Abstract
Introduction: Cannabis sativa has been used for centuries in treating pain. However, the analgesic role of many of its constituents including terpenes is unknown. This research examined the contributions of terpenes (volatile oil) and cannabinoids in cannabis-mediated analgesia in rats.
Methods: Animals received intraperitoneal administration of either vehicle, 10.0 or 18.0 mg/kg morphine, or various doses of the extract without terpenes, isolated terpenes, Δ9-tetrahydrocannabinol (THC), or the full extract. Thirty minutes later animals were tested on hotplate and tail-flick tests of thermal nociception. One week later, rats received a second administration of test articles and were tested 30 min later in the abdominal writhing test of inflammatory nociception.
Results: In the thermal assays, hotplate and tail-flick latencies for morphine-treated rats were dose dependent and significantly higher than vehicle-treated animals. All the cannabinoid compounds except for the isolated terpenes produced dose-dependent increases in hotplate and tail-flick latencies. In the inflammatory nociceptive assay, animals treated with vehicle and isolated terpenes demonstrated increased abdominal writhing, whereas all the cannabinoid compounds significantly decreased abdominal writhing responses.
Conclusions: Overall, THC alone produced robust analgesia equivalent to the full cannabis extract, whereas terpenes alone did not produce analgesia. These data suggest the analgesic activity of cannabis is largely mediated by THC, whereas terpenes alone do not cause alterations in cannabis-mediated analgesia.
Keywords: analgesia, nociception, terpenes, THC, volatile oil
Introduction
Cannabis sativa has been used for more than four centuries to treat a variety of medical conditions including pain.1,2 Although the cannabis plant as a whole has analgesic properties, isolated constituents of cannabis such as Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) have been shown to be an effective treatment for acute and chronic pain.3–5 However, these cannabinoids may not be the only contributors to the overall efficacy of cannabis-mediated analgesia. More than 565 constituents have been identified in cannabis. Whether these play a significant role in cannabis-mediated analgesic is unknown.6
One class of constituents whose medicinal contributions are unknown are the terpenes. Terpenes are fragrant volatile oils found in the resin of plants that act as natural attractants or repellants.7 There has been speculation that terpenes are responsible for some of the analgesic properties of the plant. However, the contribution of terpenes in nociception are yet unknown.
This study aims to determine whether terpenes in and from cannabis extracts contribute to the analgesic activity of cannabis by evaluating the plant extract with and without terpenes, isolated terpenes, and isolated THC across a battery of nociceptive assays. These include two thermal and one inflammatory pain assays.
Materials and Methods
Subjects
Male Sprague-Dawley rats (200–250 g; Harlan, Indianapolis, IN) were housed in pairs and maintained under a 12-h light/dark cycle in a temperature and humidity controlled vivarium. Food and water were provided ad libitum. Animals were handled twice daily for 5 days before behavioral testing to reduce experimenter stress. Rats were sequentially tested on the hotplate and tail-flick assays. After these tests, rats were handled once daily for 6 days and then enrolled in the abdominal writhing assay. Experimenters were blinded to all treatment conditions. All experimental procedures were approved by the University of Mississippi Institutional Animal Care and Use Committee (protocol #15-025).
Experimental drug preparation
Four products were prepared from high potency C. sativa (THC-rich plant) for this study (cannabis extract with terpenes, terpenes, extract without terpenes, and the major cannabinoid, Δ9-THC). To make the total extract, the dried powdered plant material was extracted by maceration in hexanes for 17 h. The hexanes solution was drained and evaporated under vacuum to yield the total extract.
Group 1 preparation
To prepare the first group (total extract under reflux), a portion of the extract was heated with 500 mL of distilled water under reflux for 3 h, then the cooled mixture was extracted with dichloromethane, and these extracts dried over anhydrous sodium sulfate. The solvent was then evaporated under vacuum to make sure that all the solvent was out of the product. This reflux step was carried out to decarboxylate the extract (convert all the cannabinoid acids into natural ones) and to subject the extract to the same conditions used to remove the volatile oil from the extract used to treat group 2. In addition, reflux allows the evaporated terpenes to condensate back to the extract. The product was the extract with terpenes.
