Abstract
Background
Diarrhoea and soil‐transmitted helminth (STH) infections represent a large disease burden worldwide, particularly in low‐income countries. As the aetiological agents associated with diarrhoea and STHs are transmitted through faeces, the safe containment and management of human excreta has the potential to reduce exposure and disease. Child faeces may be an important source of exposure even among households with improved sanitation.
Objectives
To assess the effectiveness of interventions to improve the disposal of child faeces for preventing diarrhoea and STH infections.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE, Embase, and 10 other databases. We also searched relevant conference proceedings, contacted researchers, searched websites for organizations, and checked references from identified studies. The date of last search was 27 September 2018.
Selection criteria
We included randomized controlled trials (RCTs) and non‐randomized controlled studies (NRS) that compared interventions aiming to improve the disposal of faeces of children aged below five years in order to decrease direct or indirect human contact with such faeces with no intervention or a different intervention in children and adults.
Data collection and analysis
Two review authors selected eligible studies, extracted data, and assessed the risk of bias. We used meta‐analyses to estimate pooled measures of effect where appropriate, or described the study results narratively. We assessed the certainty of the evidence using the GRADE approach.
Main results
Sixty‐three studies covering more than 222,800 participants met the inclusion criteria. Twenty‐two studies were cluster RCTs, four were controlled before‐and‐after studies (CBA), and 37 were NRS (27 case‐control studies (one that included seven study sites), three controlled cohort studies, and seven controlled cross‐sectional studies). Most study sites (56/69) were in low‐ or lower middle‐income settings. Among studies using experimental study designs, most interventions included child faeces disposal messages along with other health education messages or other water, sanitation, and hygiene (WASH) hardware and software components. Among observational studies, the main risk factors relevant to this review were safe disposal of faeces in the latrine or defecation of children under five years of age in a latrine.
Education and hygiene promotion interventions, including child faeces disposal messages (no hardware provision)
Four RCTs found that diarrhoea incidence was lower, reducing the risk by an estimated 30% in children under six years old (rate ratio 0.71, 95% confidence interval (CI) 0.59 to 0.86; 2 trials, low‐certainty evidence). Diarrhoea prevalence measured in two other RCTs in children under five years of age was lower, but evidence was low‐certainty (risk ratio (RR) 0.93, 95% CI 0.84 to 1.04; low‐certainty evidence).
Two controlled cohort studies that evaluated such an intervention in Bangladesh did not detect a difference on diarrhoea prevalence (RR 0.91, 95% CI 0.64 to 1.28; very low‐certainty evidence). Two controlled cross‐sectional studies that evaluated the Health Extension Package in Ethiopia were associated with a lower two‐week diarrhoea prevalence in 'model' households than in 'non‐model households' (odds ratio (OR) 0.26, 95% CI 0.16 to 0.42; very low‐certainty evidence).
Programmes to end open defecation by all (termed community‐led total sanitation (CLTS) interventions plus adaptations)
Four RCTs measured diarrhoea prevalence and did not detect an effect in children under five years of age (RR 0.92, 95% CI 0.79 to 1.07; moderate‐certainty evidence). The analysis of two trials did not demonstrate an effect of the interventions on STH infection prevalence in children (pooled RR 1.03, 95% CI 0.64 to 1.65; low‐certainty evidence).
One controlled cross‐sectional study compared the prevalence of STH infection in open defecation‐free (ODF) villages that had received a CLTS intervention with control villages and reported a higher level of STH infection in the intervention villages (RR 2.51, 95% CI 1.74 to 3.62; very low‐certainty evidence).
Sanitation hardware and behaviour change interventions, that included child faeces disposal hardware and messaging
Two RCTs had mixed results, with no overall effect on diarrhoea prevalence demonstrated in the pooled analysis (RR 0.79, 95% CI 0.49 to 1.26; very low‐certainty evidence).
WASH hardware and education/behaviour change interventions
One RCT did not demonstrate an effect on diarrhoea prevalence (RR 1.15, 95% CI 0.93 to 1.41; very low‐certainty evidence).
Two CBAs reported that the intervention reduced diarrhoea incidence by about a quarter in children under five years of age, but evidence was very low‐certainty (rate ratio 0.77, 95% CI 0.71 to 0.84). Another CBA reported that the intervention reduced the prevalence of STH in an intervention village compared to a control village, again with GRADE assessed at very low‐certainty (OR 0.17, 95% CI 0.02 to 0.73).
Case‐control studies
Pooled results from case‐control studies that presented data for child faeces disposal indicated that disposal of faeces in the latrine was associated with lower odds of diarrhoea among all ages (OR 0.73, 95% CI: 0.62 to 0.85; 23 comparisons; very low‐certainty evidence). Pooled results from case‐control studies that presented data for children defecating in the latrine indicated that children using the latrine was associated with lower odds of diarrhoea in all ages (OR 0.54, 95% CI 0.33 to 0.90; 7 studies; very low‐certainty evidence).
Authors' conclusions
Evidence suggests that the safe disposal of child faeces may be effective in preventing diarrhoea. However, the evidence is limited and of low certainty. The limited research on STH infections provides only low and very‐low certainty evidence around effects, which means there is currently no reliable evidence that interventions to improve safe disposal of child faeces are effective in preventing such STH infections.
While child faeces may represent a source of exposure to young children, interventions generally only address it as part of a broader sanitation initiative. There is a need for RCTs and other rigorous studies to assess the effectiveness and sustainability of different hardware and software interventions to improve the safe disposal of faeces of children of different age groups.
23 September 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (27 Sep, 2018) were included and four ongoing studies identified
Plain language summary
Interventions to improve child faeces disposal and prevent diarrhoea and soil‐transmitted helminths
What was the aim of this review?
The aim of this Cochrane Review was to assess the impact of improved disposal of child faeces on diarrhoea and soil‐transmitted helminth (STH) infection. We collected and analysed all relevant studies and found 63 studies covering over 222,800 participants.
Key messages
We found some evidence that interventions to promote safe disposal of child faeces were protective against diarrhoea. However, the evidence was mixed and its certainty was very low to moderate. We found no evidence that such interventions were protective against STH infections, but the evidence was very limited and the certainty was low to very low. More research is needed to study the health impact of different types of interventions to improve child faeces disposal.
What was studied in this review?
Diarrhoea and STH infections affect millions of people worldwide, particularly in low‐income countries. Diarrhoea and STHs are transmitted through human faeces so the safe containment and management of human excreta has the potential to significantly reduce exposure and disease. An often‐neglected source of exposure is from the unsafe disposal of child faeces. Research has shown that even in settings with improved sanitation, child faeces are thrown into refuse piles or elsewhere and not disposed of in latrines as considered safe by the World Health Organization (WHO) and United Nations Children's Fund (UNICEF).
We included 26 studies with experimental designs and 37 observational studies in this review. Most included studies were conducted in low‐ and middle‐income countries.
What were the main results of the review?
Results from studies using experimental study designs suggest that:
Education and hygiene promotion interventions that included child faeces disposal messages may reduce diarrhoea incidence by about 30% but did not show an effect on diarrhoea prevalence (low‐certainty evidence).
Evidence from interventions that addressed child faeces as part of a wider intervention aimed at ending open defecation by all household members did not detect an effect on diarrhoea prevalence (moderate‐certainty evidence) or STH infection (low‐certainty evidence).
Sanitation hardware (for example, faeces scoopers, potties) and behaviour change interventions (for example, to increase use of latrines) had mixed results on diarrhoea prevalence, but no effect was demonstrated in the combined analysis (very low‐certainty evidence).
Interventions that addressed safe disposal of child faeces education as part of a wider water, sanitation, and hygiene hardware intervention did not demonstrate an effect on diarrhoea prevalence (one study; very low‐certainty evidence). Although diarrhoea incidence (two studies) and STH prevalence (one study) were lower, the evidence was very low‐certainty so we do not know if this is a true effect.
Results from observational studies (where researchers observe the effect of a treatment without trying to change who is or is not exposed to it) showed mixed results of education and hygiene promotion interventions, with two studies in Bangladesh showing no effect on diarrhoea prevalence (very low‐certainty evidence) and two studies in Ethiopia reducing diarrhoea prevalence (very low‐certainty evidence). One study evaluating an intervention aimed at ending open defecation found an increase in STH infection the intervention arm (very low‐certainty evidence). Pooled results from other studies that presented data for child faeces disposal indicate that disposal of faeces in the latrine may decrease the odds of diarrhoea by about a quarter among all ages (very low‐certainty evidence). Children using the latrine to defecate may reduce the odds of diarrhoea by about half in all ages (very low‐certainty evidence). However, given the very low‐certainty evidence we are unsure about the effects of these risk factors on diarrhoea.
How up to date was this review?
We searched for available studies up to 27 September 2018.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Education and hygiene promotion intervention compared with no intervention for preventing diarrhoea in low‐ and middle‐income countries | ||||||
|
Patient or population: adults and children Settings: LMICs Intervention: education and hygiene promotion intervention that includes promotion of safe child faeces disposal among other promoted behaviours Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea prevalence Cluster RCTs |
3 episodes per person per year | 2.79 episodes per person per year (2.52 to 3.12) | RR 0.93 (0.84 to 1.04) | 12,040 (2 studies) | ⊕⊕⊝⊝ Lowa,b,c,d | The intervention may make little or no difference to diarrhoea prevalence. |
|
Diarrhoea incidence Cluster RCTs |
3 episodes per person per year | 2.13 episodes per person per year (1.77 to 2.58) | Rate ratio 0.71 (0.59 to 0.86) | 2549 (2 studies) | ⊕⊕⊝⊝ Lowa,d,e,f | The intervention may reduce diarrhoea incidence. |
|
Diarrhoea prevalence Controlled cohort studies: Sanitation Hygiene Education and Water Supply in Bangladesh (SHEWA‐B) intervention |
3 episodes per person per year | 2.73 episodes per person per year (1.92 to 3.84) | RR 0.91 (0.64 to 1.28) | ˜2000 (2 studies) | ⊕⊝⊝⊝ Very lowa,g,h,i | We are uncertain whether or not the intervention reduces diarrhoea prevalence. |
|
Diarrhoea prevalence Controlled cross‐sectional studies: Health Extension Package intervention (Ethiopia) |
3 episodes per person per year | 0.78 episodes per person per year (0.48 to 1.26)j | OR 0.26 (0.16 to 0.42) | 1660 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,d,k | We are uncertain whether or not the intervention reduces diarrhoea prevalence. |
| *The assumed risk for diarrhoea is taken from Walker 2012 and represented an estimated mean for the incidence of diarrhoea in LMICs. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008) CI: confidence interval; LMICs: low‐ and middle‐income countries; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: the outcome was self‐reported diarrhoea, and was susceptible to bias as all studies were unblinded. bNo serious inconsistency. cDowngraded one level for indirectness: only two studies in low‐income countries. Both conducted in rural settings, one in Rwanda and one in Democratic Republic of Congo. Diarrhoea was only measured in children aged < 3 years in Haggerty 1994 DRC. dNo serious imprecision. eNo serious inconsistency: there was considerable statistical heterogeneity (I² = 82%); however, there was consistency in the direction of the effect. Possible reasons for heterogeneity included the location of the studies; Stanton 1987 BGD was conducted in urban Bangladesh and Hashi 2017 ETH in rural Ethiopia. Furthermore, the studies used different definitions of diarrhoea and different age groups (aged less than six years for Stanton 1987 BGD and less than five years for Hashi 2017 ETH). fDowngraded one level for indirectness: only two studies, one in an urban Asian setting (Bangladesh) and one in an African rural setting (Ethiopia). gDowngraded one level for inconsistency: substantial statistical heterogeneity (I² = 55%). hDowngraded one level for indirectness: only two studies, both conducted in Bangladesh and evaluating the same intervention that was specifically tailored to Bangladesh. iDowngraded one level for imprecision: small sample size and large CIs which included important effects in both directions. jCalculated using the OR as an approximation for RR. kDowngraded one level for indirectness: only two studies, both conducted in rural Ethiopia and evaluating an intervention specifically designed for Ethiopia.
Summary of findings 2. Summary of findings table 2.
| CLTS or CLTS adaptation intervention compared with no intervention for preventing diarrhoea and STHs | ||||||
|
Patient or population: adults and children Settings: LMICs Intervention: CLTS or CLTS adaptation interventions, aiming to end open defecation by all Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea prevalence RCTs |
3 episodes per person per year | 2.76 episodes per person per year (2.37 to 3.21) | RR 0.92 (0.79 to 1.07) | 16,033 (4 studies) | ⊕⊕⊕⊝ Moderatea,b,c,d | The intervention probably makes little or no difference to diarrhoea prevalence. |
|
STH infection (any helminth) RCTs |
4.8 out of 100 people with any helminths | 4.9 out of 100 people with any helminths (3.07 to 7.92) | RR 1.03 (0.64 to 1.65) | 3480 (2 studies) | ⊕⊕⊝⊝ Lowb,e,f,g | The intervention may make little or no difference to STH infection. |
|
STH infection (any helminth) Controlled cross‐sectional study |
4.8 out of 100 people with any helminths | 12 of 100 people with any helminths (8.4 to 17.4) | RR 2.51 (1.74 to 3.62) | 341 (1 study) | ⊕⊝⊝⊝ Very lowb,d,e,h | We are uncertain whether or not the intervention increases STH infection. |
| *The assumed risk for diarrhoea is taken from Walker 2012 and represented an estimated average for the incidence of diarrhoea in LMICs. The assumed risk for any helminth in stool is an average of the control group risks of Cameron 2013 INA (control group risk: 3.9%) Patil 2014 IND (control group risk: 5.6%). The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008) CI: confidence interval; CLTS: community‐led total sanitation; LMICs: low‐ and middle‐income countries; RCT: randomized controlled trial; RR: risk ratio; STH: soil‐transmitted helminth. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: the outcome was measured as self‐reported diarrhoea, and is susceptible to bias as all studies were unblinded. bNo serious inconsistency. cNo serious indirectness: four studies, all conducted in rural settings of low‐ and lower middle‐income settings; two in Africa and two in Asia. dNo serious imprecision. eNo serious risk of bias: although assessors and participants were not blinded to the intervention, the outcome was objective. fDowngraded one level for indirectness: only two RCTs assessed the impact of CLTS/CLTS adaptation interventions on STH. Both studies were conducted in rural Asia (in Indonesia and India). gDowngraded one level for imprecision: small sample size and large CIs which include important effects in both directions. hDowngraded two levels for serious indirectness: only one small study conducted in rural Philippines. This single controlled cross‐sectional study compared the parasitological status of school‐age and preschool‐age children in two open defecation‐free villages and two villages that did not benefit from CLTS. It was not possible to make broad generalizations to other settings.
Summary of findings 3. Summary of findings table 3.
| Sanitation hardware and behaviour change intervention compared with no intervention for preventing diarrhoea | ||||||
|
Patient or population: adults and children Settings: LMICs Intervention: sanitation hardware and behaviour change interventions, which include child faeces management hardware and promotion Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea prevalence RCTs |
3 episodes per person per year | 2.37 episodes per person per year (1.47 to 3.78) | RR 0.79 (0.49 to 1.26) | 9558 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,c,d | We are uncertain whether or not the intervention reduces diarrhoea prevalence. |
| *The assumed risk for diarrhoea is taken from Walker 2012 and represented an estimated mean for the incidence of diarrhoea in LMICs. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008). CI: confidence interval; LMICs: low‐ and middle‐income countries; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: the outcome was measured as self‐reported diarrhoea, and was susceptible to bias as both studies were unblinded. bDowngraded one level for serious inconsistency: considerable statistical heterogeneity (I² = 90%). There were large effects in Bangladesh but not in Kenya. cDowngraded one level for indirectness: only two studies, both conducted in rural areas, one in Bangladesh and one in Kenya. dNo serious imprecision. The 95% CI of the pooled effect included important effects in both directions, but this imprecision was a result of the heterogeneity between studies.
Summary of findings 4. Summary of findings table 4.
| WASH hardware and education/behaviour change interventions compared with no intervention for preventing diarrhoea and STHs | ||||||
|
Patient or population: adults and children Settings: LMICs Intervention: WASH hardware interventions that included child faeces disposal messaging in their education or behaviour change component Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea prevalence RCTs |
3 episodes per person per year | 3.45 episodes per person per year (2.79 to 4.23) | RR 1.15 (0.93 to 1.41) | 3650 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c,d | We are uncertain whether or not the intervention reduces diarrhoea prevalence. |
|
Diarrhoea incidence CBAs |
3 episodes per person per year | 2.31 episodes per person per year (2.13 to 2.52) | Rate ratio 0.77 (0.71 to 0.84) | 1028 (2 studies) | ⊕⊝⊝⊝ Very lowa,b,d,e | We are uncertain whether or not the intervention reduces diarrhoea incidence. |
|
STH infection (any helminth) CBAs |
4.8 out of 100 people with any helminths | 0.82 of 100 people with any helminths (0.096 to 3.5) | OR 0.17 (0.02 to 0.73)f | 99 (1 study) | ⊕⊝⊝⊝ Very lowb,g,h | We are uncertain whether or not the intervention reduces STH infection. |
| *The assumed risk for diarrhoea is taken from Walker 2012 and represented an estimated mean for the incidence of diarrhoea in LMICs. The assumed risk for any helminth in stool was a mean of the control group risks of Cameron 2013 INA (control group risk: 3.9%) and Patil 2014 IND (control group risk: 5.6%). The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008). CBA: controlled before‐and‐after study; CI: confidence interval; LMICs: low‐ and middle‐income countries; OR: odds ratio; RCT: randomized controlled trial; RR: risk ratio; STH: soil‐transmitted helminth; WASH: water, sanitation, and hygiene. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for serious risk of bias: the outcome was measured as self‐reported diarrhoea, and was susceptible to bias as all studies were unblinded. bNo serious inconsistency. cDowngraded two levels for serious indirectness: this single RCT from Zimbabwe evaluated the provision of a WASH hardware and behaviour change intervention. It was not possible to make broad generalizations to other settings. dNo serious imprecision. eDowngraded one level for indirectness: only two studies, both in rural Bangladesh. fCalculated using the OR as an approximation for RR. gDowngraded two levels for serious indirectness: only one study that was conducted in rural Indonesia. This CBA study compared STH infection in one control village and one intervention village, where residents received a latrine constructed with local materials and health education. It was not possible to make broad generalizations to other settings. hDowngraded one level for imprecision: small sample size and large CIs.
Summary of findings 5. Summary of findings table 5.
| Disposal of child faeces in a latrine vs elsewhere for preventing diarrhoea (findings from case‐control studies) | ||||||
|
Patient or population: adults and children Settings: all settings Intervention: child faeces disposal in latrine Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea Case‐control studies: child faeces disposal in latrine |
3 episodes per person per year | 2.19 episodes per person per year (1.86 to 2.55)a | OR 0.73 (0.62 to 0.85) | 32,957 (17 studies) | ⊕⊝⊝⊝ Very lowb,c,d | We are uncertain whether or not the intervention reduces diarrhoea. |
| *The assumed risk for diarrhoea was taken from Walker 2012 and represented an estimated mean for the incidence of diarrhoea in LMICs. The corresponding risk (and its 95% CI) was based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008). CI: confidence interval; LMIC: low‐ and middle‐income countries; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aCalculated using the OR as an approximation for RR. bDowngraded one level for serious inconsistency: substantial statistical heterogeneity (I² = 71%), which was not completely explained by the subgroup analyses. cNo serious indirectness: these 17 studies were from a variety of low‐, middle‐, and high‐income countries, in urban, rural, and periurban areas. dNo serious imprecision.
Summary of findings 6. Summary of findings table 6.
| Defecation of children in a latrine vs elsewhere for preventing diarrhoea (findings from case‐control studies) | ||||||
|
Patient or population: adults and children Settings: LMIC Intervention: defecation of children in latrine Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE)** | Comments | |
| Risk with no intervention | Risk with intervention | |||||
|
Diarrhoea Case‐control studies: defecation of children in latrine |
3 episodes per person per year | 1.62 episodes per person per year (0.99 to 2.70)a | OR 0.54 (0.33 to 0.90) | 2996 (7 studies) | ⊕⊝⊝⊝ Very lowb,c,d | We are uncertain whether or not the intervention reduces diarrhoea. |
| *The assumed risk for diarrhoea was taken from Walker 2012 and represented an estimated mean for the incidence of diarrhoea in LMICs. The corresponding risk (and its 95% CI) was based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **RCTs begin as high‐certainty evidence and observational studies as low‐certainty evidence (Guyatt 2008). CI: confidence interval; LMIC: low‐ and middle‐income countries; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aCalculated using the OR as an approximation for RR. bDowngraded one level for serious inconsistency: substantial statistical heterogeneity (I² = 68%), which was not completely explained by the subgroup analyses. cNo serious indirectness: seven studies from a variety of LMICs, in mostly urban settings. dNo serious imprecision.
Background
Epidemiology and transmission of diarrhoeal disease and soil‐transmitted helminth infection
Despite advances in prevention and treatment, diarrhoea and soil‐transmitted helminth (STH) infections still represent a large disease burden, particularly in low‐income countries. Diarrhoeal diseases account for an estimated 1.65 million deaths annually worldwide and rank eighth globally for leading causes of death among all ages (GBD 2018). Among children under the age of five years, diarrhoea kills more than 440,000 children annually, making it the fifth leading cause of death in that age group (GBD 2018). Over five billion people worldwide, including one billion school‐aged children (aged five to 14 years), are at risk of infection with at least one STH species (Pullan 2012). The three STHs responsible for most infections are Ascaris lumbricoides, Trichuris trichiura, and hookworms (Ancylostoma duodenale or Necator americanus), with 819 million, 464.6 million, and 438.9 million people infected in 2010, respectively (Pullan 2014).
The pathogens that cause diarrhoea are mainly transmitted via the faecal–oral route (Byers 2001). Pathogens from contaminated faeces can be passed on to a new susceptible host via contaminated hands, drinking water, soil, flies, or by ingesting contaminated food (Wagner 1958). The settings, pathogens, and their prevalence in different populations will determine the importance of each transmission route (Brown 2013). The symptoms of diarrhoea and course of disease vary with age, nutritional status, and immune status of the infected person, and the causative pathogens (Clasen 2010). The main characteristics of infection are changes in stool consistency, increases in volume or fluidity, and increased frequency of defecation (Thapar 2004). The three clinical presentations of diarrhoea are: acute watery diarrhoea lasting several hours or days; acute bloody diarrhoea (dysentery); and persistent diarrhoea lasting 14 days or more (Heymann 2008). The direct threat from acute watery diarrhoea is dehydration, and loss of fluids and electrolytes. Severe dehydration can result in death if untreated (Keusch 2006).
STHs are transmitted via ingestion of STH eggs (A lumbricoides and T trichiura) or larvae (A duodenale), or via penetration of third‐stage larvae (hookworms) (Bethony 2006). The larvae go through several developmental stages in the human host and, depending on the species, the adult parasites can settle in different parts of the gastrointestinal (GI) tract, where they can live for several years, mating and producing eggs that are passed in the faeces (Bethony 2006). The eggs (A lumbricoides and T trichiura) and larvae (hookworm) can survive in the soil for several months (eggs) or several weeks (larvae), depending on the environmental conditions, including humidity, soil moisture, and temperature (Brooker 2006). Morbidity caused by STHs is linked to the intensity of infection, which is the number of worms per human host measured by the number of eggs per gram of faeces (Bethony 2006). STHs infections can have several clinical features, which can be classified into acute manifestations linked to larval migrations through the skin and intestines, and acute and chronic manifestations associated with parasite presence in the GI tract (Bethony 2006).
An additional risk of contamination of the environment with faeces, including those of children, is that it may result in extended exposure of children to faecal pathogens which may lead to environmental enteric dysfunction (EED), a disorder of the small intestine that is characterized by villous atrophy, crypt hyperplasia, inflammatory cell infiltrate, increased permeability, and malabsorption (Humphrey 2009; Mbuya 2016). EED is thought to lead to under nutrition and growth faltering (Humphrey 2009; Lin 2013; Mbuya 2016).
In addition to the direct health consequences of diarrhoeal diseases and STHs infections, they have longer‐term impacts on human development due to malabsorption and malnutrition (resulting in stunting and chronic anaemia), and on capacity (via lower cognition, school absenteeism and inability to work), which in turn can have impacts on development and poverty (Harhay 2010). STHs are believed to be one of the main causes of physical and intellectual growth retardation in the world (Bethony 2006).
Furthermore, enteric infections or stunting can predispose to obesity and associated comorbidities (diabetes, hypertension, cardiovascular diseases), increasing healthcare costs which in turn contributes to poverty (Guerrant 2013).
Sanitation and disposal of child faeces
As the aetiological agents associated with diarrhoea and STHs are transmitted through faeces, the safe collection and disposal of human excreta has the potential to reduce exposure and disease. When BMJ readers were asked to vote on the "greatest medical advance" since 1840, they chose the sanitary revolution (the introduction of clean water and sewage disposal) over antibiotics, anaesthesia, vaccines, and germ theory (Ferriman 2007). Large‐scale efforts have been made to increase coverage of improved sanitation, most recently as part of the Millennium Development Goal (MDG) sanitation target of halving the proportion of the population without access to basic sanitation by 2015 (UN 2013). However, this target was missed by almost 700 million people and 2.4 billion people were still without improved sanitation in 2015, including almost one billion people practicing open defecation (WHO/UNICEF 2015a). The post‐2015 sustainable development goals (SDGs) include goal 6 "Ensure availability and sustainable management of water and sanitation for all" with target 6.2 aiming, by 2030, to "achieve access to adequate and equitable sanitation and hygiene for all and end open defecation, paying special attention to the needs of women and girls and those in vulnerable situations" (UN 2016).
A series of published systematic reviews has consistently concluded that sanitation interventions are effective in preventing diarrhoea and STH infections. Esrey 1991 reported a 22% median reduction in diarrhoea from 11 observational studies and 36% reduction from five rigorous studies. They also reported reduction in Ascaris and hookworm from water supply and sanitation interventions, especially on the reduction in disease intensity (egg counts). Fewtrell 2005 reported a pooled risk ratio (RR) for diarrhoea of 0.68 (95% confidence interval (CI) 0.53 to 0.87) from two intervention studies. Waddington 2009 reported a pooled RR for diarrhoea of 0.63 (95% CI 0.43 to 0.93) from six controlled studies among children. Clasen 2010 found a consistent protective effect against diarrhoea among 13 intervention studies but noted that nearly all involved water or hygiene (various hygiene promotion, for example handwashing with soap, safe household water storage, etc.) interventions in addition to sanitation (interventions to introduce or expand the provision or use of facilities for excreta disposal). Norman 2010 reported that sewerage led to a 30% reduction in diarrhoea (RR 0.70, 95% CI 0.58 to 0.85) among 17 observational studies. Ziegelbauer 2012 reported that sanitation interventions were protective against Ascaris, Trichuris, and hookworm, while Strunz 2014 found that access to sanitation was associated with reduced odds of infection with any STH, Ascaris,and Trichuris but not hookworm. Freeman 2017 found that sanitation was associated with 12% lower odds of diarrhoea (OR 0.88, 95% CI 0.83 to 0.92; 27 studies), when restricted to the 16 intervention studies, the protective effect doubled to 23% (OR 0.77, 95% CI 0.66 to 0.91). Freeman 2017 also found that sanitation was associated with lower odds of infection of Ascaris, Trichuris, hookworm, and Strongyloides stercoralis. Wolf 2018 found that sanitation interventions were associated with 25% reduction in diarrhoeal morbidity (RR 0.75, 95% CI 0.63 to 0.88; 22 studies).
However, these reviews focused on interventions to improve coverage, use, or functionality of sanitation facilities or services. Only one systematic review specifically addressed the disposal of child faeces, another source of exposure even among households with improved sanitation. The review, with different inclusion criteria to the current one, concluded that the health impact of improving child faeces disposal was inconclusive (Morita 2016). Our rationale for focusing on child faeces disposal was that the unsafe disposal of child faeces may represent a more important health risk to children, caregivers, and other community members than faeces of adults. This is because young children have the highest incidence of enteric infections (Walker 2012), and their faeces are most likely to contain infectious agents (Feachem 1983). Young children are more likely to defecate in places where susceptible children could be exposed (Lanata 1998). This exposure is worse for other young children due to the amount of time they spend on the ground and their exploratory behaviours, including putting fingers and fomites in their mouths, and common behaviours such as geophagia (intentional consumption of soil) (Moya 2004; Ngure 2013; Young 2011). Perhaps for these reasons, the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) Joint Monitoring Programme for Water Supply and Sanitation (JMP), which was charged with assessing progress toward the MDG sanitation targets, treated disposal of child faeces that were not deposited in a latrine or buried as unsanitary (WHO/UNICEF 2006). The JMP, which will also monitor progress towards SDGs, will classify the following methods for disposal of child stools as appropriate methods: the child using an improved toilet/latrine or the caretaker putting/rinsing stools into an improved toilet/latrine. Disposal with solid waste will only be considered appropriate if solid waste is stored, collected and disposed of in a sanitary manner (WHO/UNICEF 2018).
Only one recent peer‐reviewed study has summarized the evidence on the impact of child faeces disposal on human health. However, it had different inclusion criteria to the current review, resulting in far fewer studies (eight) and included no quantitative analysis (Morita 2016). In an unpublished review and meta‐analysis of 10 observational studies published between 1987 and 2001, Gil 2004 found that child faeces disposal behaviours considered risky (open defecation, stool disposal in the open, stools not removed from soil, stools seen in household soil, and children seen eating faeces) were associated with a 23% increase in risk of diarrhoeal diseases (RR 1.23, 95% CI 1.15 to 1.32); in contrast, behaviours considered safe (use of latrines, nappies, potties, toilets, washing nappies) were borderline protective (RR 0.93, 95% CI 0.86 to 1.00).
One observational study in rural Bangladesh found that disposal of child faeces in closed spaces, such as pit latrines, was associated with a 35% reduction in helminthiasis in children under two years of age compared with disposal in open spaces (Roy 2011). This indicated that safe disposal of child faeces may also play a role in the control of STH infections.
Furthermore, one study analysing Demographic and Health Surveys (DHS) data from 34 countries found that household child faeces disposal practices were strongly associated with child growth. The study found that improved child faeces disposal (child faeces disposed into improved latrine) practices were associated with reduced levels of child stunting and underweight and increases in height‐for‐age Z (HAZ) and weight‐for‐age Z (WAZ) scores (Bauza 2017), indicating that child faeces disposal may also be a determining factor for nutritional outcomes. Another cohort study in rural Bangladesh found that children from households that disposed of their children's faeces unsafely had higher scores of enteropathy and growth faltering, and greater odds of being wasted (George 2016), again supporting the possibly important role of safe child faeces disposal.
Prevalence of safe child faeces disposal
Safe disposal of child faeces has been defined in different ways, predominantly involving disposal of the faeces in a latrine (WHO/UNICEF 2018; UNICEF 2012; WSP 2015), but also sometimes involving burying (WHO/UNICEF 2006). However, it was deemed that burying of faeces or throwing faeces in garbage should not be considered safe or improved disposal in an expert consultation (Bain 2015). Another definition of safe disposal of child faeces categorized safe disposal (disposal into any latrine) further into improved disposal if the latrine in which the faeces end up was considered improved (WSP 2015). In addition to disposal in an improved latrine, the JMP will consider disposal with solid waste as appropriate if the solid waste is stored, collected and disposed of in a sanitary manner (WHO/UNICEF 2018). None of these definitions are supported by high‐quality evidence. The definitions of safe disposal of child faeces involve the child if the child defecates in a latrine directly or involves the caregiver disposing the faeces of the child safely into a latrine. The caregiver thus plays an important role, especially for younger children who are too young to be able to use a latrine, both to dispose of the faeces and also to train the child to use a latrine.
Data on child faeces disposal practices has been collected through DHS and Multiple Indicator Cluster surveys (MICS) since the start of these surveys in 1986 and 1995 (Bain 2015). The core question asked to caregivers of children under two (MICS) or under five (DHS) years of age is "The last time [name] passed stools, what was done to dispose of the stools?" (WHO/UNICEF 2006; WHO/UNICEF 2018).
Worldwide, safe disposal of child faeces is suboptimal. A report by the World Bank Water and Sanitation Program (WSP) presenting analysis from the latest available MICS/DHS surveys found that in 15 out of 26 locations more than 50% of households reported that the faeces of their youngest child under three years of age were disposed of unsafely (not into a latrine) (WSP 2015), and the percentage of faeces ending up in improved latrines was even lower. Worldwide, child faeces disposal was safer in urban settings, in households with improved sanitation, for older children, and in richer households (WSP 2015).
Description of the intervention
The interventions relevant to this Cochrane Review aim to improve the safe collection or disposal of faeces of children aged below five years in order to decrease direct or indirect human contact with such faeces. They may act by: improving the defecation site of the child, so the child defecates directly in the latrine; or improving collection and disposal of child faeces in a latrine (see Figure 1).
1.
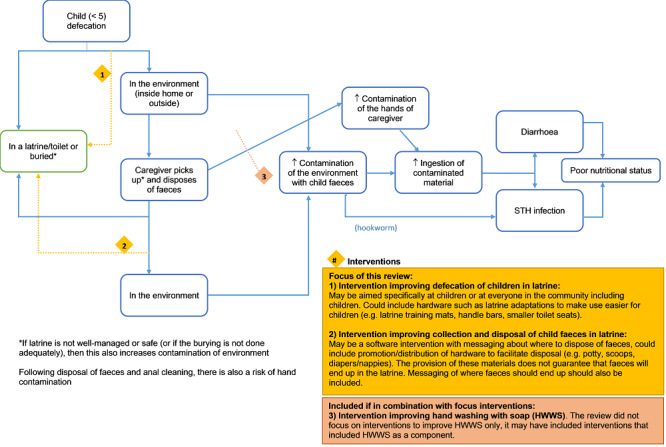
Logic model. Abbreviations: HWWS: hand washing with soap; STH: soil‐transmitted helminth.
Interventions could include the provision of hardware (e.g. nappies, potties, faecal collection devices, cleaning products to remove faeces, child‐friendly squatting slabs, or latrines used by children), software (e.g. promotion of safe disposal practices), or both. These interventions may be combined with or included in other interventions, such as hygiene promotion interventions (e.g. promotion of hand hygiene, food hygiene, etc.) or sanitation interventions (sanitation hardware provision or behaviour change messaging to end open defecation, or both).
It is important to note that these interventions may not completely reduce exposure to child faeces, as child faeces management involves a series of steps which present risks of exposure to pathogens in child faeces (Majorin 2017; Miller‐Petrie 2016), including the defecation place of the child, where the faeces are disposed and how, and what hygiene behaviours are conducted. In addition, practices for child faeces disposal may differ depending on the caregiver, defecation place, or season. Furthermore, interventions seeking to improve child faeces disposal by providing hardware may not succeed in changing the behaviour of the caregivers, so the hardware (e.g. potties or scoops) may not be used or may not be used as intended, disposing of the child faeces in the open rather than in the latrine or toilet.
We categorized the results of this Cochrane Review into different types of intervention, in order to make them comparable to one another. The interventions were categorized as shown in Table 7, and as described below.
1. Summary of intervention categories.
| Intervention category | Child faeces component of intervention | Other intervention components |
| Education and hygiene promotion interventions | Software only | None or limited hardware |
| Community‐led total sanitation interventions + adaptations | Software only | Software only – focus on ending open defecation |
| Sanitation hardware and behaviour change interventions | Software + hardware (potties/scoops) | Software + hardware (sanitation only) |
| WASH hardware and education/behaviour change interventions | Software only | Software + hardware (e.g. hand pumps, latrines, water treatment solution, soap, handwashing facilities, protected infant play space) |
| Daycare centre‐based hygiene hardware and education interventions | Software + hardware | Software + hardware |
WASH: water, sanitation, and hygiene.
1. Education and hygiene promotion interventions
These were software‐only interventions that had no or limited (e.g. soap, chlorine, drinking container) hardware components. These interventions included safe child faeces disposal promotion, as their only promoted intervention or among other interventions (promotion of other WASH behaviours (e.g. hand washing with soap, safe water storage behaviours, use of latrines) or other public health behaviours (e.g. exclusive breastfeeding for children under six months of age, maternal nutrition during pregnancy, disposal of animal faeces, safe waste disposal, use of bed nets, immunizations)). While some of the interventions promoted child potties or dirt throwers/scoops, no child faeces disposal hardware was provided as part of the interventions. The intervention delivery method varied across all the interventions (e.g. education in health centres, mass‐media campaigns, community‐based volunteer groups, household visits). The messages on child faeces differed across interventions, but included one or more of the following messages.
Disposal of faeces in a latrine when available.
Use of latrines by everyone, including children.
Burying the faeces or constructing a specific pit to dispose of child faeces.
Covering faeces with leaf or paper prior to burying them.
Disposal of child faeces in a contained waste disposal sites, as opposed to uncollected waste.
Use of chamber pots/potties.
Use a dirt thrower/scoop to remove child faeces.
Not letting dogs or pigs eat children's faeces.
Discouraging children from defecating around households.
Keeping the home environment free from faeces.
Washing babies in a particular place after defecation.
2. Community‐led total sanitation interventions plus adaptations
These interventions also had no hardware component, but their principal goal was to end open defecation by all household members (i.e. latrine use by all), with few other behaviours targeted for change. CLTS is an approach that aims to change behaviour in a community through stimulating a collective sense of disgust and shame that triggers the whole community to stop practicing open defecation; once communities succeed in ending open defecation, they are rewarded open defecation‐free (ODF) certification (Kar 2008). CLTS does not encourage hardware subsidies; however, some of the included studies used CLTS techniques but also provided subsidies for building latrines and some included strengthening of the sanitation supply chain.
In this category of studies, it was not always clear whether children aged less than five years were specifically targeted in the triggering activities to end open defecation and none of the interventions included child faeces management hardware. A review of CLTS processes and protocols in Sub‐Saharan Africa said that most countries' CLTS programmes require children's faeces to be safely disposed of. However, only two out of 15 countries reviewed had an indicator for child faeces disposal (Thomas 2013).
3. Sanitation hardware and behaviour change interventions
These interventions included a hardware and software component to improve the sanitation behaviours of everyone in the household. These interventions included providing child faeces management hardware, potties and sani‐scoops (e.g. dustpans), as well as sanitation hardware (improvements to latrines or new latrines). The software component of these interventions included messages to encourage mothers to safely manage child faeces and to dispose of faeces in latrines.
4. Water, Sanitation, and Hygiene hardware and education/behaviour change interventions
These were interventions that addressed child faeces disposal education as part of a wider water (e.g. building of hand pumps or provision of chlorine for water treatment) or sanitation (e.g. provision of latrines) or hygiene (handwashing facilities), or a combination of these, hardware intervention. The educational messages on child faeces disposal in different interventions included the following.
Disposal of child's faeces soon after defecation.
Importance of everyone using latrines, including young children.
Not disposing of used nappies in the garden or bushes or in waterways.
Use handy tool (e.g. shovel) to collect and dispose of faeces and keep the tool clean.
"Child faeces are more harmful than the adult."
Wash hands after disposing of child faeces.
5. Daycare centre‐based hygiene hardware and education interventions
These were studies conducted in the USA, which aimed to improve several hygiene behaviours in daycare centres. They also included some hygiene equipment, including nappy changing equipment and instructions on how to dispose of nappies.
How the intervention might work
The intervention might work through reducing exposure to child faeces, which are currently mostly ending up in the environment. This reduced exposure to faeces would reduce possible ingestion of faecal pathogens (bacteria, viruses, protozoa, and worm eggs) or penetration of hookworm larvae, leading to reduced diarrhoea and soil‐transmitted infections, which in turn would improve nutritional status (see Figure 1).
Objectives
To assess the effectiveness of interventions to improve the disposal of child faeces for preventing diarrhoea and STH infections.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) that were either individually‐ or cluster‐randomized, and the following types of non‐randomized controlled studies (NRS): quasi‐RCTs, non‐RCTs, controlled before‐and‐after studies, interrupted time series studies, historically controlled studies, case‐control studies, cohort studies, and cross‐sectional studies (see definitions in Appendix 1). We included NRS as based on a previous review, Gil 2004, we assumed that there would be no or very few RCTs assessing the effect of improved disposal of child faeces for preventing diarrhoea and STH infection. Despite the risk of confounding, NRS studies contribute useful additional information to that provided by RCTs, as the interventions evaluated in the RCTs mostly evaluate interventions to improve WASH and other behaviours rather than just child faeces disposal and thus do not give measures of effect of improving child faeces disposal itself. We excluded non‐controlled studies, such as case reports or case series, due to the importance of control groups to determine the effect of the intervention on the outcomes of interest.
Types of participants
Adults and children.
Types of interventions
Intervention
All interventions aiming to improve the safe collection or disposal of faeces of children aged below five years in order to decrease direct or indirect human contact with such faeces. For NRS, this extended to interventions that occurred in the course of usual healthcare or daily life, or those that were deliberately introduced. This included, but was not limited to, safe disposal practices as defined by the JMP, namely direct defecation into a latrine, disposal of stools in a latrine, or burying of stools (WHO/UNICEF 2006). Interventions could include the provision of hardware (e.g. nappies, potties, faecal collection devices, cleaning products to remove faeces, child‐friendly squatting slabs, or latrines used by children), software (e.g. promotion of safe disposal practices), or both. We included interventions that combined the safe disposal of child faeces with other interventions, such as hygiene promotion interventions.
Control
Participants that continued their usual practices of child faeces disposal instead of the intervention, or who received a different type of intervention (e.g. a health promotion intervention).
Types of outcome measures
Primary outcomes
Diarrhoea episodes among individuals, whether or not confirmed by microbiological examination. We defined an episode according to the case definitions used in each reviewed study. A third of the included studies used the WHO definition, which is the passage of three or more loose or liquid stools per day or more than usual for the individual (WHO 2013), while others used other definitions, which are defined in the results section. We treated this outcome as dichotomous, whether an individual had one or more episodes of diarrhoea.
Infection with one or more of the following species of STHs: Ascaris lumbricoides (round worm), Trichuris trichiura (whip worm), Ancylostoma duodenale, orNecator americanus (hookworm). We defined infection as the presence of eggs, or juvenile nematodes, or both in the stools of the participants. We included any accepted diagnostic techniques.
Secondary outcomes
Dysentery (bloody diarrhoea).
Severe diarrhoea (clinical features associated with greater severity of diarrhoea illness include: high stool frequency or stool output and persistent diarrhoea (Bhandari 2002)).
Persistent diarrhoea (diarrhoea lasting 14 days or longer).
Clinical visits for diarrhoea.
Intensity of STH infection (number of eggs per gram of stool).
Presence of pathogenic microbes in stool assays.
Anthropometry (weight‐for‐age and height‐for‐age).
Serology.
Other markers of infection and disease.
Mortality.
Use and adoption of the intervention (behaviour change).
Adverse events.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and ongoing).
Electronic searches
The search terms are detailed in Appendix 2 and included terms for "faeces disposal" or "sanitation" and for "child". We did not include specific terms for study designs or outcomes to ensure relevant studies were not missed.
We searched the following databases:
the Cochrane Infectious Diseases Group (CIDG) Specialized Register (27 September 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (27 September 2018);
MEDLINE (27 September 2018);
Embase (27 September 2018);
Global Health (5 October 2018);
Web of Science (27 September 2018);
LILACS (27 September 2018);
POPLINE (27 September 2018).
Also, we examined Chinese‐language databases available in the China National Knowledge Infrastructure (25 January 2015) and the Wan Fang Portal (11 January 2015) using the search terms detailed in Appendix 2 or their Chinese language equivalents. We searched the metaRegister of Controlled Trials (mRCT), ClinicalTrials.gov (clinicaltrials.gov), and the WHO International Clinical Trials Registry Platform Search Portal (www.who.int/trialsearch) using "sanitation" and "hygiene" as search terms, as well as an index to theses in the UK (ethos.bl.uk) (27 September 2018). We searched the Open Grey (www.opengrey.eu) database for grey literature (27 September 2018).
Searching other resources
Conference proceedings
We searched the following organizations' conference proceedings: International Water Association and Water, Engineering and Development Centre, Loughborough University, UK.
Researchers and organizations
We contacted individuals working in the field, and contacted or searched websites of the following organizations for other potential published and unpublished studies:
Water, Sanitation and Health Programme of the WHO;
World Bank WSP;
UNICEF Water, Environment and Sanitation;
Environmental Health Project (US Agency for International Development (USAID));
IRC International Water and Sanitation Centre;
Global Water, Sanitation and Hygiene (Centers for Disease Control and Prevention);
International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B);
USAID;
UK Department for International Development (DFID);
Asian Development Bank (ADB);
WASHplus (www.washplus.org/);
Sustainable Sanitation Alliance (www.susana.org/);
community‐led total sanitation (CLTS);
the sanitation updates blog (sanitationupdates.wordpress.com/); and
the STEPS Centre at the Institute of Development Studies University of Sussex (steps‐centre.org).
Reference lists
We checked the reference lists of studies identified by the above methods.
Data collection and analysis
Selection of studies
One review author (FM) examined titles of all identified studies removing those that were clearly ineligible and off‐topic. Two researchers (among FM, Lyndsey Gray (LG), BT, Christian Landon (CL), and Czarina Cooper (CC)) independently examined abstracts and selected all potentially eligible studies based on the inclusion criteria. If a title or abstract could not be rejected with certainty due to lack of information, we obtained the full‐text article for further assessment. GC reviewed the results of the Chinese database search, undertaking the same process as FM, LG, BT, CL, and CC. We obtained full copies of all studies agreed by either reviewer to potentially fall within the inclusion criteria. Two researchers (FM and LG, BT, CL, or CC) independently determined whether each study met the inclusion criteria using a form. When we agreed, we either included or excluded the study. If we were unable to agree, we consulted review author Thomas Clasen (TC) who made the final decision. One review author (FM) corresponded with authors in case data needed to assess eligibility was not obvious in the study or if data were missing from the report. Any studies that FM or the second reviewer (LG, BT, CL, or CC) suggested to include but which was ultimately excluded through discussion or by a third review author (TC or FM) was presented with the reason for exclusion in the Characteristics of excluded studies table. We checked study reports to ensure that multiple publications of the same study were only included once.
Data extraction and management
Two review authors (FM and BT) independently extracted data from the included studies using a data extraction form after it was piloted on two included studies (items included in the form are presented in Appendix 3). In case of discrepancy, we discussed the data and consulted TC, if necessary, who made the final decision. One review author (FM) entered and analysed the agreed data in Review Manager 5 (Review Manager 2014), and a second review author (BT) independently cross‐checked a sample of the data.
