Abstract
Influenza virus infection is a serious threat to humans and animals, with the potential to cause severe pneumonia and death. Annual vaccination strategies are a mainstay to prevent complications related to influenza. However, protection from the emerging subtypes of influenza A viruses (IAV) even in vaccinated individuals is challenging. Innate immune cells are the first cells to respond to IAV infection in the respiratory tract. Virus replication-induced production of cytokines from airway epithelium recruits innate immune cells to the site of infection. These leukocytes, namely, neutrophils, monocytes, macrophages, dendritic cells, eosinophils, natural killer cells, innate lymphoid cells, and γδ T cells, become activated in response to IAV, to contain the virus and protect the airway epithelium while triggering the adaptive arm of the immune system. This review addresses different anti-influenza virus schemes of innate immune cells and how these cells fine-tune the balance between immunoprotection and immunopathology during IAV infection. Detailed understanding on how these innate responders execute anti-influenza activity will help to identify novel therapeutic targets to halt IAV replication and associated immunopathology.
1. Introduction
Respiratory viruses infect millions around the world each year causing a range of symptoms and claiming thousands of lives [1–3]. Some viruses such as respiratory syncytial virus are lethal to the very young but harmless to most adults [4]. Other viruses, such as rhinovirus, cause essentially the same symptoms (predominantly a runny nose) in any age group [5]. Still others like influenza A virus (IAV) can cause severe infections in patients across age groups during one season, mild symptoms in another year or be lethal in yet another season [6]. In fact, IAV infections cause seasonal epidemics and global pandemics and are a major cause for public health concern. Although generally milder than pandemics, seasonal influenza epidemics can cause around 650,000 deaths globally each year [7]. Despite mainstay vaccination strategies to minimize IAV infections, influenza pandemics have occurred once every 10-30 years, primarily due to cross-species transmission and antigenic shifts in the virus [8, 9].
As a member of the Orthomyxoviridae family, IAV is an enveloped virus with an octasegmented negative sense, single-stranded RNA genome [6]. At present, 18 hemagglutinin (HA) and 11 neuraminidase (NA) subtypes of IAV are documented to circulate in nature [10]. Only two HA subtypes of IAV (H1N1 and H3N2) and two lineages of influenza B viruses (Victoria and Yamagata) cause annual epidemics in humans [6]. However, IAVs dominate, inducing more severe morbidity and mortality compared to influenza B viruses, therefore, will be the focus of this review [11–13].
Rapid changes in IAV surface antigens through antigenic shift have resulted in three pandemics during the 20th century [14]. Avian IAV subtypes (H5N1, H7N1, H7N2, H7N3, H7N9, H9N2, and H10N8) can cross the species barrier to infect humans [8, 15, 16] and cause severe, lethal disease. In fact, H5N1 highly pathogenic avian influenza (HPAI) and the H7N9 low pathogenic avian influenza viruses pose a serious public health concern due to their respective high fatality rates of 52.79% and 39.42% [17]. The most severe H1N1 influenza pandemic occurred in 1918 claiming over 50 million lives [18] while the last H1N1 pandemic in 2009 (pH1N1) is estimated to have claimed ~200,000 lives across the globe [19]. While annual vaccinations are highly encouraged by public health agencies, poor adherence and low efficacy increase the need for better strategies to understand host responses during IAV pathogenesis in order to delineate other mechanisms which enhance antiviral immunity.
Primary and secondary immune barriers play a crucial role in safeguarding the host against influenza. Physical barriers of the immune system including soluble components like mucus, collectins, and antimicrobial peptides provide the first line of defense by mitigating virus exposure to underlying airway epithelial cells which are the principal site for IAV replication [20, 21]. Upon breaching these physical barriers, IAVs bind to sialic acid receptors on airway epithelial cells and enter these cells to complete replicative cycles within, destroying infected cells in the process [22, 23]. Predilections for specific sialic acid linkages may restrict IAV to the upper respiratory tract. However, alterations in the sialic acid linkage preferences, particularly in reassortant viruses, can render IAV more adept at lower respiratory tract infection and dissemination [24, 25]. Infection of the epithelium activates the innate branch of the immune system which consists of humoral/soluble as well as cellular components.
Infected airway epithelial cells trigger innate immune responses in two ways. First, viral RNAs within infected cells are sensed by pattern recognition receptors such as toll-like receptors, nucleotide-binding oligomerization domain-like receptors, or retinoic acid-inducible gene (RIG)-I like receptors. Signaling through these receptors induces the production of antiviral soluble factors, including interferons (IFNs), which act on adjacent healthy cells in a paracrine manner to trigger antiviral gene transcription. Activation of mediators like protein kinase R, 2′5′-oligoadenylate synthetase, RNaseL, cleavage and polyadenylation factor [26, 27] in otherwise healthy cells induces an antiviral state thereby limiting viral replication [28]. IFNs also induce expression of interferon-stimulated genes (like myxovirus-1) that have strong anti-influenza activity [29]. Furthermore, infected alveolar epithelial cells in the airway secrete proinflammatory cytokines like TNFα, IL-1β, IL-6, CCL2, CCL5, CXCL8, and CXCL10 [30] in superfluity, which support innate immune cell recruitment [20, 31] to contain viral spread. However, the production of inflammatory cytokines is not always protective as cytokine storms arise when they are secreted in excess as observed during HPAI H5N1 virus infection [32–34] often leading to severe pneumonia and death. Secondly, phagocytosis of infected cells by antigen-presenting cells (APCs) can activate adaptive immune responses [20, 28] that help to eliminate the infection.
Innate immune cells provide the first line of cellular defense to combat IAV infection. Leukocytes like neutrophils, monocytes, eosinophils, natural killer (NK) cells, innate lymphoid cells (ILCs), and γδ T cells provide anti-influenza host protection by releasing preformed cytokines and granule contents that either directly or indirectly help the host to eliminate the threat posed by replicating virus. On the other hand, macrophages and dendritic cells (DCs) can phagocytose IAV-infected cells and present viral antigens to T cells, initiating adaptive immune responses including B cell-mediated humoral immunity. The aim of this review is to provide insight into the function of these innate leukocytes in immune protection and repair of damaged airways upon IAV infection.
2. Neutrophils: The First Recruits
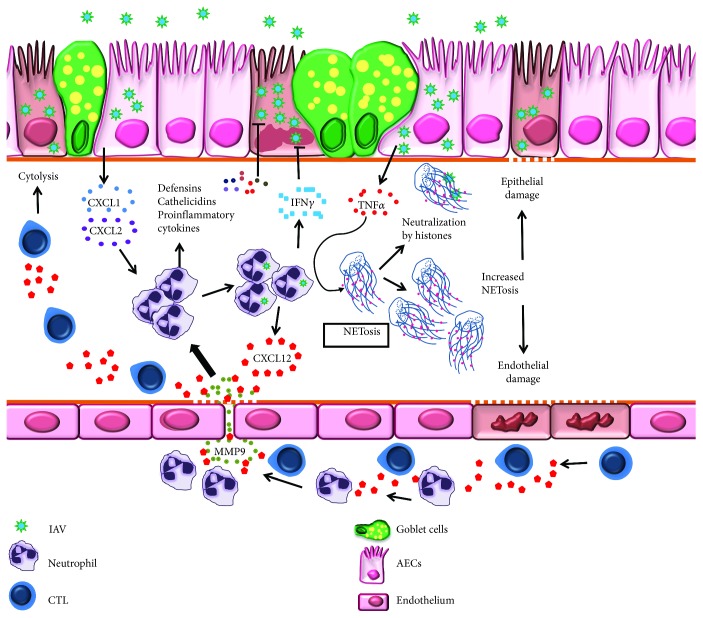
Neutrophils are among the first innate cells to be recruited during IAV infection. Neutrophils are granulocytes derived from hematopoietic stem cells influenced by IL-3, M-CSF, and G-CSF. While neutrophils are first to respond to any noxious stimuli, their role during influenza is complex and accumulation in the lungs is impacted both by virus strain and dose [35, 36]. Secretion of several chemotactic factors such as CCL3 [37], CXCL-1 (IL-8 in humans), and CXCL-2 [38] acts as chemotactic signals for neutrophil generation and recruitment. Neutrophils release MMP-9 to digest type IV collagen in the pulmonary endothelial basement membrane [37] and enter the lung tissue through CXCR2 engagement [39] (Figure 1).
Figure 1.
Schematic representation of neutrophil-mediated host defense mechanisms during influenza virus infection. Influenza A virus- (IAV)-infected airway epithelium releases neutrophil chemoattractants: CXCL1 and CXCL2. Neutrophils traffic into infected lungs by digesting endothelial basement membrane collagen. During trafficking, they release CXCL12-loaded vesicles/membranes which provide signals for CD8+ T cell migration and effector function. Once in the lungs, neutrophils secrete antimicrobial peptides and cytokines, including IFNγ, to inhibit IAV replication. TNFα produced by infected airway epithelium triggers the formation of neutrophil extracellular traps (NETs) which neutralize IAV particles. Enhanced NETosis damages airway epithelium and endothelium leading to severe pneumonia.
Neutropenia has been demonstrated to increase the pulmonary virus titer and mortality rate upon IAV infection [40] suggesting a protective role for neutrophils during influenza. Phagocytosis, release of granular contents, and production of cytokines are major effector functions of neutrophils. These granulocytes contain cationic antimicrobial peptides like defensins and cathelicidins that neutralize IAV [41, 42]. Cathelicidins have anti-influenza activity in vivo through the reduction of viral replication and hindering the production of inflammatory mediators [41], showcasing a promising role for neutrophil antimicrobial peptides during virus infections. Furthermore, proinflammatory cytokines produced by neutrophils limit virus replication and halt progression to severe disease [43, 44] (Figure 1). Neutrophils are susceptible to IAV infection [45–47], although infection has been demonstrated to be both productive (for pH1N1) [48] and abortive (for seasonal H1N1 and WSN33) [45, 49]. These discrepancies might be related to virus subtypes/strains used. Interestingly, mice treated with β-defensin had reduced IAV burden and an associated reduction in neutrophils in the lungs [21], underscoring the importance of a prudently adjudicated immune response to host protection. Nonetheless, IAV-infected neutrophils upregulate IFNs and other antiviral factors [45, 46] that limit viral replication (Figure 1).
