Abstract
Diabetic patients have an increased prevalence of blood pressure (BP) circadian rhythm disruption, which is associated with increased risk of target organ damage and detrimental cardiovascular events. Limited information is available regarding the role of clock genes in the disruption of the BP circadian rhythm in diabetes due to the lack of a diabetic animal model that allows real-time monitoring of clock gene oscillation. Here, we generated a novel diabetic db/db-mPer2Luc mouse model by crossing the type 2 diabetic db/db mice with the mPer2Luc knock-in mice. The daily rhythms of BP, heart rate, locomotor activity, and food and water intake were acquired by radiotelemetry or metabolic chambers. The daily oscillation of mPer2 bioluminescence was recorded by LumiCycle in real-time in tissue explants and by IVIS system in vivo. Our results showed that the db/db-mPer2Luc mice were obese, diabetic and glucose intolerant. The db/db-mPer2Luc mice displayed a compromised BP daily rhythm, which was associated with the disruption of the daily rhythms in baroreflex sensitivity, locomotor activity, and metabolism, but not heart rate or food and water intake. The phase of the mPer2 daily oscillation was advanced to different extents in the explanted peripheral tissues from the db/db-mPer2Luc mice relative to that in the control mice. In contrast, no phase shift was detected in the mPer2 daily oscillation in the explanted suprachiasmatic nucleus (SCN). Moreover, the advanced phase shift of the mPer2 daily oscillation was also detected in the liver, kidney and submandibular gland in vivo in the db/db-mPer2Luc mice. In conclusion, the diabetic db/db-mPer2Luc mouse is a novel animal model that allows real-time monitoring of mPer2 circadian rhythms ex vivo and in vivo. The results from db/db-mPer2Luc mice suggest that the desynchrony of mPer2 daily oscillation in the peripheral tissues contributes to the loss of BP daily oscillation in diabetes.
Keywords: Diabetes, circadian rhythm, blood pressure, baroreflex sensitivity, locomotor activity, metabolism, Per2, clock gene, db/db mice
Introduction
Diabetes is one of the most common metabolic disorders, and its prevalence is rising to epidemic levels worldwide (Collaboration 2016). Cardiovascular diseases are the major common causes of mortality and morbidity in diabetic patients (Rask-Madsen and King 2013). Accumulated evidence from human and rodent studies suggests that disruption of normal circadian rhythms is associated with increased incidence of obesity and type 2 diabetes (Bass and Takahashi 2010). Night or rotating shift workers have a higher prevalence of type 2 diabetes (Gan, Yang et al. 2015). Imposing chronic jet-lag or light at night in mice disrupts food intake pattern and is associated with higher body weight (Fonken, Workman et al. 2010, Oike, Sakurai et al. 2015) and leptin resistance (Kettner, Mayo et al. 2015). Food intake at the “wrong” time (i.e., during the rest period) and increased snacking has been demonstrated to be critical for the development of obesity and diabetic metabolic disorder, independent of total caloric intake and macronutrient quality (Hatori, Vollmers et al. 2012). In humans, genetic variations in BMAL1, a core clock gene, are associated with increased incidence of type 2 diabetes and hypertension (Woon, Kaisaki et al. 2007, Corella, Asensio et al. 2016, Uemura, Katsuura-Kamano et al. 2016). In mice, either global (Rudic, McNamara et al. 2004, Kondratov, Kondratova et al. 2006) or tissue-specific Bmal1 deletion in liver (Lamia, Storch et al. 2008, Jacobi, Liu et al. 2015) or pancreas (Marcheva, Ramsey et al. 2010) causes impaired glucose homeostasis and/or insulin resistance. Taken together, a large body of evidence indicates a critical role of the circadian rhythm disruption in the pathogenesis of obesity, diabetes, and metabolic disorders. In contrast, much less research has been conducted on the impact of diabetes on the circadian rhythm in particular related to diabetic cardiovascular complications.
Blood pressure (BP) in humans undergoes a daily oscillation that is characterized by lowest levels at night (i.e., a “nocturnal dip”) and a peak before awakening (morning surge) (Millar-Craig, Bishop et al. 1978). The importance of the BP circadian rhythm is highlighted by a meta-analysis that demonstrated the early morning BP surge is associated with a 40% higher risk of acute myocardial infarction, a 29% higher risk of sudden cardiac death, and a 49% higher risk of stroke (Cohen, Rohtla et al. 1997). The results of a cross-sectional analysis of a 20,000-patient database revealed that up to 70% of diabetic patients are non-dippers (Gorostidi, Sobrino et al. 2007), in which the decrease in BP during the nocturnal sleep period is less than 10% of the daytime values. The non-dipping BP rhythm is the most common form of BP circadian rhythm disruption in diabetic patients and is associated with increased risks of target organ damage and detrimental cardiovascular outcomes (Routledge, McFetridge-Durdle et al. 2007, Eguchi 2011, Yano and Kario 2012, Ayala, Moya et al. 2013). Importantly, the non-dipping BP is emerging as an independent predictor of future cardiovascular events (Friedman and Logan 2009, Cuspidi, Vaccarella et al. 2010). The nocturnal laboratory animals, such as mice, have a BP daily oscillation that is opposite to that in humans, i.e., BP is low during the day (rest phase) and peaks at night (active phase). In agreement with these studies, we and others recently reported that the db/db mice, a widely-used diabetic mouse model (Herberg and Coleman 1977), are not only hypertensive but are also non-dippers (Park, Bivona et al. 2008, Su, Guo et al. 2008, Goncalves, Tank et al. 2009, Senador, Kanakamedala et al. 2009). Moreover, we and others have demonstrated that either global or selective deletion of Bmal1 in smooth muscle or adipocytes abolishes or attenuates BP circadian rhythm (Curtis, Cheng et al. 2007, Xie, Su et al. 2015, Yang, Chen et al. 2016, Chang, Xiong et al. 2018). Furthermore, the daily oscillations of the mRNA encoding many core clock genes and clock target genes are altered in isolated tissues from db/db mice (Kudo, Akiyama et al. 2004, Su, Guo et al. 2008, Caton, Kieswich et al. 2011, Su, Xie et al. 2012, Nernpermpisooth, Qiu et al. 2015, Grosbellet, Dumont et al. 2016). However, most of these studies investigated the core clock genes and clock target genes oscillations by real-time PCR or Western blot quantification of mRNAs or proteins in tissues collected every 4 to 6 hours in only one day. Consequently, the time resolution of circadian rhythm analysis is limited due to the limited sampling intervals and duration. One of the major reasons accounting for these limitations is that increasing the time resolution of clock gene oscillation requires a significant increase in time points of tissue collections which is cost prohibitive.
To overcome this barrier and to stimulate the research on the BP circadian rhythm in diabetes, we crossed the db/db mice with the mPer2Luc knock-in mice and generated a novel db/db-mPer2Luc mouse model. The db/db mouse is an extensively used monogenic type 2 diabetic mouse model. The syndrome in db/db mice is similar to that in maturity-onset diabetes in humans, characterized by obesity, infertility, hyperphagia and marked hyperglycemia (Ktorza, Bernard et al. 1997). Diabetes in db/db mice is caused by a spontaneous point mutation in the “leptin receptor” gene (lepr), resulting in abnormal splicing of the gene transcript, leading to defective in leptin signaling (Chen, Charlat et al. 1996, Lee, Proenca et al. 1996). The db/db-mPer2Luc mice are expected to not only be diabetic but to allow quantitatively measuring of mPer2 protein oscillation by mPer2Luc bioluminescence monitoring in real-time ex vivo and in vivo (Yoo, Yamazaki et al. 2004, Tahara, Kuroda et al. 2012) due to a reporter gene luciferase fused in-frame to the 3’ end of the endogenous mPer2 gene (Yoo, Yamazaki et al. 2004). We reported here for the first time that the db/db-mPer2Luc mice are obese and diabetic and that they are non-dippers although they are normotensive. The compromised daily rhythm in BP in the db/db-mPer2Luc mice is associated with the disruption of daily rhythms in baroreflex sensitivity, locomotor activity, and metabolism. Moreover, by monitoring mPer2Luc bioluminescence in various peripheral and suprachiasmatic nucleus or nuclei (SCN) tissues ex vivo and in vivo, we demonstrated that there is a desynchrony of mPer2Luc bioluminescence daily oscillations in peripheral tissues but not in the SCN in the db/db-mPer2Luc mice.
Materials and methods
Generation of the db/db-mPer2Luc mice
The heterozygous leptin receptor (Leprdb) mutation db/+ mice on the C57BL/KsJ background (Stock No: 000642; also known as C57BL/KsJ-db/+) and the homozygous mPer2Luc mice on the C57BL/6J background (Stock No: 006852; also known as C57BL/6J-mPer2Luc) were purchased from the Jackson Laboratory. Since the homozygous C57BL/KsJ-db/db mice are infertile, the heterozygous male C57BL/KsJ-db/+ mice and homozygous female C57BL/6J-mPer2Luc mice were used as breeders to generate the homozygous diabetic db/db-mPer2Luc mice and heterozygous non-diabetic db/+-mPer2Luc control mice (Figure S1A). Of note, both db/db-mPer2Luc and db/+-mPer2Luc control mice have a mixed C57BL/KsJ and C57BL/6J background. The genotyping protocol for the db/db mice is listed in the Jackson Laboratory website. The genotyping protocol for the mPer2Luc mice was described previously (Yoo, Yamazaki et al. 2004). The representative agarose gels for PCR genotyping of the mPer2Luc and db/db mice are shown in Figure S1B and S1C. The mice were fed normal chow diet and housed under 12:12 light: dark condition. Only the 4-6 month-old male db/db-mPer2Luc and age- and gender-matched db/+-mPer2Luc control mice were used in the current study. All animal procedures were approved by the Institutional Animal Care and Use Committee.