Type of data extracted
Randomized controlled trials randomized by cluster
For cluster RCTs, we extracted the number of participants enrolled and the number analysed in each treatment group for each outcome. We noted whether or not the authors reported adjusting for clustering in the analysis. We endeavoured to collect intracluster correlation coefficients (ICC) for cluster RCTs but only four of the trials reported this measure. In addition, we extracted data on the study setting, study design, study participants, details of the interventions and control groups and activities, details of outcomes measured in the study and their measures of effect, and when and how they were measured. When an RCT included several arms with a relevant intervention but only had one control group, we extracted data for the study arm most relevant to this review.
Non‐randomized studies
For NRS, we extracted details on the features of the design, the confounding factors considered in the study, methods used to control for confounding, data on the risk of bias specific for NRS (see Assessment of risk of bias in included studies), the total numbers of participants included in the study and in each comparison group, and the measures of effect and CIs.
Assessment of risk of bias in included studies
Two review authors (BT and FM) independently applied the risk of bias criteria using an assessment form. In case of disagreement, we discussed the issue to make the final decision. For each study, we justified reasons for the level of risk of bias and included it in the 'Risk of bias' table.
For RCTs, we used the Cochrane tool to assess the risk of bias, which includes methods of random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessment; incomplete outcome data; and selective reporting (Higgins 2011a). For each domain, we followed the definitions of low risk, unclear risk, and high risk described in Higgins 2011a.
For cluster RCTs, we also assessed the risk of bias specific to this study design.
Recruitment bias. We qualified the study at high risk of bias when the participants and staff were aware of which cluster the intervention or control was; unclear risk of bias when the information was not collected or reported; or low risk of bias if clusters were not known to be intervention or control during participant recruitment.
Baseline imbalance. We assessed a study at high risk of bias when there were large differences in baseline characteristics and they were not adjusted for in the analysis; low risk of bias where statistical methods were used to match the clusters at the design stage or to adjust for imbalances in the analysis, or in case there were no substantial differences in baseline characteristics; or unclear risk of bias if it was not mentioned in the report.
Loss of clusters. We qualified studies at high risk of bias where more than 10% of clusters were lost to follow‐up; low risk of bias where less than 10% of clusters were lost to follow‐up; or unclear risk of bias if loss to follow‐up was not mentioned.
Incorrect analyses. We assessed studies at high risk of bias if they did not analyse the data adjusting for clustering; low risk of bias where there were no unit‐of analysis errors in the study and if clustering was adjusted for in the analysis; or unclear risk of bias if it was not reported in the study.
Comparability with individually randomized RCTs. We analysed cluster‐RCTs separately from other study designs.
For controlled before‐and‐after studies, controlled cohort studies, and cross‐sectional studies, we used the EPOC criteria to assess the risk of bias (EPOC 2013). This tool includes random sequence generation, allocation concealment, incomplete outcome data (less than 10% loss to follow‐up or no difference between arms was considered low, more than 10% was considered high, and if it was not mentioned or reported, it was considered as unclear), selective outcome reporting, and other biases that were similar to the RCT 'Risk of bias' tool, as well as the following additional domains.
Similarity of baseline characteristics. Important baseline characteristics for this study included: access and type of sanitation facilities, water access and quality, age, wealth, and hygiene practices. We qualified the studies as high risk of bias where there were substantial differences; low risk of bias if baseline characteristics were reported and there was no substantial difference; or unclear risk of bias if it was not reported or unknown.
Similarity of baseline outcome measurements. We gave high risk of bias scores when large differences were present and they were not adjusted for in the analysis; low risk of bias scores to studies if participant outcomes were measured prior to the intervention and there were no substantial differences; or unclear risk of bias if it was not mentioned in the report.
Adequate protection against contamination? We qualified a study as high risk of bias if it was likely that the control group received the intervention; low risk of bias if it was unlikely that the control group received the intervention; or unclear risk of bias in case it was possible contamination could have occurred.
Adequate allocation of intervention concealment during the study. We qualified studies as high risk of bias if the outcomes were not assessed blindly; low risk of bias if the authors explicitly reported that the primary outcomes were assessed blindly or the outcomes were objective; or unclear risk of bias if it was not specified in the paper.
We also added a domain to assess whether the studies appropriately adjusted for confounders. The following confounders related to child faeces disposal and diarrhoea or STHs infections were considered important for this review: access to or ownership of a sanitation facility, type of sanitation facility (improved or unimproved according to the JMP classification (WHO/UNICEF 2014), use of sanitation facility, wealth, age, water access, season, water quality, animal ownership, household size, educational level, attendance to school or preschool by the children, shoe‐wearing, and hygiene practices. We qualified studies as low risk of bias if they controlled for at least one of the listed confounders in the design (e.g. matching) or the analysis (e.g. multivariable statistical modelling). We qualified studies as high risk of bias if no adjustment for confounding variables was conducted and unclear risk of bias where it was not mentioned in the paper.
For case‐control studies, we assessed the quality of the studies using the Newcastle Ottawa scale (NOS) (Wells 2013). The scale is divided into eight items grouped into three domains: selection, comparability, and ascertainment of exposure. For each item in the selection and exposure ascertainment domains a total of one 'star' can be awarded to a study; in the comparability domain two stars can be awarded. For one star in the comparability domain, the study had to control for access to or ownership of a sanitation facility. For two stars, the study had to control for at least one other important confounding variable, such as type of sanitation facility (improved or unimproved) use of sanitation facility, wealth, age, water access, season, water quality, animal ownership, household size, educational level, attendance to school or preschool by the children, shoe‐wearing, and hygiene practices.
Measures of treatment effect
For RCTs with dichotomous outcomes, we calculated risk ratios (RR) with 95% confidence intervals (CIs) where raw data were available. If not, we used the effect measures reported, along with the 95% CI. For continuous variables, we extracted the mean differences (MD). We calculated or extracted standard errors and 95% CI from these studies.
For NRS, we reported measures of effect adjusted for confounders from the studies. If several adjusted estimates were reported, we used the estimate adjusting for the most confounders. We specified the confounders that were adjusted for in the study and whether it was done in the design or in the analysis. In case the effect measures extracted were expressed in different metrics, we converted them into a common measure, RR for controlled cohorts and cross‐sectional studies and odds ratio (OR) for case‐control studies; if they were all the same, we combined them using the effect measure used in the reports. If no adjusted measures could be obtained from the studies, we used unadjusted measures reported in the study or calculated RR or OR (for case‐controls) and 95% CI from the raw data.
Unit of analysis issues
We searched for both individually and cluster‐RCTs, however we identified no individually‐RCTs that met our inclusion criteria. For cluster‐RCTs, we assessed whether clustering was properly accounted for in the analysis and used the adjusted measure of effect reported. When the studies did not adjust for clustering or measures of effect needed to be calculated, we extracted or calculated unadjusted measures of effect and CIs, the mean cluster sizes and calculated adjusted measures of effect that accounted for clustering using the inflating standard error method using ICC from other similar studies (Higgins 2011b). We added details of ICCs used in the footnotes of the forest plots.
Dealing with missing data
If studies had missing data needed for assessment of eligibility or analysis, one review author (FM) attempted to contact authors to obtain the data. We report the number of participants in each study and the number of participants who were lost to follow‐up.
Assessment of heterogeneity
We assessed heterogeneity by visually examining the CIs in the forest plot and by using the Chi² test and I² statistic (Higgins 2003). We considered a significance level of P less than 0.1 for the Chi² test to be significant and indicate potential heterogeneity. To estimate the degree of heterogeneity, we classified an estimate of the I² statistic greater than 50% to indicate substantial heterogeneity and greater than 75% to indicate considerable heterogeneity (Deeks 2011). We prespecified in the protocol that if there were sufficient studies (more than 10) and substantial heterogeneity, we would investigate causes of heterogeneity using subgroup analysis (Majorin 2014).
Assessment of reporting biases
We tried to minimize reporting bias by using a comprehensive search strategy including published and unpublished studies. We compared the outcomes listed in the methods and those reported in the results sections. We assessed the potential of publication bias using funnel plots of case‐control studies included in the analysis of safe disposal of child faeces, as they were the only analysis that had sufficient studies (more than 10).
Data synthesis
We analysed the data using Review Manager 5 (Review Manager 2014). If there was more than one study with comparable participants, interventions, and outcomes, we conducted a meta‐analysis to estimate a pooled measure of effect. We used random‐effects models to pool the data. The comparisons made were between those with the intervention and those without or with a different intervention. Due to differences in potential risk of bias of different study designs (Reeves 2011), we only pooled results of similar study designs.
We stratified the case‐control analyses according to the level of quality of the studies, according to the numbers of stars it received.
When there were not enough similar studies to pool them, we described them in the text organizing them by type of intervention, outcome, and study design.
'Summary of findings' tables
One review author (FM) assessed the methodological certainty of each outcome across the included studies using GRADE guidelines (Guyatt 2011). We summarized the methodological certainty in Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
The 'Summary of findings' tables present the following outcomes.
Diarrhoea episodes.
Infections with one or more species of STHs.
We used the following criteria to grade the certainty in the 'Summary of findings' tables.
For study limitations: we downgraded studies one level for serious risk of bias if the outcome was self‐reported or not objective and susceptible to bias due to the studies being unblinded. As most environmental interventions, including sanitation, are difficult or impossible to blind, studies that met other criteria for low risk of bias were nevertheless downgraded unless the outcome was objective.
For inconsistency of results: we downgraded studies if there was substantial (I² greater than 50%) statistical heterogeneity and this could not be explained through subgroup analyses.
For indirectness of evidence: we downgraded if there were limited populations or settings in the included the studies, which did not allow us to make generalizations about the findings to other settings relevant to this review.
For imprecision: we downgraded if the studies had a small sample size and large CIs that included important effects in both directions
Subgroup analysis and investigation of heterogeneity
Only case‐control studies had sufficient comparisons, as prespecified in our protocol (greater than 10), for subgroup analyses. In the case‐control analyses, we conducted subgroup analyses to investigate the effects of:
safe child faeces disposal on outcomes in different age groups, children aged under five years versus all ages;
different case‐definitions;
intervention site (urban versus rural);
intervention settings (low‐, middle‐ or high‐income country);
different methods to ascertain child faeces disposal behaviour: observations versus survey questionnaire.
Sensitivity analysis
We conducted sensitivity analyses to check robustness of the choice of analysis method (random‐effects model versus fixed‐effect) for the main health outcomes.
Results
Description of studies
Results of the search
The searches identified 38,731 records (34,200 from English databases, 3613 from Chinese databases, and 918 from other sources). We screened the titles and abstracts and obtained 935 full texts, of which 78 reports of 63 studies met the inclusion criteria (see Figure 2).
2.

PRISMA diagram.
Included studies
Study designs
The 63 included studies covered at least 222,846 participants (see Characteristics of included studies table). Of these studies, 22 were cluster‐RCTs, four were CBAs, and 37 were NRS (27 case‐control studies (one which included seven study sites), three controlled cohort studies, and seven controlled cross‐sectional studies) (see Appendix 1 for study design definitions).
Twenty‐four included studies had insufficient information or had no comparable studies to be included in the quantitative analysis. We have described these in this review, but have not included them in the analyses. We contacted 36 authors of included studies for additional details on their study, of whom 23 replied.
Randomized controlled trials
Out of the 22 cluster‐RCTs, 10 were education and hygiene promotion interventions that included child faeces management instructions exclusively (Yeager 2002 PER), or among other targeted hygiene, sanitation, or other public health behaviours (Altmann 2018 TCD; Barrios 2008 PHI; Haggerty 1994 DRC; Hashi 2017 ETH; Jinadu 2007 NGR; Nair 2017 IND; Sarrassat 2018 BUR; Sinharoy 2017 RWA; Stanton 1987 BGD). Among these, Altmann 2018 TCD and Hashi 2017 ETH also provided WASH kits or soap and Sarrassat 2018 BUR was a mass radio campaign.
Five studies focused on ending open defecation throughout the target community using either CLTS (Pickering 2015 MLI) or TSSM, which included CLTS‐messaging and sanitation marketing (Briceño 2015 TAN; Cameron 2013 INA), or India's Total Sanitation Campaign (TSC), which included subsidies and latrine promotion (Dickinson 2015 IND and Patil 2014 IND, which also included additional TSSM support including CLTS messaging).
Four studies evaluated sanitation hardware and behaviour change interventions, which included the provision of child sanitation hardware (potties and sani‐scoops) and behaviour messaging (Caruso 2019 IND; Christensen 2015a KEN; Luby 2018 BGD; Null 2018 KEN). Three of these trials were from WASH Benefits (WASH B) study, one from the pilot in Kenya (Christensen 2015a KEN), and on the main outcomes from Kenya (Null 2018 KEN) and Bangladesh (Luby 2018 BGD). The WASH B studies included several study arms, for this review we included only the sanitation versus control results as they were most relevant.
One study, the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial, evaluated a WASH hardware and behaviour change intervention (Humphrey 2019 ZIM).
Two studies included child faeces disposal in their multicomponent interventions in daycare centres (Butz 1990 USA; Kotch 2007 USA).
Controlled before‐and‐after studies
Ahmed 1993 BGD consisted of an education intervention on sanitation, food, and personal hygiene.
The other three CBAs were WASH hardware and education interventions that included instructions for children to use toilets constructed in its WASH intervention (Aziz 1990 BGD), or included child faeces disposal messaging in their health education component along with providing hand pumps (Alam 1989 BGD), or providing latrines (Park 2016 INA).
Non‐randomized studies
Controlled cohort studies
Two controlled cohort studies were education and hygiene promotion interventions that evaluated the Sanitation Hygiene Education and Water Supply in Bangladesh (SHEWA‐B) intervention in Bangladesh. The intervention included child faeces disposal in its hygiene education component (Huda 2012 BGD; Luby 2014 BGD). The third controlled cohort was a WASH hardware and education interventions that compared wards that received a community‐based health project and WASH‐focused activities, which included messages about child faeces disposal in its mothers' groups and children's club meetings, with wards that only received the community‐based health project (Hoq 2016 BGD).
Controlled cross‐sectional studies
Six controlled cross‐sectional studies were education and hygiene promotion interventions (Berhe 2014 ETH; Fisher 2011 BGD; Gebru 2014 ETH; Mathew 2004 ZIM; Oguro 2016 MYA; Waterkeyn 2005 ZIM). Two cross‐sectional studies compared "model" and "non‐model" families from the Ethiopian Health Extension Package (HEP) (Berhe 2014 ETH; Gebru 2014 ETH). Model families were those that fully implemented the HEP, whereas non‐model families did not fully implement the HEP. The HEP consisted of health promotion in four main categories: family health services, infectious disease prevention and control, hygiene and environmental sanitation, and health education and communication. The maternal and child health package (in the family health services category) included messaging about safe child stool disposal (the stool should be cleared and disposed of in a pit latrine, or should be covered with a leaf or paper and be buried) (HEP 2003).
Two studied the behaviour change as a result of community health clubs, which provided participatory health education classes on various health topics (Mathew 2004 ZIM; Waterkeyn 2005 ZIM). One of the lessons included child faeces disposal in a latrine. One study investigated the behaviour change and health effect of the BRAC WASH programme (a WASH programme of BRAC, which is a non‐governmental development organization based in Bangladesh) (Fisher 2011 BGD), which provided hygiene education including child faeces disposal in a latrine in its sanitation messaging. One study compared behaviour change in two villages that received a Women's Health Volunteer Group (WVG) intervention with two villages that did not (Oguro 2016 MYA).
One controlled cross‐sectional study evaluated a CLTS intervention by comparing the parasitology and nutritional status of children in two villages that benefited from CLTS and attained ODF status with two other villages that did not benefit from CLTS (Belizario 2015 PHI).
Case‐control studies
In the case‐control studies, three studies included two risk factors related to child faeces disposal, and one study had seven different study sites (Baker 2016 BGD), thus making a total of 29 comparisons. Six studies could not be included in the analyses as they either had insufficient or no data or could not be compared to the other case‐control studies (Arvelo 2009 USA; Bassal 2016 ISR; Chiang 2005 TWN; Daniels 1990 LES; Menon 1990 USA; Nanan 2003 PAK).
Study participants and settings
Randomized controlled trials
Most RCTs (19/22) were conducted in low‐ or lower middle‐income settings, apart from Butz 1990 USA and Kotch 2007 USA, which were conducted in daycare centres in the USA and Yeager 2002 PER, which was conducted in urban Peru.
Stanton 1987 BGD was conducted in urban Bangladesh; Barrios 2008 PHI in rural Philippines; Cameron 2013 INA in rural Indonesia; Caruso 2019 IND, Dickinson 2015 IND, Nair 2017 IND, and Patil 2014 IND in rural India; and Luby 2018 BGD in rural Bangladesh. Altmann 2018 TCD was conducted in Chad, Briceño 2015 TAN in rural Tanzania, Christensen 2015a KEN and Null 2018 KEN in rural Kenya, Haggerty 1994 DRC in rural Democratic Republic of Congo (DRC), Hashi 2017 ETH in rural Ethiopia, Jinadu 2007 NGR in rural Nigeria, Pickering 2015 MLI in rural Mali, Sarrassat 2018 BUR in rural Burkina Faso, Sinharoy 2017 RWA in rural Rwanda, and Humphrey 2019 ZIM in rural Zimbabwe.
Apart from Stanton 1987 BGD, which collected diarrhoea morbidity data in children aged less than six years, Jinadu 2007 NGR, which collected data on children aged five year or less and Butz 1990 USA, which included children aged between one month and seven years in daycare centres, all other studies collected data for children aged less than five years.
Controlled before‐and‐after studies
Three studies were conducted in rural Bangladesh and collected data for children aged less than five years (Aziz 1990 BGD), less than 23 months (Alam 1989 BGD), and less than 19 months (Ahmed 1993 BGD). Park 2016 INA was conducted in rural Indonesia and collected data on STH in children aged between three and 13 years.
Non‐randomized studies
Controlled cohort studies
The three cohort studies were conducted in Bangladesh. Huda 2012 BGD included only rural populations, while Luby 2014 BGD included both urban and rural areas and Hoq 2016 BGD was in periurban areas. Huda 2012 BGD and Luby 2014 BGD studied outcomes in children aged below five years, while Hoq 2016 BGD measured outcomes in children aged below two years.
Controlled cross‐sectional studies
Berhe 2014 ETH and Gebru 2014 ETH were conducted in rural Ethiopia and measured outcomes in children aged less than five years. Mathew 2004 ZIM and Waterkeyn 2005 ZIM were conducted in rural Zimbabwe and did not specify the age of the children whose defecation or faeces disposal behaviour were collected. Fisher 2011 BGD covered children aged less than five years in rural Bangladesh. Belizario 2015 PHI was conducted in rural Philippines and measured STH prevalence in children that were aged between two and 15 years. Oguro 2016 MYA was conducted in Myanmar and measured behaviour change reported by caregivers of children aged less than five years.
Case‐control studies
Most of the case‐control study sites (23/33) occurred in low‐ or lower middle‐income countries apart from Chompook 2006 THA; Genthe 1997 SAF; Heller 2003 BRA; Knight 1992 MAL; and Strina 2012 BRA, which were in upper middle‐income countries, and Abalkhail 1995 KSA; Arvelo 2009 USA; Bassal 2016 ISRChiang 2005 TWN; and Menon 1990 USA, which were in high‐income countries.
In general, included studies considered cases and controls only aged less than five years or younger age groups. The exceptions were Arvelo 2009 USA, which did not specify the age of the children in the daycare centres; Chompook 2006 THA, which included all ages (median age: five years in cases and controls); Clemens 1987 BGD included children aged less than six years; Cummings 2012 UGA, which only collected data on cases and controls aged more than 10 years (median age in cases: 26 years, in controls: 33 years); Genthe 1997 SAF, which included preschool children (age range 0.2 to 67.2 months); Nanan 2003 PAK, who considered cases and controls aged between four and 71 months; Oketcho 2012 TAN aged between six and 60 months; and Strina 2012 BRA aged less than 10 years.
Most of the case‐control studies (11 studies) recruited cases from healthcare settings and controls from the community (of those Menon 1990 USA; Mertens 1992 SRI; and Traoré 1994b BUR had both community and hospital controls), eight recruited cases and controls from healthcare settings, seven recruited cases and controls from the community, and Arvelo 2009 USA recruited cases and controls from among licensed daycare centres.
Interventions
Education and hygiene promotion interventions
A summary of the study designs, settings, and outcome measures of the education and hygiene promotion interventions is presented in Table 8.
2. Summary of the study designs, settings, and outcome measures of the education and hygiene promotion interventions.
| Study (setting) | Summary of the intervention | Outcomes used in reviewa | |||
| Diarrhoea | Anthropometry | Behaviour change | Other | ||
| RCTs | |||||
| Altmann 2018 TCD (not specified) | Intervention in health centres to children admitted for OTP. The intervention group received routine OTP services (as the control group) plus WASH kit and promotion, which included messaging to bury children's stool. The household WASH kit given at admission contained a safe drinking water storage container with a lid, water disinfection consumables (180 chlorine tablets), 12 bars of soap for hand washing, a plastic cup with handle (to be reserved for the child to facilitate safe drinking water practice), and a laminated leaflet with pictures representing the main hygiene messages. They also received a promotion session on the kit use at each weekly visit to the health centre and 2 extra home visits for assessing and reinforcing adherence. Promotion at health centre included key messages on:
The household WASH kit was designed to last for 3 months (2 months during treatment in the OTP and 1 month after the end of the treatment). |
Longitudinal prevalence (narratively) | Recovery rate from SAM (narratively) | — | Death rate (narratively) |
| Barrios 2008 PHI (rural) | Hygiene promotion programme that focused on improving hand washing and stool disposal behaviours. Delivered by midwives and health workers in small group meetings and in home visits. For the disposal of child faeces, caretakers were encouraged to use toilets (any type) as the final site of faeces disposal. When a toilet was not available, burying faeces ≥ 10 m away from water sources and living areas was discussed. The main message was the sanitary disposal of faeces, regardless of where a child defecated. | — | — | Observed faeces in the yard | — |
| Haggerty 1994 DRC (rural) | Education intervention to improve personal and domestic hygiene behaviours including: disposal of animal faeces, hand washing before meal preparation and after defecation/washing hands and buttocks of young children after defecation, disposal of children's faeces (emphasized digging or improving pit latrines). The messages were delivered by female community volunteers in village‐wide meetings and small group discussions. | 7‐day recall ≥ 1 episodes of diarrhoea at any time during the surveillance period. |
— | — | — |
| Hashi 2017 ETH (rural) | Health education and provision of soap (white bars). The health education consisted of 12 sessions on key WASH messages (hand washing with soap, water storage behaviour, latrine availability and use, safe waste disposal) and demonstration of hand washing with soap. Messages to dispose of children's waste properly were delivered via demonstrations and instructions. The messages were to dispose of children's waste properly in the waste disposal site (in a waste container at the corner/back of the house) as opposed to the garbage (uncollected waste) and in a latrine (if they had 1) but never in the open field, garbage, or around utensils and kitchen. |
Incidence (2‐week recall) | — | — | — |
| Jinadu 2007 NGR (rural) | Educational intervention programme to promote the hygienic disposal of children's faeces:
|
— | — | Potty use Faeces observed in the yard/house Latrine use by children |
— |
| Nair 2017 IND (rural) | Intervention involving community‐based female worker (Suposhan Karyakarta, or SPK) carrying home visits with individual families and participatory meetings with groups of women, to improve health and nutrition in the first 1000 days of life. The training to prepare SPK to home visits included: advising caregivers to place the child's faeces in a pit latrine, or if no latrines are available (the case for > 90% of households in the trial areas), to bury them in a shallow hole away from their living area and any waterway rather than disposing of them in the open field or the household compound. | — | LAZ at 18 months Other anthropometry measures (WHZ, WAZ, MUAC, stunting, wasting, underweight) (narratively) |
— | Mortality (narratively) |
| Sarrassat 2018 BUR (rural) | Mass radio campaign targeted at women of reproductive age and caregivers of children aged < 5 years, on 17 childcare behaviours, including safe child faeces disposal. The radio campaign included short spots (1 minute' duration, broadcast approximately 10 times per day) and interactive long‐format programmes (2 hours' duration, broadcast 5 days per week, followed by phone‐ins to allow listeners to comment). All materials were produced in the predominant local languages of each intervention cluster. Behaviours covered by spots changed weekly. The long‐format programme covered 2 behaviours a day and changed daily. Safe child stool disposal was covered in 3 weeks of spots and 94 long‐format modules. The recommendation was for all faeces (including the faeces of babies and small children) to be disposed of after defecation in a hygienic way. Either by using latrines or by using pots for young children or burying the stools outside the house/compound. | — | — | Safe disposal of last children's stools | All‐cause postneonatal mortality in children aged < 5 years (narratively) All‐cause mortality in children aged < 5 years (narratively) |
| Sinharoy 2017 RWA (rural) | Community‐Based Environmental Health Promotion Programme, which used the community health club approach to promote healthy practices. The study evaluated 2 versions of the programme: a lite (8 education sessions) and classic (20 sessions). Education sessions include: personal hygiene, handwashing, diarrhoea, water sources, safe storage of drinking water, treatment of drinking water, and sanitation. Both lite and classic interventions included messages on child sanitation under the topic of sanitation ("zero open defecation"). The participants were recommended the following:
|
Prevalence, 7‐day recall | HAZ (used in analysis) WHZ |
Safe child (aged < 3 years) faeces disposal Presence of faeces (human or animal) in compound |
— |
| Stanton 1987 BGD (urban) | Educational intervention emphasizing 3 messages:
The intervention was delivered in the community over 8 weeks through small group discussions, larger demonstrations, community wide planning and action meeting, posters, games, pictorial stories, and flexi flans (flannel board with movable characters). |
Incidence (2‐week recall) | Weight for age Height for age Weight for height (narratively) |
Open defecation of children aged < 5 years | Mortality (narratively) |
| Yeager 2002 PER (urban) | Hygiene promotion for potty use and keeping the home environment free from faeces. The intervention was delivered through routine health services, and using video presentations, leaflets including 4 steps to potty training and counselling by health staff during consultations | — | — | Potty use Latrine use by children Safe disposal of child faeces |
— |
| CBA studies | |||||
| Ahmed 1993 BGD (rural) | Participatory behaviour change intervention "Porichchhanna Jibon" (clean life). The campaign was developed in partnership with the community. The intervention involved teaching the germ theory of disease then encouraging mothers to identify their problems and to find solutions through group participation and discussion. The interventions developed were:
|
Trends in daily diarrhoea prevalence (narratively) | Weight for age (narratively) | — | — |
| Controlled cohort studies | |||||
| Huda 2012 BGD (rural) | SHEWA‐B, a large‐scale hygiene promotion intervention which engages local residents to develop their own community action plans, including targets for improvements in latrine coverage and use, access to arsenic‐free water and improved hygiene practices. Community hygiene promoters are trained to deliver 11 key messages including "use hygienic latrine by all family members including children" and "dispose of children's faeces into hygienic latrines" using household visits, courtyard meetings and different activities for example hygiene fairs, village theatre, and group discussions in tea stalls. Promoters used flip charts and flash cards. | Diarrhoea prevalence (2‐day recall) | — | Safe disposal of child faeces (observed) | — |
| Luby 2014 BGD (rural and urban) | Improved SHEWA‐B. Changes in the intervention included a mass media campaign including radio spots across 6 regional channels from November 2011 to February 2012 encouraging HWWS before food, after defecation, and after cleaning a child and video spots on 5 television stations (November–February 2012) encouraging HWWS, using sanitary latrines for defecation, discarding child faeces, and keeping latrines clean to reduce bad smells and flies. A second series of videos encouraged testing tube‐wells for arsenic and using arsenic free water for cooking and drinking. The intervention target population also expanded to include urban households. | Diarrhoea prevalence (2‐day recall) | HAZ WAZ WHZ (narratively) |
Safe disposal of child faeces (observed) | — |
| Controlled cross‐sectional studies | |||||
| Berhe 2014 ETH (rural) | The HEP is implemented by full‐time female health extension workers who provide training to households. The packages include interventions in 4 main categories: family health services, infectious disease prevention and control, hygiene and environmental sanitation, and health education and communication. The maternal and child health package (in the family health services category) includes safe child stool disposal (the stool should be cleaned and disposed in a pit latrine, or shall be covered with a leaf or paper and be buried) (HEP 2003). | Diarrhoea prevalence (2‐week recall) | — | Safe disposal of child faeces | — |
| Fisher 2011 BGD (rural) | BRAC hygiene education intervention, trained field workers provide WASH education to separate clusters of men, women, adolescents, and children at least once every 3 months. The education uses pictorial flip chart with a total of 39 messages covering multiple aspects of cleanliness, clean water, and sanitation. Villagers are also encouraged to learn the '19 Messages to Remember', concerning hand washing, sanitation (includes child faeces disposal in latrine), and safe water. | Diarrhoea prevalence in last month (narratively) | — | Safe disposal of child faeces | — |
| Gebru 2014 ETH (rural) | HEP intervention, as in Berhe 2014 ETH. | Diarrhoea prevalence (2‐week recall) | — | Safe disposal of child faeces | — |
| Mathew 2004 ZIM (rural) | Community health clubs – structured weekly course of participatory health education classes. 15 health topics covered using PHAST techniques, within the hygiene lesson covering disposal of toddler's faeces in a latrine. | — | — | Percentage of children (aged < 5 years) present at the time of observations not using a latrine (narratively) | — |
| Oguro 2016 MYA (rural) | WVGs were established by organizing women and training them using a participatory approach. The activities of the WVGs after 3 years of being established included:
The WVG encouraged latrine use by children aged < 5 years to villagers as part of a programme to promote sanitation education and promoted appropriate disposal (flushing in a latrine) of child faeces. |
— | — | Appropriate disposal of child stool (narratively) | — |
| Waterkeyn 2005 ZIM (rural) | CHC intervention, as Mathew 2004 ZIM. | — | — | Observed child faeces in the yard (narratively) | — |
CBA: controlled before‐and‐after; HAZ: height‐for‐age Z score; HEP: Health Extension Package; HWWS: handwashing with soap; PHAST: Participatory Hygiene and Sanitation Transformation; OTP: Outpatient Therapeutic feeding Program; LAZ: length‐for‐age Z score; MUAC: mid‐upper‐arm‐circumference; RCT: randomized controlled trial; SAM: severe acute malnutrition; SHEWA‐B: Sanitation Hygiene Education and Water Supply in Bangladesh; WASH: water, sanitation, and hygiene; WAZ: weight‐for‐age Z score; WHZ: weight‐for‐height Z score; WVG: Women's Health Volunteer Group. aNone of the education and hygiene promotion interventions measured soil‐transmitted helminth outcomes.
Randomized controlled trials
The 10 education and hygiene promotion interventions included different messages on child faeces disposal (Characteristics of included studies table).
Yeager 2002 PER focused on promoting the use of a potty for children aged 15 to 47 months and to keep the home environment free of faeces through the routine health service. Although the intervention described what messages were promoted to train children to defecate in potties, there were no details in the report as to where potties should have been emptied.
Altmann 2018 TCD evaluated a WASH package given alongside routine Outpatient Therapeutic feeding Program (OTP) for severe acute malnutrition. The WASH package consisted of a WASH kit (safe drinking water storage container, water disinfection tablets, soap bars, and a plastic cup with handle) and promotion, which included messaging to bury children's stools.
Barrios 2008 PHI focused its intervention messages on hand washing and stool disposal aiming to ensure the sanitary disposal of faeces in a latrine or burying in case no latrine was available, regardless of where the child defecated.
Haggerty 1994 DRC promoted the disposal of animal faeces, hand washing at different key moments, and disposal of children's faeces, emphasizing digging or improving pit latrines.
Hashi 2017 ETH provided health education and soap (white bars). The health education consisted of 12 sessions on key WASH messages (hand washing with soap, water storage behaviour, latrine availability and use, safe waste disposal including child faeces disposal) and demonstration of hand washing with soap.
Jinadu 2007 NGR promoted the hygienic disposal of children's faeces by educating mothers to use chamber pots for disposal (although no details on final disposal site are provided in the paper), discouraging children from defecating around households, and also promoting the construction of ventilated improved pit (VIP) latrines and educating mothers to wash their hands after using the toilet and cleaning up children's faeces.
Nair 2017 IND used community‐based female workers (Suposhan Karyakarta, or SPK) to conduct home visits with individual families and participatory meetings with groups of women, to improve health and nutrition in the first 1000 days of life. This included advising caregivers to place the child's faeces in a pit latrine or if no latrines were available to bury them in a shallow hole away from their living area and any waterway rather than disposing of them in the open field or the household compound.
Sarrassat 2018 BUR evaluated a mass radio campaign targeted at women of reproductive age and caregivers of children aged less than five years, on 17 childcare behaviours, including safe child faeces disposal (using latrines or using potties for young children or burying the stools outside the house/compound).
Sinharoy 2017 RWA evaluated two versions of the Community‐Based Environmental Health Promotion Programme ('lite' and 'classic'), which involved community health clubs that promoted healthy behaviours. The lite version included eight topics, and the classic version included 20 topics. Topics range from handwashing, diarrhoea, water sources, and sanitation to specific diseases. In the sanitation topic (included in both the lite and classic version), it promoted that children should defecate into chamber pots, that their faeces should be buried if there is no latrine (cat sanitation), and that one should never let the dog or pig eat children's faeces after defecation.
Stanton 1987 BGD promoted proper hand washing before food preparation, defecation away from the house and in a proper site, and suitable disposal of waste and faeces. The final disposal site for child faeces was not specified in the paper.
Controlled before‐and‐after studies
Ahmed 1993 BGD generated the intervention messages through participation with the community and thus contained a large amount of target behaviours, including the use of a dirt thrower to immediately remove child or animal faeces from the compound and to construct a pit to dispose of faeces and other dirty material from the compound.
Controlled cohort studies
The SHEWA‐B programme promoted the disposal of children's faeces into hygienic latrines and the importance of everyone in the household, including children, using the latrine, among other messages in their educational component (Huda 2012 BGD; Luby 2014 BGD).
Controlled cross‐sectional studies
In the HEP programme in Ethiopia (Berhe 2014 ETH; Gebru 2014 ETH), education on child faeces disposal was included in the maternal and child health package, emphasizing cleaning faeces and disposing of them in a pit latrine or burying the faeces (HEP 2003). The HEP includes health promotion and education on 16 packages in four main categories: family health services, disease prevention and control, hygiene and environmental sanitation, and health education and communication.
The CHC (Mathew 2004 ZIM; Waterkeyn 2005 ZIM), and BRAC WASH (Fisher 2011 BGD), programmes promoted the disposal of children's faeces into hygienic latrines, among other messages in their educational component.
In Oguro 2016 MYA, as part of the sanitation education, the WVG encouraged latrine use by children aged less than five years to villagers and promoted appropriate disposal (flushing in a latrine) of child faeces.
Community‐led total sanitation interventions plus adaptations
A summary of the interventions, settings and outcome measures of the CLTS interventions plus adaptations is presented in Table 9.
3. Summary of the study designs, settings, and outcome measures of the CLTS interventions plus adaptations.
| Study (setting) | Summary of intervention | Outcomes used in review | ||||
| Diarrhoea | STH | Anthropometry | Behaviour change | Other | ||
| RCTs | ||||||
| Briceño 2015 TANa (rural) | TSSM uses CLTS (triggering of community to increase demand for improved sanitation and promote open defecation‐free communities) and sanitation marketing to increase demand for improved sanitation. Also strengthens the supply of sanitation goods and services to local markets to make these products more affordable and accessible. Sanitation marketing messages concentrated on positive aspirational messages rather than shame tactics. No subsidies were used. | Prevalence, 7‐ and 14‐day recall | — | WAZ HAZ |
Safe child faeces disposal | Mortality (narratively) |
| Cameron 2013 INA (rural) | TSSM which includes CLTS to stop open defecation, social sanitation marketing to increase availability of products and services, and strengthening the enabling environment at policy and institutional levels. | Prevalence (2‐, 7‐, and 14‐day recall) | Ascaris, Trichuris, and hookworm infections | WAZ HAZ (narratively) |
Open defecation of children aged < 5 years Safe disposal of child faeces |
Dysentery |
| Dickinson 2015 IND (rural) | The Bhadrak Total Sanitation Campaign promoted community‐wide latrine adoption (i.e. an end to open defecation) through a number of participatory activities to create a sense of disgust and shame about open defecation. The campaign subsidized materials and labour for latrine construction for households eligible for government of India subsidies (i.e. below poverty line households) and masons to guide households and organized sanitation marts in each village. Messages were also given on the benefits of latrines, both health and non‐health (convenience of time‐saving, privacy, dignity). Messages to improve child faeces disposal practices were included. | Prevalence (2‐week recall) (narratively) | — | MUAC HAZ WAZ (narratively) |
Children aged < 5 years walking time to open defecation site (narratively) | |
| Patil 2014 IND (rural) | India Total Sanitation Campaign (subsidies and promotion of individual household latrines) and Nirmal Vatika (additional subsidies) and support from Water and Sanitation Program through the TSSM project, which included creation of enabling environment + capacity building to implement CLTS‐based behaviour change methods. | Prevalence (7‐day recall) | Any helminth A lumbricoides |
WAZ HAZ |
No open defecation of children aged < 5 years Safe disposal of child faeces |
Presence of pathogenic microbes in stool assays (narratively) |
| Pickering 2015 MLI (rural) | CLTS which uses participatory methods to eliminate the practice of open defecation in rural households and promote building of toilets. No hardware or subsidies was provided to households. | Prevalence (2‐day or 2‐week recall) | — | HAZ WAZ |
Potty use Open defecation by children aged < 5 years |
Dysentery Mortality (narratively) |
| Controlled cross‐sectional studies | ||||||
| Belizario 2015 PHI (rural) | CLTS: key messages delivered by the community leaders and volunteers to households included:
The criteria for declaring open defecation‐free status included the following:
Messages about child faeces disposal and use of toilets by children were included during the CLTS activities in the villages. |
— | STH prevalence STH intensity Prevalence of Ascaris Intensity of Ascaris Prevalence of Trichuris Intensity of Trichuris Prevalence of hookworm Intensity of hookworm (narratively) |
Weight for age Height for age BMI for age (10–15 years old) (narratively) |
— | — |
BMI: body mass index; CLTS: community‐led total sanitation; HAZ: height‐for‐age Z score; MUAC: mid‐upper‐arm‐circumference; RCT: randomized controlled trial; STH: soil‐transmitted helminth; TSSM: Total Sanitation and Sanitation Marketing; WAZ: weight‐for‐age Z score.
aBriceño 2015 TAN had two intervention arms: TSSM and TSSM plus hand washing with soap. In all analyses we use the results for the TSSM arm only.
Randomized controlled trials
Briceño 2015 TAN; Cameron 2013 INA; Dickinson 2015 IND; Patil 2014 IND; and Pickering 2015 MLI focused on ending open defecation, including by children in their intervention using CLTS messaging. CLTS aimed to change the behaviour in a community through stimulating a collective sense of disgust and shame that triggered the whole community to stop practicing open defecation; once communities succeeded in ending open defecation, they were rewarded ODF certification (Kar 2008). Briceño 2015 TAN; Cameron 2013 INA; Dickinson 2015 IND; and Patil 2014 IND also had other components to increase demand for sanitation as part of the TSSM project (Briceño 2015 TAN; Cameron 2013 INA; Patil 2014 IND), and in India the TSC also included subsidies for latrine construction (Dickinson 2015 IND; Patil 2014 IND). In the criteria for ODF certification in Mali, among other indicators was that "all family members must use the latrine or a child potty" (Pickering 2015 MLI).
Controlled cross‐sectional studies
In the CLTS intervention in the Philipines (Belizario 2015 PHI), community leaders and volunteers delivered the following key messages to households: 1. the shame of having open defecation in the village and the importance of attaining ODF status in the village; 2. the importance for each household to possess its own sanitary toilet; and 3. the need for households to ensure solid waste management and disposal, as well as maintain sanitary conditions in animal facilities in the backyard (e.g. pig pens). Messages about child faeces disposal and use of toilets by children were also included.
Sanitation hardware and behaviour change interventions
A summary of the interventions, settings, and outcome measures of the sanitation hardware and behaviour change interventions is presented in Table 10.
4. Summary of the study designs, settings, and outcome measures of the sanitation hardware and behaviour change interventions.
| Study (setting) | Summary of intervention | Outcomes used in review | |||
| Diarrhoea | Anthropometry | Behaviour change | Other | ||
| RCTs | |||||
| Caruso 2019 IND (rural) | "Sundara Grama", a multilevel behaviour change intervention that included the following activities.
|
— | — | Safe child faeces disposal | — |
| Christensen 2015a KEN; Christensen 2015b KEN (rural) | Pilot of the WASH‐B study in Kenya. Study included 2 pilot RCTs, 1 testing individual arms (water, sanitation, and hygiene) (Christensen 2015a KEN) and the other the combination of the 3 (WASH), the combination plus nutrition (WASH +) and nutrition alone (Christensen 2015b KEN). In the analyses, we include results for the sanitation only arm and WASH arm. The sanitation arm included hardware: compounds received a faeces disposal sani‐scooper tool similar to a dustpan with a metal paddle (1 for each household in the compound), a plastic child potty (1 for each household in the compound with a child aged < 3 years), and improvements to their existing latrine (consisting of a plastic latrine slab with a built‐in drop‐hole cover if the latrine floor was not concrete and simple mud walls, roof, and door if not present) or construction of a new latrine if they had none. In addition there were monthly household visits for behaviour change communication. The sanitation intervention's primary behaviour change messages emphasized preventing faecal contamination of the environment and safe removal of faeces (human and animal) from the environment facilitated by the potty, sani‐scooper, and latrine. It also focused on contamination pathways, behaviours that could lead to exposure, and motivators and barriers of the targeted behaviours. |
— | — | Safe child faeces disposal Faeces observed in the compound |
— |
| Luby 2018 BGD (rural) | WASH Benefits study consisting of 6 intervention arms: water quality, sanitation, hygiene, combined WASH, nutrition, and combine WASH + nutrition. In the review, we used results for the sanitation arm. Sanitation arm consisted of: provision of free child potties, sani‐scoop hoes to remove faeces from household environments, and latrine upgrades or construction if did not have 1. Local promoters encouraged mothers to teach their children to use the potties, to safely dispose of faeces in latrines, and to regularly remove animal and human faeces from the compound. |
Prevalence (7‐day recall) | LAZ WAZ Other anthropometry measures (narratively) |
From Parez 2018:
|
Mortality (narratively) |
| Null 2018 KEN (rural) | WASH Benefits study consisting of 6 intervention arms: water quality, sanitation, hygiene, combined WASH, nutrition, and combine WASH + nutrition. In the review use results for the sanitation arm. Sanitation arm consisted of: provision of free child potties, sani‐scoops to remove faeces from household environments, and latrine upgrades or construction if did not have 1. Local promoters visited study compounds to deliver behaviour change messages on the use of latrines for defecation and the removal of human and animal faeces from the compound. |
Prevalence (7‐day recall) | LAZ WAZ Other anthropometry measures (narratively) |
Safe disposal of child faeces | Mortality (narratively) |
RCT: randomized controlled trial; WASH‐B: Water, Sanitation, and Hygiene – Benefits.
In the WASH Benefits trials (Luby 2018 BGD; Null 2018 KEN), and the pilot study in Kenya (Christensen 2015a KEN; Christensen 2015b KEN), the sanitation arm included the provision of hardware (faeces disposal sani‐scooper, a plastic child potty, and improvements to their existing latrine or construction of a new latrine if they had none). In addition, there was behaviour change communication, which emphasized preventing faecal contamination of the environment and safe removal of faeces (human and animal) from the environment facilitated by the potty, sani‐scooper, and latrine.
Caruso 2019 IND evaluated a multilevel behaviour change intervention the "Sundara Grama", which aimed to increase latrine use and safe disposal of child faeces. The intervention included activities at the community level (a traditional folk dance, a transect walk, community meeting, recognition of positive deviants, village map painting), group level (mother's group meeting), and household level (household visits and latrine repairs). The mother's group meeting was for mothers and caregivers of children aged under five years, to provide action knowledge and hardware (potties and scoops) to enable the safe disposal of child faeces. The importance of child faeces disposal was also mentioned during the folk dance performance and other activities.
WASH hardware and education/behaviour change interventions
A summary of the interventions, settings, and outcome measures of the WASH hardware and education/behaviour change interventions is presented in Table 11.
5. Summary of the study designs, settings, and outcome measures of the WASH hardware and education/behaviour change interventions.
| Study (setting) | Summary of intervention | Outcomes used in review | ||||
| Diarrhoea | STH | Anthropometry | Behaviour change | Other | ||
| RCTs | ||||||
| Humphrey 2019 ZIM (rural) | 3 intervention arms. Only included the WASH vs non‐WASH results in review.
|
Mean prevalence of diarrhoea (7‐day recall) | — | Mean LAZ score Mean WAZ scores Weight‐for‐length Z scores MUAC‐for‐age Z scores Head circumference‐for‐age Z scores Stunting (LAZ score < –2) Severely stunted (LAZ score < –3) Underweight (WAZ scores < –2) Wasted (weight‐for‐height Z scores < –2) (narratively) |
Dispose of water from cleaning infant nappies with faeces in a latrine (narratively) | Cumulative mortality (narratively) |
| CBA studies | ||||||
| Alam 1989 BGD (rural) | Hand pumps were provided with a ratio of 4–6 households (3 times more than control) + health education (main objectives: promotion of consistent and exclusive use of hand pump water, improvement of water handling and storage practices, disposal of child's faeces soon after defecation, washing hands before handling food, and rubbing hands in ash or using soap after defecation). | Diarrhoea incidence (7‐day recall) | — | — | — | — |
| Aziz 1990 BGD (rural) | 148 new hand pumps (1 pump to 30 people on average) + free maintenance, 92% of households received a double‐pit water‐sealed latrine, hygiene education emphasising exclusive use of the pump water for all personal and domestic use, and the need for all members of the household, including young children to use the latrines. | Diarrhoea incidence (7‐day recall) | — | Anthropometry (weight for age, height for age, weight for height) (narratively) | — | Dysentery, persistent diarrhoea (narratively) |
| Park 2016 INA (rural) | Budi's Amphibious Latrine (BALatrines) (simple squat latrines with a septic tank or pit) were constructed and all residents were given health education regarding hygiene, sanitation, and prevention of STH infections. The health education included many messages about preventing soil‐transmitted helminthiases. For mothers of small children, the messages included not disposing of used nappies in the garden, bushes, or waterways. Children were also told that they should stay away from any faeces they might find around their home, and that they should report any symptoms (diarrhoea, fever, etc.) to a parent or teacher. | — | STH infection (presence of helminth eggs in stool (narratively) | — | — | — |
| Controlled cohort studies | ||||||
| Hoq 2016 BGD (periurban) | Community‐based health project + WASH‐focused activities. WASH‐focused activities included:
Child faeces disposal messages (included in both intervention and control but this was done in more detail in the children's clubs and mother's group meetings):
|
— | — | Weight for age (underweight defined as WAZ < –2) MUAC (acute malnutrition defined as MUAC < 125 mm) (narratively) |
— | — |
CBA: controlled before‐and‐after; IYCF: infant and young child feeding; LAZ: length‐for‐age Z score; MUAC: mid‐upper‐arm‐circumference; RCT: randomized controlled trial; STH: soil‐transmitted helminth; WASH: water, sanitation, and hygiene; WAZ: weight‐for‐age Z score.