In addition to these conventional roles, neutrophils also utilize more sophisticated strategies to safeguard the host during influenza. One such scheme is the production of neutrophil extracellular traps (NETs) through NETosis, a release of web like structures of nucleic acids coated with histones [50] which capture viral particles [51, 52] thereby preventing viral dissemination. Inflammatory mediators like TNFα and cathelicidins in the pulmonary environment trigger NETosis [53, 54] (Figure 1). Arginine in histones within NETs has differential aggregation properties for IAV wherein seasonal strains are more likely to be inhibited compared to pH1N1 [55]. However, mechanisms that dictate strain preferences for NETosis in response to IAV are unknown. Increased NET production, as observed in severe cases of H7N9 and pH1N1 infection [56], augmented damage to the pulmonary endothelia and epithelia [56, 57] leading to severe pneumonia suggesting that uncontrolled NET production may contribute to disease severity.
Neutrophils were also shown to guide CD8+ T cell activation and recruitment into the lungs during influenza emphasizing the reliance of adaptive immune cells on those from the innate branch. By presenting IAV antigens on the cell surface, neutrophils function as APCs to activate CD8+ T cells within the lungs [58]. In addition, neutrophil membrane-bound CXCL12 is used as a beacon by CD8+ T cells during migration into the IAV-infected lungs [59] (Figure 1). The importance of neutrophils in innate immune defenses against IAV is well defined. Yet, uncontrolled trafficking of neutrophils as observed during infection with highly pathogenic H5N1 and 1918 pandemic IAV [44, 60] results in the production of excessive reactive oxygen species [61, 62] that causes oxidative stress-mediated pulmonary damage. Additionally, neutrophil CXCL12 trails can recruit CD8+ T cells [59] in excess which may boost/perpetuate inflammation, lung damage and severe pathology through cytolysis of infected epithelia during IAV infection. Thus, the abundance of neutrophils in the IAV-infected lungs may dictate their precise role in immunoprotection or immunopathology.
3. Monocytes: Recruited in Time of Need
Monocytes, peripheral blood phagocytes, are recruited into the lungs during IAV infection. Based on cell surface receptors, CD14 and CD16, circulating human monocytes can be subdivided into three distinct subsets: classical (CD14++/CD16−), intermediate (CD14++/CD16+), and nonclassical (CD14+/CD16++) [63–65]. Compared to peripheral circulation (where classical monocytes are predominant), intermediate monocytes predominate in airways [66]. Similarly, murine monocytes (CD11b+CD115+) are divided into three subsets based on the expression of Ly6C as classical (Ly6C++), intermediate (Ly6C+), and nonclassical (Ly6C−) [67, 68].
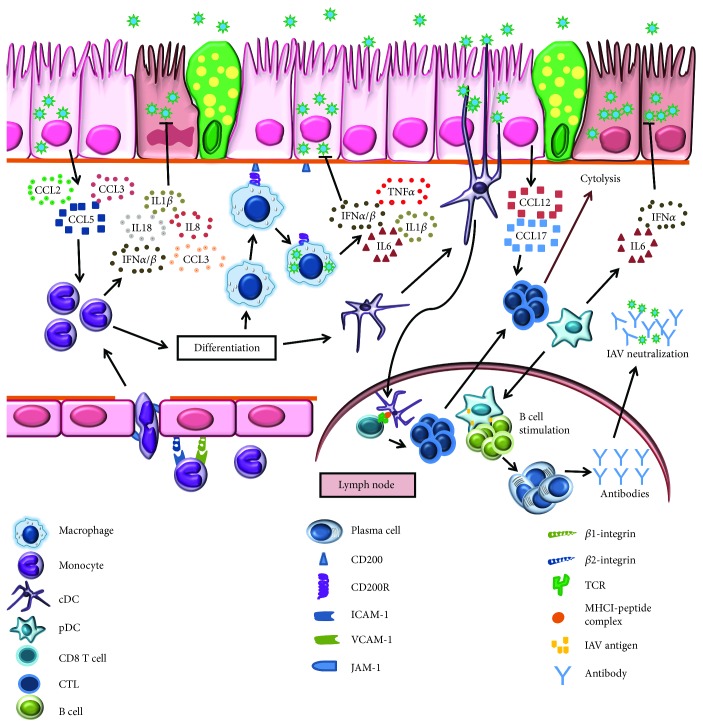
The recruitment of these circulatory monocytes across the endothelial/epithelial barrier into the lungs has been extensively studied [69]. IAV-infected alveolar macrophages and epithelial cells secrete monocyte attracting chemokines, CCL2, CCL3, and CCL5 [69–71]. However, transepithelial migration of monocytes occurs through the CCL2/CCR2 axis, wherein CCR2-expressing monocytes move chemotactically towards CCL2 [69]. Interaction between monocyte integrins and endothelial adhesion molecules mediates transendothelial migration of monocytes [69, 72–75]. During IAV infection, monocyte transmigration across the endothelial barrier is predominantly dependent on interactions between VCAM-1 and β1 integrin (CD49d) and ICAM-1 interactions with β2 integrin (CD11a/CD18 or CD11b/CD18) [69] and promoted by TNFα secreted by infected alveolar macrophages (AMs) [69, 76], integrin-associated protein (CD47) [69, 76, 77], and junctional adhesion molecule 3 [69, 76, 78] (Figure 2).
Figure 2.
Schematic representation of monocyte-, macrophage-, and dendritic cell- (DC-) controlled immunoprotection and immunopathology during influenza virus infection. Influenza A virus- (IAV-) infected airway epithelium produces monocyte chemoattractants: CCL2, CCL3, and CCL5. Monocytes interact with endothelium through β1 integrin, VCAM-1, and β2 integrin, ICAM-1, binding, and subsequent entry into the lung tissue is assisted by JAM. These activated monocytes secrete inflammatory cytokines that inhibit IAV replication. Monocytes exert anti-IAV activity by differentiating into phagocytic cells like macrophages and DCs. IAV infection downregulates CD200-CD200R activating macrophages. These activated macrophages release an array of inflammatory cytokines that limit IAV replication. Both conventional and plasmacytoid DCs (cDCs and pDCs) exert anti-IAV activity in lungs. IAV-activated cDCs migrate to lymph node and present antigen to CD8+ T cells. Chemokines, CCL12 and CCL17, secreted by infected airway epithelium provide signals for the activated cytotoxic T cells trafficking into the lungs where they lyse IAV-infected cells. The other DC subset, pDC migrate to lymph node and stimulate B cell differentiation into antibody secreting plasma cells. These antibodies neutralize IAV particles conferring humoral protection.
Similar to neutrophils, monocytes are susceptible to IAV infection [79–81] irrespective of the subset [31], and infection rates may vary with the IAV subtype with the highest infectivity for H5N1 and H7N9 than pH1N1 [81]. Virus-infected monocytes secrete inflammatory cytokines and chemokines [31, 80] (Figure 2) in a virus dose-dependent manner [31]. Infected monocytes produce large amounts of IFNs [80, 82, 83] well known for their anti-influenza activity in addition to serving as a source for an array of cytokines and chemokines; for example, U937 promonocytic cells produce IL-1β, IL-8, IL-18, CCL3 [82], and IFN-α/β [80, 83]. Importantly, the microenvironment of infected lungs drives monocyte differentiation into macrophages and DCs (Figure 2) which play a pivotal role in phagocytosis of virus-infected cells thereby priming the adaptive immune system. During airway inflammation, migration and differentiation of blood monocytes contribute to the pool of pulmonary macrophages [20]. The absence of CD14 on monocytes/macrophages alters the immunopathogenesis during complex disease-disease interactions that occur when IAV infects hosts with underlying allergic states [84] suggesting that the function of each subset is regulated by the inflammatory milieu during influenza. The role of monocyte-derived macrophages in protection from IAV infection is described elsewhere [85, 86].
Although monocytes provide immunoprotection against IAV infection, excessive infiltration of monocytes can induce immunopathology and mice exhibiting uncontrolled IAV replication have excessive monocyte recruitment into the lungs [87]. Conversely, monocyte-deficient CCR2−/− mice demonstrate increased survival following IAV infection [87–89], suggesting that monocytes can drive IAV-induced host pathology. Moreover, disease severity correlates with increased monocyte-derived cytokines [87] which in turn leads to immunopathology. Overall, the role in immune protection or pathology is determined by the number of infiltrating monocytes and the cytokine milieu.
4. Pulmonary Macrophages: Sentinels in Command?
The pulmonary macrophage population consists of alveolar macrophages (AMs) and interstitial macrophages (IMs) [86, 90]. Macrophages are phenotypically classified as proinflammatory (M1) and anti-inflammatory (M2). This classification is best suited for monocyte-derived macrophages wherein different cytokines and growth factors are used for differentiation but not AMs [86]. In relation to IAV, AMs are best studied while the role of IMs is ambiguous due to phenotypic similarity with monocytes and technical difficulty in liberating them from the lung tissue [91]. Irrespective of lower phagocytic ability compared to AMs [92], owing to their location in the interstitium, IMs phagocytose pathogens that have evaded AMs thereby providing a second line of defense within the tissue. The potential antiviral activity of IMs needs to be explored further.
As tissue resident professional phagocytes that safeguard the airway against intruding pulmonary pathogens, AMs maintain lung homeostasis [93, 94]. Cytokines, TGF-β and GM-CSF, promote differentiation of fetal monocytes into AMs [95–97]. With a prolonged half-life of about 1-5 weeks, the AM population is primarily maintained via self-renewal [98–100]. Pulmonary homeostasis is conserved in part by interaction of these AMs with alveolar epithelial cells (AECs). At steady state, AECs that express CD200 engage surface CD200R on AMs controlling the production of inflammatory cytokines [101]. However, IAV infection lowers the expression of CD200 and CD200R [101] compromising the CD200-CD200R axis which leads to macrophage activation and production of inflammatory mediators (Figure 2).