Group 2 preparation
The next drug group was the extract without volatile oil. The total extract was hydrodistilled to collect the oil, using a volatile oil preparation apparatus. Fifteen grams of the total cannabis extract and 500 mL of water were hydrodistilled for 3 h to produce the extract without the volatile oil and the volatile oil separately. After hydrodistillation, the extract without volatile oil was separated from water using methylene chloride following the same procedure under group 1 preparation. The product was the extract without terpenes.
Group 3 preparation
The steam condensed volatile oil collected from the hydrodistillation process to prepare group 2 preparation was used to treat group 3. The volatile oil was analyzed by gas chromatography to determine its terpenes composition and ensure all THC was removed from this product.
Group 4 preparation
The final drug group was administered Δ9-THC. Δ9-THC was acquired from the NIDA Drug Supply Program.
Treatment groups
Doses of the extract without terpenes (4.2, 8.4, and 12.7 mg/kg), isolated terpenes (0.19, 0.39, and 0.57 mg/kg), and THC (2.5, 5.0, and 7.5 mg/kg) were tested at the concentrations found in the full extract (4.8, 9.7, and 14.5 mg/kg). Drugs were dissolved in 10% cremaphor, 10% ethanol, and 80% water. Morphine (Sigma-Aldrich, St. Louis, MO) was diluted in 0.9% physiological saline and tested at 10.0 and 18.0 mg/kg. All test compounds were administered intraperitoneally in a volume of 2 mL/kg.
Hotplate
The hotplate assay was used to characterize the antinociceptive properties of test compounds against thermal nociception. Animals were administered test compounds 30 min before testing. Rats were placed into an acrylic enclosure positioned on top of a heated plate (Model #52-8570; Harvard Apparatus) maintained at 54°C. The latency to flutter or lick a hindpaw or perform an escape response (i.e., jumping out of the apparatus) was recorded. A 35-sec cutoff score was used to prevent tissue damage.
Tail flick
The tail-flick assay was used to characterize the antinociceptive properties of test compounds against thermal nociception. Animals were confined in a restrainer with their tails positioned in a groove above a radiant heat source (IITC #33; IITC Life Science, Inc., Woodland Hills, CA). The latency to flick or remove the tail from the light stimulus was recorded. The intensity of the light was adjusted to yield a baseline latency around 5 sec in control animals. A 20-sec cutoff was used to prevent tissue damage.
Abdominal writhing
The abdominal writhing assay was used to characterize the anti-inflammatory antinociceptive effects of test compounds. Animals were administered test compounds and 30 min later injected with 0.4% acetic acid solution in a volume of 10 mL/kg and immediately confined to observation chambers (42 cm×20 cm×21 cm). The number of writhes or stretches over 30 min were recorded.
Data analysis
Data were analyzed using SPSS software. Group differences in all tests were analyzed using one-way analyses of variance (ANOVAs) followed by Fisher's LSD post hoc test. Data significant on Levene's test of homogeneity of variance were analyzed using a negative binomial regression. Saline and vehicle treatment groups for both hotplate and tail-flick tests were not significantly different in latencies; therefore. these treatment groups were combined for all analyses. It is common that 15–20% of animals tested in the abdominal writhing assay to be nonresponders to the nociceptive stimulus.8 Indeed, two rats in the vehicle group did not respond to the acetic acid administration. This prompted the removal of two nonresponders from each treatment group before data analysis.