Randomized controlled trials
In the WASH arm of the SHINE study, households were provided with VIP latrines, two handwashing stations, a plastic mat and play yard, and monthly deliveries of soap and chlorine (Humphrey 2019 ZIM). Behaviour change modules were delivered by village health workers, in the WASH group the messages included information about child faeces disposal, hand washing with soap at key times, protection of infants from geophagia and ingestion of animal faeces, chlorination of drinking water (especially for infants), and hygienic preparation of complementary food.
Controlled before‐and‐after studies
Aziz 1990 BGD included the provision of water and sanitation infrastructure as well as hygiene education, which included the need for children to use the toilets constructed.
Alam 1989 BGD provided hand pumps to communities as well as health education on use of hand pump water, improvement of water handling and storage practices, disposal of child's faeces soon after defecation (with no details on how or where), and washing hands before handling food.
Park 2016 INA provided simple squat latrines with a septic tank or pit to households and gave health education regarding hygiene, sanitation, and prevention of STH infections. The health education component consisted of many messages, including hand washing, boiling water, food hygiene, and sanitation. The messages included not disposing of used nappies in the garden, bushes or waterways (if the nappies were not disposable) and for children to stay away from any faeces around their home.
Controlled cohort studies
Hoq 2016 BGD included several messages regarding child faeces disposal in both the intervention and control wards in the mass awareness behaviour change campaign. However, in the intervention wards this was done in additional mediums including mother's group meetings and child clubs. The child faeces disposal messages were: 1. throw the child faeces in the latrine immediately after defecation; 2. use handy tool (shovel, etc.) to collect and dispose the faeces. Keep the tool clean; 3. encourage the children and start practicing defecation in the latrine instead of defecating on yard; 4. "child faeces are more harmful than the adult" as the mothers believed that children faeces were less harmful; and 5. wash hands after dispose of child faeces.
Interventions in daycare centres
Of the two studies in daycare centres in the USA, Butz 1990 USA included advice on handwashing and nappy‐changing practices and instructions to dispose of gloves, disposable pads, and nappies in plastic bags and centres were given supplies (gloves, nappy changing pads, hand rinse solution). Kotch 2007 USA provided nappy changing, handwashing, and food‐preparation equipment with impermeable, seamless surfacing and automatic faucets and foot‐activated, roll‐out waste bins for nappy disposal. A summary of the interventions, settings, and outcome measures of the interventions in daycare centres is presented in Table 12.
6. Summary of the study designs, settings, and outcome measures of the daycare centre‐based hygiene hardware and education interventions.
| Study (setting) | Summary of intervention | Outcomes used in review | ||||
| Diarrhoea | STH | Anthropometry | Behaviour change | Other | ||
| Butz 1990 USA (Urban (daycare centres)) | Instruction to daycare providers on modes of transmission of pathogens, instructions of handwashing, and use of vinyl gloves and disposable nappy changing pads at each nappy change. Providers were instructed to dispose of gloves, disposable pads, and nappies in plastic bags and given supplies (gloves, nappy changing pads, hand rinse solution). | Prevalence (narratively). Symptoms recorded daily | — | — | — | — |
| Kotch 2007 USA (Rural and urban (daycare centres)) | Staff in childcare centres were trained using the 'Keep It Clean' training module to improve and standardize the hand‐washing, sanitation, nappy changing, and food‐preparation procedures. Nappy changing, hand‐washing, and food‐preparation equipment with impermeable, seamless surfacing for food preparation, nappy changing, and hand washing were provided. In addition, automatic faucets and foot‐activated, roll‐out waste bins for nappy disposal were provided. | Diarrhoea incidence (2‐week recall) (narratively) | — | — | — | — |
STH: soil‐transmitted helminth.
Case‐control studies
Among the case‐control studies, child faeces disposal variables were categorized into safe and unsafe disposal differently (Characteristics of included studies table). The most common categorization of child faeces disposal was disposal into a latrine versus elsewhere (10 comparisons of which one included both disposal in a latrine after defecation elsewhere and defecation in a latrine). In some studies, the authors classifies the defecation in a latrine as well as disposal in a latrine as safe in the same variable, whereas other studies presented separate variables for disposal in a latrine and defecation in a latrine. Thus, we pooled studies that had variables of safe disposal into a latrine (which in some cases included defecation into a latrine) and separately pooled studies with variables of defecation into a latrine.
Some definitions of safe disposal were more specific, including only certain disposal places as safe, such as Baker 2016 BGD only considered certain types of latrines in which the faeces were disposed of as safe: hanging latrines and bucket latrines were considered open disposal. Baltazar 1989 PHI defined sanitary disposal as child defecated in a nappy and faeces were thrown away in washing, child used chamber pot/piece of paper and faecal matter was thrown in the toilet or child used the toilet, whereas unsanitary was when the faeces were deposited elsewhere than latrine or the child defecated outside (regardless of where faecal matter was finally thrown away). Mertens 1992 SRI defined unsanitary stool disposal as stools passed, or disposed of, in or out of the yard without being disposed within one day in a latrine or in a covered rubbish pit, while proper disposal was stools passed in a potty and later disposed of in a latrine or in a covered pit.
Asfaha 2018 ETH did not specify what they considered to be "safe" disposal. Ghosh 1994 IND and Ghosh 1997 IND did not define what they considered indiscriminate disposal of stools. Strina 2012 BRA did not define what they considered to be inadequate/adequate disposal of excreta of children.
In the studies with variables including defecation in a latrine, Chompook 2006 THA categorized data into children always using latrines versus not/sometimes using latrines. Clemens 1987 BGD considered the latrine or some other specially designated place versus open defecation. Knight 1992 MAL grouped defecation in a nappy and latrine as safe, whereas Maung 1992a MYA and Traoré 1994b BUR categorized data into defecation in pots and latrines versus elsewhere. Mediratta 2010b ETH and Oketcho 2012 TAN categorized defecation into the latrine or elsewhere.
In Arvelo 2009 USA, the risk factor relevant to this review was whether daycare centres had lined, lidded bins for nappy disposal (the unit of analysis was the daycare centre). In Bassal 2016 ISR, the risk factor relevant to this review was children who were not toilet trained and used nappies versus children who were toilet trained and did not use nappies. In Chiang 2005 TWN, the risk factor relevant to the review was open defecation of children aged less than five years but the reference category was not provided. Daniels 1990 LES collected data on disposal of child faeces in latrines in cases and controls but did not provide data separately for both groups. In Menon 1990 USA, the risk factor of interest was whether households had dirty nappies in the yard. Nanan 2003 PAK studied whether cases and controls were from Water and Sanitation Extension Programme (WASEP) villages, which included in its intervention education on the safe disposal of faeces (adult, child, and household animals). Thus, these six studies could not be compared with the other case‐control studies and were excluded from the analyses.
Primary outcome measures
Diarrhoea
For the 50 studies that measured diarrhoea as an outcome, 18 used the WHO's definition (passage of three or more loose or liquid stools per day or more than usual for the individual) for the case definition of diarrhoea (Characteristics of included studies table). Other studies defined diarrhoea as: softer than usual, one to five stools per day; watery, one to five stools per day; softer than usual, five to 10 stools per day; watery, five to 10 stools per day; watery more than 10 stools per day; or dysentery (Ahmed 1993 BGD), three or more soft liquid stools within 12 hours or a single soft or liquid stool with blood, pus, or mucous (Abalkhail 1995 KSA), three or more loose/watery stools in a 24‐hour period or having a stool with blood or mucous (Briceño 2015 TAN; Cameron 2013 INA; Mertens 1992 SRI; Patil 2014 IND), at least three loose or watery stools within 24 hours or at least one stool with blood (Luby 2018 BGD; Null 2018 KEN), the passage of three or more liquid or semi‐liquid stools in a 24‐hour period or the passage of at least one liquid or semi‐liquid stool with blood or mucous (Hashi 2017 ETH), occurrence of loose, unformed bowel movements at twice the normal frequency (infants, one to two stools per day; and older children, one stool per day) (Butz 1990 USA), passage of at least three liquid, watery mucoid stools with or without blood during the past 24 hours. For infants aged up to three months, an increase in the frequency and a change in the consistency of stools which was of concern to mothers (Ghosh 1997 IND), mother's own definition using local term to describe diarrhoea (Haggerty 1994 DRC), any loose, watery stool that if contained would assume the shape of the container (Kotch 2007 USA), caretaker reported increase in the stool fluidity and frequency of passing stool for at least two days (Oketcho 2012 TAN) or as reported by the mother and examined by a doctor (Traoré 1994a BUR).
Baker 2016 BGD included criteria qualifying the episode to be moderate or severe. Cummings 2012 UGA used acute watery diarrhoea in an area with laboratory‐confirmed cholera cases.
Other definitions required laboratory testing to confirm shigella (Arvelo 2009 USA; Chiang 2005 TWN; Chompook 2006 THA), rotavirus (Menon 1990 USA; Strina 2012 BRA), or campylobacter (Bassal 2016 ISR). Maung 1992a MYA used persistent diarrhoea and protein energy malnutrition.
Eight studies did not provide a case definition for diarrhoea (Baltazar 1989 PHI; Berhe 2014 ETH; Dikassa 1993 DRC; Dickinson 2015 IND; Gebru 2014 ETH; Ghosh 1994 IND; Godana 2013 ETH; Heller 2003 BRA).
Soil‐transmitted infections
Belizario 2015 PHI and Patil 2014 IND both assessed the presence of STH in stool samples using the Kato‐Katz technique. Park 2016 INA used the Impankaew faecal flotation technique. Cameron 2013 INA did not specify STH diagnosis technique.
Excluded studies
The 44 studies that were discussed but subsequently excluded are described in the Characteristics of excluded studies table. The other studies that were excluded without requiring discussion have reasons summarized in Figure 2.
Ongoing studies
Four studies appeared to meet our inclusion criteria but are still ongoing are presented in the Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias of trials and non‐randomized studies apart from case‐control studies are summarized in Table 13, Table 14 and in the Characteristics of included studies table.
7. Summary of risk of bias of cluster‐RCTs.
| Study ID | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Recruitment bias | Baseline imbalance | Loss of clusters | Incorrect analysis |
| Altmann 2018 TCD | L | L | H | H | L | L | H | L | L | L |
| Barrios 2008 PHI | L | U | H | H | H | H | H | H | L | H |
| Briceño 2015 TAN | U | U | H | H | L | L | H | H | L | L |
| Butz 1990 USA | U | U | H | H | U | L | H | L | L | H |
| Cameron 2013 INA | L | L | H | H | L | L | H | L | L | L |
| Caruso 2019 IND | L | L | H | H | U | L | H | L | L | L |
| Christensen 2015a KEN; Christensen 2015b KEN | L | L | H | H | H | H | L | L | L | L |
| Dickinson 2015 IND | L | L | H | H | L | L | L | L | L | L |
| Haggerty 1994 DRC | U | U | H | H | L | H | L | L | L | L |
| Hashi 2017 ETH | L | L | H | H | L | L | H | L | L | L |
| Humphrey 2019 ZIM | L | L | H | H | L | U | H | L | L | L |
| Jinadu 2007 NGR | U | U | H | H | U | L | H | U | L | H |
| Kotch 2007 USA | U | U | H | U | L | L | H | H | L | L |
| Luby 2018 BGD | L | L | H | H | L | U | L | L | L | L |
| Nair 2017 IND | L | L | H | L | L | L | H | L | L | L |
| Null 2018 KEN | L | L | H | H | L | U | L | L | L | L |
| Patil 2014 IND | L | L | H | H | L | L | H | L | L | L |
| Pickering 2015 MLI | L | L | H | H | L | L | L | L | L | L |
| Sarrassat 2018 BUR | L | L | H | H | L | L | H | L | L | L |
| Sinharoy 2017 RWA | L | L | H | H | L | L | L | L | L | L |
| Stanton 1987 BGD | L | U | H | H | U | L | L | L | U | H |
| Yeager 2002 PER | U | U | H | H | U | L | H | L | U | H |
H: high risk of bias; L: low risk of bias; U: unclear risk of bias.
8. Summary of risk of bias of other prospective studies (CBAs, cohorts, cross‐sectional studies).
| Study ID | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Incomplete outcome data (attrition bias) | selective reporting (reporting bias) | Other bias | Similarity of baseline outcome measurements | Similarity of baseline characteristics | Adequate allocation of intervention concealment | Adequate protection against contamination | Confounders adequately adjusted for in analysis/ design |
| Controlled before‐and‐after studies | ||||||||||
| Ahmed 1993 BGD | H | H | U | L | — | H | H | H | L | H |
| Alam 1989 BGD | H | H | L | L | — | U | U | H | H | H |
| Aziz 1990 BGD | H | H | U | L | — | L | U | H | L | H |
| Park 2016 INA | H | H | L | L | — | L | U | L | L | H |
| Controlled cohort studies | ||||||||||
| Hoq 2016 BGD | H | H | U | L | — | L | H | H | U | H |
| Huda 2012 BGD | H | H | U | L | — | U | L | H | L | H |
| Luby 2014 BGD | H | H | U | L | — | U | L | H | L | H |
| Controlled cross‐sectional studies | ||||||||||
| Belizario 2015 PHI | H | H | L | L | — | U | U | L | L | H |
| Berhe 2014 ETH | H | H | L | L | — | U | U | L | H | L |
| Fisher 2011 BGD | H | H | L | L | H | U | U | H | U | H |
| Gebru 2014 ETH | H | H | L | L | — | U | U | U | H | L |
| Mathew 2004 ZIM | H | H | U | U | — | U | U | H | U | H |
| Oguro 2016 MYA | H | H | U | U | — | U | U | U | U | L |
| Waterkeyn 2005 ZIM | H | H | L | L | — | U | U | H | L | H |
CBA: controlled before‐and‐after; H: high risk of bias; L: low risk of bias; U: unclear risk of bias.
Allocation (selection bias)
Random sequence generation was at low risk of selection bias in 16 of the cluster RCTs and unclear risk in the other six. Concealment was at low risk in fourteen studies and unclear risk in eight. All CBAs, cohort, and cross‐sectional studies were at high risk.
Blinding (performance bias and detection bias)
All cluster RCTs were at high risk for blinding participants and personnel. Apart from one study at unclear risk (Kotch 2007 USA) and one study at low risk (Nair 2017 IND), all other cluster RCTs were at high risk for blinding of outcome assessment.
Incomplete outcome data (attrition bias)
Barrios 2008 PHI and Christensen 2015a KEN were at high risk for incomplete outcome data, five RCTs were at unclear risk (Butz 1990 USA; Caruso 2019 IND; Jinadu 2007 NGR; Stanton 1987 BGD; Yeager 2002 PER), and the remaining 15 at low risk.
Two CBAs were at unclear risk (Ahmed 1993 BGD; Aziz 1990 BGD), and two at low risk (Alam 1989 BGD; Park 2016 INA). The three cohort studies were at unclear risk. Of the cross‐sectional studies, two were at unclear risk (Mathew 2004 ZIM; Oguro 2016 MYA), and five at low risk.
Selective reporting (reporting bias)
Three RCTs were at high risk of selective reporting (Barrios 2008 PHI; Christensen 2015a KEN; Haggerty 1994 DRC), three were at unclear risk (Humphrey 2019 ZIM; Luby 2018 BGD; Null 2018 KEN), while the other 16 RCTs were at low risk.
All CBAs, cohorts, and cross‐sectional studies were at low risk apart from Mathew 2004 ZIM and Oguro 2016 MYA, which were at unclear risk.
Risk of bias specific to cluster‐randomized controlled trials
Fourteen cluster‐RCTs were at high risk and the remaining eight at low risk for recruitment bias. For baseline imbalance, three CRCTs were at high risk, Jinadu 2007 NGR at unclear risk, and the rest at low risk. For loss of clusters, two studies were at unclear risk (Stanton 1987 BGD; Yeager 2002 PER), and all other cluster‐RCTs were at low risk. For incorrect analysis, five cluster‐RCTs were at high risk, while the remaining 17 were at low risk.
Risk of bias specific to non‐randomized studies (except case‐control studies)
For similarity of baseline outcome measurements, Ahmed 1993 BGD was at high risk, Alam 1989 BGD at unclear risk, and Aziz 1990 BGD and Park 2016 INA at low risk. The cohort and cross‐sectional studies were at unclear risk apart from Hoq 2016 BGD, which was at low risk. For similarity of baseline characteristics, Ahmed 1993 BGD was at high risk while the three other CBAs were at unclear risk. In the cohort studies, Huda 2012 BGD and Luby 2014 BGD were at low risk and Hoq 2016 BGD at high risk. The seven cross‐sectional studies were at unclear risk. For adequate allocation of intervention concealment, all CBAs apart from Park 2016 INA and the three cohorts were at high risk. Three of the cross‐sectional studies were at high risk, Gebru 2014 ETH and Oguro 2016 MYA were at unclear, and Berhe 2014 ETH and Belizario 2015 PHI at low risk. For adequate protection against contamination, Alam 1989 BGD was at high risk while the three other CBAs were at low risk. Hoq 2016 BGD was at unclear risk and the two other cohorts studies were at low risk. Berhe 2014 ETH and Gebru 2014 ETH were at high risk, while Fisher 2011 BGD; Mathew 2004 ZIM; and Oguro 2016 MYA were at unclear risk and Belizario 2015 PHI and Waterkeyn 2005 ZIM at low risk. For adequate adjustment for confounders, the four CBAs, the cohort studies, and four cross‐sectional studies were at high risk. Berhe 2014 ETH; Gebru 2014 ETH; and Oguro 2016 MYA were at low risk.
Risk of bias of the case‐control studies
The case‐control studies risk of bias are presented in Table 15. In addition a funnel plot investigating the potential publication bias of case‐control studies was conducted (Figure 3). The funnel plot appeared to be fairly symmetrical, indicating a low risk of publication bias. However, given the studies were observational, and the investigators may have collected data on many risk factors, they may not always present the results of the effect of child faeces disposal if it was not an important risk factor.
9. Risk of bias of case‐control studies.
| Study ID | Selection | Comparability of cases and controls on the basis of the design or analysisa | Exposure | Total number of 'stars' (*) | |||||
| Case definition adequate | Representativeness of cases | Selection of Controls | Definition of controls | Ascertainment of exposure | Same method of ascertainment for cases and controls? | Non‐response rate | |||
| Abalkhail 1995 KSA | *Yes physician at health centre. | *Cases were incident cases during the study period. | *Controls selected from residential neighbours of cases. | *Controls had no history of hospitalization for diarrhoeal diseases. | *Analysis adjusted for maternal education, child and maternal age and family size. | No mention of blinding of interviewers to case/control status. | *Yes structured questionnaire and observations. | Cases = 7 no response for child faeces disposal, controls = 17 no response for child faeces disposal. | 6 |
| Arvelo 2009 USA | *Yes laboratory confirmed then calculated attack rate. | 11% of licensed daycare centres (LDCs) did not participate in investigation – no reason described. | LDCs controls selected from LDCs. | Lower attack rate < 2%. | No control in analysis or design. | No detail of blinding. | *Yes interviews and inspections. | *For exposure of interest, no non‐response. | 3 |
| Asfaha 2018 ETH | Self‐reports | *Random sample of 200 cases from 250. | *Community controls, randomly selected from potential controls. | *Without diarrhoea in the preceding of 2 weeks. | No control for confounders in analysis of risk factor of interest (child faeces disposal). | *"Both data collectors and supervisors did not participate in the survey; were unaware of diarrhoeal disease status of the study groups and were provided only the identification numbers of households and name and age of the child." | *Yes | *Same for both groups. | 6 |
| Baker 2016 BGD; Baker 2016 GMB; Baker 2016 IND; Baker 2016 KEN; Baker 2016 MLI; Baker 2016 MOZ; Baker 2016 PAK | *Yes, GEMS clinician. | "Each site restricted enrolment to about the first nine eligible cases per age stratum per fortnight to maintain a manageable work flow throughout the study." | *Community controls | *"No diarrhoea in the previous 7 days." | *Matched for age and adjusted for wealth. | "Case enrolment interviews took place at the SHC whereas control caretakers were interviewed at home," so assume knew status | No, initial interview was in health centre for cases and in home for controls, although at 60 days, also did a household visit to both | Unclear what the non‐response rate for child faeces disposal was | 4 |
| Baltazar 1989 PHI | *Cases were brought to the clinic. | *"All cases seen at the clinics on a "morbidity day" during the recruitment period that satisfied this definition were included in the study. | "Children aged <2 years who were brought to the clinic because of an acute respiratory infection." | *Had not had diarrhoea during the previous 24 hours. | *Adjusted for toilet facilities) and *water supply, sex, education of head of household and mother, feeding practices, level of health service utilization, number of children under 5 in household. | Did not specify if they were blinded to status of case/control | *Yes, structured questionnaire. | *No missing values for child faeces disposal. | 7 |
| Bassal 2016 ISR | *Stool samples | *Randomly selected from all eligible cases. | *Community controls, identified from Israeli population register. | *No diarrhoea in past 2 weeks. | *Controls for age and number of children in household. | Structured interview, no mention of blinding. | *Yes, structured interview. | *Same for both groups (response rate was 86.3% for cases and 85% for controls). | 7 |
| Chiang 2005 TWN | *Cases were confirmed by laboratory test. | No details on how the cases were selected. | Hospital/clinic control | *Children who went to the clinics for vaccination and showing no symptoms of diarrhoea or fever. | *Matched for age. | Did not specify if they were blinded to status of case/control. | *Yes, semi‐structured questionnaire. | No details on missing values for child defecation variable | 4 |
| Chompook 2006 THA1 | *Yes laboratory diagnosis. | "All shigellosis cases ascertained from the population‐based surveillance study were eligible to be included in a matched case‐control study. However, during the peak of the shigellosis season in June 2001, only 14 of the 50 shigellosis cases were recruited into the study." | *Community control. "For each case enrolled, two matched controls were randomly selected from the population list of the health centre where the case resided." | *"Individuals free from diarrhoea or dysentery during the four weeks prior to recruitment were eligible to participate in the study as controls." | *Study controls for age in design. | "Un‐blinded status of the investigator visiting the households and conducting interviews to the case/control status of the participant." | *Yes, questionnaire and observations. | *No missing values for child defecation variable. | 6 |
| Clemens 1987 BGD | Self‐reports | Potential for selection bias – not all cases and based on reported diarrhoea incidence. | *Community controls | *No history of diarrhoea in the 3 months. | Study did not control in design or analysis for any of the confounders. | *Structured observations blinded to history of diarrhoea. | *Yes questionnaire and observations. | Defecation of ambulatory children was only observed in 15 case and 15 control families. | 4 |
| Cummings 2012 UGA | *Medical records | Not stated if consecutive/representative | *Community; however, no description on how they selected them. | *Had not experienced diarrhoea from April to time of investigation. | *Sanitation included in analysis and *Controls for age, gender, and water treatment practices. |
Did not specify if health workers were blind to case/control status. | *Yes, questionnaire | *No non‐response rate reported. | 7 |
| Daniels 1990 LES | *Hospital nurse | *Consecutive cases recruited. | Hospital controls with ARI/trauma. | *No diarrhoea at recruitment | *Sanitation is main exposure, *Age, education of mother |
Interview not blinded as nurse did interview at hospital | *Yes, questionnaire and for subsample second interview at home with observations of facilities. | No information on non‐response. | 6 |
| Dikassa 1993 DRC | *Hospital | Incident case identified at hospital – no details on whether it was consecutive. | *Community; however, not described how they selected them. | *No history of hospitalization for diarrhoea | No statistical difference in sanitation but it was not matched for in design. *Matches for education in analysis and age of child in design. |
Not specified that interviewers were blinded to case/control status. | *Yes, structured interview and observations. | Numbers not described – no information about non‐respondents | 5 |
| Genthe 1997 SAF | *Yes hospital staff. | No detail on how "a sample was drawn from pre‐school children who were brought to the day hospitals with diarrhoea." | *Community controls | *"Children who suffered from diarrhoea during the preceding 14 days at the time of the visit were excluded as controls." | *Study controlled for age in design. | "It was not possible to blind the interviewer to the disease status of the child under study." | *Yes, interviews and spot check observations. | Non‐respondents not described. | 5 |
| Ghosh 1994 IND | Self‐reports to surveillance worker. | Not stated how chose the case families out of the 980 study families. | *Neighbouring families | *No history of diarrhoea in the study period. | *Adjusted for age in matching when selected controls. | Not specified that interviewers were blinded to case/control. | *Yes, observations | *No missing data for indiscriminate disposal. | 5 |
| Ghosh 1997 IND | Self‐reports to surveillance worker. | Not stated how chose the 90 case families out of the 1027 study families. | *Neighbouring families | *No history of diarrhoea in the study period. | *Adjusted for age in matching when selected controls. | Not specified that interviewers were blinded to case/control. | *Yes, observations | *No missing data for indiscriminate disposal. | 5 |
| Godana 2013 ETH | Self‐report | *Appropriate sample selected at random. | *Community controls | *No diarrhoea in previous 2 weeks. | *Controls for latrine ownership, *Controls for source of water and whether treat water. |
No mention of blinding. | *Yes, questionnaire | different rates of non‐response for infant faeces disposal (33.7% missing in cases vs 20.4% missing in controls). | 6 |
| Heller 2003 BRA | *Physician at health centre | All cases diagnosed during study period were included (although 29% couldn't be found). | *Community controls | No mention of history of outcome. | *Adjust in analysis for: child's age, ownership of fridge, water reservoir. | *Double blinded interviews were planned but in some situations the participant status was obvious for the respondent. | *Yes, home interviews | Not known how many missing answers for child faeces disposal. | 5 |
| Knight 1992 MAL | *Doctor/health assistant | *Register each child with diarrhoea or other illness | Hospital controls with ARI mainly | *No diarrhoea | *Controlled for SES, educational level of main caregiver, health centre of recruitment, interviewer, birth order of child and number of people living in house. | *Interviewing team were unaware of the case/control status of the child | *Yes, questionnaire, direct observations and water quality testing. | No information on non‐respondents for child faeces disposal. | 6 |
| Maung 1992a MYA | *Yes, seen at hospital/health centre. | Cases were selected from among admitted children but did not say how they were selected | *Community control | *No diarrhoea in past 2 months and no PEM. | *Matched for age and sex in selection. | Interview not blinded to case/ control status. | *Yes, house interviews and observations. | Non‐responders are different for child defecation variable (13 missing in cases and 7 missing for controls). | 5 |
| Mediratta 2010a ETH | *Assessed at outpatient department in hospital. | *"Cases with acute diarrhoea were consecutively enrolled from the OPD and inpatient paediatric ward." | Controls selected from outpatient and inpatient ward. | *"Did not present with acute diarrhoea for ≥ 14 days before the date of interview." | *Study controls for age in the design of the study. | Structure interview not blind "the clinical presentation of illness, food and fluid intake, and treatment given by physicians were recorded for all the cases." | *Yes, structured questionnaire | *No missing respondents for child faeces disposal variable. | 6 |
| Menon 1990 USA | *Nurses and ELISA confirmation of rectal swabs. | *"The nursing staff at the outpatient department and emergency room were instructed to obtain a rectal swab from every child less than two years of age who presented with diarrhoea." | *Hospital and community controls but only a few community controls (n = 24) so using hospital controls in primary analyses. | *Controls who had diarrhoea during the 2‐week period were excluded. | *Study matched for age and sex. | Interview but no mention of blinding. | *Yes, interviews and observations | *No non‐responses for nappies. | 7 |
| Mertens 1992 SRI | *Medical professionals | *All children aged < 5 years presenting to hospitals. | *Hospital and community controls but used hospital controls for main analysis. | *Controls had a control disease: acute conditions including respiratory tract infections, malaria, fever of unknown origin, and otitis. | *Controls for age, hand washing, water source, and distance. | *Structured interview blinded to status. | *Yes, questionnaires and observations | Rate of non‐response for child excreta disposal behaviour was different and not described (94 responses (6.6%) missing for cases and 40 (1.8%) for controls). | 7 |
| Nanan 2003 PAK | *Diagnosed at health centre | *All eligible cases recruited within time of recruitment. | Health centre controls | *No diarrhoea | *Study controls for age | *Structured interview blind to exposure "Interviewers at the health centre were blinded to the exposure status of cases and controls, and staff from WASEP were blinded as to whether a village included in the study was associated with a case or a control." | *Yes structured interview | *No missing data for WASEP variable | 7 |
| Oketcho 2012 TAN | *Admission to paediatric infectious disease ward and caretaker reported increase in stool fluidity. | *Consecutive; "all children meeting the case criteria and those meeting control criteria admitted at the same time of the same age group and residing in Morogoro region were included in the study." | Hospital controls | *No history of diarrhoea in the previous 2 weeks. | No control in design or analysis. | Structure interview, no mention of blinding and improbable as interview took place at hospital. | *Yes, structured interview | No description of non‐responders for child defecation. | 4 |
| Strina 2012 BRA | *Stool laboratory examination | *Seems that all confirmed rotavirus diarrhoea were cases. | Hospital controls | *No history of diarrhoea in the preceding 3 weeks. | *Study adjusts for age and gender in design. | Structured interview but infer not blind; "information about the house and the peridomestic environment was collected by direct observation, together with information, for cases, about the episode itself." | *Yes (interview with caregiver at hospital and home visit for another interview + observations). | Different rates of no children aged < 2 years in the household (33% missing in controls vs 21% missing in cases). | 5 |
| Traoré 1994a BUR | *Yes caregiver and doctor | All cases presenting to hospital with diarrhoea or dysentery or both but 28% could not be found for interview. | *Community and hospital controls. Main analysis using community control. |
*Not admitted to hospital and for those at hospital no diarrhoea/ dysentery. | *Controls for age, water source, SES (radio ownership), and household size. | *Interviewers were not blind to whether child had been to hospital but were blind to whether had diarrhoea/not. | *Yes, questionnaire and spot checks | *In cases only 2 answers missing for disposal (0.3%) and 3 missing for defecation (0.4%) and 0 missing for community controls. | 7 |
| Wijewardene 1992 SRI | *Yes (stated in the limitations that all children were clinically examined and cross‐checked for recent visits to doctor, and child welfare cards available were examined). | *"The first hundred consecutive families with children aged < 5 years with an acute episode of diarrhoea." | *Community cases and controls | *No diarrhoea episode in last 6 months. | *Controls for use of shared/public latrines vs private and *Controlled for age in matching and other relevant confounders in regression. |
No mention of blinding | *Yes, questionnaire and observations of the facilities | *From table 1, appeared there were no missing values for child faeces disposal. | 8 |
ARI: acute respiratory infection; ELISA: enzyme‐linked immunosorbent assay; GEMS: Global Enteric Multicenter Study; LDC: licensed daycare centre; n: number; OPD: outpatient department; PEM: protein‐energy malnutrition; SES: socioeconomic status; SHC: sentinel health centre; WASEP: Water and Sanitation Extension Programme. aRisk factors listed in the column are those relevant to the review (prespecified in the protocol). For a full list of confounders adjusted for in the analysis, see Table 16 and Table 17. 1The thesis reported that child faeces disposal was insignificant but no data were presented. *These are the 'stars' given based on the Newcastle‐Ottawa quality assessment scale. Total number of stars are provided in the appropriate column.
3.

Funnel plot of case‐control studies that included the disposal of child faeces in latrine versus elsewhere as a risk factor for diarrhoea (including severe and cholera).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Education and hygiene promotion interventions
Randomized controlled trials
Barrios 2008 PHI; Jinadu 2007 NGR; and Yeager 2002 PER did not measure health impacts of the interventions. Nair 2017 IND did not measure the impact of the intervention on diarrhoea, the primary outcome was children's length‐for‐age Z score at 18 months of age. Sarrassat 2018 BUR did not measure the impact of the intervention on diarrhoea, rather on all‐cause postneonatal mortality in children aged under five years and all‐cause mortality in children aged under five years. None of the education and hygiene promotion interventions measured STH outcomes.
Non‐randomized studies
Mathew 2004 ZIM; Oguro 2016 MYA; and Waterkeyn 2005 ZIM did not measure health impacts of their programmes.
Diarrhoea
Randomized controlled trials
Five RCTs evaluated the impact of education and hygiene promotion interventions on diarrhoea. Two studies showed no effect on diarrhoea prevalence (RR 0.93, 95% CI 0.84 to 1.04; Analysis 1.1). Haggerty 1994 DRC found the intervention reduced the risk of children aged three to 35 months with one or more episodes of diarrhoea at any time during the surveillance period by 11% but it was not statistically demonstrated at P 0.05 level when adjusted for clustering using the inflating standard errors method. Sinharoy 2017 RWA found no effect of the 'lite' or 'classic' community health club intervention on diarrhoea in the previous seven days in children aged less than five years.
1.1. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 1 Diarrhoea prevalence – randomized controlled trials (RCTs).
Two studies reduced diarrhoea incidence by about 30% (RR 0.71 95% CI 0.59 to 0.86; Analysis 1.2). Hashi 2017 ETH reduced diarrhoea incidence by 35% (rate ratio 0.65, 95% CI 0.57 to 0.73) and Stanton 1987 BGD reduced diarrhoea incidence by 22% (rate ratio 0.78, 95% CI 0.71 to 0.86). There was high heterogeneity in Analysis 1.2, which could not be further investigated through subgroup analyses as there are only two studies. Possible reasons for heterogeneity included the location of the studies; Stanton 1987 BGD was conducted in urban Bangladesh and Hashi 2017 ETH in rural Ethiopia. Furthermore, the studies used different definitions of diarrhoea and different age groups (aged less than six years for Stanton 1987 BGD and aged less than five years for Hashi 2017 ETH).
1.2. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 2 Diarrhoea incidence – RCTs.
Altmann 2018 TCD measured longitudinal prevalence of diarrhoea as a secondary outcome and did not detect a difference between the intervention and control groups (absolute difference –1.7, 95% CI –4.5 to 1.0). The data were not in a format that could be pooled with the other studies.
Controlled before‐and‐after studies
Ahmed 1993 BGD only presented trends in daily diarrhoea prevalence in the education intervention and control groups in graphs. It seemed that, although for a portion of the intervention the prevalence of diarrhoea was lower than the control group, by the end of the study the prevalence was similar between groups.
Controlled‐cohort studies
The SHEWA‐B evaluation did not demonstrate a difference in diarrhoea prevalence in children aged less than five years (recall two days) in intervention and control groups during the first 24 months of the evaluation (10.5% with intervention versus 10.3% with control; P = 0.67) (Luby 2014 BGD). In the last 18 months of the evaluation, they found that children in the intervention group had less diarrhoea in rural areas (9% with intervention versus 12% with control; RR 0.80; P = 0.033); however, the evaluation found no impact in the urban slums exposed to the intervention compared to control slums (7% with intervention versus 6% with control; RR 1.12; P = 0.348). The pooled effect showed no difference in diarrhoea between intervention and control areas (RR 0.91, 95% CI 0.64 to 1.28; Analysis 1.3).
1.3. Analysis.
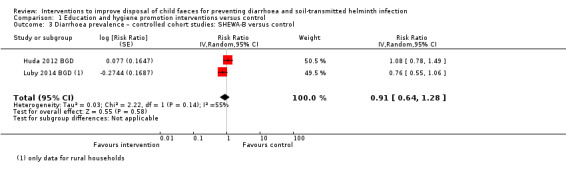
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 3 Diarrhoea prevalence – controlled cohort studies: SHEWA‐B versus control.
Controlled cross‐sectional studies
Fisher 2011 BGD found that among households in the BRAC villages, five children had diarrhoea during the month preceding data collection compared to six in the control village, which was reported as less, significant at the P 0.05 level, but provided no additional data (P = 0.027).
Berhe 2014 ETH and Gebru 2014 ETH studied the difference in two‐week diarrhoea prevalence in model and non‐model households of the HEP and found that being a model family decreased the odds of having diarrhoea by about three‐quarters (OR 0.26, 95% CI 0.16 to 0.42; Analysis 1.4).
1.4. Analysis.
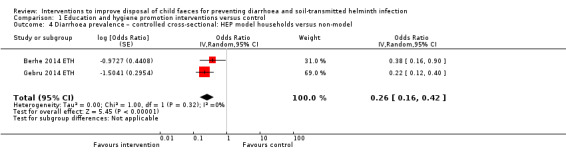
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 4 Diarrhoea prevalence – controlled cross‐sectional: HEP model households versus non‐model.
Severe diarrhoea
Controlled before‐and‐after studies
Ahmed 1993 BGD only presented trends in daily severe diarrhoea prevalence in the intervention and control sites and it seems that although for a portion of the intervention the prevalence of severe diarrhoea was lower, by the end of the study the prevalence was similar between groups.
Anthropometry
Randomized controlled trials
Stanton 1987 BGD and Sinharoy 2017 RWA reported no differences in the intervention and control groups on anthropometry. Nair 2017 IND found that fewer children were underweight at 18 months in the intervention than the control arm (OR 0.81, 95% CI 0.66 to 0.99). However, the intervention did not have any impact on other child anthropometry measures (length‐for‐age Z score, weight‐for‐height Z score, WAZ score, mid‐upper arm circumference, stunting, or wasting). Analysis 1.5 shows the pooled effects of Nair 2017 IND and Sinharoy 2017 RWA on height/length‐for‐age.
1.5. Analysis.
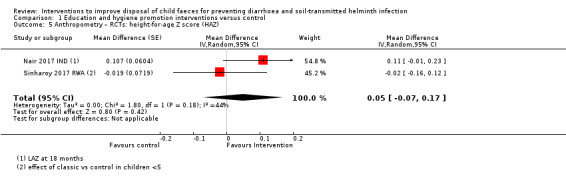
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 5 Anthropometry – RCTs: height‐for‐age Z score (HAZ).
Altmann 2018 TCD found no differences in the relapse rates to SAM at two months (absolute difference –0.4%, 95% CI –7.2 to 6.4) and six months (–1.0%, 95% CI –4.0 to 2.0).
Controlled before‐and‐after studies
Ahmed 1993 BGD reported that percentages of severely malnourished children (–3 SD WAZ) reduced over time in the intervention compared to the control site (at end of the study the percentage of children –3 SD WAZ score was approximately 21.5% in the intervention group and 35.5% in the control group; P < 0.0001).
Cohort studies
Luby 2014 BGD did not detect a difference in nutritional status in HAZ score, WAZ, or weight‐for‐height Z (WHZ) score in the intervention and control groups.
Mortality
Randomized controlled trials
Stanton 1987 BGD reported that rates of child and infant death were similar in the intervention and control groups. Nair 2017 IND found that fewer infants died in the intervention than the control (OR 0.63, 95% CI 0.39 to 1.00). Altmann 2018 TCD did not detect a difference in death rate between the intervention and control groups. Sarrassat 2018 BUR did not detect an intervention effect on all‐cause postneonatal mortality in children aged under five years or all‐cause mortality in children aged under five years.
Behaviour change
Randomized controlled trials
Six RCTs reported behavioural outcomes after the education and hygiene promotion intervention. For three of the studies, this was the main outcome (Barrios 2008 PHI; Jinadu 2007 NGR; Yeager 2002 PER), while for the other three studies it was as intermediate outcome (Sarrassat 2018 BUR; Sinharoy 2017 RWA; Stanton 1987 BGD). Different behaviours related to child faeces disposal were measured in the different interventions.
Analysis 1.6 shows the impact of the interventions on latrine use by children aged less than five years. Jinadu 2007 NGR reported an increase in latrine use by children aged 25 to 60 months, while Yeager 2002 PER observed no effect of the intervention on latrine use by children aged 15 to 47 months. Stanton 1987 BGD found no decrease in open defecation in the living area by ambulatory children (67% in the intervention group versus 63% in control group).
1.6. Analysis.
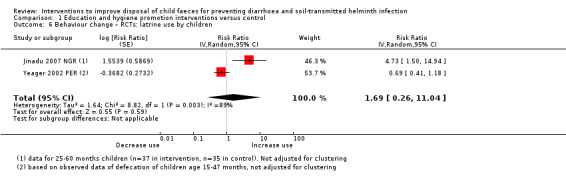
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 6 Behaviour change – RCTs: latrine use by children.
Analysis 1.7 presents data on potty use of children after the intervention, which was higher in households in the intervention arm in Jinadu 2007 NGR compared to the control arm, but did not show a difference between intervention and control households in Yeager 2002 PER.
1.7. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 7 Behaviour change – RCTs: potty use by children.
Analysis 1.8 shows the impacts of interventions on child faeces disposal behaviours. Safe child faeces disposal practices were not different between intervention and control arms in Sarrassat 2018 BUR or Yeager 2002 PER. Sinharoy 2017 RWA also found no impact of the 'classic' or 'lite' intervention on safe disposal (Analysis 1.9).
1.8. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 8 Behaviour change – RCTs: safe disposal of child faeces.
1.9. Analysis.
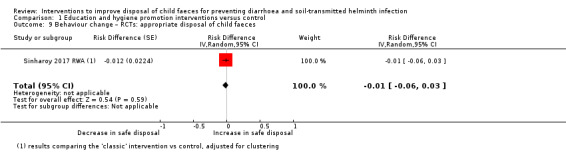
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 9 Behaviour change – RCTs: appropriate disposal of child faeces.
Analysis 1.10 shows the impact of interventions on faeces observed in the yard. Barrios 2008 PHI found no effect on faeces visible in the yard, Jinadu 2007 NGR reported an increase in no child faeces observed in the yard. There was no obvious difference between study arms, in either intervention ('classic' and 'lite') in faeces observed in compounds in Sinharoy 2017 RWA (Analysis 1.11). It is important to note that studies observing fewer faeces in the yard, might not necessarily be an indicator of increased safe disposal as the child faeces may not have been disposed of in a latrine but rather been thrown elsewhere.
1.10. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 10 Behaviour change – RCTs: faeces not observed in yard/ HH.
1.11. Analysis.
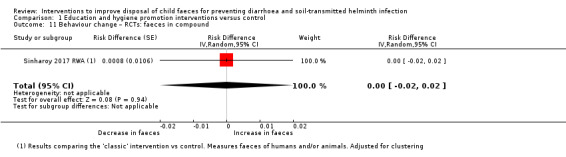
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 11 Behaviour change – RCTs: faeces in compound.
Controlled‐cohort studies
Huda 2012 BGD and Luby 2014 BGD found no impact of the SHEWA‐B intervention on child faeces disposal behaviour at mid‐study and end of the study compared to controls (Analysis 1.12).
1.12. Analysis.
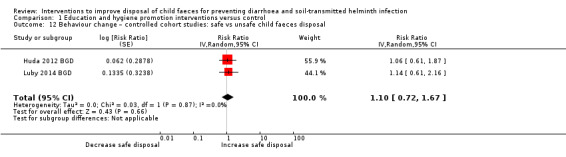
Comparison 1 Education and hygiene promotion interventions versus control, Outcome 12 Behaviour change – controlled cohort studies: safe vs unsafe child faeces disposal.
Controlled cross‐sectional studies
Berhe 2014 ETH; Fisher 2011 BGD; and Gebru 2014 ETH found an increase in safe disposal of child faeces in the intervention areas compared to the control areas (Analysis 1.13). Although Gebru 2014 ETH did not specify what they considered to be safe disposal, it was assumed that their definition included burying of faeces as well as disposal in the latrines as that is what is promoted in the HEP. Thus when calculating the risk of safe disposal for Berhe 2014 ETH, the same classification of safe disposal was used, although restricting the definition of safe disposal to just defecation in a latrine and disposal in a latrine; it also showed that intervention increased safe disposal. Oguro 2016 MYA found that the presence of a WVG did not have a significant effect on the proportion of appropriately disposed faeces compared to the control villages (OR 3.57, 95% CI 0.53 to 23.65).
1.13. Analysis.

Comparison 1 Education and hygiene promotion interventions versus control, Outcome 13 Behaviour change – controlled cross‐sectional studies: safe vs unsafe child faeces disposal.
Mathew 2004 ZIM found that in CHC areas, a lower percentage of children were not using a latrine compared to control areas (approximately 54% in CHC area versus 83% in control areas); however, no statistical analysis was presented and insufficient data were provided to perform an analysis. In Waterkeyn 2005 ZIM, there was no difference detected in observing child faeces in the yard in CHC households versus control households (in Tsolotsho: 4% in CHC households versus 0% in control households; P = 0.0807; in Makoni: 16% in CHC households versus 23% in control household; P = 0.0972).
Community‐led total sanitation interventions plus adaptations
Diarrhoea
Randomized controlled trials
The pooled effect of the CLTS interventions plus adaptations revealed no effect on diarrhoea prevalence (RR 0.92, 95% CI 0.79 to 1.07; Analysis 2.1). Pickering 2015 MLI did not find a difference in child diarrhoea prevalence between intervention and control groups with either a two‐day (22.5% with intervention versus 24.1% with control; P = 0.486) or two‐week recall period (31.2% with intervention versus 32.0% with control; P = 0.787). Patil 2014 IND did not find a difference in diarrhoea prevalence (seven‐day recall) between the intervention and control (7.4% with intervention versus 7.7% with control; P = 0.687). Briceño 2015 TAN found no decrease in diarrhoea prevalence between the sanitation arm and the control arm, but found a decrease in diarrhoea in the sanitation and handwashing combined arm (12.5% with sanitation and handwashing versus 16.8% with control for 14‐day recall). Diarrhoea symptoms in the past seven days did not show a difference between either treatment (TSSM and HWWS combined or TSSM alone) and control groups. Cameron 2013 INA found that the intervention group had lower diarrhoea prevalence compared to control children (2.4% with intervention versus 3.8% with control: P = 0.07 for seven‐day recall and 1.6% with intervention versus 3.1% with control; P = 0.025 for two‐day recall). Dickinson 2015 IND did not present data in a way that could be pooled with the other studies, but found that the TSC was associated with decreased diarrhoea rates (point estimate –0.21); however, these effects were not statistically significant at the P 0.05 level.
2.1. Analysis.
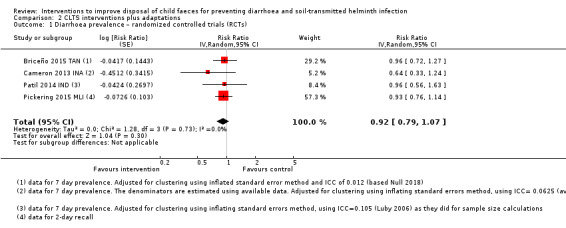
Comparison 2 CLTS interventions plus adaptations, Outcome 1 Diarrhoea prevalence – randomized controlled trials (RCTs).