Pigs [102] and mice [103, 104] intranasally administered with clodronate liposome to deplete AMs have higher lung virus titers and severe disease characterized by impaired clearance of surfactant proteins, cellular debris, dead cells, pulmonary edema, and inflammation [102, 103]. Moreover, transgenic mice selectively depleted of AMs exhibit severe influenza [104, 105] suggesting a critical role for AMs in limiting virus-induced airway injury. However, the possible protective role of IMs may not be underestimated as they have more potent activators of antigen-specific T cells compared to AMs [90]. As most other leukocytes, AMs too secrete type I IFNs during IAV infection [106, 107] (Figure 2). IAV infection of AMs showed species specificity where human AMs are not infected while the murine counterparts exhibit abortive infection [108, 109]. Since IAVs fail to infect human AMs [108], an alternative mechanism like phagocytosis of apoptotic cells may also trigger AM activation and cytokine secretion during infection [110].
Owing to the contribution of AMs in maintaining lung tissue integrity and pulmonary homeostasis, their role in protecting AECs from IAV-induced damage is important. Type I AECs are responsible for gas exchange, and their impairment during infection leads to pulmonary dysfunction. On the other hand, type II AECs can self-renew or divide to replenish damaged type I AECs [111]. Engagement of CysLT, a cell surface cysteinyl leukotriene receptor on type I AEC, enhances their susceptibility to IAV infection [104]. However, AMs suppress the CysLT pathway in type I AEC [104] conferring the protection to AECs during infection. Peroxisome proliferator-activated receptor gamma (PPAR-γ), highly expressed in AMs and critical during their development [112], restricts AMs from excessive proinflammatory cytokine production [113] thereby maintaining lung homeostasis. Parallelly, PPAR-γ stimulation lessens IAV-associated inflammation within airways [114] and increases the secretion of tissue remodeling (MMP7 and MMP9) and epi-endothelial growth factors (EGF and VEGF) [115] suggesting a critical role for AMs as regulators of wound healing and tissue repair upon IAV infection.
The contribution of macrophages to immunopathology is evident from the fact that CCR2−/− mice are protected from IAV-induced pulmonary tissue destruction and mortality [89]. Furthermore, protective mechanisms in these macrophage deficient mice are traced to delayed migration of T cells [89] suggesting that macrophages alter cellular immune responses. The role of macrophages in IAV-mediated pathology is based on findings from monocyte-derived macrophages (MDMs). The induction of macrophage-derived cytokines, IFN-α/β, TNFα, CCL2, CCL3, CCL5, and CXCL10, is greater for highly pathogenic H5N1 virus than seasonal influenza viruses [116, 117] suggesting that these inflammatory mediators may contribute to IAV-induced immunopathology. In contrast, MDMs infected with H5N1 virus showed lower expression of cytokines, namely, IFN-α/β, TNFα, CCL5, and CCL8 at early times postinfection [118]. This may be representative of an immune evasion strategy of IAVs to prolong uncontrolled replication and replication-induced pathology to gain a foothold in the host. Cumulatively, these observations suggest that pulmonary dysfunction observed in fatal cases of influenza depends on how macrophages respond to the invading virus.
5. Dendritic Cells: Calling for Help
Dendritic cells (DCs) are professional APCs that patrol the body surfaces (skin, gut, or airway) for intruding microbes or insults. They play a key role in host immunity by bridging innate and adaptive arms of the immune system [119, 120]. DCs are broadly classified as CD11c+ conventional DCs (cDCs) and CD11c− plasmacytoid DCs (pDCs) [121, 122]. Furthermore, two subsets of cDCs have been identified in the airways: CD103+CD11b− (CD103+ cDCs) and CD103−CD11b+ (CD11b+ cDCs) cells [123]; the latter of which resides in the lamina propria which lies immediately beneath the airway epithelium [124, 125]. In a steady state, the major DC population in the airway mucosa is CD103+ cDCs followed by CD11b+ cDCs and pDCs [124, 126, 127]. Humans also have more myeloid DCs (similar to cDCs in mice) in the airways than pDCs [66]. The CD103+ cDCs can extend their dendrites or traverse the alveolar epithelia into the airway for surveillance and antigen capture [123] (Figure 2).
Acute IAV infection leads to a decrease in cDCs and pDCs in peripheral circulation [128–130] and a sustained increase in the respiratory tract [129, 130] (Figure 2) suggesting active trafficking of DC populations during influenza. Following intranasal inoculation of IAV (X31 H3N2) in mice, pulmonary cDC subsets demonstrated sustained increase while it was transient for pDC. Moreover, draining lymph nodes (DLNs) contained only cDCs [127] suggesting maturation and migration of cDCs to DLNs following IAV infection (Figure 2). Antimicrobial peptide, β-defensin, induces a reduction in lung cDCs during pH1N1 infection [21], emphasizing the complex interaction and dependence between the soluble and cellular factors of the innate immune system. Depletion of CD103+ cDCs aggravated disease severity [127] suggesting a crucial role during influenza. Therefore, the totality of cDCs' contribution to lung health during IAV infection remains unknown, and since these subsets of cDCs secrete inflammatory mediators [131], a possible role in recruiting other leukocytes during influenza needs to be more fully explored.
Compared to other DC subsets, CD103+ cDCs are highly efficient at viral antigen uptake and migration to DLNs [131] and are the most efficient cross-presenters of the immune system [132] as they differ in their antigen processing and presentation capabilities [133]. While CD103+ cDCs process and present IAV antigens to CD8+ T cells efficiently [127, 131], IAV-activated CD11b+ cDCs fail to prime CD8+ T cells [127] (Figure 2). This might be related to the ability of these cells to support IAV replication ex vivo; CD103+ cDCs support productive IAV infection while CD11b+ cDCs do not [134], providing an explanation for CD103+ cDC's superior antigen-presenting capacity. However, whether CD103+ cDCs support IAV replication in vivo is unclear with data suggesting that viral antigen is acquired through phagocytosis instead to allow cross-presentation to CD8+ T cells [132].
The effective function of cDCs in priming the T cell-mediated immune response depends on (1) trafficking of IAV-activated DCs into DLNs, (2) presentation of antigen to specific CD8+ T cells, and (3) lung homing capacity of activated CD8+ T cells. Migration of pulmonary cDCs into DLNs depends on expression of CCR7, as ccr7-deficient mice lack DC trafficking into DLNs [133, 135]. Both CD103+ cDCs and CD11b+ cDCs express CCR7 [131] confirming the migratory nature of these cDCs. Once in the DLNs, these DCs prime IAV antigen-specific CD8+ T cells, which migrate into infected lungs in a CCR4-dependent manner [136] to exert anti-influenza activity (Figure 2).
Following IAV infection, pDCs produce large amounts of chemoattractants, importantly CXCL1, CCL2, CCL5, and CXCL10 [137] (Figure 2). Mice selectively depleted of pDCs exhibited delayed recruitment of T cells into airways [138] suggesting that they regulate accumulation of T cells during early IAV infection. The ability of pDCs to support IAV replication is dose dependent [139, 140]; susceptibility observed with higher multiplicity of infections (MOIs) [139, 140] might not correlate with infection in humans. Since, at lower MOIs, pDCs do not support IAV replication [139], they fail to prime CD4+ or CD8+ T cells directly [127]. However, pDCs can uptake IAV antigens from virus-infected cells and upregulate CCR7 expression in response [141] suggesting that they too can migrate to DLNs and cross-present acquired IAV antigens. During IAV infection, pDCs induce differentiation of B cells into antibody-secreting plasma cells through IFNα and IL-6 [142] and the depletion of pDCs abrogates antibody secretion [127]. The activation of lung-resident B cells during pH1N1 infection [143] may be led by these DC subsets that encounter viral antigen thereby providing dual protection at the site of infection as well as by couriering a call for help (Figure 2).
As with most factors of the immune response, moderation is required for health and excessive cytokine levels can be detrimental to the host. Highly pathogenic H5N1 virus-infected pDCs produce higher amounts of IFNα than those infected with less virulent seasonal H1N1 and H3N2 strains [139, 144]. While IFNα secreted from pDCs does have antiviral functions [139, 145] (Figure 2), prolific production during IAV infection results in uncontrolled inflammation and host pathology [146], alluding that pDCs may also contribute to the cytokine storm during infection. As an antithesis to sublethal IAV infection, lethal infection causes the accumulation of pDCs in DLNs with enhanced expression of Fas ligand (FasL) [147, 148] which engages with Fas, a membrane protein of the death receptor family [149] expressed on IAV-specific CD8+ T cells eliminating them via Fas-mediated apoptosis [147, 148]. Conversely, since IAV infection in pDC-depleted mice leads to enhanced accumulation of inflammatory cells, particularly CD11b+ cDCs and macrophages which produce massive amounts of proinflammatory cytokines (TNFα and IL-6) [150], pDCs may be important immunoregulators during lethal IAV infection. The protective versus deleterious effect of pDCs depends on the infectious dose.
6. Eosinophils: Additional Sources of Host Defense
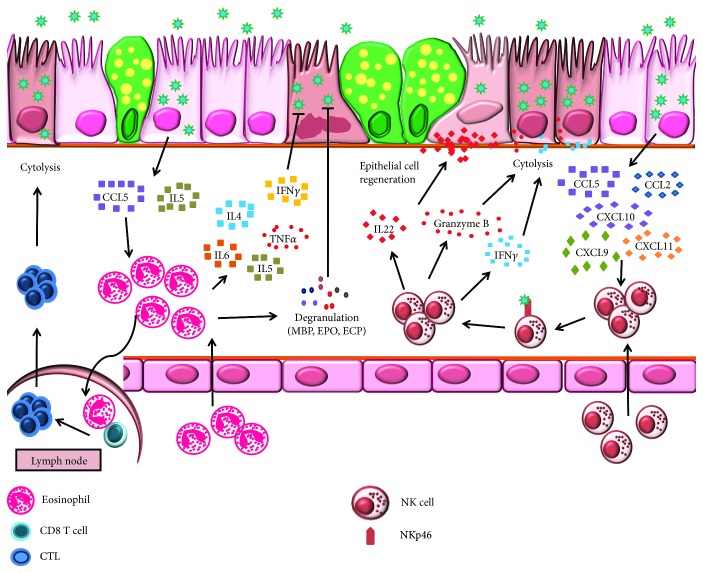
Eosinophils are not usually considered mediators of anti-influenza immunity. However, epidemiologic data associated with the 2009 H1N1 pandemic suggested that asthmatics, presumably with pulmonary eosinophilia, were less likely to suffer from IAV-induced morbidity and mortality [151–153]. Pediatric influenza patients who developed acute pneumonia demonstrated a rise in the serum IL-5 levels and peripheral eosinophilia [154], suggesting that eosinophil recruitment may be necessary for late-stage anti-influenza host defense. Pulmonary eosinophilia has also been documented in IAV-infected mice [155–158] suggesting that cytokines like CCL5 [30] or IL-5 [155] produced during IAV infection may drive eosinophil migration (Figure 3). Based on known functions of eosinophils during parasite infections and allergy [159], it may be likely that their influx into the lung during mid-late infection is in support of reparative processes required after IAV-induced cytopathology.
