27 |
| DBS_Z_white | 0.79±0.64 | 0.60±0.63 | 0.66±0.62 | 0.80±0.70 | 0.71±0.67 | 0.64±0.58 | 0.65±0.59 | 0.72±0.68 |
| DBS_Z_gray_ratio | 0.45±0.33 | 0.48±0.34 | 0.42±0.27* | 0.64±0.49 | 0.47±0.34 | 0.46±0.33 | 0.48±0.35 | 0.45±0.32 |
| DBS_Z_white_ratio | 0.56±0.64 | 0.43±0.49 | 0.43±0.44* | 0.71±0.89 | 0.49±0.60 | 0.48±0.51 | 0.48±0.48 | 0.49±0.64 |
Note: *P<0.05.
Discussion
The present multicenter study using different MRI scanners of different magnetic field strengths demonstrated that combined volume measures of MTL and DBS were more helpful than single volume measures of MTL for differentiation of DLB and AD in VBM.
The present results of less atrophy of MTL structures in DLB than in AD agreed well with previous investigations.16,18 Our previous single-center study15 using VSRAD revealed that a target VOI in the white matter limited to the midbrain plus pons, which corresponded to DBS_Z_white in the present study, exhibited an AUC of 0.74, sensitivity of 60%, specificity of 90%, and accuracy of 75% for the differentiation of DLB and AD. However, the accuracy of DBS_Z_white alone in the training data set was just 54.6% in the present multicenter study. The lower accuracy may be due to the use of different MRI scanners of different magnetic field strengths at multiple sites. Although DBS_Z_white_ratio showed better accuracy (66.4%) than DBS_Z_white in the training data set, this accuracy was almost equal to that of MTL_Z alone (65.2%). In the test data set, these accuracies decreased to below 60%, and the accuracy of DBS_Z_white_ratio was the same as that of MTL_Z. During image acquisition, pulsatile and respiratory motion resulting from the cardiac cycle and respiratory activity may produce some distortions of the brain stem.25 Cerebrospinal fluid has also been found to flow in both rostral and caudal directions during the cardiac cycle. This cardiac-synchronized flow in the cerebrospinal fluid can result in brain stem pulsation. These distortions and pulsations may fluctuate with the differences in not only the MR scanners, but also the magnetic field strength. In VSRAD analysis, differences in volume measures of white matter and differences in cutoff values for significant gray matter atrophy have been reported between 1.5-T and 3.0-T MRI data.26 These factors may cause segmentation fluctuations in VBM of the brain stem and may at least partly explain the variable results in brain stem atrophy mainly in gray matter or mainly in white matter among previous VBM studies of DLB. The present decision tree analysis revealed that the influences of segmentation fluctuations on differential performance are reduced by the combination of MTL_Z, DBS_Z_gray_ratio, and DBS_Z_white_ratio. Compared with MTL_Z alone, this combination showed better accuracy of 8.2% and 6.2% in the training and test data sets, respectively.
The previous VBM studies27 of DLB patients with visual hallucination revealed gray matter atrophy mainly in the occipital, parietal, and frontal cortices, the MTL, and cholinergic structures such as the substantia innominata. Janzen et al28 demonstrated gray matter atrophy in the pedunculopontine nucleus in patients with DLB with visual hallucination. Our results partly agree with these observations. The DLB patients with visual hallucination showed greater atrophy in the MTL with less atrophy in DBS, resulting in a significantly lower DBS_Z_gray_ratio and DBS_Z_white_ratio.
Another study29 revealed less atrophy in the temporoparietal cortex, hippocampus, and amygdala in DLB patients with REM sleep behavior disorder than in those without REM sleep behavior disorder. The presence of REM sleep behavior disorder is associated with a higher likelihood of DLB and less severe AD pathology in the MTL. The present results showed less MTL atrophy in patients with REM sleep behavior disorder than in those without REM sleep behavior disorder, though the difference did not reach statistically significant.
