Abstract
MicroRNAs (miRNAs) are non-coding RNA molecules that are generally encoded by endogenous genes and exert suppressive effects on post-transcriptional regulation of their target genes by translation repression or degradation of mRNA. This subsequently mediates activation or blocking of downstream signaling pathways associated with oral malignancies. Aberrant levels of certain miRNAs have been identified in cell experiments, clinical carcinomatous specimens, saliva, serum or plasma samples of patients with oral malignancies. miRNAs are associated with multiple aspects of oral cancer, including tumor growth, cellular proliferation, apoptosis, migration, invasion, metastasis, glycometabolism, radiosensitivity and chemosensitivity. miRNAs have the potential to be used in clinical applications as minimally invasive or non-invasive tools for early diagnosis and prognosis by the detection of serum, plasma and saliva levels, and may provide a new ancillary or additional reference index of traditional pathological grading and clinical staging. Furthermore, miRNAs may be used as prognostic biomarkers or targets for novel therapies for oral cancer.
Keywords: oral carcinoma, microRNA, target gene, biomarker, non-invasive method
1. Introduction
Oral cancer is a common and fatal malignancy among head and neck malignant neoplasms; the number of new cases of oral cancer globally was 354,864 in 2018 (1). At present, principal treatments of oral cancer include extensive exeresis of the primary carcinoma, with or without neck dissection, and pre- or postoperative adjuvant chemotherapy and radiotherapy (2). However, the overall 5-year survival rate of patients with oral cancer was 65%, and the overall 5-year survival rate of patients with advanced oral cancer was as low as 27% between 2007 and 2013 in the USA (3). Despite the application of reconstructive radical resection and postoperative radiotherapy or chemotherapy, the 5-year survival rate of patients with terminal oral cancer has not improved effectively over the past years (4–6). Furthermore, the dysphagia, maxillofacial malformation and dysarthria induced by the aforementioned therapies negatively affect the quality of life and psychology of patients (7). Therefore, there is a requirement to identify more effective treatment strategies to improve the survival rate and reduce complications of patients with oral cancer.
MicroRNAs (miRNAs/miRs) have attracted increasing attention over the past years as their roles in malignant tumors, where they regulate target genes and downstream signaling pathways, have been recognized (8). Non-coding RNA, composed of 18–22 nucleotides, silences corresponding target genes by binding to the 3′-untranslated regions (3′UTRs) of mRNA to mediate the biological behavior of cancer cells (9). Dysregulation of miRNA in hepatoma, lymphoma and colorectal, ovarian and pancreatic cancer has been previously reported (10–15). In oral carcinoma, miRNAs are associated with oral carcinomatous cell proliferation, apoptosis, invasion, metastasis, epithelial-mesenchymal transition (EMT), chemoresistance, radioresistance and cell cycle arrest. The abnormal expression of miRNA detected in tumor, serum and saliva samples obtained from patients with oral cancer has clinical significance in prognosis prediction and the development of effective treatments (16–19). The present review discusses recent developments with regard to miRNAs and their potential clinical applications in oral cancer.
2. Biogenesis and function of miRNA
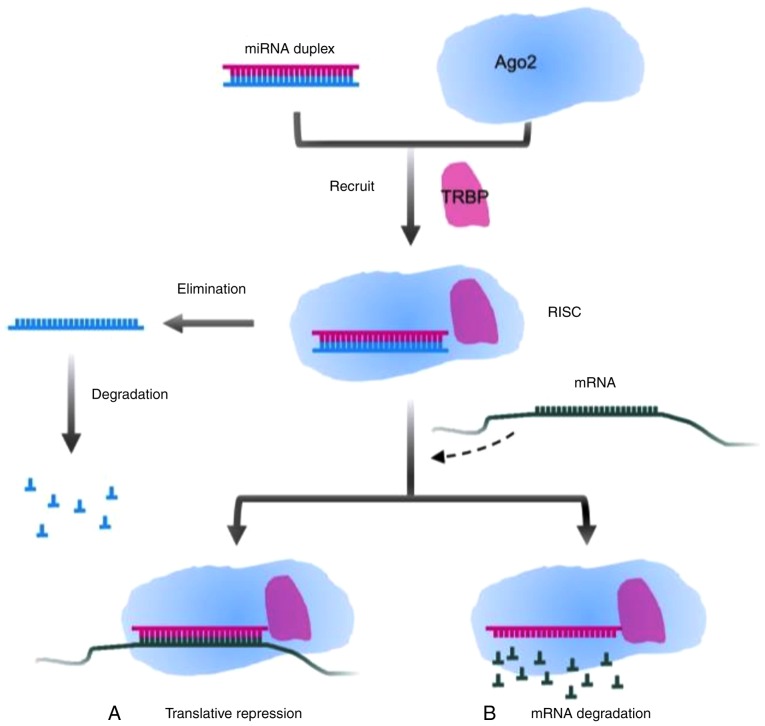
The biogenesis of miRNA involves processes in the nucleus and cytoplasm. In the nucleus, primary miRNA (pri-miRNA), a type of miRNA that contains 300–1,000 nucleotides, is generally issued from the miRNA gene and is transcribed by RNA polymerase II. Subsequently the long transcript, pri-miRNA, is cleaved by the drosha ribonuclease III (DROSHA)/DiGeorge syndrome chromosomal region 8 (DGCR8) complex into a ~70-nt structure termed precursor miRNA (pre-miRNA), which has lost a 7-methyl guanine nucleoside in the 5′-capped end and a 3′poly-(A) tail, but has a conserved stem-loop. Subsequently, the aforementioned stem-loop is sheared by RNA III enzyme Dicer and double-strand RNA-binding domain protein after the GTP-binding nuclear protein Ran/exportin-5 (RanGTP/XPO5) complex carries pre-miRNA to the cytoplasm, to form a double-strand miRNA molecule consisting of 22 nucleotides. Transactivation response element RNA binding protein (TRBP) recruits the mature miRNA to RNA-induced silencing complex (RISC) together with argonaute 2 (Ago2) that has endonuclease activity. A single strand of the double-strand miRNA is conserved in the RISC, and another one is degraded (20,21). Partial or complementary pairing between the RISC and the 3′-UTR of target mRNA functionally plays a role in repressing the translation of mRNA or degrading target mRNA (22,23). The mechanisms are presented in Figs. 1 and 2.
Figure 1.
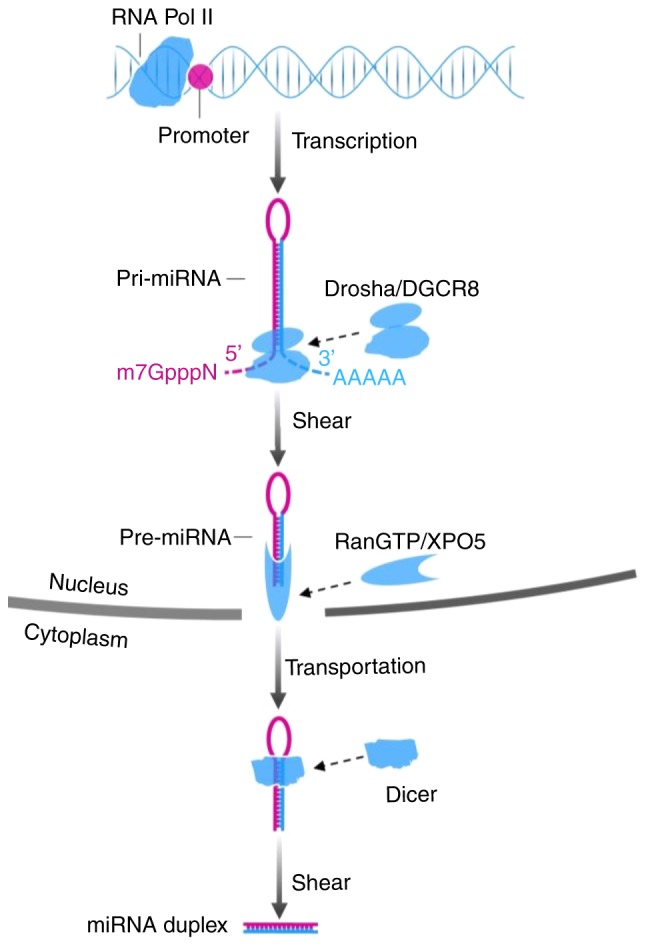
Biogenesis of miRNA. Pri-miRNA is transcribed by RNA Pol II. Drosha/DGCR8 shears the 7-methyl guanine nucleoside (m7GpppN) and 3′poly-(A) tail (AAAAA), which forms pre-miRNA. The RanGTP/XPO5 complex functions as a carrier for nucleocytoplasmic transport of pre-miRNA. miRNA duplex is generated after Dicer cleaves the stem-loop of pre-miRNA. miRNA, microRNA; pri-miRNA, primary miRNA; RNA Pol II, RNA polymerase II; pre-miRNA, precursor miRNA; DGCR8, Drosha/DiGeorge syndrome chromosomal region 8; RanGTP/XPO5, GTP-binding nuclear protein Ran/exportin-5.
Figure 2.
Modulatory mode of miRNA and target mRNA. TRBP recruits the miRNA duplex and Ago2, forming RISC. One strand of miRNA degrades and the other remains, which targets mRNA. There are two ways to silence the target genes: (A) Translative repression by incomplete pairing and (B) degradation of target mRNA by complementary pairing. miRNA, microRNA; TRBP, transactivation response element RNA binding protein; Ago2, argonaute 2; RISC, RNA-induced silencing complex.