Figure 3.
Immunoprotection and immunopathology mediated by eosinophils and natural killer (NK) cells during influenza. CCL5 and IL-5 released by IAV-infected airway epithelium mediate eosinophil trafficking into the lungs. Eosinophils undergo piecemeal degranulation and secrete inflammatory cytokines that exert anti-IAV activity. Additionally, IAV-infected eosinophils migrate to draining lymph nodes to present viral antigen to CD8+ T cells. Once activated, cytotoxic T cells traffic to the lungs to lyse IAV-infected cells. NK cell recruitment is driven by CCL2, CCL5, CXCL9, CXCL10, and CXCL11 secreted by IAV-infected airway epithelium. Engagement of IAV with NK cell receptor NKp46 activates NK cell effector functions. Perforins, granzyme B, and IFNγ released by NK cell destroy IAV-infected cells while IL-22 aids in the regeneration of damaged epithelium.
Eosinophils are conducive to IAV infection and undergo piecemeal degranulation in response to IAV [160]. This selective release of granule proteins such as major basic protein, eosinophil peroxidase, eosinophil cationic protein, and eosinophil-derived neurotoxin, in addition to immunoregulatory cytokines and chemokines [161, 162] at the infection site, may either immunomodulate other leukocytes or help contain the virus in situ (Figure 3). Additionally, mice suffering from acute allergy are protected from IAV-induced airway damage [157] suggesting that eosinophils are important mediators during anti-influenza immunity in special populations of patients such as those with a TH2 bias. Our findings that eosinophils were capable of trafficking to DLNs following IAV infection and their putative function in IAV antigen presentation in the context of MHCI to activate CD8+ T cells [160] forecast multifaceted functions for eosinophils during influenza pathogenesis [163].
7. Natural Killer Cells: On-Site Killing
As large granular lymphocytes representing about 10% of lung resident lymphocytes, NK cells accumulate in the respiratory tract in response to IAV infection [164–167]. This increase correlates with an initial decrease in circulatory NK cells [166, 168] suggesting that during early IAV infection NK cells are recruited directly from blood [164] (Figure 3). NK cell recruitment to the site of infection depends on expression of chemokine receptors, namely, CCR2, CCR5, and CXCR3 [164, 167, 169] through interaction with ligands like CCL2, CCL5, CXCL9, CXCL10, and CXCL11 [164, 167, 169] (Figure 3).
Primary effector functions (cytolysis of infected cells and cytokine secretion [170]) of IAV-stimulated NK cells are facilitated through cytotoxic receptors, NKp46, NKp44, and NKp30, of which viral HA binds directly to sialic acids expressed on NKp46 [171–173] (Figure 3). Viral subtypes may dictate the strength of NK cell activation wherein H5N1 and 1918 H1N1 viruses induce stronger responses compared to the pH1N1 virus [174], suggesting that the difference in receptor binding preference of the infecting virus differentially regulates NK cell activation. Cytolysis of IAV-infected cells is achieved by degranulation and release of perforin and granzyme, as well as by lytic activity of IFNγ secreted by activated NK cells [174] (Figure 3).
Epithelial cytopathology is a principal outcome of IAV infection of the respiratory tract which NK cells can mitigate through IL-22 production [165]. Airway epithelial cells expressing IL-22R [175] respond to IL-22 through epithelial regeneration which reduces inflammation [165]. This demonstrated the pivotal role of the IL-22-IL-22R axis in maintaining epithelial integrity and tissue homeostasis upon IAV infection (Figure 3). In vitro studies suggest that NK cell apoptosis triggered during IAV replication within these cells [171] may be an immune evasion strategy empoyed by IAV. Since the full spectrum of interaction between NK cells and IAV is yet to be elucidated, additional studies that focus on NK cells are warranted.
Cells that coexpress the NK cell marker and the T cell receptor, termed NKT cells, have gained more attention from influenza virologists in recent years. These cells provide immunoprotection during IAV infection by reducing inflammation, primarily the accumulation of inflammatory monocytes [176], reducing immunosuppressive activity of myeloid-derived suppressor cells [177], or through the production of IL-22 that controls lung epithelial damage [178].
8. Innate Lymphoid Cells: Immediate Depots of Cytokines
The ILC family consists of cytotoxic NK cells and three noncytotoxic members, ILC1, ILC2, and ILC3, that are innate counterparts of T cells that do not express antigen receptors [179]. Various insults activate ILC subsets; ILC1 responds to viruses and intracellular bacteria, ILC2 to extracellular parasites and allergens, and ILC3 to extracellular bacteria and fungi [179–182].
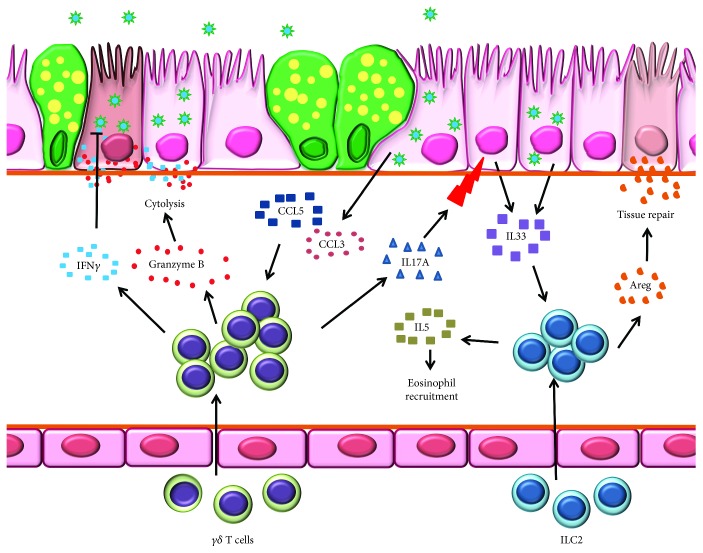
Although ILC2s are known to provide antihelminthic immunity, recent findings demonstrate their influx into lungs following IAV infection [183] most likely in response to alveolar epithelial- and macrophage-derived IL-33 [155, 184–187] sensed through surface-expressed IL-33R [188] (Figure 4). As potent producers of IL-5, ILC2s may regulate eosinophil infiltration during influenza [155, 188]. Within the lungs, ILC2s increase expression of genes encoding amphiregulin and extracellular matrix proteins asporin, decorin, and dermatopontin [188]. Strikingly, ILC2-derived amphiregulin is involved in tissue repair and remodeling [188] (Figure 4) suggesting that ILC2s are capable of lung tissue homeostasis during influenza. As major contributors of IFNγ, a crucial cytokine in the anti-influenza armamentarium, ILC1s induce cytolysis of IAV-infected cells [179–182]. However, IFNγ can also enhance influenza disease severity possibly through suppression of ILC2 function [189].
Figure 4.
Innate lymphoid cells (ILCs) and γδ T cells are important during early stages of influenza virus infection. Schematic representation of immunoprotection and immunopathology mediated by ILCs in the IAV-infected lungs. γδ T cell migration into the infected lungs is driven along the CCR5-CCL5 axis. γδ T cells destroy infected cells through the secretion of IFNγ and perforin-granzyme B pathway and secrete IL-17A to trigger airway epithelium release of IL-33, a chemoattractant for ILC2. In addition, IL-33 secreted by IAV-infected epithelium enhances the trafficking of ILC2 into the lungs. Activated ILC2s release amphiregulin (Areg) which helps to repair tissue damage and IL-5 which induces eosinophil trafficking.
9. γδ T Cells: Holding Down the Fort
Innate-like T cells expressing γ and δ chains as receptors, γδ T cells constitute around 1-5% of blood lymphocytes. Given that γδ T cells respond antecedent to αβ T cells during infection [190], they may serve a pivotal role in early-stage antiviral host defense during influenza. Intravenous adoptive transfer of γδ T cells to IAV-infected mice inhibits viral replication and controls disease progression [191] suggesting that these cells can traffic to lungs (Figure 4). Moreover, IAV-activated γδ T cells express high levels of chemokine receptors CXCR5, CCR1, and CCR5 [192] allowing their migration towards CCL3 and CCL5 [193] abundant IAV-infected lungs [116] (Figure 4). As a means of self-promotion, IAV-activated γδ T cells produce preferred chemokines including CCL5 [192] to chaperon circulatory γδ T cell recruitment into the lungs. The production of CCL5 is dependent on virus subtypes (higher for avian viruses than human viruses [192]), indicating that infection with avian viruses promotes more robust γδ T cell infiltration.
Similar to other innate lymphocytes, γδ T cells exert anti-influenza activity either by direct killing [194, 195] or noncytolytic inhibition of virus replication through the secretion of IFNγ [192, 196] (Figure 4). The inhibition of human H1N1 virus replication is achieved by both noncytolytic and cytolytic mechanisms [192, 196]. In contrast, avian IAVs are resistant to the antiviral activity of IFNγ. The cytotoxicity of γδ T cells involves cytolytic granules (perforin and granzymes), NKG2D, TRAIL, and Fas-FasL pathways [194, 195]. Furthermore, γδ T cells effectively kill cells infected with various IAV subtypes [192, 195, 196] suggesting their role in the heterosubtypic immune response. It has been argued that IAV-infected macrophages and sentinel DCs alter the mevalonate pathway which in turn liberates isopentyl pyrophosphate (IPP), an antigen for γδ T cells [196]. The production of IPP activates γδ T cells, thereby conferring immune protection independent of incoming virus subtypes.