Animal characterization
Body composition (lean mass and fat mass) was assessed by NMR spectroscopy (Echo MRITM-100H, Houston, TX, USA). Non-fasting blood glucose level was measured between Zeitgeber Time (ZT; ZT0 is defined as light on and ZT12 is defined as light off) ZT9 to ZT10 by using StatStrip® XepressTM glucometer (NOVA® biomedical, Waltham, MA, USA). Non-fasted plasma insulin level was determined between ZT10 to ZT11 by an ELISA according to the manufacturer’s instructions (Chrystal Chem, Downers Grove, IL, USA). Intraperitoneal glucose tolerance test (IPGTT) was performed at ZT3 after 6-hour fasting by injecting 1 mg/kg glucose.
Radiotelemetry measurement of BP, heart rate, and locomotor activity
The db/db-mPer2Luc and control mice were chronically instrumented in the left common carotid artery with a radiotelemetry probe (TA11PA-C10, Data Sciences International, St. Paul, MN, USA) as described previously (Su, Guo et al. 2008, Su, Xie et al. 2013, Xie, Su et al. 2015). After 10-day recovery from the surgery, BP, heart rate, and locomotor activity were recorded for three consecutive days.
Baroreflex sensitivity analysis
Spontaneous baroreflex sensitivity was analyzed by sequence techniques using Hemolab software (http://www.haraldstauss.com/HemoLab/HemoLab.html). At least four consecutive sequences where the systolic arterial pressure and pulse interval were positively correlated (r2>0.80) were counted. Baroreflex sensitivity was calculated as the average slope of the systolic pressure-pulse interval relationships as described previously (Xie, Su et al. 2015).
Metabolic chamber measurement of locomotor activity, food and water intake, respiratory exchange ratio (RER) and energy expenditure (EE)
The locomotor activity, food and water intake, RER, and EE were determined by indirect gas calorimetry LabMaster system (TSE System, Bad Homburg, Germany; also known as metabolic chambers). The mice were exposed to a 12:12 light:dark cycle and were individually housed in the acclimation cages for seven days and then transferred to the metabolic chambers. The oxygen consumption and carbon dioxide production in the metabolic chambers were measured every 30 minutes for three consecutive days. RER and EE were calculated by the accompanied TSE PhenoMaster software.
Real-time monitoring of mPer2 oscillations in explant tissues by LumiCycle
The procedure for real-time monitoring of mPer2 oscillations in explant tissues by LumiCycle was adapted from the previous report (Yamazaki and Takahashi 2005). Briefly, the aorta, mesenteric artery (MA), kidney, liver, white adipose tissue (WAT), thymus, lung, adrenal gland (AG), and brain were isolated from mice between ZT10 and ZT11. The aorta was cleaned, cut open longitudinally, and denuded of endothelium cells. The MA was dissected to remove fat tissues. The kidney, liver, WAT, thymus, and lung were cut into small pieces, with a diameter varying between approximately 2 and 6 mm depending upon the tissue. The total AG was used. The brain containing the SCN was cut into 250 μm thick sections by using NVSL manual advance vibroslice (World Precision Instruments, Sarasota, FL, USA). Each tissue was cultured in a well-sealed 35-mm Petri dish containing Dulbecco’s Modified Eagle Medium (DMEM) and 0.1 mM D-luciferin (Gold Biotechnology Inc., St. Louis, MO). Details of the medium constituent were described previously (Yamazaki and Takahashi 2005). The light emission from the cultured tissues was measured with photon-counting photomultiplier tubes that count photons for 1 min over a 10 min interval using a LumiCycle 32 system (Actimetrics, Wilmette, IL, USA) as described (Yamazaki and Takahashi 2005). The bioluminescence data obtained from the explanted tissues were analyzed using LumiCycle Analysis software (Actimetrics, Wilmette, IL, USA). To detrend the signal drift over time, the 24-hour moving average was subtracted from the raw data. To eliminate the influence of exposure to environmental lighting before recording, the first 12-hour data collected in the explant culture were excluded. The data collected from 12 hours to 36 hours in the culture were used to determine the oscillation amplitude and acrophase. The data collected from 12 hours to 120 hours in the culture were used to determine the oscillation period by the dampened sine-curve fitting method. The data with a goodness of fit >0.8 were used for analysis in all the tissues except in kidney where data with a goodness of fit > 0.7 were used due to the rapid dampening of the oscillation.
In vivo imaging of mPer2 time-of-day variation in the kidney, liver, and submandibular gland (SG)
The procedure for in vivo imaging of mPer2 time-of-day variation in the kidney, liver, and SG was adapted from the previous report (Tahara, Kuroda et al. 2012). Briefly, at ZT5, 11, 17 and 23, mice were anesthetized with 2.5-4% isoflurane and subcutaneously injected with D-luciferin (15 mg/kg body weight in PBS). The mice were imaged 7 minutes later for dorsal side up and 10 minutes later for later ventral side up for 5 seconds by using the IVIS spectrum (IVIS spectrum in vivo imaging system, PerkinElmer, Waltham, MA, USA). Bioluminescence from the liver was quantified (photon/s/cm2/sr) by setting the region of interest to the same shape and size using Living Image software (IVIS Imaging System). The bioluminescence intensity was expressed as an absolute value or as the percentage of the average value throughout the day as described (Tahara, Kuroda et al. 2012).
Cosinor analysis of circadian rhythm
The daily rhythms of BP, heart rate, locomotor activity, food and water intake, RER and EE were analyzed by using Cosinor analysis as previously reported (Refinetti, Lissen et al. 2007). Briefly, a cosine wave with a known period (24 hours) was fitted by the least squares to the data as an estimate of the pattern of the smooth rhythm. The model equation was written as xi=M+Acos (θi+φ), where M is mesor, A is amplitude, φ is acrophase, and θi is trigonometric angles corresponding to the sampling time.
Quantitative analysis of mRNA expression
Mesenteric arteries were isolated from db/db-mPer2Luc and control mice at ZT5 and ZT17. RNA extraction, cDNA synthesis, and real-time PCR were carried out as described (Guo, Su et al. 2005, Xie, Su et al. 2015). The real-time PCR primers for each gene are described in Table S1.
Statistical analysis
All data were expressed as mean ± SEM. For comparison of 1 parameter between 2 strains of mice, unpaired 2-tail Student’s t-test was used. For comparison of one parameter across a time period between 2 strains of mice, 2-way ANOVA with repeated measures and Bonferroni’s post-test were performed. For comparison of multiple parameters between 2 strains of mice, regular 2-way ANOVA with Bonferroni’s post-test was performed. P < 0.05 was defined as statistically significant.
Results
Db/db-mPer2Luc mice are obese and diabetic
The db/db mouse is an extensively used monogenic type 2 diabetic mouse model. The syndrome in db/db mice is similar to that in maturity-onset diabetes in humans, characterized by obesity, infertility, hyperphagia and marked hyperglycemia (Ktorza, Bernard et al. 1997). The diabetic phenotype of the db/db mice, however, varies depending on the genetic background. Currently, there are two db/db mouse models: one is on the C57BL/KsJ background with severe hyperglycemia and temporarily elevated plasma insulin; the other one is on the C57BL/6J background with transient hyperglycemia and marked hyperinsulinemia (Hummel, Coleman et al. 1972). To study the disruption of circadian rhythms in type 2 diabetes, we crossed the C57BL/KsJ-db/db mice that have severe diabetes with the mPer2Luc mice that contain a knock-in luciferase gene fused to mouse Period2 (mPer2) as a clock gene reporter (Yoo, Yamazaki et al. 2004), and generated a novel db/db-mPer2Luc mice. Since the mPer2Luc mice are on the C57BL/6J background, the generated db/db-mPer2Luc mice have a mixed background (C57BL/KsJ and C57BL/6J). It is unclear to what extent the db/db-mPer2Luc mice retain obesity and diabetes. Therefore we first characterized this novel mouse model with respect to obesity, hyperglycemia, hyperinsulinemia and insulin resistance.
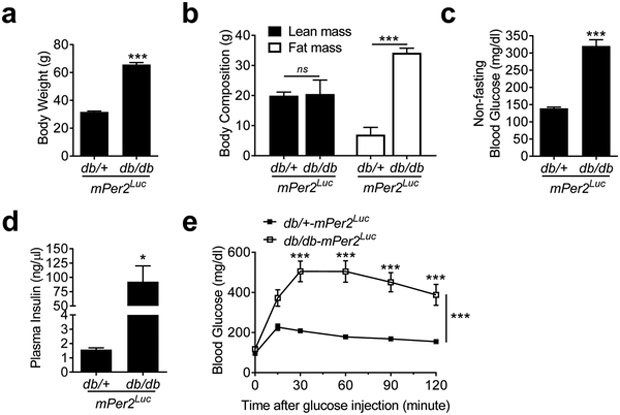
The db/db-mPer2Luc mice had significantly increased body weight when compared to their littermate db/+-mPer2Luc control mice (Figure 1a). The body weight increase was mostly attributable to an increased fat mass as the lean mass was comparable between the db/db-mPer2Luc and control mice (Figure 1b). The non-fasting blood glucose and plasma insulin levels in the db/db-mPer2Luc mice were also markedly elevated relative to those in the control mice (Figure 1c and 1d). Moreover, the db/db-mPer2Luc mice exhibited a severely impaired glucose tolerance (Figure 1e). These results indicate that the db/db-mPer2Luc mice manifest the common characteristics of type 2 diabetes, e.g. obesity, hyperglycemia, hyperinsulinemia, and impaired glucose tolerance.