This study was possibly limited by several factors. First, the diagnoses were not pathologically confirmed. Second, the association of volumetric results and clinical features was not investigated in all DLB patients. The trend observed in the present study might have been statistically significant with a higher number of patients. Third, the present VBM results were not compared with other imaging biomarkers such as dopamine transporter SPECT/PET, metaiodobenzylguanidine myocardial scintigraphy,30,31 and brain perfusion SPECT or FDG-PET.32 These comparisons with other biomarkers would clarify the significance of the present findings.
Conclusion
On VBM of 3D T1-weighted images, gray matter atrophy in the MTL was less in DLB patients than in AD patients. VBM of the DBS in addition to that of the MTL increases the differentiation of AD and DLB by up to 8.2%. Automatic VBM software would be useful for this differential diagnosis.
Acknowledgments
This study was supported by an Intramural Research Grant (30-10) for Neurological and Psychiatric Disorders from the National Center of Neurology and Psychiatry (Japan).
Disclosure
TO, TG, and KS are employees of Dai Nippon Printing Co., Ltd. Dai Nippon Printing Co., Ltd is a consignment contractor between companies. TO, TG, and KS have a patent US 10285657 B2 issued, patents JP 2016-64004 A, EP 3199102 A1, and CN 106659424 A pending. TO, TG and KS report and served for outsourcing contract between companies for software development-related work, call center-related business, system-related work, work related to creating support materials, and printing related business. The authors report no other conflicts of interest in this work.
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1 [DOI] [PubMed] [Google Scholar]
- 2.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24 [DOI] [PubMed] [Google Scholar]
- 3.Galasko D, Hansen LA, Katzman R, et al. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch Neurol. 1994;51(9):888–895. doi: 10.1001/archneur.1994.00540210060013 [DOI] [PubMed] [Google Scholar]
- 4.Fujishiro H, Iseki E, Higashi S, et al. Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett. 2010;486(1):19–23. doi: 10.1016/j.neulet.2010.09.036 [DOI] [PubMed] [Google Scholar]
- 5.Matsuda H. Voxel-based morphometry of brain MRI in normal aging and Alzheimer’s disease. Aging Dis. 2013;4(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Burton EJ, Karas G, Paling SM, et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage. 2002;17(2):618–630. [PubMed] [Google Scholar]
- 7.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088 [DOI] [PubMed] [Google Scholar]
- 8.Brenneis C, Wenning GK, Egger KE, et al. Basal forebrain atrophy is a distinctive pattern in dementia with Lewy bodies. Neuroreport. 2004;15(11):1711–1714. doi: 10.1097/01.wnr.0000136736.73895.03 [DOI] [PubMed] [Google Scholar]
- 9.Borroni B, Premi E, Formenti A, et al. Structural and functional imaging study in dementia with Lewy bodies and Parkinson’s disease dementia. Parkinsonism Relat Disord. 2015;21(9):1049–1055. doi: 10.1016/j.parkreldis.2015.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Peraza LR, Colloby SJ, Firbank MJ, et al. Resting state in Parkinson’s disease dementia and dementia with Lewy bodies: commonalities and differences. Int J Geriatr Psychiatry. 2015;30(11):1135–1146. doi: 10.1002/gps.4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roquet D, Noblet V, Anthony P, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7(1):9437. doi: 10.1038/s41598-017-08667-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colloby SJ, Elder GJ, Rabee R, O’Brien JT, Taylor JP. Structural grey matter changes in the substantia innominata in Alzheimer’s disease and dementia with Lewy bodies: a DARTEL-VBM study. Int J Geriatr Psychiatry. 2017;32(6):615–623. doi: 10.1002/gps.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(Pt 3):708–719. doi: 10.1093/brain/awl388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JE, Park B, Song SK, Sohn YH, Park HJ, Lee PH. A comparison of gray and white matter density in patients with Parkinson’s disease dementia and dementia with Lewy bodies using voxel-based morphometry. Mov Disord. 2010;25(1):28–34. [DOI] [PubMed] [Google Scholar]