3. miRNAs in oncogenesis, diagnosis and prognosis of oral cancer
Previous studies suggested that common potentially malignant disorders and precancerous conditions, including oral leukoplakia (OL) and oral lichen planus (OLP), were correlated with aberrant miRNA, but the mechanism of malignant transformation by miRNA remains unclear (24,25). However, investigating unknown neoplastic transformations for precancerous lesions or conditions may provide novel insight into the mechanisms of tumorigenesis. In addition, the significant differential expression of miRNA between normal tissue, potential malignant disorders and oral cancerous specimens indicates that miRNA may be used as an independent prognostic marker (26,27). Ultimately, a minimally invasive or non-invasive method, such as the detection of miRNA in saliva or serum, may be applied in preoperative prediction and postoperative follow-up (28–30). Indeed, a number of recent studies demonstrated that the aberrant expression of different miRNAs, such as miR-195-5p, miR-375, miR-143, miR-26b, miR-155-5p and miR-483-5p, was associated with oral cancer (31–36). Therefore, miRNAs may have prognostic and diagnostic value in oral cancer and may serve as targets for novel therapeutic strategies.
Latent mechanism of tumorigenesis by miRNAs
Previous studies identified that the miR-31 expression level in oral potential malignant disorder (OPMD) is higher than that in normal oral mucosa, which is correlated with higher expression of vascular endothelial growth factor and lower expression of E-cadherin in OPMD. miR-31 expression was further upregulated in patients with recurrent OPMD and malignant transformation (37). Another previous study suggested that aberrant expression of miR-200c was associated with oral submucous fibrosis (OSF). The overexpression of miR-200c inhibited collagen gel contraction and migration and invasion of fibrotic buccal mucosal fibroblasts induced by arecoline via inhibition of zinc finger E-box binding homeobox 1 (ZEB1). Additionally, reverse transcription-quantitative PCR (RT-qPCR) analysis revealed that miR-200c was downregulated in 25 OSF samples compared with 25 normal mucosae (38). Brito et al (39) concluded that higher expression of miR-21 in OL was associated with increased mitotic figures, incremental nuclear/cytoplasmic ratio and hyperchromasia. Nylander et al (40) found that miR-21 was upregulated in 30 patients diagnosed with multifocal OLP compared with 10 healthy subjects, and in agreement, another study demonstrated that upregulation of miR-21 served a tumor-promoting role in oral cancer and upregulation of miR-21 was observed in 60 of 79 individuals with the disease (41). Aghbari et al (26) identified that miR-27b and miR-137 levels were downregulated in tissue and saliva samples of patients with OLP compared with those in normal controls. Among OLP subgroups, it was demonstrated that miR-137 exhibited the lowest expression level in the erosive type, suggesting that it serve as a biomarker for monitoring potential malignant transformation. The level of miR-375 in progressive lesions was significantly downregulated compared with that in non-progressive control lesions, and miR-375 expression was significantly downregulated in tissues following the transformation of premalignant lesions (including verrucous hyperplasia and verrucopapillary hyperkeratosis) into carcinoma, by comparison of premalignant lesions and oral carcinoma in situ (27). While only 2–3 and 0.4–2% of patients with OL and OLP, respectively, exhibit malignant transformation (42,43), further investigation of the roles of miR-21, miR-27b, miR-137, miR-200c and miR-375 may provide novel insights into pathways involved in the development of oral cancer.
Aberrant expression of miRNA in oral cancer tissues
Dysregulation of specific miRNAs has been previously reported in oral cancer. Wang et al (31) reported that miR-195-5p was significantly downregulated in 40 oral cancerous tissues compared with non-tumor tissues. Another study reported a distinct downregulation of miR-375 in 44 cancerous tissues compared with that in normal mucosae (32). Furthermore, a previous study revealed that miR-143 was downregulated in cancerous tissues compared with that in corresponding adjacent non-cancerous tissues in 81.6% (40/49) of patients (33). miR-802 was downregulated in 60.0% (12/20) of tongue squamous cell carcinoma (TSCC) cases compared with that in normal tissues (44). The expression levels of miR-137 (n=25) and miR-204-5p (n=52) were downregulated in oral cancer samples compared with those in matched normal tissues (45,46). Moreover, upregulation of specific miRNAs was observed in oral cancer tissues. Upregulation of miR-183 was identified in 68.3% (41/60) of TSCC tissues compared with adjacent non-cancerous tissues (47). The miR-373-3p expression level in oral cancerous tissues (n=63) was increased compared with that in adjacent non-cancerous tissues (48). The miR-155 expression level was upregulated in oral squamous cell carcinoma (OSCC) tissues (n=46) compared with that in normal oral mucosa, and the expression level was increased with increasing Tumor-Node-Metastasis (TNM) stage (49). miR-31, miR-182, miR-200a and miR-141 were significantly upregulated in cancerous tissues in 10 patients compared with adjacent non-cancerous tissues (50). The expression of miR-24 was significantly increased in TSCC tissues of 84 patients compared with adjacent non-cancerous tissues (51). Liu et al (52) demonstrated that 67% (10/15) of patients with primary oral cancer had increased miR-1275 expression in tumor tissues compared with that in adjacent tissues.
Aberrant expression of miRNA in serum, plasma and saliva
Previous studies showed that RNA in saliva is protected from degradation by binding to macromolecules such as apoptotic bodies and RISC, a mechanism also observed in plasma and serum RNAs (53–55). Park et al (56) analyzed saliva by immunoblotting analysis using an antibody against Ago2, and demonstrated that Ago2 was present in saliva, where it may confer stability to miRNAs. Furthermore, Park et al (56) found lower levels of miR-125a and miR-200a in the saliva of patients with oral cancer (n=12) compared with those in healthy controls (n=12), suggesting that the aforementioned miRNAs may serve as stable biomarkers of the disease. Liu et al (28) reported that the level of miR-31 in the saliva of patients with OSCC (n=45) prior to surgery was significantly increased compared with that in healthy subjects (n=24). Moreover, the miR-31 level in saliva samples was higher compared with that in plasma samples. The upregulation of miR-31 was detected with high sensitivity even in very small tumors, and the ability to detect miR-31 levels in the saliva of patients with small tumors was not significantly different compared with patients with advanced tumors, suggesting that salivary miR-31 may be utilized to detect and diagnose oral cancer lesions in high-risk populations. Zahran et al (29) reported a significant upregulation in salivary miR-21 and miR-184 levels in patients with oral cancer compared with those in healthy controls. Specifically, a four-fold increase in miR-21, with 65% specificity and 65% sensitivity, and a three-fold increase in miR-184, with 75% specificity and 80% sensitivity, were observed. The expression of salivary miR-145 was significantly decreased in patients with OSCC compared with clinically healthy controls, with 70% specificity and 60% sensitivity. Ries et al (30) reported that miR-3651 and miR-494 levels were upregulated, while the miR-186 level was significantly downregulated in whole blood samples of patients with recurrent tumors compared with non-recurrent controls. Therefore, miRNAs may be promising candidates for the development of diagnostic tools for oral cancer.
miRNAs as feasible biomarkers of pathology, metastasis and prognosis
Certain miRNAs are known to be downregulated in tumor tissues compared with noncancerous tissues. Lower levels of miR-195-5p were associated with higher pathological differentiation grade (31). In comparison with patients without lymph node metastases (n=26), patients with lymph node metastases (n=18) had significantly downregulated miR-375. Furthermore, the overall survival of patients with oral cancer with low miR-375 expression (n=19) was lower than that of patients with high miR-375 expression (n=25) (32). Cao et al (34) revealed that advanced clinical stage and large tumor size of oral cancer were associated with low miR-26b expression. The 5-year survival rates of patients with low and high miR-26b levels were 26.7 and 53.3%, respectively.
On the other hand, certain miRNAs are upregulated in tumor tissues compared with adjacent non-cancerous tissues. Upregulation of miR-183 in patients with oral cancer markedly shortened the overall survival time and increased the risk of poor prognosis by 5.666 times. Upregulation of miR-21 resulted in a higher risk of short survival time (47). The expression of miR-373-3p was higher in primary tumors with metastases compared with that in tumors with no metastases (48). The upregulation of miR-24 was associated with advanced clinical stage in patients with oral cancer (51). A positive correlation between a high miR-155-5p level and cervical lymphatic metastases was observed, and the survival analysis of carcinomatous recurrence and metastasis identified an association between high miR-155-5p expression and a poor survival rate (n=73); miRNA-155-5p may be considered as a specific factor resulting in a worse prognosis (35).
Serum miR-483-5p was higher in patients with oral cancer (n=101) compared with healthy controls (n=103); the survival rates of patients with high miR-483-5p serum level (n=43; >3.23-fold higher compared with healthy controls) were decreased compared with that of patients with lower miR-483-5p serum levels (n=42; <3.23-fold higher compared with healthy controls), and multivariate analyses for overall survival suggested that a high miR-483-5p serum level was an independent prognostic indicator (36). Furthermore, patient follow-up revealed that patients with higher blood miR-372 levels had more extensive primary tumors, a greater tendency of node metastases, a more terminal stage and higher mortality rates (57). Additionally, miR-372 was downregulated in plasma and saliva among postoperative patients compared with preoperative patients (57). By measuring salivary miR-31, it was determined that 86.4% (19/22) of patients exhibited a significant decrease in miR-31 levels following tumor resection (28). miR-372 and miR-31 may therefore serve as biomarkers for the evaluation of surgical efficacy (28,57). Sun et al (58) suggested that serum miR-9 is an independent prognostic factor for oral cancer, as downregulated miR-9 was associated with lymph node metastases, advanced TNM stage and poor prognosis. Patients with low serum miR-9 expression had a worse disease-free survival rate compared with patients with high miR-9 expression (26.5 and 54.6%, respectively). The overall survival rate of patients with low and high miR-9 expression was 42.9 and 67.3%, respectively. By determining levels of miRNAs in the serum or saliva of patients, miRNAs may be used in minimally or non-invasive methods to predict lymph node metastasis and assess the prognosis of patients with oral cancer. Table I presents the aberrant levels of other miRNAs in the saliva, blood, serum and plasma (28–30,59–68).