Fig. 1. The db/db-mPer2Luc mice are obese and diabetic.
Body weight (a; N = 12), body composition (b; N = 4-6), non-fasting blood glucose (c; N = 12), and plasma insulin (d; N = 4-5) were measured between ZT9 and ZT11 in the db/db-mPer2Luc and control db/+-mPer2Luc mice. Glucose tolerance test (e; N = 11-12) was performed at ZT3 after 6-hour fasting. All data were expressed as mean ± SEM. *, P < 0.05; ***, P < 0.001; ns, not significant.
Db/db-mPer2Luc mice have a compromised BP daily rhythm that is associated with the disruption of the daily rhythms in baroreflex sensitivity but not heart rate
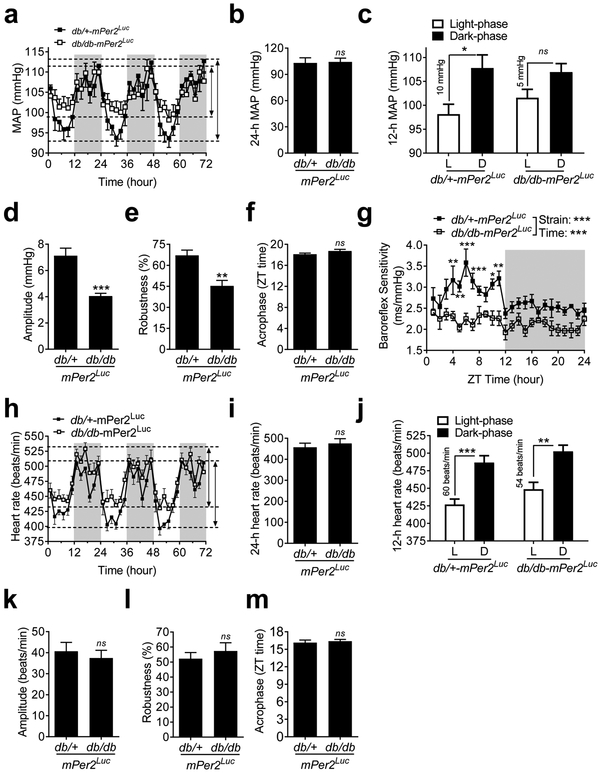
To determine whether the BP daily rhythm is disrupted in the db/db-mPer2Luc mice, we recorded BP by radiotelemetry under normal 12:12 light-dark cycle for 72 consecutive hours. We found that the daily oscillations of mean arterial pressure (MAP), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were diminished in the db/db-mPer2Luc mice compared to that in the control mice (Figure 2a; Figure S2a and S2d). The compromised daily rhythms of the MAP, SBP, and DBP were primarily caused by the decreased dipping during the inactive light phase with no change during the active dark phase in the db/db-mPer2Luc mice relative to the control mice (Figure 2a; Figure S2a and S2d). Quantitative analysis of the daily (24-hour) average of MAP, SBP, and DBP showed no difference between the db/db-mPer2Luc and control mice (Figure 2b; Figure S2b and S2e), indicating that the db/db-mPer2Luc mice are normotensive, unlike the C57BL/KsJ-db/db mice (Park, Bivona et al. 2008, Su, Guo et al. 2008, Goncalves, Tank et al. 2009, Senador, Kanakamedala et al. 2009). Further quantitative analysis of the BP during either the light or dark phase (12-hour) revealed a 50% reduction in the difference between the light phase and the dark phase in the MAP, SBP, and DBP in the db/db-mPer2Luc mice compared with that in the control mice (Figure 2c; Figure S2c and S2f). Cosinor analysis of the oscillations showed that the amplitude (half of the range of oscillation) and robustness of daily rhythms in the MAP, SBP, and DBP were significantly attenuated in the db/db-mPer2Luc mice compared with that in the control mice (Figure 2d and 2e; Table S2). Interestingly, no differences were found in the acrophase (the time when the cycle peaks) between the db/db-mPer2Luc and control mice (Figure 2f; Table S2).
Fig. 2. The daily rhythms of blood pressure (BP) and baroreflex sensitivity but not heart rate are disrupted in the db/db-mPer2Luc mice.
BP and heart rate were recorded by radiotelemetry in the db/db-mPer2Luc and control db/+-mPer2Luc mice. a. The 72-hour recording of mean arterial pressure (MAP). The light grey box indicates the dark-phase and the length of the arrowhead lines indicates the BP difference between the light and dark phase in the two mouse strains. b. The 24-hour MAP. c. The 12-hour MAP during the light phase (L) and dark phase (D). d., e. and f.: The amplitude, robustness, and acrophase of the MAP daily oscillation. g. The spontaneous baroreflex sensitivity over the 24-hour day. h. The 72-hour recording of heart rate. i. The 24-hour heart rate. j. The 12-hour heart rate during the light phase (L) and dark phase (D). k., l., and m.: The amplitude, robustness, and acrophase of heart rate daily oscillation. All data were expressed as mean ± SEM (N = 6). *, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant.
Baroreflex is an important rapid negative feedback mechanism for maintaining normal BP. Therefore we investigated whether the compromised BP daily rhythm in the db/db-mPer2Luc mice is associated with an alteration of the time-of-day variations in baroreflex sensitivity. We analyzed spontaneous baroreflex sensitivity by sequence techniques in the db/db-mPer2Luc and control mice as previously described (Xie, Su et al. 2015). In the db/+-mPer2Luc control mice, baroreflex sensitivity was significantly higher during the light phase than during the dark phase (Figure 2g). In contrast, such time-of-day variations of baroreflex sensitivity were abolished in the db/db-mPer2Luc mice. This result implicates the loss of daily variation in baroreflex sensitivity contributes to the compromised BP daily rhythm.
Because heart rate is an important factor that determines the cardiac output and BP level (Reule and Drawz 2012), we investigated whether the daily heart rate oscillation is also altered in the db/db-mPer2Luc mice. We found that the daily heart rate, the difference between light phase and dark phase heart rate, and its rhythmicity, including amplitude, robustness, and acrophase, were not significantly altered in the db/db-mPer2Luc mice compared to that in the control mice (Figure 2h-2m).
The compromised BP daily rhythm is associated with the disruption of daily rhythms in locomotor activity and metabolism but not in food and water intake in the db/db-mPer2Luc mice
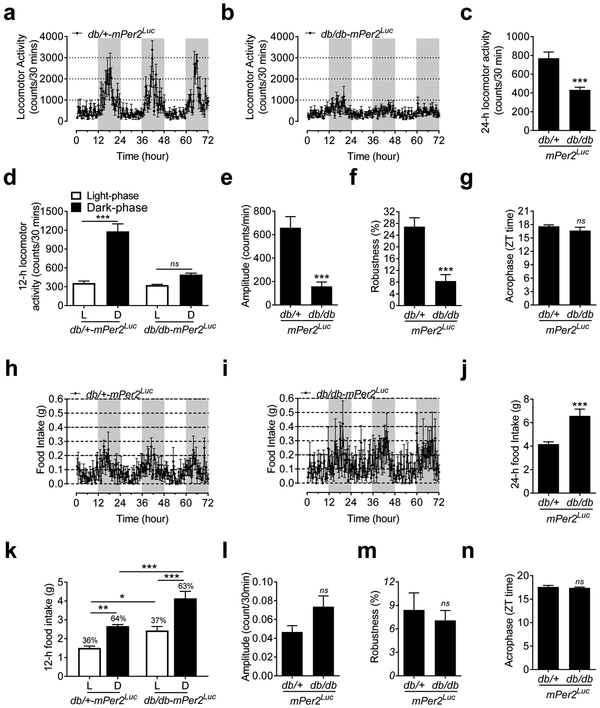
Behavioral factors such as locomotor activity, food and water intake as well as metabolism may affect central and peripheral clock function through the release of neurotransmitters and hormones and thus impinge on BP circadian rhythm (Rudic and Fulton 2009). Therefore, the daily rhythms in locomotor activity, food and water intake, and metabolism were monitored by indirect calorimetry (also known as a metabolic chamber) in the db/db-mPer2Luc and control mice every 30 minutes over 72 consecutive hours under 12: 12 light: dark condition. We also used radiotelemetry to monitor locomotor activity independently to confirm the indirect calorimetry data. The results from both indirect calorimetry and radiotelemetry data consistently showed that the daily oscillation in locomotor activity was abolished in the db/db-mPer2Luc mice compared with that in the control mice (Figure 3a and 3b; Figure S3a and S3b). While the absolute counts regarding the daily locomotor activity from indirect calorimetry (Figure 3c) and radiotelemetry (Figure S3c) were not consistent, both methods showed a loss of the locomotor activity daily oscillation in the db/db-mPer2Luc mice (Figure 3d; Figure S3d). Cosinor analysis revealed that the amplitude and robustness of the locomotor activity daily oscillations were largely diminished in the db/db-mPer2Luc mice (Figure 3e and 3f; Figure S3e and S3f). Interestingly, in agreement with the compromised BP daily rhythm in the db/db-mPer2Luc mice (Figure 2f), there were also no differences in the acrophase of the locomotor activity daily oscillation between the db/db-mPer2Luc and control mice (Figure 3g; Figure S3g).
