Abstract
N-acetylcysteine is a commonly used antioxidant that is broadly effective despite its limited ROS reactivity. Chemoprotection by N-acetylcysteine frequently results from inactivation of primary toxicants or reactive electrophiles arising as metabolites or lipid peroxidation products.
Graphical Abstract
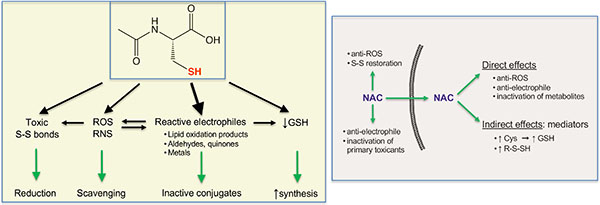
Reactive oxygen species (ROS) are linked to the development of many human diseases and biological injury by numerous xenobiotics. Oxidative damage is the first mechanism that is often tested for toxicants. There is also a frequent projection of the established ROS mechanism for one member to a broader group to which this chemical belongs. However, the biological significance of oxidative processes is not always easy to establish, as oxidants could be a cause or result of cellular injury. The role of ROS is tested through genetic manipulations of oxidative stress-protective proteins and addition of small antioxidants. In general, genetic approaches produce weaker protective effects than small antioxidants, which can reflect different anti-ROS specificity. Another possibility is that chemical antioxidants have ROS-unrelated chemoprotective activities. N-Acetylcysteine (NAC) is the most frequently used antioxidant (>76000 hits in the Google Scholar search for “antioxidant and N-acetylcysteine”). The chemoprotective effectiveness of NAC has been reported for numerous disease models, xenobiotics and other cell stress-inducing conditions, which is commonly taken as evidence for the causal involvement of ROS. However, the direct contribution of NAC and other small thiols to inactivation of the main primary ROS, H2O2 and O2•−, is very small, which is dominated by highly effective enzymatic processes.1,2 Reaction rates for NAC with the major ROS are slow in general and almost undetectable for superoxide radical. The relative effectiveness of thiols in detoxification of oxidants is related to pKa of their SH groups, as the rates of reactions with the majority of ROS (H2O2, O2•−, CO3•−, •NO2, ONOO−, HOCl) is higher for the thiolate anion (RS−) than RSH. Since NAC has a significantly higher pKa (9.5) than Cys (8.3) or glutathione (8.7), the concentration of the active RS− form at physiological pH is much lower for NAC. At pH 7.4, only 0.8% of NAC is present in the RS− form in comparison to 12.6% for Cys. However, higher pKa of the SH group in NAC offers its advantage in scavenging a highly toxic hydroxyl radical (•OH) that reacts with thiols through the hydrogen abstraction. High antioxidant activity of NAC despite its weak direct ROS reactivity can be reconciled by a recently outlined mechanism for NAC-induced production of hydropersulfides (R-S-SH).3 Persulfides are considered as hyperactivated thiols due to a heightened reactivity of their outer sulfur atom with oxidants and electrophiles. Formation of hydropersulfides from NAC involved its deacetylation to Cys that underwent further enzymatic reactions, suggesting that the yield of persulfides can be cell type-specific and subjected to metabolic controls.
Other activities that can contribute to chemoprotection by NAC include replenishment of glutathione through cellular delivery of Cys (formed after NAC deacetylation) and the reduction of disulfide bonds in proteins.1 Restoration of hepatic glutathione is a known mechanism in the action of NAC as antidote for acetaminophen overdosing. There is also a strong evidence that a direct breakup of excessive disulfide bonds is responsible for the clinical effectiveness of NAC as a mucolytic agent. Cys-rich lung mucus-forming mucins are oxidized during inflammation, leading to abnormal disulfide bridging between domains. The resulting stiffening of mucins impairs movement of the mucocilliary escalator, which leads to thickening of the mucus and obstructed airflow and infections. NAC has a higher disulfide-reducing activity than Cys or glutathione due to its stronger nucleophilicity. The importance of glutathione replenishment is likely limited to the conditions with a massive production of oxidants in order to cause a significant drop in cellular glutathione with its normal content of 3–10 mM. Glutathione biosynthesis is regulated by a negative feedback mechanism, making it difficult to boost glutathione above its physiological levels via the addition of NAC. The breakup of disulfide bridges in cellular proteins by NAC cannot be specific toward pathological S-S bonds and this lack of selectivity accounts for toxic effects of NAC, including mortality in the cases of severe overdosing in clinical settings.