Soil‐transmitted helminth
Randomized controlled trials
Two of the CLTS RCTs reported on the impact of the interventions on STHs and found no effect on any STH infection (RR 1.03, 95% CI 0.64 to 1.65; Analysis 2.2) or on A lumbricoides (RR 1.01, 95% CI 0.60 to 1.71; Analysis 2.3). Patil 2014 IND did not find a difference in helminth prevalence between intervention and control groups (any helminth: 5.9% with intervention versus 5.6% with control; A lumbricoides: 4.3% with intervention versus 4.4% with control). Cameron 2013 INA did not detect a difference in the probability of having any helminth between the children in the treatment and control groups (4.0% with intervention versus 3.9% with control; P = 0.889), A lumbricoides (3.4% with intervention versus 3.3% with control; P = 0.881), T Trichuris (0% with intervention versus 0.1% with control; P = 0.319), or hookworm (0.6% with intervention versus 0.5% with control; P = 0.733).
2.2. Analysis.
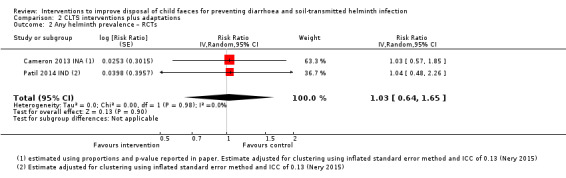
Comparison 2 CLTS interventions plus adaptations, Outcome 2 Any helminth prevalence – RCTs.
2.3. Analysis.

Comparison 2 CLTS interventions plus adaptations, Outcome 3 Ascaris lumbricoides prevalence – RCTs.
Controlled cross‐sectional studies
Belizario 2015 PHI found that in villages with CLTS, the prevalence of STH was 42% (67.4% in Buenavista and 4.9% in Caubang), whereas in villages without CLTS, the prevalence of STH was 16.8% (16.7% in Bitoon and16.8% in Saub). Prevalence in CLTS versus non‐CLTS villages of Ascaris was 22% versus 11%, for Trichiuris was 34% versus 8.9%, and for hookworm was 4% versus 0%.
Dysentery
Randomized controlled trials
Pickering 2015 MLI did not detect a difference in prevalence of blood in stools between intervention and control groups using a two‐day recall period (1.2% with intervention versus 1.4% with control; P = 0.481), but the two‐week prevalence was lower in the intervention than control villages (prevalence ratio (PR): 0.68, 95% CI 0.48 to 0.97; P = 0.031). Cameron 2013 INA found lower prevalence of mucous or blood in stools (seven‐day prevalence) in intervention versus control (0.8% with intervention versus 2% with control; P = 0.034). Overall the pooled effect showed no effect of the interventions (RR 0.69, 95% CI 0.35 to 1.34; Analysis 2.4).
2.4. Analysis.

Comparison 2 CLTS interventions plus adaptations, Outcome 4 Dysentery – RCTs.
Intensity of soil‐transmitted helminth infection (number of eggs per gram of stool)
Randomized controlled trials
Cameron 2013 INA did not detect a difference in infection intensity between intervention and control groups.
Controlled cross‐sectional studies
Belizario 2015 PHI found that the prevalence of moderate–heavy intensity infections was 14.5% in CLTS villages compared to 2.8% in non‐CLTS villages.
Presence of pathogenic microbes in stool assays
Randomized controlled trials
Patil 2014 IND did not detect a difference in prevalence of any protozoan present in intervention and control (21.7% with intervention versus 25.7% with control) or entamoeba histolytica (3.3% with intervention versus 2.5% with control). They found lower prevalence of Giardia Lamblia (18.4% with intervention versus 23.2% with control; MD 4.8%; P = 0.047).
Anthropometry
Randomized controlled trials
Patil 2014 IND and Cameron 2013 INA reported finding no differences in the intervention and controls groups on anthropometry. Pickering 2015 MLI found that children aged less than five years in intervention villages were taller than those in control villages by a mean of 0.17 in HAZ score (95% CI 0.04 to 0.31) and did not find a difference in WAZ scores (mean 0.09 WAZ score, 95% CI –0.03 to 0.20), when restricting the analysis to younger children a larger effect was found on HAZ. Briceño 2015 TAN did not find a difference between the sanitation only arm and the control group (there was a decrease in weight for age by 0.075 SDs off a mean WAZ score of –1.03 (P < 0.05) and weight‐for‐height by 0.097 SDs from a mean WHZ score of 0.055 (P < 0.05) in the combined arm of the intervention (hand washing with soap and sanitation) compared to the control group). The pooled effect on HAZ (MD 0.06, 95% CI –0.07 to 0.19; 3 studies with usable data) and WAZ scores (MD 0.04, 95% CI –0.04 to 0.11) did not demonstrate an effect (Analysis 2.5; Analysis 2.6). Dickinson 2015 IND could not be pooled due to the analysis presented in the paper. The study reported that mid‐upper‐arm‐circumference (MUAC) Z scores were 0.20 to 0.30 SDs higher in treatment villages relative to controls after the sanitation campaign. HAZ had increased by about 0.37 to 0.52 SDs (P < 0.01) and WAZ increased by 0.26 to 0.31 SDs (P < 0.05) in treatment villages relative to controls after the sanitation campaign.
2.5. Analysis.

Comparison 2 CLTS interventions plus adaptations, Outcome 5 Anthropometry: height‐for‐age Z score (HAZ) – RCTs.
2.6. Analysis.
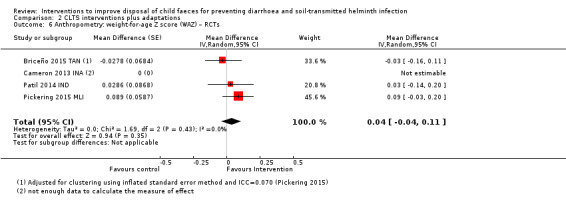
Comparison 2 CLTS interventions plus adaptations, Outcome 6 Anthropometry: weight‐for‐age Z score (WAZ) – RCTs.
Cross‐sectional studies
Belizario 2015 PHI examined the nutritional status of subgroups of children (weight for age and height for age for two‐ to five‐year olds and six‐ to nine‐year olds and BMI for age and height for age for 10‐ to 15‐year olds). The study did not identify a difference between CLTS villages and non‐CLTS villages apart from BMI for age (10‐ to 15‐year olds, n = 120). About 2.5% of children in CLTS villages were stunted compared to 21.3% in the non‐CLTS villages.
Mortality
Randomized controlled trials
Pickering 2015 MLI did not find a difference in all‐cause mortality between intervention and control groups but fewer households in the intervention group reported to have had a diarrhoeal‐related death (16 total diarrhoeal deaths with intervention versus 34 with control; PR 0.46, 95% CI 0.26 to 0.83) and child diarrhoeal deaths (11 child diarrhoea deaths in intervention versus 23 in control; PR 0.47, 95% CI 0.23 to 0.98) than controls.
Briceño 2015 TAN did not find a difference in the mortality of children aged less than five years in control and intervention groups.
Behaviour change
Randomized controlled trials
All the CLTS intervention studies reported on behavioural outcomes as intermediate outcomes of their intervention.
Analysis 2.7 shows the effects of the CLTS interventions on open defecation by children aged less than five years. Cameron 2013 INA; Patil 2014 IND; and Pickering 2015 MLI reported a significant difference in no open defecation by children aged less than five years in intervention arms compared to control.
2.7. Analysis.
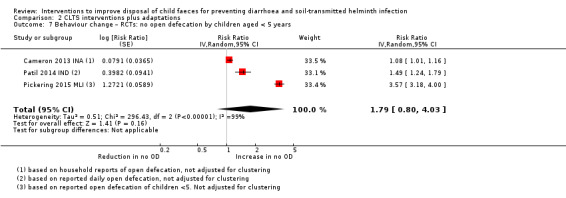
Comparison 2 CLTS interventions plus adaptations, Outcome 7 Behaviour change – RCTs: no open defecation by children aged < 5 years.
Analysis 2.8 shows the impacts of CLTS interventions on child faeces disposal behaviours (the data for Cameron 2013 INA were not in a usable format). Safe child faeces disposal practices was higher in the intervention than control arms in Briceño 2015 TAN (safe disposal also improved in the hand washing and sanitation combination arm) and Patil 2014 IND.
2.8. Analysis.

Comparison 2 CLTS interventions plus adaptations, Outcome 8 Behaviour change – RCTs: safe disposal of child faeces.
Pickering 2015 MLI found that potty use of children after the intervention was higher in intervention arms compared to control arms (Analysis 2.9).
2.9. Analysis.
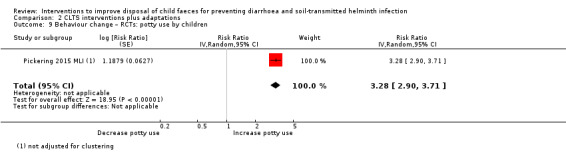
Comparison 2 CLTS interventions plus adaptations, Outcome 9 Behaviour change – RCTs: potty use by children.
Dickinson 2015 IND measured the reported time spent walking to defecation sites and found that children (aged less than five years) in the intervention arm experienced time savings of about 2.2 minutes per defecation trip.
Sanitation hardware and behaviour change interventions
The WASH‐B studies measured effects of the interventions on health outcomes, including diarrhoea and anthropometry (Luby 2018 BGD; Null 2018 KEN). The pilot study of the WASH‐B intervention, Christensen 2015a KEN, and the Sundara Grama intervention, Caruso 2019 IND, only measured the effect of their intervention on behaviour change.
Diarrhoea
Randomized controlled trials
Pooled results from the WASH‐B sanitation arms showed no effect on diarrhoea prevalence (RR 0.79, 95% CI 0.49 to 1.26; Analysis 3.1). However, the two trials had disparate effects on diarrhoea. In Bangladesh, Luby 2018 BGD found that the seven‐day diarrhoea prevalence was lower among index children and children aged under three years at enrolment who received the sanitation intervention compared to the control arm (PR 0.61, 95% CI 0.46 to 0.81). In Kenya, however, Null 2018 KEN found no effect of the sanitation intervention on diarrhoea prevalence.
3.1. Analysis.
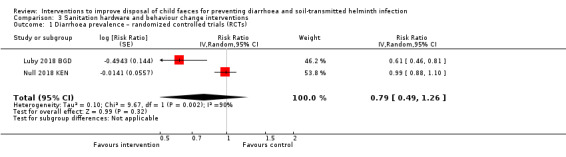
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 1 Diarrhoea prevalence – randomized controlled trials (RCTs).
Soil‐transmitted helminth
Randomized controlled trials
The WASH‐B studies also assessed the impact of the intervention on STH prevalence in a subset of children (results pending publication) and on STH presence in household soil. In Kenya, the authors found that the combined sanitation intervention group (sanitation and WASH households) had no impact on STH prevalence in household soil (17.0% with intervention versus 18.9% with control), concentration of STH eggs in soil or single STH species or viable eggs (Steinbaum 2017 (see under Null 2018 KEN)).
Anthropometry
Randomized controlled trials
The WASH‐B sanitation arms had no effects on anthropometry outcomes. Luby 2018 BGD and Null 2018 KEN did not find a difference in length‐for‐age Z scores (pooled MD –0.04, 95% CI –0.12 to 0.04; Analysis 3.2) or in WAZ scores in children in sanitation intervention arms (pooled MD –0.04, 95% CI –0.11 to 0.04; Analysis 3.3). Both studies also found no impact of the sanitation arms on other anthropometry outcomes (weight‐for‐length Z scores, head circumference, stunting, severe stunting, wasting, and underweight).
3.2. Analysis.
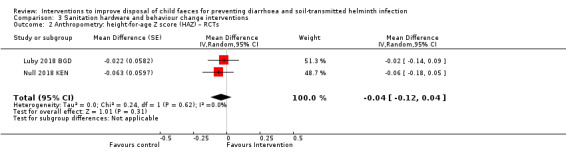
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 2 Anthropometry: height‐for‐age Z score (HAZ) – RCTs.
3.3. Analysis.

Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 3 Anthropometry: weight‐for‐age Z score (WAZ) – RCTs.
Mortality
Randomized controlled trials
The sanitation arms of the WASH‐B studies had no impact on all‐cause mortality (Luby 2018 BGD; Null 2018 KEN).
Behaviour change
All sanitation hardware and behaviour change RCTs reported on behavioural outcomes. For Christensen 2015a KEN and Caruso 2019 IND it was the main outcome. While for Luby 2018 BGD and Null 2018 KEN it was as intermediate outcomes of their intervention.
Analysis 3.4 shows the impacts of interventions on child faeces disposal behaviours. Safe child faeces disposal practices were higher in the intervention than control arms in Luby 2018 BGD (although adjusted risk difference (RD) did not show a difference, RD 20, 95% CI –11 to 51) and Null 2018 KEN. In Null 2018 KEN, safe child faeces disposal improved from baseline (19% safely disposed) more in year one (77% ) than at the end of the study (37%) in the sanitation arm. In both pilot RCTs in Kenya, child faeces disposal was higher in the intervention arm compared to the control arm (Analysis 3.5). In the sanitation only arm, a difference was not detected (RD 0.10, 95% CI –0.21 to 0.42; Christensen 2015a KEN), whereas in the combined WASH arm, appropriate child faeces disposal was 47.8 percentage points higher than in the control arm (RD 0.47, 95% CI 0.372 to 0.571; Christensen 2015b KEN). The intention‐to‐treat difference analysis in Caruso 2019 IND found an increase in reported safe disposal of child faeces of 20.4% (95% CI 11.7% to 29.2%; P < 0.001) in the intervention group at the end of the study after accounting for the increase in safe disposal of child faeces observed in the control group.
3.4. Analysis.
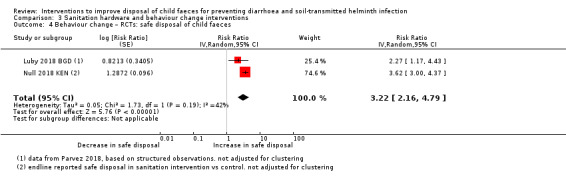
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 4 Behaviour change – RCTs: safe disposal of child faeces.
3.5. Analysis.
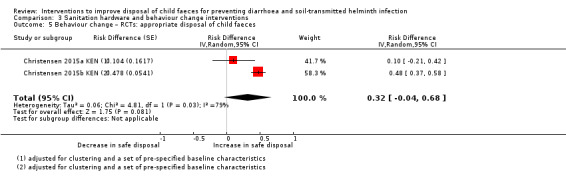
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 5 Behaviour change – RCTs: appropriate disposal of child faeces.
Luby 2018 BGD found that potty use of children after the intervention was higher in intervention arms compared to control arms (although adjusted RD in the sanitation arm did not demonstrate an effect, RD 22, 95% CI –18 to 61; Analysis 3.6). Luby 2018 BGD also observed some use of the sani‐scoops for cleaning human faeces in the sanitation (27%) and combined WASH arms (25% in the WASH arm, 38% in the WASH–nutrition arm) of the study, while this behaviour was not observed in the control arm.
3.6. Analysis.
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 6 Behaviour change – RCTs: potty use by children.
Christensen 2015a KEN and Christensen 2015b KEN observed human faeces in the compound was lower in the intervention arms compared to the control arms (Analysis 3.7); however, this was only statistically significant at the 0.05 level in the combined WASH trial. Luby 2018 BGD also observed fewer human faeces in the compounds in the sanitation intervention arm compared to the control; however, this was not reported as statistically significant at the 0.05 level.
3.7. Analysis.
Comparison 3 Sanitation hardware and behaviour change interventions, Outcome 7 Behaviour change – RCTs: faeces in compound.
WASH hardware and education/behaviour change interventions
Diarrhoea
Randomized controlled trials
Humphrey 2019 ZIM found that at 12 and 18 months, the prevalence of diarrhoea was not different between WASH and non‐WASH groups(Analysis 4.1).
4.1. Analysis.
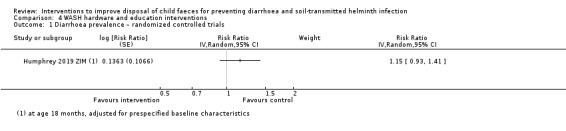
Comparison 4 WASH hardware and education interventions, Outcome 1 Diarrhoea prevalence – randomized controlled trials.
Controlled before‐and‐after studies
The pooled effect of the CBAs evaluating WASH hardware and education interventions reduced diarrhoea incidence by about a quarter (rate ratio 0.77, 95% CI 0.71 to 0.84; two studies; Analysis 4.2).
4.2. Analysis.
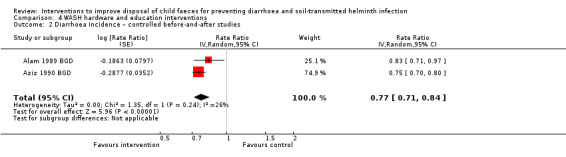
Comparison 4 WASH hardware and education interventions, Outcome 2 Diarrhoea incidence – controlled before‐and‐after studies.
Soil‐transmitted helminth
Controlled before‐and‐after studies
Park 2016 INA found that the odds of STH reinfection (participants in both arms were given albendazole if found to be infected at baseline) were lower in the intervention village compared to the control (OR 0.17, 95% CI 0.02 to 0.73, P = 0.014).
Dysentery and persistent diarrhoea
Controlled before‐and‐after studies
Aziz 1990 BGD found that children had 27% less dysentery (incidence density ratio (IDR) 0.73, 95% CI 0.61 to 0.88) and 40% less persistent diarrhoea (IDR 0.58, 95% CI 0.52 to 0.65) in the intervention than controls.
Anthropometry
Randomized controlled trials
Humphrey 2019 ZIM found that WASH interventions had no effect on the mean infant length‐for‐age Z score or any other growth measurements except for mean head‐circumference‐for‐age Z scores in adjusted analyses (Z score difference 0.08, 95% CI 0.01 to 0.15); however, this effect was driven entirely by the infant and young child feeding plus WASH group.
Controlled before‐and‐after studies
Aziz 1990 BGD did not find a difference in nutritional status in the intervention and control groups.
Cohort studies
Hoq 2016 BGD did not detect a difference in the rate of change in underweight children (WAZ < 2) over time. However, the study found a difference in the rate of change in acute malnutrition (MUAC < 125 mm), which was significantly higher in the integrated WASH intervention site (0.02%, 95% CI 0.014% to 0.026%) compared to the comparison site (0.006%, 95% CI 0.002% to 0.010%) (Chi² test = 20, P = 0.0001).
Mortality
Randomized controlled trials
Humphrey 2019 ZIM found that cumulative mortality at 18 months was similar between WASH and non‐WASH groups (adjusted PR 0.96, 95% CI 0.72 to 1.30).
Behaviour change
Randomized controlled trials
Humphrey 2019 ZIM found that 77% of mothers in the WASH groups reported to dispose of water from cleaning infant nappies with faeces in a latrine compared with 32% in non‐WASH groups.
Daycare centre‐based hygiene hardware and education interventions
Diarrhoea
Two interventions were conducted in daycare centres in the USA. Butz 1990 USA found that symptoms of diarrhoea were lower in intervention daycare centres (OR 0.715, 95% CI 0.54 to 0.72). Kotch 2007 USA found that children in the intervention daycare centres had fewer episodes of diarrhoea compared to the control group (0.90 diarrhoea illnesses per 100 child‐days with intervention versus 1.58 diarrhoea illnesses per 100 child‐days with control; P < 0.001).
Case‐control studies: disposal of child faeces in the latrine versus elsewhere
Diarrhoea
Pooled results from case‐control studies that presented data for child faeces disposal indicated that disposal of faeces in the latrine decreased the odds of diarrhoea by about a quarter among all ages (OR 0.73, 95% CI 0.62 to 0.85; 23 comparisons) and children aged less than five years (OR 0.72, 95% CI 0.61 to 0.85; 20 comparisons) (Analysis 5.1). See Table 16 for more information on those studies. In subgroup analyses, it seemed the effect of disposal of faeces in a latrine differed according to the type of diarrhoea, with a larger reduction in acute (possibly bloody) diarrhoea than moderate‐to‐severe diarrhoea (Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6). Although studies with no specified case definition also had a lower OR. The quality of the studies, as indicated by the number of stars obtained when applying the NOS risk of bias criteria, also seemed to differ among groups, with higher quality subgroups having lower ORs. The effect of safe disposal on diarrhoea did not seem to differ according to the data collection method, income level of the country, or setting where the study was conducted (Analysis 5.2; Analysis 5.5; Analysis 5.6).
5.1. Analysis.

Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 1 Diarrhoea (including severe and cholera): subgrouped by age group.
10. Case‐control studies: disposal elsewhere versus latrine.
| Study ID (setting) | Age group (years) | Outcome | Specific comparison | Measure of effect (95% CI) | What was it adjusted for? |
| Abalkhail 1995 KSA (urban) | < 3 | Diarrhoea | Disposal of child faeces elsewhere vs in latrine. | OR (adj): 1.46 (1.03 to 2.08) | Paternal education, child and maternal age and family size |
| Asfaha 2018 ETH (rural) | < 5 | Diarrhoea | Unsafe vs safe child stool disposal (no definition of safe/unsafe). | OR: 2.69 (1.88 to 3.87) | — |
| Baker 2016 BGD (rural) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with a pit or sewer. Hanging latrines and bucket latrines were considered open disposal. | OR (adj): 1.26 (1.05 to 1.52) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 GMB (rural) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 0.85 (0.38 to 1.88) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 IND (urban) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 1.11 (0.92 to 1.35) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 KEN (rural) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 1.02 (0.87 to 1.2) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 MLI (urban) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 2.01 (0.51 to 7.82) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 MOZ (rural) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 0.65 (0.32 to 1.3) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baker 2016 PAK (periurban) | < 5 | MSD | Disposal of child faeces in the open vs in any type of latrine with pit/sewer. | OR (adj): 0.82 (0.63 to 1.07) | Adjusted for wealth quintiles and the presence of both parents in the home. |
| Baltazar 1989 PHI (urban and rural) | < 2 | Diarrhoea | Unsanitary vs sanitary disposal of child faeces (sanitary = child defecated in a nappy and faeces thrown away in washing, child used chamber pot/piece of paper and faecal matter was thrown in the toilet or child used the toilet). | OR (adj): 1.34 (0.93 to 1.92) | Water supply, toilet facilities, sex, education of head of household and mother, feeding practices, level of health service utilization, number of children aged < 5 years in household. |
| Cummings 2012 UGAa (rural) | > 10 | Cholera | Not disposing of child faeces in latrine vs using latrine to dispose of faeces in cases vs control. | OR (adj): 15.76 (1.54 to 161.25) | Reside in household with another case, did not use chlorine tablet to disinfect water, ate roadside food, girls, age group (10–17 years old), no latrine in household, did not wash hands after defecation, did not store water in sealed container, eats mostly cold meals, and drinks local alcoholic beverage. |
| Dikassa 1993 DRC (urban) | < 3 | Diarrhoea | Not disposing of child faeces in latrine vs using latrine to dispose of faeces. | OR (adj): 3.61 (1.32 to 9.85) | Garbage disposal, caretaker hygiene, maternal education |
| Genthe 1997 SAF (urban/ periurban) | Preschool children | Diarrhoea | Open disposal of stools vs disposal into any form of sanitation. | OR: 1 (0.53 to 1.88) | — |
| Ghosh 1994 IND (rural) | < 3 | Diarrhoea case families | Indiscriminate disposal of child stools. | OR: 2.22 (1.08 to 4.56) | — |
| Ghosh 1997 IND (rural) | < 4 | Diarrhoea case families | Indiscriminate disposal of child stools. | OR (adj): 1.99 (0.97 to 4.08) | Bottle feeding, cleaning feeding container without soap, using pond water for cleaning feeding container, storing drinking water in wide mouthed vessel (bucket). |
| Godana 2013 ETHb (rural) | < 5 | Acute diarrhoea | Child faeces disposal elsewhere vs in latrine. | OR (calc): 2.49 (1.64 to 3.77) | — |
| Heller 2003 BRA (urban) | < 5 | Diarrhoea | Faeces disposal from swaddle elsewhere vs in toilet/latrine. | OR (adj): 1.45 (0.99 to 2.12) | Fruit and green hygiene, mother's religion, superficial presence of wastewater in street, refuse storage, domestic reservoir (2 categories), child's age, refuse disposal, number of children, near stream existence, own a fridge, cockroach presence, flooding in lot, mosquito presence, refuse collection frequency, domestic water reservoir (3 categories), faeces disposal from swaddle (no swaddle use vs latrine/toilet) + interaction terms for wastewater in street* refuse storage, domestic reservoir (no storage vs covered + clean)*cockroach, domestic reservoir (vessel storage vs covered+clean)*cockroach, domestic water storage (3 different categories)* cockroach, cockroach*mosquito. |
| Maung 1992a MYA (urban) | 1–59 months | Persistent diarrhoea + PEM | Faeces were disposed of around house vs latrine. | OR: 1.88 (0.6 to 5.96) | — |
| Mediratta 2010a ETH (urban) | < 5 | Acute diarrhoea | Disposal of stool elsewhere (garbage, buried, left on ground) vs in latrine. | OR: 0.78 (0.53 to 1.15) | — |
| Mertens 1992 SRI (rural) | < 5 | diarrhoea | Unsanitary vs sanitary disposal of < 5 child stools. Unsanitary stool disposal = stools passed, or disposed of, in or out of the yard without being later (within 1 day) disposed of in a latrine or in a covered rubbish pit. Proper = stools passed in a potty and later disposed of in a latrine or in a covered pit. | OR (adj): 1.42 (1.01 to 1.98) | Child's age, recruitment clinic, the distance from the home to the clinic, handwashing before a meal, water quantity, occupation of the head of the household, main type of water source used, and distance to the water source. |
| Strina 2012 BRA (urban) | < 10 | Rotavirus diarrhoea | Inadequate vs adequate disposal of excreta of children aged ≤ 2 years (no definition). | OR (adj): 1.34 (0.98 to 1.83) | Age and gender |
| Traoré 1994a BUR (urban) | < 3 | Diarrhoea/dysentery | Disposal elsewhere vs in latrines. | OR (adj): 1.5 (1.09 to 2.06) | Age, mother's religion, father's occupation, source of drinking water, possession of a radio‐cassette, whether the child was reported to eat soil, whether the mother practised "lavements" (anal purging) on the child, number of people in the household. |
| Wijewardene 1992 SRI (urban) | < 5 | Acute diarrhoea | Children's faeces not disposed in latrine in cases vs controls. | OR (adj): 2.28 (1.09 to 4.78) | Household size, source of water, disposal of garbage, adult defecation site, mother's education, mother's lack of knowledge regarding infectivity of diarrhoea, mother's lack of knowledge of mode of spread of diarrhoea, families that keep cooked food, feeding bottle and children's drinking cups uncovered. |
adj: adjusted; calc: calculated crude value; CI: confidence interval; MSD: Moderate‐to‐severe diarrhoea; OR: odds ratio; PEM: protein‐energy malnutrition. aCummings 2012 UGA reported a CI of 1.54 to 161.25; however, the closest we could enter was 161.26. bCalculated a crude OR as could not obtain as narrow CIs as what was reported in the paper.
5.2. Analysis.
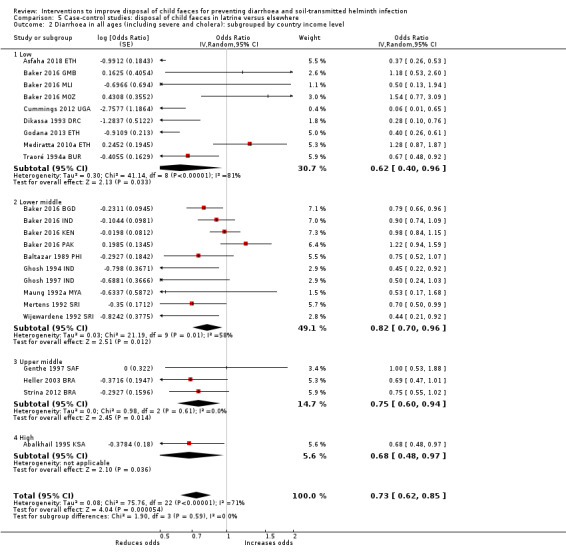
Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 2 Diarrhoea in all ages (including severe and cholera): subgrouped by country income level.
5.3. Analysis.
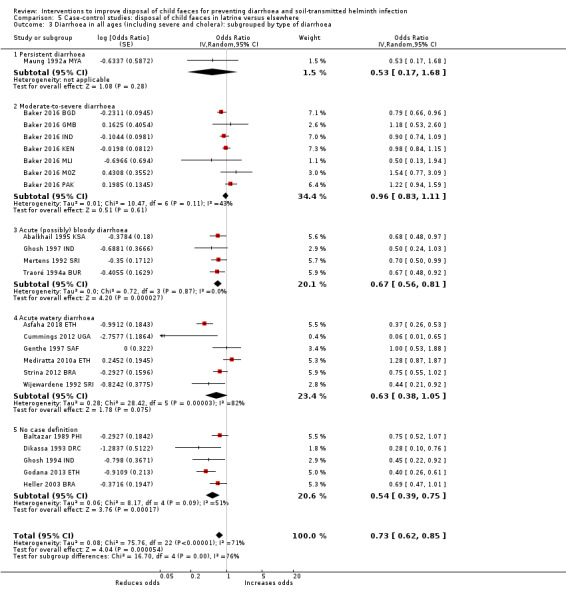
Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 3 Diarrhoea in all ages (including severe and cholera): subgrouped by type of diarrhoea.
5.4. Analysis.
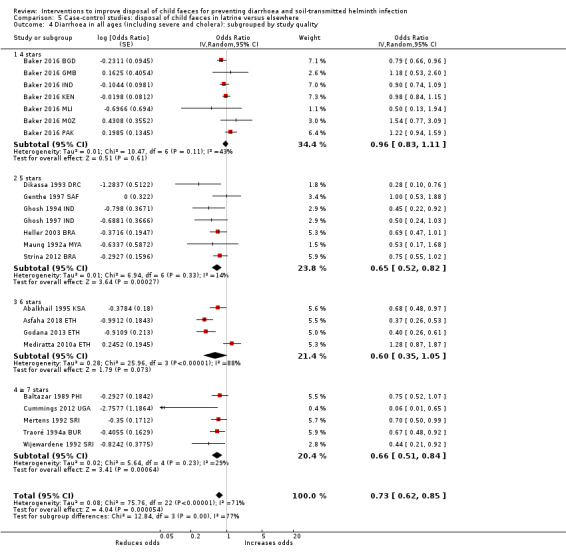
Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 4 Diarrhoea in all ages (including severe and cholera): subgrouped by study quality.
5.5. Analysis.

Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 5 Diarrhoea in all ages (including severe and cholera): subgrouped by setting.
5.6. Analysis.
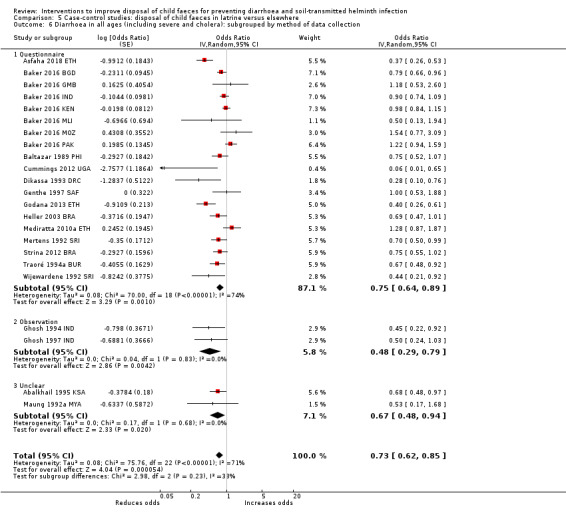
Comparison 5 Case‐control studies: disposal of child faeces in latrine versus elsewhere, Outcome 6 Diarrhoea in all ages (including severe and cholera): subgrouped by method of data collection.
Case‐control studies: defecation of children in the latrine versus elsewhere
Diarrhoea
Pooled results from case‐control studies that presented data for children defecating in the latrine indicated that children using the latrine reduced the odds of diarrhoea by about half in all ages (OR 0.54, 95% CI 0.33 to 0.90; 7 studies); the corresponding pooled point estimate for children aged less than five years was similar, although the confidence intervals were wide (OR 0.54, 95% CI 0.28 to 1.07; 5 studies) (Analysis 6.1). See Table 17 for more information about these studies. In subgroup analyses, persistent diarrhoea had lower ORs than acute and acute watery diarrhoea (Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6). The quality of the studies also seemed to change the observed association between children defecating in a latrine and diarrhoea. As the quality of the studies improved, as indicated by the number of stars, the association became closer to null. The effect of child defecation in the latrine on diarrhoea also seemed to differ according to the data collection method and country income level.
6.1. Analysis.

Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 1 Diarrhoea: case‐control studies: subgrouped by age group.
11. Case‐control studies: defecation elsewhere versus in latrine.
| Study ID (setting) | Age group | Outcome | Specific comparison | Measure of effect (95% CI) | What was it adjusted for? |
| Chompook 2006 THA (Semi‐urban) | All | Shigellosis | Child not/sometimes using latrine vs using latrine. | OR 1.27 (0.84 to 1.93) | — |
| Clemens 1987 BGD (urban) | < 6 years | Diarrhoea ≥ 1.7 times rate expected | Open defecation in the family living area rather than latrine or some other specially designated place in cases vs controls. | OR 8 (1.52 to 42.04) | — |
| Knight 1992 MAL (rural) | 4 to 59 months | Diarrhoea | Indiscriminate defecation of child (not in latrine or nappy) vs defecation in nappy/latrine. | OR (adj) 1.33 (0.52 to 3.42) | SES, educational level of main caregiver, health centre of recruitment, interviewer, birth order of child and number of people living in house. |
| Maung 1992b MYA (urban) | 1 to 59 months | Persistent diarrhoea + PEM | Child defecated on the floor vs in pot/latrine. | OR 3.76 (1.68 to 8.42) | — |
| Mediratta 2010b ETH (urban) | < 5 | Acute diarrhoea | Defecation elsewhere vs in latrines. | OR 0.88 (0.33 to 2.34) | — |
| Oketcho 2012 TAN (urban) | 6 to 60 months | Diarrhoea | Use of latrine by children vs defecation elsewhere. | OR 7.38 (1.91 to 28.58) | — |
| Traoré 1994b BUR (urban) | < 3 | Diarrhoea / dysentery | Defecation elsewhere vs in pots/latrines. | OR (adj) 1.1 (0.78 to 1.57) | Age, mother's religion, father's occupation, source of drinking water, possession of a radio‐cassette, whether the child was reported to eat soil, whether the mother practised "lavements" (anal purging) on the child, number of people in the household. |
adj: adjusted; CI: confidence interval; OR: odds ratio; PEM: protein‐energy malnutrition; SES: socioeconomic status.
6.2. Analysis.
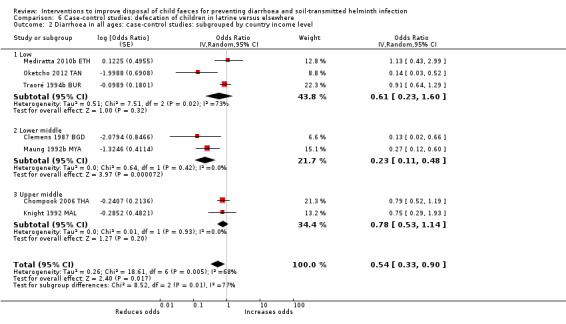
Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 2 Diarrhoea in all ages: case‐control studies: subgrouped by country income level.
6.3. Analysis.
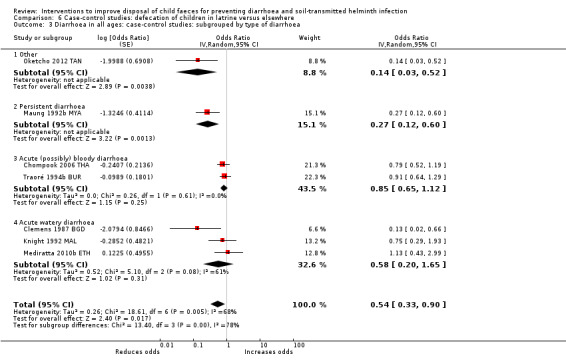
Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 3 Diarrhoea in all ages: case‐control studies: subgrouped by type of diarrhoea.
6.4. Analysis.
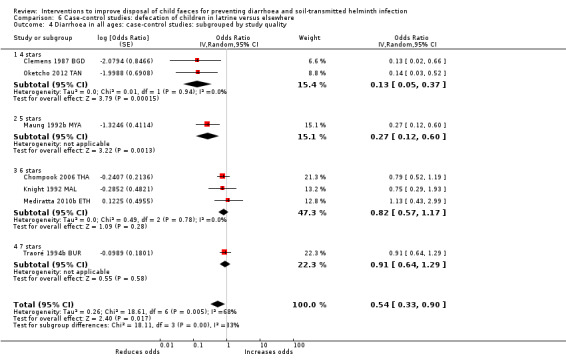
Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 4 Diarrhoea in all ages: case‐control studies: subgrouped by study quality.
6.5. Analysis.
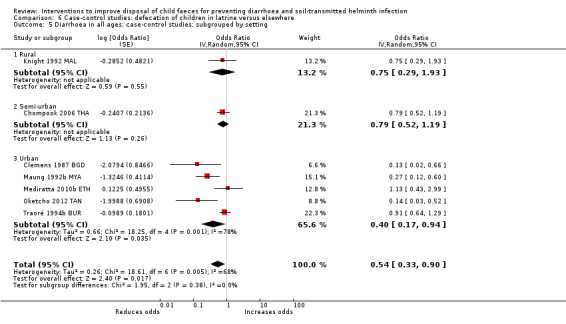
Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 5 Diarrhoea in all ages: case‐control studies: subgrouped by setting.
6.6. Analysis.
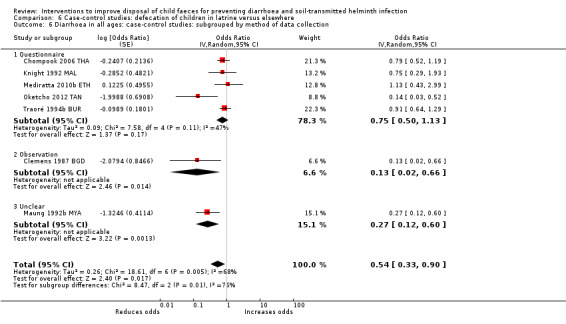
Comparison 6 Case‐control studies: defecation of children in latrine versus elsewhere, Outcome 6 Diarrhoea in all ages: case‐control studies: subgrouped by method of data collection.
Case‐control studies: other interventions
Arvelo 2009 USA did not show a difference in lidded bins for nappy disposal between case and control licensed daycare centres (OR 2.0, 95% CI 0.5 to 8.1). Bassal 2016 ISR found that the odds for infection with campylobacter among children who were not toilet trained and used nappies were higher than among those who did not use nappies (OR 7.36, 95% CI 1.66 to 32.70; P < 0.01). Chiang 2005 TWN found that open defecation of children increased the odds of being a case (OR 6.32, 95% CI 0.7 to 54.5, adjusted for ethnicity and living residence). Daniels 1990 LES found that among both the cases and controls, 50% of latrine owners reported that they disposed of the child's stools in the latrine; however, this was not shown separately for cases and controls. Menon 1990 USA did not find a difference in the number of dirty nappies in the yards of case households compared to controls (OR 3.5, 95% CI 0.88 to 13.93). Nanan 2003 PAK found that cases were more likely to come from non‐WASEP villages than controls (OR 1.33, 95% CI 1.0 to 1.8).
Clinical visits for diarrhoea, serology, and other markers of infection and disease
No included study reported on these outcomes.
Adverse events
No study reported adverse events related to the child faeces disposal components of the interventions.
Sensitivity analyses
The fixed‐effect and random‐effects analyses were similar and did not change the conclusions of the analyses. The random‐effects method measures were more conservative, having larger CIs.
Discussion
See Table 1; Table 2; Table 3; Table 4; Table 5; Table 6.
Summary of main results
While numerous studies met the review's inclusion criteria, we consider the evidence linking the safe disposal of child faeces with diarrhoea or STH infection to be limited. Few studies focused solely on interventions aimed at improving the collection or disposal of child faeces. Of the 22 RCTs, only one focused exclusively on improving child faeces disposal behaviour, and that study only measured behaviour change. Nine other RCTs included child faeces disposal as one of the messages in their education and hygiene promotion intervention, only seven of those included health outcomes. Of the other RCTs, five measured the health impacts of their intervention to end open defecation of the whole community including children as well as indicators of child faeces disposal behaviour change, one evaluated a WASH hardware and behaviour change intervention, four included child faeces disposal hardware (potties and sani‐scoops) within its sanitation intervention, and two were based in daycare facilities. Of the four CBAs, one included child faeces disposal as part of several messages in its education and hygiene promotion intervention, while the other three provided WASH hardware along with education that included child sanitation messages. The health impacts of the child faeces disposal component of these interventions can thus not be measured.
The three cohort studies and four of the seven cross‐sectional studies included in the review also measured the health effect of combined interventions, while three only measured the behaviour change after the CHC or women volunteer group intervention.
The most direct evidence supporting the protective effect from safe child faeces disposal in a latrine on diarrhoea came from the case‐control studies. Twenty‐seven case‐control studies were included, with 21 of them being used in the quantitative analyses. The evidence from these studies suggested that disposing of child faeces in a latrine was associated with reduced odds of diarrhoea (OR 0.73, 95% CI 0.62 to 0.85; very low‐certainty evidence). These studies also suggested that children defecating in a latrine rather than elsewhere was associated with reduced odds of diarrhoea (OR 0.54, 95% CI 0.33 to 0.90; very low‐certainty evidence). It is important to note that we classified safe child faeces disposal as disposal into any latrine or as defined by the study authors. It is unclear from current evidence whether there is a difference in effect between disposing of faeces in improved versus unimproved latrines.
Only four studies (two RCTs, one CBA, and one cross‐sectional) reported impacts of their intervention on STH infection. Both RCTs were interventions aiming to stop open defecation generally (not safe disposal of child faeces specifically) and neither study found a health impact on helminth infection. Both RCTs reported reduction in open defecation of children and Patil 2014 IND reported improved disposal of child faeces in the intervention arm. However, Patil 2014 IND found that the intervention led to a small increase in latrine construction accompanied with a small decrease of open defecation and that these improvements were not sufficient to see an improvement in health outcomes (both diarrhoea and STH). In Cameron 2013 INA, the intervention led to a moderate increase in toilet construction, with associated decreases in open defecation in households that did not have access to sanitation at baseline, which suggested an improvement in behaviour due to the toilet construction. While, the intervention was associated with lower diarrhoea prevalence in the intervention communities, there was no effect on STH infection. This could be because diarrhoea prevalence was measured through self‐reports, which could have been biased due to non‐blinding while the STH infections were diagnosed from stools, thus a more objective measure. Alternatively, as STH eggs can survive longer in the environment than diarrhoea‐causing pathogens, it may take longer to observe an impact on STH. The CBA study, Park 2016 INA, found that providing simple pit latrines and hygiene education reduced the prevalence of STH in the intervention village compared to the control village. It should be noted that this study had a small sample size and no intermediary outcomes, such as behaviour change, were measured to support the conclusions of the study. In the cross‐sectional study, which compared STH prevalence and nutritional outcomes in two villages that were ODF after a CLTS campaign with control villages, found that STH prevalence was higher in the CLTS villages (Belizario 2015 PHI). Again, this study did not report indicators of the campaign success, such as indicators of latrine use or child sanitation, which could have explained the findings.
Overall completeness and applicability of evidence
Most of the included studies were conducted in low‐ or lower middle‐income countries, while some were in upper middle‐ or high‐income countries. Most study sites were in rural areas (64%).
Few studies investigated specific hardware for safe child faeces disposal. Two studies promoted potties (Jinadu 2007 NGR; Yeager 2002 PER), and potties were one of the criteria of the ODF certification in CLTS in Mali (all family members had to use the latrine or a child potty) (Pickering 2015 MLI). However, it was unclear how much focus there was on safe disposal of child faeces as part of the triggering of activities in the paper. The sanitation hardware and behaviour change studies provided potties and sani‐scoops (Caruso 2019 IND; Christensen 2015a KEN; Christensen 2015b KEN; Luby 2018 BGD; Null 2018 KEN). The studies did find improvements in child faeces disposal at follow‐up and some use of the hardware. Ahmed 1993 BGD included messaging to use a dirt thrower to dispose of child faeces. Butz 1990 USA and Kotch 2007 USA included some nappy changing equipment in their intervention and instructions to dispose of nappies in plastic bags (Butz 1990 USA), and roll‐out waste bins for nappy disposal (Kotch 2007 USA). No other included study had a hardware component and none encompassed different hardware solutions for different age groups (e.g. nappies for babies, latrine slabs for latrine training).
Few studies included details of the behaviour change messaging that was provided and only a few based their interventions on theory and behavioural frameworks and developed them through formative research (Caruso 2019 IND; Humphrey 2019 ZIM; Luby 2018 BGD; Null 2018 KEN; Yeager 2002 PER).
Certainty of the evidence
The certainty of evidence of the RCTs was very low, low, or moderate due to the risk of bias, the indirectness of the evidence, heterogeneity, and imprecision. The CBAs, cohort studies, and cross‐sectional studies were all very low‐certainty evidence due to risk of bias, heterogeneity, indirectness, and imprecision. The certainty of evidence for case‐control studies was very low due to heterogeneity (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
Potential biases in the review process
We endeavoured to identify all eligible studies by conducting searches with no time or language restrictions. The high number of studies resulting from the search criteria meant that it was not possible for two review authors to check the titles, so only one author went through all titles excluding those that were clearly irrelevant. This could have biased the findings as some relevant findings could have been missed by the single author. However, with knowledge of this risk, we sought only to exclude titles that were clearly irrelevant (e.g. dental hygiene; chemical pollution; non‐relevant infectious diseases such as tuberculosis, malaria, or dengue; surgery; pharmacology; etc.) and kept anything that was unclear or possibly relevant for abstract screening, which two review authors conducted.
Agreements and disagreements with other studies or reviews
There are only two previous reviews on the safe disposal of child faeces. Gil 2004 was conducted in the early 2000s, and included 10 observational studies and no intervention studies. It reported that child faeces disposal behaviours considered risky (open defecation, stool disposal in the open, stools not removed from soil, stools seen in household soil, and children seen eating faeces) were associated with a 23% increase in risk of diarrhoea (RR 1.23, 95% CI 1.15 to 1.32); however, behaviours considered safe (use of latrines, nappies, potties, toilets, washing nappies) were borderline protective (RR 0.93, 95% CI 0.86 to 1.00). An unpublished update of that systematic review, Scott 2008, found a further four papers. Two papers found that unsafe disposal of child faeces (not in a latrine) increased the risk of diarrhoea (Heller 2003 BRA; Tumwine 2002), while two papers did not demonstrate an association between presence of human faeces in the compound and bloody diarrhoea (Brooks 2003), and between potty use and typhoid fever (Ram 2007). Although we identified and included substantially more studies in our review, the results were not inconsistent with this previous research. Both found safe disposal of child faeces to be protective against diarrhoea.