Fig. 3. The daily rhythm of locomotor activity but not food intake is disrupted in the db/db-mPer2Luc mice.
Locomotor activity and food intake were recorded by indirect calorimetry. a. The 72-hour recording of locomotor activity in the control mice where the light grey box indicates the dark-phase. b. The 72-hour recording of locomotor activity in the db/db-mPer2Luc mice where the light grey box indicates the dark-phase. c. The 24-hour locomotor activity. d. The 12-hour locomotor activity during the light phase (L) and dark phase (D). e., f., and g: The amplitude, robustness, and acrophase of locomotor activity daily oscillation. h. The 72-hour recording of food intake in the control mice where the light grey box indicates the dark-phase. i. The 72-hour recording of food intake in the db/db-mPer2Luc mice where the light grey box indicates the dark-phase. j. The 24-hour food intake. k. The 12-hour food intake during the light phase (L) and dark phase (D). l., m., and n: The amplitude, robustness, and acrophase of the food intake daily rhythm. All data were expressed as mean ± SEM (N = 6). *, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant.
In contrast to the locomotor activity, the food and water intake daily oscillations appeared to be preserved in the db/db-mPer2Luc mice (Figure 3h and 3i; Figure S4a and S4b), although the db/db-mPer2Luc mice consumed more food and water than the control mice (Figure 3j; Figure S4c). Since the db/db-mPer2Luc mice consumed more food and water proportionally during both the light and dark phase than the control mice (Figure 3k; Figure S4d), the percentages of daily food and water intake during the light and dark phase were similar between two strains of mice (Figure 3k; Figure S4d). In accordance with these findings, there were also no differences in robustness and acrophase in food and water intake daily oscillations (Figure 3m and 3n; Figure S4f and S4g). Interestingly, there was a trend towards an increased daily oscillation amplitude in food intake (Figure 3l) and a significant increase in water intake (Figure S4e) in the db/db-mPer2Luc mice.
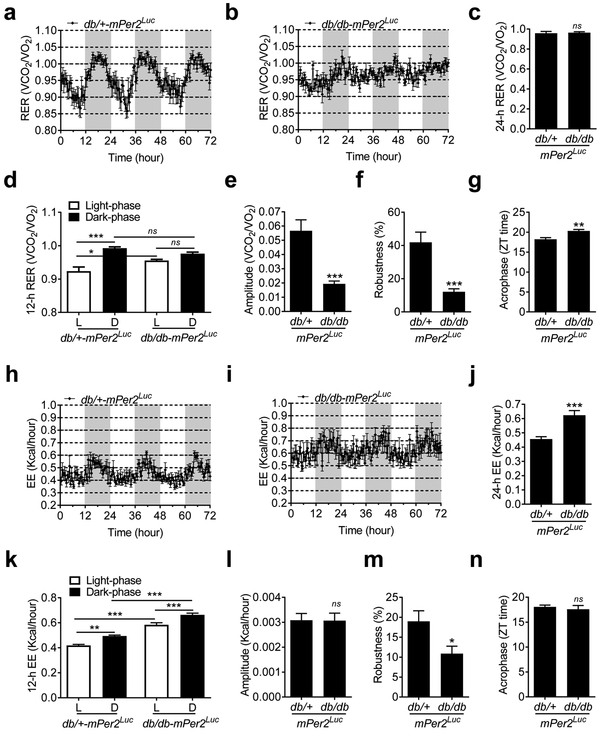
The respiratory exchange ratio (RER) and energy expenditure (EE) daily oscillations were acquired by the metabolic chamber. The RER is calculated as the ratio between the volume of carbon dioxide (VCO2) produced and the volume of oxygen (VO2) used in metabolism. It is an indicator of fuel sources (Even and Nadkarni 2012). The EE is calculated as the total daily energy expenditure (calories) in the metabolic chamber, including basal and physical activity expenditure, thermoregulation, and the thermic effects of food (Even and Nadkarni 2012). The RER daily oscillation was disrupted in the db/db-mPer2Luc mice compared with the control mice (Figure 4a vs. 4b). Although both strains of mice had a similar average RER (Figure 4c), the db/db-mPer2Luc mice lost the RER daily oscillation compared to the control mice (Figure 4d). In agreement with these findings, the amplitude and robustness of the RER daily oscillation were suppressed (Figure 4e and 4f), and the acrophase was delayed in the db/db-mPer2Luc mice (Figure 4g). In contrast, the EE daily oscillation was preserved in both strains of mice (Figure 4h and 4i), although the daily EE level was higher in the db/db-mPer2Luc than the control mice (Figure 4j). Both strains of mice exhibited a similar EE daily oscillation pattern (Figure 4k). In agreement with these findings, there was no difference in amplitude and acrophase between the db/db-mPer2Luc and control mice (Figure 4l and 4n). However, the robustness was suppressed in the db/db-mPer2Luc mice (Figure 4m).
Fig. 4. The daily rhythms of respiratory exchange ratio (RER) but not energy expenditure (EE) is disrupted in the db/db-mPer2Luc mice.
RER and EE were recorded by indirect calorimetry. a. The 72-hour recording of the RER in the control db/+-mPer2Luc mice. The light grey box indicates the dark-phase. b. The 72-hour recording of the RER in the db/db-mPer2Luc mice. The light grey box indicates the dark-phase. c. The 24-hour RER. d. The 12-hour RER during the light phase (L) and dark phase (D). e., f., and g.: The amplitude, robustness, and acrophase of the RER daily rhythm. h. The 72-hour recording of the EE in the control db/+-mPer2Luc mice. The light grey box indicates the dark-phase. i. The 72-hour recording of the EE in the diabetic db/db-mPer2Luc mice. The light grey box indicates the dark-phase. j. The 24-hour EE. k. The 12-hour EE during the light phase (L) and dark phase (D). l., m., and n: The amplitude, robustness, and acrophase of the EE daily rhythm. All data were expressed as mean ± SEM (N = 6). *, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant.
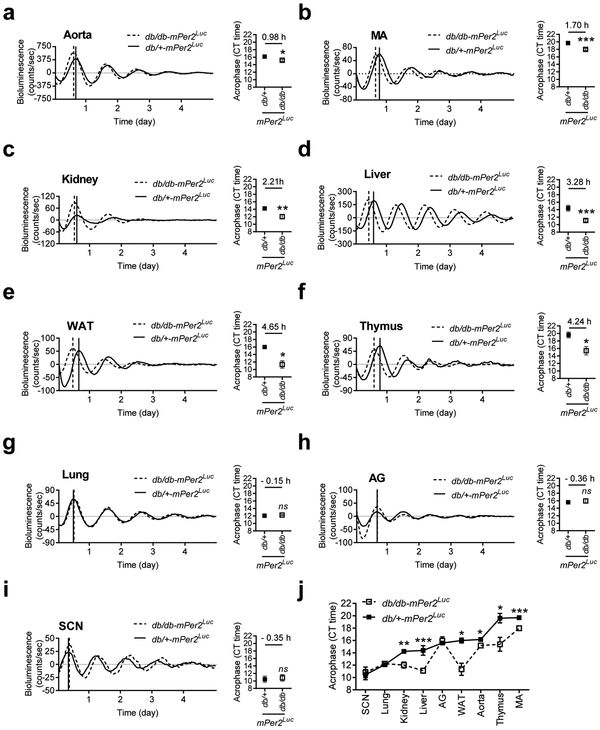
Ex vivo LumiCycle recording reveals that the phases of mPer2 daily oscillation are shifted to different extents in various peripheral tissues but not the SCN from the db/db-mPer2Luc mice
Multiple systems coordinate to maintain the normal physiological BP circadian rhythm (Coffman 2011). To investigate in which tissue the clock genes are altered in the db/db-mPer2Luc mice that may contribute to the compromised BP circadian rhythm, we monitored mPer2 bioluminescence in real-time in peripheral and central SCN tissues in explant organ culture in the db/db-mPer2Luc and control mice. In the various tissues from the control mice, the acrophases of mPer2 oscillation varied but were orchestrated in a specific order (Figure 5a through 5j), with the earliest peak shown by the SCN (10.47 ± 0.82 hours) and later peaks shown by the lung (12.08 ± 0.24 hours), kidney (14.23 ± 0.11 hours), liver (14.39 ± 0.77 hours), adrenal gland (15.59 ± 0.20 hours), white adipose tissue (WAT; 15.59 ± 0.39 hours), aorta (16.17 ± 0.24 hours), thymus (19.61 ± 0.77 hours), and mesenteric arteries (MA; 19.69 ± 0.29 hours).
Fig. 5. The phases of mPer2 protein daily oscillation are desynchronized in various explanted peripheral tissues from the db/db-mPer2Luc mice.