- 15.Nakatsuka T, Imabayashi E, Matsuda H, Sakakibara R, Inaoka T, Terada H. Discrimination of dementia with Lewy bodies from Alzheimer’s disease using voxel-based morphometry of white matter by statistical parametric mapping 8 plus diffeomorphic anatomic registration through exponentiated Lie algebra. Neuroradiology. 2013;55(5):559–566. doi: 10.1007/s00234-013-1138-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantarci K, Ferman TJ, Boeve BF, et al. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012;79(10):553–560. doi: 10.1212/WNL.0b013e31826357a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarci K, Lesnick T, Ferman TJ, et al. Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology. 2016;87(22):2317–2323. doi: 10.1212/WNL.0000000000003371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi R, Ishii K, Miyamoto N, et al. Measurement of gray and white matter atrophy in dementia with Lewy bodies using diffeomorphic anatomic registration through exponentiated lie algebra: A comparison with conventional voxel-based morphometry. AJNR Am J Neuroradiol. 2010;31(10):1873–1878. doi: 10.3174/ajnr.A2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson R, O’Brien JT, Barber R, Blamire AM. Patterns of gray matter atrophy in dementia with Lewy bodies: a voxel-based morphometry study. Int Psychogeriatr. 2012;24(4):532–540. doi: 10.1017/S1041610211002171 [DOI] [PubMed] [Google Scholar]
- 21.Harper L, Bouwman F, Burton EJ, et al. Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry. 2017;88(11):908–916. doi: 10.1136/jnnp-2016-314978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda H, Mizumura S, Nemoto K, et al. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer disease. AJNR Am J Neuroradiol. 2012;33(6):1109–1114. doi: 10.3174/ajnr.A2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De’ath G, Fabricius KE. Classification and regression trees: a powerful technique for ecological data analysis. Ecology. 2000;81(11):3178–3192. doi: 10.1890/0012-9658(2000)081[3178:CARTAP]2.0.CO;2 [DOI] [Google Scholar]
- 25.Ford AA, Colon-Perez L, Triplett WT, Gullett JM, Mareci TH, Fitzgerald DB. Imaging white matter in human brainstem. Front Hum Neurosci. 2013;7:400. doi: 10.3389/fnhum.2013.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sone D, Imabayashi E, Maikusa N, Ogawa M, Sato N, Matsuda H. Voxel-based specific regional analysis system for Alzheimer’s disease (VSRAD) on 3-tesla normal database: diagnostic accuracy in two independent cohorts with early Alzheimer’s disease. Aging Dis. 2018;9(4):755–760. doi: 10.14336/AD.2017.0818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Structural and functional neuroimaging in patients with Parkinson’s disease and visual hallucinations: A critical review. Parkinsonism Relat Disord. 2015;21(7):683–691. doi: 10.1016/j.parkreldis.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Janzen J, van ‘t Ent D, Lemstra AW, Berendse HW, Barkhof F, Foncke EM. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J Neurol. 2012;259(1):147–154. doi: 10.1007/s00415-011-6149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray ME, Ferman TJ, Boeve BF, et al. MRI and pathology of REM sleep behavior disorder in dementia with Lewy bodies. Neurology. 2013;81(19):1681–1689. doi: 10.1212/01.wnl.0000435299.57153.f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nuvoli S, Palumbo B, Malaspina S, Madeddu G, Spanu A. 123I-ioflupane SPET and 123I-MIBG in the diagnosis of Parkinson’s disease and parkinsonian disorders and in the differential diagnosis between Alzheimer’s and Lewy’s bodies dementias. Hell J Nucl Med. 2018;21(1):60–68. doi: 10.1967/s002449910707 [DOI] [PubMed] [Google Scholar]
- 31.Nuvoli S, Spanu A, Piras MR, et al. 123I-ioflupane brain SPECT and 123I-MIBG cardiac planar scintigraphy combined use in uncertain parkinsonian disorders. Medicine (Baltimore). 2017;96(21):e6967. doi: 10.1097/MD.0000000000006967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuvoli S, Spanu A, Madeddu G. Brain SPECT with perfusion radiopharmaceuticals and dopaminergic system radiocompounds in dementia disorders. Curr Alzheimer Res. 2017;14(2):143–153. [DOI] [PubMed] [Google Scholar]