The second review was published in 2016 (Morita 2016). This review differed from ours in that it used different inclusion criteria, resulting in far fewer studies (eight) compared to our 63 studies. Both reviews agreed that none of the included studies that reported health outcomes focused exclusively on improving child faeces disposal and that there is a need for RCTs to evaluate the health impact of safe child faeces disposal interventions.
Our results are also generally consistent with recent reviews of the effects of sanitation generally against diarrhoeal disease. Freeman and colleagues reported improved sanitation to reduce the odds of diarrhoeal disease by 12% compared to unimproved sanitation (OR 0.88, 95% CI 0.83 to 0.92; 27 studies), when restricted to 16 intervention studies, the protective effect doubled to 23% (OR 0.77, 95% CI 0.66 to 0.91) (Freeman 2017).
Authors' conclusions
Implications for practice.
While child faeces may represent an important source of pathogen exposure, there is little research on the health effects of interventions to improve the safe disposal of child faeces, except as part of a larger sanitation initiative. The available evidence suggests that children should be encouraged to use latrines and that child faeces should be disposed of in a latrine. However, the evidence is of very low certainty, thus we are unsure about the effect of these interventions.
Implications for research.
Randomized controlled trials (RCTs) that study the health impact of different hardware and software interventions aimed specifically at improving the safe disposal of child faeces of different age groups will help to clarify the potential for child faeces management to prevent diarrhoea and soil‐transmitted helminth (STH) infections. These studies should be conducted in different settings to improve external validity. Additionally, since these studies cannot normally be blinded, measuring effects using objective outcomes, such as pathogens in stools or anthropometry, will also reduce potential risk of bias associated with reported diarrhoea. The RCTs should include intermediate measures to study the impact of the intervention on possible transmission routes, such as contamination of water, soil, and hands, to increase the plausibility of the findings. Additionally, the studies should measure behaviour change over longer periods and within entire communities.
Future studies should consider the various steps involved in the management of child faeces, as there are several points which may cause exposure, including the place of defecation, cleaning practices, place of disposal, and subsequent handwashing.
Some studies did not explain their definition of safe disposal. Water, sanitation, and hygiene (WASH) interventions should be more explicit about their 'hygiene' or 'sanitation' education interventions to outline what messages were included and how these were developed. Additionally community‐led total sanitation (CLTS) studies should be more clear on whether children were specifically included in their efforts to end open defecation. None of the five interventions aiming to eliminate open defecation explicitly described the messages that were given to the communities about child faeces disposal or the use of latrines by children. We would recommend that interventions that use CLTS messaging to eliminate open defecation be more explicit about their contents and how they address the needs of different age groups, including children. In addition, CLTS interventions should include child faeces disposal in their manuals and in the indicators that are measured for communities to be considered open defecation‐free, as this is not consistently done.
Acknowledgements
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank Aurelie Jeandron for assessing the eligibility of the Russian literature; Lyndsey Gray, Christian Landon, and Czarina Cooper for doing abstract and full‐text eligibility assessments; and Christianne Esparza and her colleagues for assistance in finding articles.
Appendices
Appendix 1. Study design definitions
Quasi‐randomized controlled trial (quasi‐RCT): a study with an experimental design where participants are allocated to different interventions using a quasi‐random method, such as date of birth, alternation, and medical record number.
Non‐RCT: a study with an experimental design where participants are allocated to different interventions using a non‐random method.
Controlled before‐and‐after study: a study where observations are made in a control and intervention group, before and after the implementation of an intervention.
Interrupted time series study: a study in which observations are done at multiple time points before and after an intervention (interruption). The design of the study enables researchers to see if the intervention has an effect that is greater than underlying trend over time.
Historically controlled study: a study comparing a group of participants receiving an intervention with a similar group from the past that did not.
Cohort study: a study that follows a defined group of people (cohort) over a period of time to examine interventions received and subsequent outcomes. A 'prospective' cohort study recruits participants before an intervention and follows them whereas a 'retrospective' cohort study recruits participants from the past using records from the past that describe the interventions received and follows them in the past using the records.
Case‐control study: a study that compares participants with a certain outcome (cases) with people from the same source population without the outcome (controls) and examines the associations between the outcome and prior exposures (e.g. receiving an intervention).
Cross‐sectional study: a study where information on past or current interventions and health outcomes are collected for a group of people at a particular time point in order to study associations between outcomes and exposure to interventions.
We adopted these definitions from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Appendix 2. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINE | Embase | Global Health | Web of Science | LILACS | POPLINE |
| 1 | feces OR faeces OR faecal OR fecal OR stool* OR excreta* OR excrement OR diarrhoea OR diarrhea OR defaecation OR defecation OR human waste | feces OR faeces OR faecal OR fecal OR stool* OR excreta* OR excrement OR diarrhoea OR diarrhea OR defaecation OR defecation OR human waste | (f?eces or f?ecal or stool$ or excreta$ or excrement or diarrh?ea or defa?cation or human waste) adj3 (management or dispos$ or remov$ or cleansing or cleaning or washing)) | (f?eces or f?ecal or stool$ or excreta$ or excrement or diarrh?ea or defa?cation or human waste) adj3 (management or dispos$ or remov$ or cleansing or cleaning or washing)) | (f?eces or f?ecal or stool* or excreta* or excrement or diarrh?ea or defa?cation or human waste) adj3 (management or dispos*or remov* or cleansing or cleaning or washing)) | F$eces OR f$ecal OR stool* OR excreta* OR excrement OR diarrh$ea OR defecation OR human waste | feces or faeces or fecal or faecal or stool$ or excreta$ or excrement or diarrhea or diarrhoea or defecation or defaecation or human waste | feces OR faeces OR faecal OR fecal OR stool* OR excreta* OR excrement OR diarrhea OR diarrhoea OR defaecation OR defecation OR human waste |
| 2 | management OR dispos*OR remov* OR cleansing OR cleaning OR washing | management OR dispos*OR remov* OR cleansing OR cleaning OR washing | sanitation or potty or potties or diaper$ or nappy or nappies or latrine$ or toilet$ or cloth$ diaper$ or swaddle or wrap$ | sanitation or potty or potties or diaper$ or nappy or nappies or latrine$ or toilet$ or cloth$ diaper$ or swaddle or wrap$ | sanitation or potty or potties or diaper* or nappy or nappies or latrine* or toilet* or cloth* or diaper* or swaddle or wrap* | management OR dispos*OR remov* OR cleansing OR cleaning OR washing | management or dispos$ or remov$ or cleansing or cleaning or washing | management OR dispos* OR remov* OR cleansing OR cleaning OR washing |
| 3 | 1 AND 2 | 1 AND 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 AND 2 | 1 AND 2 | 1 AND 2 |
| 4 | sanitation OR potty OR potties OR diaper* OR nappy OR nappies OR latrine* OR toilet* OR cloth* OR diaper* OR swaddle OR wrap* | sanitation OR potty OR potties OR diaper* OR nappy OR nappies OR latrine* OR toilet* OR cloth* OR diaper* OR swaddle OR wrap* | exp Sanitation/ | exp sanitation/ or exp environmental sanitation/ | exp sanitation/ | sanitation OR potty OR potties OR diaper* OR nappy OR nappies OR latrine* OR toilet* OR cloth OR diaper* OR swaddle OR wrap* | child$ or babies or baby or infant$ or toddler$ or neonate$ or preschool or pre‐school | sanitation OR potty OR potties OR diaper* OR nappy OR nappies OR latrine* OR toilet* OR cloth OR diaper* OR swaddle OR wrap* |
| 5 | 3 OR 4 | 3 OR 4 | 3 or 4 | 3 or 4 | 3 or 4 | 3 OR 4 | 3 AND 4 | 3 OR 4 |
| 6 | child* OR babies OR baby OR infant* OR toddler* OR neonate* OR preschool OR pre‐school | [Sanitation] | child$ or babies or baby or infant$ or toddler$ or neonate$ or pre?school | child$ or babies or baby or infant$ or toddler$ or neonate$ or pre?school | child* or babies or baby or infant* or toddler* or neonate* or pre?school | child* OR babies OR baby OR infant* OR toddler* OR neonate* OR preschool OR pre$school | Keywords : sanitation OR Hygiene | |
| 7 | 5 and 6 | 5 OR 6 | exp child/ or exp child, preschool/ or exp infant/ | exp child/ | exp children/ | 5 AND 6 | 5 OR 6 | |
| 8 | child* OR babies OR baby OR infant* OR toddler* OR neonate* OR preschool OR pre‐school | 6 or 7 | 6 or 7 | Exp infants/ | child* OR babies OR baby OR infant* OR toddler* OR neonate* OR preschool OR pre‐school | |||
| 9 | [child] | 5 and 8 | 5 and 8 | 6 or 7 or 8 | Keywords : child OR infant | |||
| 10 | [infant] | 5 and 9 | 8 OR 9 | |||||
| 11 | 8 OR 9 OR 10 | 7 AND 10 | ||||||
| 12 | 7 AND 11 |
aCochrane Infectious Diseases Group Specialized Register.
Appendix 3. Items for data extraction
| Study data |
| Person extracting data |
| Date of extraction |
| Study ID |
| Report ID (if different from study ID) |
| Reference citation |
| Study author details |
| Publication type |
| Publication status |
| Notes (e.g. questions for authors, statistical concerns) |
| Study eligibility: (if answer no to one of the criteria, exclude) |
| Type of study: RCT or NRS with control group (quasi‐RCTs, non‐RCTs, controlled before‐and‐after studies, interrupted time series studies, historically controlled studies, case‐control studies, cohort studies and cross‐sectional studies) |
| Participants: adults or children |
| Type of intervention: hardware or software interventions that reduce the direct or indirect contact with child (aged < 5 years) faeces? |
| Type of comparison: no intervention or other intervention? |
| Type of outcome: diarrhoea episodes; infections with ≥ 1 species of STHs; intensity of infection with ≥ 1 species of STH; dysentery; severe diarrhoea; persistent diarrhoea; clinical visits for diarrhoea; presence of pathogenic microbes in stools; anthropometry; serology; other markers of infection and disease; adverse events; mortality; or behaviour change? |
| If excluded, reasons for exclusion |
| Characteristics of included studies |
| Country and district, state, or town |
| Setting (hospital, school, community, urban, or rural) |
| Season |
| Design |
| Description of design |
| Was it a multicentre study? |
| Funding source |
| Duration of study (start and end date of study) |
| Duration of participation (start of recruitment until last follow‐up time point) |
| Ethical approval if needed |
| Missing data and reasons |
| Unit of randomization and whether the analysis adjusted for clustering if cluster design |
| Participants |
| Population demographics |
| Study inclusion criteria |
| Study exclusion criteria |
| Method of participant recruitment |
| Total number of participants recruited |
| Withdrawals, exclusions, loss to follow‐up |
| Age |
| Sex |
| Household size |
| Education level |
| Socioeconomic level |
| Pre‐ and postintervention water quality |
| Sanitation type and coverage |
| Hygiene practices |
| Type of water supply and coverage |
| Baseline child faeces disposal sites |
| Prevalence of open defecation |
| Deworming history in the study population |
| Solid waste disposal practices |
| Animal ownership |
| School or preschool attendance |
| Shoe wearing practices |
| Intervention group |
| Description of intervention |
| Number of participants |
| Cointerventions? |
| Who delivered the intervention? |
| Format and timing of delivery? |
| Coverage and uptake of child faeces collection and disposal practices |
| Compliance to intervention |
| Control group |
| Description of control |
| Number of participants |
| Cointervention? |
| Outcomes |
| Case definition for health outcomes |
| Measuring/diagnosis method (if self‐reported include recall period) |
| Time points measured |
| Effect estimate and 95% CI and raw numbers (for NRS record adjusted and unadjusted measures with confounders adjusted for; for cluster RCT specify if effect estimate is adjusted for clustering) |
| List of outcomes measured in study |
| Key conclusions of authors |
| Explanations of unexpected findings |
| Risk of bias assessment |
| RCTs (high, low, or unclear risk) |
| Random sequence generation? |
| Allocation concealment? |
| Blinding of participants and personnel? |
| Blinding of outcome assessment? |
| Incomplete outcome data? |
| Selective reporting? |
| Other risks of bias? |
| Cluster‐RCTs (high, low, or unclear risk) |
| Recruitment bias? |
| Baseline imbalance? |
| Loss of clusters? |
| Incorrect analyses? |
| NRS except case‐control and interrupted time series (high, low, or unclear risk) |
| Random sequence generation? |
| Allocation concealment? |
| Baseline outcome measures similar? |
| Baseline characteristics similar? |
| Incomplete outcome data? |
| Adequate allocation of intervention concealment? |
| Adequate protection against contamination? |
| Selective reporting? |
| Other risks of bias? |
| Confounders adequately adjusted for in analysis or design? (describe adjustment method) |
| Methods to identify and measure confounders |
| List all confounders considered in study |
| Interrupted time series (high, low, or unclear risk) |
| Intervention independent from other changes? |
| Prespecified shape of the intervention? |
| Intervention likely to affect the data collection? |
| Knowledge of the allocated interventions was adequately prevented? |
| Incomplete outcome data? |
| Selective outcome reporting? |
| Other risk of bias? |
| Case‐control studies |
| ‐ Selection |
| Is the case definition adequate? |
| Representativeness of the cases |
| Selection of controls |
| Definition of controls |
| ‐ Comparability |
| Comparability of cases and controls on the basis of the design or analysis |
| ‐ Exposure |
| Ascertainment of exposure |
| Same method of ascertainment for cases and controls |
| Non‐response rate |
| NRS: non‐randomized study; RCT: randomized controlled trial; STH: soil‐transmitted helminth. |
Data and analyses
Comparison 1. Education and hygiene promotion interventions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea prevalence – randomized controlled trials (RCTs) | 2 | Risk Ratio (Random, 95% CI) | 0.93 [0.84, 1.04] | |
| 2 Diarrhoea incidence – RCTs | 2 | Rate Ratio (Random, 95% CI) | 0.71 [0.59, 0.86] | |
| 3 Diarrhoea prevalence – controlled cohort studies: SHEWA‐B versus control | 2 | Risk Ratio (Random, 95% CI) | 0.91 [0.64, 1.28] | |
| 4 Diarrhoea prevalence – controlled cross‐sectional: HEP model households versus non‐model | 2 | Odds Ratio (Random, 95% CI) | 0.26 [0.16, 0.42] | |
| 5 Anthropometry – RCTs: height‐for‐age Z score (HAZ) | 2 | Mean Difference (Random, 95% CI) | 0.05 [‐0.07, 0.17] | |
| 6 Behaviour change – RCTs: latrine use by children | 2 | Risk Ratio (Random, 95% CI) | 1.69 [0.26, 11.04] | |
| 7 Behaviour change – RCTs: potty use by children | 2 | Risk Ratio (Random, 95% CI) | 1.37 [0.57, 3.30] | |
| 8 Behaviour change – RCTs: safe disposal of child faeces | 2 | Risk Ratio (Random, 95% CI) | 1.01 [0.93, 1.08] | |
| 9 Behaviour change – RCTs: appropriate disposal of child faeces | 1 | Risk Difference (Random, 95% CI) | ‐0.01 [‐0.06, 0.03] | |
| 10 Behaviour change – RCTs: faeces not observed in yard/ HH | 2 | Risk Ratio (Random, 95% CI) | 1.09 [0.61, 1.94] | |
| 11 Behaviour change – RCTs: faeces in compound | 1 | Risk Difference (Random, 95% CI) | 0.00 [‐0.02, 0.02] | |
| 12 Behaviour change – controlled cohort studies: safe vs unsafe child faeces disposal | 2 | Risk Ratio (Random, 95% CI) | 1.10 [0.72, 1.67] | |
| 13 Behaviour change – controlled cross‐sectional studies: safe vs unsafe child faeces disposal | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 13.1 BRAC | 1 | Risk Ratio (Random, 95% CI) | 4.25 [1.91, 9.46] | |
| 13.2 HEP | 2 | Risk Ratio (Random, 95% CI) | 1.36 [0.98, 1.89] |
Comparison 2. CLTS interventions plus adaptations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea prevalence – randomized controlled trials (RCTs) | 4 | Risk Ratio (Random, 95% CI) | 0.92 [0.79, 1.07] | |
| 2 Any helminth prevalence – RCTs | 2 | Risk Ratio (Random, 95% CI) | 1.03 [0.64, 1.65] | |
| 3 Ascaris lumbricoides prevalence – RCTs | 2 | Risk Ratio (Random, 95% CI) | 1.01 [0.60, 1.71] | |
| 4 Dysentery – RCTs | 2 | Risk Ratio (Random, 95% CI) | 0.69 [0.35, 1.34] | |
| 5 Anthropometry: height‐for‐age Z score (HAZ) – RCTs | 4 | Mean Difference (Random, 95% CI) | 0.06 [‐0.07, 0.19] | |
| 6 Anthropometry: weight‐for‐age Z score (WAZ) – RCTs | 4 | Mean Difference (Random, 95% CI) | 0.04 [‐0.04, 0.11] | |
| 7 Behaviour change – RCTs: no open defecation by children aged < 5 years | 3 | Risk Ratio (Random, 95% CI) | 1.79 [0.80, 4.03] | |
| 8 Behaviour change – RCTs: safe disposal of child faeces | 3 | Risk Ratio (Random, 95% CI) | 1.29 [1.02, 1.64] | |
| 9 Behaviour change – RCTs: potty use by children | 1 | Risk Ratio (Random, 95% CI) | 3.28 [2.90, 3.71] |
Comparison 3. Sanitation hardware and behaviour change interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea prevalence – randomized controlled trials (RCTs) | 2 | Risk Ratio (Random, 95% CI) | 0.79 [0.49, 1.26] | |
| 2 Anthropometry: height‐for‐age Z score (HAZ) – RCTs | 2 | Mean Difference (Random, 95% CI) | ‐0.04 [‐0.12, 0.04] | |
| 3 Anthropometry: weight‐for‐age Z score (WAZ) – RCTs | 2 | Mean Difference (Random, 95% CI) | ‐0.04 [‐0.11, 0.04] | |
| 4 Behaviour change – RCTs: safe disposal of child faeces | 2 | Risk Ratio (Random, 95% CI) | 3.22 [2.16, 4.79] | |
| 5 Behaviour change – RCTs: appropriate disposal of child faeces | 2 | Risk Difference (Random, 95% CI) | 0.32 [‐0.04, 0.68] | |
| 6 Behaviour change – RCTs: potty use by children | 1 | Risk Ratio (Random, 95% CI) | 1.69 [1.08, 2.65] | |
| 7 Behaviour change – RCTs: faeces in compound | 3 | Risk Difference (Random, 95% CI) | ‐0.08 [‐0.13, ‐0.03] |
Comparison 4. WASH hardware and education interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea prevalence – randomized controlled trials | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2 Diarrhoea incidence – controlled before‐and‐after studies | 2 | Rate Ratio (Random, 95% CI) | 0.77 [0.71, 0.84] |
Comparison 5. Case‐control studies: disposal of child faeces in latrine versus elsewhere.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea (including severe and cholera): subgrouped by age group | 23 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 All ages | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 1.2 Aged ≤ 5 years | 20 | Odds Ratio (Random, 95% CI) | 0.72 [0.61, 0.85] | |
| 2 Diarrhoea in all ages (including severe and cholera): subgrouped by country income level | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 2.1 Low | 9 | Odds Ratio (Random, 95% CI) | 0.62 [0.40, 0.96] | |
| 2.2 Lower middle | 10 | Odds Ratio (Random, 95% CI) | 0.82 [0.70, 0.96] | |
| 2.3 Upper middle | 3 | Odds Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] | |
| 2.4 High | 1 | Odds Ratio (Random, 95% CI) | 0.68 [0.48, 0.97] | |
| 3 Diarrhoea in all ages (including severe and cholera): subgrouped by type of diarrhoea | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 3.1 Persistent diarrhoea | 1 | Odds Ratio (Random, 95% CI) | 0.53 [0.17, 1.68] | |
| 3.2 Moderate‐to‐severe diarrhoea | 7 | Odds Ratio (Random, 95% CI) | 0.96 [0.83, 1.11] | |
| 3.3 Acute (possibly) bloody diarrhoea | 4 | Odds Ratio (Random, 95% CI) | 0.67 [0.56, 0.81] | |
| 3.4 Acute watery diarrhoea | 6 | Odds Ratio (Random, 95% CI) | 0.63 [0.38, 1.05] | |
| 3.5 No case definition | 5 | Odds Ratio (Random, 95% CI) | 0.54 [0.39, 0.75] | |
| 4 Diarrhoea in all ages (including severe and cholera): subgrouped by study quality | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 4.1 4 stars | 7 | Odds Ratio (Random, 95% CI) | 0.96 [0.83, 1.11] | |
| 4.2 5 stars | 7 | Odds Ratio (Random, 95% CI) | 0.65 [0.52, 0.82] | |
| 4.3 6 stars | 4 | Odds Ratio (Random, 95% CI) | 0.60 [0.35, 1.05] | |
| 4.4 ≥ 7 stars | 5 | Odds Ratio (Random, 95% CI) | 0.66 [0.51, 0.84] | |
| 5 Diarrhoea in all ages (including severe and cholera): subgrouped by setting | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 5.1 Rural | 10 | Odds Ratio (Random, 95% CI) | 0.65 [0.49, 0.87] | |
| 5.2 Urban | 10 | Odds Ratio (Random, 95% CI) | 0.74 [0.61, 0.90] | |
| 5.3 Periurban/urban and rural | 3 | Odds Ratio (Random, 95% CI) | 0.98 [0.70, 1.38] | |
| 6 Diarrhoea in all ages (including severe and cholera): subgrouped by method of data collection | 23 | Odds Ratio (Random, 95% CI) | 0.73 [0.62, 0.85] | |
| 6.1 Questionnaire | 19 | Odds Ratio (Random, 95% CI) | 0.75 [0.64, 0.89] | |
| 6.2 Observation | 2 | Odds Ratio (Random, 95% CI) | 0.48 [0.29, 0.79] | |
| 6.3 Unclear | 2 | Odds Ratio (Random, 95% CI) | 0.67 [0.48, 0.94] |
Comparison 6. Case‐control studies: defecation of children in latrine versus elsewhere.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea: case‐control studies: subgrouped by age group | 7 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 All ages | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 1.2 Aged ≤ 5 years | 5 | Odds Ratio (Random, 95% CI) | 0.54 [0.28, 1.07] | |
| 2 Diarrhoea in all ages: case‐control studies: subgrouped by country income level | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 2.1 Low | 3 | Odds Ratio (Random, 95% CI) | 0.61 [0.23, 1.60] | |
| 2.2 Lower middle | 2 | Odds Ratio (Random, 95% CI) | 0.23 [0.11, 0.48] | |
| 2.3 Upper middle | 2 | Odds Ratio (Random, 95% CI) | 0.78 [0.53, 1.14] | |
| 3 Diarrhoea in all ages: case‐control studies: subgrouped by type of diarrhoea | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 3.1 Other | 1 | Odds Ratio (Random, 95% CI) | 0.14 [0.03, 0.52] | |
| 3.2 Persistent diarrhoea | 1 | Odds Ratio (Random, 95% CI) | 0.27 [0.12, 0.60] | |
| 3.3 Acute (possibly) bloody diarrhoea | 2 | Odds Ratio (Random, 95% CI) | 0.85 [0.65, 1.12] | |
| 3.4 Acute watery diarrhoea | 3 | Odds Ratio (Random, 95% CI) | 0.58 [0.20, 1.65] | |
| 4 Diarrhoea in all ages: case‐control studies: subgrouped by study quality | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 4.1 4 stars | 2 | Odds Ratio (Random, 95% CI) | 0.13 [0.05, 0.37] | |
| 4.2 5 stars | 1 | Odds Ratio (Random, 95% CI) | 0.27 [0.12, 0.60] | |
| 4.3 6 stars | 3 | Odds Ratio (Random, 95% CI) | 0.82 [0.57, 1.17] | |
| 4.4 7 stars | 1 | Odds Ratio (Random, 95% CI) | 0.91 [0.64, 1.29] | |
| 5 Diarrhoea in all ages: case‐control studies: subgrouped by setting | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 5.1 Rural | 1 | Odds Ratio (Random, 95% CI) | 0.75 [0.29, 1.93] | |
| 5.2 Semi‐urban | 1 | Odds Ratio (Random, 95% CI) | 0.79 [0.52, 1.19] | |
| 5.3 Urban | 5 | Odds Ratio (Random, 95% CI) | 0.40 [0.17, 0.94] | |
| 6 Diarrhoea in all ages: case‐control studies: subgrouped by method of data collection | 7 | Odds Ratio (Random, 95% CI) | 0.54 [0.33, 0.90] | |
| 6.1 Questionnaire | 5 | Odds Ratio (Random, 95% CI) | 0.75 [0.50, 1.13] | |
| 6.2 Observation | 1 | Odds Ratio (Random, 95% CI) | 0.13 [0.02, 0.66] | |
| 6.3 Unclear | 1 | Odds Ratio (Random, 95% CI) | 0.27 [0.12, 0.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abalkhail 1995 KSA.
| Methods | Case‐control study | |
| Participants | Cases: children aged < 3 years admitted to 20 primary HCs for primary diagnosis of diarrhoea with infectious origin, n = 319 (after excluding 3), mean age 13.1 months, 45.3% girls. Controls: children aged < 3 years with no history of hospitalization for diarrhoeal diseases, selected randomly from the nearest residential neighbours, n = 312 (after excluding 13). mean age 19.2 months, 52.6% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea (≥ 3 soft liquid stools within 12 hours or a single soft or liquid stool with blood, pus, or mucous) | |
| Notes | Location: urban Makkah area, 20 primary HCs, Saudi Arabia Length of recruitment: 3 months (October 1994 to January 1995) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Ahmed 1993 BGD.
| Methods | CBA study | |
| Participants | Number: 370 families (after lost 17: 9 deaths and 8 left the study area) Inclusion criteria: families with a child aged < 19 months Intervention group: mean age of children 8.8 months and 51% girls. Control group: mean age 8.9 months and 56% girls |
|
| Interventions | 1 intervention site (5 contiguous villages): participatory behaviour change intervention, campaign called "Porichchhanna Jibon" (clean life). The campaign was developed in partnership with the community. The intervention involved teaching the germ theory of disease then encouraging mothers to identify their problems and to find solutions through group participation and discussion. Interventions were developed, implemented, and adopted by community.
1 control site (5 contiguous villages) where a structured observation study was taking place. |
|
| Outcomes | Diarrhoea daily prevalence and severe diarrhoea daily prevalence. Mothers were asked to recall the presence or absence of diarrhoea according to their own perceptions day‐by‐day. If diarrhoea was reported, the mother was asked if the stool was: softer than usual, 1–5 stools; watery, 1–5 stools; softer than usual, 5–10 stools; watery, 5–10 stools; > 10 watery stools per day; or dysentery. Diarrhoea was recategorized into 2 levels: any diarrhoea and severe diarrhoea (all reported watery stools and dysentery). Severe diarrhoea = all reported watery stools and dysentery. Daily prevalence = number of children sick with diarrhoea over total children observed. Anthropometry (weight for age) Awareness, understanding, and adoption of each message Cleanliness observations |
|
| Notes | Location: 10 rural villages, Bangladesh Length of study: 9 months (October 1985 to July 1986) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization, researchers chose the community for intervention as the poorer, less hygienic site. |
| Allocation concealment (selection bias) | High risk | Investigators could foresee assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified how many child days are missing in analysis. |
| Selective reporting (reporting bias) | Low risk | Report on all outcomes specified in methods. |
| Other bias | Unclear risk | _ |
| Similarity of baseline outcome measurements | High risk | There were baseline imbalances in all outcomes and the study did not adjust for it in analysis. |
| Similarity of baseline characteristics | High risk | There were baseline imbalances in crowding, mother and father education, father occupation, land and animal ownership and the study did not adjust for it in analysis. |
| Adequate allocation of intervention concealment during the study | High risk | Outcomes were not assessed blindly. |
| Adequate protection against contamination | Low risk | Unlikely that the control group received the intervention. Quote: "The intervention site was 5 km away from the control site and accessible by a 2‐hr boat ride most of the year, and by foot over narrow foot paths in about 1.5hr during the driest months." Comment: the intervention was delivered by members of the community so likely they would know participants. |
| Confounders adequately adjusted for in analysis/design | High risk | No adjustments for any confounders. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Alam 1989 BGD.
| Methods | CBA study | |
| Participants | Number: 623 children (after excluded 27 in intervention group and 50 in control group) Inclusion criteria: HHs with children aged 6–23 months, with > 6 months' observations per year |
|
| Interventions | Intervention site (3 subunits): hand pumps were provided with a ratio of 4–6 HHs (3 times more than control) + health education (main objectives: promotion of consistent and exclusive use of hand pump water, improvement of water handling and storage practices, disposal of child's faeces soon after defecation, washing hands before handling food and rubbing hands in ash or using soap after defecation). Control site (2 subunits): no project input. |
|
| Outcomes | Incidence of diarrhoea among children aged 6–23 months. Diarrhoea: ≥ 3 loose motions in 24‐hour period whether or not blood was present. An episode was considered new if there was an interval of ≥ 48 hours between symptoms (recall = 7 days). Observed sources of water, faeces visible in the yard, handwashing before food and after defecation |
|
| Notes | Location: 5 subunits (paras) in a village in rural Bangladesh Length: 3 years (July 1980 to June 1983) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation not random. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of child‐periods excluded in the analysis in both groups (54 vs 55). |
| Selective reporting (reporting bias) | Low risk | Report on outcomes prespecified in methods. |
| Other bias | Unclear risk | ‐ |
| Similarity of baseline outcome measurements | Unclear risk | No mention of baseline risk. |
| Similarity of baseline characteristics | Unclear risk | The intervention and control "populations were comparable in terms of education, HH size and sanitation conditions". but no data presented). |
| Adequate allocation of intervention concealment during the study | High risk | Quote: "Workers' knowledge of which area was intervention and control." |
| Adequate protection against contamination | High risk | Allocation by community – adjacent paras and in the control group some HHs installed hand pumps. Quote: "Over the years of the project some households in the control area purchased their own hand pumps privately." |
| Confounders adequately adjusted for in analysis/design | High risk | No analysis adjusting for confounders. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Altmann 2018 TCD.
| Methods | Cluster RCT | |
| Participants | Number: 20 HCs (1626 children aged 6 to 59 months) Inclusion criteria: all new admissions of children aged 6 to 59 months to the HCs for OTP. Routine criteria for OTP admission included children aged 6 to 59 months with a WHZ score < −3 or a MUAC < 115 mm or the presence of mild or moderate bilateral oedema, or a combination of these. |
|
| Interventions | Intervention (10 HCs, 850 children): WASH kit plus promotion, which included messaging to bury children's stool. The HH WASH kit given at admission contained a safe drinking water storage container with a lid, water disinfection consumables (180 chlorine tablets), 12 bars of soap for hand washing, a plastic cup with handle (to be reserved for the child to facilitate safe drinking water practice), and a laminated leaflet with pictures representing the main hygiene messages. They also received a promotion session on the kit use at each weekly visit to the HC and 2 extra home visits for assessing and reinforcing adherence. Promotion at HC included key messages on:
The HH WASH kit was designed to last for 3 months (2 months during treatment in the OTP and 1 month after the end of the treatment). The intervention group also received the routine OTP services (as the control group). Control (10 HCs, 776 children): routine OTP services (implemented as per the national guideline for nutrition rehabilitation) and basic hygiene education and care practice sessions during HC visits. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Tertiary outcomes:
|
|
| Notes | Location: 20 HCs, in Mondo and Mao districts, Kanem region, Chad Length of study: recruitment: April to December 2015, 6‐month follow‐up phase finished May 2016 Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly extracted one letter of the alphabet and we assigned within each pair the intervention to the HC with the first letter of its name closest to this letter." |
| Allocation concealment (selection bias) | Low risk | Participants and investigators could not foresee assignment due to random selection. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quotes: "Masking of participants was not possible because of the nature of the intervention." "It was not possible to blind research staff, but they rotated so they covered different groups." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was not possible to blind research staff, but they rotated so they covered different groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in the 2 arms. |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | Quote: "Recruitment started 1 month after allocation of each HC to either group." |
| Baseline imbalance | Low risk | Quote: "Health centers were stratified in pairs (intervention and control) according to the monthly number of SAM admissions (historic data from the year 2013) to obtain a balanced number of enrolments in the two arms." |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Analysis adjusted for clustering. |
Arvelo 2009 USA.
| Methods | Case‐control study | |
| Participants | Case LDC: LDC with a secondary attack rate of shigellosis ≥ 2% (median 5%; range 2–25%), n = 18 Control LDCs: LDC with a secondary attack rate < 2% (median 0; range 0–1.2%), n = 21 |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Daycare centre with a secondary attack rate of shigellosis (shigellosis case was defined as a person with any Shigella species isolated from stool) ≥ 2%. | |
| Notes | Location: 39 LDCs in Kansas City metropolitan area, USA Length: 2 months (October to November 2005) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Asfaha 2018 ETH.
| Methods | Case‐control study (community‐based, not‐matched) | |
| Participants | Case: 0–59 months children with diarrhoea in the preceding of 2 weeks during a house‐to‐house survey, n = 199 (0.5% non‐response) Control: 0–59 months children without diarrhoea in the preceding of 2 weeks during a house to house survey, n = 398 (0.5% non‐response) |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: having ≥ 3 loose or watery stools in a 24‐hour period, as reported by the mother/caretaker of the child. | |
| Notes | Location: Medebay Zana district, northwest Tigray, Ethiopia Length: 1.5 months (1 October 2015 to 15 November 2015) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Aziz 1990 BGD.
| Methods | CBA study | |
| Participants | Number: exact numbers not presented, on average complete data available for 405 children Inclusion criteria: HHs with children aged < 5 years |
|
| Interventions | Intervention (2 villages): 148 new hand pumps (1 pump: 30 people on average) + free maintenance, 92% of HHs received a double pit water sealed latrine, hygiene education emphasising exclusive use of the pump water for all personal and domestic use and the need for all members of the HH, including young children to use the latrines. Control (3 villages): no intervention provided. ORS was given to sick children + referral to hospital if sick. |
|
| Outcomes | Diarrhoea incidence, case definition: ≥ 3 loose motions in a 24‐hour period. Recall period 7 days, an episode was considered complete after 2 diarrhoea‐free days. Dysentery incidence, case definition: blood was present in the stools. Persistent diarrhoea incidence, case definition: episodes of duration > 14 days Days of diarrhoea Anthropometry (weight for age, height for age, weight for height) (Hasan 1989; reference is listed under Aziz 1990 BGD)) Hand pump distance and use, defecation of children or disposal of their faeces in latrine (only reported in intervention arm) |
|
| Notes | Location: 5 villages in rural Bangladesh Length: 3 years (January 1984 to December 1987) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomized allocation. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total number of children or loss to follow‐up not reported. |
| Selective reporting (reporting bias) | Low risk | Report on outcomes prespecified in methods. |
| Similarity of baseline outcome measurements | Low risk | Diarrhoea and anthropometry measures were similar at baseline. |
| Similarity of baseline characteristics | Unclear risk | Quote: "The two areas were comparable with respect to most sociodemographic and economic characteristics although the control area was slightly better off in terms of female education and socio‐economic level." Comment: however, no data presented. |
| Adequate allocation of intervention concealment during the study | High risk | Quote: "Project staff and the community under investigation knew that the aim of the study was to decrease the diarrhoea incidence." |
| Adequate protection against contamination | Low risk | The 2 areas were 5 km apart. |
| Confounders adequately adjusted for in analysis/design | High risk | No adjustments in the analysis. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 BGD.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case: children aged 0–59 months belonging to the demographic surveillance system population at the site, not currently enrolled as a case (previously enrolled and pending 60‐day visit) seeking care at HC with moderate‐to‐severe diarrhoea, n = 1374 (1.4% LTFU compared to all cases enrolled at site) Control: child with no diarrhoea in the previous 7 days, residing in demographic surveillance system area, matched to the case for age (SD 2 months for 0–11 and 12–23 months, SD 4 months for 24–59 months, not exceeding the stratum boundaries of the case), sex, residence (lives in the same or nearby village/neighbourhood as the case), and time (enrolled within 14 days of presentation of the case), n = 2428 (1.5% LTFU compared to all controls enrolled at site) |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 abnormally loose stools in the previous 24 hours. Diarrhoea episode had to be acute (onset within 7 days of study enrolment) and be a new episode (onset after ≥ 7 diarrhoea‐free days). Moderate‐to‐severe: child met ≥ 1 of the following criteria:
|
|
| Notes | Location: 1 rural sentinel HC, Mirzapur, Bangladesh Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 GMB.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 910 (11.6% LTFU), controls n = 1456 (7.2% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 5 rural sentinel HCs, Basse, The Gambia Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 IND.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 1505 (4% LTFU), controls n = 1967 (2.3% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 2 urban sentinel HCs, Kolkata, West Bengal, India Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 KEN.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 1419 (3.9% LTFU), controls n = 1841 (2.2% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 11 rural sentinel HCs, Nyanza Province, Kenya Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 MLI.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 1786 (12.1% LTFU), controls n = 1891 (8.4% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 9 urban sentinel HCs, Bamako, Mali Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 MOZ.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 602 (11.6%), controls n = 1182 (8.8% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 5 rural sentinel HCs, Manhiça, Mozambique Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baker 2016 PAK.
| Methods | Case‐control study (prospective, age‐stratified, matched) | |
| Participants | Case and control definitions were the same as Baker 2016 BGD. Cases n = 996 (20.8% LTFU), controls n = 1625 (11.6% LTFU). | |
| Interventions | Same as Baker 2016 BGD | |
| Outcomes | Same as Baker 2016 BGD | |
| Notes | Location: 7 periurban sentinel HCs, Karachi (Bin Qasim Town), Pakistan Length: 3 years (1 December 2007 to 3 March 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Baltazar 1989 PHI.
| Methods | Case‐control study | |
| Participants | Cases: children aged < 2 years brought to clinic for diarrhoea, n = 275 (after excluding 6), 68% aged < 1 year Controls: children aged < 2 years brought to clinic for ARI without diarrhoea in past 24 hours, n = 381 (after excluding 3), 73% aged < 1 year |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea (no case definition) Rectal swabs for diagnosis of diarrhoea pathogens and carried out a subgroup analysis for laboratory‐confirmed cases. |
|
| Notes | Location: 16 clinics, Cebu area (urban and rural), Phillippines Length of recruitment: 5 months (June–October 1985) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Barrios 2008 PHI.
| Methods | Cluster RCT | |
| Participants | Number: 495 respondents (enrolment rate 90%) Inclusion criteria: HHs with children aged < 5 years |
|
| Interventions | Interventions (2 barangays (smallest local government unit)): hygiene promotion programme that focused on improving hand washing and stool disposal behaviours. Midwives and barangay health workers delivered the educational sessions in small group meetings and in home visits. Activities to promote the behaviours included demonstrations of proper hand washing, a drawing activity with a brief story‐board of the negative effects of improper stool disposal. For the disposal of child faeces, caretakers were encouraged to use toilets (any type) as the final site of faeces disposal. When a toilet was not available, burying faeces ≥ 10 m away from water sources and living areas was discussed. The main message was the sanitary disposal of faeces, regardless of where a child defecated. Control intervention (2 barangays): caregivers received education on signs and symptoms of dehydration and the importance of oral rehydration during diarrhoea. Control with no contact (2 barangays): no contact, no treatment |
|
| Outcomes | Diarrhoea (measured but not reported on) Handwashing behaviour Stool disposal behaviour: observed faeces in the yard Knowledge, attitudes, beliefs on hand washing and stool disposal |
|
| Notes | Location: 6 rural barangays in Basista, Philippines Length of study: 2 months Publication status: PhD thesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment to one of three experimental conditions was achieved by a simple sample draw with replacement." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind to the intervention although 1 of the control groups had a placebo intervention, the other control to which behaviours were compared received no intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Midwives who delivered the intervention also collected data on outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details on LTFU and not reporting data on both control groups at end of study. |
| Selective reporting (reporting bias) | High risk | Collected data on diarrhoea but no results presented. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Participants were recruited once the clusters had been randomly allocated. |
| Baseline imbalance | High risk | Only 3 demographic variables presented. |
| Loss of clusters | Low risk | No mention of loss of barangays. |
| Incorrect analysis | High risk | No adjustments for clustering. |
Bassal 2016 ISR.
| Methods | Case‐control study (matched) | |
| Participants | Cases: children aged 1–5 years, living in central Israel, having diarrhoea, and a positive stool culture for Campylobacter. Cases diagnosed in the community and reported to the Israel Center for Disease Control between August 2009 and April 2010 were identified. n = 113, mean age 2.5 (SD 1.3), 40.7% girls. Control: healthy children with no history of diarrhoea 2 weeks before the interview (each case was matched by gender, age (SD 3 months), and neighbourhood (the streets surrounding the case house, in which a control was available, from Israeli Population Register), n = 113, mean age 2.5 (SD 1.3), 40.7% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Campylobacter diarrhoea: diarrhoea with positive stool culture for Campylobacter | |
| Notes | Location: Central Israel (Ashdod to Hadera) Length of recruitment: August 2009 to April 2010 Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Belizario 2015 PHI.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 341 respondents Inclusion criteria: school‐aged children (6–15 years old) and preschool aged children (2–5 years old) enrolled in public elementary schools and daycare centres and residing in the selected villages |
|
| Interventions | Intervention (150 respondents): 2 villages with CLTS + mass drug administration. Key messages delivered by the community leaders and volunteers to HHs included:
The criteria for declaring ODF status included the following:
Messages about child faeces disposal and use of toilets by children were included during the CLTS activities in the villages. Control (191 respondents): 2 villages with no CLTS + mass drug administration |
|
| Outcomes | STH prevalence (stool samples were processed using the Kato‐Katz technique) STH intensity Prevalence of Ascaris Intensity of Ascaris Prevalence of Trichuris Intensity of Trichuris Prevalence of hookworm Intensity of hookworm Weight for age Height for age BMI for age (10–15 years old) Haemoglobin status (anaemia) |
|
| Notes | Location: 4 villages in the province of Southern Leyte in Eastern Province, Philippines Length of study: 1 month (August 2013) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No random allocation to intervention/control. |
| Allocation concealment (selection bias) | High risk | No allocation concealment/NA to the study design. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | — |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing data was similar across intervention (9.6%) and control villages (11.6%). |
| Selective reporting (reporting bias) | Low risk | All important outcomes specified in methods were reported on. |
| Other bias | High risk | No adjustments for clustering. Did not measure use of sanitation facilities at the time of the study. |
| Similarity of baseline outcome measurements | High risk | NA, not relevant to design. |
| Similarity of baseline characteristics | High risk | NA, not relevant to design. |
| Adequate allocation of intervention concealment during the study | Low risk | Outcome measures were objective (STH in stool/anthropometry). |
| Adequate protection against contamination | Low risk | CLTS and non‐CLTS villages were far from one another. |
| Confounders adequately adjusted for in analysis/design | High risk | No analysis adjusting of confounders. They said the villages were selected to be similar (in number of school‐aged children and preschool‐aged children, number of HHs, presence of elementary schools and daycare centres, sources of livelihood, security, accessibility, and willingness of community leaders to collaborate). |
| Recruitment bias | Unclear risk | — |
| Baseline imbalance | Unclear risk | — |
| Loss of clusters | Unclear risk | — |
| Incorrect analysis | Unclear risk | — |
Berhe 2014 ETH.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 650 HHs (866 children aged < 5 years) (model HHs had 1% non‐response) Inclusion criteria: HHs that had ≥ 1 child aged < 5 years in 12 gotts. For model families (intervention): HHs that fully implemented the HEP. For non‐model families (control): HHs that did not fully implement the HEP. |
|
| Interventions | Intervention (327 respondents): HHs who had implemented the HEP packages fully. The HEP was implemented by full‐time female health extension workers, who trained HHs to implement packages. The packages included interventions in 4 main categories: family health services, infectious disease prevention and control, hygiene and environmental sanitation, and health education and communication. The maternal and child health package (in the family health services category) includes safe child stool disposal (the stool should be cleaned and disposed in a pit latrine, or shall be covered with a leaf or paper and be buried) (HEP 2003). Control (323 respondents): non‐model families |
|
| Outcomes | 2‐week diarrhoea prevalence (having diarrhoea in the 2 weeks prior to the interview, no additional details on case definition) WASH and nutritional behaviours including child stool disposal method |
|
| Notes | Location: 12 gotts, Tula subcity, Ethiopia Length of study: 1 month (January 2012) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random allocation to model or non‐model HHs. |
| Allocation concealment (selection bias) | High risk | Non‐random allocation to model or non‐model HHs. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 99% response rate in model HHs and 100% in non‐model HHs. |
| Selective reporting (reporting bias) | Low risk | Report on main outcomes specified in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA, no baseline |
| Similarity of baseline characteristics | Unclear risk | NA, no baseline. |
| Adequate allocation of intervention concealment during the study | Low risk | Quote: "Data collectors were blinded regarding whether each HH was model or non‐model in order to reduce interviewer bias." |
| Adequate protection against contamination | High risk | Quote: "The absence of clear demarcation between model and non‐model with reference to distance (closeness of model and non‐model) may have created information contamination as well as diarrhoeal disease transmission to the model HH members and vice versa." |
| Confounders adequately adjusted for in analysis/design | Low risk | Multivariate analysis. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Briceño 2015 TAN.
| Methods | Cluster RCT (factorial design) | |
| Participants | Number: 3619 HHs (5768 children aged < 5 years) (97.2% response rate) Inclusion criteria: HH was present during the period of listing; had been living in the village since the beginning of 2009 or earlier; and had ≥ 1 child under the age of 5 years. |
|
| Interventions | Interventions: 3 arms (TSSM only, hand‐washing promotion only, combined TSSM and HWWS)
Control (46 wards): no intervention |
|
| Outcomes | Access to an improved latrine and open defecation practice Caregiver handwashing practices Diarrhoea (7‐ and 14‐day recall): ≥ 3 loose/watery stools in a 24‐hour period or having a stool with blood or mucous Anaemia Anthropometry (weight for age, height for age, weight for height, head circumference) Abrasions, bruising, scrapes |
|
| Notes | Location: 181 rural wards, in 10 districts, Tanzania Length of study: 46 months (February 2009 to December 2012) Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details apart from "randomly assigned." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Ensured interviewers were blinded to the intervention status of each village." Comment: however, not possible to completely blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "3,619 completed interviews from 3,724 attempted (97.2% response rate)." |
| Selective reporting (reporting bias) | Low risk | Report on prespecified outcomes in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Recruited participants after their villages had received intervention/not (no baseline). |
| Baseline imbalance | High risk | No baseline |
| Loss of clusters | Low risk | 9 wards (< 10%) were reassigned and lost after they were randomized. |
| Incorrect analysis | Low risk | Quote: "Standard errors are clustered at the ward level." |
Butz 1990 USA.
| Methods | Cluster RCT | |
| Participants | Number: 114 children (aged 1 month to 7 years) attending 24 FDCHs Inclusion criteria: all children attending FDCHs Intervention group: 69% aged ≤ 36 months and 57% girls; control group: 62% aged ≤ 36 months and 42% girls. |
|
| Interventions | Intervention (12 FDCHs): instruction to daycare providers on modes of transmission of pathogens, instructions of handwashing, use of vinyl gloves and disposable nappy changing pads at each nappy change. Providers were instructed to dispose of gloves, disposable pads, and nappies in plastic bags and given supplies (gloves, nappy changing pads, hand rinse solution). Control (12 FDCHs): no education but received biweekly nurse visits for symptom data collection. |
|
| Outcomes | Diarrhoea longitudinal prevalence (diarrhoea symptom days/childcare days). Diarrhoea: occurrence of loose, unformed bowel movements at twice the normal frequency (infants: 1–2 stools per day; older children: 1 stool per day). Symptoms recorded daily Longitudinal prevalence of vomiting and runny nose Absence from daycare home (reasons for absenteeism not recorded) |
|
| Notes | Location: 24 FDCHs in urban Baltimore, USA Length of study: 12 months (4 January 1988 to 31 December 1988) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "FDCHs were randomly assigned to control or intervention group." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Daycare providers were aware that the intervention programme was being tested in certain homes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Daycare providers recorded the symptoms." Comment: daycare providers not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10.6% of missing/absent days excluded in analysis, with no information on whether they were from intervention or control FDCHs. |
| Selective reporting (reporting bias) | Low risk | Reported main outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Staff were aware of which cluster were intervention and control. |
| Baseline imbalance | Low risk | No significant baseline imbalances. |
| Loss of clusters | Low risk | Only 2 clusters lost (1 control and 1 intervention) = 8.3%. |
| Incorrect analysis | High risk | Not adjusted for clustering in analyses. |
Cameron 2013 INA.
| Methods | Cluster RCT | |
| Participants | Number: 2500 HHs at end of study Inclusion criteria: HHs with children aged < 2 years (and HH with children aged < 5 years where too few HH with aged < 2 years found) |
|
| Interventions | Intervention (80 subvillages): TSSM which included CLTS to stop open defecation, social sanitation marketing to increase availability of products and services and strengthening the enabling environment at policy and institutional levels. Control (80 subvillages): no intervention |
|
| Outcomes | Changes in perceptions of consequences of poor sanitation Sanitation improvements (toilet construction and access to improved sanitation) Open defecation practices Diarrhoea prevalence (2‐, 7‐, or 14‐day recall): ≥ 3 stools per day and the stools were loose or watery, or blood or mucous (or both) visible in stool Symptoms: nausea, vomiting, water or soft stools, mucous or blood in stool, refusal to eat, bruising, abrasion, itchy skin or scalp Intestinal parasite infections (Ascaris, Trichuris, hookworm infections) Anthropometry (stunting and wasting) Iron‐deficiency anaemia Cognitive and motor development (communication skills, mobility skills, and social‐personal skills for age) Water source Handwashing practices ARIs |
|
| Notes | Location: 160 rural subvillages, East Java, Indonesia Length of study: 30 months (August 2008 to February 2011) Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random number generator in STATA, the IE [impact evaluation] team randomly selected 10 treatment and 10 control villages in each district." |
| Allocation concealment (selection bias) | Low risk | Quote: "Once the IE team received the sub‐village lists from the district offices for all 20 villages, they told district offices which villages were in the treatment group and which were in the control group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "179 could not be contacted (86 households in the control group and 93 households in the treatment group)" (8.5% LTFU). |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes specified in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Seemed the baseline data collection occurred after assignment to intervention and control. |
| Baseline imbalance | Low risk | Quotes: "For the key outcome variables (household water and sanitation condition, as well as children's health variables), balance is achieved." "demographic and socio‐economic characteristics are also similar across treatment and control groups." |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Incorrect analysis | Low risk | In multivariate analysis adjust for clustering. |
Caruso 2019 IND.
| Methods | Cluster RCT | |
| Participants | Number: HHs in 66 villages (406 HHs with children aged < 5 years) Inclusion criteria: villages that had not been declared ODF by the Government of India, had 50–150 HHs, and minimum 60% latrine coverage. In selected villages, all HHs that owned latrines (regardless of functionality) were eligible for inclusion. |
|
| Interventions | Intervention (33 villages): "Sundara Grama," a multilevel behaviour change intervention that included the following activities.