The bioluminescence of mPer2 protein daily oscillation was recorded by LumiCycle in explanted central SCN and peripheral tissues from the db/db-mPer2Luc and control db/+-mPer2Luc mice. The mPer2 oscillation acrophase of the tissues was calculated using the LumiCycle analysis software. In the representative mPer2 bioluminescence real-time recording (left panel), the solid vertical line indicates the acrophase of the non-diabetic db/+-mPer2Luc control mice, whereas the dotted vertical line indicates the acrophase of the diabetic db/db-mPer2Luc mice. In the acrophase (right panel), the number above the symbol indicates the difference of the acrophase between two strains of mice. All data were expressed as mean ± SEM from the aorta (a; N = 7-11), mesentery artery (MA; b; N = 8-12), kidney (c; N = 4-5), liver (d; N = 6-12), white fat tissue (WAT; e; N = 3-4), thymus (f; N = 3-5), lung (g; N = 4-6), adrenal gland (h; N = 3-6), and suprachiasmatic nucleus (I; SCN; N = 6-11). *, P < 0.05; **, P < 0.01, ***, P < 0.001; ns, not significant.
In the tissues from the db/db-mPer2Luc mice, the acrophases of mPer2 oscillations were significantly advanced to different extents relative to the corresponding control in a tissue-specific manner (Figure 5j). The aorta, MA, and kidney, which are crucial for BP and cardiovascular homeostasis, had a 0.98 ± 0.40, 1.70 ± 0.42, and 2.21 ± 0.56 hour phase advance, respectively (Figure 5a to 5c). The liver and WAT, two tissues that are crucial for energy metabolism, had a 3.28 ± 0.77 and 4.65 ± 1.21 hour phase advance (Figure 5d and 5e). The thymus, a primary lymphoid organ, had a 4.24 ± 1.59 hour phase advance (Figure 5f). In contrast, the lung and adrenal gland had no significant phase shift (Figure 5g and 5h). Interestingly, the SCN that has long been believed to be a major regulator of BP circadian rhythm, had also no significant phase shift (Figure 5i). In contrast to the shift in the acrophase in tissues from the db/db-mPer2Luc mice, no consistent change was detected in period and amplitude of mPer2 luciferase oscillations in most peripheral tissues from the db/db-mPer2Luc mice (Table S3).
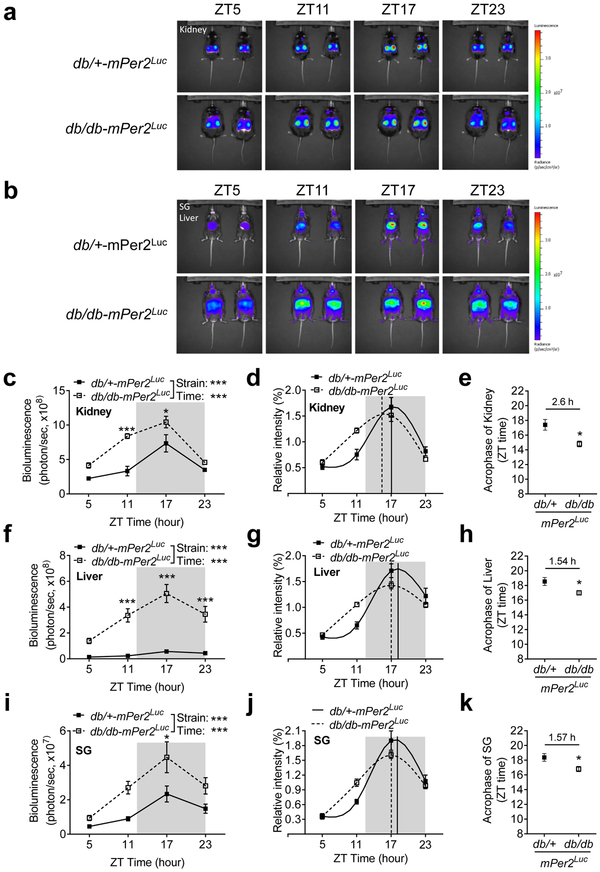
In vivo imaging verifies that the phase of mPer2 oscillation is also advanced in the kidney, liver, and submandibular gland (SG) in the db/db-mPer2Luc mice
To investigate whether the phase advance of the mPer2 oscillation observed in the explant tissue culture represents in vivo tissue oscillation, we used IVIS spectrum and monitored the mPer2 oscillations of the kidney, liver, and SG in the intact db/db-mPer2Luc and control mice. The in vivo mPer2 bioluminescence images were obtained with 6 hours interval at ZT5, ZT11, ZT17, and ZT23, respectively. In accordance with the result from the ex vivo LumiCycle recording (Figure 5c and 5d), the in vivo mPer2 bioluminescence of the kidney, liver, and SG exhibited apparent time-of-day variations. The lowest absolute bioluminescence intensity was detected at ZT5 and the highest absolute bioluminescence intensity was detected at ZT17 in all three tissues (Figure 6a and 6b). The absolute bioluminescence intensities were significantly higher in the db/db-mPer2Luc mice as compared with the control mice at ZT11 and ZT17 in the kidney (Figure 6c), at ZT11, ZT17, and ZT23 in the liver (Figure 6f) and at ZT17 in the SG (Figure 6i).
Fig. 6. The in vivo imaging shows a phase shift in mPer2 oscillation in the kidney, liver, and submandibular gland (SG) in the db/db-mPer2Luc mice.
The in vivo imaging of mPer2 bioluminescence by the IVIS spectrum show a time-of-day variation in the kidney, liver, and SG. a. Representative in vivo imaging of the mPer2 bioluminescence in the kidney in the db/db-mPer2Luc (upper panel) and control mice (lower panel). b. Representative in vivo imaging of the mPer2 bioluminescence in the SG and liver in the db/db-mPer2Luc (upper panel) and control mice (lower panel). The absolute bioluminescence intensity detected in the kidney (c), liver (f), and SG (i). The relative bioluminescence intensity obtained by normalizing to the average of the four-time points’ data in the kidney (d), liver (g), and SG (j). The brown color solid vertical line indicates the acrophase of the control db/+-mPer2Luc mice, whereas the blue dotted vertical line indicates the acrophase of the db/db-mPer2Luc mice. The acrophase of the two strains of mice in the kidney (e), liver (h), and SG (k) where the number above the symbol indicates the difference of the acrophase between the two strains of mice. All data were expressed as mean ± SEM (N = 4-5). *, P < 0.05; ***, P < 0.001.
To better quantify the mPer2 oscillation in all three tissues between the two mouse strains, we normalized the absolute mPer2 bioluminescence intensities to the average of the four ZT time points absolute mPer2 bioluminescence intensities, in accordance with a previous report (Tahara, Kuroda et al. 2012). The resulting analysis revealed that the relative mPer2 bioluminescence signal from the db/db-mPer2Luc mice peaked earlier in all three tissues than those of the control mice (Figure 6d, 6g, and 6j). Moreover, cosinor analysis further illustrated that the phase of the mPer2 oscillation was significantly advanced in all three tissues in the db/db-mPer2Luc mice compared with that in control mice, with 2.60 ± 0.82, 1.54 ± 0.59, and 1.571 ± 0.61 hour advance in the kidney, liver, and SG (Figure 6e, 6h, and 6k), respectively.
The time-of-day variations in gene expressions are altered in the mesenteric arteries from the db/db-mPer2Luc mice
Db/db mice exhibit alterations in the daily mRNA expressions of clock genes and BP regulatory genes as we have previously shown (Su, Xie et al. 2012). In addition, we have demonstrated that smooth muscle BMAL1 participates in the control of the BP daily rhythm by regulating one of the contraction regulatory proteins Rho-kinase 2 (ROCK2) in wild-type mice (Xie, Su et al. 2015). To test whether any putative clock-controlled blood pressure-associated genes are dysregulated in db/db-mPer2Luc mice, we determined mRNA expressions of Bmal1 and several contractile regulatory genes in the MA at ZT5 and ZT17. As shown in Figures S5a through S5e, Bmal1, ROCK1, calponin-1, tropomyosin-2, and smooth muscle protein-22α (SM22α) mRNA expression exhibited a significant time-of-day variation. Importantly, an attenuation or loss of the time-of-day variations was found in the db/db-mPer2Luc mice compared with the control mice. In contrast, no time-of-day variations were detected in ROCK2, calponin-2, calponin-3, and tropomyosin-1 mRNA in either genotype (Figures S5c, e, f, and g).
Discussion
The current study describes a novel type 2 diabetic db/db-mPer2Luc mouse model. The major new findings are: 1) the db/db-mPer2Luc mice are obese, hyperglycemic, and glucose-intolerant and thus resemble type 2 diabetic patients; 2) the db/db-mPer2Luc mice are normotensive but exhibit a compromised BP daily rhythm, which is associated with the disruption of daily rhythms in baroreflex sensitivity, locomotor activity, and metabolism, but not heart rate or food and water intake; 3) a desynchrony of peripheral tissue oscillation is caused by the various extents of phase advances of the mPer2 oscillation ex vivo of many tissues except the central SCN pacemaker; 4) the similar desynchrony of mPer2 phase is also observed in vivo in the kidney, liver, and SG.