Subtypes of IAV differ in the degree of disease severity they elicit in the host. Apart from engaging the T cell receptor, IAV HA protein activates γδ T cells [197, 198] through α-2,3 and α-2,6 sialic acid receptor engagement on the cell surface [198] suggesting that HA-mediated differential activation of γδ T cells might be related to the degree of protection provided by these immune cells to IAVs with different sialic acid preferences.
The rapid secretion of IL-17A by γδ T cells from neonatal mice promotes AECs to produce IL-33 (Figure 4), protecting neonates from severe influenza [199]. Since γδ T cell-mediated IL-33 production serves as a cue for IL-33R expressing ILC recruitment [188], which in turn promotes tissue repair through amphiregulin secretion [199], the complex cell-cell interactions that occur even in the innate cell compartment of the immune system are necessary and important to anti-influenza host protection (Figure 4). These observations suggested that IL-17A and the timing of its release can modulate the balance between protection and pathogenicity in a host infected with IAV.
10. Conclusion
Despite annual vaccination strategies, IAV infections pose a continuous threat to human health. Development of better therapeutic options is required to tackle the growing burden of IAV infections. This requires a thorough understanding of virus pathogenesis and contribution of immediate responders during infection. Innate immune cells are critical to primary immunity against IAV infection at the respiratory barrier. However, the balance between innate immune cell-induced protection and pathology is governed by their abundance, the potency of secreted immune mediators, infecting viral strain/subtype, and immune status of the host (Figure 5). Hence, the identification of key regulators in innate leukocytes that mediate protection against IAV may provide broader options for therapeutic interventions.
Figure 5.
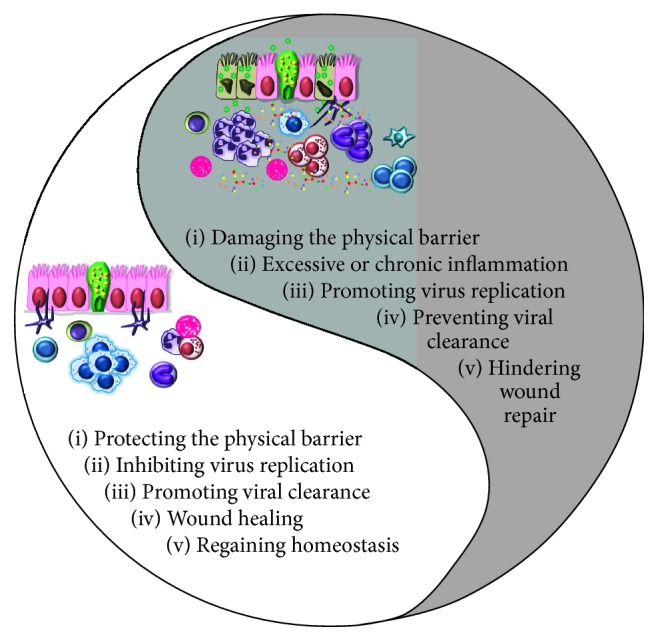
Yin and Yang of innate immune cell functions during influenza virus infection. At steady state, innate immune cells corporate to maintain pulmonary homeostasis while clearing IAV effectively with minimal tissue damage. When infected with a highly pathogenic virus or when immediate immune responses are unable to clear the infection, the immune system goes on overdrive causing excessive proinflammatory cytokine production and uncontrolled inflammation which can be pathogenic and detrimental to the host.
Acknowledgments
The authors would like to thank Samarasinghe Group members, Kim LeMessurier, Maneesha Palipane, and Meenakshi Tiwary, for their comments and feedback on the manuscript. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01-AI125481 to AES.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Ye S., Wang T. Laboratory epidemiology of respiratory viruses in a large children’s hospital. Medicine. 2018;97(30, article e11385) doi: 10.1097/MD.0000000000011385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaunt E. R., Harvala H., McIntyre C., Templeton K. E., Simmonds P. Disease burden of the most commonly detected respiratory viruses in hospitalized patients calculated using the disability adjusted life year (DALY) model. Journal of Clinical Virology. 2011;52(3):215–221. doi: 10.1016/j.jcv.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visseaux B., Burdet C., Voiriot G., et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS One. 2017;12(7, article e0180888) doi: 10.1371/journal.pone.0180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karron R. A., Black R. E. Determining the burden of respiratory syncytial virus disease: the known and the unknown. The Lancet. 2017;390(10098):917–918. doi: 10.1016/S0140-6736(17)31476-9. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs S. E., Lamson D. M., St. George K., Walsh T. J. Human rhinoviruses. Clinical Microbiology Reviews. 2013;26(1):135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knipe D. M., Howley P. M., Griffin D. E., Knipe, Howley . Fields virology. In: Wright P., Neumann G., Kawaoka Y., editors. Orthomyxoviruses. 6th. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. pp. 1186–1243. [Google Scholar]
- 7.World Health Organization. Influenza (Seasonal) Geneva: WHO; 2018. [Google Scholar]
- 8.Chan J. F.-W., To K. K. W., Chen H., Yuen K. Y. Cross-species transmission and emergence of novel viruses from birds. Current Opinion in Virology. 2015;10:63–69. doi: 10.1016/j.coviro.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster R. G., Govorkova E. A. Continuing challenges in influenza. Annals of the New York Academy of Sciences. 2014;1323(1):115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong S., Zhu X., Li Y., et al. New world bats harbor diverse influenza A viruses. PLoS Pathogens. 2013;9(10, article e1003657) doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caini S., Kroneman M., Wiegers T., el Guerche-Séblain C., Paget J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza and Other Respiratory Viruses. 2018;12(6):780–792. doi: 10.1111/irv.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu P., Presanis A. M., Bond H. S., Lau E. H. Y., Fang V. J., Cowling B. J. A joint analysis of influenza-associated hospitalizations and mortality in Hong Kong, 1998-2013. Scientific Reports. 2017;7(1):p. 929. doi: 10.1038/s41598-017-01021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daley A., Nallusamy R., Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. Journal of Paediatrics and Child Health. 2000;36(4):332–335. doi: 10.1046/j.1440-1754.2000.00533.x. [DOI] [PubMed] [Google Scholar]
- 14.Cox N. J., Subbarao K. Global epidemiology of influenza: past and present. Annual Review of Medicine. 2000;51(1):407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 15.Kalthoff D., Globig A., Beer M. (Highly pathogenic) avian influenza as a zoonotic agent. Veterinary Microbiology. 2010;140(3-4):237–245. doi: 10.1016/j.vetmic.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Wong S. S. Y., Yuen K. Avian influenza virus infections in humans. Chest. 2006;129(1):156–168. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Influenza at the Human-Animal Interface: Monthly Risk Assessment and Summary. Geneva: WHO; 2019. [Google Scholar]
- 18.Taubenberger J. K., Morens D. M. 1918 influenza: the mother of all pandemics. Emerging Infectious Diseases. 2006;12(1):15–22. doi: 10.3201/eid1209.05-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonsen L., Spreeuwenberg P., Lustig R., et al. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Medicine. 2013;10(11, article e1001558) doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreijtz J. H. C. M., Fouchier R. A. M., Rimmelzwaan G. F. Immune responses to influenza virus infection. Virus Research. 2011;162(1-2):19–30. doi: 10.1016/j.virusres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 21.LeMessurier K. S., Lin Y., McCullers J. A., Samarasinghe A. E. Antimicrobial peptides alter early immune response to influenza A virus infection in C57BL/6 mice. Antiviral Research. 2016;133:208–217. doi: 10.1016/j.antiviral.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan W., Dong Z., Li F., et al. Visualizing influenza virus infection in living mice. Nature Communications. 2013;4(1, article 2369) doi: 10.1038/ncomms3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manicassamy B., Manicassamy S., Belicha-Villanueva A., Pisanelli G., Pulendran B., Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tse H., To K. K. W., Wen X., et al. Clinical and virological factors associated with viremia in pandemic influenza A/H1N1/2009 virus infection. PLoS One. 2011;6(9, article e22534) doi: 10.1371/journal.pone.0022534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taubenberger J. K., Morens D. M. The pathology of influenza virus infections. Annual Review of Pathology. 2008;3(1):499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hale B. G., Randall R. E., Ortin J., Jackson D. The multifunctional NS1 protein of influenza A viruses. The Journal of General Virology. 2008;89(10):2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 27.Kochs G., Garcia-Sastre A., Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. Journal of Virology. 2007;81(13):7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Liu S., Goraya M. U., Maarouf M., Huang S., Chen J. L. Host immune response to influenza A virus infection. Frontiers in Immunology. 2018;9:p. 320. doi: 10.3389/fimmu.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haller O., Kochs G. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. Journal of Interferon & Cytokine Research. 2011;31(1):79–87. doi: 10.1089/jir.2010.0076. [DOI] [PubMed] [Google Scholar]
- 30.Matsukura S., Kokubu F., Kubo H., et al. Expression of RANTES by normal airway epithelial cells after influenza virus A infection. American Journal of Respiratory Cell and Molecular Biology. 1998;18(2):255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- 31.Hoeve M. A., Nash A. A., Jackson D., Randall R. E., Dransfield I. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One. 2012;7(1, article e29443) doi: 10.1371/journal.pone.0029443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong M. D., Simmons C. P., Thanh T. T., et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Medicine. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peiris J. S. M., Yu W. C., Leung C. W., et al. Re-emergence of fatal human influenza A subtype H5N1 disease. The Lancet. 2004;363(9409):617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To K. F., Chan P. K. S., Chan K. F., et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. Journal of Medical Virology. 2001;63(3):242–246. doi: 10.1002/1096-9071(200103)63:3<242::AID-JMV1007>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Tate M. D., Ioannidis L. J., Croker B., Brown L. E., Brooks A. G., Reading P. C. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011;6(3, article e17618) doi: 10.1371/journal.pone.0017618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tate M. D., Brooks A. G., Reading P. C. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respiratory Research. 2008;9(1):p. 57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley L. M., Douglass M. F., Chatterjee D., Akira S., Baaten B. J. G. Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathogens. 2012;8(4, article e1002641) doi: 10.1371/journal.ppat.1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden F. G., Fritz R., Lobo M. C., Alvord W., Strober W., Straus S. E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. The Journal of Clinical Investigation. 1998;101(3):643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wareing M. D., Shea A. L., Inglis C. A., Dias P. B., Sarawar S. R. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunology. 2007;20(3):369–378. doi: 10.1089/vim.2006.0101. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa H. Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. Journal of Virology. 2008;82(6):2772–2783. doi: 10.1128/JVI.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlow P. G., Svoboda P., Mackellar A., et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6(10, article e25333) doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daher K. A., Selsted M. E., Lehrer R. I. Direct inactivation of viruses by human granulocyte defensins. Journal of Virology. 1986;60(3):1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tate M. D., Deng Y. M., Jones J. E., Anderson G. P., Brooks A. G., Reading P. C. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. Journal of Immunology. 2009;183(11):7441–7450. doi: 10.4049/jimmunol.0902497. [DOI] [PubMed] [Google Scholar]