Control (33 villages): no intervention |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Location: 66 villages in Puri district, Odisha, India Length of study: trial: 14 months (October 2017 to March 2019) Publication status: unpublished report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Village allocation was performed by a study investigator using a computer‐generated randomization sequence generated in Stata v.14." |
| Allocation concealment (selection bias) | Low risk | Randomization was done using a computer‐generated randomization sequence at the start of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the intervention, neither participants nor study investigators will be blinded to treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Due to the nature of the intervention, neither participants nor study investigators will be blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only preliminary unpublished data thus far. |
| Selective reporting (reporting bias) | Low risk | Reported on main outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | Randomization was conducted prior to baseline. However, all eligible participants were recruited so unlikely to have affected recruitment. |
| Baseline imbalance | Low risk | No baseline imbalance. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Analysis accounted for clustering. |
Chiang 2005 TWN.
| Methods | Case‐control study | |
| Participants | Cases: children aged < 5 years in Hualian County with shigellosis (confirmed by laboratory test) from hospitals and clinics. n = 46, 50% girls Controls: children aged < 5 years who visited the same hospitals/clinics ± 10 days of the cases, for vaccination (excluding those with diarrhoea symptoms or fever within 10 days of house visit/survey), matched for age group (0–1, 1–3, 3–5 years). n = 92, 41.3% girls |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Shigella: symptoms of diarrhoea, abdominal pain, fever, nausea, mucous stool, tenesmus etc, and tested positive for Group B or D Shigella | |
| Notes | Location: hospital and clinics in Hualien County, Taiwan Length of recruitment: 10 months (1 August 2001 to 31 May 2002) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Chompook 2006 THA.
| Methods | Case‐control study (matched) | |
| Participants | Cases: attended health facility with diarrhoea and shigella isolated from rectal swab, n = 139 (after 53 not enrolled: not resident, not found, moved away, died, or time‐constraints), median age 5 years, 57% girls. Controls: individuals free from diarrhoea or dysentery during the 4 weeks prior to recruitment, matched for sex and age with the cases (within 3 months for children aged < 2 years; within 6 months for children aged < 5 years; within 12 months for children aged < 16 years old; and within 5 years for people aged ≥ 16 years), randomly selected from the population list of the HC where the case resided. n = 264 (after 7 moved and 2 refused), median age 5 years, 58% girls. |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea (≥ 3 loose stools, or ≥ 1 watery, bloody, or mucoid stool in the 24 hours prior to visiting the health facility) with isolated Shigella | |
| Notes | Location: semi‐urban, Kaengkhoi District, Saraburi Province, Thailand Length of recruitment: 2 years surveillance for Shigella (2000–2002) Publication status: journal and PhD thesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Christensen 2015a KEN.
| Methods | Cluster RCT | |
| Participants | Number: 113 HHs at end of study (after 14.4% LTFU from baseline) Inclusion criteria: pregnant women in their second/third trimester and caregivers of children aged < 3 months |
|
| Interventions | Interventions (3 arms)
Control (baseline: 30 HHs, end of study: 24 HHs, 9 villages): no intervention |
|
| Outcomes | Child illness and growth (not sufficiently powered for it) Uptake of interventions:
|
|
| Notes | Location: 34 rural villages near Bungoma, Western Kenya Length of study: 6 months (November 2011 to May 2012) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each village was assigned a randomly generated number using Stata, and intervention assignments were made to villages in ascending numerical order. |
| Allocation concealment (selection bias) | Low risk | Centrally allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and some of the outcomes were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LTFU was high and different across arms. |
| Selective reporting (reporting bias) | High risk | Did not report on some of the measures collected. However, authors stated that the conclusions were not affected. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Assignment of individuals to clusters was done before randomization by having village elders define the boundaries of their village and specify in which village all potentially eligible respondents lived. |
| Baseline imbalance | Low risk | There were some significant baseline imbalances in child faeces disposal practices. The authors adjusted for baseline imbalance (presented in supplementary table 3), which did not change the conclusions. |
| Loss of clusters | Low risk | No loss of clusters |
| Incorrect analysis | Low risk | Used robust standard errors |
Christensen 2015b KEN.
| Methods | Cluster RCT | |
| Participants | Number: 323 HHs at end of study (after 12% LTFU from baseline) Inclusion criteria: caregivers of children aged 4–16 months |
|
| Interventions | Interventions (3 arms):
Control (baseline: 101 HHs, end of study: 94 HHs, 10 villages): no intervention |
|
| Outcomes | Child illness and growth (not sufficiently powered for it) Uptake of interventions:
|
|
| Notes | Location: 28 rural villages near Kakamega, in Western Kenya Length of study: 6 months (November 2011 to May 2012) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each village was assigned a randomly generated number using STATA, and intervention assignments were made to villages in ascending numerical order. |
| Allocation concealment (selection bias) | Low risk | Centrally allocated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and some of the outcomes were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LTFU was high and different across arms. |
| Selective reporting (reporting bias) | High risk | Did not report on some of the measures collected. However, authors stated that the conclusions were not affected. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Assignment of individuals to clusters was done before randomization by having village elders define the boundaries of their village and specify in which village all potentially eligible respondents lived. |
| Baseline imbalance | Low risk | There were some significant baseline imbalances in child faeces disposal practices. The authors adjusted for baseline imbalance (presented in supplementary table 3), which did not change the conclusions. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Used robust standard errors. |
Clemens 1987 BGD.
| Methods | Case‐control study (community‐based, cases and control selected from families in diarrhoea surveillance) | |
| Participants | Case families: sentinel families with diarrhoea rate 1.7 times expected rate for similar aged children during 3‐month observation, n = 45 Control families: sentinel families without any episodes of childhood diarrhoea during the 3‐month period of observation, n = 53 |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 unformed stools in any 24‐hour period during the 2‐week interval. Stipulated that a child could have a maximum of 1 episode in any 1 recall period and a new episode began only after a round without diarrhoea (or in the first round) and ended with the next diarrhoea‐free round (data collected fortnightly). | |
| Notes | Location: Dhaka slums, Bangladesh Length of recruitment: 3 months' fortnightly histories of diarrhoea + observations in sentinel families (October 1984 to January 1985) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Cummings 2012 UGA.
| Methods | Case‐control study (unmatched) | |
| Participants | Cases: people aged > 10 years who met the UMOH's outbreak case definition admitted to a cholera treatment centre in Moroto during April–June 2010; and resided in 1/15 selected villages in Nadunget, n = 99, median age 26 years, 64.6% female. Controls: people aged > 10 years who had not experienced any form of diarrhoea from April 2010 to the time of investigation, resided in 1/15 selected villages in Nadunget, n = 99, median age 33 years, 51.5% female. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Cholera: acute watery diarrhoea in an area with laboratory‐confirmed cholera cases | |
| Notes | Location: rural Karamoja subregion, north‐east Uganda Length of recruitment: 3 months (April–June 2010) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Daniels 1990 LES.
| Methods | Case‐control study (clinic‐based) | |
| Participants | Cases: children aged < 5 years who presented to the participating health facilities with diarrhoea, n = 803 (after excluding 3), 43.5% aged < 12 months, 48.8% girls. Controls: the same age range who reported with either respiratory infections or trauma, but without diarrhoea. Children also had to: be accompanied by a parent or guardian who had been responsible for the child for the previous 3 months, be living in a HH within Mohale's Hoek district, have no congenital abnormality or chronic illness, and the accompanying adult had to consent to his or her child's inclusion in the study. n = 810 (after excluding 4). 54.6% < 12 months, 52.4% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: as defined by the mother, with ≥ 3 loose or watery stools in previous 24 hours | |
| Notes | Location: 4 health facilities in rural Mohale's Hoek district, Lesotho Length of recruitment: 6 months (8 December 1987 to 6 June 1988) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Dickinson 2015 IND.
| Methods | Cluster RCT | |
| Participants | Number: 1086 HHs (baseline) (1572 children aged < 5 years), 1050 HHs (end of study) (1256 children aged < 5 years) Inclusion criteria: HHs with ≥ 1 child aged < 5 years |
|
| Interventions | Intervention (20 villages, baseline: 797 children from 534 HHs; end of study: 641 children from 529 HHs): The Bhadrak Total Sanitation Campaign promoted community‐wide latrine adoption (i.e. an end to open defecation) through a number of participatory activities. The purpose of these activities was to create a sense of disgust and shame about open defecation and a desire for an immediate village‐wide end to open defecation. The 'Walk of Shame' component consisted of a march through the village during which campaign motivators pointed out areas where people had openly defecated. The 'Fecal Calculation' component had participants estimate the volume of faeces generated by the village over a given period of time. The 'Spatial Mapping' activity had participants examine the spatial distribution of houses, open defecation hot spots, and drinking water sources to understand community exposure. These activities were meant to call attention to the level of contamination in the village and the collective nature of the problem: unless everyone stopped open defecation by using latrines, everyone would continue to be exposed to faecal matter. The goal was to induce entire villages to commit to becoming ODF by a collectively agreed‐on date. The campaign subsidized materials and labour for latrine construction for HHs eligible for government of India subsidies (i.e. below poverty line HHs). The intervention also supplied masons to guide HHs and organized sanitation marts (production centres) operated by local non‐governmental organizations in each village. Messages were also given on the benefits of latrines, both health and non‐health (convenience of time‐saving, privacy, dignity). Messages to improve child faeces disposal practices where included (according to personal communication). Control (20 villages, baseline: 775 children from 552 HHs; end of study: 615 children from 521 HHs): no intervention. |
|
| Outcomes | Diarrhoea (in past 2 weeks) MUAC Height for age Weight for age Walking time to defecation sites (women, men, children aged < 5 years) Satisfaction with sanitation facilities |
|
| Notes | Location: 40 rural villages in Bhadrak district in Orissa, India Length of study: 14 months (August 2005 to September 2006) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a public lottery. |
| Allocation concealment (selection bias) | Low risk | Participants and investigators could not foresee assignment due to lottery. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and some outcomes were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The % of attrition was similar in intervention (20%) and control (21%) groups, mostly due to children turning 5 years of age between baseline and end of study. |
| Selective reporting (reporting bias) | Low risk | Report on all outcomes listed in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Participants were recruited prior to random allocation of village to intervention. |
| Baseline imbalance | Low risk | No substantial differences in baseline characteristics were observed in general, treatment and control villages are similar, with few significant differences in observable covariates (treatment villages had lower population density, televisions, and latrines) and baseline characteristics were included in analysis. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Incorrect analysis | Low risk | Reported measures are adjusted for clustering at village level. However, it was unclear whether it was also adjusted at HH level if > than 1 child. |
Dikassa 1993 DRC.
| Methods | Case‐control study (matched) | |
| Participants | Cases: children aged < 3 years admitted to hospital and admission for primary diagnosis of diarrhoea of infectious origin, n = 107 (after excluding 6), mean age 11.9 months, 39.3% girls Controls: age‐matched children who were the nearest residential neighbours of the cases recruited for the study and who had no history of hospitalization for diarrhoeal disease, n = 107 (after excluding 6), mean age 10.5 months, 41.1% girls |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Severe diarrhoea, all cases were identified by the first author (no case definition). The severity of diarrhoea was assessed based on evident dehydration of the child requiring hospitalization. | |
| Notes | Location: 2 hospitals, urban Kinshasa, Democratic Republic of the Congo Length of recruitment: 8 months (March–November 1988) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Fisher 2011 BGD.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 107 respondents (1.8% non‐response) Inclusion criteria: caregivers of a child aged < 5 years |
|
| Interventions | Intervention (2 villages, 80 respondents): BRAC hygiene education intervention; trained field workers provided water, sanitation, and hygiene education to separate clusters of men, women, adolescents, and children at least once every 3 months. The education used pictorial flip chart with a total of 39 messages covering multiple aspects of cleanliness, clean water, and sanitation. Villagers are also encouraged to learn the '19 Messages to Remember', concerning hand washing, sanitation (included child faeces disposal in latrine), and safe water. Control (1 village, 27 respondents): no BRAC intervention. |
|
| Outcomes | Diarrhoea in previous month: ≥ 3 loose or watery stools within a 24‐hour period (WHO definition) Behaviour change: comparison between disposal of child faeces in latrine (child used latrine + faeces disposed in latrine) vs elsewhere for the last time the child defecated Knowledge and practices covered in BRAC |
|
| Notes | Location: 3 rural villages, Mymensingh District, Bangladesh Length of study: not specified Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention not allocated randomly. |
| Allocation concealment (selection bias) | High risk | No details on concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1.8% non‐response. |
| Selective reporting (reporting bias) | Low risk | Report on outcomes from methods. |
| Other bias | High risk | Small sample size with only 1 control village. |
| Similarity of baseline outcome measurements | Unclear risk | NA, no baseline. |
| Similarity of baseline characteristics | Unclear risk | NA, no baseline. |
| Adequate allocation of intervention concealment during the study | High risk | Allocation to intervention occurred prior to study and the interviews were conducted by BRAC field workers (presumably aware of allocation of intervention). |
| Adequate protection against contamination | Unclear risk | Control village was 7 km away from the other 2 villages but unclear whether it was nearby to another BRAC village. |
| Confounders adequately adjusted for in analysis/design | High risk | No analysis controlling for confounders. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Gebru 2014 ETH.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 794 respondents (96.2% response rate) Inclusion criteria: HHs with ≥ 1 child aged < 5 years in 11 randomly selected kebeles. For model families (intervention), all HHs graduated (trained) health extension programme (HEP). For non‐model families (control), all non‐graduated HHs. |
|
| Interventions | Intervention (265 respondents): health promotion and education. Female and male HH heads who had graduated as model families after being given basic training on the 16 HEP packages for 96 hours (maternal and child health package included safe child stool disposal HEP 2003). Control (529 respondents): non‐model‐families. |
|
| Outcomes | 2‐week diarrhoea prevalence (adapted WHO questionnaire but no additional details on case definition) Possible environmental and behavioural risk factors for diarrhoea, including proper vs improver child stool disposal method (no definition of proper disposal) |
|
| Notes | Location: 11 rural kebeles, Sheko district, South West Ethiopia Length of study: 1 month (31 January to 29 February 2012) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The model HHs were not allocated to the intervention at random. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 96.2% response rate. |
| Selective reporting (reporting bias) | Low risk | Report on outcomes from methods. |
| Other bias | Unclear risk | _ |
| Similarity of baseline outcome measurements | Unclear risk | NA, no baseline. |
| Similarity of baseline characteristics | Unclear risk | NA, no baseline. |
| Adequate allocation of intervention concealment during the study | Unclear risk | Allocation to intervention occurred prior to study but no mention of whether data collectors were blind to whether HH was model/non‐model. |
| Adequate protection against contamination | High risk | No specification about whether the model and non‐model HHs were in the same kebeles. |
| Confounders adequately adjusted for in analysis/design | Low risk | Analysis of diarrhoea risk factors controls for wealth, education, and handwashing. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Genthe 1997 SAF.
| Methods | Case‐control study | |
| Participants | Cases: a sample was drawn from preschool children who were brought to the day hospitals with diarrhoea, n = 169, median age 12 months, 50.6% girls. Controls: selected according to age (± 6 months) and type of water supply from the immediate neighbourhood of the case and who had not had diarrhoea during the preceding 14 days of the visit. Controls were matched for the time of occurrence of the case as well as the dates for interviews and observational studies, n = 166. median age 18 months, 47.3% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 loose or watery stools in a period of 24 hours (WHO definition) | |
| Notes | Location: 2 day hospitals, urban townships, Kliayelitsha, Cape Flats, South Africa Length of recruitment: 2 × 3‐month periods (wet and dry seasons) in 1993–1994 Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Ghosh 1994 IND.
| Methods | Case‐control study (nested in a community longitudinal study following up of children aged < 3 years with twice a week active surveillance for diarrhoea) | |
| Participants | Cases: families with a child aged < 3 years with diarrhoea, n = 105 (initially 76 but 29 controls developed diarrhoea and became a case). Controls: families with an age‐matched child aged < 3 years without diarrhoea in neighbourhood, n = 47 (initially 76 but 29 controls developed diarrhoea and became a case). |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea (no case definition), data collected twice per week | |
| Notes | Location: rural West Bengal, India Length of recruitment: 12 months Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Ghosh 1997 IND.
| Methods | Case‐control study (nested in a community longitudinal study following up of children aged < 4 years with twice a week active surveillance for diarrhoea) | |
| Participants | Cases: families with a child aged < 4 years with diarrhoea, n = 108 (initially 90 but 18 control families became cases). Controls: neighbourhood families with a study child of similar age but without diarrhoea within preceding 7 months (if control family developed diarrhoea in following 6 months it became a case family instead of a control family), n = 72 (initially 90 but 18 control families became cases). |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: passage of ≥ 3 liquid, watery mucoid stools with or without blood during the past 24 hours. For infants aged up to 3 months, an increase in the frequency and a change in the consistency of stools which was of concern to mothers. | |
| Notes | Location: 3 rural villages in West Bengal, India Length of recruitment: 24 months (July 1992 to June 1994) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Godana 2013 ETH.
| Methods | Case‐control study (community‐based, unmatched) | |
| Participants | Cases: children aged < 5 years, resident in a Derashe rural area, with a report of diarrhoea by mother or caretaker in the 2 weeks preceding the survey, n = 199 (after 5 non‐responders), 57.8% < 12 months Controls: children aged < 5 years without diarrhoea in the preceding 2 weeks, randomly chosen from the resident population in the rural kebele, n = 393 (after 15 non‐responders), 57.5% < 12 months |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: report of diarrhoea by mother or caretaker in the 2 weeks preceding survey | |
| Notes | Location: 5 rural kebeles, Derashe District, Southern Nations Nationalities and Peoples Region, Ethiopia Length of recruitment: 2 months (January and February 2012) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Haggerty 1994 DRC.
| Methods | Cluster RCT | |
| Participants | Number: 1764 (after excluding 190 children with < 9 weeks' diarrhoea morbidity data) Inclusion criteria: children aged 3–35 months |
|
| Interventions | Intervention (9 villages): education intervention to improve personal and domestic hygiene behaviour including: disposal of animal faeces; hand washing before meal preparation and after defecation/washing hands and buttocks of young children after defecation; disposal of children's faeces (emphasized digging or improving pit latrines). The messages were delivered by female community volunteers in village‐wide meetings and small group discussions. Control (9 villages): education to continue breastfeeding and give rice water during diarrhoea by community volunteers selected and trained in the same way as intervention. |
|
| Outcomes | Diarrhoea incidence, duration of diarrhoeal episodes, number of diarrhoea days. Weekly visit (7‐day recall). The mother's own definition of diarrhoea was used, employing the local word ("pulu‐pulu") to describe diarrhoea. For each day that diarrhoea occurred, the mother was asked if the child was febrile, whether there was blood in the stool and what (if any) treatment was used. A gap of ≥ 2 diarrhoea‐free days was used to define a new episode of diarrhoea. Observed hygiene practices (data not presented) Child growth (data not presented) |
|
| Notes | Location: 18 rural villages, in Bandundu province, Democratic Republic of the Congo Length of study: 14 months (October 1987 to December 1988) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following the baseline diarrhoeal and observational studies, all sites were ranked from lowest to highest according to age‐adjusted mean days of diarrhoea […] and then one in each pair was chosen at random to receive the intervention, the other to serve as a control." |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control sites also received a placebo intervention but the intervention was clearly different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 10% had < 9 complete weeks of diarrhoea data. |
| Selective reporting (reporting bias) | High risk | Did not report on behaviour change in the study although it was specified in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Low risk | Clusters were not known to be intervention or control during participant recruitment. |
| Baseline imbalance | Low risk | Matched clusters according to mean days of diarrhoea. |
| Loss of clusters | Low risk | No reported loss of clusters. |
| Incorrect analysis | Low risk | Clusters were adjusted for in analysis. |
Hashi 2017 ETH.
| Methods | Cluster RCT | |
| Participants | Number: 1199 children after excluding 25 children who had migrated Inclusion criteria: a HH was considered eligible for the study if they had ≥ 1 child aged 1–59 months living in the home and were not a model health extension HH. |
|
| Interventions | Intervention (12 subkebelles): health education and provision of soap (white bars). The health education was provided by clinical nurse professionals (field workers) and consisted of 12 sessions on key WASH messages and demonstration of HWWS. Primary caretakers were instructed to keep their water storage container clean and covered, to have a latrine and utilize it properly, and to wash their hands and children's hands ideally with soap after defecation, and before meal preparation and eating. Caregivers received the message to dispose of their children's waste properly via demonstrations and instructions. The messages were to dispose of child waste properly in the waste disposal site (in a waste container at the corner/back of the house) as opposed to the garbage (uncollected waste) and in a latrine (if they had 1) but never in the open field, garbage, or around utensils and kitchen. Control (12 subkebelles): no intervention |
|
| Outcomes | Diarrhoea incidence (diarrhoea defined as passage of ≥ 3 liquid or semi‐liquid stool or the passage of ≥ 1 liquid or semi‐liquid stool with blood or mucous) at 2‐week recall Bacteriological quality of drinking water at HH level |
|
| Notes | Location: 24 rural subkebelles, Jigjiga district, Somali region, Eastern Ethiopia Length of study: 6 months (1 February to 30 July 2015) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used lottery to allocate the 2 kebelles groups to intervention or control and then used computer‐generated numbers to pick the subkebelles within the kebelles. |
| Allocation concealment (selection bias) | Low risk | Used public lottery to allocate the kebelles but then the assignment was already known when randomly selecting the subkebelles. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind to the intervention and personnel could have inferred it. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors could have inferred intervention allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses to follow‐up in both groups. |
| Selective reporting (reporting bias) | Low risk | Reported on both prespecified outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | The subkebelles were assigned to intervention group prior to recruitment. |
| Baseline imbalance | Low risk | No apparent imbalance in baseline characteristics and analysis controlled for possible confounders. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Incorrect analysis | Low risk | Quote: "Generalized estimating equation with log link Poisson distribution family was used to compute adjusted incidence rate ratio and the corresponding 95% confidence interval of the dependent variable (longitudinal incidence of diarrhoea) and covariates." |
Heller 2003 BRA.
| Methods | Case‐control study | |
| Participants | Cases: children aged < 5 years resident in Betim area attending a HC for diarrhoea, n = 997, mean age 1.72 years, 47.1% girls Controls: children aged < 5 years resident in Betim area chosen randomly from a register (used by municipality with purpose of housing taxes), n = 999, mean age 2.63 years, 49.8% girls |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: the attendant physician diagnosis of diarrhoea was assumed as the case definition. | |
| Notes | Location: 29 HCs in urban area of Betim in Minais Gerais State in South‐East Brazil Length of recruitment: 5 months (November 1993 to April 1994) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Hoq 2016 BGD.
| Methods | Controlled cohort study | |
| Participants | Number: 2037 (total number of children registered in study period) Inclusion criteria: children aged < 2 years |
|
| Interventions | Intervention (2 wards): community‐based health project + WASH‐focused activities. Community‐based health project included:
WASH‐focused activities included:
Child faeces disposal messages (included in both intervention and control but this was done in more detail in the children's clubs and mother's group meetings):
Control (7 wards): community‐based health project activities only (same as intervention, included same child faeces disposal messages in mass awareness behaviour change communication campaign). |
|
| Outcomes | Weight for age (underweight defined as WAZ < –2) MUAC (acute malnutrition defined as MUAC < 125 mm) |
|
| Notes | Location: 9 wards in periurban area of Kurigam Municipality, Bangladesh Length of study: 3 years (November 2011 to December 2014) Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not random allocation. Out of 9 wards, chose the 2 with highest prevalence of acute malnutrition to do the WASH intervention. |
| Allocation concealment (selection bias) | High risk | No concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | — |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | — |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of LTFU. |
| Selective reporting (reporting bias) | Low risk | Report on nutritional outcomes specified in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Low risk | Prevalence in the outcomes was different. Measured rate of change in outcome so accounted for it in analysis. |
| Similarity of baseline characteristics | High risk | No baseline measures, although authors stated that the comparison site had similar ecological and demographic characteristics, childcare practices, and hygiene behaviour and sanitation coverage and then they confirmed at the end of the first year of the project. |
| Adequate allocation of intervention concealment during the study | High risk | Outcomes were assessed in centres in the wards, they would have known the allocation of the wards (same implementers). |
| Adequate protection against contamination | Unclear risk | No details of how far the intervention and control wards were. |
| Confounders adequately adjusted for in analysis/design | High risk | No confounders included in the analyses. |
| Recruitment bias | Unclear risk | — |
| Baseline imbalance | Unclear risk | — |
| Loss of clusters | Unclear risk | — |
| Incorrect analysis | Unclear risk | — |
Huda 2012 BGD.
| Methods | Controlled cohort study | |
| Participants | Number: 1699 HHs for structured observations and 1000 HHs for diarrhoea surveillance. Inclusion criteria: HH with a child aged < 5 years and a guardian of the child agreed to participate in the study. |
|
| Interventions | Intervention (50 communities): SHEWA‐B, a large‐scale hygiene promotion intervention which engaged local residents to develop their own community action plans, including targets for improvements in latrine coverage and use, access to arsenic‐free water, and improved hygiene practices. Community hygiene promoters were trained to deliver 11 key messages including "use hygienic latrine by all family members including children" and "dispose of children's faeces into hygienic latrines" using HH visits, courtyard meetings, and different activities, e.g. hygiene fairs, village theatre, group discussions in tea stalls. Promoters used flip charts and flash cards. Control (50 communities): no major water, sanitation, hygiene programme ongoing. |
|
| Outcomes | Diarrhoea prevalence. Diarrhoea: passage of ≥ 3 loose or watery stools in 24‐hour period. Monthly visits to ask about episodes of diarrhoea in previous 2 days. Acute respiratory illness Observed hygiene behaviours including child faeces disposal, considered appropriate if faeces were observed to be disposed in a toilet or in a specific pit. |
|
| Notes | Location: 100 rural villages across Bangladesh Length of study: 24 months (October 2007 to September 2009) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention not randomly allocated. |
| Allocation concealment (selection bias) | High risk | Intervention communities were allocated prior to enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of respondents not reported for the health outcomes. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes prespecified in the methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | From figure it appears the baseline diarrhoea prevalence was slightly different but no data presented. |
| Similarity of baseline characteristics | Low risk | No major differences at baseline. |
| Adequate allocation of intervention concealment during the study | High risk | Although the community monitors were not aware of the hypothesis, they were aware of allocation to intervention/control group. |
| Adequate protection against contamination | Low risk | Selected subdistricts in which (quote) "Department of Public Health Engineering of the Government of Bangladesh, who were responsible for implementing SHEWA‐B and confirmed that there was no similar intervention ongoing." |
| Confounders adequately adjusted for in analysis/design | High risk | No confounders adjusted for in analyses. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Humphrey 2019 ZIM.
| Methods | Cluster RCT | |
| Participants | Number: 5280 pregnant women in 211 clusters Inclusion criteria: women were eligible if they permanently resided in a study cluster and were confirmed pregnant. |
|
| Interventions | Intervention: 3 arms
Control (52 clusters): standard of care messages, which consisted of village health workers promoting exclusive breastfeeding to 6 months of age, advised on neonatal care, and promoted uptake of Ministry of Health and Child Care services, including antenatal care, immunizations, and family planning. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Location: rural districts of Chirumanzu and Shurugwi, Zimbabwe Length of study: 18 months' follow‐up (recruitment: November 2012 to March 2015, end of follow‐up: July 2017) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was random. |
| Allocation concealment (selection bias) | Low risk | Quote: "the final allocation was selected at a public randomization event attended by elected representatives of the study districts." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking of participants and fieldworkers was not possible because of the obvious visual differences between interventions, but investigators were blinded to treatment groups until the final analysis of each prespecified outcome." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking of participants and fieldworkers was not possible because of the obvious visual differences between interventions, but investigators were blinded to treatment groups until the final analysis of each prespecified outcome." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Reported on primary outcomes but future publications will cover additional prespecified outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | Participants were prospectively enrolled into the study once the clusters had already been randomized to intervention groups. |
| Baseline imbalance | Low risk | Most baseline characteristics of enrolled HHs were similar across groups. |
| Loss of clusters | Low risk | < 10% (only 2 clusters lost in the IYCF group) clusters LTFU. |
| Incorrect analysis | Low risk | Accounted for clustering. |
Jinadu 2007 NGR.
| Methods | Cluster RCT | |
| Participants | Number: 514 Inclusion criteria: mothers of children aged < 5 years Intervention group: 65.8% aged ≤ 12 months; control group: 65.9% aged ≤ 12 months |
|
| Interventions | Intervention (5 villages): educational intervention programme to promote the hygienic disposal of children's faeces:
Control (5 villages): no health promotion activities |
|
| Outcomes | Hygienic behaviours: child defecation pattern, HHs with sanitary latrines, HH using potties, HH where mothers HWWS after cleaning child faeces and defecation, HH with no children's faeces lying around. | |
| Notes | Location: 10 rural villages in Osun State, Nigeria Length of study: 12 months Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding and no mention of relation of interviewers in relation to trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Present outcomes prespecified in the methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Clusters were known to be intervention or control during participant recruitment (only selected participants to measure outcomes after intervention had been implemented). |
| Baseline imbalance | Unclear risk | No baseline. |
| Loss of clusters | Low risk | No reported loss of villages. |
| Incorrect analysis | High risk | No correction for clustering. |
Knight 1992 MAL.
| Methods | Case‐control study (matched) | |
| Participants | Cases: child aged 4–59 months resident in Tumpat, Malaysia, who presented at a HC with ≥ 3 loose stools in 24 hours and duration of diarrhoea < 2 weeks (and without measles, malaria, urinary tract infection, ARI, acute otitis media, or antibiotics use in the previous 2 weeks), n = 98 (after 2 left area). Controls: randomly selected from children resident in Tumpat, Malaysia, registered at a HC usually within 1 week of their respective case child, with a condition other than diarrhoea, and age (± 6 weeks for children aged < 1 year, ± 3 months for children aged 1 year, ± 6 months for children aged ≥ 2 years) and sex matched to case child and who did not have skin infection, conjunctivitis, or worm infestation as their provisional diagnosis, n = 98. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 loose stools in 24 hours | |
| Notes | Location: 5 HCs, Tumpat rural district, Malaysia Length of recruitment: 2 months (February and March 1989) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Kotch 2007 USA.
| Methods | Cluster RCT | |
| Participants | Number: 388 children Inclusion criteria: children were expected to remain assigned to the same classroom throughout the 7‐month study period and be 36 months of age at the end of data collection and that ≥ 1 family contact could participate in a telephone survey in English. Siblings were allowed to participate when they also attended the study centre and met the eligibility criteria. Intervention group: mean age of children = 21.26 months and 6.39 boys per class. Control group: mean age = 21.41 months and 3.61 boys per class. |
|
| Interventions | Intervention (23 childcare centres): staff were trained using the 'Keep It Clean' training module to improve and standardize the handwashing, sanitation, nappy changing, and food‐preparation procedures. Nappy changing, handwashing, and food‐preparation equipment with impermeable, seamless surfacing were provided. In addition, automatic faucets and foot‐activated, roll‐out waste bins for nappy disposal were provided. Control (23 childcare centres): staff were trained using the 'Keep It Clean' training module but received no equipment. |
|
| Outcomes | Severe diarrhoea incidence: any loose, watery stool that if contained would assume the shape of the container. A separate episode of diarrhoea was defined by an interval of 7 diarrhoea‐free days. Survey every 2 weeks. Number of days sick Number of days child absent for centre because of illness Number of days parents missed work because of child illness Sick days of caregivers in centres Nappy and food preparations practices |
|
| Notes | Location: 46 childcare centres in 21 counties, NC, USA Length of study: 7 months' follow‐up (December 2002 to July 2003) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details. Quote: "from each pair 1 centre was randomly selected as intervention centre." |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding specified although as the outcome was assessed by telephone by the survey research unit at UNC, it could have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 121 children LTFU from 388 children in total (31% LTFU) but the numbers were similar in intervention and control groups (59 control and 62 intervention LTFU, not significant). |
| Selective reporting (reporting bias) | Low risk | Report on prespecified outcomes in paper. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Appeared the directors recruiting the children were aware of which cluster the centre was in. |
| Baseline imbalance | High risk | Baseline imbalances in mean classroom enrolment, mean number of children participating in the study per classroom, mean number of boys enrolled in the classroom, and mean number of boys participating in the study per classroom. Because the direction of the differences, more boys and more total children in intervention classrooms and did not adjust in analysis. |
| Loss of clusters | Low risk | No loss of centres reported. |
| Incorrect analysis | Low risk | Adjusted for clustering at class level by adding random effect. |
Luby 2014 BGD.
| Methods | Controlled cohort study | |
| Participants | Number: 1000 urban HHs and 1000 rural HHs for diarrhoea surveillance, 1000 HHs for anthropometry and 1000 HHs for structured observations Inclusion criteria: HH with a child aged < 5 years and a guardian of the child agreed to participate in the study. |
|
| Interventions | Intervention: SHEWA‐B, improved from findings in Huda 2012 BGD. Changes in the intervention included a mass media campaign including radio spots across 6 regional channels from November 2011 to February 2012 encouraging HWWS before food, after defecation, and after cleaning a child and video spots on 5 television stations (November 2011 to February 2012) encouraging HWWS, using sanitary latrines for defecation and discarding child faeces and keeping latrines clean to reduce bad smells and flies. A second series of videos encouraged testing tube‐wells for arsenic and using arsenic‐free water for cooking and drinking. The intervention target population also expanded to include urban HHs. Control: no major water, sanitation, hygiene programme ongoing. |
|
| Outcomes | Diarrhoea prevalence. Diarrhoea: the passage of ≥ 3 loose or watery stools within 24‐hour period. Monthly visits to ask about episodes of diarrhoea in previous 2 days. Acute respiratory illness Anthropometry Observed hygiene and sanitation behaviours including child faeces disposal, considered appropriate if faeces were observed to be disposed in a toilet or in a specific pit. Water quality |
|
| Notes | Location: rural villages and urban slums across Bangladesh Length of study: 60 months in total (October 2007 to September 2012). This study reported from 2011 to 2012. Publication status: report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention not randomly allocated. |
| Allocation concealment (selection bias) | High risk | Intervention communities were allocated prior to enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of respondents is not reported for the health outcomes. |
| Selective reporting (reporting bias) | Low risk | Report on all outcomes prespecified in methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | In figure it looks like the baseline diarrhoea prevalence was different but no data presented. |
| Similarity of baseline characteristics | Low risk | No major differences at baseline; however, the control and intervention HHs at follow‐up were different. |
| Adequate allocation of intervention concealment during the study | High risk | Were aware of allocation to intervention/control group. |
| Adequate protection against contamination | Low risk | Selected subdistricts in which "Department of Public Health Engineering of the Government of Bangladesh, who were responsible for implementing SHEWA‐B and confirmed that there was no similar intervention ongoing." |
| Confounders adequately adjusted for in analysis/design | High risk | No confounders adjusted for in analyses. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Luby 2018 BGD.
| Methods | Cluster RCT | |
| Participants | Number: 14,425 children (7331 in year 1, 7094 in year 2) with diarrhoea data at year 1 or 2 in all arms. Children who were in utero or aged < 3 years at enrolment Inclusion criteria: children of enrolled pregnant women (index children) were eligible for inclusion if their mother was planning to live in the study village for the next 2 years, regardless of where she gave birth. Only 1 pregnant woman (in the first 2 trimesters of her pregnancy) was enrolled per compound, but if she gave birth to twins, both children were enrolled. Children aged < 3 years at enrolment and lived in the compound were included in diarrhoea measurements. |
|
| Interventions | Intervention: 6 intervention arms
Control (180 clusters, each consisting of 8 compounds): no intervention |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Tertiary outcome:
|
|
| Notes | Location: rural villages, Gazipur, Kishoreganj, Mymensingh, and Tangail districts, Bangladesh Length of study: 37 months (recruitment 31 May 2012 to 7 July 2013 followed by 2 years' follow‐up) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator by a coinvestigator at University of California, Berkeley." |
| Allocation concealment (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator by a coinvestigator at University of California, Berkeley." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Interventions included distinct visible components so neither participants nor data collectors were masked to intervention assignment, although the data collection and intervention teams were different individuals." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Interventions included distinct visible components so neither participants nor data collectors were masked to intervention assignment, although the data collection and intervention teams were different individuals." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was fairly balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Reported on primary outcomes but future publications will cover additional prespecified outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Participants were enrolled prior to knowing allocation of intervention. |
| Baseline imbalance | Low risk | Baseline characteristics of enrolled HHs were similar across group. |
| Loss of clusters | Low risk | No reported loss of cluster. |
| Incorrect analysis | Low risk | Low, accounted for clustering. |
Mathew 2004 ZIM.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 115 respondents Inclusion criteria: no details |
|
| Interventions | Intervention (2 villages): CHCs: structured weekly course of participatory health education classes. 15 health topics covered using PHAST techniques, within the hygiene lesson cover disposal of toddler's faeces in a latrine. Control (2 villages): no CHCs |
|
| Outcomes | Knowledge of risks and practices including: percentage of children aged < 5 years present at the time of observations not using a latrine. | |
| Notes | Location: 4 rural villages, Bikita district, Zimbabwe Length of study: not specified Publication status: PhD thesis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention not randomly allocated. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Non‐response data not reported. |
| Selective reporting (reporting bias) | Unclear risk | Tool for observations not available. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA, not relevant to design. |
| Similarity of baseline characteristics | Unclear risk | NA, not relevant to design. |
| Adequate allocation of intervention concealment during the study | High risk | No blinding. |
| Adequate protection against contamination | Unclear risk | No details about distance or possibility for contamination. |
| Confounders adequately adjusted for in analysis/design | High risk | No adjustments for any confounders. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Maung 1992a MYA.
| Methods | Case‐control study | |
| Participants | Cases: children aged 1–59 months admitted to paediatric wards of the North Okkalapa General Hospital, or presented at the urban HC or at the emergency department of the North Okkalapa General Hospital, for persistent diarrhoea and PEM, n = 67. Controls: age‐ and sex‐matched apparently healthy children within the neighbourhood of the case children (usually within the same street, selected from houses with structural appearances similar to that of the cases). The control children had no diarrhoea or PEM in the last 2 months, n = 67. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Persistent diarrhoea: passage of watery or loose stools (with or without mucous) > 3 times/day on most days lasting ≥ 14 days during the last 2 months, with an interval of ≤ 6 days during which loose motions were < 3 times/day. PEM: children with kwashiorkor, marasmic kwashiorkor or marasmus, or children with weight‐for‐age < 2 SD below the median National Centre for Health Statistics reference. |
|
| Notes | Location: town hospital and urban HC, Yangon region, Myanmar Length of recruitment: not specified Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Maung 1992b MYA.
| Methods | Case‐control study | |
| Participants | Cases: children aged 1–59 months admitted to paediatric wards of the North Okkalapa General Hospital, or presented at the urban HC or at the emergency department of the North Okkalapa General Hospital, for persistent diarrhoea and PEM, n = 67. Controls: age‐ and sex‐matched apparently healthy children within the neighbourhood of the case children (usually within the same street, selected from houses with structural appearances similar to that of the cases). The control children had no diarrhoea or PEM in the last 2 months, n = 67. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Persistent diarrhoea: passage of watery or loose stools (with or without mucous) > 3 times/day on most days lasting ≥ 14 days during the last 2 months, with an interval of not more than 6 days during which loose motions were < 3 times/day PEM: children with kwashiorkor, marasmic kwashiorkor or marasmus, or children with weight‐for‐age < 2 SD below the median National Centre for Health Statistics reference |
|
| Notes | Location: town hospital and urban HC, Yangon region, Myanmar Length of recruitment: not specified Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Mediratta 2010a ETH.
| Methods | Case‐control study (clinic based) | |
| Participants | Cases: children aged < 5 years with acute diarrhoea were consecutively enrolled from the outpatient department and inpatient paediatric ward, n = 220, mean age 1.57 years, 35% girls Controls: selected from children with other conditions who did not present with acute diarrhoea for ≥ 14 days before the date of interview. Match the cases with 1:1 ratio for age (within 6 months), sex, within 2 weeks from the date of the case visit and the same ward, n = 220, mean age 1.51 years, 35% girls |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 liquid stools within a 24‐hour period Acute diarrhoea: having diarrhoea for < 14 days |
|
| Notes | Location: University of Gondar Referral and Teaching Hospital in the North Gondar Zone, Ethiopia Length of recruitment: 6 months (July 2007 to January 2008) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Mediratta 2010b ETH.
| Methods | Case‐control study (clinic based) | |
| Participants | Cases: children aged < 5 years with acute diarrhoea were consecutively enrolled from the outpatient department and inpatient paediatric ward, n = 220, mean age 1.57 years , 35% girls Controls: selected from children with other conditions who did not present with acute diarrhoea for ≥ 14 days before the date of interview. Match the cases with 1:1 ratio for age (within 6 months), sex, within 2 weeks from the date of the case visit and the same ward, n = 220. mean age 1.51 years, 35% girls |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 liquid stools within a 24‐hour period Acute diarrhoea: having diarrhoea for < 14 days |
|
| Notes | Location: University of Gondar Referral and Teaching Hospital in the North Gondar Zone, Ethiopia Length of recruitment: 6 months (July 2007 to January 2008) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Menon 1990 USA.
| Methods | Case‐control study | |
| Participants | Cases: Apache children aged < 2 years residing on the White Mountain reservation, seen at the Whiteriver Indian Hospital with rotavirus diarrhoea, n = 45 (after 1 refused, 27 respondents were not available and 5 cases were dropped as had no matched control). Hospital controls: children aged < 2 years residing on the White Mountain reservation, matched for sex and age within 2 months, chosen from outpatient and inpatient records for a variety of other non‐diarrhoeal illnesses, and visited the hospital within 2 weeks of the date of diagnosis of the case, n = 45. Neighbourhood controls: children aged < 2 years within same age group, same sex, and neighbourhood (area served by same water supply system), n = 24. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Rotavirus diarrhoea: ≥ 3 loose or watery stools during the previous 24 hours which tested positive (2+) for rotavirus antigen using the enzyme‐linked immunosorbent assay. | |
| Notes | Location: 1 hospital on White Mountain reservation in east‐central Arizona, USA Length of recruitment: 7 months (1 May to 15 December 1985) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Mertens 1992 SRI.