Results
Hotplate latency
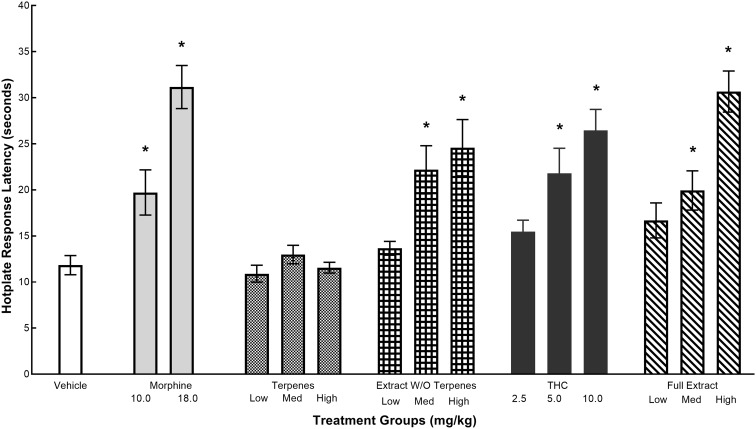
The effects of various test articles on hotplate latencies are given in Figure 1. Hotplate latencies for the morphine group were dose dependent and significantly higher than the vehicle group. All the cannabinoid compounds except for the terpenes produced dose-dependent increase in hotplate latencies. Consistent with these findings, a one-way ANOVA of hotplate data revealed a significant main effect for treatment groups, F(14,161)=10.54, p<0.0001. Post hoc analyses revealed the mean hotplate response latency for 10.0 and 18.0 mg/kg morphine was significantly higher than the vehicle (ps<0.005). Mean hotplate response latency for the extract without the terpenes, THC, and the full extract was also significantly higher than the vehicle at the medium and high doses (ps<0.005) but not at the low doses (ps>0.090). Response latencies in the terpenes group was not significantly different than vehicle at any dose (ps>0.701).
FIG. 1.
The effects of morphine, volatile oils, extract without volatile oils, THC, and full extract on the hotplate response latencies. Values represent mean (±SEM) latency to flutter or lick hindpaw, or to perform an escape response. *Significant decrease (p<0.05) compared with cremaphor. Sample sizes were n=10–14 per group. SEM, standard error of the mean; THC, Δ9-tetrahydrocannabinol.
Tail-flick latency
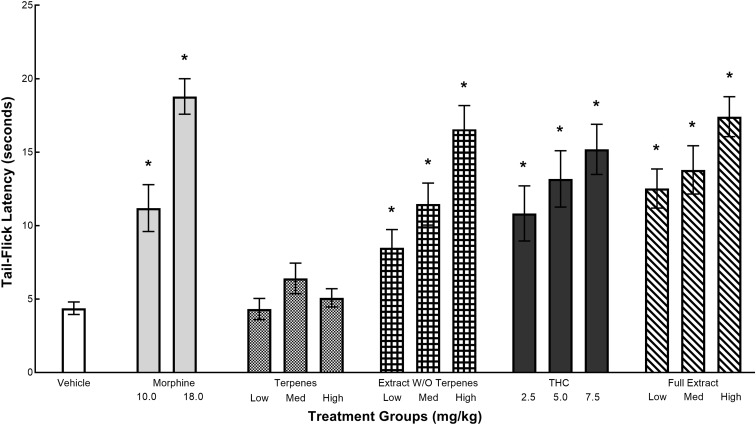
The effects of various test articles on tail-flick latencies are given in Figure 2. Tail-flick latencies for the morphine group were dose dependent and significantly higher than the vehicle group. All the cannabinoid compounds except for the terpenes produced dose-dependent increases in tail-flick latencies. Consistent with these findings, a one-way ANOVA revealed a significant main effect for treatment groups, F(14,165)=9.77, p<0.0001. Post hoc analyses revealed that the mean tail-flick latency for 10.0 and 18.0 mg/kg morphine was significantly higher than that of the vehicle (ps<0.001). Mean tail-flick latency for the extract without the terpenes, THC, and the full extract was also significantly higher than that of the vehicle at across all doses (ps<0.039). Response latencies in the terpenes group was not significantly different from vehicle at any dose (ps>0.347).
FIG. 2.
The effects of morphine, volatile oils, extract without volatile oils, THC, and full extract on tail-flick withdrawal latencies. Values represent mean (±SEM) latency of tail withdrawal. *Significant decrease (p<0.05) compared with cremaphor. Samples sizes were n=10–18 per group.