- 44.Perrone L. A., Plowden J. K., García-Sastre A., Katz J. M., Tumpey T. M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathogens. 2008;4(8, article e1000115) doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malachowa N., Freedman B., Sturdevant D. E., et al. Differential ability of pandemic and seasonal H1N1 influenza A viruses to alter the function of human neutrophils. mSphere. 2018;3(1) doi: 10.1128/mSphereDirect.00567-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivan F. X., Tan K. S., Phoon M. C., et al. Neutrophils infected with highly virulent influenza H3N2 virus exhibit augmented early cell death and rapid induction of type I interferon signaling pathways. Genomics. 2013;101(2):101–112. doi: 10.1016/j.ygeno.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y., Lu M., Lau L. T., et al. Neutrophils may be a vehicle for viral replication and dissemination in human H5N1 avian influenza. Clinical Infectious Diseases. 2008;47(12):1575–1578. doi: 10.1086/593196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z., Huang T., Yu F., et al. Infectious progeny of 2009 A (H1N1) influenza virus replicated in and released from human neutrophils. Scientific Reports. 2015;5(1, article 17809) doi: 10.1038/srep17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassidy L. F., Lyles D. S., Abramson J. S. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. Journal of Clinical Microbiology. 1988;26(7):1267–1270. doi: 10.1128/jcm.26.7.1267-1270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yipp B. G., Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 51.Jenne C. N., Wong C. H. Y., Zemp F. J., et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host & Microbe. 2013;13(2):169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Saitoh T., Komano J., Saitoh Y., et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host & Microbe. 2012;12(1):109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Hemmers S., Teijaro J. R., Arandjelovic S., Mowen K. A. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One. 2011;6(7, article e22043) doi: 10.1371/journal.pone.0022043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tripathi S., Verma A., Kim E. J., White M. R., Hartshorn K. L. LL-37 modulates human neutrophil responses to influenza A virus. Journal of Leukocyte Biology. 2014;96(5):931–938. doi: 10.1189/jlb.4A1113-604RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoeksema M., Tripathi S., White M., et al. Arginine-rich histones have strong antiviral activity for influenza A viruses. Innate Immunity. 2015;21(7):736–745. doi: 10.1177/1753425915593794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L., Liu L., Zhang Y., et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza A infection. The Journal of Infectious Diseases. 2018;217(3):428–437. doi: 10.1093/infdis/jix475. [DOI] [PubMed] [Google Scholar]
- 57.Narasaraju T., Yang E., Samy R. P., et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. The American Journal of Pathology. 2011;179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hufford M. M., Richardson G., Zhou H., et al. Influenza-infected neutrophils within the infected lungs act as antigen presenting cells for anti-viral CD8+ T cells. PLoS One. 2012;7(10, article e46581) doi: 10.1371/journal.pone.0046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim K., Hyun Y. M., Lambert-Emo K., et al. Neutrophil trails guide influenza-specific CD8+ T cells in the airways. Science. 2015;349(6252, article aaa4352) doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tumpey T. M., Garcia-Sastre A., Taubenberger J. K., et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. Journal of Virology. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yatmaz S., Seow H. J., Gualano R. C., et al. Glutathione peroxidase-1 reduces influenza A virus-induced lung inflammation. American Journal of Respiratory Cell and Molecular Biology. 2013;48(1):17–26. doi: 10.1165/rcmb.2011-0345OC. [DOI] [PubMed] [Google Scholar]
- 62.Vlahos R., Stambas J., Bozinovski S., Broughton B. R. S., Drummond G. R., Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathogens. 2011;7(2, article e1001271) doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas G. D., Hamers A. A. J., Nakao C., et al. Human blood monocyte subsets: a new gating strategy defined using cell surface markers identified by mass cytometry. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(8):1548–1558. doi: 10.1161/ATVBAHA.117.309145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ziegler-Heitbrock L. Blood monocytes and their subsets: established features and open questions. Frontiers in Immunology. 2015;6:p. 423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziegler-Heitbrock L., Ancuta P., Crowe S., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 66.Baharom F., Thomas S., Rankin G., et al. Dendritic cells and monocytes with distinct inflammatory responses reside in lung mucosa of healthy humans. Journal of Immunology. 2016;196(11):4498–4509. doi: 10.4049/jimmunol.1600071. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds G., Haniffa M. Human and mouse mononuclear phagocyte networks: a tale of two species? Frontiers in Immunology. 2015;6:p. 330. doi: 10.3389/fimmu.2015.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J., Zhang L., Yu C., Yang X. F., Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker Research. 2014;2(1):p. 1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herold S., von Wulffen W., Steinmueller M., et al. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. Journal of Immunology. 2006;177(3):1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- 70.Bußfeld D., Kaufmann A., Meyer R. G., Gemsa D., Sprenger H. Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactivated influenza A virus. Cellular Immunology. 1998;186(1):1–7. doi: 10.1006/cimm.1998.1295. [DOI] [PubMed] [Google Scholar]
- 71.Sprenger H., Meyer R. G., Kaufmann A., Bussfeld D., Rischkowsky E., Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. Journal of Experimental Medicine. 1996;184(3):1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zen K., Parkos C. A. Leukocyte-epithelial interactions. Current Opinion in Cell Biology. 2003;15(5):557–564. doi: 10.1016/S0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 73.Kwiatkowski R., Artrip J. H., Ankersmit J., et al. Importance of CD49d-VCAM interactions in human monocyte adhesion to porcine endothelium. Xenotransplantation. 1998;5(1):67–74. doi: 10.1111/j.1399-3089.1998.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 74.Li X. C., Miyasaka M., Issekutz T. B. Blood monocyte migration to acute lung inflammation involves both CD11/CD18 and very late activation antigen-4-dependent and independent pathways. The Journal of Immunology. 1998;161(11):6258–6264. [PubMed] [Google Scholar]
- 75.Meerschaert J., Furie M. B. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. The Journal of Immunology. 1995;154(8):4099–4112. [PubMed] [Google Scholar]
- 76.Rosseau S., Selhorst J., Wiechmann K., et al. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. Journal of Immunology. 2000;164(1):427–435. doi: 10.4049/jimmunol.164.1.427. [DOI] [PubMed] [Google Scholar]
- 77.Brown E. J., Frazier W. A. Integrin-associated protein (CD47) and its ligands. Trends in Cell Biology. 2001;11(3):130–135. doi: 10.1016/S0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 78.Woodfin A., Voisin M. B., Beyrau M., et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature Immunology. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamichhane P. P., Puthavathana P. PR8 virus harbouring H5N1 NS gene contributed for THP-1 cell tropism. Virus. 2018;29(4):548–552. doi: 10.1007/s13337-018-0499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamichhane P. P., Boonnak K., Changsom D., et al. H5N1 NS genomic segment distinctly governs the influenza virus infectivity and cytokine induction in monocytic cells. Asian Pacific Journal of Allergy and Immunology. 2018;36(1):58–68. doi: 10.12932/AP0870. [DOI] [PubMed] [Google Scholar]
- 81.Lee A. C. Y., To K. K. W., Zhu H., et al. Avian influenza virus A H7N9 infects multiple mononuclear cell types in peripheral blood and induces dysregulated cytokine responses and apoptosis in infected monocytes. The Journal of General Virology. 2017;98(5):922–934. doi: 10.1099/jgv.0.000751. [DOI] [PubMed] [Google Scholar]
- 82.Vongsakul M., Kasisith J., Noisumdaeng P., Puthavathana P. The difference in IL-1β, MIP-1α, IL-8 and IL-18 production between the infection of PMA activated U937 cells with recombinant vaccinia viruses inserted 2004 H5N1 influenza HA genes and NS genes. Asian Pacific Journal of Allergy and Immunology. 2011;29(4):349–356. [PubMed] [Google Scholar]
- 83.Ludwig S., Ehrhardt C., Neumeier E. R., Kracht M., Rapp U. R., Pleschka S. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. The Journal of Biological Chemistry. 2001;276(14):10990–10998. doi: 10.1074/jbc.M009902200. [DOI] [PubMed] [Google Scholar]
- 84.Palipane M., Snyder J. D., LeMessurier K. S., Schofield A. K., Woolard S. N., Samarasinghe A. E. Macrophage CD14 impacts immune defenses against influenza virus in allergic hosts. Microbial Pathogenesis. 2019;127:212–219. doi: 10.1016/j.micpath.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cline T. D., Beck D., Bianchini E. Influenza virus replication in macrophages: balancing protection and pathogenesis. The Journal of General Virology. 2017;98(10):2401–2412. doi: 10.1099/jgv.0.000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicol M. Q., Dutia B. M. The role of macrophages in influenza A virus infection. Future Virology. 2014;9(9):847–862. doi: 10.2217/fvl.14.65. [DOI] [Google Scholar]
- 87.Lin S. J., Lo M., Kuo R. L., et al. The pathological effects of CCR2+ inflammatory monocytes are amplified by an IFNAR1-triggered chemokine feedback loop in highly pathogenic influenza infection. Journal of Biomedical Science. 2014;21(1):p. 99. doi: 10.1186/s12929-014-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin K. L., Suzuki Y., Nakano H., Ramsburg E., Gunn M. D. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. The Journal of Immunology. 2008;180(4):2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 89.Dawson T. C., Beck M. A., Kuziel W. A., Henderson F., Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. The American Journal of Pathology. 2000;156(6):1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaynagetdinov R., Sherrill T. P., Kendall P. L., et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. American Journal of Respiratory Cell and Molecular Biology. 2013;49(2):180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liegeois M., Legrand C., Desmet C. J., Marichal T., Bureau F. The interstitial macrophage: a long-neglected piece in the puzzle of lung immunity. Cellular Immunology. 2018;330:91–96. doi: 10.1016/j.cellimm.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Fathi M., Johansson A., Lundborg M., Orre L., Sköld C. M., Camner P. Functional and morphological differences between human alveolar and interstitial macrophages. Experimental and Molecular Pathology. 2001;70(2):77–82. doi: 10.1006/exmp.2000.2344. [DOI] [PubMed] [Google Scholar]