| Methods | Case‐control study | |
| Participants | Cases: all children aged < 5 years presenting with diarrhoea to 1 of 5 hospitals, n = 2458 (only visited 1415), mean age 20.6 months, 45.6% girls. Hospital controls: children with a control disease, frequency matched for age with the cases (within a range of 5 months), n = 4140 (only visited HH of 2279), mean age 23.3 months, 48.8% girls. Community controls: a random sample of children aged < 5 years was recruited from the community in the catchment areas of the hospitals, using multistage sampling, and applying the same exclusion criteria as the clinic controls, n = 1659, mean age 25.8 months, 47.6% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea defined as ≥ 3 loose or watery stools in the previous 24 hours, or as stools with blood or mucous | |
| Notes | Location: 5 rural hospitals and community, district of Kurunegala, Sri Lanka Length of recruitment: 14 months (January 1987 to March 1988) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Nair 2017 IND.
| Methods | Cluster RCT | |
| Participants | Number: 2633 children found at 18 months Inclusion criteria: pregnant women in their third trimester. Their children were followed up to 18 months of age. Exclusion criteria: stillbirths and neonatal deaths; infants whose mothers died; those with congenital abnormalities, multiple births, and mother and infant pairs who migrated out of the study area permanently during the trial period. |
|
| Interventions | Intervention (60 clusters, each consisted of a geographical cluster with a population of around 1000 people each to approximate the catchment area of an Anganwadi worker):
In addition, 5 participatory meetings were held in both the intervention and control arm, with village health sanitation and nutrition committees to strengthen the capacity of the committees to assess community health needs, prepare and implement village health plans, and monitor the provision of local health and nutrition services. Control (60 clusters): only the participatory meetings with the village health sanitation and nutrition committees. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Location: 120 clusters, West Singhbhum and Kendujhar, 2 adjoining rural districts of Jharkhand and Odisha in eastern India Length of study: 27 months (randomization: July 2013; recruitment and data collection: 1 October 2013 to 31 December 2015) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clusters were randomly allocated to treatment or control using lottery method. |
| Allocation concealment (selection bias) | Low risk | Clusters were randomly allocated to treatment or control using lottery method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and the intervention team were not blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the data collection team and data manager were masked to allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data similar across arms. |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes in methods. Although the outcomes were changed in the trial registration this was prior to data collection. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | Clusters were randomized prior to recruitment of participants. |
| Baseline imbalance | Low risk | No baseline imbalance. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Adjusted for clustering using random effects. |
Nanan 2003 PAK.
| Methods | Case‐control study (clinic based) | |
| Participants | Cases: children aged 4– 71 months with diarrhoea (episode‐based) that attended the recruitment centres during the study period, had been resident in the same village for the previous 2 weeks, and were accompanied by a parent or guardian who was willing to participate in the study, n = 454 (after excluding 54), 63% aged < 24 months, 45% girls. Controls: children aged 4–71 months with any complaint other than diarrhoea and without a skin condition or worm infestation that attended the recruitment centres during the study period, had been resident in the same village for the previous 2 weeks, and were accompanied by a parent or guardian who was willing to participate in the study, frequency matched on the HC of recruitment and time of diagnosis (selected within 24 hours of a case), n = 349 (after excluding 125), 49% aged < 24 months, 38% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: ≥ 3 loose, watery stools in the last 24 hours | |
| Notes | Location: 6 Aga Khan Health services, Pakistan (AKHS,P) centres, Ghizer and Gilgit districts, Pakistan Length of recruitment: 2 months (July–September 2001) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Null 2018 KEN.
| Methods | Cluster RCT | |
| Participants | Number: 6494 children with diarrhoea data at year 1 or 2 in all arms. Children who were in utero or aged < 3 years at enrolment Inclusion criteria: children of enrolled pregnant women (index children) were eligible for inclusion if their mother was planning to live in the study village for the next 2 years, regardless of where she gave birth. Only 1 pregnant woman (in the first 2 trimesters of her pregnancy) was enrolled per compound, but if she gave birth to twins, both children were enrolled. Children aged < 3 years at enrolment and lived in the compound were included in diarrhoea measurements. |
|
| Interventions | Intervention : 6 intervention arms
Control (158 clusters): no intervention, monthly visits by community‐based health promoter to measure the child's MUAC. Passive control (80 clusters): no activity apart from data collection. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Tertiary outcome:
|
|
| Notes | Location: rural villages in Bungoma, Kakamega, and Vihiga counties in Kenya's western region Length of study: 42 months (recruitment: 27 November 2012 to 21 May 2014 with 2 years' follow‐up) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator with reproducible seed at the University of California, Berkeley." |
| Allocation concealment (selection bias) | Low risk | Quote: "Clusters were randomly allocated to treatment using a random number generator with reproducible seed at the University of California, Berkeley." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Blinding of participants was not possible. Participants were informed of their treatment assignment after baseline data collection and might have known the treatment assignment of nearby villages." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The health promoters and staff who delivered the interventions were not involved in data collection, but the data collection team could have inferred treatment status if they saw intervention materials in study communities." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up fairly balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Reported on primary outcomes but future publications will cover additional prespecified outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Participants were enrolled prior to knowing allocation of intervention. |
| Baseline imbalance | Low risk | Baseline characteristics of enrolled HHs were similar across groups. |
| Loss of clusters | Low risk | No reported loss of cluster. |
| Incorrect analysis | Low risk | Accounted for clustering in analysis. |
Oguro 2016 MYA.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 188 respondents Inclusion criteria: aged 15–49 years, living in the experimental or control villages, ≥ 1 child aged ≤ 5 years, able to communicate in the Myanmar language, and no serious mental illness. |
|
| Interventions | Intervention (2 villages): WVGs were established by organizing women and training them using a participatory approach. The activities of the WVGs after 3 years of being established included:
Control (2 villages): no WVGs |
|
| Outcomes | Appropriate disposal of child stool (flushed in latrine) vs inappropriate (left in the open, thrown in garbage) Any antenatal care Knowledge of danger signs Knowledge of modern contraceptive methods Acceptable first aid Knowledge of malaria prevention |
|
| Notes | Location: 4 villages in Meiktila Township, Mandalay Division Length of study: 2 months (February–March 2007) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No random allocation. |
| Allocation concealment (selection bias) | High risk | No allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all respondents reported on child faeces disposal and it was unclear why they were missing. |
| Selective reporting (reporting bias) | Unclear risk | Authors did not specify what the main outcomes were. They stated there were 102 questions in questionnaire but only presented 6 outcome measures. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA, not relevant to design. |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | Outcomes were not objective but it was unclear whether the data collectors were blinded to the allocation of intervention. |
| Adequate protection against contamination | Unclear risk | No details of how far the villages were to one another. |
| Confounders adequately adjusted for in analysis/design | Low risk | Adjusted for wealth in the logistic regression. |
| Recruitment bias | Unclear risk | — |
| Baseline imbalance | Unclear risk | — |
| Loss of clusters | Unclear risk | — |
| Incorrect analysis | Unclear risk | — |
Oketcho 2012 TAN.
| Methods | Case‐control study (clinic‐based) | |
| Participants | Cases: children aged 6–60 months admitted to the paediatric infectious diseases ward and the caretaker reported increase in the stool fluidity and frequency of passing stool for ≥ 2 days, n = 151. Controls: children aged 6–60 months admitted to the ward for management of non‐infectious diseases, without diarrhoea within the previous 2 weeks. All children meeting the case and control criteria admitted at the same time of the same age group and residing in Morogoro region were included in the study, n = 152. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Diarrhoea: caretaker reported increase in the stool fluidity and frequency of passing stool for ≥ 2 days | |
| Notes | Location: urban, Morogoro Regional Hospital, Tanzania Length of recruitment: 8 months (January–September 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Park 2016 INA.
| Methods | CBA study | |
| Participants | Number: 99 children Inclusion criteria: children aged 3–13 years Mean age: intervention group 7.1 (SD 3.2, range 3–13) years; control group 8.4 (SD 3, range 4–13). Intervention group 54% girls; control group 65.7% girls |
|
| Interventions | Intervention site (1 village, 50 children): Budi's Amphibious Latrine (BALatrines) (simple squat latrines with a septic tank or pit) were constructed and all residents were given health education regarding hygiene, sanitation, and prevention of STH infections. The health education included many messages about preventing soil‐transmitted helminthiases: appropriate hand washing; boiling water before home use; not drinking river water; peeling fruit; cooking vegetables; avoiding street food; not defecating in waterways, paddy fields, or gardens; keeping domestic animals in cages not close to waterways; etc. Regarding latrines, the messages mentioned that they should be ≥ 10 m away from wells, and that it is best for them to flush and cover after use. Messages about recognizing signs and symptoms of soil‐transmitted helminthiases were also included. For mothers of small children, the messages included not disposing of used nappies in the garden or bush or in waterways. 2 messages directed specifically at children were that they should stay away from any faeces they might find around their home, and that they should report any symptoms (diarrhoea, fever, etc.) to a parent or teacher. All children who were found to have STH infection at a baseline were treated with albendazole 400 mg. Control site (1 village, 49 children): no intervention. All children who were found to have STH infection at a baseline were treated with albendazole 400 mg. |
|
| Outcomes | STH infection (presence of helminth eggs in stool, diagnosed using Impankaew method (simple faecal flotation)) | |
| Notes | Location: 2 villages in the Gunungpati subdistrict, Semarang, Central Java, Indonesia Length: 8 months (no specific dates) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No random allocation. |
| Allocation concealment (selection bias) | High risk | No concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It seemed there was no LTFU. However, there was no information about how many non‐respondents were at baseline/recruitment. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes outlined in the methods. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Low risk | Prevalence of STH infection was statistically not different at baseline. |
| Similarity of baseline characteristics | Unclear risk | No baseline characteristics apart from child age and sex. However, sanitation coverage and other measures would be important. |
| Adequate allocation of intervention concealment during the study | Low risk | Outcome measures were objective (STH in stool). |
| Adequate protection against contamination | Low risk | Quote: "Although they are in the same sub‐district, the two villages are not in close proximity to each other." |
| Confounders adequately adjusted for in analysis/design | High risk | No confounders adjusted for. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Patil 2014 IND.
| Methods | Cluster RCT | |
| Participants | Number: 3039 HHs (5209 children aged < 5 years) (after 15.3% LTFU) Inclusion criteria: HH with ≥ 1 child aged < 24 months at enrolment. For follow‐up, the HH had to have ≥ 1 child aged 21–45 months and were living in the village at the time of baseline. Mean age: intervention group 21.9 months; control group 22.1 months |
|
| Interventions | Intervention (40 villages): India Total Sanitation Campaign (subsidies and promotion of individual HH latrines) and Nirmal Vatika (additional subsidies) and support from WSP through TSSM project, which included creation of enabling environment + capacity building to implement CLTS‐based behaviour change methods. Control (40 villages): no intervention. |
|
| Outcomes | Toilet coverage, defecation behaviours (including daily open defecation by children (aged < 5 years), hygienic child faeces disposal) Diarrhoea: ≥ 3 loose or watery stools in 24 hours or a single stool with blood/mucous. 7‐day recall in questionnaire at baseline and at end of study. Highly credible gastrointestinal illness Acute lower respiratory illness Bruising/abrasions and itchy skin/scalp (negative control outcomes) Anthropometry (weight for age, height for age, weight for height, MUAC) Anaemia Water quality Child stool parasitology (including helminth present in stool,Ascaris lumbricoides present in stool) |
|
| Notes | Location: 80 rural villages in 2 neighbouring districts in Madhya Pradesh, India Length of study: 23 months (25 May 2009 to 25 April 2011) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used public lottery to assign villages to arms. |
| Allocation concealment (selection bias) | Low risk | Used public lottery to assign villages to arms. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants possible but outcomes were self‐reported so could have been affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Field interviewers were not informed of group assignment, but it was possible for them to identify intervention villages during interviews of Block officers or the village secretary." Comment: incomplete blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was not differential by randomized group and no missing values for main outcomes. |
| Selective reporting (reporting bias) | Low risk | Report on main outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | Follow‐up data which were the data used for analysis were measured in newly recruited HHs that belonged to either intervention or control arms. |
| Baseline imbalance | Low risk | No major imbalance and the analysis adjusted for the 3 characteristics that had slight imbalance between groups. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Adjusted for clustering in the analyses. |
Pickering 2015 MLI.
| Methods | Cluster RCT | |
| Participants | Number: 6319 children aged < 5 years at end of study (4031 HHs) (after 11.1% LTFU) Inclusion criteria: HHs with ≥ 1 child aged < 10 years |
|
| Interventions | Interventions (60 villages, 2365 HHs): CLTS which used participatory methods to eliminate the practice of open defecation in rural HHs and promote building of toilets. No hardware or subsidies was provided to HHs. Control (61 villages, 2167 HHs ): no intervention |
|
| Outcomes | Diarrhoea (2‐day and 2‐week prevalence): ≥ 3 loose or watery stools per 24 hours Symptoms: loose stool by chart, blood in stool, vomit, fever, cough, congestion, difficulty breathing, earache, and bruising (negative controls) Anthropometry (height for age, weight for age) Self‐reported all‐cause and cause‐specific mortality Sanitation access and defecation behaviours (including open defecation by children and use of potty) Drinking water quality Hand hygiene |
|
| Notes | Location: 121 villages in Koulikoro district, Mali Length of study: 24 months (April 2011 to May 2013) Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One of the study investigators (MLA) used a computer‐generated algorithm that randomly assigned villages (1:1) to treatment and control groups." |
| Allocation concealment (selection bias) | Low risk | Quote: "The algorithm generated a random number for each village, which was then used to sort villages and assigned the first 60 to the intervention group and the remaining 61 to the control group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Masking of participants was not possible because of the nature of the intervention." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Field staff were not informed of village treatment status, but could have inferred this during the follow‐up from the presence of signage showing village certification of an open defecation free status." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentage LTFU (11.8% of HHs in control group and 10.4% in intervention group). |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in methods were reported. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Low risk | The participants were unaware whether they were randomized to CLTS or control villages. |
| Baseline imbalance | Low risk | No substantial differences in baseline characteristics were observed. Quote: "access to sanitation and an improved water source were similar across groups. Baseline diarrhoeal and respiratory illness symptoms were at higher prevalence in villages assigned to the CLTS intervention." |
| Loss of clusters | Low risk | No loss of villages reported. |
| Incorrect analysis | Low risk | In the analysis used (quote) "robust standard errors (the Huber‐White Sandwich estimator) to account for correlated outcomes at the village level." |
Sarrassat 2018 BUR.
| Methods | Cluster RCT | |
| Participants | Number: pregnancy histories were completed for 102,684 women at end of study. At baseline, 5043 mothers completed the behavioural questionnaire and 5670 mothers at end of study. Inclusion criteria:
|
|
| Interventions | Intervention (7 geographical areas): mass radio campaign targeted at women of reproductive age and caregivers of children aged < 5 years, on 17 childcare behaviours, including safe child faeces disposal. The radio campaign included short broadcasts (1‐minute duration, broadcast approximately 10 times per day) and interactive long‐format programmes (2‐hour duration, broadcast 5 days per week, followed by phone‐ins to allow listeners to comment). All materials were produced in the predominant local languages of each intervention cluster. Behaviours covered by broadcasts changed weekly. The long‐format programme covered 2 behaviours per day and changed daily. Safe child stool disposal was covered in 3 weeks of broadcasts and 94 long‐format modules. Control (7 geographical areas): no radio campaign |
|
| Outcomes | Primary outcome:
Secondary outcome:
Intermediate outcomes:
|
|
| Notes | Location: 14 distinct geographical areas centred around a community FM radio station across Burkina Faso. Each clusters included about 40,000 inhabitants. Length of study: December 2011 to March 2015. Baseline (December 2011 to February 2012); end of study (November 2014 to March 2015). Intervention ran from March 2012 to January 2015. Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Participants and investigators could not foresee assignment due to random allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The nature of the intervention precluded formal masking of respondents and interviewers." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The nature of the intervention precluded formal masking of respondents and interviewers." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No difference across arms. |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | High risk | Quote: "Randomisation was done before baseline survey." |
| Baseline imbalance | Low risk | Quote: "Pair matched randomisation based on geography and radio listenership" and then analysis included adjusting for a confounder score. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Conducted cluster‐level analyses. |
Sinharoy 2017 RWA.
| Methods | Cluster RCT | |
| Participants | Number: 10,793 children aged < 5 years at end of study (7934 HHs) (after 18.6% of children < 5 years LTFU) Inclusion criteria: all HHs with a child aged < 5 years in the study area |
|
| Interventions | Interventions, 2 arms testing 2 different versions of the CBEHPP, which used the CHC approach to promote healthy practices.
Both the lite and classic intervention included messages on child sanitation under the topic of sanitation (zero open defecation). The participants were mainly recommended the following:
Control (50 villages), baseline: 2948 HHs (4523 children aged < 5 years); end of study: 2723 HHs (3782 children aged < 5 years): no intervention |
|
| Outcomes | Diarrhoea (7‐day recall) Height‐for‐age or LAZ score WHZ or weight‐for‐length Z score Colony‐forming units of thermotolerant (faecal) coliforms per 100 mL water Intermediary outcomes:
|
|
| Notes | Location: 150 villages in Rusizi district, Western Rwanda Length of study: 32 months (May 2013 to December 2015) Publication status: published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We assessed villages for eligibility then randomly selected 150 [villages] for the study using a simple random sampling routine in STATA. We stratified villages by wealth index and by the proportion of children younger than 2 years with caregiver‐reported diarrhoea within the past 7 days. We randomly allocated these villages to three study groups: no intervention (control; n = 50), eight community health club sessions (Lite intervention; n = 50), or 20 community health club sessions (Classic intervention; n = 50)." |
| Allocation concealment (selection bias) | Low risk | Quote: "used Stata to randomly order the villages and divide them into three groups with approximately the same number of villages in each group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and some outcomes were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No difference in attrition between intervention groups." |
| Selective reporting (reporting bias) | Low risk | Report on outcomes specified in methods apart from clinical data for diarrhoea and malaria and data for infant and child mortality, but authors stated that these outcomes will be reported elsewhere. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | — |
| Similarity of baseline characteristics | Unclear risk | — |
| Adequate allocation of intervention concealment during the study | Unclear risk | — |
| Adequate protection against contamination | Unclear risk | — |
| Confounders adequately adjusted for in analysis/design | Unclear risk | — |
| Recruitment bias | Low risk | Conducted baseline first then allocated villages to intervention arms. |
| Baseline imbalance | Low risk | Conducted stratification on average fraction of children aged < 2 years with caregiver‐reported diarrhoea in the previous 7 days; and mean wealth index. |
| Loss of clusters | Low risk | No loss of clusters. |
| Incorrect analysis | Low risk | Used generalized estimating equations to account for village‐level clustering. |
Stanton 1987 BGD.
| Methods | Cluster RCT | |
| Participants | Number: 1923 families, 1350 with children aged < 6 years (after 0.8% emigrated) Inclusion criteria: families with children aged < 6 years |
|
| Interventions | Intervention (25 slums): educational intervention emphasizing 3 messages: proper hand washing before food preparation, defecation away from the house and in a proper site, and suitable disposal of waste and faeces. The intervention was delivered in the community over 8 weeks through small group discussions, larger demonstrations, community wide planning and action meeting, posters, games, pictorial stories, flexi flans (flannel board with movable characters). Control (26 slums): community health workers continued to provide the primary healthcare services. |
|
| Outcomes | Diarrhoea incidence in 6 months following intervention and 1 year following intervention. Diarrhoea: ≥ 3 unformed stools in any 24‐hour period during the 2‐week interval. stipulated that a child could have a maximum of 1 episode in any 1 recall period, and that a new episode began only after a round without diarrhoea (or in the first round) and ended with the next diarrhoea‐free round. Nutritional status (weight for age, height for age, weight for height) (Stanton 1988) Hygiene behaviour change: hand washing before serving food, child defecate in living area, garbage and faeces seen in living area, child observed to put garbage in mouth. |
|
| Notes | Location: Dhaka slums, Bangladesh Length of study: 18 months (October 1984 to March 1986). Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random number table. |
| Allocation concealment (selection bias) | Unclear risk | No detail on how allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This study was not performed in a double‐blinded fashion." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This study was not performed in a double‐blinded fashion." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Similar attrition in both groups. Quote: "equivalent percentages of intervention and control communities immigrated (19% in intervention vs. 23% in control) or emigrated (38% in intervention vs. 37% in control)" but unclear number of children who provided full histories of diarrhoea. |
| Selective reporting (reporting bias) | Low risk | Report on all outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Low risk | Participants were recruited in clusters prior to randomization. |
| Baseline imbalance | Low risk | Similar baseline characteristics and matched at design stage. Quote: "grouped the ordered communities into 25 adjacent pairs and one remaining community…within each stratum (pair), one community was assigned to intervention and one to control." |
| Loss of clusters | Unclear risk | No mention of loss of clusters, although did not present the single control slum that was not matched. |
| Incorrect analysis | High risk | Although reported on analysis using cluster as individuals, did not present data and quote unadjusted data as final. |
Strina 2012 BRA.
| Methods | Case‐control study (clinic‐based) | |
| Participants | Cases: children (aged < 10 years) presenting with diarrhoea as a main complaint in 5 health facilities of Salvador and tested positive for rotavirus in stool sample, n = 390, 39.0% < 12 months, 43.3% girls. Controls: children without diarrhoea selected from children attending the same health facilities, at well‐baby consultations or because of other health problems not related to diarrhoea, such as orthopaedic procedures or evaluation before a surgical operation. Controls were frequency matched to cases by age and health insurance, n = 1674, 31.2% < 12 months, 47.5% girls. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Rotavirus diarrhoea: children with diarrhoea who tested positive for rotavirus in stool | |
| Notes | Location: urban, 5 health facilities, Salvador, Brazil Length of recruitment: 21 months (November 2002 to August 2004) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Traoré 1994a BUR.
| Methods | Case‐control study | |
| Participants | Cases: children aged ≤ 36 months, resident in Bobo‐Dioulasso and admitted to hospital at Sanou Souro Hospital during the period of the study, with symptoms which included diarrhoea or dysentery, or both, as reported by the mother, n = 757 (1056 cases in total but 28% LTFU), 49% < 12 months, 45% girls Hospital controls: any child aged ≤ 36 months, resident in Bobo‐Dioulasso and admitted to hospital at Sanou Souro Hospital during the period of the study without symptoms of diarrhoea or dysentery, n = 631 (72% follow‐up), 40% < 12 months, 46% girls Neighbourhood controls: these were neighbours of children admitted to hospital with symptoms of diarrhoea or dysentery, or both, matched for age group, n = 1405, 47% < 12 months, 53% girls |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: as reported by mother and examined by a doctor; dysentery: bloody or mucoid stools | |
| Notes | Location: urban Bobo‐Dioulasso, Burkina Faso Length of recruitment: 2.5 months (15 January 1990 to 31 March 1991) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Traoré 1994b BUR.
| Methods | Case‐control study | |
| Participants | Cases: children aged ≤ 36 months, resident in Bobo‐Dioulasso and admitted to hospital at Sanou Souro Hospital during the period of the study, with symptoms which included diarrhoea or dysentery, or both, as reported by the mother, n = 757 (1056 cases in total but 28% LTFU), 49% < 12 months, 45% girls. Hospital controls: any child aged ≤ 36 months, resident in Bobo‐Dioulasso and admitted to hospital at Sanou Souro Hospital during the period of the study without symptoms of diarrhoea or dysentery, n = 631 (72% follow‐up), 40% < 12 months, 46% girls. Neighbourhood controls: these were neighbours of children admitted to hospital with symptoms of diarrhoea or dysentery, or both, matched for age group, n = 1405, 47% < 12 months, 53% girls. |
|
| Interventions | Risk factors of interest:
|
|
| Outcomes | Diarrhoea: as reported by mother and examined by a doctor; dysentery: bloody or mucoid stools | |
| Notes | Location: urban Bobo‐Dioulasso, Burkina Faso Length of recruitment: 2.5 months (15 January 1990 to 31 March 1991) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Waterkeyn 2005 ZIM.
| Methods | Controlled cross‐sectional study | |
| Participants | Number: 908 respondents Inclusion criteria: intervention survey respondents had to be members of health clubs, control group respondents came from areas with no health clubs matched with regard to demography, cultural practices, levels of sanitation and water coverage. |
|
| Interventions | Intervention (382 respondents from Makoni and 354 from Tsholotsho): CHCs – structured weekly course of participatory health education classes. The training materials used for health promotion consisted of 14 sets of illustrated cards. The different topics were reflected in a ‘membership card' which provided an outline of the syllabus: 1. mapping of village, 2 disease identification, 3. balanced diet, 4. nutrition plans, 5. Diarrhoea, 6. salt sugar solution, 7. home hygiene, 8. water sources, 9. drinking water, 10. water storage, 11. hand washing, 12. bilharzia, 13. skin and eye diseases, 14. worms, 15. sanitation ladder, 16. sanitation story, 17. malaria, 18. respiratory diseases, 19. tuberculosis, and 20. AIDs and STDs. Within the hygiene lesson cover: disposal of toddler's faeces in a latrine. Control (113 respondents from Makoni and 59 from Tsholotsho): no CHCs |
|
| Outcomes | 20 observable indicators of behaviour change including child faeces in yard | |
| Notes | Location: rural wards in Makoni (21 intervention wards) and Tsholotsho districts (3 intervention wards), Zimbabwe Length of study: 7 months (August 2000 to March 2001) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention not randomly allocated. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It seemed they observed hygiene indicators in all HHs. |
| Selective reporting (reporting bias) | Low risk | Behaviours prespecified were reported. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA, not relevant to design. |
| Similarity of baseline characteristics | Unclear risk | NA, not relevant to design. |
| Adequate allocation of intervention concealment during the study | High risk | No blinding. |
| Adequate protection against contamination | Low risk | Control areas were "far removed from health clubs areas (typically 30–50km away)." |
| Confounders adequately adjusted for in analysis/design | High risk | No adjustments for any confounders. |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Wijewardene 1992 SRI.
| Methods | Case‐control study (community‐based) | |
| Participants | Cases: families with 1 child aged < 5 years having acute diarrhoea in previous 6 months (identified through community visits), n = 100. Controls: families with ≥ 1 child aged < 5 years that did not have a single episode of diarrhoea during the previous 6 months, matched for age of child, occupation, and ethnic group of father, n = 100. |
|
| Interventions | Risk factor of interest:
|
|
| Outcomes | Acute diarrhoea for children aged > 1 years: ≥ 3 loose stools in 24 hours for ≤ 7 days | |
| Notes | Location: Urban, Galle municipality, Sri Lanka Length of recruitment: no details Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NA |
| Allocation concealment (selection bias) | Unclear risk | NA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | NA |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NA |
| Selective reporting (reporting bias) | Unclear risk | NA |
| Other bias | Unclear risk | NA |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | Unclear risk | NA |
| Baseline imbalance | Unclear risk | NA |
| Loss of clusters | Unclear risk | NA |
| Incorrect analysis | Unclear risk | NA |
Yeager 2002 PER.
| Methods | Cluster RCT | |
| Participants | Number: 722 HHs (postintervention) Inclusion criteria: HH had to have an eligible child (aged 15–47 months) |
|
| Interventions | Intervention (4 clusters): hygiene promotion for potty use and keeping the home environment free from faeces. The intervention was delivered through routine health services, and using video presentations, leaflets including 4 steps to potty training and counselling by health staff during consultations. Control (4 clusters): no intervention |
|
| Outcomes | Observed behaviours: use of potties, defecation behaviour of children, hygiene behaviours afterwards, disposal behaviour of faeces | |
| Notes | Location: San Juan de Lurigancho district, Lima, Peru Length of study: 17 months (October 1996 to March 1998) Publication status: journal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Quote: "One of these groups was then selected at random as the intervention group." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of non‐response. |
| Selective reporting (reporting bias) | Low risk | Report on main outcomes. |
| Other bias | Unclear risk | — |
| Similarity of baseline outcome measurements | Unclear risk | NA |
| Similarity of baseline characteristics | Unclear risk | NA |
| Adequate allocation of intervention concealment during the study | Unclear risk | NA |
| Adequate protection against contamination | Unclear risk | NA |
| Confounders adequately adjusted for in analysis/design | Unclear risk | NA |
| Recruitment bias | High risk | For end of study data collection, field workers would have known allocation of cluster. |
| Baseline imbalance | Low risk | The implementers had matched the zones. |
| Loss of clusters | Unclear risk | No loss of clusters reported. |
| Incorrect analysis | High risk | No statistical calculations. |
ARI: acute respiratory infection; BMI: body mass index; CBA: controlled before‐and‐after; CBEHPP: Community‐Based Environmental Health Promotion Programme; CHC: community health club; CLTS: community‐led total sanitation; FDCH: family daycare home; HC: health centre; HEP: health extension package; HH: household; HWWS: handwashing with soap; IYCF: infant and young child feeding; LAZ: length‐for‐age Z score; LDC: licensed daycare centre; LNS: lipid‐based nutrient supplement; LTFU: lost to follow‐up; MUAC: mid‐upper‐arm‐circumference; n: number of participants; NA: not applicable; ODF: open defecation‐free; OTP: Outpatient Therapeutic feeding Program; ORS: oral rehydration solution; PEM: protein‐energy malnutrition; PHAST: Participatory Hygiene and Sanitation Transformation; RCT: randomized controlled trial; SAM: severe acute malnutrition; SD: standard deviation; SHEWA‐B: Sanitation Hygiene Education and Water Supply in Bangladesh; STH: soil‐transmitted helminth; TSSM: Total Sanitation and Sanitation Marketing; UMOH: Uganda Ministry of Health; WASEP: Water and Sanitation Extension Programme; WASH: water, sanitation, and hygiene; WAZ: weight‐for‐age Z score; WHO: World Health Organization; WHZ: weight‐for‐height Z score; WVG: Women's Health Volunteer Group.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Assefa 2010 | Study design not eligible. |
| Babu 2015 | No control group. |
| Ban 2015 | Intervention not specific to child sanitation. |
| Blum 1990 | Unclear whether child faeces disposal or use of latrines by children was included in the intervention. |
| Boehm 2016 | No relevant outcomes. |
| Bohnert 2016 | Primary school‐based intervention. |
| Carnell 2014 | Outcomes not eligible. |
| Clarke 2016 | Intervention did not seem to include child sanitation. |
| Clasen 2015 | In the intervention there was no messaging done on child faeces disposal or toilet use behaviour change. |
| Ditai 2016 | Intervention not eligible, only included alcohol hand rub. |
| Dumba 2013 | Unclear whether child faeces disposal or use of latrines by children was included in the intervention. |
| Erismann 2017 | School‐based intervention (children aged ≥ 8 years) so no focus on sanitation for children aged < 5 years. |
| Francis 2016 | Intervention not eligible, water filter with no child sanitation component. |
| Freeman 2015 | In the intervention there was no messaging done on child faeces disposal or toilet use behaviour change. |
| Galiani 2016 | Intervention not eligible, handwashing education only, no child sanitation component. |
| Garn 2016 | Intervention not eligible: WASH in primary schools. |
| Gelaye 2014 | No control and intervention was not eligible: primary school intervention. |
| Gorter 1998 | Study design and intervention not eligible. |
| Greenland 2016 | Intervention not eligible, it only included the toilets and service. No specific behaviour change messaging. |
| Gungoren 2007 | Unclear whether child faeces disposal or use of latrines by children was included in the intervention. |
| Hartinger 2016 | Intervention not eligible, no child sanitation component. |
| Hunter 2004 | Risk factor was contact with toileting child or changing nappy (yes vs no) not about the disposal of the faeces and where the faeces end up. |
| Hürlimann 2018 | Unclear whether the intervention included child faeces disposal messaging. Author did not reply. |
| IOB/UNICEF 2011 | Insufficient detail provided on whether child faeces disposal or use of latrines by children was included in the intervention whether there was a control group. |
| Islam 2018 | Study design not eligible (no control), baseline data from the WASH‐B study. |
| JDC/IHI 2012 | Unclear whether child faeces disposal was included in the intervention. |
| Kaatano 2015 | Study design not eligible (before and after study without a control) and unclear whether the intervention included child sanitation. |
| Lamichhane 2018 | Study design not eligible. Analysis of data from the Nepal Demographic Health Survey 2011. |
| Law 2016 | Intervention not eligible: a psychological intervention aimed at improving toilet behaviour of children (aged 4–7 years) with faecal incontinence. |
| Liu 2017 | Unclear whether the intervention included messaging on child faeces disposal. The author did not reply. |
| Messou 1997 | Unclear whether child faeces disposal or use of latrines by children was included in the intervention. |
| Nerkar 2015 | Intervention not eligible: focused on toilet construction and watershed management. No focus on child faeces management. |
| Njuguna 2016 | Risk factor was hand washing after child faeces disposal, not child faeces disposal itself. |
| Olayo 2014 | Intervention and outcomes not eligible. |
| Park 2018 | Unclear whether intervention and outcomes were eligible. Author did not reply. |
| Raso 2018 | Unclear whether intervention included child faeces disposal messaging. Author did not reply. |
| Reese 2017 | Intervention not eligible. Exchange with author confirmed that the intervention did not include specific messaging about child faeces disposal. |
| Sarkar 2014 | Risk factor was not specific to child sanitation, it was use of the latrine by all household members. |
| Slayton 2016 | Intervention not eligible, antimicrobial hand towel with no child sanitation component. |
| Taha 2000 | Intervention and outcome not eligible. |
| Trinies 2016 | Intervention not eligible: WASH intervention based in primary school. |
| Yeasmin 2017 | Intervention, study design, and outcomes not eligible. |
| Yentur 2015 | Risk factor not eligible. |
| Zomer 2015 | Intervention not eligible: focused on hand hygiene only, including after nappy changing but nothing about faeces disposal. |
WASH: water, sanitation, and hygiene.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000523707.
| Trial name or title | The effectiveness and acceptability of the 'BALatrine': a culturally acceptable latrine intervention in resource limited environments |
| Methods | Cluster RCT |
| Participants | Estimated: 4000 |
| Interventions | The intervention is a household latrine (BALatrine) plus health education/promotion on hygiene and sanitation. The BALatrine is a simple squat latrine. |
| Outcomes | Primary outcome
Secondary outcome
|
| Starting date | September 2016 |
| Contact information | Prof Donald Stewart, Griffith University, South Brisbane |
| Notes | Location: Wonosobo, Central Java, Indonesia Trial registration number: ACTRN12613000523707 |
ISRCTN10419317.
| Trial name or title | The impact of improved sanitation on the diarrhoeal reduction of under‐five children in Democratic Republic of Congo |
| Methods | Cluster‐RCT |
| Participants | All children in estimated 720 households |
| Interventions | Intervention: sanitation campaign to increase latrine coverage using CLTS principle and borehole drilling. Child faeces disposal messaging is included in the CLTS triggering. Control: borehole drilling |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | December 2014 |
| Contact information | Dr Seungman Cha, Korea International Cooperation Agency |
| Notes | Trial registration number: ISRCTN10419317 Location: Bandundu province (Democratic Republic of Congo) |
ISRCTN16961836.
| Trial name or title | Efficacy of a behavioural intervention based on food consumption, nutritional state and micronutrient deficiency in under five children, Angola |
| Methods | RCT |
| Participants | All children living in participating hamlets aged < 36 months old and their primary caregivers. Estimated: 2182 |
| Interventions | Nutrition arm: participants receive 12 personalized home‐based counselling visits divided in blocks of 3 monthly visits after baseline and each follow‐up time point (6, 12, and 18 months). These visits involve the delivering of 11 key recommendations and messages for promoting infant and young children optimal feeding practices regarding breastfeeding, complementary feeding (dietary diversity, meal frequency, and quantity of food), responsive feeding, feeding during and after illness; hygiene and food safety. Participants also attend 4 community group meetings at baseline, 6, 12, and 18 months, which focus on the key messages along theoretical and practical sessions. WASH arm: participants receive 12 personalized home‐based counselling visits divided in blocks of 3 monthly visits after baseline and each follow‐up time point (6, 12, and 18 months). These visits involve the delivering of 11 key recommendations and messages for promoting optimal parental hygiene and health practices regarding infant personal hygiene, hand washing (supplies, techniques, critical moments), safe drinking water (treatment, collection, storage), house surrounding environment, safely disposal of faeces, and malaria prevention. Participants also attend 4 community group meetings at baseline, 6, 12, and 18 months, which focus on the key messages along theoretical and practical sessions. Control arm: no educational package between assessments. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | November 2014 |
| Contact information | Miguel Brito Rua Direita do Caxito Hospital Geral do Bengo – Caxito, Província do Bengo Angola |
| Notes | Trial registration number: ISRCTN16961836 Location: Angola |
NCT02754583.
| Trial name or title | SWIFT: Sanitation, Water, and Instruction in Face‐washing for Trachoma |
| Methods | Cluster‐RCT |
| Participants | Estimated: 220,000 |
| Interventions | A series of 3 cluster‐RCTs to assess several alternative strategies for trachoma control in communities that have been treated with many years of mass azithromycin distributions. The first trial ('WUHA') compares communities that receive a comprehensive WASH package (including promotion to households that the faeces of children aged < 5 years should be deposited in a latrine) to those that receive no intervention. The second trial ('TAITU‐A') compares communities randomized to targeted antibiotic treatment vs those randomized to mass antibiotics for trachoma. The third trial ('TAITU‐B') compares communities randomized to targeted antibiotics vs those randomized to delayed antibiotics. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | November 2015 |
| Contact information | Jeremy D Keenan – University of California San Francisco Proctor Foundation |
| Notes | Location: Ethiopia Trial registration number: NCT02754583 |
CLTS: community‐led total sanitation; RCT: randomized controlled trial; rRNA: ribosomal ribonucleic acid; STH: soil‐transmitted helminth; WASH: water, sanitation, and hygiene.
Differences between protocol and review
In the published protocol, Majorin 2014, we prespecified that if there were a sufficient number of included studies (more than 10) we would investigate causes of heterogeneity using subgroup analysis. However, we investigated causes of heterogeneity using subgroup analysis even when there were fewer than 10 studies.
We pooled comparable studies together if there was more than one study, even when the I² statistic value was greater than 75%.
One review author assessed the certainty of the evidence rather than two as planned in the protocol.
Contributions of authors
TC and FM planned the review.
FM drafted the protocol.
FM screened titles.
FM, BT, and GC screened abstracts and full texts.
FM contacted study authors for additional information.
FM, BT, and GC extracted data.
FM entered the data and BT checked a sample.
FM drafted the review.
All authors provided comments on the review, and read and approved the final review version.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Bill & Melinda Gates Foundation, USA.
This research is supported in part by a grant from the foundation
-
Department for International Development, UK.
Project number 300342‐104
Declarations of interest
FM has no known conflicts of interest.
BT has no known conflicts of interest.
GC has no known conflicts of interest.
TC has no known conflicts of interest.
New
References
References to studies included in this review
Abalkhail 1995 KSA {published data only}
- Abalkhail B, Bahnassy AA, Al Abasi H. Maternal knowledge, attitudes, and practices as predictors of diarrhoeal disease in Saudi children. Journal of the Egyptian Public Health Association 1995;70(5‐6):559‐77. [PubMed] [Google Scholar]
Ahmed 1993 BGD {published data only}
- Ahmed NU, Zeitlin MF, Beiser AS, Super CM, Gershoff SN. A longitudinal study of the impact of behavioural change intervention on cleanliness, diarrhoeal morbidity and growth of children in rural Bangladesh. Social Science & Medicine 1993;37(2):159‐71. [DOI] [PubMed] [Google Scholar]
Alam 1989 BGD {published data only}
- Alam N, Wojtyniak B, Henry FJ, Rahaman MM. Mothers' personal and domestic hygiene and diarrhoea incidence in young children in rural Bangladesh. International Journal of Epidemiology 1989;18(1):242‐7. [DOI] [PubMed] [Google Scholar]
Altmann 2018 TCD {published data only}
- Altmann M, Altare C, Spek N, Barbiche JC, Dodos J, Bechir M, et al. Effectiveness of a household water, sanitation and hygiene package on an outpatient program for severe acute malnutrition: a pragmatic cluster‐randomized controlled trial in Chad. American Journal of Tropical Medicine and Hygiene 2018;98(4):1005‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arvelo 2009 USA {published data only}
- Arvelo W, Hinkle CJ, Nguyen TA, Weiser T, Steinmuller N, Khan F, et al. Transmission risk factors and treatment of pediatric shigellosis during a large daycare center‐associated outbreak of multidrug resistant Shigella sonnei: implications for the management of shigellosis outbreaks among children. Pediatric Infectious Disease Journal 2009;28(11):976‐80. [DOI] [PubMed] [Google Scholar]
Asfaha 2018 ETH {published data only}
- Asfaha KF, Tesfamichael FA, Fisseha GK, Misgina KH, Weldu MG, Welehaweria NB, et al. Determinants of childhood diarrhea in Medebay Zana District, Northwest Tigray, Ethiopia: a community based unmatched case‐control study. BMC Pediatrics 2018;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aziz 1990 BGD {published data only}
- Aziz KM, Hoque BA, Hasan KZ, Patwary MY, Huttly SR, Rahaman MM, et al. Reduction in diarrhoeal diseases in children in rural Bangladesh by environmental and behavioural modifications. Transactions of the Royal Society of Tropical Medicine & Hygiene 1990;84(3):433‐8. [DOI] [PubMed] [Google Scholar]
- Hasan KZ, Briend A, Aziz KM, Hoque BA, Patwary MY, Huttly SR. Lack of impact of a water and sanitation intervention on the nutritional status of children in rural Bangladesh. European Journal of Clinical Nutrition 1989;43(12):837‐43. [PubMed] [Google Scholar]
Baker 2016 BGD {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, Eijk A, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clinical Infectious Diseases 2012;55(S4):S232‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case‐control study. Lancet 2013;382(9888):209‐22. [DOI] [PubMed] [Google Scholar]
- Long KZ, Faruque AS, Ahmed T, Gunanti IR, Hoque ME, Das SK, et al. Household sanitation and hygiene indicators of enteric pathogen transmission and childhood diarrheal exposure risk in Mirzapur, Bangladesh. Tropical Medicine & International Health 2015:437.
Baker 2016 GMB {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016 IND {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016 KEN {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016 MLI {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016 MOZ {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhampossa T, Mandomando I, Acacio S, Quintó L, Vubil D, Ruiz J, et al. Diarrheal disease in rural Mozambique: burden, risk factors and etiology of diarrheal disease among children aged 0–59 months seeking care at health facilities. PLOS One 2015;10(5):e0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2016 PAK {published data only}
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene‐specific risk factors for moderate‐to‐severe diarrhea in young children in the Global Enteric Multicenter Study, 2007–2011: case‐control study. PLOS Medicine 2016;13(5):e1002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baltazar 1989 PHI {published data only}
- Baltazar JC, Solon FS. Disposal of faeces of children under two years old and diarrhoea incidence: a case‐control study. International Journal of Epidemiology 1989;18(4 Suppl 2):S16‐9. [DOI] [PubMed] [Google Scholar]
Barrios 2008 PHI {unpublished data only}
- Barrios M. Behavioral Determinants of Diarrhea Among Children Under Five Years of Age in the Rural Philippines: Hand Washing, Stool Disposal, and the Effects of Demographic, Psychosocial, and Social Ecological Factors [PhD thesis]. Los Angeles (CA): University of California Los Angeles, 2008. [Google Scholar]
Bassal 2016 ISR {published data only}
- Bassal R, Ovadia A, Bromberg M, Stein M, Shainberg B, Loewenthal S, et al. Risk factors for sporadic infection with Campylobacter Spp. among children in Israel: a case‐control study. Pediatric Infectious Disease Journal 2016;35(3):249‐52. [DOI] [PubMed] [Google Scholar]
Belizario 2015 PHI {published data only}
- Belizario VY Jr, Liwanag HJ, Naig JR, Chua PL, Madamba MI, Dahildahil RO. Parasitological and nutritional status of school‐age and preschool‐age children in four villages in Southern Leyte, Philippines: lessons for monitoring the outcome of community‐led total sanitation. Acta Tropica 2015;141(Pt A):16‐24. [DOI] [PubMed] [Google Scholar]
Berhe 2014 ETH {published data only}
- Berhe F, Berhane Y. Under five diarrhea among model household and non model households in Hawassa, South Ethiopia: a comparative cross‐sectional community based survey. BMC Public Health 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Briceño 2015 TAN {published and unpublished data}
- Briceño B, Coville A, Gertler P, Martinez S. Are there synergies from combining hygiene and sanitation promotion campaigns: evidence from a large‐scale cluster‐randomized trial in rural Tanzania. PLOS One 2017;12(11):e0186228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceño B, Coville A, Martinez S. Promoting handwashing and sanitation: evidence from a large‐scale randomized trial in rural Tanzania. World Bank, Policy Research Working Paper 7164. 2015. documents.worldbank.org/curated/en/545961468165561161/pdf/WPS7164.pdf (accessed prior to 9 August 2019).
Butz 1990 USA {published data only}
- Butz AM, Larson E, Fosarelli P, Yolken R. Occurrence of infectious symptoms in children in day care homes. American Journal of Infection Control 1990;18(6):347‐53. [DOI] [PubMed] [Google Scholar]
Cameron 2013 INA {unpublished data only}
- Borja‐Vega C. The effects of the Total Sanitation and Sanitation Marketing programme on gender and ethnic groups in Indonesia. Waterlines 2014;33(1):55‐70. [Google Scholar]
- Cameron L, Shah M, Olivia S. Impact evaluation of a large‐scale rural sanitation project in Indonesia. World Bank, Policy research working paper. Impact evaluation series 83. 2013. documents.worldbank.org/curated/en/783781468044120966/pdf/wps6360.pdf (accessed prior to 9 August 2019).