Abdominal writhing
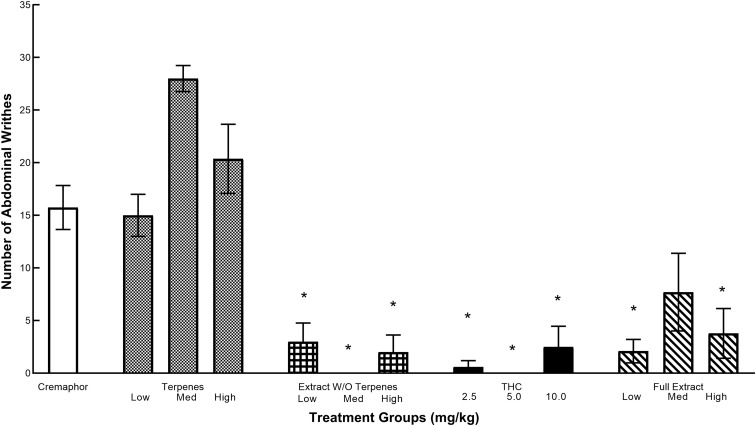
The effects of various test articles on abdominal writhing are given in Figure 3. Animals treated with vehicle and the volatile oil demonstrated increased abdominal writhing. All the cannabinoid compounds significantly decreased this writhing response. A one-way ANOVA was carried out, and as Levene's test was significant, data were further analyzed using a negative binomial regression. Results from this analysis indicated that treatment was a significant predictor of the number of abdominal writhes [χ2 (12)=121.722, p<0.0001]. Wald chi-square hypothesis test revealed significant treatment effects across all doses of the extract without terpenes and the full extract as well as the low and high THC doses compared with the vehicle group (ps<0.006), whereas the medium THC dose and all doses of the terpenes were not significant (ps>0.149).
FIG. 3.
The effects of morphine, volatile oils, extract without volatile oils, THC, and full extract in the abdominal writhing test. Values represent the mean number of writhes after an intraperitoneal injection of 0.7% acetic acid over a 30-min test session. *Significant decrease (p<0.05) compared with cremaphor. Sample sizes were n=5–10 per group.
Analysis of the terpene (volatile oil) fraction of the extract
Steam distillation of 15 g of the total extract using the volatile oil collection unit resulted in a significant amount of volatile oil (0.6 mL of the oil or 4% v/w). Gas chromatography with flame ionization detection (GC/FID) analysis of the volatile oil showed the presence of the following terpenes and their percent content in the oil: α-pinene 4.12%, β-pinene 2.15%, β-myrcene 2.38%, limonene 0.50%, terpinolene 1.47%, linalool 1.93%, α-terpineol 0.59%, β-caryophyllene 23.30%, α-humulene 5.51%, and caryophyllene oxide 1.44%.
Discussion
This study sought to determine whether terpenes (volatile oils) and other cannabis constituents contribute to cannabis-mediated analgesia. To answer this question, four cannabinoid products (full extract, THC, extract without terpenes, and terpenes) were prepared and evaluated at three doses in three nociceptive assays: two thermal nociception assays and one inflammatory nociception assay.
In the thermal assays, the full extract, THC, and the extract without terpenes produced dose-dependent increases in responses latencies, whereas the terpenes treatment groups did not. Isolated THC produced antinociception equivalent to the full extract, suggesting that THC alone is largely responsible for the analgesic actions of cannabis. Furthermore, these data indicate that terpenes have no significant role in the production of cannabis-mediated analgesia.
On the test of inflammatory nociception, a similar pattern of antinociception was observed with the full extract, THC, and the extract without terpenes. Each produced a decrease in abdominal writhes indicative of analgesia. Terpenes alone produced abdominal writhing equivalent to the vehicle group, suggesting that terpenes do not affect inflammatory nociception.
Our findings demonstrate that THC produced robust antinociception equivalent to the whole extract in models of thermal and inflammatory nociception. Thus, other cannabinoid constituents including terpenes do not add to the analgesic actions of cannabis beyond that of isolated THC. This analgesia across several pain models suggest a range of clinical applications for THC.
The current studies fit well with the existing literature on the role of cannabinoid modulation of nociception. For example, THC has been shown to attenuate neuropathy in a rodent model of diabetic-induced neuropathy.9 Although there is evidence that CBD can have analgesic actions of its own,10 the extracts used in this study are from a high THC variety that contains a small amount of CBD. A study to ascertain the contribution of CBD to the analgesic activity of cannabis needs to be carried out using extracts of the mixed THC:CBD variety, containing significant amounts of both cannabinoids. As only one-time point postdrug administration was tested, the duration of these antinociceptive effects cannot be addressed and is a limitation to this study. Future time-course studies should be conducted to evaluate the duration of these antinociceptive effects. These studies are currently underway in this laboratory.