Table I.
Dysregulation of miRNAs associated with oral cancer detected in saliva, blood, serum and plasma.
| Author, year | miRNA | Dysregulation | Sample | (Refs.) |
|---|---|---|---|---|
| Liu et al, 2012 | miR-31 | Upregulation | Saliva | (28) |
| Zahran et al, 2015 | miR-21 | Upregulation | Saliva | (29) |
| miR-184 | Upregulation | Saliva | ||
| Ries et al, 2017 | miR-186 | Downregulation | Blood | (30) |
| miR-3651 | Upregulation | Blood | ||
| miR-494 | Upregulation | Blood | ||
| Yang et al, 2011 | miR-181 | Upregulation | Plasma | (59) |
| Wong et al, 2018 | miR-184 | Upregulation | Plasma | (60) |
| Lu et al, 2012 | miR-10b | Upregulation | Plasma | (61) |
| miR-196a | Downregulation | Plasma | ||
| miR-196b | Downregulation | Plasma | ||
| miR-582-5p | Downregulation | Plasma | ||
| miR-15b | Downregulation | Plasma | ||
| miR-301 | Downregulation | Plasma | ||
| miR-148b | Downregulation | Plasma | ||
| miR-128a | Downregulation | Plasma | ||
| miR-503 | Downregulation | Plasma | ||
| miR-31 | Downregulation | Plasma | ||
| Kao et al, 2015 | miR-21 | Upregulation | Plasma | (62) |
| miR-31 | Upregulation | Plasma | ||
| miR-146 | Upregulation | Plasma and saliva | ||
| miR-184 | Upregulation | Plasma | ||
| miR-372 | Upregulation | Plasma | ||
| Liu et al, 2013 | miR-196a | Upregulation | Plasma | (63) |
| Lin et al, 2010 | miR-24 | Upregulation | Plasma | (64) |
| Lu et al, 2015 | miR-196a | Upregulation | Plasma | (65) |
| miR-196b | Upregulation | Plasma | ||
| Liu et al, 2017 | miR-187* | Upregulation | Plasma | (66) |
| Lo et al, 2012 | miR-27b | Downregulation | Plasma | (67) |
| Ries et al, 2014 | miR-494 | Upregulation | Blood | (68) |
| miR-3162 | Upregulation | Blood | ||
| miR-3651 | Upregulation | Blood | ||
| miR-186 | Downregulation | Blood | ||
| let-7 | Downregulation | Blood |
miRNAs associated with oral cancer detected in saliva, blood, serum and plasma may serve as tumor biomarkers. The upregulation of miR-31, miR-494, miR-3651 and miR-196a, and the downregulation of miR-186 are associated with tumor recurrence. High expression levels of miR-181 and miR-196a indicate a poor prognosis. miRNA/miR, microRNA.
4. Partial subtypes of oral cancer and miRNAs
Mucoepidermoid carcinoma
As a salivary gland-derived malignancy, mucoepidermoid carcinoma may occur in the oral cavity. Shin et al (69) reported that the overexpression of miR-127-3p led to an increase in the number of cells in the G1 phase, indicating that miR-127-3p resulted in G1/S cell cycle arrest in vitro. miR-127-3p-induced cell cycle arrest in mucoepidermoid carcinoma MC-3 cells was associated with the increase of cyclin-dependent kinase inhibitor 1A and interferon α inducible protein 27 expression via the regulation of Sp1 transcription factor (69). Binmadi et al (70) found that miR-302a was significantly increased in mucoepidermoid carcinoma tissues compared with normal tissues. Furthermore, upregulated miR-302a induced invasion of mucoepidermoid carcinoma cells in vitro.
Adenoid cystic carcinoma
Adenoid cystic carcinoma (ACC) generally occurs in the minor salivary glands and has a poor long-term prognosis due to perineural invasion and lung metastasis (71). Wang et al (72) analyzed the expression of miR-130a in 21 patients with ACC and corresponding normal salivary gland tissues. Compared with that in normal salivary gland tissues, the expression of miR-130a in ACC tissues increased by 1.58–29.1 times. In addition, the level of miR-130a was negatively correlated with N-myc downstream-regulated gene 2 in ACC tissues. Chen et al (73) analyzed miRNAs during the metastasis of ACC cells and found that the expression levels of miR-4487, miR-4430 and miR-486-3p were upregulated, and the expression levels of miR-5191, miR-3131 and miR-211-3p were downregulated. Andreasen et al (74) found that high expression levels of miR-21, miR-181a-2 and miR-152 in patients with ACC was associated with a decreased overall survival rate, and high expression of miR-374c was associated with an improved relapse-free survival rate. Wang et al (75) found that an miR-21 inhibitor significantly reduced the resistance of lung metastatic salivary adenoid cystic carcinoma cells (SACC-LM) to simvastatin. Furthermore, the combination of simvastatin and miR-21 inhibitor decreased the proliferation of SACC-LM cells (75).
5. Conceivable therapeutic value of miRNAs
Tumor suppressor miRNAs
Yang et al (76) found that when a recombinant lentivirus carrying the miR-381-3p gene was transduced into SCC-9 and Tca-8113 cell lines, miR-381-3p-overexpressing cells demonstrated downregulation of fibroblast growth factor receptor 2. Furthermore, the percentage of cells at the G1/G0 phase was increased and the number of cells at the S phase was decreased. In addition, the apoptotic rate was significantly increased and the number of colonies was decreased in SCC-9 and Tca-8113 cells overexpressing miR-381-3p compared with those in the negative control group. EMT, an important mechanism of invasion or metastasis of cancer, which involves the downregulation of E-cadherin and increases metastasis and invasion as a consequence of loss of intercellular adhesion, may be regulated by miRNAs (77). ZEB was reported as an EMT-related transcription factor, which was shown to directly combine with the E-cadherin promoter and inhibit its transcription (77,78). Hashiguchi et al (79) identified an association between miR-205 and the EMT phenotype in SQUU-B cells and demonstrated that overexpression of miR-205 downregulated ZEB1, ZEB2 and N-cadherin, and upregulated E-cadherin. Another study reported that overexpression of miR-375 significantly upregulated SCC-4 cell radiation-induced apoptosis by directly regulating the insulin-like growth factor 1 receptor (IGF1R) (32). Tumor suppressor miRNAs, and their respective target genes and downstream signaling pathways, are presented in Table II (80–111).
Table II.
Tumor suppressor miRNAs, and their respective target genes or signaling pathways in oral cancer.
| Author, year | miRNA | Target gene/pathway | Possible role | (Refs.) |
|---|---|---|---|---|
| Shi et al, 2015 | miR-375 | KLF5 | Proliferation, apoptosis | (80) |
| Wu et al, 2017 | miR-375 | SLC7A11 | Proliferation, invasion | (81) |
| Ji et al, 2017 | miR-138 | AKT1 | Invasion | (82) |
| Xu et al, 2015 | miR-138 | YAP-1 | Proliferation | (83) |
| Kim et al, 2018 | miR-203 | Bmi1 | Apoptosis | (84) |
| Lim et al, 2017 | miR-203 | SEMA6A | Apoptosis | (85) |
| Lin et al, 2016 | miR-203 | PIK3CA | Proliferation, chemosensitivity | (86) |
| Xie et al, 2018 | miR-200c | ZEB1 | EMT | (87) |
| Zhao et al, 2015 | miR-222 | ABCG2 | Invasion, chemosensitivity | (88) |
| Wang et al, 2017 | miR-15b | TRIM14 | Chemoresistance, EMT | (89) |
| Li et al, 2017 | miR-124 | CCL-2, IL-8 | Proliferation | (90) |
| Lin et al, 2017 | miR-485-5p | PAK1 | EMT, chemosensitivity | (91) |
| Lin et al, 2014 | miR-639 | FOXC1 | EMT | (92) |
| Liu et al, 2017 | miR-27b | FZD7 | Proliferation | (93) |
| Min et al, 2014 | miR-148a | Wnt10b | Migration, invasion | (94) |
| Nagai et al, 2018 | miR-205-5p | TIMP2 | Invasion | (95) |
| Qiao et al, 2017 | miR-524-5p | ILK, TGF-β/Smad (−) | Proliferation, invasion | (96) |
| Qiu et al, 2016 | miR-22 | CD147 | Proliferation, metastasis | (97) |
| Rastogi et al, 2017 | miR-377 | HDAC9, NR4A1, Nur77 | Growth, migration, apoptosis | (98) |
| Ruan et al, 2018 | miR-30a-5p | FAP | Proliferation, invasion | (99) |
| Sakha et al, 2014 | miR-1246 | DENND2D | Motility, invasion | (100) |
| Shang et al, 2017 | miR-9 | CDK4/6 | Apoptosis, cell arrest | (101) |
| Shi et al, 2015 | miR-146a | SOX-2 | Invasion | (102) |
| Wang et al, 2016 | miR-188 | SIX1 | Proliferation, invasion | (103) |
| Wang et al, 2017 | miR-139-5p | HOXA9 | Proliferation, invasion | (104) |
| Wang et al, 2017 | miR-376c-3p | HOXB7 | Proliferation | (105) |
| Wang et al, 2018 | miR-655 | MTDH, PTEN/AKT (−) | Proliferation, invasion | (106) |
| Wang et al, 2018 | miR-1294 | c-Myc (−) | Growth, migration | (107) |
| Weng et al, 2016 | miR-494-3p | Bmi1 | Radiosensitivity | (108) |
| Xu et al, 2016 | miR-340 | Glut | Glucose metabolism | (109) |
| Zeng et al, 2016 | miR-27a-3p | YAP-1 | EMT | (110) |
| Li et al, 2018 | miR-218-5p | CD44 | Invasion | (111) |
Tumor suppressor miRNAs inhibit their respective target genes, which inhibit cellular proliferation, growth, motility, migration, invasion, metastasis, glucose metabolism, EMT, promote cell arrest and apoptosis and increase chemosensitivity and radiosensitivity. The (−) symbol indicates inhibition of downstream signaling pathways. miRNA/miR, microRNA; EMT, epithelial-mesenchymal transition; KLF5, kruppel like factor 5; SLC7A11, solute carrier family 7 member 11; AKT1, AKT serine/threonine kinase 1; YAP-1, yes associated protein 1; SEMA6A, semaphorin 6A; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α; ABCG2, ATP-binding cassette subfamily G member 2; TRIM14, tripartite motif containing 14; CCL-2, C-C motif chemokine ligand 2; IL-8, interleukin-8; PAK1, p21 (RAC1) activated kinase 1; FOXC1, forkhead box C1; FZD7, frizzled class receptor 7; Wnt10b, wingless-type MMTV integration site family, member 10b; TIMP2, TIMP metallopeptidase inhibitor 2; ILK, integrin-linked kinase; BSG, basigin; HDAC9, histone deacetylase 9; NR4A1, nuclear receptor subfamily 4 group A member 1; FAP, fibroblast activation protein α; DENND2D, DENN domain containing 2D; CDK4/6, cyclin-dependent kinase 4/6; SOX-2, sex determining region Y box 2; SIX1, sine oculis-related homeobox 1; HOXA, homeobox A9; HOXB7, homeobox B7; MTDH, metadherin; Glut1, glucose transporter-1. Bmi1, B lymphoma Mo-MLV insertion region 1 homolog.