The db/db mice have been used extensively for studying the pathogenesis of obesity and diabetes. Interestingly, the diabetic phenotype of db/db mice varies depending on the genetic background. The hyperglycemia is more severe when the leptin receptor mutation is expressed on a C57BL/KsJ background than on a C57BL/6J background (Leiter, Coleman et al. 1981). Probably because of its severe diabetic phenotype, the C57BL/KsJ-db/db mice are most commonly used. Interestingly, the db/db-mPer2Luc mice have a significantly higher body weight than the age-matched C57BL/KsJ-db/db mice (65.72 ± 1.38 g vs. 47.07 ± 1.05 g; N=12; P<0.001). However, the hyperglycemia in the db/db-mPer2Luc mice is much less severe than that in the C57BL/KsJ-db/db mice (320.3 ± 18.46 mg/dl vs. 585.9 ± 9.163 mg/dl; n=12; P<0.001). These results suggest that the db/db-mPer2Luc mice more closely resemble the C57BL/6J-db/db mice (Hummel, Coleman et al. 1972) and mimic diabetic patients with obesity, moderate hyperglycemia, and glucose intolerance.
In agreement with their moderate diabetic phenotypes, the db/db-mPer2Luc mice are normotensive, which contrasts with the hypertensive C57BL/KsJ-db/db mice (Park, Bivona et al. 2008, Su, Guo et al. 2008, Goncalves, Tank et al. 2009, Senador, Kanakamedala et al. 2009). Despite this difference, the db/db-mPer2Luc mice also exhibit non-dipping BP, similar to the C57BL/KsJ-db/db mice (Park, Bivona et al. 2008, Su, Guo et al. 2008, Goncalves, Tank et al. 2009, Senador, Kanakamedala et al. 2009), which is typified by a lack of BP fall during the inactive light phase. Although leptin signaling is implicated in obesity-associated hypertension (Simonds, Pryor et al. 2014), such non-dipping BP in the db/db-mPer2Luc and C57BL/KsJ-db/db mice is unlikely to be mediated directly by the loss of function mutation in the leptin receptor since the disruption of BP circadian rhythm was only detectable in mice older than 11-weeks (Senador, Kanakamedala et al. 2009). The mechanism by which diabetes induces non-dipping BP is unclear. In particular, it is unclear whether hyperglycemia, insulin resistance, or both are responsible for the disrupted BP circadian rhythm. While this important mechanistic issue remains to be elucidated, the current study demonstrates for the first time that the disrupted BP daily rhythm in the db/db-mPer2Luc mice is associated with the loss of the daily rhythm in spontaneous baroreflex sensitivity but not heart rate. Baroreflex is a critical mechanism for maintaining the BP homeostasis, and baroreflex sensitivity exhibit daily variations in humans (Hossmann, Fitzgerald et al. 1980, Di Rienzo, Parati et al. 2001). Interestingly, the observed loss of baroreflex sensitivity daily variation resembles the loss of baroreflex sensitivity daily variation we reported in the smooth muscle Bmal1 knockout mice (Xie, Su et al. 2015), indicating that dysfunction of clock genes in the db/db-mPer2Luc mice may cause loss of baroreflex sensitivity daily variation thus contributes to the decreased nocturnal BP decline phenotype.
We have previously reported that the daily locomotor activity rhythm is lost in the C57BL/KsJ-db/db mice (Su, Guo et al. 2008). In accordance with this finding, the current study illustrated that this locomotor rhythm was similarly abolished in the db/db-mPer2Luc mice (Su, Guo et al. 2008). We speculate that the loss of the locomotor activity rhythm in both strains of db/db mice results from their severe obesity, i.e., that they are too heavy to move around. Although the loss of locomotor activity rhythm may potentially contribute to the loss of the BP daily rhythm [47], the loss of locomotor activity mainly occurred during the night in the db/db-mPer2Luc mice, whereas the loss of BP dipping occurred during the day. Therefore, it is unlikely that the loss of locomotor activity accounts for the disrupted BP daily rhythm in the db/db-mPer2Luc mice.
In humans (van Moorsel, Hansen et al. 2016) and rodents (Oosterman, Foppen et al. 2015, Sun, Wang et al. 2015), RER displays time-of-day variations, with higher values during the active phase indicating the preferential use of carbohydrates and lower values during the inactive phase indicating the preferential use of fats. In the C57BL-KsJ-db/db mice, RER was decreased at one specific time of the day (Osborn, Sanchez-Alavez et al. 2010, Choi, Kim et al. 2015). However, it is surprising that it has not been reported whether the daily rhythm of RER is disrupted in the db/db mice. One of the very intriguing findings from the current study is that the db/db-mPer2Luc mice lost the RER time-of-day variations, mainly due to an increased RER during the inactive light phase as compared to control mice. These results suggest that the flexibility to use different sources of fuel is compromised in the diabetic db/db-mPer2Luc mice. Moreover, there is a temporal correlation between increased RER and decreased BP decline as both occurred during the inactive light phase. However, it is unclear whether the increased RER during the light phase caused the compromised BP dipping in the db/db-mPer2Luc mice.
Accumulated evidence from the animal and human studies during the last decade suggests that the BP circadian rhythm is regulated by multiple organs and systems, including the neuroendocrine system, kidneys, and vasculature (resistance arteries) (Rudic and Fulton 2009). It is long believed that the BP circadian rhythm, just like other physiological and behavioral circadian rhythms, is mostly controlled by the master pacemaker in the SCN. However, the current study demonstrated that the phase of mPer2 protein daily oscillation was not significantly altered in the SCN tissue from the db/db-mPer2Luc mice compared to controls. These results confirm previous reports that there is a little or no change of the SCN mPer2 mRNA daily oscillation in the C57BL-KsJ-db/db mice (Kudo, Akiyama et al. 2004, Nernpermpisooth, Qiu et al. 2015, Grosbellet, Dumont et al. 2016). These results are also consistent with previous reports that peripheral clock gene oscillations are altered in some tissues from diabetic patients (Ando, Takamura et al. 2009, Pappa, Gazouli et al. 2013) and db/db mice (Kudo, Akiyama et al. 2004, Caton, Kieswich et al. 2011, Su, Xie et al. 2012, Nernpermpisooth, Qiu et al. 2015). In addition, in db/db mice, the alternations of peripheral clock expression occur as early as 6-8 weeks of age- (Kudo, Akiyama et al. 2004, Caton, Kieswich et al. 2011) whereas the disruption of BP circadian rhythm is not detectable in db/db mice until 11-weeks or older, indicating that peripheral clock impairment precedes the disruption of the BP circadian rhythm. Taken together, these results suggest that the peripheral oscillators, in contrast to the master SCN pacemaker, are strongly affected by diabetes and may be responsible for the disruption of BP circadian rhythm.
Perhaps one of the most important findings from the current study is that the phase of the mPer2 protein daily oscillation was advanced into various extents in a tissue-specific manner in peripheral tissues, in the absence of any change in the phase in the SCN. This finding was revealed by monitoring mPer2 protein oscillation in real-time in our novel db/db-mPer2Luc mice. , In agreement with the important role of BMAL1 in the renal, smooth muscle, and fat tissues in regulation of BP rhythm under physiological conditions (Tokonami, Mordasini et al. 2014, Xie, Su et al. 2015, Chang, Xiong et al. 2018), we found that the phase of mPer2 protein oscillation was advanced in the WAT, kidney, MA, and aorta from the db/db-mPer2Luc mice to 4.6, 2.21, 1.71, and 0.99 hours, respectively. These results are also consistent with the previous studies reporting mPer2 mRNA daily oscillation was altered in these tissues from the C57BL-KsJ-db/db mice (Su, Guo et al. 2008, Caton, Kieswich et al. 2011, Su, Xie et al. 2012, Nernpermpisooth, Qiu et al. 2015). In contrast, it was surprising that the phase of mPer2 protein daily oscillation in the adrenal gland, an important source of hormones that regulate the BP circadian rhythm, was not significantly changed in the C57BL-KsJ-db/db mice relative to control mice. It was also surprising that the phase of mPer2 protein daily oscillation in the thymus, an important organ that produces T lymphocytes, was advanced up to 4.23 hours. This result is consistent with the recent report that T lymphocytes play a critical role in angiotensin II-induced hypertension (Guzik, Hoch et al. 2007), and suggests that clock genes in T lymphocytes may be crucially involved in the disruption of the BP circadian rhythm in diabetes.
Obesity and diabetes in mice can be induced by a high fat (HF) diet, which also altered activity, feeding, and molecular circadian rhythms (Kohsaka, Laposky et al. 2007, Hatori, Vollmers et al. 2012). Although the causes of obesity and diabetes in HF diet-fed mice and db/db-mPer2Luc mice are different, it is interesting to note that there are some similarities in respect to the effects of HF diet and leptin receptor mutation (db/db mice) on the mPer2 rhythm. For example, using ex vivo bioluminescent analyses, Pendergast et al. demonstrated that HF diet-fed mice exhibit a 1-4 hour phase advances in the mPer2 rhythms of two organs but not the SCN (Pendergast, Branecky et al. 2013). Using the same ex vivo assay, the current study obtained the similar results in the db/db-mPer2Luc mice, suggesting that the disruption of peripheral clocks in the absence of a change in the central pacemaker by obesity or diabetes is not model-specific. In addition, a ~ 4-hour phase advance of the mPer2 oscillation in liver explants without a phase shift in explants of lung is observed in HF-fed mice (Pendergast, Branecky et al. 2013, Branecky, Niswender et al. 2015). The current study also obtained the similar results in the db/db-mPer2Luc mice, suggesting that different peripheral tissues have different sensitivity to obesity and diabetes. Despite these similarities, it should be pointed out that there were some differences in respect to the effects of HF diet and leptin receptor mutation (db/db mice) on the mPer2 rhythm. For example, the mPer2 rhythm in the aorta explants showed a ~ 1-hour phase advance in the db/db-mPer2Luc mice, but no change in the HF diet-fed mice (Pendergast, Branecky et al. 2013).