- 93.Kopf M., Schneider C., Nobs S. P. The development and function of lung-resident macrophages and dendritic cells. Nature Immunology. 2015;16(1):36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 94.Hussell T., Bell T. J. Alveolar macrophages: plasticity in a tissue-specific context. Nature Reviews Immunology. 2014;14(2):81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 95.Yu X., Buttgereit A., Lelios I., et al. The cytokine TGF-β promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47(5):903–912.e4. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 96.van de Laar L., Saelens W., de Prijck S., et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity. 2016;44(4):755–768. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 97.Guilliams M., de Kleer I., Henri S., et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. Journal of Experimental Medicine. 2013;210(10):1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomez Perdiguero E., Klapproth K., Schulz C., et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tarling J. D., Lin H. S., Hsu S. Self-renewal of pulmonary alveolar macrophages: evidence from radiation chimera studies. Journal of Leukocyte Biology. 1987;42(5):443–446. doi: 10.1002/jlb.42.5.443. [DOI] [PubMed] [Google Scholar]
- 100.Sawyer R. T. The cytokinetic behavior of pulmonary alveolar macrophages in monocytopenic mice. Journal of Leukocyte Biology. 1986;39(1):89–99. doi: 10.1002/jlb.39.1.89. [DOI] [PubMed] [Google Scholar]
- 101.Snelgrove R. J., Goulding J., Didierlaurent A. M., et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nature Immunology. 2008;9(9):1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 102.Kim H. M., Lee Y. W., Lee K. J., et al. Alveolar macrophages are indispensable for controlling influenza viruses in lungs of pigs. Journal of Virology. 2008;82(9):4265–4274. doi: 10.1128/JVI.02602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tate M. D., Pickett D. L., van Rooijen N., Brooks A. G., Reading P. C. Critical role of airway macrophages in modulating disease severity during influenza virus infection of mice. Journal of Virology. 2010;84(15):7569–7580. doi: 10.1128/JVI.00291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cardani A., Boulton A., Kim T. S., Braciale T. J. Alveolar macrophages prevent lethal influenza pneumonia by inhibiting infection of type-1 alveolar epithelial cells. PLoS Pathogens. 2017;13(1, article e1006140) doi: 10.1371/journal.ppat.1006140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schneider C., Nobs S. P., Heer A. K., et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathogens. 2014;10(4, article e1004053) doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J., Nikrad M. P., Travanty E. A., et al. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One. 2012;7(3, article e29879) doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu W. C. L., Chan R. W. Y., Wang J., et al. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. Journal of Virology. 2011;85(14):6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ettensohn D. B., Frampton M. W., Nichols J. E., Roberts N. J., Jr. Human alveolar macrophages may not be susceptible to direct infection by a human influenza virus. The Journal of Infectious Diseases. 2016;214(11):1658–1665. doi: 10.1093/infdis/jiw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Londrigan S. L., Short K. R., Ma J., et al. Infection of mouse macrophages by seasonal influenza viruses can be restricted at the level of virus entry and at a late stage in the virus life cycle. Journal of Virology. 2015;89(24):12319–12329. doi: 10.1128/JVI.01455-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trinchieri G. Type I interferon: friend or foe? Journal of Experimental Medicine. 2010;207(10):2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ward H. E., Nicholas T. E. Alveolar type I and type II cells. Australian and New Zealand Journal of Medicine. 1984;14(5, Supplement 3):731–734. doi: 10.1111/j.1445-5994.1984.tb04928.x. [DOI] [PubMed] [Google Scholar]
- 112.Schneider C., Nobs S. P., Kurrer M., Rehrauer H., Thiele C., Kopf M. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nature Immunology. 2014;15(11):1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 113.Chawla A. Control of macrophage activation and function by PPARs. Circulation Research. 2010;106(10):1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moseley C. E., Webster R. G., Aldridge J. R. Peroxisome proliferator-activated receptor and AMP-activated protein kinase agonists protect against lethal influenza virus challenge in mice. Influenza and Other Respiratory Viruses. 2010;4(5):307–311. doi: 10.1111/j.1750-2659.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang S., Zhu B., Cheon I. S., et al. PPAR-γ in macrophages limits pulmonary inflammation and promotes host recovery following respiratory viral infection. Journal of Virology. 2019;93(9) doi: 10.1128/JVI.00030-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou J., Law H. K. W., Cheung C. Y., Ng I. H. Y., Peiris J. S. M., Lau Y. L. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. The Journal of Infectious Diseases. 2006;194(1):61–70. doi: 10.1086/504690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheung C. Y., Poon L. L. M., Lau A. S., et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? The Lancet. 2002;360(9348):1831–1837. doi: 10.1016/S0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 118.Friesenhagen J., Boergeling Y., Hrincius E., Ludwig S., Roth J., Viemann D. Highly pathogenic avian influenza viruses inhibit effective immune responses of human blood-derived macrophages. Journal of Leukocyte Biology. 2012;92(1):11–20. doi: 10.1189/jlb.0911479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waithman J., Mintern J. D. Dendritic cells and influenza A virus infection. Virulence. 2012;3(7):603–608. doi: 10.4161/viru.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heath W. R., Carbone F. R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nature Immunology. 2009;10(12):1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 121.Shortman K., Liu Y. J. Mouse and human dendritic cell subtypes. Nature Reviews Immunology. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 122.Liu Y. J., Kanzler H., Soumelis V., Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nature Immunology. 2001;2(7):585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 123.Sung S.-S. J., Fu S. M., Rose C. E., Jr., Gaskin F., Ju S. T., Beaty S. R. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. The Journal of Immunology. 2006;176(4):2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 124.Lambrecht B. N., Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annual Review of Immunology. 2012;30(1):243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 125.Beaty S. R., Rose C. E., Jr., Sung S. S. J. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. Journal of Immunology. 2007;178(3):1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 126.Helft J., Ginhoux F., Bogunovic M., Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunological Reviews. 2010;234(1):55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 127.GeurtsvanKessel C. H., Willart M. A. M., van Rijt L. S., et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. Journal of Experimental Medicine. 2008;205(7):1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lichtner M., Mastroianni C. M., Rossi R., et al. Severe and persistent depletion of circulating plasmacytoid dendritic cells in patients with 2009 pandemic H1N1 infection. PLoS One. 2011;6(5, article e19872) doi: 10.1371/journal.pone.0019872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gill M. A., Long K., Kwon T., et al. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. The Journal of Infectious Diseases. 2008;198(11):1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gill M. A., Palucka A. K., Barton T., et al. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. The Journal of Infectious Diseases. 2005;191(7):1105–1115. doi: 10.1086/428589. [DOI] [PubMed] [Google Scholar]
- 131.Ho A. W. S., Prabhu N., Betts R. J., et al. Lung CD103+ dendritic cells efficiently transport influenza virus to the lymph node and load viral antigen onto MHC class I for presentation to CD8 T cells. The Journal of Immunology. 2011;187(11):6011–6021. doi: 10.4049/jimmunol.1100987. [DOI] [PubMed] [Google Scholar]
- 132.Helft J., Manicassamy B., Guermonprez P., et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. Journal of Clinical Investigation. 2012;122(11):4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.del Rio M.-L., Rodriguez-Barbosa J. I., Kremmer E., Förster R. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. The Journal of Immunology. 2007;178(11):6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 134.Moltedo B., Li W., Yount J. S., Moran T. M. Unique type I interferon responses determine the functional fate of migratory lung dendritic cells during influenza virus infection. PLoS Pathogens. 2011;7(11, article e1002345) doi: 10.1371/journal.ppat.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim T. S., Braciale T. J. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One. 2009;4(1, article e4204) doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mikhak Z., Strassner J. P., Luster A. D. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. Journal of Experimental Medicine. 2013;210(9):1855–1869. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Iparraguirre A., Tobias J. W., Hensley S. E., et al. Two distinct activation states of plasmacytoid dendritic cells induced by influenza virus and CpG 1826 oligonucleotide. Journal of Leukocyte Biology. 2008;83(3):610–620. doi: 10.1189/jlb.0807511. [DOI] [PubMed] [Google Scholar]
- 138.Wolf A. I., Buehler D., Hensley S. E., et al. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. Journal of Immunology. 2009;182(2):871–879. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- 139.Thitithanyanont A., Engering A., Ekchariyawat P., et al. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-α and TLR ligands. Journal of Immunology. 2007;179(8):5220–5227. doi: 10.4049/jimmunol.179.8.5220. [DOI] [PubMed] [Google Scholar]
- 140.Vangeti S., Gertow J., Yu M., et al. Human blood and tonsil plasmacytoid dendritic cells display similar gene expression profiles but exhibit differential type I IFN responses to influenza A virus infection. Journal of Immunology. 2019;202(7):2069–2081. doi: 10.4049/jimmunol.1801191. [DOI] [PubMed] [Google Scholar]