Tumor-promoting miRNAs
Fu et al (49) demonstrated that miR-155 targeted the cyclin-dependent kinase inhibitor 1B (CDKN1B) 3′-UTR by a luciferase reporter assay. Furthermore, downregulation of miR-155 led to an increase in CDKN1B and inhibited cell proliferation and cell cycle progression in oral cancer Tca8113 cells (49). A previous study demonstrated that the mRNA and protein levels of vimentin and N-cadherin were upregulated in SCC-9 and UM1 cells transfected with miR-373-3p, respectively, while E-cadherin and CK18 were downregulated, suggesting that miR-373-3p may stimulate the EMT phenotype (48). The repression of the negative regulator of the Wnt signaling pathway, Dickkopf-related protein 1, and the nuclear accumulation of β-catenin promoted the Wnt signaling pathway, which facilitated proliferation of SCC-9 and UM1 cells (48). Dysregulation of the Wnt signaling pathway in tumors may result in chemoresistance. Protein phosphatase 2 regulatory subunit Bα (PPP2R5A) is a repressor of Wnt signaling, and in oral cancer cells, miR-218 increased cisplatin resistance via the Wnt signaling pathway by repressing PPP2R5A expression (112). Zhuang et al (112) demonstrated that when UM1 and Cal27 cells were transfected with miR-218 mimics, the miR-218 overexpression resulted in increased levels of β-catenin, indicating activation of Wnt signaling, and enhanced cell viability. Furthermore, blocking the effect of miR-218 reversed chemoresistance in resistant cells. Jiang et al (113) revealed that low expression of miR-222 increased the chemosensitivity of oral cancer cells to cis-diaminedichloroplatinum (CDDP) and that the combination of antisense-miR-222 and CDDP may be an effective curative strategy by upregulating the expression of p53, a modulator of apoptosis. Table III presents tumor-promoting miRNAs with their respective target genes and downstream signaling pathways (112–129).
Table III.
Tumor-promoting miRNAs and their respective target genes or signaling pathways in oral cancer.
| Author, year | miRNA | Target gene/pathway | Possible role | (Refs.) |
|---|---|---|---|---|
| Zhuang et al, 2017 | miR-218 | PPP2R5A,Wnt (+) | Cisplatin resistance | (112) |
| Jiang et al, 2014 | miR-222 | PUMA | Cisplatin resistance | (113) |
| Du et al, 2017 | miR-221 | TIMP3 | Chemosensitivity | (114) |
| Zhou et al, 2016 | miR-221/222 | PTEN | Proliferation, invasion, apoptosis | (115) |
| Zheng et al, 2015 | miR-24 | PTEN, Akt (+) | Cisplatin resistance | (116) |
| Cheng et al, 2016 | miR-455-5p | UBE2B | Proliferation | (117) |
| Guo et al, 2015 | miR-96 | MTSS1 | Proliferation, metastasis | (118) |
| Hu et al, 2016 | miR-497 | SMAD7 | Metastasis | (119) |
| Kawakubo-Yasukochi et al, 2018 | miR-200c-3p | CHD9, WRN | Invasion | (120) |
| Li et al, 2018 | miR-182-5p | CAMK2N1 | Proliferation | (121) |
| Lin et al, 2016 | miR-187 | BARX2 | Metastasis | (122) |
| Liu et al, 2015 | miR-92b | NLK, NF-κB (+) | Proliferation, apoptosis | (123) |
| Lu et al, 2018 | miR-654-5p | GRAP, Ras-ERK (+) | Metastasis | (124) |
| Peng et al, 2018 | miR-134 | PDCD7 | Proliferation, migration | (125) |
| Qiao et al, 2017 | miR-27a-3p | SFRP1, Wnt/β-catenin (+) | EMT | (126) |
| Zhao et al, 2017 | miR-24 | PTEN | Unknown | (127) |
| Zheng et al, 2016 | miR-21 | CADM1 | Chemosensitivity | (128) |
| Chen et al, 2016 | miR-211 | TCF-12 | Antioxidant activity | (129) |
Οncogenic miRNAs silence their respective target genes, which facilitates cellular proliferation, growth, migration, invasion, metastasis, antioxidant activity and EMT, inhibit apoptosis of cancer cells and reduce chemosensitivity. The (+) symbol indicates activation of downstream signaling pathways. Unknown refers to target genes or possible roles of miRNAs that are not reported in previous studies. miRNA, microRNA; EMT, epithelial-mesenchymal transition; PPP2R5A, protein phosphatase 2 regulatory subunit Bα; TIMP3, tissue inhibitor of metalloproteinase 3; PTEN, phosphatase and tensin homolog; UBE2B, ubiquitin conjugating enzyme E2B; MTSS1, metastasis suppressor 1; SMAD7, SMAD family member 7; CHD9, chromodomain helicase DNA binding protein 9; WRN, Werner syndrome RecQ like helicase; CAMK2N1, calcium/calmodulin-dependent protein kinase II inhibitor 1; BARX2, BarH-like homeobox 2; NLK, nemo-like kinase; GRAP, GRB2-related adaptor protein; PDCD7, programmed cell death 7; SFRP1, secreted frizzled-related protein 1; CADM1, cell adhesion molecule 1; TCF-12, transcription factor 12.
Recent conventional experimental methods
Bioinformatics analysis may be used to predict the pairing sequences of miRNAs and target genes, which may be verified by luciferase reporter assays. Western blotting and RT-qPCR may subsequently be used to detect protein and miRNA expression, respectively. Frequently used oral cancer cell lines include SCC-9, SCC-4, Tca-8113 and Cal27 (45,48,49,51). Cell experiments demonstrated that specific miRNAs served an anticancer role such as miR-138, miR-200c, miR-15b, miR-485-5p and miR-340 (83,87,89,91,109); whereas other miRNAs stimulated oral cancer by regulating cellular proliferation, apoptosis, invasion, EMT, metastasis, radiosensitivity, chemosensitivity and glucose metabolism, such as miR-221, miR-455-5p, miR-27a-3p, miR-21 and miR-10a (114,117,126,128,130). The results obtained from in vitro cell experiments may provide novel therapeutic targets for potential clinical application.
Experimental conclusion in oral carcinoma
miRNAs are promising therapeutic targets, as they serve important roles in cancer. Theoretically, by silencing tumor-promoting miRNAs and inducing the expression of tumor-suppressing miRNAs synchronously, it is possible to treat oral carcinoma. Specific miRNAs have multiple target genes, for example, miR-375 targets IGF1R (32), platelet-derived growth factor subunit A (131), Kruppel-like factor 5 (80) and solute carrier family 7 member 11 (81); miR-138 targets AKT serine/threonine kinase 1 (82) and yes-associated protein 1 (83); miR-203 targets B lymphoma Mo-MLV insertion region 1 homolog (84), semaphorin 6A (85) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (86); and miR-221 targets tissue inhibitor of metalloproteinase 3 (114) and phosphatase and tensin homolog (PTEN) (115). A possible treatment strategy may involve anticancer drugs that selectively regulate the expression of such miRNAs. Certain genes or signaling pathways are regulated by two or more miRNAs, for instance, tripartite motif containing 14 is targeted by miR-195-5p (31) and miR-15b (89); IGF1R is targeted by miR-98 (132) and miR-375 (32); ZEB1 is targeted by miR-205 (79) and miR-200c (87); and PTEN is targeted by miR-221/222 (115) and miR-24 (116). Therefore, the combination of more specific inhibitors or activators of cancer-associated genes or signaling pathways may be a suitable therapeutic strategy. With regard to controversial miRNAs, including miR-222, which served an ambivalent role in disparate experiments, more research is required to verify their roles in oral cancer (88,113,115).
6. Conclusions
On the basis of current research, the aberrant expression of miRNAs has been demonstrated to be significantly associated with oral cancer. As either a tumor marker or a therapeutic target, miRNA has potential to diagnose or treat oral cancer and improve survival. miRNA serves important roles in the occurrence, development, therapy and prognosis of oral cancer, and is a promising target for clinical application. In terms of the mechanism of malignant transformation or oncogenicity, prognostic and diagnostic value, and potential as a therapeutic target, miR-31 seems to be a promising candidate for clinical application. miR-31 is differentially expressed in normal mucosa, OPMD and oral cancer, and may be detected with high sensitivity in tissue, saliva and plasma. Furthermore, miR-31 may be used to evaluate surgical efficacy. However, miR-375 and miR-203 may be superior therapeutic targets, as they target multiple genes that regulate additional factors and malignant biological properties in oral cancer.