Another interesting finding of the current study is that the mPer2 phase shifts observed in vitro from tissues explanted from the db/db-mPer2Luc mouse reflected phase shifts observed in vivo. This raises the question whether the mPer2 phase shifts in the db/db-mPer2Luc mice might be caused directly by leptin receptor mutation within cells or indirectly by hyperphagia, obesity, and diabetes. Although it is currently uncertain, there is some evidence to support all possibilities. First, leptin is an adipocyte-derived hormone that binds to the leptin receptor and promotes weight loss by reducing appetite and food intake and by increasing energy expenditure (Kelesidis, Kelesidis et al. 2010). Serum leptin levels display diurnal variations in both humans and rodents. There is also evidence that leptin can directly regulate clock gene oscillations. For example, leptin can phase advance the electrical activity rhythm in the rat SCN in vitro (Prosser and Bergeron 2003). Moreover, leptin is implicated in the regulation of hypertension in obesity (Simonds, Pryor et al. 2014). Thus, leptin receptor mutation in various tissues may directly advance the mPer2 oscillations in vivo. Second, consistent with a previous report (Ktorza, Bernard et al. 1997), the current study demonstrated that the db/db-mPer2Luc mice consumed more food and water than the control mice. Although food intake pattern was not altered in the db/db-mPer2Luc mice, it is possible that the increased food intake due to impaired leptin signaling in the db/db-mPer2Luc mice alters circadian rhythms. In fact, evidence that increased food intake affects behavioral, metabolism, and molecular circadian rhythms has been demonstrated in HF diet-fed mice (Kohsaka, Laposky et al. 2007, Hatori, Vollmers et al. 2012, Pendergast, Branecky et al. 2013, Branecky, Niswender et al. 2015) and db/db mice (Kennedy, Ellacott et al. 2010). Thus, hyperphagia may mediate leptin receptor mutation-associated phase advance of the mPer2 oscillation in vivo. Third, the current study demonstrated hyperglycemia, hyperinsulinemia, and glucose intolerance in the db/db-mPer2Luc mice. Since both glucose and insulin have been shown to alter clock gene expression rhythms in vitro and in vivo (Hirota, Okano et al. 2002, Dang, Sun et al. 2016), it is likely that obesity and diabetes resulting from leptin receptor mutation may also have an indirect effect on mPer2 phase advances in these tissues in vivo. Nevertheless, future studies are required to distinguish these potential mechanisms.
In conclusion, the current study described a novel diabetic db/db-mPer2Luc mouse model that allows real-time measurement of diabetes-induced dysregulation of clock genes and disruption of the BP circadian rhythm. Using this novel db/db-mPer2Luc mouse model, we have revealed that disruption of the BP circadian rhythm in diabetes is associated with the loss of daily rhythms in baroreflex sensitivity, locomotor activity, metabolism, and a tissue-specific phase advance of the peripheral oscillators but not the central SCN pacemaker. These findings provide preclinical evidence for a potentially significant role of peripheral clock gene desynchrony in linking diabetes to compromised BP and metabolism circadian rhythms. The results from the current study may lead to the identification of synchronizing various oscillators as a novel therapeutic strategy against diabetic cardiovascular complications and thus improve the prognosis of diabetic patients.
Supplementary Material
Acknowledgments
The authors thank Mrs. Ming Zhang for the animal breeding, Dr. Wendy Katz for assistance with the indirect calorimetry measurements, and Dr. Marilyn Duncan and Mr. Mark Schwarcz for editing the manuscript. This work was supported by the US NIH Grants HL125228 and HL106843 (to M.C.G. and Z.G.), the US Department of Veteran Affairs (VA Merit Award to Z.G.), and the Institutional Development Award (IDeA) from the US National Institute of General Medical Sciences of NIH, under grant number P20GM103527.
Footnotes
Conflict of Interest Statement
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Ando H, Takamura T, Matsuzawa-Nagata N, Shima K, Eto T, Misu H, Shiramoto M, Tsuru T, Irie S and Fujimura A (2009). "Clock gene expression in peripheral leucocytes of patients with type 2 diabetes." Diabetologia 52(2): 329–335. [DOI] [PubMed] [Google Scholar]
- Ayala DE, Moya A, Crespo JJ, Castineira C, Dominguez-Sardina M, Gomara S, Sineiro E, Mojon A, Fontao MJ, Hermida RC and I. Hygia Project (2013). "Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes." Chronobiol Int 30(1-2): 99–115. [DOI] [PubMed] [Google Scholar]
- Bass J and Takahashi JS (2010). "Circadian integration of metabolism and energetics." Science 330(6009): 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branecky KL, Niswender KD and Pendergast JS (2015). "Disruption of Daily Rhythms by High-Fat Diet Is Reversible." PLoS One 10(9): e0137970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton PW, Kieswich J, Yaqoob MM, Holness MJ and Sugden MC (2011). "Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice." Diabetes Obes Metab 13(12): 1097–1104. [DOI] [PubMed] [Google Scholar]
- Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD and Chen YE (2018). "Bmal1 in Perivascular Adipose Tissue Regulates Resting Phase Blood Pressure Through Transcriptional Regulation of Angiotensinogen." Circulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI and Morgenstern JP (1996). "Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice." Cell 84(3): 491–495. [DOI] [PubMed] [Google Scholar]
- Choi HM, Kim HR, Kim EK, Byun YS, Won YS, Yoon WK, Kim HC, Kang JG and Nam KH (2015). "An age-dependent alteration of the respiratory exchange ratio in the db/db mouse." Lab Anim Res 31(1): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman TM (2011). "Under pressure: the search for the essential mechanisms of hypertension." Nat Med 17(11): 1402–1409. [DOI] [PubMed] [Google Scholar]
- Cohen MC, Rohtla KM, Lavery CE, Muller JE and Mittleman MA (1997). "Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death." Am J Cardiol 79(11): 1512–1516. [DOI] [PubMed] [Google Scholar]
- Collaboration, N. R. F. (2016). "Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4· 4 million participants." The Lancet 387(10027): 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corella D, Asensio EM, Coltell O, Sorli JV, Estruch R, Martinez-Gonzalez MA, Salas-Salvado J, Castaner O, Aros F, Lapetra J, Serra-Majem L, Gomez-Gracia E, Ortega-Azorin C, Fiol M, Espino JD, Diaz-Lopez A, Fito M, Ros E and Ordovas JM (2016). "CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial." Cardiovasc Diabetol 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS and Fitzgerald GA (2007). "Circadian variation of blood pressure and the vascular response to asynchronous stress." Proc Natl Acad Sci U S A 104(9): 3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi C, Vaccarella A, Leonetti G and Sala C (2010). "Ambulatory blood pressure and diabetes: targeting nondipping." Current diabetes reviews 6(2): 111–115. [DOI] [PubMed] [Google Scholar]
- Dang F, Sun X, Ma X, Wu R, Zhang D, Chen Y, Xu Q, Wu Y and Liu Y (2016). "Insulin post-transcriptionally modulates Bmal1 protein to affect the hepatic circadian clock." Nat Commun 7: 12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G and Pedotti A (2001). "Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life." American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 280(3): R744–R751. [DOI] [PubMed] [Google Scholar]
- Eguchi K (2011). "Ambulatory blood pressure monitoring in diabetes and obesity—a review." International journal of hypertension 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even PC and Nadkarni NA (2012). "Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives." Am J Physiol Regul Integr Comp Physiol 303(5): R459–476. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A and Nelson RJ (2010). "Light at night increases body mass by shifting the time of food intake." Proc Natl Acad Sci U S A 107(43): 18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman O and Logan AG (2009). "Can nocturnal hypertension predict cardiovascular risk?" Integr Blood Press Control 2: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, Li L, Cao S, Dong X, Gong Y, Shi O, Deng J, Bi H and Lu Z (2015). "Shift work and diabetes mellitus: a meta-analysis of observational studies." Occup Environ Med 72(1): 72–78. [DOI] [PubMed] [Google Scholar]
- Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J and Gross V (2009). "Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction." Hypertension 53(2): 387–392. [DOI] [PubMed] [Google Scholar]
- Gorostidi M, Sobrino J, Segura J, Sierra C, de la Sierra A, Hernandez del Rey R, Vinyoles E, Galceran JM, Lopez-Eady MD, Marin R, Banegas JR, Sarria A, Coca A, Ruilope LM and A. R. i. Spanish Society of Hypertension (2007). "Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross-sectional analysis of a 20,000-patient database in Spain." J Hypertens 25(5): 977–984. [DOI] [PubMed] [Google Scholar]
- Grosbellet E, Dumont S, Schuster-Klein C, Guardiola-Lemaitre B, Pevet P, Criscuolo F and Challet E (2016). "Circadian phenotyping of obese and diabetic db/db mice." Biochimie 124: 198–206. [DOI] [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Daugherty A, Smart E and Gong MC (2005). "COX-2 up-regulation and vascular smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice." Cardiovasc Res 67(4): 723–735. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG (2007). "Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction." J Exp Med 204(10): 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH and Panda S (2012). "Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet." Cell Metab 15(6): 848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg L and Coleman DL (1977). "Laboratory animals exhibiting obesity and diabetes syndromes." Metabolism 26(1): 59–99. [DOI] [PubMed] [Google Scholar]