Although our studies did not reveal significant effect on terpene antinociception, a literature exists demonstrating the benefits from individual terpenes across various pain models. For example, terpenes such as β-caryophyllene, β-myrcene, and α-pinene have been shown to alleviate neuropathic11 and inflammatory pain.12–14 However, many of these studies evaluated these effects using plants containing a significant amount of the terpene being studied. Although this study evaluated a combination of terpenes present in cannabis on acute pain, future studies to evaluate the role of mixed terpenes in chronic pain models should be examined. To our knowledge, this is the first study to report that terpenes do not contribute to cannabis-mediated analgesia.
Conclusions
Botanical products possessing a wide array of poorly understood constituents have the potential to interact with organ systems leading to a host of side effects. The work herein demonstrates that cannabis extracts can not only produce robust analgesia without the terpene-containing volatile oils, but isolated THC appears to be all that is required to produce such effects.
Acknowledgments
This project was supported by funding from National Center for Natural Products Research and the Sally McDonnell Barksdale Honors College.
Abbreviations Used
- ANOVA
analysis of variance
- CBD
cannabidiol
- SEM
standard error of the mean
- THC
Δ9-tetrahydrocannabinol
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Harris HM, Rousseau MA, Wanas AS, Radwan MM, Caldwell S, Sufka KJ, ElSohly MA (2019) The role of cannabinoids and terpenes in cannabis-mediated analgesia in rats, Cannabis and Cannabinoid Research 4:3, 177–182, DOI: 10.1089/can.2018.0054.
References
- 1. Chiou LC, Hu SS, Ho Y. Targeting the cannabinoid system for pain relief? Acta Anaesthesiol Taiwan. 2013;51:161–170 [DOI] [PubMed] [Google Scholar]
- 2. Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1998;58:315–348 [DOI] [PubMed] [Google Scholar]
- 3. Hill KP, Palastro MD, Johnson B, et al. Cannabis and pain: a clinical review. Cannabis Cannabinoid Res. 2017;2:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris HM, Sufka KJ, Gul W, et al. Effects of delta-9-tetrahydrocannabinol and cannabadiol on cisplatin-induced neuropathy in mice. Planta Med. 2016;82:1169–1172 [DOI] [PubMed] [Google Scholar]
- 5. Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ElSohly MA, Radwan MM, Gul W, et al. Phytochemistry of Cannabis sativa L. Prog Chem Org Nat Prod. 2017;103:1–36 [DOI] [PubMed] [Google Scholar]
- 7. Paduch R, Kandefer-Szerszeń M, Trytek M, et al. Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp. 2007;55:315–327 [DOI] [PubMed] [Google Scholar]
- 8. Collier HO, Dinneen LC, Johnson CA, et al. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams J, Haller VL, Stevens DL, et al. Decreased basal endogenous opioid levels in diabetic rodents: effects on morphine and delta-9-tetrahydrocannabinoid-induced antinociception. Eur J Pharmacol. 2008:584:78–86 [DOI] [PubMed] [Google Scholar]
- 10. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia. 2004;59:440–452 [DOI] [PubMed] [Google Scholar]
- 11. Klauke AL, Racz I, Pradier B, et al. The cannabinoid CB(2) receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropscyhopharmacol. 2014:24:608–620 [DOI] [PubMed] [Google Scholar]
- 12. Ghelardini C, Galetotti N, Di Cesare Mannelli L, et al. Local anaestheic activity of beta-caryophyllene. Farmco. 2001:56:387–389 [DOI] [PubMed] [Google Scholar]
- 13. Lorenzetti BB, Souza GE, Sarti SJ, et al. Myrcene mimis the peripheral analgesic activity of lemongrass tea. J Ethnopharmacol. 1991;34:43–48 [DOI] [PubMed] [Google Scholar]
- 14. Baron EP. Medicinal properties of cannabinoids, terpenes, and flavonids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache. 2018;58:1139–1186 [DOI] [PubMed] [Google Scholar]