Caruso 2019 IND {unpublished data only}
- Caruso BA, Sclar GD, Routray P, Majorin F, Nagel C, Clasen T. A cluster‐randomized multi‐level intervention to increase latrine use and safe disposal of child feces in rural Odisha, India: the Sundara Grama research protocol. BMC Public Health 2019;19:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2005 TWN {published data only}
- Chiang TY, Jiang DD, Shaw CK. A matched case‐control study of shigellosis in children under the age of five in Hualien County. Taiwan Journal of Public Health 2005;24(5):431‐9. [Google Scholar]
Chompook 2006 THA {published and unpublished data}
- Chompook P. The Epidemiology of Shigellosis in Thailand [PhD thesis]. London (UK): University of London, 2004. [Google Scholar]
- Chompook P, Todd J, Wheeler JG, Seidlein L, Clemens J, Chaicumpa W. Risk factors for shigellosis in Thailand. International Journal of Infectious Diseases 2006;10(6):425‐33. [DOI] [PubMed] [Google Scholar]
Christensen 2015a KEN {published data only}
- Christensen G, Dentz HN, Pickering AJ, Bourdier T, Arnold BF, Colford JM Jr, et al. Pilot cluster randomized controlled trials to evaluate adoption of water, sanitation, and hygiene interventions and their combination in rural western Kenya. American Journal of Tropical Medicine and Hygiene 2015;92(2):437‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2015b KEN {published data only}
- Christensen G, Dentz HN, Pickering AJ, Bourdier T, Arnold BF, Colford JM Jr, et al. Pilot cluster randomized controlled trials to evaluate adoption of water, sanitation, and hygiene interventions and their combination in rural western Kenya. American Journal of Tropical Medicine and Hygiene 2015;92(2):437‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clemens 1987 BGD {published data only}
- Clemens JD, Stanton BF. An educational intervention for altering water‐sanitation behaviors to reduce childhood diarrhea in urban Bangladesh. I. Application of the case‐control method for development of an intervention. American Journal of Epidemiology 1987;125(2):284‐91. [DOI] [PubMed] [Google Scholar]
Cummings 2012 UGA {published data only}
- Cummings MJ, Wamala JF, Eyura M, Malimbo M, Omeke ME, Mayer D, et al. A cholera outbreak among semi‐nomadic pastoralists in northeastern Uganda: epidemiology and interventions. Epidemiology and Infection 2012;140(8):1376‐85. [DOI] [PubMed] [Google Scholar]
Daniels 1990 LES {published data only}
- Daniels DL, Cousens SN, Makoae LN, Feachem RG. A case‐control study of the impact of improved sanitation on diarrhoea morbidity in Lesotho. Bulletin of the World Health Organization 1990;68(4):455‐63. [PMC free article] [PubMed] [Google Scholar]
Dickinson 2015 IND {published data only}
- Dickinson KL, Patil SR, Pattanayak SK, Poulos C, Yang JH. Nature's call: impacts of sanitation choices in Orissa, India. Economic Development and Cultural Change 2015;64(1):1‐29. [Google Scholar]
Dikassa 1993 DRC {published data only}
- Dikassa L, Mock N, Magnani R, Rice J, Abdoh A, Mercer D, et al. Maternal behavioural risk factors for severe childhood diarrhoeal disease in Kinshasa, Zaire. International Journal of Epidemiology 1993;22(2):327‐33. [DOI] [PubMed] [Google Scholar]
Fisher 2011 BGD {published data only}
- Fisher S, Kabir B, Lahiff E, MacLachlan M. Knowledge, attitudes, practices and implications of safe water management and good hygiene in rural Bangladesh: assessing the impact and scope of the BRAC WASH programme. Journal of Water and Health 2011;9(1):80‐93. [DOI] [PubMed] [Google Scholar]
Gebru 2014 ETH {published data only}
- Gebru T, Taha M, Kassahun W. Risk factors of diarrhoeal disease in under‐five children among health extension model and non‐model families in Sheko district rural community, Southwest Ethiopia: comparative cross‐sectional study. BMC Public Health 2014;14:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genthe 1997 SAF {published data only}
- Genthe B, Strauss N, Vundule C, Maforah F, Seager J. The effect of water supply, handling and usage on water quality in relation to health indices in developing communities. Urbanisation and Health Newsletter. WRC Report No. 562/1/96, 1997. www.ircwash.org/sites/default/files/Genthe‐1996‐Effect.pdf (accessed prior to 9 August 2019). [PubMed]
Ghosh 1994 IND {published data only}
- Ghosh S, Sengupta PG, Mandal SK, Manna B, Sikder SN, Sirkar BK. Maternal behaviour and feeding practices as determinants of childhood diarrhoea: some observations amongst rural Bengalee mothers. Indian Journal of Public Health 1994;38(2):77‐80. [PubMed] [Google Scholar]
Ghosh 1997 IND {published data only}
- Ghosh S, Sengupta PG, Gupta DN, Mondal SK, Goswami M, Bhattacharya SK, et al. Maternal knowledge on risk behavioural practices and it's association with diarrhoea in a rural community of West Bengal, India. Journal of Communicable Diseases 1998;30(4):251‐5. [PubMed] [Google Scholar]
- Ghosh S, Sengupta PG, Mondal SK, Banu MK, Gupta DN, Sircar BK. Risk behavioural practices of rural mothers as determinants of childhood diarrhoea. Journal of Communicable Diseases 1997;29(1):7‐14. [PubMed] [Google Scholar]
Godana 2013 ETH {published data only}
- Godana W, Mengistie B. Determinants of acute diarrhoea among children under five years of age in Derashe District, Southern Ethiopia. Rural and Remote Health 2013;13(3):2329. [PubMed] [Google Scholar]
Haggerty 1994 DRC {published data only}
- Haggerty PA, Manunebo MN, Ashworth A, Muladi K, Kirkwood BR. Methodological approaches in a baseline study of diarrhoeal morbidity in weaning‐age children in rural Zaire. International Journal of Epidemiology 1994;23(5):1040‐9. [DOI] [PubMed] [Google Scholar]
- Haggerty PA, Muladi K, Kirkwood BR, Ashworth A, Manunebo M. Community‐based hygiene education to reduce diarrhoeal disease in rural Zaire: impact of the intervention on diarrhoeal morbidity. International Journal of Epidemiology 1994;23(5):1050‐9. [DOI] [PubMed] [Google Scholar]
Hashi 2017 ETH {published data only}
- Hashi A, Kumie A, Gasana J. Hand washing with soap and WASH educational intervention reduces under‐five childhood diarrhoea incidence in Jigjiga District, Eastern Ethiopia: a community‐based cluster randomized controlled trial. Preventive Medicine Reports 2017;6:361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heller 2003 BRA {published data only}
- Heller L, Colosimo EA, Antunes CM. Environmental sanitation conditions and health impact: a case‐control study. Revista Da Sociedade Brasileira de Medicina Tropical 2003;36(1):41‐50. [DOI] [PubMed] [Google Scholar]
- Heller L, Colosimo EA, Antunes CM. Setting priorities for environmental sanitation interventions based on epidemiological criteria: a Brazilian study. Journal of Water and Health 2005;3(3):271‐81. [DOI] [PubMed] [Google Scholar]
Hoq 2016 BGD {published data only}
- Hoq M, Brogan J. Evaluation of an integrated health‐nutrition‐WASH project to reduce malnutrition prevalence in children under two in Bangladesh. Field Exchange Emergency Nutrition Network ENN, January 2016. www.ennonline.net/fex/51/healthnutwashbangladesh (accessed prior to 9 August 2019).
Huda 2012 BGD {published data only}
- Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Social Science & Medicine 2012;75(4):604‐11. [DOI] [PubMed] [Google Scholar]
Humphrey 2019 ZIM {published data only}
- Humphrey JH, Mbuya MN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, et al. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster‐randomised trial. Lancet Global Health 2019;7(1):e132‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team, Humphrey JH, Jones AD, Manges A, Mangwadu G, Maluccio JA, et al. The Sanitation Hygiene Infant Nutrition Efficacy (SHINE) trial: rationale, design, and methods. Clinical Infectious Diseases 2015;61(Suppl 7):S685‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jinadu 2007 NGR {published data only}
- Jinadu MK, Adegbenro CA, Esmai AO, Ojo AA, Oyeleye BA. Health promotion intervention for hygienic disposal of children's faeces in a rural area of Nigeria. Health Education Journal 2007;66(3):222‐8. [Google Scholar]
Knight 1992 MAL {published data only}
- Knight SM, Toodayan W, Caique WC, Kyi W, Barnes A, Desmarchelier P. Risk factors for the transmission of diarrhoea in children: a case‐control study in rural Malaysia. International Journal of Epidemiology 1992;21(4):812‐8. [DOI] [PubMed] [Google Scholar]
Kotch 2007 USA {published data only}
- Kotch JB, Isbell P, Weber DJ, Nguyen V, Savage E, Gunn E, et al. Hand‐washing and diapering equipment reduces disease among children in out‐of‐home child care centers. Pediatrics 2007;120(1):e29‐36. [DOI] [PubMed] [Google Scholar]
Luby 2014 BGD {unpublished data only}
- Luby SP, Unicomb L, Halder AK, Nurul Huda TM, Sultana S, Bulbul T, et al (SHEWA‐B evaluation team). SHEWA‐B Health Impact Study Report. September 2014. www.unicef.org/evaldatabase/files/WASH_SHEWA‐Bangladesh_health_impact_eval.pdf (accessed 18 April 2016).
Luby 2018 BGD {published data only}
- Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Global Health 2018;6(3):e302‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez SM, Azad R, Rahman M, Unicomb L, Ram PK, Naser AM, et al. Achieving optimal technology and behavioral uptake of single and combined interventions of water, sanitation hygiene and nutrition, in an efficacy trial (WASH benefits) in rural Bangladesh. Trials 2018;19:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Ashraf S, Unicomb L, Mainuddin AK, Parvez SM, Begum F, et al. WASH Benefits Bangladesh trial: system for monitoring coverage and quality in an efficacy trial. Trials 2018;19:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicomb L, Begum F, Leontsini E, Rahman M, Ashraf S, Naser AM, et al. WASH Benefits Bangladesh trial: management structure for achieving high coverage in an efficacy trial. Trials 2018;19:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 2004 ZIM {unpublished data only}
- Mathew B. Ensuring Sustained Beneficial Outcomes for Water and Sanitation (WATSAN) Programmes in the Developing World [PhD thesis]. Cranfield (UK): Institute of Water and Environment, Cranfield University, 2004. [Google Scholar]
Maung 1992a MYA {published data only}
- Maung K, Khin M, Wai NN, Hman NW, Myint TT, Butler T. Risk factors for the development of persistent diarrhoea and malnutrition in Burmese children. International Journal of Epidemiology 1992;21(5):1021‐9. [DOI] [PubMed] [Google Scholar]
Maung 1992b MYA {published data only}
- Maung K, Khin M, Wai NN, Hman NW, Myint TT, Butler T. Risk factors for the development of persistent diarrhoea and malnutrition in Burmese children. International Journal of Epidemiology 1992;21(5):1021‐9. [DOI] [PubMed] [Google Scholar]
Mediratta 2010a ETH {published data only}
- Mediratta RP, Feleke A, Moulton LH, Yifru S, Sack RB. Risk factors and case management of acute diarrhoea in North Gondar Zone, Ethiopia. Journal of Health, Population & Nutrition 2010;28(3):253‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mediratta 2010b ETH {published data only}
- Mediratta RP, Feleke A, Moulton LH, Yifru S, Sack RB. Risk factors and case management of acute diarrhoea in North Gondar Zone, Ethiopia. Journal of Health, Population, and Nutrition 2010;28(3):253‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 1990 USA {published data only}
- Menon S, Santosham M, Reid R, Almeido‐Hill J, Sack RB, Comstock GW. Rotavirus diarrhoea in Apache children: a case‐control study. International Journal of Epidemiology 1990;19(3):715‐21. [DOI] [PubMed] [Google Scholar]
Mertens 1992 SRI {published data only}
- Mertens TE, Jaffar S, Fernando MA, Cousens SN, Feachem RG. Excreta disposal behaviour and latrine ownership in relation to the risk of childhood diarrhoea in Sri Lanka. International Journal of Epidemiology 1992;21(6):1157‐64. [DOI] [PubMed] [Google Scholar]
Nair 2017 IND {published data only}
- Nair N, Tripathy P, Sachdev HS, Pradhan H, Bhattacharyya S, Gope R, et al. Effect of participatory women's groups and counselling through home visits on children's linear growth in rural eastern India (CARING trial): a cluster‐randomised controlled trial. Lancet Global Health 2017;5(10):e1004‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nanan 2003 PAK {published data only}
- Nanan D, White F, Azam I, Afsar H, Hozhabri S. Evaluation of a water, sanitation, and hygiene education intervention on diarrhoea in northern Pakistan. Bulletin of the World Health Organization 2003;81(3):160‐5. [PMC free article] [PubMed] [Google Scholar]
Null 2018 KEN {published data only}
- Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster‐randomised controlled trial. Lancet. Global Health 2018;6(3):e316‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaum L, Mboya J, Mahoney R, Otuke J, Njenga S, Null C, et al. Effect of a sanitation intervention on soil transmitted helminth prevalence and concentration in household soil: a cluster randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):25‐6. [Google Scholar]
- Stewart CP, Kariger P, Fernald L, Pickering AJ, Arnold CD, Arnold BF, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on child development in rural Kenya (WASH Benefits Kenya): a cluster‐randomised controlled trial. Lancet. Child and Adolescent Health 2018;2(4):269‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oguro 2016 MYA {published data only}
- Oguro M, Horiuchi S. A cross‐sectional study of community‐based maternal and child health interventions involving women's health volunteer groups in rural Myanmar. Public Health Nursing 2016;33(5):449‐59. [DOI] [PubMed] [Google Scholar]
Oketcho 2012 TAN {published data only}
- Oketcho R, Nyaruhucha CN, Taybali S, Karimuribo ED. Influence of enteric bacteria, parasite infections and nutritional status on diarrhoea occurrence among 6–60 months old children admitted at a Regional Hospital in Morogoro, Tanzania. Tanzania Journal of Health Research 2012;14(2):104‐14. [DOI] [PubMed] [Google Scholar]
Park 2016 INA {published data only}
- Park MJ, Laksono B, Clements A, Sadler R, Stewart D. Worm‐free children: an integrated approach to reduction of soil‐transmitted helminth infections in Central Java. Reviews on Environmental Health 2016;31(1):111‐3. [DOI] [PubMed] [Google Scholar]
Patil 2014 IND {published data only}
- Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM Jr, et al. The effect of India's total sanitation campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLOS Medicine 2014;11(8):e1001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pickering 2015 MLI {published data only}
- Pickering AJ. Effect of a community‐led sanitation intervention on child diarrhoea and child growth in rural Mali. Field Exchange Emergency Nutrition Network ENN. 2016. www.ennonline.net/fex/52/sanitationchildgrowth (accessed prior to 9 August 2019).
- Pickering AJ, Alzua ML, Djebbari H. Impact of a community‐led total sanitation intervention on child health in rural Mali: evidence from a cluster randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2014; Vol. 5, issue Suppl 1.
- Pickering AJ, Djebbari H, Lopez C, Coulibaly M, Alzua ML. Effect of a community‐led sanitation intervention on child diarrhoea and child growth in rural Mali: a cluster‐randomised controlled trial. Lancet Global Health 2015;3(11):e701‐11. [DOI] [PubMed] [Google Scholar]
Sarrassat 2018 BUR {published data only}
- Sarrassat S, Meda N, Badolo H, Ouedraogo M, Some H, Bambara R, et al. Effect of a mass radio campaign on family behaviours and child survival in Burkina Faso: a repeated cross‐sectional, cluster‐randomised trial. Lancet Global Health 2018;6(3):e330‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinharoy 2017 RWA {published data only}
- Sinharoy SS, Schmidt WP, Wendt R, Mfura L, Crossett E, Grépin KA, et al. Effect of community health clubs on child diarrhoea in western Rwanda: cluster‐randomised controlled trial. Lancet Global Health 2017;5(7):e699‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 1987 BGD {published data only}
- Stanton BF, Clemens JD. An educational intervention for altering water‐sanitation behaviors to reduce childhood diarrhea in urban Bangladesh. II. A randomized trial to assess the impact of the intervention on hygienic behaviors and rates of diarrhea. American Journal of Epidemiology 1987;125(2):292‐301. [DOI] [PubMed] [Google Scholar]
- Stanton BF, Clemens JD, Khair T. Educational intervention for altering water‐sanitation behavior to reduce childhood diarrhea in urban Bangladesh: impact on nutritional status. American Journal of Clinical Nutrition 1988;48(5):1166‐72. [DOI] [PubMed] [Google Scholar]
Strina 2012 BRA {published data only}
- Strina A, Rodrigues LC, Cairncross S, Ferrer SR, Fialho AM, Leite JPG, et al. Factors associated with rotavirus diarrhoea in children living in a socially diverse urban centre in Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 2012;106(7):445‐51. [DOI] [PubMed] [Google Scholar]
Traoré 1994a BUR {published data only}
- Traoré E, Cousens S, Curtis V, Mertens T, Tall F, Traoré A, et al. Child defecation behaviour, stool disposal practices, and childhood diarrhoea in Burkina Faso: results from a case‐control study. Journal of Epidemiology and Community Health 1994;48(3):270‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Traoré 1994b BUR {published data only}
- Traoré E, Cousens S, Curtis V, Mertens T, Tall F, Traoré A, et al. Child defecation behaviour, stool disposal practices, and childhood diarrhoea in Burkina Faso: results from a case‐control study. Journal of Epidemiology and Community Health 1994;48(3):270‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waterkeyn 2005 ZIM {published and unpublished data}
- Waterkeyn J. Cost‐effective Health Promotion and Hygiene Behaviour Change through Community Health Clubs in Zimbabwe [PhD thesis]. London (UK): London School of Hygiene & Tropical Medicine, 2006. [Google Scholar]
- Waterkeyn J, Cairncross S. Creating demand for sanitation and hygiene through Community Health Clubs: a cost‐effective intervention in two districts in Zimbabwe. Social Science & Medicine 2005;61(9):1958‐70. [DOI] [PubMed] [Google Scholar]
Wijewardene 1992 SRI {published data only}
- Wijewardene K, Fonseka P, Wijayasiri WA. Risk factors contributing to acute diarrhoeal disease in children below five years. Ceylon Medical Journal 1992;37(4):116‐9. [PubMed] [Google Scholar]
Yeager 2002 PER {published data only}
- Yeager BA, Huttly SR, Diaz J, Bartolini R, Marin M, Lanata CF. An intervention for the promotion of hygienic feces disposal behaviors in a shanty town of Lima, Peru. Health Education Research 2002;17(6):761‐73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Assefa 2010 {unpublished data only}
- Assefa B. Impacts of WASH on Community‐Managed Acute Child Malnutrition in Ethiopia [MSc thesis]. Loughborough (UK): Loughborough University, 2010. [Google Scholar]
Babu 2015 {published data only}
- Babu MC, Tandur B, Sharma D, Murki S. Disposable diapers decrease the incidence of neonatal infections compared to cloth diapers in a level II neonatal intensive care unit. Journal of Tropical Pediatrics 2015;61(4):250‐4. [DOI] [PubMed] [Google Scholar]
Ban 2015 {published data only}
- Ban HQ, Li T, Shen J, Li J, Peng PZ, Ye HP, et al. Effects of multiple cleaning and disinfection interventions on infectious diseases in children: a group randomized trial in China. Biomedical & Environmental Sciences 2015;28(11):779‐87. [DOI] [PubMed] [Google Scholar]
Blum 1990 {published data only}
- Blum D, Emeh RN, Huttly SR, Dosunmu‐Ogunbi O, Okeke N, Ajala M, et al. The Imo State (Nigeria) Drinking Water Supply and Sanitation Project, 1. Description of the project, evaluation methods, and impact on intervening variables. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(2):309‐15. [DOI] [PubMed] [Google Scholar]
Boehm 2016 {published data only}
- Boehm AB, Wang D, Ercumen A, Shea M, Harris AR, Shanks OC, et al. Occurrence of host‐associated fecal markers on child hands, household soil, and drinking water in rural Bangladeshi households. Environmental Science & Technology Letters 2016;3(11):393‐8. [PMC free article] [PubMed] [Google Scholar]
Bohnert 2016 {published data only}
- Bohnert K, Chard AN, Mwaki A, Kirby AE, Muga R, Nagel CL, et al. Comparing sanitation delivery modalities in urban informal settlement schools: a randomized trial in Nairobi, Kenya. International Journal of Environmental Research & Public Health 2016;13(12):E1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carnell 2014 {published data only}
- Carnell MA, Dougherty L, Pomeroy AM, Karim AM, Mekonnen YM, Mulligan BE. Effectiveness of scaling up the 'three pillars' approach to accelerating MDG 4 progress in Ethiopia. Journal of Health, Population, and Nutrition 2014;32(4):549‐63. [PMC free article] [PubMed] [Google Scholar]
Clarke 2016 {published data only}
- Clarke NE, Clements AC, Amaral S, Richardson A, McCarthy JS, McGown J, et al. (S)WASH‐D for Worms: a pilot study investigating the differential impact of school‐ versus community‐based integrated control programs for soil‐transmitted helminths. PLOS Neglected Tropical Diseases 2018;12(5):e0006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke NE, Clements AC, Bryan S, McGown J, Gray D, Nery SV. Investigating the differential impact of school and community‐based integrated control programmes for soil‐transmitted helminths in Timor‐Leste: the (S)WASH‐D for Worms pilot study protocol. Pilot Feasibility Study 2016;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clasen 2015 {published data only}
- Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil‐transmitted helminth infection, and child malnutrition in Odisha, India: a cluster‐randomised trial. Lancet Global Health 2015;2(11):e645‐53. [DOI] [PubMed] [Google Scholar]
Ditai 2016 {published data only}
- Ditai J, Abeso J, Mudoola M, Faragher B, Adengo M, Dusabe Richards J, et al. A pilot cluster randomised trial of alcohol‐based hand rub to prevent community neonatal sepsis in rural Uganda. RCOG World Congress; 2016 Jun 20‐22; Birmingham (UK).
Dumba 2013 {published data only}
- Dumba R, Kaddu JB, Wabwire‐Mangen F. Design and implementation of participatory hygiene and sanitation transformation (PHAST) as a strategy to control soil‐transmitted helminth infections in Luweero, Uganda. African Health Sciences 2013;13(2):512‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erismann 2017 {published data only}
- Erismann S, Diagbouga S, Schindler C, Odermatt P, Knoblauch AM, Gerold J, et al. School children's intestinal parasite and nutritional status one year after complementary school garden, nutrition, water, sanitation, and hygiene interventions in Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;97(3):904‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2016 {published data only}
- Francis MR, Sarkar R, Roy S, Jaffar S, Mohan VR, Kang G, et al. Effectiveness of membrane filtration to improve drinking water: a quasi‐experimental study from rural Southern India. American Journal of Tropical Medicine and Hygiene 2016;95(5):1192‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2015 {published data only}
- Freeman MC, Majorin F, Boisson S, Routray P, Torondel B, Clasen T. The impact of a rural sanitation programme on safe disposal of child faeces: a cluster randomised trial in Odisha, India. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;110(7):386‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galiani 2016 {published data only}
- Galiani S, Gertler P, Ajzenman N, Orsola‐Vidal A. Promoting handwashing behavior: the effects of large‐scale community and school‐level interventions. Health Economics 2016;25(12):1545‐59. [DOI] [PubMed] [Google Scholar]
Garn 2016 {published data only}
- Garn JV, Brumback BA, Drews‐Botsch CD, Lash TL, Kramer MR, Freeman MC. Estimating the effect of school water, sanitation, and hygiene improvements on pupil health outcomes. Epidemiology 2016;27(5):752‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelaye 2014 {published data only}
- Gelaye B, Kumie A, Aboset N, Berhane Y, Williams MA. School‐based intervention: evaluating the role of water, latrines and hygiene education on trachoma and intestinal parasitic infections in Ethiopia. Journal of Water, Sanitation and Hygiene for Development 2014;4(1):120‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gorter 1998 {published data only}
- Gorter AC, Sandiford P, Pauw J, Morales P, Pérez RM, Alberts H. Hygiene behaviour in rural Nicaragua in relation to diarrhoea. International Journal of Epidemiology 1998;27(6):1090‐100. [DOI] [PubMed] [Google Scholar]
Greenland 2016 {published data only}
- Greenland K, Witt Huberts J, Wright RL, Hawkes L, Ekor C, Biran A. A cross‐sectional survey to assess household sanitation practices associated with uptake of "Clean Team" serviced home toilets in Kumasi, Ghana. Environment and Urbanization 2016;28(2):583‐98. [Google Scholar]
Gungoren 2007 {published data only}
- Gungoren B, Latipov R, Regallet G, Musabaev E. Effect of hygiene promotion on the risk of reinfection rate of intestinal parasites in children in rural Uzbekistan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(6):564‐9. [DOI] [PubMed] [Google Scholar]
Hartinger 2016 {published data only}
- Hartinger SM, Lanata CF, Hattendorf J, Verastegui H, Gil AI, Wolf J, et al. Improving household air, drinking water and hygiene in rural Peru: a community‐randomized‐controlled trial of an integrated environmental home‐based intervention package to improve child health. International Journal of Epidemiology 2016;45(6):2089‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunter 2004 {published data only}
- Hunter PR, Hughes S, Woodhouse S, Syed Q, Verlander NQ, Chalmers RM, et al. Sporadic cryptosporidiosis case‐control study with genotyping. Emerging Infectious Diseases 2004;10(7):1241‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hürlimann 2018 {published data only}
- Hürlimann E, Silué KD, Zouzou F, Ouattara M, Schmidlin T, Yapi RB, et al. Effect of an integrated intervention package of preventive chemotherapy, community‐led total sanitation and health education on the prevalence of helminth and intestinal protozoa infections in Cote d'Ivoire. Parasites and Vectors 2018;11(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
IOB/UNICEF 2011 {unpublished data only}
- Policy and Operations Evaluation Department (IOB) of the Netherlands Ministry of Foreign Affairs, UNICEF Evaluation Office, UNICEF Mozambique. Impact evaluation of drinking water supply and sanitation interventions in rural Mozambique. 2011. www.unicef.org/evaldatabase/files/283729_B85_IOB_360_BW_WEB_Mozambique‐Final.pdf (accessed 18 April 2016).
Islam 2018 {published data only}
- Islam M, Ercumen A, Ashraf S, Rahman M, Shoab AK, Luby SP, et al. Unsafe disposal of feces of children <3 years among households with latrine access in rural Bangladesh: association with household characteristics, fly presence and child diarrhea. PLOS One 2018;13(4):e0195218. [DOI] [PMC free article] [PubMed] [Google Scholar]
JDC/IHI 2012 {unpublished data only}
- Jimat Development Consultants, Ifakara Health Institute. Government of Tanzania/UNICEF 7 Learning Districts Strategy (2007–2011). www.unicef.org/evaldatabase/files/TNZ‐2011‐004‐1.pdf (accessed 18 April 2016).
Kaatano 2015 {published data only}
- Kaatano GM, Siza JE, Mwanga JR, Min DY, Yong TS, Chai JY, et al. Integrated schistosomiasis and soil‐transmitted helminthiasis control over five years on Kome Island, Tanzania. Korean Journal of Parasitology 2015;53(5):535‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamichhane 2018 {published data only}
- Lamichhane P, Sharma A, Mahal A. Does safe disposal of child faeces matter? An assessment of access to improved sanitation and child faeces disposal behaviour and diarrhoea in rural Nepal. International Health 2018;10(4):277‐84. [DOI] [PubMed] [Google Scholar]
Law 2016 {published data only}
- Law E, Yang JH, Coit MH, Chan E. Toilet school for children with failure to toilet train: comparing a group therapy model with individual treatment. Journal of Developmental & Behavioral Pediatrics 2016;37(3):223‐30. [DOI] [PubMed] [Google Scholar]
Liu 2017 {published data only}
- Liu C, Lu L, Zhang L, Luo R, Sylvia S, Medina A, et al. Effect of deworming on indices of health, cognition, and education among schoolchildren in rural China: a cluster‐randomized controlled trial. American Journal of Tropical Medicine and Hygiene 2017;96(6):1478‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Messou 1997 {published data only}
- Messou E, Sangaré SV, Josseran R, Corre C, Guelain J. Effect of improvement of sanitary conditions and of domestic hygiene on incidence of enteric for ascaridiasis and ankylostomiasis of children aged 2 to 4 years in rural area of Côte d'Ivoire [Impact de l'assainissement et de l’hygiène domestique sur l’incidence de l’ascaridiose et de l’ankylostomose chez les enfants de 2 à 4 ans dans les zones rurales de Côte d’Ivoire]. Bulletin de la Société de Pathologie Exotique 1997;90(1):48‐50. [PubMed] [Google Scholar]
Nerkar 2015 {published data only}
- Nerkar SS, Pathak A, Lundborg CS, Tamhankar AJ. Can integrated watershed management contribute to improvement of public health? A cross‐sectional study from Hilly Tribal Villages in India. International Journal of Environmental Research and Public Health 2015;12(3):2653‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Njuguna 2016 {published data only}
- Njuguna C, Njeru I, Mgamb E, Langat D, Makokha A, Ongore D, et al. Enteric pathogens and factors associated with acute bloody diarrhoea, Kenya. BMC Infectious Diseases 2016;16:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olayo 2014 {published data only}
- Olayo R, Wafula C, Aseyo E, Loum C, Kaseje D. A quasi‐experimental assessment of the effectiveness of the Community Health Strategy on health outcomes in Kenya. BMC Health Services Research 2014;14(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2018 {published data only}
- Park MJ, Jung HS, Stewart DE. Reducing the exposure of children in rural Indonesia to environmental contamination by human waste a pilot study. Therapeutic Research 2018;39(6):565‐70. [Google Scholar]
Raso 2018 {published data only}
- Raso G, Essé C, Dongo K, Ouattara M, Zouzou F, Hürlimann E, et al. An integrated approach to control soil‐transmitted helminthiasis, schistosomiasis, intestinal protozoa infection, and diarrhea: protocol for a cluster randomized trial. JMIR Research Protocols 2018;7(6):e145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reese 2017 {published data only}
- Reese H, Routray P, Sinharoy S, Torondel B, Chang H, Clasen T. Effectiveness of a combined household‐level piped water and sanitation intervention in rural Odisha, India on health: a matched cohort study. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):25. [Google Scholar]
Sarkar 2014 {published data only}
- Sarkar R, Kattula D, Francis MR, Ajjampur SS, Prabakaran AD, Jayavelu N, et al. Risk factors for cryptosporidiosis among children in a semi urban slum in southern India: a nested case‐control study. American Journal of Tropical Medicine and Hygiene 2014;91(6):1128‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slayton 2016 {published data only}
- Slayton RB, Murphy JL, Morris J, Faith SH, Oremo J, Odhiambo A, et al. A cluster randomized controlled evaluation of the health impact of a novel antimicrobial hand towel on the health of children under 2 years old in rural communities in Nyanza Province, Kenya. American Journal of Tropical Medicine and Hygiene 2016;94(2):437‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taha 2000 {published data only}
- Taha AZ, Sebai ZA, Shahidullah M, Hanif M, Ahmed HO. Assessment of water use and sanitation behavior in a rural area of Bangladesh. Archives of Environmental Health 2000;55(1):51‐7. [DOI] [PubMed] [Google Scholar]
Trinies 2016 {published data only}
- Trinies V, Garn JV, Chang HH, Freeman MC. The impact of a school‐based water, sanitation, and hygiene program on absenteeism, diarrhea, and respiratory infection: a matched‐control trial in Mali. American Journal of Tropical Medicine and Hygiene 2016;94(6):1418‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeasmin 2017 {published data only}
- Yeasmin F, Luby SP, Saxton RE, Nizame FA, Alam MU, Dutta NC, et al. Piloting a low‐cost hardware intervention to reduce improper disposal of solid waste in communal toilets in low‐income settlements in Dhaka, Bangladesh. BMC Public Health 2017;17:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yentur 2015 {published data only}
- Yentur Doni N, Yildiz Zeyrek F, Simsek Z, Gurses G, Sahin İ. Risk factors and relationship between intestinal parasites and the growth retardation and psychomotor development delays of children in Sanliurfa, Turkey. Turkiye Parazitolojii Dergisi 2015;39(4):270‐6. [DOI] [PubMed] [Google Scholar]
Zomer 2015 {published data only}
- Zomer TP, Erasmus V, Looman CW, Tjon‐A‐Tsien A, Beeck EF, Graaf JM, et al. A hand hygiene intervention to reduce infections in child daycare: a randomized controlled trial. Epidemiology and Infection 2015;143(12):2494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12613000523707 {unpublished data only}
- ACTRN12613000523707. The effectiveness and acceptability of the 'BALatrine': a culturally acceptable latrine intervention in resource limited environments. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000523707 (first received 10 May 2013).
ISRCTN10419317 {published data only}
- Cha S, Lee J, Seo D, Park BM, Mansiangi P, Bernard K, et al. Effects of improved sanitation on diarrheal reduction for children under five in Idiofa, DR Congo: a cluster randomized trial. Infectious Diseases of Poverty 2017;6(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN10419317. The impact of improved sanitation on the diarrhoeal reduction of under‐five children in Democratic Republic of Congo. www.isrctn.com/ISRCTN10419317 (first received 13 March 2015).
ISRCTN16961836 {published data only}
- ISRCTN16961836. Efficacy of a behavioural intervention based on food consumption, nutritional state and micronutrient deficiency in under five children, Angola. www.isrctn.com/ISRCTN16961836 (first received 5 August 2016).
NCT02754583 {published data only}
- NCT02754583. Sanitation, Water, and Instruction in Face‐washing for Trachoma (SWIFT). clinicaltrials.gov/ct2/show/NCT02754583 (first posted 28 April 2016).
Additional references
Bain 2015
- Bain R, Luyendijk R. Are burial or disposal with garbage safe forms of child faeces disposal? An expert consultation. Waterlines 2015;34(3):241‐54. [Google Scholar]
Bauza 2017
- Bauza V, Guest JS. The effect of young children's faeces disposal practices on child growth: evidence from 34 countries. Tropical Medicine & International Health 2017;22(10):1233‐48. [DOI] [PubMed] [Google Scholar]
Bethony 2006
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil‐transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 2006;367(9521):1521‐32. [DOI] [PubMed] [Google Scholar]
Bhandari 2002
- Bhandari N, Bahl R, Taneja S, Strand T, Mølbak K, Ulvik RJ, et al. Substantial reduction in severe diarrheal morbidity by daily zinc supplementation in young north Indian children. Pediatrics 2002;109(6):e86. [DOI] [PubMed] [Google Scholar]
Brooker 2006
- Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil‐transmitted helminth infections. Advances in Parasitology 2006;62:221‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 2003
- Brooks JT, Shapiro RL, Kumar L, Wells JG, Phillips‐Howard PA, Shi YP, et al. Epidemiology of sporadic bloody diarrhea in rural Western Kenya. American Journal of Tropical Medicine and Hygiene 2003;68(6):671‐7. [PubMed] [Google Scholar]
Brown 2013
- Brown J, Cairncross S, Ensink JH. Water, sanitation, hygiene and enteric infections in children. Archives of Disease in Childhood 2013;98(8):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Byers 2001
- Byers KE, Guerrant RL, Farr BM. Faecal‐oral transmission. In: Thomas JC, Webber DJ editor(s). Epidemiological Methods for the Study of Infectious Diseases. Oxford (UK): Oxford University Press, 2001:228‐48. [Google Scholar]
Clasen 2010
- Clasen TF, Bostoen K, Schmidt WP, Boisson S, Fung IC, Jenkins MW, et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007180.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
EPOC 2013
- Cochrane Effective Practice, Organisation of Care. Suggested risk of bias criteria for EPOC reviews. epoc.cochrane.org (accessed 31 August 2013).
Esrey 1991
- Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bulletin of the World Health Organization 1991;69:609‐21. [PMC free article] [PubMed] [Google Scholar]
Feachem 1983
- Feachem RG, Bradley DJ, Garelick H, Mara DD. Sanitation and Disease: Health Aspects of Wastewater and Excreta Management. Chichester (UK): John Wiley & Sons, 1983. [Google Scholar]
Ferriman 2007
- Ferriman A. BMJ readers choose the "sanitary revolution" as greatest medical advance since 1840. BMJ 2007;334:111. [Google Scholar]
Fewtrell 2005
- Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta‐analysis. Lancet Infectious Diseases 2005;5(1):42‐52. [DOI] [PubMed] [Google Scholar]
Freeman 2017
- Freeman MC, Garn JV, Sclar GD, Boisson S, Medlicott K, Alexander KT, et al. The impact of sanitation on infectious disease and nutritional status: a systematic review and meta‐analysis. International Journal of Hygiene and Environmental Health 2017;220(6):928‐49. [DOI] [PubMed] [Google Scholar]
GBD 2018
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infectious Diseases 2018;18:1211‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
George 2016
- George CM, Oldja L, Biswas S, Perin J, Sack RB, Ahmed S, et al. Unsafe child feces disposal is associated with environmental enteropathy and impaired growth. Journal of Pediatrics 2016;176:43‐9. [DOI] [PubMed] [Google Scholar]
Gil 2004
- Gil A, Lanata C, Kleinau E, Penny M. Children's faeces disposal practices in developing countries and interventions to prevent diarrhoeal diseases. 2004. www.ehproject.org/PDF/Strategic_papers/SR11‐Child%20Excreta%20Format.pdf (accessed prior to 9 August 2019).
Guerrant 2013
- Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut – a triple burden of diarrhoea, stunting and chronic disease. Nature Reviews Gastroenterology and Hepatology 2013;10:220‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ. GRADE: what is “quality of evidence” and why is it important to clinicians?. BMJ 2008;336:995‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Harhay 2010
- Harhay MO, Horton J, Olliaro PL. Epidemiology and control of human gastrointestinal parasites in children. Expert Review of Anti‐infective Therapy 2010;8(2):219‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
HEP 2003
- Federal Democratic Republic of Ethiopia, Ministry of Health. HEP training materials, 2003. cnhde.ei.columbia.edu/training/documents/Maternal_and_Child_Health.pdf (accessed 20 April 2016).
Heymann 2008
- Heymann D, editor. Control of Communicable Diseases Manual. Washington (DC): American Public Health Association, 2008. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Humphrey 2009
- Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374(9694):1032‐5. [DOI] [PubMed] [Google Scholar]
Kar 2008
- Kar K, Chambers R. Handbook on community‐led total sanitation. 2008. www.communityledtotalsanitation.org/sites/communityledtotalsanitation.org/files/media/cltshandbook.pdf (accessed 9 May 2016).
Keusch 2006
- Keusch GT, Fontaine O, Bhargava A, Boschi‐Pinto C, Bhutta ZA, Gotuzzo E, et al. Chapter 19. Diarrheal Diseases. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. editor(s). Disease Control Priorities in Developing Countries. 2nd Edition. Washington (DC): World Bank, 2006. [Google Scholar]
Lanata 1998
- Lanata CF, Huttly SR, Yeager BA. Diarrhea: whose feces matter? Reflections from studies in a Peruvian shanty town. Pediatric Infectious Disease Journal 1998;17(1):7‐9. [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin A, Arnold BF, Afreen S, Goto R, Huda TM, Haque R, et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. American Journal of Tropical Medicine and Hygiene 2013;89(1):130‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luby 2006
- Luby SP, Agboatwalla M, Painter J, Altaf A, Billhimer W, Keswick B, et al. Combining drinking water treatment and hand washing for diarrhoea prevention, a cluster randomised controlled trial. Tropical Medicine & International Health 2006;11(4):479‐89. [DOI] [PubMed] [Google Scholar]
Majorin 2017
- Majorin F, Torondel B, Routray P, Rout M, Clasen T. Identifying potential sources of exposure along the child feces management pathway: a cross‐sectional study among urban slums in Odisha, India. American Journal of Tropical Medicine and Hygiene 2017;97(3):861‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbuya 2016
- Mbuya MNN, Humphrey JH. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Maternal & Child Nutrition 2016;12(Suppl 1):106‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller‐Petrie 2016
- Miller‐Petrie MK, Voigt L, McLennan L, Cairncross S, Jenkins MW. Infant and young child feces management and enabling products for their hygienic collection, transport, and disposal in Cambodia. American Journal of Tropical Medicine and Hygiene 2016;94(2):456‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morita 2016
- Morita T, Godfrey S, George CM. Systematic review of evidence on the effectiveness of safe child faeces disposal interventions. Tropical Medicine & International Health 2016;21(11):1403‐19. [DOI] [PubMed] [Google Scholar]
Moya 2004
- Moya J, Bearer CF, Etzel RA. Children's behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004;113(4 Suppl):996‐1006. [PubMed] [Google Scholar]
Nery 2015
- Nery SV, McCarthy JS, Traub R, Andrews RM, Black J, Gray D, et al. A cluster‐randomised controlled trial integrating a community‐based water, sanitation and hygiene programme, with mass distribution of albendazole to reduce intestinal parasites in Timor‐Leste: the WASH for WORMS research protocol. BMJ Open 2015;5(12):e009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngure 2013
- Ngure FM, Humphrey JH, Mbuya MN, Majo F, Mutasa K, Govha M, et al. Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. American Journal of Tropical Medicine and Hygiene 2013;89(4):709‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2010
- Norman G, Pedley S, Takkouche B. Effects of sewerage on diarrhoea and enteric infections: a systematic review and meta‐analysis. Lancet Infectious Diseases 2010;10(8):536‐44. [DOI] [PubMed] [Google Scholar]
Pullan 2012
- Pullan RL, Brooker SJ. The global limits and population at risk of soil‐transmitted helminth infections in 2010. Parasites and Vectors 2012;5:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pullan 2014
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites and Vectors 2014;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ram 2007
- Ram PK, Naheed A, Brooks WA, Hossain MA, Mintz ED, Breiman RF, et al. Risk factors for typhoid fever in a slum in Dhaka, Bangladesh. Epidemiology and Infection 2007;135:458‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2011
- Reeves BC, Deeks JJ, Higgins JP, Wells GA. Chapter 13: Including non‐randomized studies. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roy 2011
- Roy E, Hasan KZ, Haque R, Fazlul Haque AKM, Siddique AK, Bradley Sack R. Patterns and risk factors for helminthiasis in rural children aged under 2 in Bangladesh. South African Journal of Child Health 2011;5(3):78‐84. [Google Scholar]
Scott 2008
- Scott B. Children's stool disposal – a review of prevalence of practice and its relationship with health, and recommendations for filling the evidence gaps (as supplied in 2008). Review on file.
Strunz 2014
- Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil‐transmitted helminth infection: a systematic review and meta‐analysis. PLOS Medicine 2014;11(3):e1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thapar 2004
- Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet 2004;363(9409):641‐53. [DOI] [PubMed] [Google Scholar]
Thomas 2013
- Thomas A, Bevan J. Developing and monitoring protocol for the elimination of open defecation in Sub‐Saharan Africa. 2013. www.ircwash.org/sites/default/files/thomas_and_bevan_elimination_of_open_defecation_in_sub‐saharan_africa.pdf (accessed prior to 9 August 2019).
Tumwine 2002
- Tumwine JK, Thompson J, Katua‐Katua M, Mujwajuzi M, Johnstone N, Wood E, Porras I. Diarrhoea and effects of different water sources, sanitation and hygiene behaviour in East Africa. Tropical Medicine and International Health 2002;7(9):750‐6. [DOI] [PubMed] [Google Scholar]
UN 2013
- United Nations. Millenium Development Goals. www.un.org/millenniumgoals/environ.shtml (accessed 13 March 2014).
UN 2016
- United Nations. Sustainable Development Goals. sustainabledevelopment.un.org/sdg6 (accessed 18 March 2016).
UNICEF 2012
- UNICEF. Pneumonia and diarrhoea: tackling the deadliest diseases for the world's poorest children. 2012. www.unicef.org/publications/index_65491.html (accessed prior to 9 August 2019).
Waddington 2009
- Waddington H, Snilstveit B, White H, Fewtrell L. Water, sanitation and hygiene interventions to combat childhood diarrhoea in developing countries. International Initiative for Impact Evaluation 2009;1:1‐119. [Google Scholar]
Wagner 1958
- Wagner EG, Lanoix JN. Excreta disposal for rural areas and small communities. 9th Edition. Geneva: World Health Organization, 1958. [PubMed] [Google Scholar]
Walker 2012
- Walker CL, Perin J, Aryee MJ, Boschi‐Pinto C, Black RE. Diarrhea incidence in low‐ and middle‐income countries in 1990 and 2010: a systematic review. BMC Public Health 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2013
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 9 August 2013).
WHO 2013
- World Health Organization. Diarrhoeal disease. www.who.int/mediacentre/factsheets/fs330/en/index.html (accessed 9 August 2013).
WHO/UNICEF 2006
- World Health Organization, United Nations Children's Fund. Core questions on drinking water and sanitation for household surveys. www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf. Geneva: World Health Organization, (accessed 13 March 2014).
WHO/UNICEF 2014
- World Health Organization, United Nations Children's Fund. Types of drinking‐water sources and sanitation. 2014. www.wssinfo.org/definitions‐methods/watsan‐categories/ (accessed 12 January 2014).
WHO/UNICEF 2015a
- World Health Organization, United Nations Children's Fund. Joint Monitoring Programme for Water Supply and Sanitation. Progress on sanitation and drinking water – 2015 update and MDG assessment. World Health Organization and United Nations Children's Fund (accessed prior to 9 August 2019).
WHO/UNICEF 2015b
- World Health Organization, United Nations Children's Fund. JMP Green Paper: Global monitoring of water, sanitation and hygiene post‐2015 Draft. Updated October 2015. www.wssinfo.org/fileadmin/user_upload/resources/JMP‐Green‐Paper‐15‐Oct‐2015.pdf (accessed 10 May 2016).
WHO/UNICEF 2018
- World Health Organization, United Nations Children's Fund. Core questions on drinking water, sanitation and hygiene for household surveys: 2018 update. New York: United Nations Children’s Fund (UNICEF) and World Health Organization 2018.
Wolf 2018
- Wolf J, Hunter PR, Freeman MC, Cumming O, Clasen T, Bartram J, et al. Impact of drinking water, sanitation and handwashing with soap on childhood diarrhoeal disease: updated meta‐analysis and meta‐regression. Tropical Medicine & International Health 2018;23(5):508‐25. [DOI] [PubMed] [Google Scholar]
WSP 2015
- Water and Sanitation Program. Management of child feces: current disposal practices. 2015. www.wsp.org/sites/wsp.org/files/publications/WSP‐CFD‐Summary‐Brief.pdf (accessed 18 March 2016).
Young 2011
- Young SL, Sherman PW, Lucks JB, Pelto GH. Why on earth?: evaluating hypotheses about the physiological functions of human geophagy. Quarterly Review of Biology 2011;86(2):97‐120. [DOI] [PubMed] [Google Scholar]
Ziegelbauer 2012
- Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil‐transmitted helminth infection: systematic review and meta‐analysis. PLOS Medicine 2012;9(1):e1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Majorin 2014
- Majorin F, Torondel B, Ka Seen Chan G, Clasen T. Interventions to improve disposal of child faeces for preventing diarrhoea and soil‐transmitted helminth infection. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD011055] [DOI] [PMC free article] [PubMed] [Google Scholar]