- Hirota T, Okano T, Kokame K, Shirotani-Ikejima H, Miyata T and Fukada Y (2002). "Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts." J Biol Chem 277(46): 44244–44251. [DOI] [PubMed] [Google Scholar]
- Hossmann V, Fitzgerald GA and Dollery CT (1980). "Circadian rhythm of baroreflex reactivity and adrenergic vascular response." Cardiovasc Res 14(3): 125–129. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Coleman DL and Lane PW (1972). "The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains." Biochem Genet 7(1): 1–13. [DOI] [PubMed] [Google Scholar]
- Jacobi D, Liu S, Burkewitz K, Kory N, Knudsen NH, Alexander RK, Unluturk U, Li X, Kong X and Hyde AL (2015). "Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness." Cell metabolism 22(4): 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelesidis T, Kelesidis I, Chou S and Mantzoros CS (2010). "Narrative review: the role of leptin in human physiology: emerging clinical applications." Ann Intern Med 152(2): 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AJ, Ellacott KL, King VL and Hasty AH (2010). "Mouse models of the metabolic syndrome." Dis Model Mech 3(3–4): 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettner NM, Mayo SA, Hua J, Lee C, Moore DD and Fu L (2015). "Circadian Dysfunction Induces Leptin Resistance in Mice." Cell Metab 22(3): 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW and Bass J (2007). "High-fat diet disrupts behavioral and molecular circadian rhythms in mice." Cell Metab 6(5): 414–421. [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV and Antoch MP (2006). "Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock." Genes & development 20(14): 1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktorza A, Bernard C, Parent V, Penicaud L, Froguel P, Lathrop M and Gauguier D (1997). "Are animal models of diabetes relevant to the study of the genetics of non-insulin-dependent diabetes in humans?" Diabetes Metab 23 Suppl 2: 38–46. [PubMed] [Google Scholar]
- Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T and Shibata S (2004). "Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver." Diabetologia 47(8): 1425–1436. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Storch K-F and Weitz CJ (2008). "Physiological significance of a peripheral tissue circadian clock." Proceedings of the national academy of sciences 105(39): 15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI and Friedman JM (1996). "Abnormal splicing of the leptin receptor in diabetic mice." Nature 379(6566): 632–635. [DOI] [PubMed] [Google Scholar]
- Leiter EH, Coleman DL and Hummel KP (1981). "The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. III. Effect of H-2 haplotype and sex." Diabetes 30(12): 1029–1034. [DOI] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S and Vitaterna MH (2010). "Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes." Nature 466(7306): 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar-Craig M, Bishop C and Raftery E (1978). "Circadian variation of blood-pressure." The Lancet 311(8068): 795–797. [DOI] [PubMed] [Google Scholar]
- Nernpermpisooth N, Qiu S, Mintz JD, Suvitayavat W, Thirawarapan S, Rudic DR, Fulton DJ and Stepp DW (2015). "Obesity alters the peripheral circadian clock in the aorta and microcirculation." Microcirculation 22(4): 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nernpermpisooth N, Qiu S, Mintz JD, Suvitayavat W, Thirawarapan S, Rudic DR, Fulton DJ and Stepp DW (2015). "Obesity alters the peripheral circadian clock in the aorta and microcirculation." Microcirculation 22(4): 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike H, Sakurai M, Ippoushi K and Kobori M (2015). "Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work." Biochem Biophys Res Commun 465(3): 556–561. [DOI] [PubMed] [Google Scholar]
- Oosterman JE, Foppen E, van der Spek R, Fliers E, Kalsbeek A and la Fleur SE (2015). "Timing of fat and liquid sugar intake alters substrate oxidation and food efficiency in male Wistar rats." Chronobiol Int 32(2): 289–298. [DOI] [PubMed] [Google Scholar]
- Osborn O, Sanchez-Alavez M, Brownell SE, Ross B, Klaus J, Dubins J, Beutler B, Conti B and Bartfai T (2010). "Metabolic characterization of a mouse deficient in all known leptin receptor isoforms." Cell Mol Neurobiol 30(1): 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa KI, Gazouli M, Anastasiou E, Iliodromiti Z, Antsaklis A and Anagnou NP (2013). "Circadian clock gene expression is impaired in gestational diabetes mellitus." Gynecological Endocrinology 29(4): 331–335. [DOI] [PubMed] [Google Scholar]
- Park S, Bivona BJ, Feng Y, Lazartigues E and Harrison-Bernard LM (2008). "Intact renal afferent arteriolar autoregulatory responsiveness in db/db mice." Am J Physiol Renal Physiol 295(5): F1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD and Yamazaki S (2013). "High-fat diet acutely affects circadian organisation and eating behavior." Eur J Neurosci 37(8): 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA and Bergeron HE (2003). "Leptin phase-advances the rat suprachiasmatic circadian clock in vitro." Neurosci Lett 336(3): 139–142. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C and King GL (2013). "Vascular complications of diabetes: mechanisms of injury and protective factors." Cell Metab 17(1): 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R, Lissen GC and Halberg F (2007). "Procedures for numerical analysis of circadian rhythms." Biol Rhythm Res 38(4): 275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reule S and Drawz PE (2012). "Heart rate and blood pressure: any possible implications for management of hypertension?" Curr Hypertens Rep 14(6): 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge FS, McFetridge-Durdle JA, Dean CR and Canadian Hypertension S (2007). "Night-time blood pressure patterns and target organ damage: a review." Can J Cardiol 23(2): 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD and Fulton DJ (2009). "Pressed for time: the circadian clock and hypertension." J Appl Physiol 107(4): 1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis A-M, Boston RC, Panda S, Hogenesch JB and FitzGerald GA (2004). "BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis." PLoS biology 2(11): e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senador D, Kanakamedala K, Irigoyen MC, Morris M and Elased KM (2009). "Cardiovascular and autonomic phenotype of db/db diabetic mice." Exp Physiol 94(6): 648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG Jr., Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS and Cowley MA (2014). "Leptin mediates the increase in blood pressure associated with obesity." Cell 159(6): 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Guo Z, Randall DC, Cassis L, Brown DR and Gong MC (2008). "Hypertension and disrupted blood pressure circadian rhythm in Type 2 diabetic db/db mice." Am J Physiol Heart Circ Physiol 295(4): H1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Xie Z, Guo Z, Duncan MJ, Lutshumba J and Gong MC (2012). "Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice." Am J Physiol Heart Circ Physiol 302(3): H621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Xie Z, Liu S, Calderon LE, Guo Z and Gong MC (2013). "Smooth muscle-selective CPI-17 expression increases vascular smooth muscle contraction and blood pressure." Am J Physiol Heart Circ Physiol 305(1): H104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang Y, Song Y, Cheng XR, Xia S, Rahman MR, Shi Y and Le G (2015). "Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice." Biochem Biophys Res Commun 458(1): 86–91. [DOI] [PubMed] [Google Scholar]
- Tahara Y, Kuroda H, Saito K, Nakajima Y, Kubo Y, Ohnishi N, Seo Y, Otsuka M, Fuse Y, Ohura Y, Komatsu T, Moriya Y, Okada S, Furutani N, Hirao A, Horikawa K, Kudo T and Shibata S (2012). "In vivo monitoring of peripheral circadian clocks in the mouse." Curr Biol 22(11): 1029–1034. [DOI] [PubMed] [Google Scholar]
- Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez ML and Firsov D (2014). "Local renal circadian clocks control fluid-electrolyte homeostasis and BP." J Am Soc Nephrol 25(7): 1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H, Katsuura-Kamano S, Yamaguchi M, Arisawa K, Hamajima N, Hishida A, Kawai S, Oze I, Shinchi K, Takashima N, Suzuki S, Nakahata N, Mikami H, Ohnaka K, Kuriyama N, Kubo M, Tanaka H and Japan G Multi-Institutional Collaborative Cohort Study (2016). "Variant of the clock circadian regulator (CLOCK) gene and related haplotypes are associated with the prevalence of type 2 diabetes in the Japanese population." J Diabetes 8(5): 667–676. [DOI] [PubMed] [Google Scholar]
- van Moorsel D, Hansen J, Havekes B, Scheer FA, Jorgensen JA, Hoeks J, Schrauwen-Hinderling VB, Duez H, Lefebvre P, Schaper NC, Hesselink MK, Staels B and Schrauwen P (2016). "Demonstration of a day-night rhythm in human skeletal muscle oxidative capacity." Mol Metab 5(8): 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M and Gauguier D (2007). "Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes." Proc Natl Acad Sci U S A 104(36): 14412–14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z and Gong MC (2015). "Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation." J Clin Invest 125(1): 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S and Takahashi JS (2005). "Real-time luminescence reporting of circadian gene expression in mammals." Methods Enzymol 393: 288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G and FitzGerald GA (2016). "Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival." Sci Transl Med 8(324): 324ra316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano Y and Kario K (2012). "Nocturnal blood pressure and cardiovascular disease: a review of recent advances." Hypertens Res 35(7): 695–701. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M and Takahashi JS (2004). "PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues." Proc Natl Acad Sci U S A 101(15): 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.