There are a number of challenges in the experimental research and clinical application of miRNA. The transcriptional activation of miRNA and the regulation of indispensable components (containing Drosha/DGCR8, Dicer, XPO5 and TRBP) in the maturation process of oncogenic or antineoplastic miRNA through signaling pathways, and the interference of signaling pathways by mature miRNA, form a series of feedback loops, which may either contribute to tumorigenesis or be used for effective treatments. Further clinical trials that explore specific or highly sensitive miRNA closely associated with oral cancer are required to identify biomarkers with prognostic value. Combining multiple miRNAs for diagnosis and therapy is also a promising strategy that requires further examination, and investigating the association between oral cancer subtypes and miRNA may facilitate the development of targeted medicine in oral cancer. Identifying the detection threshold of different miRNAs in serum, specimen and saliva may aid in predicting the risk of malignant transformations and in evaluating the risk of tumor metastasis or relapse. In addition to further elucidating the mechanisms and anticancer strategies targeted at miRNA, the potential resistance and complications of new antitumor drugs are novel challenges to overcome in order to identify more effective treatments for oral cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Chongqing Municipal Health Bureau (grant no. 2017HBRC004) and the Natural Science Foundation Project of CQ CSTC (grant no. cstc2018jcyjAX0763).
Availability of data and materials
Not applicable.
Authors' contributions
YL designed the review and revised the manuscript. CF wrote the manuscript. Both authors reviewed the final version and approved it for publication.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Jr, Sun J, Zhu HG, Tu WY, Li J, Cai YL, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–751. doi: 10.1200/JCO.2012.43.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadighi S, Keyhani A, Harirchi I, Garajei A, Aghili M, Kazemian A, Motiee Langroudi M, Zendehdel K, Nikparto N. Neoadjuvant chemotherapy for locally advanced squamous carcinoma of oral cavity: A pilot study. Acta Med Iran. 2015;53:380–386. [PubMed] [Google Scholar]
- 6.Bossi P, Lo Vullo S, Guzzo M, Mariani L, Granata R, Orlandi E, Locati L, Scaramellini G, Fallai C, Licitra L. Preoperative chemotherapy in advanced resectable OCSCC: Long-term results of a randomized phase III trial. Ann Oncol. 2014;25:462–466. doi: 10.1093/annonc/mdt555. [DOI] [PubMed] [Google Scholar]
- 7.Valdez JA, Brennan MT. Impact of oral cancer on quality of life. Dent Clin North Am. 2018;62:143–154. doi: 10.1016/j.cden.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 9.Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18:5–18. doi: 10.1038/nrc.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Cao XY, Li YN, Qiu YY, Li YN, Li W, Wang H. Reversal of cisplatin resistance by microRNA-139-5p-independent RNF2 downregulation and MAPK inhibition in ovarian cancer. Am J Physiol Cell Physiol. 2018;315:C225–C235. doi: 10.1152/ajpcell.00283.2017. [DOI] [PubMed] [Google Scholar]
- 11.Gong R, Lv X, Liu F. MiRNA-17 encoded by the miR-17-92 cluster increases the potential for steatosis in hepatoma cells by targeting CYP7A1. Cell Mol Biol Lett. 2018;23:16. doi: 10.1186/s11658-018-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhl R, Rana S, Kelley K, Espinosa-Diez C, Hudson C, Lanciault C, Thomas CR, Jr, Liana Tsikitis V, Anand S. MicroRNA-451a regulates colorectal cancer proliferation in response to radiation. BMC Cancer. 2018;18:517. doi: 10.1186/s12885-018-4370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Sun Y, Wang H, Li H, Zhang M, Zhou L, Meng X, Wu Y, Liu P, Liu X, et al. MicroRNA-221 induces autophagy through suppressing HDAC6 expression and promoting apoptosis in pancreatic cancer. Oncol Lett. 2018;16:7295–7301. doi: 10.3892/ol.2018.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastasiadou E, Stroopinsky D, Alimperti S, Jiao AL, Pyzer AR, Cippitelli C, Pepe G, Severa M, Rosenblatt J, Etna MP, et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas. Leukemia. 2019;33:132–147. doi: 10.1038/s41375-018-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anastasiadou E, Faggioni A, Trivedi P, Slack FJ. The nefarious nexus of noncoding RNAs in cancer. Int J Mol Sci. 2018;19:2072. doi: 10.3390/ijms19072072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Xue X, Lan J, Gao Y, Xiong Z, Zhang H, Jiang W, Song W, Zhi Q. MicroRNA-29a upregulates MMP2 in oral squamous cell carcinoma to promote cancer invasion and anti-apoptosis. Biomed Pharmacother. 2014;68:13–19. doi: 10.1016/j.biopha.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Xu Q, Sun Q, Zhang J, Yu J, Chen W, Zhang Z. Downregulation of miR-153 contributes to epithelial-mesenchymal transition and tumor metastasis in human epithelial cancer. Carcinogenesis. 2013;34:539–549. doi: 10.1093/carcin/bgs374. [DOI] [PubMed] [Google Scholar]
- 18.Arantes LMRB, De Carvalho AC, Melendez ME, Lopes Carvalho A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev Mol Diagn. 2018;18:85–112. doi: 10.1080/14737159.2017.1404906. [DOI] [PubMed] [Google Scholar]
- 19.Chai L, Yuan Y, Chen C, Zhou J, Wu Y. The role of long non-coding RNA ANRIL in the carcinogenesis of oral cancer by targeting miR-125a. Biomed Pharmacother. 2018;103:38–45. doi: 10.1016/j.biopha.2018.01.105. [DOI] [PubMed] [Google Scholar]
- 20.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 22.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeom KH, Lee Y, Han J, Suh MR, Kim VN. Characterization of DGCR8/Pasha, the essential cofactor for Drosha in primary miRNA processing. Nucleic Acids Res. 2006;34:4622–4629. doi: 10.1093/nar/gkl458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HC, Tseng YK, Chi CC, Chen YH, Yang CM, Huang SJ, Lee YC, Liou HH, Tsai KW, Ger LP. Genetic variants in microRNA-146a (C>G) and microRNA-1269b (G>C) are associated with the decreased risk of oral premalignant lesions, oral cancer, and pharyngeal cancer. Arch Oral Biol. 2016;72:21–32. doi: 10.1016/j.archoralbio.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Philipone E, Yoon AJ, Wang S, Shen J, Ko YC, Sink JM, Rockafellow A, Shammay NA, Santella RM. MicroRNAs-208b-3p, 204–5p, 129-2-3p and 3065-5p as predictive markers of oral leukoplakia that progress to cancer. Am J Cancer Res. 2016;6:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 26.Aghbari SMH, Gaafar SM, Shaker OG, Ashiry SE, Zayed SO. Evaluating the accuracy of microRNA-27b and microRNA-137 as biomarkers of activity and potential malignant transformation in oral lichen planus patients. Arch Dermatol Res. 2018;310:209–220. doi: 10.1007/s00403-018-1805-0. [DOI] [PubMed] [Google Scholar]
- 27.Harrandah AM, Fitzpatrick SG, Smith MH, Wang D, Cohen DM, Chan EK. MicroRNA-375 as a biomarker for malignant transformation in oral lesions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:743–752.e1. doi: 10.1016/j.oooo.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Liu CJ, Lin SC, Yang CC, Cheng HW, Chang KW. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 2012;34:219–214. doi: 10.1002/hed.21713. [DOI] [PubMed] [Google Scholar]
- 29.Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C. Salivary microRNAs in oral cancer. Oral Dis. 2015;21:739–747. doi: 10.1111/odi.12340. [DOI] [PubMed] [Google Scholar]
- 30.Ries J, Baran C, Wehrhan F, Weber M, Neukam FW, Krautheim-Zenk A, Nkenke E. Prognostic significance of altered miRNA expression in whole blood of OSCC patients. Oncol Rep. 2017;37:3467–3474. doi: 10.3892/or.2017.5639. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Ren Y, Liu R, Ma J, Shi Y, Zhang L, Bu R. MiR-195-5p suppresses the proliferation, migration, and invasion of oral squamous cell carcinoma by targeting TRIM14. Biomed Res Int. 2017;2017:7378148. doi: 10.1155/2017/7378148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Li Y, Hou D, Shi Q, Yang S, Li Q. MicroRNA-375 inhibits growth and enhances radiosensitivity in oral squamous cell carcinoma by targeting insulin like growth factor 1 receptor. Cell Physiol Biochem. 2017;42:2105–2117. doi: 10.1159/000479913. [DOI] [PubMed] [Google Scholar]
- 33.Xu P, Li Y, Yang S, Yang H, Tang J, Li M. Micro-ribonucleic acid 143 (MiR-143) inhibits oral squamous cell carcinoma (OSCC) cell migration and invasion by downregulation of phospho-c-Met through targeting CD44 v3. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:43–51. doi: 10.1016/j.oooo.2015.02.486. [DOI] [PubMed] [Google Scholar]