The SH group in NAC is a soft Lewis base, making it highly reactive with soft Lewis acids such as several toxic metals. Metals are a good example when pathology-causing oxidative mechanisms established for one member are frequently but erroneously extrapolated to the entire chemical group. Generation of oxidants during Fe(II)/Fe(III) redox cycling is an established cause of pathological effects in conditions of iron overload. Redox-based toxic mechanisms are also invoked for other metals, in part or largely based on the chemoprotective activity of NAC. However, some of these metals are thermodynamically unable to undergo redox cycling [Cd(II), Hg(II), or Pt(II), for example]. A high effectiveness of NAC against toxic metals with soft Lewis acid properties (Cd, Hg, Co) is clearly related to their stable binding with the SH-group, producing inactive complexes. Formation of these complexes inside the cells prevents toxic binding of metals to protein-Cys-SH whereas their extracellular production blocks cellular uptake of metals.4
ROS cause oxidation of lipids, which releases a variety of reactive carbonyls that can diffuse through the cell and damage various targets. The most toxic lipid oxidation products are α,β-unsaturated aldehydes such as acrolein, crotonaldehyde and 4-hydroxy-2-nonenal (4HNE). Toxicity of these carbonyl compounds is caused by their very high reactivity with the protein-SH groups through Michael addition at the β-carbon of the double bond (R-CH=CH-CHO). A strong electron-shifting ability of carbonyl groups increases electrophilicity of the β-carbon and its susceptibility to Michael addition. Enzymatic processes for elimination of α,β-unsaturated aldehydes are much less efficient relative to ROS, which results in their higher levels in cells relative to ROS (low μM range for 4HNE vs. low nM for H2O2 and pM concentrations for O2•−).2 The SH-group of NAC rapidly forms Michael adducts with α,β-unsaturated aldehydes, preventing their conjugation to proteins and the resulting toxic effects.5,6 The protective effects of NAC through conjugation of toxicants at its highly nucleophilic SH group extends to a variety of other reactive electrophilic species such as quinones and epoxides.2 Scavenging of reactive electrophilic species (especially strong Michael acceptors) is a broadly applicable chemoprotective mechanism that is frequently overlooked. Overabundance of SH-reactive electrophiles and their inactivation of antioxidant enzymatic activities can also lead to elevated ROS, in which case NAC suppresses ROS via elimination of their cause. Overall, a broad chemoprotective effectiveness of NAC, especially in cell culture where extracellular media lack unoxidized thiols, can be attributed to more than one mechanism. Addition of NAC in many cases probably tests a role of reactive electrophilic species rather than primary ROS.
Funding information
Author’s research is supported by grants ES008786, ES028072 and ES020689 from the National Institute of Environmental Health Sciences.
Abbreviations
- 4HNE
4-hydroxy-2-nonenal
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
References
- 1.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, and Sergio F (2018) N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic. Res 52, 751–762. [DOI] [PubMed] [Google Scholar]
- 2.Parvez S, Long MJC, Poganik JR, Aye Y (2018) Redox signaling by reactive electrophiles and oxidants. Chem. Rev 118, 8798–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezeriņa D, Takano Y, Hanaoka K, Urano Y, and Dick TP (2018) N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem. Biol 25, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luczak MW, and Zhitkovich A (2013) Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium(VI), cadmium(II) and cobalt(II). Free Radic. Biol. Med 65, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Toranzo EG, and Castro JA (1994) Reaction of 4-hydroxynonenal with some thiol-containing radioprotective agents or their active metabolites. Free Radic. Biol. Med 17, 605–607. [DOI] [PubMed] [Google Scholar]
- 6.Higashi T, Elmeligy E, Mai Y, Noya Y, Terada K, Mazaki Y, Kuge Y, and Miwa S (2019) Glutathione and cysteines suppress cytotoxicity of gas phase of cigarette smoke by direct reacting with unsaturated carbonyl compounds in the gas phase. Biochem. Biophys. Res. Commun 509, 988–993. [DOI] [PubMed] [Google Scholar]