- 141.Lui G., Manches O., Angel J., Molens J. P., Chaperot L., Plumas J. Plasmacytoid dendritic cells capture and cross-present viral antigens from influenza-virus exposed cells. PLoS One. 2009;4(9, article e7111) doi: 10.1371/journal.pone.0007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jego G., Palucka A. K., Blanck J. P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 143.Doorley L. A., LeMessurier K. S., Iverson A. R., Palipane M., Samarasinghe A. E. Humoral immune responses during asthma and influenza co-morbidity in mice. Immunobiology. 2017;222(12):1064–1073. doi: 10.1016/j.imbio.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sandbulte M. R., Boon A. C. M., Webby R. J., Riberdy J. M. Analysis of cytokine secretion from human plasmacytoid dendritic cells infected with H5N1 or low-pathogenicity influenza viruses. Virology. 2008;381(1):22–28. doi: 10.1016/j.virol.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fonteneau J.-F., Gilliet M., Larsson M., et al. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101(9):3520–3526. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 146.Davidson S., Crotta S., McCabe T. M., Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nature Communications. 2014;5(1) doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boonnak K., Vogel L., Feldmann F., Feldmann H., Legge K. L., Subbarao K. Lymphopenia associated with highly virulent H5N1 virus infection due to plasmacytoid dendritic cell-mediated apoptosis of T cells. Journal of Immunology. 2014;192(12):5906–5912. doi: 10.4049/jimmunol.1302992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Langlois R. A., Legge K. L. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. Journal of Immunology. 2010;184(8):4440–4446. doi: 10.4049/jimmunol.0902984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waring P., Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunology & Cell Biology. 1999;77(4):312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 150.Soloff A. C., Weirback H. K., Ross T. M., Barratt-Boyes S. M. Plasmacytoid dendritic cell depletion leads to an enhanced mononuclear phagocyte response in lungs of mice with lethal influenza virus infection. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35(4):309–317. doi: 10.1016/j.cimid.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bramley A. M., Dasgupta S., Skarbinski J., et al. Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection - United States, 2009. Influenza and Other Respiratory Viruses. 2012;6(6):e134–e142. doi: 10.1111/j.1750-2659.2012.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Louie J. K., Acosta M., Winter K., et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A (H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 153.Vaillant L., la Ruche G., Tarantola A., Barboza P., epidemic intelligence team Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveillance. 2009;14(33) doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 154.Terai M., Honda T., Yamamoto S., et al. Early induction of interleukin-5 and peripheral eosinophilia in acute pneumonia in Japanese children infected by pandemic 2009 influenza A in the Tokyo area. Microbiology and Immunology. 2011;55(5):341–346. doi: 10.1111/j.1348-0421.2011.00320.x. [DOI] [PubMed] [Google Scholar]
- 155.Gorski S. A., Hahn Y. S., Braciale T. J. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS Pathogens. 2013;9(9, article e1003615) doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DiPiazza A., Nogales A., Poulton N., Wilson P. C., Martínez-Sobrido L., Sant A. J. Pandemic 2009 H1N1 influenza Venus reporter virus reveals broad diversity of MHC class II-positive antigen-bearing cells following infection in vivo. Scientific Reports. 2017;7(1, article 10857) doi: 10.1038/s41598-017-11313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Samarasinghe A. E., Woolard S. N., Boyd K. L., Hoselton S. A., Schuh J. M., McCullers J. A. The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunology and Cell Biology. 2014;92(5):449–459. doi: 10.1038/icb.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Furze R. C., Hussell T., Selkirk M. E. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infection and Immunity. 2006;74(3):1924–1932. doi: 10.1128/IAI.74.3.1924-1932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee J. J., Jacobsen E. A., McGarry M. P., Schleimer R. P., Lee N. A. Eosinophils in health and disease: the LIAR hypothesis. Clinical and Experimental Allergy. 2010;40(4):563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Samarasinghe A. E., Melo R. C. N., Duan S., et al. Eosinophils promote antiviral immunity in mice infected with influenza A virus. The Journal of Immunology. 2017;198(8):3214–3226. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spencer L. A., Szela C. T., Perez S. A. C., et al. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. Journal of Leukocyte Biology. 2009;85(1):117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cañas J. A., Sastre B., Mazzeo C., et al. Exosomes from eosinophils autoregulate and promote eosinophil functions. Journal of Leukocyte Biology. 2017;101(5):1191–1199. doi: 10.1189/jlb.3AB0516-233RR. [DOI] [PubMed] [Google Scholar]
- 163.LeMessurier K. S., Samarasinghe A. E. Eosinophils: nemeses of pulmonary pathogens? Current Allergy and Asthma Reports. 2019;19(8):p. 36. doi: 10.1007/s11882-019-0867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Helden M. J. G., Zaiss D. M. W., Sijts A. J. A. M. CCR2 defines a distinct population of NK cells and mediates their migration during influenza virus infection in mice. PLoS One. 2012;7(12, article e52027) doi: 10.1371/journal.pone.0052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar P., Thakar M. S., Ouyang W., Malarkannan S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunology. 2013;6(1):69–82. doi: 10.1038/mi.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gazit R., Gruda R., Elboim M., et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nature Immunology. 2006;7(5):517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 167.Carlin L. E., Hemann E. A., Zacharias Z. R., Heusel J. W., Legge K. L. Natural killer cell recruitment to the lung during influenza A virus infection is dependent on CXCR3, CCR5, and virus exposure dose. Frontiers in Immunology. 2018;9:p. 781. doi: 10.3389/fimmu.2018.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Forberg H., Hauge A. G., Valheim M., et al. Early responses of natural killer cells in pigs experimentally infected with 2009 pandemic H1N1 influenza A virus. PLoS One. 2014;9(6, article e100619) doi: 10.1371/journal.pone.0100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grégoire C., Chasson L., Luci C., et al. The trafficking of natural killer cells. Immunological Reviews. 2007;220(1):169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nature Immunology. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 171.Mao H., Tu W., Qin G., et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. Journal of Virology. 2009;83(18):9215–9222. doi: 10.1128/JVI.00805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Arnon T. I., Achdout H., Lieberman N., et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103(2):664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 173.Mandelboim O., Lieberman N., Lev M., et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409(6823):1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 174.Du N., Zhou J., Lin X., et al. Differential activation of NK cells by influenza A pseudotype H5N1 and 1918 and 2009 pandemic H1N1 viruses. Journal of Virology. 2010;84(15):7822–7831. doi: 10.1128/JVI.00069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zheng Y., Valdez P. A., Danilenko D. M., et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Medicine. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 176.Kok W. L., Denney L., Benam K., et al. Pivotal advance: invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. Journal of Leukocyte Biology. 2012;91(3):357–368. doi: 10.1189/jlb.0411184. [DOI] [PubMed] [Google Scholar]
- 177.De Santo C., Salio M., Masri S. H., et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. The Journal of Clinical Investigation. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Paget C., Ivanov S., Fontaine J., et al. Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. Journal of Biological Chemistry. 2012;287(12):8816–8829. doi: 10.1074/jbc.M111.304758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vivier E., Artis D., Colonna M., et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 180.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 181.Eberl G., Colonna M., di Santo J. P., McKenzie A. N. J. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237, article aaa6566) doi: 10.1126/science.aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spits H., Artis D., Colonna M., et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nature Reviews Immunology. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 183.Li B. W. S., de Bruijn M. J. W., Lukkes M., et al. T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. European Journal of Immunology. 2019;49(1):144–156. doi: 10.1002/eji.201747421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Robinson K. M., Ramanan K., Clay M. E., McHugh K. J., Rich H. E., Alcorn J. F. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunology. 2018;11(1):199–208. doi: 10.1038/mi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mirchandani A. S., Salmond R. J., Liew F. Y. Interleukin-33 and the function of innate lymphoid cells. Trends in Immunology. 2012;33(8):389–396. doi: 10.1016/j.it.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 186.Le Goffic R., Arshad M. I., Rauch M., et al. Infection with influenza virus induces IL-33 in murine lungs. American Journal of Respiratory Cell and Molecular Biology. 2011;45(6):1125–1132. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 187.Chang Y. J., Kim H. Y., Albacker L. A., et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature Immunology. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Monticelli L. A., Sonnenberg G. F., Abt M. C., et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature Immunology. 2011;12(11):1045–1054. doi: 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Califano D., Furuya Y., Roberts S., Avram D., McKenzie A. N. J., Metzger D. W. IFN-γ increases susceptibility to influenza A infection through suppression of group II innate lymphoid cells. Mucosal Immunology. 2018;11(1):209–219. doi: 10.1038/mi.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chien Y. H., Meyer C., Bonneville M. γδ T cells: first line of defense and beyond. Annual Review of Immunology. 2014;32(1):121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 191.Tu W., Zheng J., Liu Y., et al. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. Journal of Experimental Medicine. 2011;208(7):1511–1522. doi: 10.1084/jem.20110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qin G., Liu Y., Zheng J., et al. Type 1 responses of human Vγ9Vδ2 T cells to influenza A viruses. Journal of Virology. 2011;85(19):10109–10116. doi: 10.1128/JVI.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Glatzel A., Wesch D., Schiemann F., Brandt E., Janssen O., Kabelitz D. Patterns of chemokine receptor expression on peripheral blood γδ T lymphocytes: strong expression of CCR5 is a selective feature of Vδ2/Vγ9 γδ T cells. The Journal of Immunology. 2002;168(10):4920–4929. doi: 10.4049/jimmunol.168.10.4920. [DOI] [PubMed] [Google Scholar]
- 194.Li H., Xiang Z., Feng T., et al. Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cellular & Molecular Immunology. 2013;10(2):159–164. doi: 10.1038/cmi.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Qin G., Mao H., Zheng J., et al. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. The Journal of Infectious Diseases. 2009;200(6):858–865. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jameson J. M., Cruz J., Costanzo A., Terajima M., Ennis F. A. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human γδ T lymphocytes. Cellular Immunology. 2010;264(1):71–77. doi: 10.1016/j.cellimm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dong P., Ju X., Yan Y., et al. γδ T cells provide protective function in highly pathogenic avian H5N1 influenza A virus infection. Frontiers in Immunology. 2018;9:p. 2812. doi: 10.3389/fimmu.2018.02812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lu Y., Li Z., Ma C., et al. The interaction of influenza H5N1 viral hemagglutinin with sialic acid receptors leads to the activation of human γδ T cells. Cellular & Molecular Immunology. 2013;10(6):463–470. doi: 10.1038/cmi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Guo X. J., Dash P., Crawford J. C., et al. Lung γδ T cells mediate protective responses during neonatal influenza infection that are associated with type 2 immunity. Immunity. 2018;49(3):531–544.e6. doi: 10.1016/j.immuni.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]