- 34.Cao J, Guo T, Dong Q, Zhang J, Li Y. MiR-26b is downregulated in human tongue squamous cell carcinoma and regulates cell proliferation and metastasis through a COX-2-dependent mechanism. Oncol Rep. 2015;33:974–980. doi: 10.3892/or.2014.3648. [DOI] [PubMed] [Google Scholar]
- 35.Baba O, Hasegawa S, Nagai H, Uchida F, Yamatoji M, Kanno NI, Yamagata K, Sakai S, Yanagawa T, Bukawa H. MicroRNA-155-5p is associated with oral squamous cell carcinoma metastasis and poor prognosis. J Oral Pathol Med. 2016;45:248–255. doi: 10.1111/jop.12351. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Yang Y, Zhao H, Yang X, Luo Y, Ren Y, Liu W, Li N. Serum miR-483-5p: A novel diagnostic and prognostic biomarker for patients with oral squamous cell carcinoma. Tumour Biol. 2016;37:447–453. doi: 10.1007/s13277-015-3514-z. [DOI] [PubMed] [Google Scholar]
- 37.Hung KF, Liu CJ, Chiu PC, Lin JS, Chang KW, Shih WY, Kao SY, Tu HF. MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol. 2016;53:42–47. doi: 10.1016/j.oraloncology.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Lu MY, Yu CC, Chen PY, Hsieh PL, Peng CY, Liao YW, Yu CH, Lin KH. MiR-200c inhibits the arecoline-associated myofibroblastic transdifferentiation in buccal mucosal fibroblasts. J Formos Med Assoc. 2018;117:791–797. doi: 10.1016/j.jfma.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Brito JA, Gomes CC, Guimarães AL, Campos K, Gomez RS. Relationship between microRNA expression levels and histopathological features of dysplasia in oral leukoplakia. J Oral Pathol Med. 2014;43:211–216. doi: 10.1111/jop.12112. [DOI] [PubMed] [Google Scholar]
- 40.Nylander E, Ebrahimi M, Wahlin YB, Boldrup L, Nylander K. Changes in miRNA expression in sera and correlation to duration of disease in patients with multifocal mucosal lichen planus. J Oral Pathol Med. 2012;41:86–89. doi: 10.1111/j.1600-0714.2011.01063.x. [DOI] [PubMed] [Google Scholar]
- 41.Ren W, Wang X, Gao L, Li S, Yan X, Zhang J, Huang C, Zhang Y, Zhi K. MiR-21 modulates chemosensitivity of tongue squamous cell carcinoma cells to cisplatin by targeting PDCD4. Mol Cell Biochem. 2014;390:253–262. doi: 10.1007/s11010-014-1976-8. [DOI] [PubMed] [Google Scholar]
- 42.Arnaoutakis D, Bishop J, Westra W, Califano JA. Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol. 2013;49:814–817. doi: 10.1016/j.oraloncology.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Mortazavi H, Baharvand M, Mehdipour M. Oral potentially malignant disorders: An overview of more than 20 entities. J Dent Res Dent Clin Dent Prospects. 2014;8:6–14. doi: 10.5681/joddd.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Gong Z, Sun L, Ma L, Wang Q. MicroRNA-802 plays a tumour suppressive role in tongue squamous cell carcinoma through directly targeting MAP2K4. Cell Prolif. 2017 Mar 20; doi: 10.1111/cpr.12336. (Epub ahead of print). doi: 10.1111/cpr.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Liang J, Wang Q, Li Z, Du Y, Xu X. MicroRNA-137 suppresses tongue squamous carcinoma cell proliferation, migration and invasion. Cell Prolif. 2016;49:628–635. doi: 10.1111/cpr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Li F, Zhou X. MiR-204-5p regulates cell proliferation and metastasis through inhibiting CXCR4 expression in OSCC. Biomed Pharmacother. 2016;82:202–207. doi: 10.1016/j.biopha.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 47.Supic G, Zeljic K, Rankov AD, Kozomara R, Nikolic A, Radojkovic D, Magic Z. MiR-183 and miR-21 expression as biomarkers of progression and survival in tongue carcinoma patients. Clin Oral Investig. 2018;22:401–409. doi: 10.1007/s00784-017-2126-y. [DOI] [PubMed] [Google Scholar]
- 48.Weng J, Zhang H, Wang C, Liang J, Chen G, Li W, Tang H, Hou J. MiR-373-3p targets DKK1 to promote EMT-induced metastasis via the Wnt/β-catenin pathway in tongue squamous cell carcinoma. Biomed Res Int. 2017;2017:6010926. doi: 10.1155/2017/6010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu S, Chen HH, Cheng P, Zhang CB, Wu Y. MiR-155 regulates oral squamous cell carcinoma Tca8113 cell proliferation, cycle, and apoptosis via regulating p27Kip1. Eur Rev Med Pharmacol Sci. 2017;21:937–944. [PubMed] [Google Scholar]
- 50.Wang J, Wang W, Li J, Wu L, Song M, Meng Q. MiR-182 activates the Ras-MEK-ERK pathway in human oral cavity squamous cell carcinoma by suppressing RASA1 and SPRED1. Onco Targets Ther. 2017;10:667–679. doi: 10.2147/OTT.S121864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Hu C, Chi J, Li J, Peng C, Yun X, Li D, Yu Y, Li Y, Gao M, Zheng X. MiR-24 promotes the proliferation, migration and invasion in human tongue squamous cell carcinoma by targeting FBXW7. Oncol Rep. 2016;36:1143–1149. doi: 10.3892/or.2016.4891. [DOI] [PubMed] [Google Scholar]
- 52.Liu MD, Wu H, Wang S, Pang P, Jin S, Sun CF, Liu FY. MiR-1275 promotes cell migration, invasion and proliferation in squamous cell carcinoma of head and neck via up-regulating IGF-1R and CCR7. Gene. 2018;646:1–7. doi: 10.1016/j.gene.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 53.El-Hefnawy T, Raja S, Kelly L, Bigbee WL, Kirkwood JM, Luketich JD, Godfrey TE. Characterization of amplifiable, circulating RNA in plasma and its potential as a tool for cancer diagnostics. Clin Chem. 2014;50:564–573. doi: 10.1373/clinchem.2003.028506. [DOI] [PubMed] [Google Scholar]
- 54.Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. Characterization of RNA in saliva. Clin Chem. 2006;52:988–994. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsui NB, Ng EK, Lo YM. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin Chem. 2002;48:1647–1653. [PubMed] [Google Scholar]
- 56.Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh LY, Liu CJ, Wong YK, Chang C, Lin SC, Chang KW. MiR-372 inhibits p62 in head and neck squamous cell carcinoma in vitro and in vivo. Oncotarget. 2015;6:6062–6075. doi: 10.18632/oncotarget.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L, Liu L, Fu H, Wang Q, Shi Y. Association of decreased expression of serum miR-9 with poor prognosis of oral squamous cell carcinoma patients. Med Sci Monit. 2016;22:289–294. doi: 10.12659/MSM.895683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang CC, Hung PS, Wang PW, Liu CJ, Chu TH, Cheng HW, Lin SC. miR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:397–404. doi: 10.1111/j.1600-0714.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 60.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2018;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 61.Lu YC, Chen YJ, Wang HM, Tsai CY, Chen WH, Huang YC, Fan KH, Tsai CN, Huang SF, Kang CJ, et al. Oncogenic function and early detection potential of miRNA-10b in oral cancer as identified by microRNA profiling. Cancer Prev Res (Phila) 2012;5:665–674. doi: 10.1158/1940-6207.CAPR-11-0358. [DOI] [PubMed] [Google Scholar]
- 62.Kao YY, Tu HF, Kao SY, Chang KW, Lin SC. The increase of oncogenic miRNA expression in tongue carcinogenesis of a mouse model. Oral Oncol. 2015;51:1103–1112. doi: 10.1016/j.oraloncology.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Liu CJ, Tsai MM, Tu HF, Lui MT, Cheng HW, Lin SC. MiR-196a overexpression and miR-196a2 gene polymorphism are prognostic predictors of oral carcinomas. Ann Surg Oncol. 2013;20(Suppl 3):S406–S414. doi: 10.1245/s10434-012-2618-6. [DOI] [PubMed] [Google Scholar]
- 64.Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS, Chang KW. MiR-24 up-regulation in oral carcinoma: Positive association from clinical and in vitro analysis. Oral Oncol. 2010;46:204–208. doi: 10.1016/j.oraloncology.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Lu YC, Chang JT, Huang YC, Huang CC, Chen WH, Lee LY, Huang BS, Chen YJ, Li HF, Cheng AJ. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin Biochem. 2015;48:115–121. doi: 10.1016/j.clinbiochem.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 66.Liu CJ, Lin JS, Cheng HW, Hsu YH, Cheng CY, Lin SC. Plasma miR-187* is a potential biomarker for oral carcinoma. Clin Oral Investig. 2017;21:1131–1138. doi: 10.1007/s00784-016-1887-z. [DOI] [PubMed] [Google Scholar]
- 67.Lo WY, Wang HJ, Chiu CW, Chen SF. MiR-27b-regulated TCTP as a novel plasma biomarker for oral cancer: From quantitative proteomics to post-transcriptional study. J Proteomics. 2012;77:154–166. doi: 10.1016/j.jprot.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 68.Ries J, Vairaktaris E, Kintopp R, Baran C, Neukam FW, Nkenke E. Alterations in miRNA expression patterns in whole blood of OSCC patients. In Vivo. 2014;28:851–861. [PubMed] [Google Scholar]
- 69.Shin JA, Li C, Choi ES, Cho SD, Cho NP. High expression of microRNA-127 is involved in cell cycle arrest in MC-3 mucoepidermoid carcinoma cells. Mol Med Rep. 2013;7:708–712. doi: 10.3892/mmr.2012.1222. [DOI] [PubMed] [Google Scholar]
- 70.Binmadi NO, Basile JR, Perez P, Gallo A, Tandon M, Elias W, Jang SI, Alevizos I. miRNA expression profile of mucoepidermoid carcinoma. Oral Dis. 2018;24:537–543. doi: 10.1111/odi.12800. [DOI] [PubMed] [Google Scholar]
- 71.Ramer N, Wu H, Sabo E, Ramer Y, Emanuel P, Orta L, Burstein DE. Prognostic value of quantitative p63 immunostaining in adenoid cystic carcinoma of salivary gland assessed by computerized image analysis. Cancer. 2010;116:77–83. doi: 10.1002/cncr.24657. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Zhang CY, Xia RH, Han J, Sun B, Sun SY, Li J. The MYB/miR-130a/NDRG2 axis modulates tumor proliferation and metastatic potential in salivary adenoid cystic carcinoma. Cell Death Dis. 2018;9:917. doi: 10.1038/s41419-018-0966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen W, Zhao X, Dong Z, Cao G, Zhang S. Identification of microRNA profiles in salivary adenoid cystic carcinoma cells during metastatic progression. Oncol Lett. 2014;7:2029–2034. doi: 10.3892/ol.2014.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreasen S, Tan Q, Agander TK, Hansen TVO, Steiner P, Bjørndal K, Høgdall E, Larsen SR, Erentaite D, Olsen CH, et al. MicroRNA dysregulation in adenoid cystic carcinoma of the salivary gland in relation to prognosis and gene fusion status: A cohort study. Virchows Arch. 2018;473:329–340. doi: 10.1007/s00428-018-2423-0. [DOI] [PubMed] [Google Scholar]
- 75.Wang C, Li T, Yan F, Cai W, Zheng J, Jiang X, Sun J. Effect of simvastatin and microRNA-21 inhibitor on metastasis and progression of human salivary adenoid cystic carcinoma. Biomed Pharmacother. 2018;105:1054–1061. doi: 10.1016/j.biopha.2018.05.157. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Ruan H, Hu X, Cao A, Song L. miR-381-3p suppresses the proliferation of oral squamous cell carcinoma cells by directly targeting FGFR2. Am J Cancer Res. 2017;7:913–922. [PMC free article] [PubMed] [Google Scholar]
- 77.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Wong TS, Gao W, Chan JY. Transcription regulation of E-cadherin by zinc finger E-box binding homeobox proteins in solid tumors. Biomed Res Int. 2014;2014:921564. doi: 10.1155/2014/921564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashiguchi Y, Kawano S, Goto Y, Yasuda K, Kaneko N, Sakamoto T, Matsubara R, Jinno T, Maruse Y, Tanaka H, et al. Tumor-suppressive roles of ΔNp63β-miR-205 axis in epithelial-mesenchymal transition of oral squamous cell carcinoma via targeting ZEB1 and ZEB2. J Cell Physiol. 2018;233:6565–6577. doi: 10.1002/jcp.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi W, Yang J, Li S, Shan X, Liu X, Hua H, Zhao C, Feng Z, Cai Z, Zhang L, et al. Potential involvement of miR-375 in the premalignant progression of oral squamous cell carcinoma mediated via transcription factor KLF5. Oncotarget. 2015;6:40172–40185. doi: 10.18632/oncotarget.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y, Sun X, Song B, Qiu X, Zhao J. MiR-375/SLC7A11 axis regulates oral squamous cell carcinoma proliferation and invasion. Cancer Med. 2017;6:1686–1697. doi: 10.1002/cam4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji M, Wang W, Yan W, Chen D, Ding X, Wang A. Dysregulation of AKT1, a miR-138 target gene, is involved in the migration and invasion of tongue squamous cell carcinoma. J Oral Pathol Med. 2017;46:731–737. doi: 10.1111/jop.12551. [DOI] [PubMed] [Google Scholar]
- 83.Xu R, Zeng G, Gao J, Ren Y, Zhao Z, Zhang J, Tao H, Li D. miR-138 suppresses the proliferation of oral squamous cell carcinoma cells by targeting Yes-associated protein 1. Oncol Rep. 2015;34:2171–2178. doi: 10.3892/or.2015.4144. [DOI] [PubMed] [Google Scholar]
- 84.Kim JS, Choi DW, Kim CS, Yu SK, Kim HJ, Go DS, Lee SA, Moon SM, Kim SG, Chun HS, et al. MicroRNA-203 induces apoptosis by targeting Bmi-1 in YD-38 oral cancer cells. Anticancer Res. 2018;38:3477–3485. doi: 10.21873/anticanres.12618. [DOI] [PubMed] [Google Scholar]
- 85.Lim HS, Kim CS, Kim JS, Yu SK, Go DS, Lee SA, Moon SM, Chun HS, Kim S, Kim DK. Suppression of oral carcinoma oncogenic activity by microRNA-203 via down-regulation of SEMA6A. Anticancer Res. 2017;37:5425–5433. doi: 10.21873/anticanres.11970. [DOI] [PubMed] [Google Scholar]
- 86.Lin J, Lin Y, Fan L, Kuang W, Zheng L, Wu J, Shang P, Wang Q, Tan J. miR-203 inhibits cell proliferation and promotes cisplatin induced cell death in tongue squamous cancer. Biochem Biophys Res Commun. 2016;473:382–387. doi: 10.1016/j.bbrc.2016.02.105. [DOI] [PubMed] [Google Scholar]
- 87.Xie NN, Liu ZX, Wu C, Wang PL, Song GT, Chen Z. MicroRNA-200c suppresses tumor metastasis in oral squamous carcinoma by inhibiting epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2018;22:3415–3422. doi: 10.26355/eurrev_201806_15164. [DOI] [PubMed] [Google Scholar]
- 88.Zhao L, Ren Y, Tang H, Wang W, He Q, Sun J, Zhou X, Wang A. Deregulation of the miR-222-ABCG2 regulatory module in tongue squamous cell carcinoma contributes to chemoresistance and enhanced migratory/invasive potential. Oncotarget. 2015;6:44538–44550. doi: 10.18632/oncotarget.6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Guo H, Yao B, Helms J. miR-15b inhibits cancer-initiating cell phenotypes and chemoresistance of cisplatin by targeting TRIM14 in oral tongue squamous cell cancer. Oncol Rep. 2017;37:2720–2726. doi: 10.3892/or.2017.5532. [DOI] [PubMed] [Google Scholar]
- 90.Li X, Fan Q, Li J, Song J, Gu Y. MiR-124 down-regulation is critical for cancer associated fibroblasts-enhanced tumor growth of oral carcinoma. Exp Cell Res. 2017;351:100–108. doi: 10.1016/j.yexcr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Lin XJ, He CL, Sun T, Duan XJ, Sun Y, Xiong SJ. Hsa-miR-485-5p reverses epithelial to mesenchymal transition and promotes cisplatin-induced cell death by targeting PAK1 in oral tongue squamous cell carcinoma. Int J Mol Med. 2017;40:83–89. doi: 10.3892/ijmm.2017.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin Z, Sun L, Chen W, Liu B, Wang Y, Fan S, Li Y, Li J. miR-639 regulates transforming growth factor beta-induced epithelial-mesenchymal transition in human tongue cancer cells by targeting FOXC1. Cancer Sci. 2014;105:1288–1298. doi: 10.1111/cas.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu B, Chen W, Cao G, Dong Z, Xu J, Luo T, Zhang S. MicroRNA-27b inhibits cell proliferation in oral squamous cell carcinoma by targeting FZD7 and Wnt signaling pathway. Arch Oral Biol. 2017;83:92–96. doi: 10.1016/j.archoralbio.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 94.Min A, Zhu C, Peng S, Shuai C, Sun L, Han Y, Qian Y, Gao S, Su T. Downregulation of MicroRNA-148a in cancer-associated fibroblasts from oral cancer promotes cancer cell migration and invasion by targeting Wnt10b. J Biochem Mol Toxicol. 2016;30:186–191. doi: 10.1002/jbt.21777. [DOI] [PubMed] [Google Scholar]
- 95.Nagai H, Hasegawa S, Uchida F, Terabe T, Ishibashi Kanno N, Kato K, Yamagata K, Sakai S, Kawashiri S, Sato H, et al. MicroRNA-205-5p suppresses the invasiveness of oral squamous cell carcinoma by inhibiting TIMP2 expression. Int J Oncol. 2018;52:841–850. doi: 10.3892/ijo.2018.4260. [DOI] [PubMed] [Google Scholar]
- 96.Qiao B, Cai JH, King-Yin Lam A, He BX. MicroRNA-542-3p inhibits oral squamous cell carcinoma progression by inhibiting ILK/TGF-β1/Smad2/3 signaling. Oncotarget. 2017;8:70761–70776. doi: 10.18632/oncotarget.19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu K, Huang Z, Huang Z, He Z, You S. miR-22 regulates cell invasion, migration and proliferation in vitro through inhibiting CD147 expression in tongue squamous cell carcinoma. Arch Oral Biol. 2016;66:92–97. doi: 10.1016/j.archoralbio.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Rastogi B, Kumar A, Raut SK, Panda NK, Rattan V, Joshi N, Khullar M. Downregulation of miR-377 promotes oral squamous cell carcinoma growth and migration by targeting HDAC9. Cancer Invest. 2017;35:152–162. doi: 10.1080/07357907.2017.1286669. [DOI] [PubMed] [Google Scholar]
- 99.Ruan P, Tao Z, Tan A. Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. Biosci Rep. 2018;38:BSR20171027. doi: 10.1042/BSR20171027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep. 2014;6:38750. doi: 10.1038/srep38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shang A, Lu WY, Yang M, Zhou C, Zhang H, Cai ZX, Wang WW, Wang WX, Wu GQ. miR-9 induces cell arrest and apoptosis of oral squamous cell carcinoma via CDK 4/6 pathway. Artif Cells Nanomed Biotechnol. 2018;46:1754–1762. doi: 10.1080/21691401.2017.1391825. [DOI] [PubMed] [Google Scholar]
- 102.Shi Z, Johnson JJ, Jiang R, Liu Y, Stack MS. Decrease of miR-146a is associated with the aggressiveness of human oral squamous cell carcinoma. Arch Oral Biol. 2015;60:1416–1427. doi: 10.1016/j.archoralbio.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Liu H. microRNA-188 is downregulated in oral squamous cell carcinoma and inhibits proliferation and invasion by targeting SIX1. Tumour Biol. 2016;37:4105–4113. doi: 10.1007/s13277-015-4246-9. [DOI] [PubMed] [Google Scholar]
- 104.Wang K, Jin J, Ma T, Zhai H. MiR-139-5p inhibits the tumorigenesis and progression of oral squamous carcinoma cells by targeting HOXA9. J Cell Mol Med. 2017;21:3730–3740. doi: 10.1111/jcmm.13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang K, Jin J, Ma T, Zhai H. MiR-376c-3p regulates the proliferation, invasion, migration, cell cycle and apoptosis of human oral squamous cancer cells by suppressing HOXB7. Biomed Pharmacother. 2017;91:517–525. doi: 10.1016/j.biopha.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 106.Wang Q, Lv L, Li Y, Ji H. MicroRNA-655 suppresses cell proliferation and invasion in oral squamous cell carcinoma by directly targeting metadherin and regulating the PTEN/AKT pathway. Mol Med Rep. 2018;18:3106–3114. doi: 10.3892/mmr.2018.9292. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z, Yan J, Zou T, Gao H. MicroRNA-1294 inhibited oral squamous cell carcinoma growth by targeting c-Myc. Oncol Lett. 2018;16:2243–2250. doi: 10.3892/ol.2018.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weng JH, Yu CC, Lee YC, Lin CW, Chang WW, Kuo YL. miR-494-3p induces cellular senescence and enhances radiosensitivity in human oral squamous carcinoma cells. Int J Mol Sci. 2016;17(pii):E1092. doi: 10.3390/ijms17071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu P, Li Y, Zhang H, Li M, Zhu H. MicroRNA-340 mediates metabolic shift in oral squamous cell carcinoma by targeting glucose transporter-1. J Oral Maxillofac Surg. 2016;74:844–850. doi: 10.1016/j.joms.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 110.Zeng G, Xun W, Wei K, Yang Y, Shen H. MicroRNA-27a-3p regulates epithelial to mesenchymal transition via targeting YAP1 in oral squamous cell carcinoma cells. Oncol Rep. 2016;36:1475–1482. doi: 10.3892/or.2016.4916. [DOI] [PubMed] [Google Scholar]
- 111.Li X, He J, Shao M, Cui B, Peng F, Li J, Ran Y, Jin D, Kong J, Chang J, et al. Downregulation of miR-218-5p promotes invasion of oral squamous cell carcinoma cells via activation of CD44-ROCK signaling. Biomed Pharmacother. 2018;106:646–654. doi: 10.1016/j.biopha.2018.06.151. [DOI] [PubMed] [Google Scholar]
- 112.Zhuang Z, Hu F, Hu J, Wang C, Hou J, Yu Z, Wang TT, Liu X, Huang H. MicroRNA-218 promotes cisplatin resistance in oral cancer via the PPP2R5A/Wnt signaling pathway. Oncol Rep. 2017;38:2051–2061. doi: 10.3892/or.2017.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang F, Zhao W, Zhou L, Liu Z, Li W, Yu D. MiR-222 targeted PUMA to improve sensitization of UM1 cells to cisplatin. Int J Mol Sci. 2014;15:22128–22141. doi: 10.3390/ijms151222128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Du L, Ma S, Wen X, Chai J, Zhou D. Oral squamous cell carcinoma cells are resistant to doxorubicin through upregulation of miR-221. Mol Med Rep. 2017;16:2659–2667. doi: 10.3892/mmr.2017.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou L, Jiang F, Chen X, Liu Z, Ouyang Y, Zhao W, Yu D. Downregulation of miR-221/222 by a microRNA sponge promotes apoptosis in oral squamous cell carcinoma cells through upregulation of PTEN. Oncol Lett. 2016;12:4419–4426. doi: 10.3892/ol.2016.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng X, Li J, Peng C, Zhao J, Chi J, Meng X, Yun X, Li D, Yun Y, Gao M, Li Y. MicroRNA-24 induces cisplatin resistance by targeting PTEN in human tongue squamous cell carcinoma. Oral Oncol. 2015;51:998–1003. doi: 10.1016/j.oraloncology.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 117.Cheng CM, Shiah SG, Huang CC, Hsiao JR, Chang JY. Up-regulation of miR-455-5p by the TGF-β-SMAD signalling axis promotes the proliferation of oral squamous cancer cells by targeting UBE2B. J Pathol. 2016;240:38–49. doi: 10.1002/path.4752. [DOI] [PubMed] [Google Scholar]
- 118.Guo Y, Ren MS, Shang C, Zhu L, Zhong M. MTSS1 gene regulated by miR-96 inhibits cell proliferation and metastasis in tongue squamous cellular carcinoma Tca8113 cell line. Int J Clin Exp Med. 2015;8:15441–15449. [PMC free article] [PubMed] [Google Scholar]
- 119.Hu J, Xu JF, Ge WL. MiR-497 enhances metastasis of oral squamous cell carcinoma through SMAD7 suppression. Am J Transl Res. 2016;8:3023–3031. [PMC free article] [PubMed] [Google Scholar]
- 120.Kawakubo-Yasukochi T, Morioka M, Hazekawa M, Yasukochi A, Nishinakagawa T, Ono K, Kawano S, Nakamura S, Nakashima M. MiR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Mol Carcinog. 2018;57:295–302. doi: 10.1002/mc.22744. [DOI] [PubMed] [Google Scholar]
- 121.Li N, Nan CC, Zhong XY, Weng JQ, Fan HD, Sun HP, Tang S, Shi L, Huang SX. miR-182-5p promotes growth in oral squamous cell carcinoma by inhibiting CAMK2N1. Cell Physiol Biochem. 2018;49:1329–1341. doi: 10.1159/000493411. [DOI] [PubMed] [Google Scholar]
- 122.Lin SC, Kao SY, Chang JC, Liu YC, Yu EH, Tseng SH, Liu CJ, Chang KW. Up-regulation of miR-187 modulates the advances of oral carcinoma by targeting BARX2 tumor suppressor. Oncotarget. 2016;7:61355–61365. doi: 10.18632/oncotarget.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Diep C, Mao T, Huang L, Merrill R, Zhang Z, Peng Y. MicroRNA-92b promotes tumor growth and activation of NF-κB signaling via regulation of NLK in oral squamous cell carcinoma. Oncol Rep. 2015;34:2961–2968. doi: 10.3892/or.2015.4323. [DOI] [PubMed] [Google Scholar]
- 124.Lu M, Wang C, Chen W, Mao C, Wang J. miR-654-5p targets GRAP to promote proliferation, metastasis, and chemoresistance of oral squamous cell carcinoma through Ras/MAPK signaling. DNA Cell Biol. 2018;37:381–388. doi: 10.1089/dna.2017.4095. [DOI] [PubMed] [Google Scholar]
- 125.Peng SY, Tu HF, Yang CC, Wu CH, Liu CJ, Chang KW, Lin SC. MiR-134 targets PDCD7 to reduce E-cadherin expression and enhance oral cancer progression. Int J Cancer. 2018;143:2892–2904. doi: 10.1002/ijc.31638. [DOI] [PubMed] [Google Scholar]
- 126.Qiao B, He BX, Cai JH, Tao Q, King-Yin Lam A. microRNA-27a-3p modulates the Wnt/β-Catenin signaling pathway to promote epithelial-mesenchymal transition in oral squamous carcinoma stem cells by targeting SFRP1. Sci Rep. 2017;7:44688. doi: 10.1038/srep44688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Zhao J, Chi J, Gao M, Zhi J, Li Y, Zheng X. Loss of PTEN expression is associated with high microRNA-24 level and poor prognosis in patients with tongue squamous cell carcinoma. J Oral Maxillofac Surg. 2017;75:1449.e1–1449.e8. doi: 10.1016/j.joms.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 128.Zheng G, Li N, Jia X, Peng C, Luo L, Deng Y, Yin J, Song Y, Liu H, Lu M, et al. MYCN-mediated miR-21 overexpression enhances chemo-resistance via targeting CADM1 in tongue cancer. J Mol Med (Berl) 2016;94:1129–1141. doi: 10.1007/s00109-016-1417-0. [DOI] [PubMed] [Google Scholar]
- 129.Chen YF, Yang CC, Kao SY, Liu CJ, Lin SC, Chang KW. MicroRNA-211 enhances the oncogenicity of carcinogen-induced oral carcinoma by repressing TCF12 and increasing antioxidant activity. Cancer Res. 2016;76:4872–4886. doi: 10.1158/0008-5472.CAN-15-1664. [DOI] [PubMed] [Google Scholar]
- 130.Chen YH, Song Y, Yu YL, Cheng W, Tong X. MiRNA-10a promotes cancer cell proliferation in oral squamous cell carcinoma by upregulating GLUT1 and promoting glucose metabolism. Oncol Lett. 2019;17:5441–5446. doi: 10.3892/ol.2019.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cao ZH, Cheng JL, Zhang Y, Bo CX, Li YL. MicroRNA-375 inhibits oral squamous cell carcinoma cell migration and invasion by targeting platelet derived growth factor A. Mol Med Rep. 2017;15:922–928. doi: 10.3892/mmr.2016.6057. [DOI] [PubMed] [Google Scholar]
- 132.Du Y, Li Y, Lv H, Zhou S, Sun Z, Wang M. miR-98 suppresses tumor cell growth and metastasis by targeting IGF1R in oral squamous cell carcinoma. Int J Clin Exp Pathol. 2015;8:12252–12259. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.